Introduction
Glioma is a common type of primary tumor in the
central nervous system. It includes predominantly astrocytic,
oligodendroglial, ependymal, neuronal and mixed neuronal-glial
tumors. As a grade IV glioma classified by the World Health
Organization, glioblastoma multiforme is considered the most
malignant and invasive subtype with a median survival time of 14.6
months even after a three-pronged approach comprised of surgical
resection, radiation and chemotherapy (1). In fact, approximately 95% of patients
suffering from glioblastoma succumb to this disease within 5 years
after initial diagnosis (2).
Therefore, seeking novel strategies to treat glioblastoma appears
to be crucial.
In recent years, the use of phytochemicals as
antitumor agents has received increasing attention. A number of
plant-derived compounds such as puerarin, gingerol and silibinin
have been reported to have anticarcinogenic effects (3–5).
Ellagic acid (EA), as a type of natural dietary polyphenol
compound, exists in several fruits and plants such as walnuts,
blackberries, Pericarpium granati, cranberries, raspberries,
strawberries and grapes (6). As a
dimeric derivative of gallic acid, EA (its chemical structural
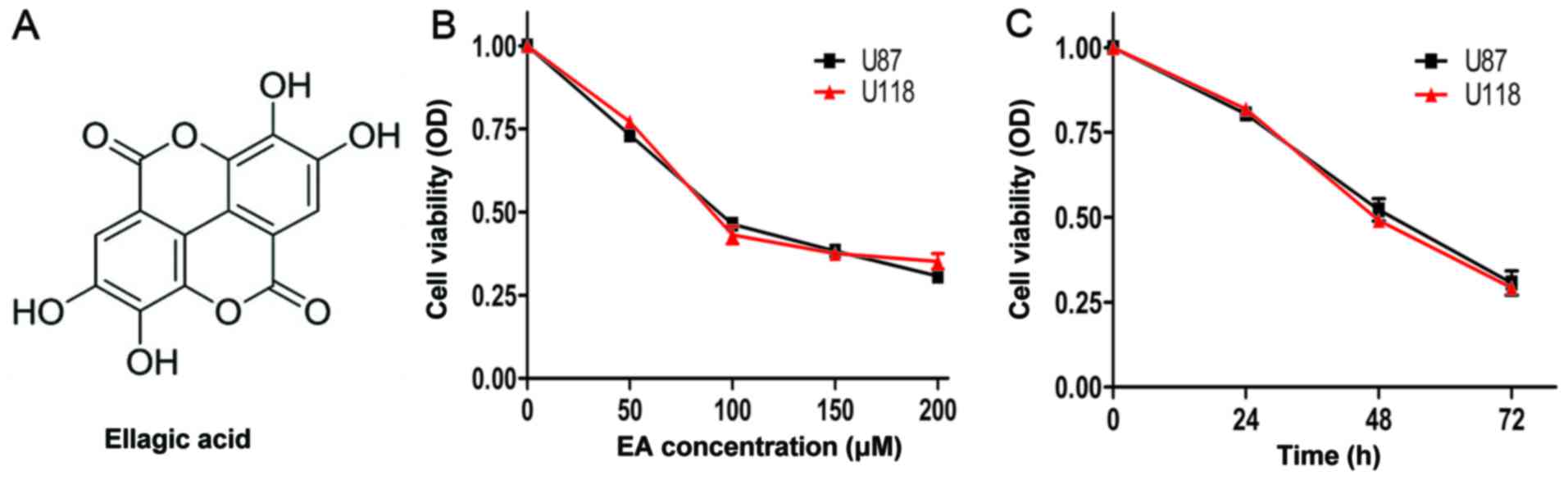
formula is shown in Fig. 1A) has
been found to have a variety of pharmacological properties, such as
antioxidant, antifibrosis, chemoprevention and anticarcinogenic
(7–9). The crude extract of EA from
Pericarpium granati has been widely applied in cooking and
clinically in traditional Chinese medicine for over two thousand
years. The anticarcinogenic effect of EA has also been revealed in
several types of tumors (10–15),
while the effect of EA on glioblastoma has rarely been reported.
Here, in the present study, we aimed to observe the
anti-glioblastoma effect of EA and investigate its molecular
mechanisms in both glioblastoma cell lines and glioblastoma
xenografts in Balb/c nude mice.
The PI3K/Akt signaling pathway plays an important
part in cell survival and proliferation, and it is usually
activated eccentrically in the later stages of human glioblastoma.
The dysregulation of the PI3K/Akt signaling axis, as well as PTEN,
in both glioblastoma initiation and progression, has illustrated
the signaling mechanisms which cause the development of
glioblastoma. What is more, Guo et al revealed that
resveratrol could suppress glioblastoma carcinogenesis in
vitro and in vivo (16).
As a highly conserved pathway throughout evolution,
the Notch signaling pathway plays a major role in tumor cell
survival, differentiation, and proliferation (17). In malignant tumors, the Notch
signaling pathway has been demonstrated to function with both
oncogenic and anticarcinogenic effects according to different cell
types (18). An increasing amount
of evidence has shown the importance of the modified Notch
signaling pathway in the growth, apoptosis and differentiation in
several malignant tumors including glioblastoma. The Notch1
signaling pathway plays critical roles in glioma formation by
facilitating exocrine lineage development and neural progenitor
cell self-renewal in in vivo experiments. Moreover, it has
been confirmed that the Notch signaling pathway is upregulated in
glioblastoma.
The cell cycle and apoptosis play an important part
in tumorigenesis. B-cell lymphoma 2 (Bcl-2) family members play a
dominant role in the process of cell apoptosis. During tumor
growth, cell cycle progression is enhanced. Signaling pathways, to
a certain extent, regulate cells to proliferate or remain
quiescent, which may link cell environment information to specific
stages of the cell cycle. The combination of cyclin-dependent
kinase (CDK) catalytic subunits and cyclin partners or CDK
inhibitors could regulate the activity of CDK. As a result, cell
proliferation is influenced by antiproliferative and mitogenic
signals through ubiquitin-dependent degeneration of cyclins or CDK
inhibitors and transcriptional regulation. Thus, identifying
molecular targets to promote suppression of the the cell cycle in
cancer cells may be an effective anticarcinogenic method.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) and
phosphate-buffered saline (PBS) were purchased from Genom Co., Ltd.
(Hangzhou, China). Fetal bovine serum (FBS) was obtained from
Tianhang Biotechnology Co., Ltd. (Deqing, China). EA was purchased
from Cayman Chemical Co. (Ann Arbor, MI, USA). DMSO was acquired
from MP Biomedicals (Solon, OH, USA). The Cell Counting Kit-8
(CCK-8) was obtained from Dojindo China Co., Ltd. (Beijing, China).
The Cell-Light EdU DNA cell proliferation kit was purchased from
RiboBio Co., Ltd. (Guangzhou, China). A bicinchoninic acid (BCA)
kit for protein determination, a cell cycle and apoptosis analysis
kit and Hoechst 33342 solution were obtained from Beyotime
Institute of Biotechnology (Jiangsu, China). Antibodies against
Bcl-2, cyclin D1, phosphorylated histone H2AX (γ-H2AX),
proliferating cell nuclear antigen (PCNA), Ki67, CDK2, CDK6,
E-cadherin, Akt, Snail, Notch1, matrix metalloproteinase (MMP)-2,
β-actin and MMP-9 were obtained from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Alex Fluor 680/790 labeled goat
anti-rabbit/mouse IgG was purchased from LI-COR Biosciences
(Lincoln, NE, USA). Unless otherwise stated, all other materials
were purchased from AntGene Biotechnology Co., Ltd. (Wuhan,
China).
Cell lines and animals
The human glioblastoma U87 and U118 cell lines were
obtained from the Shanghai Cell Bank, Chinese Academy of Sciences
(Shanghai, China). Balb/c nude mice aged 5–6 weeks were purchased
from the Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China).
All experimental procedures involving animals in our study were
performed in accordance with the guidelines of the Animal
Experiment Center of Wuhan University and approved by the Animal
Care and Use Ethics Committee of Wuhan University. All experimental
animals were housed in cages and maintained at a humidity of 50±5%
and a temperature of 27±1°C in a controlled animal facility under
diurnal lighting conditions (12-h light/dark cycle) with free
access to food and water.
Cell culture and xenografted
models
The U87 and U118 cell lines were grown and
maintained in DMEM supplemented with 10% FBS and 1% antibiotic
mixture (penicillin and streptomycin) in a constant temperature
incubator set at 37°C abounding with 5% CO2. The culture
medium was changed every 3 days. The cells were trypsinized when
they reached ~90% confluency. In order to examine the
anticarcinogenic potential of EA, a U87 MG xenograft model was
constructed. The cells were seeded in a cell culture dish and were
trypsinized when the cells reached a logarithmic growth period.
Then a cell suspension with serum-free medium at a density of
2×107 cells/ml was prepared. Subsequently,
~2×106 cells were injected subcutaneously into the
flanks of Balb/c nude mice as approved in the protocol. The mice
were randomly divided into two groups, each group containing 6
mice. The experimental group was treated with EA (40 µg/g body
weight) through gavage, from Monday to Friday for 4 weeks, once
daily. The control group was treated with normal saline by gavage
in the same way. When the experiment finished, all mice were
euthanized and tumor xenografts were dissected for final volume and
wet weight and immunohistochemistry (IHC) and western blot analysis
(WB) were performed.
Antitumor activity in vitro
CCK-8 test
The effect of EA on the cell viability of human
glioblastoma U87 and U118 cells was assessed by CCK-8 assay.
According to instructions, the cells were seeded in a 96-well plate
at a density of 0.8×104 cells/well, incubated for 12 h
and treated with various concentrations of EA (0–200 µM) for 24, 48
and 72 h, separately. At designated time-points (24, 48 and 72 h),
100 µl of EA-free medium containing 5 µl CCK-8 solution was added
into each well and incubation followed for 1 h. Then the absorbance
of the cells at 450 nm was detected on a Multiskan spectrum
microplate spectrophotometer. The experiment was repeated for three
wells in each group.
EdU staining
The procedure was performed following the product
specifications. U87 and U118 cells were seeded in a 96-well plate
and subsequently treated with or without EA for 48 h. All cells
were exposed to 10 µM 5-ethynyl-2-deoxyuridine (EdU) for 12 h in an
incubator at 37°C and then washed for 5 min twice with PBS. After
being fixed with 4% paraformaldehyde for 30 min at room
temperature, the cells were treated with 0.5% Triton X-100 for 10
min and rinsed with PBS for 5 min. Thenceforth, the cells were
treated with 100 µl of 1X Apollo® Reaction Cocktail for
30 min in the dark at room temperature. Then the cells were treated
with 0.5% Triton X-100 for 20 min and rinsed with PBS for 5 min.
Subsequently, the cells were incubated for 30 min with 1X Hoechst
33342 in order to dye the cell nuclei. The images of EdU and
Hoechst fluorescence in the cells were captured using an Olympus
fluorescence microscope (Olympus, Tokyo, Japan). Five random fields
of each well were observed. The number of EdU and Hoechst-positive
cells were counted using ImageJ software and the EdU labeling index
was calculated.
Cell cycle distribution is assessed by flow
cytometry
The effect of EA on the glioblastoma cell cycle
distribution was assessed using a cell cycle and apoptosis analysis
kit following the manufacturer's instructions. In brief, the U87
and U118 cells were treated with or without EA for 48 h. Then
~1×106 cells were trypsinized, collected by
centrifugation at 1,000 rpm for 5 min and washed using ice cold
PBS. After being fixed with ice cold 70% ethanol for 12 h at 4°C,
the cells were collected by centrifugation at 100 rpm for 5 min and
then washed with ice cold PBS. Subsequently, 0.5 ml of propidium
iodide (PI) staining solution was added and incubation followed at
37°C in the dark for 30 min. The cell cycle distribution was
analyzed on a flow cytometer (BD Biosciences, San Diego, CA,
USA).
Histone H2AX antibody detects nuclear H2AX
protein by immunofluorescent analysis
The U87 and U118 cell lines were trypsinized and
seeded in 6-well culture plates on coverslips and incubated for 12
h and then treated with EA or without for 48 h. The cells growing
on the coverslips were fixed in 4% formaldehyde in PBS for 30 min
at room temperature. For detection of γ-H2AX foci, the coverslips
were incubated with anti-γ-H2AX rabbit monoclonal antibody
overnight at 4°C. After being washed twice with PBS, the cells were
incubated with fluorescein isothiocyanate-labeled goat anti-rabbit
secondary antibody for 1 h, then washed twice with PBS. The nuclei
were counterstained with Hoechst 33342 in PBS for 30 min before
coverslips were covered by anti-fade solution and finally observed
under the Olympus fluorescence microscope. It was surmised that the
positive foci were those that were clear with easily diacritical
dots of certain brightness.
Matrigel invasion assay
Transwell filters with 8-µm pores (BD Biosciences)
were placed into 24-well culture plates. The bottom surface of the
filters was coated with 50 µl of Matrigel diluted in PBS and dried
at 37°C for 1 h. The bottom surface of the filters was then washed
with migration assay buffer (DMEM supplemented with 0.1% fatty-acid
free bovine serum albumin). Approximately 500 µl of migration assay
buffer was placed at the bottom of each filter, and 60,000 cells
suspended in migration assay buffer were placed on top of the
filters. Cells were allowed to adhere for 0.5 h before drug
treatment. Cells were then allowed to migrate for an additional
23.5 h in the cell incubator. To stop migration, filters were fixed
and stained with crystal violet. The top of the filters was wiped
clean before imaging on an Olympus fluorescence microscope. The
cell invasion was quantified by counting the number of cells per
field in five random fields.
Antitumor activity in vivo
IHC
Briefly, at the end of the animal experiment,
xenograft tissues were collected and fixed with 10% formalin, then
embedded in paraffin and sectioned. Antigens were retrieved using a
routine antigen retrieval method and washed for 5 min twice with
PBS. Ultra V Block was added to the tissues, incubated for 5 min at
room temperature and washed twice again with PBS. Then, the
sections were incubated with different primary antibodies (CDK6,
PCNA, CDK2, Ki-67, MMP-9, MMP-2, Bcl-2, cyclin D1, Snail, Akt,
Notch1 and E-cadherin) for 2 h at 37°C in a humidified chamber.
After being washed with PBS twice, a secondary antibody was added
and the sections were incubated at room temperature for 0.5 h.
Subsequently, each section was washed for 5 min with PBS thrice and
alkaline phosphatase working solution was added to the sections
followed by incubation for 20 min at 37°C according to
manufacturer's instructions. Finally, the sections were
coverslipped and imaged.
WB
Xenograft tissue lysates were subjected to SDS-PAGE,
and then gels were blotted on polyvinylidene difluoride membranes
(Millipore Corp., Billerica, MA, USA). The blots were blocked using
TBS blocking solution containing 5% powdered milk at room
temperature by shaking for 2 h and then the membranes were
incubated with a primary antibody diluted according to instructions
at 4°C overnight on a shaking table. The next day, the membranes
were washed with TBST three times and incubated with a secondary
antibody solution which was horseradish peroxidase-conjugated IgG
(1:5,000) at room temperature in the dark for 1 h. Subsequently,
the membranes were washed with TBST three times. Finally, protein
antibody complexes were measured by LI-COR Odyssey infrared imaging
system (LI-COR Biosciences).
Statistical analysis
The values are represented as the mean ± standard
deviation for three independent experiments. The two-tailed
Student's t-test was used to detect the significance between two
experimental groups, and significance in more than two groups was
determined using one-way analysis of variance. GraphPad software
(ver. 5.02; GraphPad Software, Inc., La Jolla, CA, USA) was used
for all statistical analyses. Statistical significant differences
were established when P<0.05.
Results
Antitumor activity in vitro
EA inhibits the cell viability of U87 and U118
cell lines
As shown in Fig. 1B and
C, EA markedly decreased the cell viability in the U87 and U118
cell lines in a time- and dose-dependent manner. After incubation
for 48 h, the IC50 values (concentration of the drug
resulting in a 50% reduction in cell viability) of EA against the
cell viability of U87 and U118 cell lines were 91.2 and 98.6 µM,
respectively.
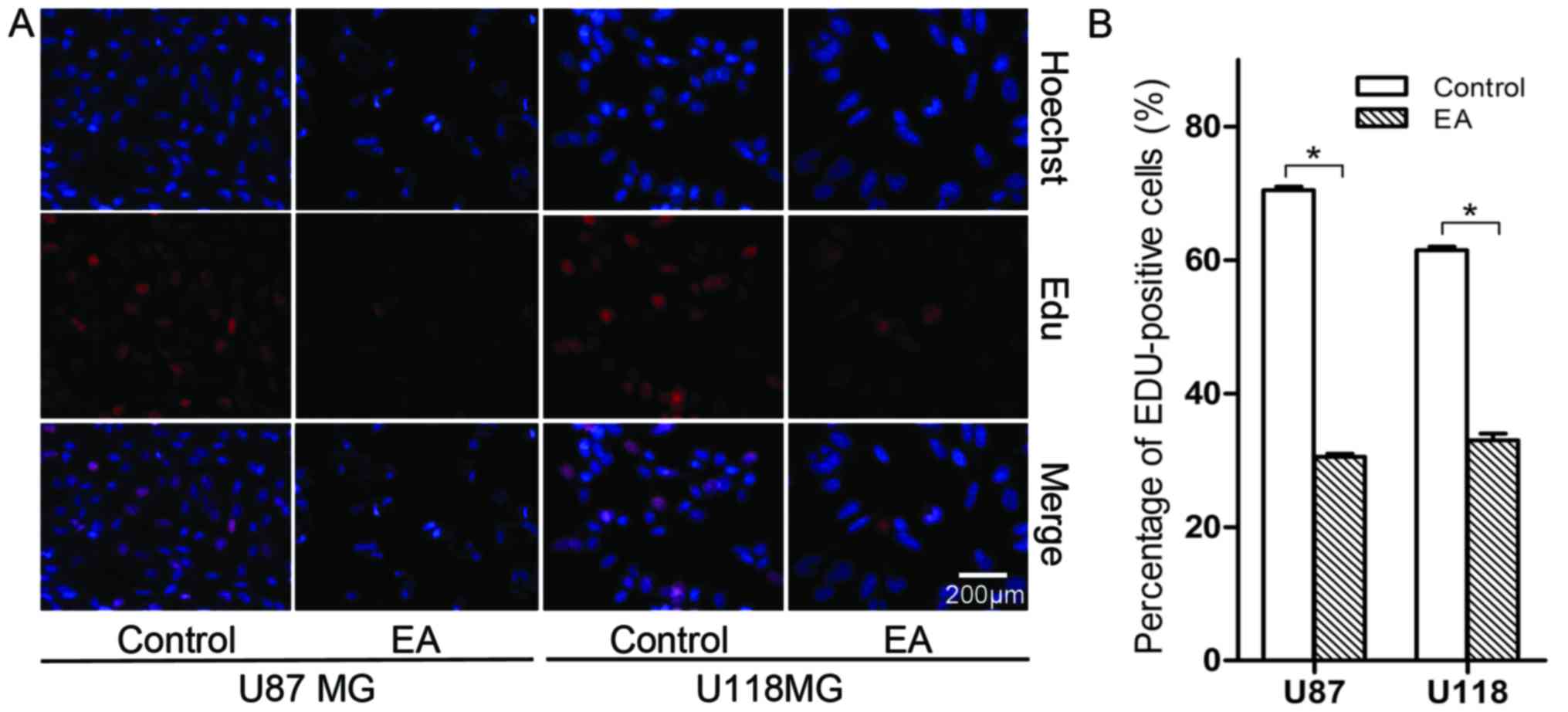
EA suppresses the proliferation of glioblastoma
cells
In the EdU assay, a marked suppression of cell
proliferation was observed in both the U87 and U118 cell lines
treated with 100 µM of EA at 48 h (Fig.
2). More specifically, the number of cell nuclei with thymidine
analog incorporated into newly synthesized DNA significantly
decreased after treatment with EA. The percentage of stained nuclei
in total cells treated with EA was lower than that noted in the
control (P<0.05).
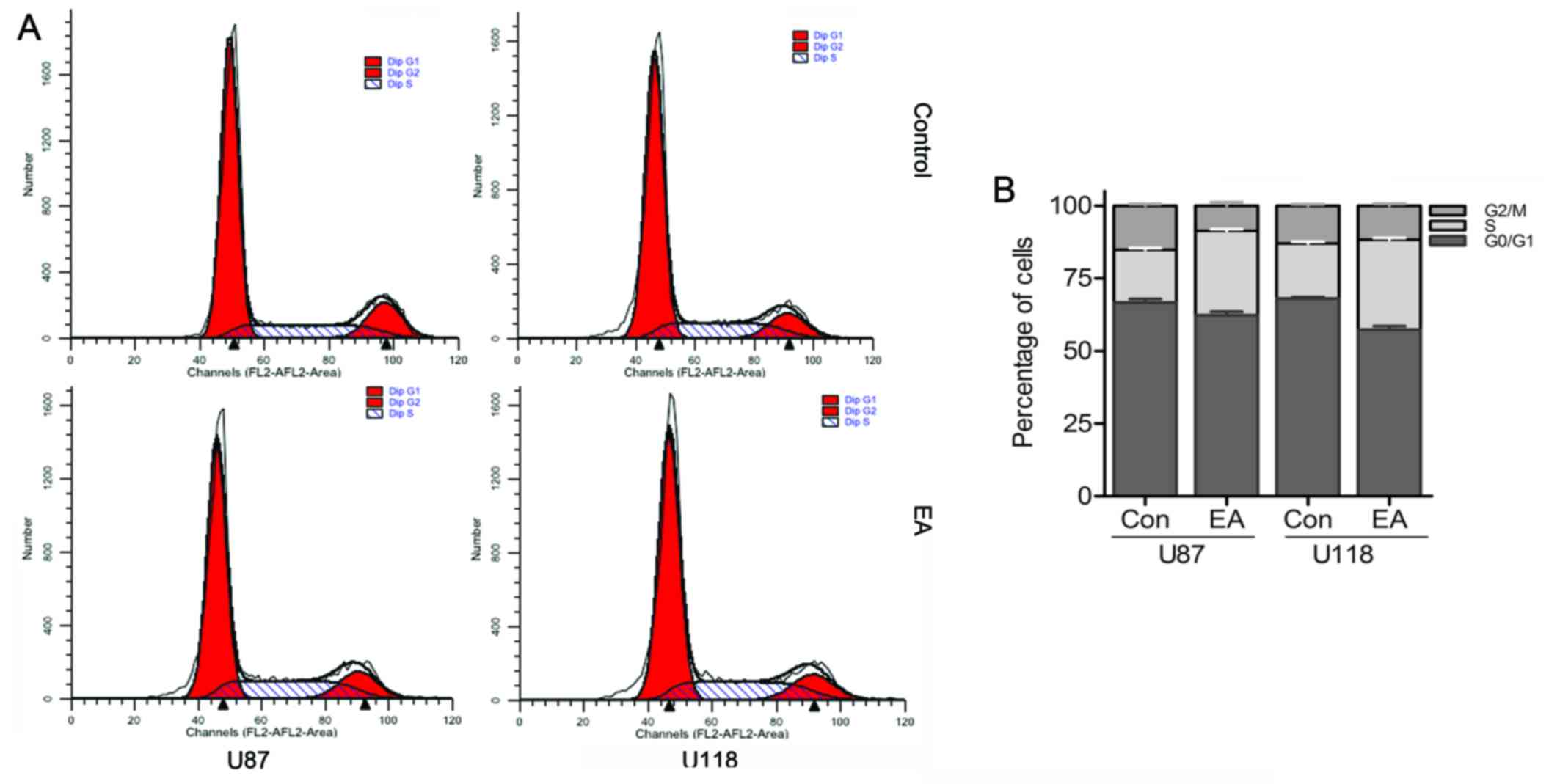
EA affects the cell cycle progression of
glioblastoma cells
For the purpose of detecting the possible mechanisms
of EA anti-proliferation, we evaluated the cell cycle distribution
of both cell lines with or without EA by flow cytometry. As shown
in Fig. 3, cultivating U87 and U118
cells with EA respectively for 48 h led to a 10.89 and 11.54%
increase separately in the percentage of cells in the S phase
compared with the control group, while accompanied by a persistent
decrease in the percentage of cells in the G1 and G2/M phases,
which revealed that EA could induce cell cycle arrest at the S
phase in both glioblastoma cell lines.
EA leads to DNA damage in glioblastoma cells
As exhibited in Fig.
4, the double-strand breaks (DSBs) and consequent γ-H2AX foci
spots induced by EA were significantly increased compared with the
control group at 48 h. The results demonstrated that EA leads to
the lethality of DSBs in glioblastoma cells.
EA inhibits the invasion of glioblastoma
cells
As shown in Fig. 5,
EA inhibited the cell invasion in U87 and U118 cells [control
(82±3), (97±6) vs. EA treatment (31±1), (50±4), P<0.05]. These
results demonstrated that EA decreases glioma cell invasion.
Antitumor activity in vivo
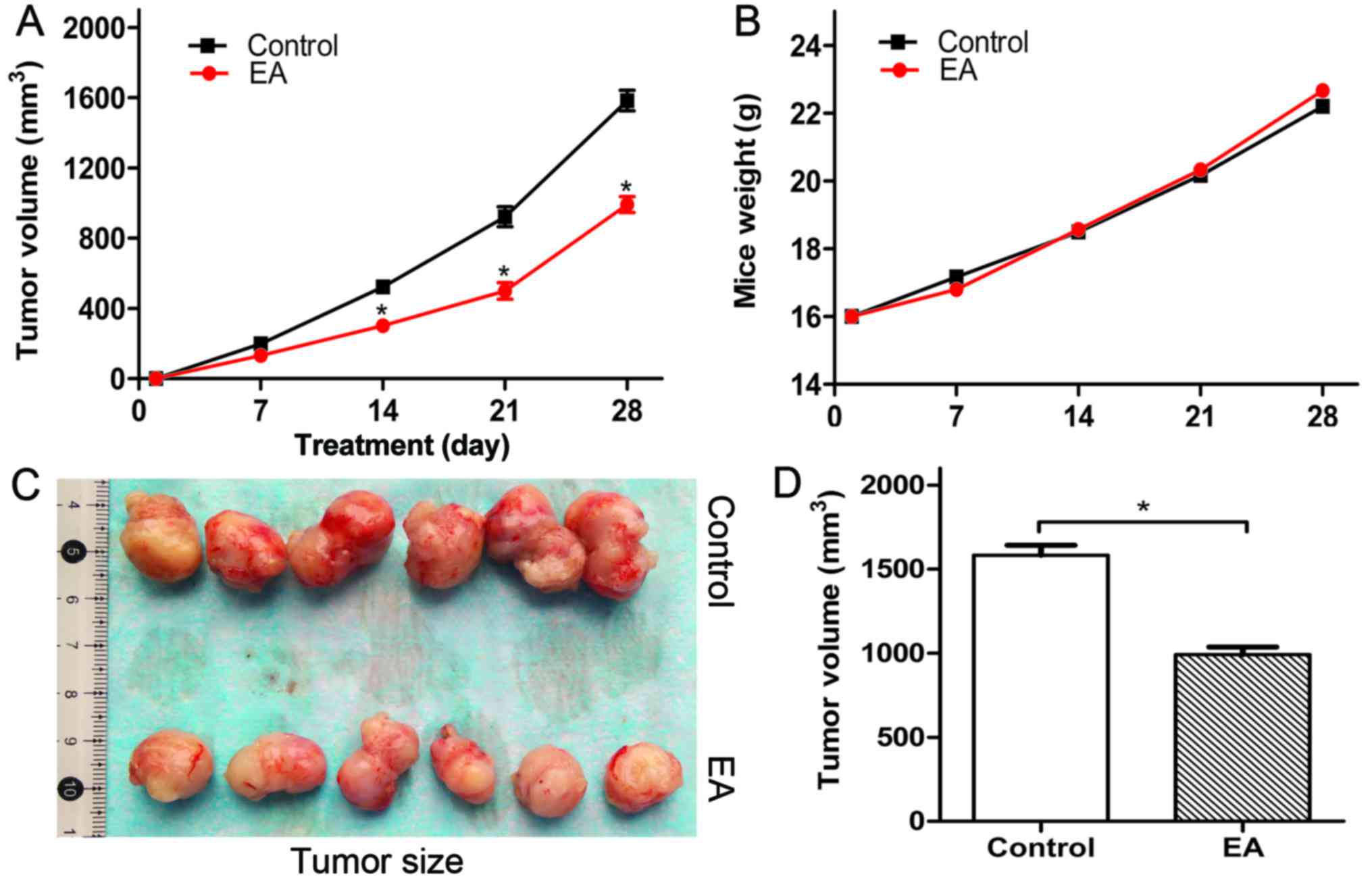
EA suppresses the growth of U87 xenografted
tumors in Balb/c nude mice
The influence of EA on the growth of glioblastoma
U87 xenografted tumors in Balb/c nude mice was evaluated. We
observed that EA suppressed the volume and size of the tumors
(Fig. 6A, C and D), while the body
weight of the tumor-bearing mice seemed not to be affected
(Fig. 6B). In addition, we observed
that there were no differences in the liver, kidney, intestine and
brain of the tumor-bearing mice compared with the control group
(data not shown). Thus, it was demonstrated that EA is a safe,
nontoxic natural dietary product. Furthermore, we detected the
influence of EA on xenograft cell proliferation by IHC and WB. As a
result, we found that EA inhibited xenograft cell proliferation
compared with the control group through the suppression of PCNA and
Ki67 (Fig. 7).
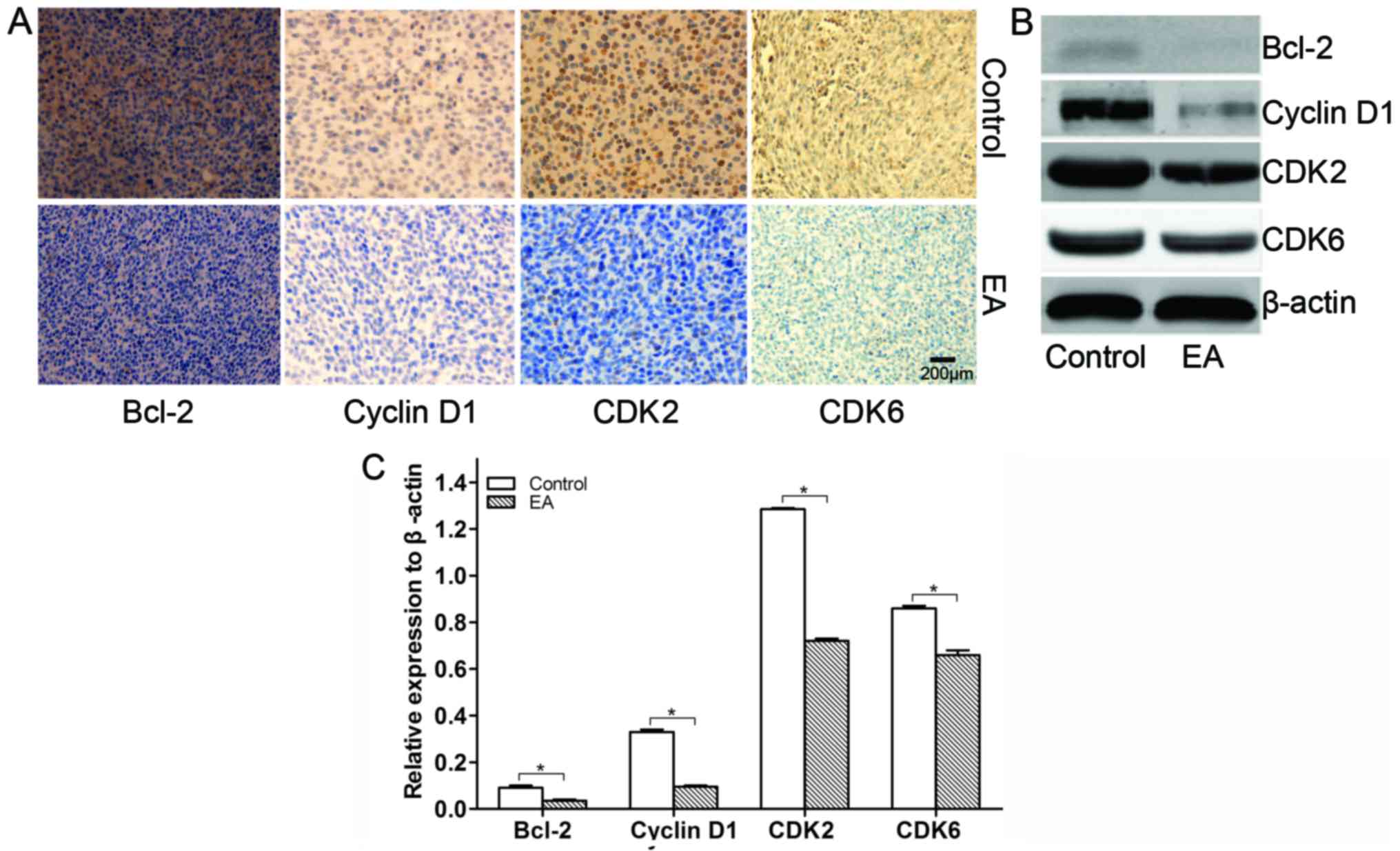
EA regulates cell cycle-related proteins and
members of the Bcl-2 family in xenograft tissues
Cell cycle-related and Bcl-2 member proteins largely
determine the survival of cells (19). In this study, the influence of EA on
the protein expression of cell cycle-related proteins and Bcl-2
family members from xenograft tissues was detected by IHC and WB.
As a result, we found that EA suppressed the expression of Bcl-2
accompanied by the suppression of the protein expression of cyclin
D1, CDK2 and CDK6 cell cycle-related proteins in the xenograft
tissues (Fig. 8). Thus, it was
revealed that EA regulates the cell cycle in U87 xenografted
tumors.
EA suppresses epithelial-mesenchymal transition
in xenograft tissues
During tumor cell invasion and metastasis,
epithelial-mesenchymal transition (EMT) involved in embryonic
development is usually activated (20). The process of EMT inhibits the
expression of E-cadherin, while it induces and enhances the
expression of transcription factors such as Snail and the
expression of MMPs (21–24). In this study, the influence of EA on
the protein expression of invasion was detected by IHC and WB. As a
result, we found that EA upregulated the protein expression level
of E-cadherin, while it suppressed the protein expression levels of
Snail, MMP-2 and MMP-9 in xenograft tissues (Fig. 9). Thus, it was revealed that EA
suppresses glioblastoma invasion by regulating the expression of
EMT-associated transcription factors and MMPs.
EA inhibits the Akt and Notch pathways
The PI3K/Akt and Notch signaling pathways play an
important role in cell survival, proliferation and progression. In
this study, the influence of EA on the protein expression levels of
Akt and Notch in xenograft tissues was determined by IHC and WB. As
a result, we found that EA regulates U87 xenograft growth by
inhibiting the Akt and Notch signaling pathways (Fig. 7).
Discussion
Glioblastoma is still a lethal human malignant tumor
with limited progress in its treatment (25). Since EA was extracted from various
plants hundreds of years ago, researchers have been increasingly
attracted by its pharmacological properties (26). Several studies have shown that EA
plays a significant role in the inhibition of cell proliferation
and invasion in tumor cells. In the present study, we treated human
glioblastoma U87 and U118 cells with EA. The results demonstrated
that EA exhibited an inhibitory effect on cell viability,
proliferation and invasion of human glioblastoma cells. Previous
studies have illustrated that cell cycle arrest is associated with
the inhibition of tumor cell proliferation. Many chemical reagents
lead to cell DNA damage that inhibit cell proliferation. The DSBs
induced by chemical reagents or ionizing radiation rapidly recruits
a large amount of phosphorylated histone H2AX named γ-H2AX, which
exerts an essential role in DNA DSB repair (27). In a set of experiments, we observed
cell cycle redistribution in both U87 MG and U118 MG cells.
Simultaneously, the increased γ-H2AX foci spots in the experimental
group at 48 h showed that EA led to DNA damage and DSBs.
Consequently, the cell cycle arrest and DNA damage induced by EA
may contribute to those inhibitory results.
The Akt signaling pathway plays a major role in the
suppression of cell apoptosis and facilitation of cell survival in
malignant tumor cells (28). Akt
signaling promotes cell survival by direct or indirect interaction
with proteins linked with proliferation and apoptosis, such as
anti-apoptotic factor Bcl-2 and cell cycle-associated protein
cyclin D1. Furthermore, we found that EA inhibited glioblastoma U87
xenografted tumor growth which was involved in the inhibition of
the Akt and Notch signaling pathways. The Akt and Notch signaling
pathways play an important role in tumor carcinogenesis. Recently,
the use of inhibitors of PI3K/Akt to treat cancer patients
clinically is being assessed and more and more researchers are
beginning to pay close attention to the therapeutic strategies in
the suppression of PI3K/Akt activity. In this study, we found that
EA suppressed tumor cell proliferation through the inhibition of
the activation of Akt in human glioblastoma U87 xenografted
tissues.
With activation of γ-secretase constitutively,
aberrant Notch signaling plays a critical role in the initiation
and progression of malignant tumors, and the inhibitors of this
signaling pathway are presently being studied in clinical trials. A
previous study showed that inhibition of Notch expression could
decrease PI3K/Akt activity, which displays its anti-proliferative
effects from another point of view (29). Thus, suppression of the Notch
signaling pathway alone or in combination with PI3K/Akt may be
conducive to obtain maximum therapeutic effects. In this study, we
found that EA inhibited tumor growth through the suppression of the
Notch1 and Akt signaling pathways.
It is widely known that tumor cells undergoing EMT
may exhibit the increase of specific secretion factors, such as
cytokines, growth factors and chemokines, which could be beneficial
for tumor progression (30). As a
family of zinc-dependent proteases, MMPs are in a position to
degrade the components of ECM, which promotes tumor invasion. In
the present study, we observed that EA suppresses glioblastoma cell
invasion in the Matrigel invasion assay, furthermore, EA inhibited
glioblastoma invasion in the U87 xenografted model by inducing
E-cadherin and suppressing the expression of transcription factors
such as Snail, MMP-2 and MMP-9. All these findings reveal that EA
suppresses metastasis in glioblastoma.
In conclusion, the suppression of the Akt and Notch
pathways by EA may act together to inhibit glioblastoma growth,
which suggests that EA, as a nontoxic compound, may possibly be
employed for the treatment of glioblastoma in the future.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (nos. 81372683 and 81572489 awarded to
Q.C.). We acknowledge the assistance of the technical staff of the
Key Laboratory of Renmin Hospital, Wuhan University, China.
Glossary
Abbreviations
Abbreviations:
|
EA
|
ellagic acid
|
|
Bcl-2
|
B-cell lymphoma 2
|
|
CDK
|
cyclin-dependent kinase
|
|
γ-H2AX
|
phosphorylated histone H2AX
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
MMPs
|
matrix metalloproteinases
|
|
PI
|
propidium iodide
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
PBS
|
phosphate-buffered saline
|
|
FBS
|
fetal bovine serum
|
|
EdU
|
5-ethynyl-2-deoxyuridine
|
|
IHC
|
immunohistochemistry
|
|
WB
|
western blot analysis
|
|
EMT
|
epithelial-mesenchymal transition
|
|
ECM
|
extracellular matrix
|
|
DSBs
|
double-strand breaks
|
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: European Organisation for Research and Treatment of
Cancer Brain Tumor and Radiotherapy Groups; National Cancer
Institute of Canada Clinical Trials Group: Radiotherapy plus
concomitant and adjuvant temozolomide for glioblastoma. N Engl J
Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cuddapah VA, Robel S, Watkins S and
Sontheimer H: A neurocentric perspective on glioma invasion. Nat
Rev Neurosci. 15:455–465. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang JA, Li JQ, Shao LM, Yang Q, Liu BH,
Wu TF, Wu P, Yi W and Chen QX: Puerarin inhibits proliferation and
induces apoptosis in human glioblastoma cell lines. Int J Clin Exp
Med. 8:10132–10142. 2015.PubMed/NCBI
|
|
4
|
Lee DH, Kim DW, Jung CH, Lee YJ and Park
D: Gingerol sensitizes TRAIL-induced apoptotic cell death of
glioblastoma cells. Toxicol Appl Pharmacol. 279:253–265. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Son YG, Kim EH, Kim JY, Kim SU, Kwon TK,
Yoon AR, Yun CO and Choi KS: Silibinin sensitizes human glioma
cells to TRAIL-mediated apoptosis via DR5 up-regulation and
down-regulation of c-FLIP and survivin. Cancer Res. 67:8274–8284.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vattem DA and Shetty K: Biological
function of ellagic acid: A Review. J Food Biochem. 29:234–266.
2005. View Article : Google Scholar
|
|
7
|
Zaveri NT: Green tea and its polyphenolic
catechins: Medicinal uses in cancer and noncancer applications.
Life Sci. 78:2073–2080. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seeram NP, Adams LS, Henning SM, Niu Y,
Zhang Y, Nair MG and Heber D: In vitro antiproliferative, apoptotic
and antioxidant activities of punicalagin, ellagic acid and a total
pomegranate tannin extract are enhanced in combination with other
polyphenols as found in pomegranate juice. J Nutr Biochem.
16:360–367. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han DH, Lee MJ and Kim JH: Antioxidant and
apoptosis-inducing activities of ellagic acid. Anticancer Res 26
(5A). 3601–3606. 2006.
|
|
10
|
Strati A, Papoutsi Z, Lianidou E and
Moutsatsou P: Effect of ellagic acid on the expression of human
telomerase reverse transcriptase (hTERT) α+β+ transcript in
estrogen receptor-positive MCF-7 breast cancer cells. Clin Biochem.
42:1358–1362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saiko P, Steinmann MT, Schuster H, Graser
G, Bressler S, Giessrigl B, Lackner A, Grusch M, Krupitza G,
Bago-Horvath Z, et al: Epigallocatechin gallate, ellagic acid, and
rosmarinic acid perturb dNTP pools and inhibit de novo DNA
synthesis and proliferation of human HL-60 promyelocytic leukemia
cells: Synergism with arabinofuranosylcytosine. Phytomedicine.
22:213–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arulmozhi V, Pandian K and Mirunalini S:
Ellagic acid encapsulated chitosan nanoparticles for drug delivery
system in human oral cancer cell line (KB). Colloids Surf B
Biointerfaces. 110:313–320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao M, Tang SN, Marsh JL, Shankar S and
Srivastava RK: Ellagic acid inhibits human pancreatic cancer growth
in Balb c nude mice. Cancer Lett. 337:210–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okla M, Kang I, Kim DM, Gourineni V, Shay
N, Gu L and Chung S: Ellagic acid modulates lipid accumulation in
primary human adipocytes and human hepatoma Huh7 cells via discrete
mechanisms. J Nutr Biochem. 26:82–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Umesalma S and Sudhandiran G: Ellagic acid
prevents rat colon carcinogenesis induced by 1, 2 dimethyl
hydrazine through inhibition of AKT-phosphoinositide-3 kinase
pathway. Eur J Pharmacol. 660:249–258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo W, Li A, Jia Z, Yuan Y, Dai H and Li
H: Transferrin modified PEG-PLA-resveratrol conjugates: In vitro
and in vivo studies for glioma. Eur J Pharmacol. 718:41–47. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Purow B: Notch inhibition as a promising
new approach to cancer therapy. Adv Exp Med Biol. 727:305–319.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koch U and Radtke F: Notch and cancer: A
double-edged sword. Cell Mol Life Sci. 64:2746–2762. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: Roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pavelic S Kraljevic, Sedic M, Bosnjak H,
Spaventi S and Pavelic K: Metastasis: New perspectives on an old
problem. Mol Cancer. 10:222011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McCarthy N: Metastasis: Route master. Nat
Rev Cancer. 9:610–611. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
McCarthy N: Metastasis: Influencing bad
behaviour. Nat Rev Cancer. 9:6092009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grossman SA and Batara JF: Current
management of glioblastoma multiforme. Semin Oncol. 31:635–644.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Landete JM: Ellagitannins, ellagic acid
and their derived metabolites: A review about source, metabolism,
functions and health. Food Res Int. 44:1150–1160. 2011. View Article : Google Scholar
|
|
27
|
Hernández L, Terradas M, Martín M, Tusell
L and Genescà A: Highly sensitive automated method for DNA damage
assessment: gamma-H2AX foci counting and cell cycle sorting. Int J
Mol Sci. 14:15810–15826. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koseoglu S, Lu Z, Kumar C, Kirschmeier P
and Zou J: AKT1, AKT2 and AKT3-dependent cell survival is cell
line-specific and knockdown of all three isoforms selectively
induces apoptosis in 20 human tumor cell lines. Cancer Biol Ther.
6:755–762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang D, Chen Q, Liu B, Li Y, Tan Y and
Yang B: Ellagic acid inhibits proliferation and induces apoptosis
in human glioblastoma cells. Acta Cir Bras. 31:143–149. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Palena C, Hamilton DH and Fernando RI:
Influence of IL-8 on the epithelial-mesenchymal transition and the
tumor microenvironment. Future Oncol. 8:713–722. 2012. View Article : Google Scholar : PubMed/NCBI
|