Introduction
Platinum-based chemotherapy including cisplatin is
the first-line therapy for the treatment of NSCLC (1). However, NSCLC remains the leading
cause of cancer-related deaths which are attributed to late
diagnosis and development of chemoresistance. Many mechanisms and
several molecules involved in chemoresistance have been
investigated, but therapeutic outcomes are still
unsatisfactory.
The TAM receptor tyrosine kinase (RTK) family,
consisting of Tyro3, Axl, MerTK is a subfamily of RTK, which has
been reported to be involved in cell growth, proliferation,
metastasis and resistance to chemotherapy (2) in various types of cancers. TAM RTKs
also play important roles in immune regulation, since they function
as phagocytic receptors in normal tissues (3,4). In
addition, structural features of TAM RTKs in the extracellular,
transmembrane and cytoplasmic kinase domains are very similar
(3), which allow them to share
various ligands including growth arrest-specific 6 (Gas6), protein
S, tubby and tulip. Gas6 has been known to bind to all three TAM
RTKs with higher affinity for Axl than the others (5). Upon activation of Axl by Gas6 binding,
tyrosine residues of the cytoplasmic tyrosine kinase domain are
autophosphorylated (6), which in
turn activate several signaling pathways mediating cell growth,
survival, proliferation, migration and inhibition of apoptosis
(3,7).
Axl, also referred to Ark, Tyro7 or Ufo, was first
isolated from chronic myelogenous leukemia cells (8). Since then, Axl overexpression has been
reported in a multitude of cancers including acute leukemia
(9), breast (10), colon (11), esophageal, lung (12), ovarian (13), prostate (14) and thyroid cancer (15). The overexpression of Axl has been
shown to be associated with epithelial-to-mesenchyaml transition
(EMT), anticancer drug resistance and angiogenesis (16–18),
whereas Axl inhibition decreased cancer cell growth and migration
and increased sensitivity to anticancer drugs (19). The overexpression of Axl is observed
in ~50% of clinical specimens of lung cancer cases and 60% of NSCLC
cell lines and is associated with increased invasion and poor
prognosis (20,21). Moreover, Axl expression mediates
resistance to anti-EGF receptor (EGFR) therapy including gefitinib
and erlotinib in NSCLC (22,23).
The inhibition of Axl with Axl-specific siRNA or monoclonal
antibodies has been reported to decrease the proliferation of NSCLC
cells in vitro and in vivo in a tumor xenograft model
(24,25) and depletion of Rac1, a downstream
effector of Axl has been shown to result in enhanced sensitivity to
anticancer drugs (26,27).
Interleukin (IL)-8 is a proinflammatory cytokine
overexpressed in many types of cancer including colon carcinoma,
melanoma (28), ovarian (29) and prostate cancer (30), and several studies recently
demonstrated that upregulation of IL-8 expression was associated
with the acquired resistance against various chemotherapeutic drugs
such as cisplatin (31,32), paclitaxel (33), and a receptor tyrosine kinase (RTK)
inhibitor, erlotinib which specifically targets epidermal growth
factor receptor (EGFR), in ovarian (33), lung (34,35)
and head and neck cancer (HNC) cells (36), respectively. Particularly, in
erlotinib-resistant HNC cells, the expression of a panel of genes
including IL-8, EGFR and Axl was found to be increased (36). In addition, Gong et al
reported that in gastric cancer, ErbB2, a member of the EGFR
family, is activated and that an ErbB2-targeting agent,
trastuzumab, decreased the expression of IL-8 (37). Therefore, IL-8 has received a lot of
attention as a potent therapeutic target to control cancer
progression as well as chemoresistance and it appears to be quite
beneficial to examine whether auto- and/or paracrine regulation of
IL-8 production is associated with the expression of some RTKs such
as EGFR and Axl or vice versa.
Luteolin, 3′,4′,5,7-tetrahydroxyflavone, is a
non-toxic flavonoid widely found in various plants and has many
biological activities including antioxidant, anti-inflammatory and
anticancer effects. Recent studies have shown that the luteolin
induced sensitization of many different cancers to therapeutic
drugs (38–40), and its anticancer effects were
mediated by diverse signaling pathways involved in cell
proliferation, angiogenesis, metastasis and apoptosis (41). However, the effect of luteolin has
not been studied yet in the expression and activation of TAM RTKs
and the association with its cytotoxicity.
In the present study, we tested the association of
TAM RTKs in the anticancer effect of luteolin in parental and
cisplatin-resistant NSCLC cells to provide a potent therapeutic
target to inhibit cell proliferation and overcome
chemoresistance.
Materials and methods
Reagents and antibodies
Luteolin was obtained from Sigma-Aldrich (St. Louis,
MO, USA). A549 and H460 cells were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA). Both control shRNA
and Axl shRNA which were annealed to gold nanoparticles were
synthesized by the domestic company, Bioneer Corp. (Daejeon,
Korea). Lipofectamine 2000 and G418 were obtained from Roche
Diagnostics Corp. (Indianapolis, IN, USA) and Gibco-BRL
(Gaithersburg, MD, USA), respectively. The plasmid, pGL3-basic
vector, and the Dual-Glo luciferase assay kit were purchased from
Promega Corp. (Madison, WI, USA). For western blot analysis,
specific antibodies against Axl, phosphor-Axl, MerTK, Tyro3 and
GAPDH, as well as secondary antibodies were obtained from Santa
Cruz Biotechnology (Dallas, TX, USA).
Cell culture and establishment of
cisplatin-resistant cells
The A549 and H460 cells were grown in RPMI-1640
medium (Gibco-BRL) containing 10% fetal bovine serum (FBS), 2 mM
L-glutamine, 10 U/ml penicillin and 10 g/ml streptomycin at 37°C in
5% CO2 in a water-saturated atmosphere.
Cisplatin-resistant cells, A549/CisR and H460/CisR, were
established by stepwise exposure of parental cells to escalating
concentrations of cisplatin (ranging from 0.5 to 2 µM).
Reverse transcription PCR
(RT-PCR)
Cells (3×105) were seeded in a 60 mm
culture dish and grown overnight. They were then treated with the
indicated concentrations of luteolin for 24 h. Total RNA was
extracted using TRI reagent and subjected to cDNA synthesis and
PCR. The specific primers were as follows: Axl sense,
5′-AACCTTCAACTCCTGCCTTCTCG-3′ and antisense,
5′-CAGCTTCTCCTTCAGCTCTTCAC-3′; GAPDH sense,
5′-GGAGCCAAAAGGGTCATCAT-3′ and antisense,
5′-GTGATGGCATGGACTGTGGT-3′.
Promoter activity assessment
The promoter reporter plasmid, pGL3-Axl, which
contains the Axl promoter region ranging from −887 to +7 bp
of the transcriptional start site was amplified by PCR and
subcloned into the pGL3-basic vector, the luciferase reporter
plasmid. The constructed promoter-reporter plasmid was
co-transfected into cells (3×105 cells in a 60-mm dish)
with Renilla luciferase vectors, pRL-SV40, as an internal
control. Luciferase activity was asessed using a Dual-Glo
luciferase assay system.
Western blot analysis
Total cell lysates were prepared from the parental
or chemoresistant cells treated with the indicated concentrations
(0, 20, 40 and 80 µM) of luteolin using lysis buffer [1% Triton
X-100, 50 mM Tris (pH 8.0), 150 mM NaCl, 1 mM phenylmethylsulphonyl
fluoride (PMSF), 1 mM Na3VO4 and protease
inhibitor cocktail]. Untreated cells were used as controls. Protein
concentrations were determined using Bio-Rad protein assays.
Proteins from the cell lysates (20–40 µg) were separated by 12%
SDS-PAGE, and electrotransferred onto nitrocellulose membranes. The
membranes were blocked for 30 min at room temperature in
Tris-buffered saline with 0.05% Tween-20 (TTBS) containing 5%
non-fat dry milk, and then incubated with TTBS containing a primary
antibody for 4 h at room temperature. After 3×10 min washes in
TTBS, the membranes were incubated with a peroxidase-conjugated
secondary antibody for 1 h. Following three additional 10-min
washes with TTBS, the protein bands of interest were visualized
using an enhanced chemiluminescence detection system (Amersham™
ECL™ Prime Western Blotting Detection Reagent; GE Healthcare,
Piscataway, NJ, USA). The density of each protein level was
measured by LAS-3000 FujiFilm Image Reader and Multi-Gauge 3.0
software and the Axl protein level was normalized with that of
GAPDH.
Cell viability measurement
To assess cell viability, the number of viable cells
was counted after trypan blue staining. Briefly, 3×103
cells were seeded into a 60-mm culture dish, grown overnight and
then treated with the indicated concentrations (0, 20, 40 and 80
µM) of luteolin for 24 h. After luteolin treatment, cells were
harvested and stained with 0.4% trypan blue solution. Dye-excluding
viable cells were counted under the microscope. Cell viability was
also expressed as a percentage of the viable cells with respect to
the untreated control cells. Additionally, the viability of cells
was assessed using Cell Counting Kit-8 (CCK-8) assay kit (Dojindo
Laboratories, Kumamoto, Japan). Cells (1×103 cells/well)
were seeded in 96-well plates and grown overnight, and then treated
with the indicated concentrations of luteolin with or without 4 µM
cisplatin for the 24 h. At the end of the treatment, 10 µl of CCK-8
solution was added and further incubated for 4 h. The absorbance at
450 nm was measured using a microplate reader (Model 680 microplate
reader; Bio-Rad Laboratories, Hercules, CA, USA). Values are
expressed as the mean ± SD for triplicate wells and normalized to
that of the control group to determine the % of viability.
Colony formation assay
Cells were seeded into 24-well plates
(1×102 cells/well) and treated with the indicated
concentrations (0, 20, 40 and 80 µM) of luteolin for 24 h.
Luteolin-treated cells were then cultured for the next 7–10 days to
form colonies. Colonies of >50 cells were stained with crystal
violet (in 60% methanol; Junsei Chemical Co., Ltd., Tokyo, Japan)
and images were acquired using the RAS-3000 Image Analysis System
(FujiFilm, Tokyo, Japan).
Ectopic expression of Axl
To ectopically express Axl, the recombinant plasmid,
pcDNA3-Axl, was constructed by cloning the Axl cDNA into the
EcoRI and BamHI sites of the pcDNA3 vector and 2 µg
of purified plasmids were transfected into the A549 or A549/Cis
cells (3×105 cells in a 100-mm dish) using Lipofectamine
2000 (Invitrogen, Carlsbad, CA, USA). To establish stable cell
lines, which constitutively express Axl, the transfected cells were
cultured in the presence of 400 µg/ml of G418. The RPMI-1640 medium
containing G418 was refreshed every three days. After three to four
weeks, the Axl-expressing cells were enriched and the Axl
expression in these cells was analyzed by western blot
analysis.
Gold nanoparticle-assisted gene
delivery system for Axl silencing
Chemically functionalized gold nanoparticles (AuNPs)
were annealed with the shRNA targeting Axl gene
(5′-TAATACGACTCACTATAGGGAAGAUUUGGAGAACACACUGA-3′)
and used as a gene delivery system (GDS) to decrease Axl
expression. Briefly, cells (3×105) were seeded in 60-mm
culture dishes, grown overnight and then incubated with 10 nM
AuNPs-Axl or control AuNPs. The cells were harvested for 24
and 48 h after transfection and used to evaluate protein expression
and cell proliferation, respectively.
Statistical analysis
Data are expressed as the means ± SD of triplicate
samples or at least three independent experiments. To determine
statistical significance, the Student's t-test was used with a
P-value threshold of <0.05.
Results
Luteolin inhibits proliferation of
both parental and cisplatin-resistant non-small lung cancer
cells
The antiproliferative effect of luteolin was first
examined in parental non-small cell lung cancer (NSCLC) A549 and
H460 cells, as well as cisplatin-resistant A549/CisR and H460/CisR
cells. As shown in Fig. 1A, the
viability of these cells was found to be decreased in a
dose-dependent manner. Next, a clonogenic assay was further
performed to confirm the cytotoxicity of luteolin in A549, H460,
A549/CisR and H460/CisR cells. These cells were treated with the
indicated concentrations of luteolin, and then allowed to grow for
the next 10 days. Luteolin treatment resulted in the dose-dependent
decrease in the colony formation (Fig.
1B). Notably, H460 and H460/CisR cells that were incubated with
80 µM luteolin failed to form visible colonies, suggesting that
luteolin seems to be more cytotoxic to H460 cells than to A549
cells. We also examined the effect of co-treatment of luteolin and
cisplatin on cell proliferation using H460/CisR cells. While 4 µM
cisplatin alone decreased the viability of the H460/CisR cells to
61% and luteolin decreased that to 67, 41 and 27% in proportion to
the concentration of luteolin, co-treatment of cells with luteolin
and cisplatin reduced that to 36, 27 and 18%, respectively
(Fig. 1C). The results demonstrated
that in the presence of cisplatin, the cytotoxicity of luteolin was
additively increased in the cisplatin-resistant cells.
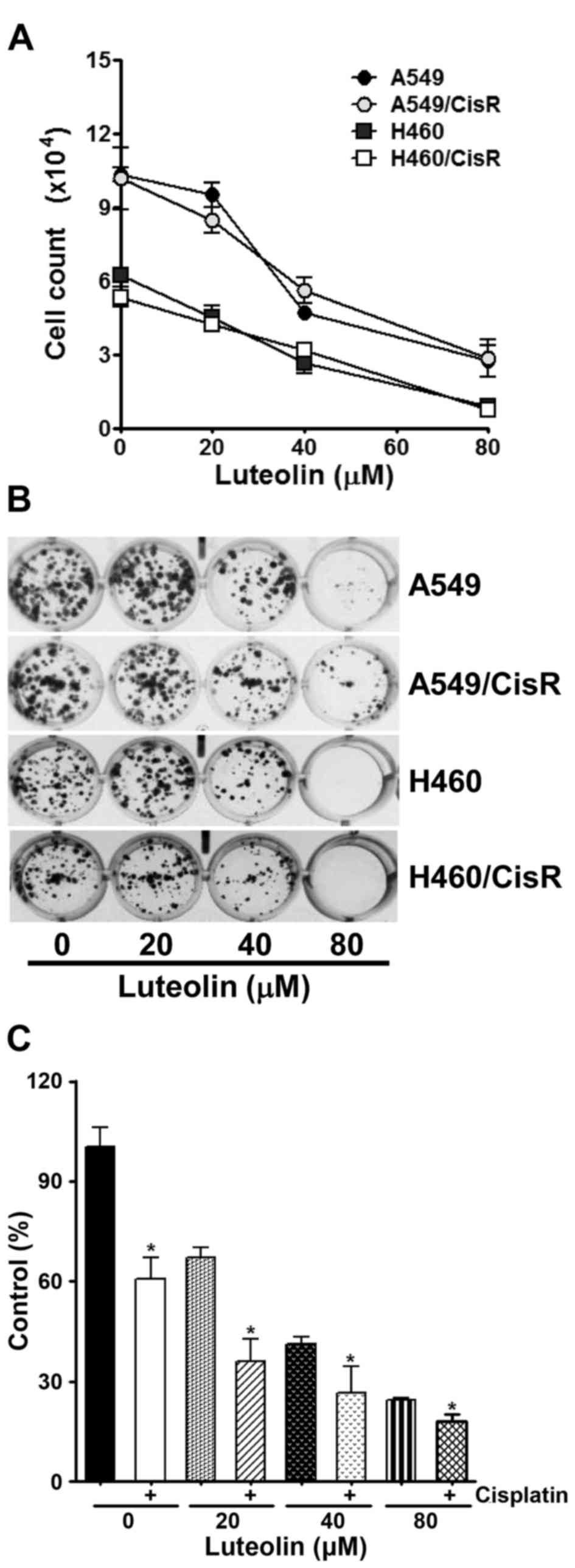 | Figure 1.Luteolin inhibits cell proliferation
of parental and cisplatin-resistant non-small lung cancer cells.
(A) A549, H460 and their cisplatin-resistant cells (A549/CisR and
H460/CisR) were seeded into 60 mm dishes (3×105
cells/dish) and grown overnight. Cells were treated with 20, 40 and
80 µM of luteolin for 24 h, and then cells were harvested and
stained with trypan blue. The number of viable cells was counted.
Data are expressed as the means ± SD of triplicate samples
conducted in three independent experiments. The asterisks indicate
the significant difference compared to the control value
(*P<0.05, vs. the untreated group). (B) Cells (2×103
cells/well) were seeded into a 24-well culture plate, grown
overnight, exposed to 20, 40 and 80 µM of luteolin, and allowed to
grow for the next 7–10 days. The colonies were visualized by
crystal violet staining. The data shown are representative of at
least three independent experiments. (C) H460/CisR cells
(1×103 cells/well) were seeded in 96-well plates, grown
overnight and then treated with the indicated concentrations of
luteolin in the absence or presence of 4 µM cisplatin for the 24 h.
To assess the cell viability, CCK-8 solution was added into each
well and the absorbance at 450 nm was assessed. Values are
normalized by the absorbance of the control group and expressed as
the mean ± SD for triplicate wells. The asterisks indicate the
significant difference compared to the value of cells without
cisplatin treatment (*P<0.05). |
Luteolin downregulates the expression
of RTKs and inhibits Axl activation upon its ligand binding
Since TAM RTKs have been reported to be associated
with oncogenesis, proliferation, survival and anti-apoptosis
(9,10,42,43),
we next assessed the effect of luteolin on the expression of TAM
RTKs. Cells were incubated with the indicated concentrations of
luteolin for 24 h and the protein levels of each TAM RTK was
measured by western blot analysis. We found that in the A549 and
A549/CisR cells, luteolin treatment dose-dependently decreased the
expression levels of all three TAM RTKs, Tyro3, Axl and MerTK.
Similarly in the H460 and H460/CisR cells, the expression levels of
both Axl and Tyro3 were decreased by luteolin (Fig. 2A). Notably, in the H460 cells, MerTK
was undetectable by western blot analysis.
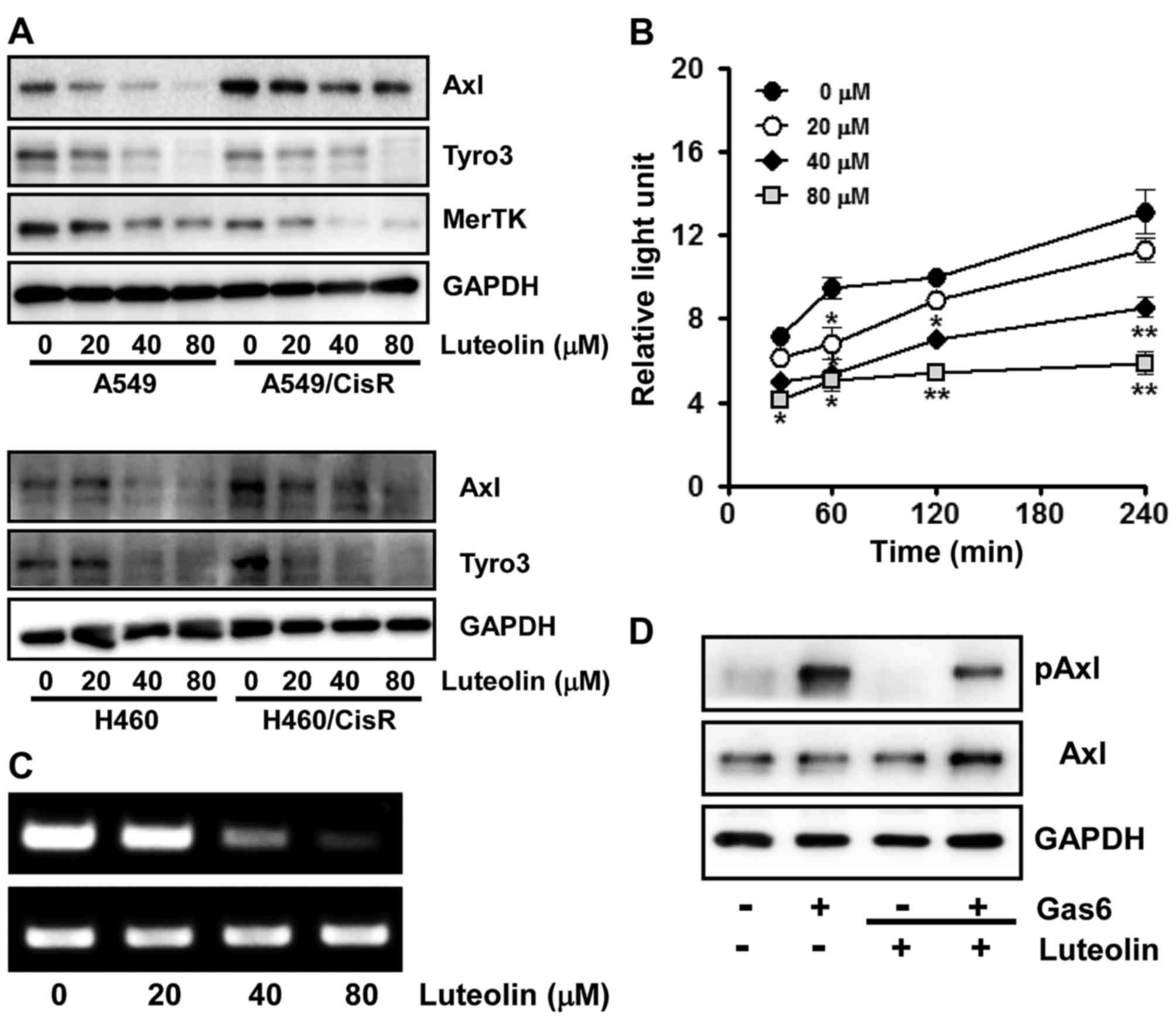 | Figure 2.Luteolin downregulates the expression
of TAM RTKs and inhibits Axl activation upon Gas6 stimulation in
non-small lung cancer cells. (A) A549, H460, A549/CisR and
H460/CisR cells (3×105 cells/dish) were seeded onto
60-mm dishes, grown overnight, treated with 20, 40 and 80 µM of
luteolin for 24 h, and then harvested. The total cell lysates were
prepared to determine Tyro3, Axl and MerTK protein levels by
western blot analysis. GAPDH was used as a loading control and the
results shown are a representative of at least three independent
experiments. (B) For RT-PCR, H460 cells (3×105 cells)
were seeded into 60 mm culture dishes, grown overnight and treated
with the indicated concentrations of luteolin for 24 h. Total RNAs
from the cells were isolated and used to determine Axl mRNA levels.
GAPDH mRNA was also amplified by RT-PCR as an internal control. The
data shown are a representative of three independent experiments
The asterisks indicate a significant difference compared to the
control value (*P<0.05, **P<0.01, vs. the untreated group).
(C) To assess the effect of luteolin on Axl promoter activity, the
H460 cells (3×104 cells) were transfected with pGL3-Axl
using Lipofectamine 2000. The cells were then incubated with 20, 40
and 80 µM of luteolin for 30, 60, 120 and 240 min and total cell
lysates were used to measure luciferase activity. Data are
expressed as the means ± SD of triplicate samples conducted in
three independent experiments. (D) H460 cells (3×105
cells/dish) were pre-incubated with 40 µM of luteolin for 60 min
and stimulated with Gas6 (250 ng/ml) for 20 min. Phospho- and total
Axl protein levels were determined by western blot analysis. Total
Axl protein level was used as a loading control. Results shown are
representative of three independent experiments. |
Consistent with the western blot results, the
inhibitory effect of luteolin on the Axl gene expression was
further demonstrated by RT-PCR and assessment of Axl
promoter activity. As shown in Fig.
2B, the mRNA level of Axl was decreased by luteolin treatment
in a dose-dependent manner. Additionally, H460 cells transfected
with pGL3-Axl, the Axl promoter-luciferase reporter plasmid,
were treated with 0, 20, 40 and 80 µM of luteolin for the indicated
time periods and luciferase activity was found to be
dose-dependently decreased by luteolin treatment (Fig. 2C), indicating that luteolin
suppresses Axl expression at the transcriptional level.
Since growth arrest-specific gene 6 (Gas6) binds to
all three TAM RTKs and is highly specific to Axl (5), we next observed the effect of luteolin
on Axl activation upon ligand binding. Serum-starved H460 cells
were pre-treated with luteolin and then stimulated with Gas6. As
illustrated in Fig. 2D,
Gas6-induced Axl phosphorylation was significantly inhibited by
luteolin, indicating that luteolin suppresses tyrosine kinase
activity of Axl which is mediated by autophosphorylation of
tyrosine residues in the intracellular kinase domain in response to
ligand binding (6).
Luteolin affects the expression of
Axl, but not IL-8, to exert its antiproliferative effect
To validate the association of Axl in the
antiproliferative effects of luteolin, we examined its cytotoxicity
toward the H460 cells in which the Axl protein was overexpressed or
knocked down, respectively. As shown in Fig. 3A, cells which were transfected with
pcDNA3-Axl containing Axl cDNA for ectopic expression of Axl
were found to be a less sensitive to luteolin than cells
transfected with the pcDNA3 vector (Fig. 3A). Colony formation assay results
also showed that clonogenicity of Axl-overexpressing cells was less
affected by luteolin treatment compared with the cells transfected
with pcDNA3, since luteolin treatment led H460/pc-DNA3 cells to
form less colonies and the size of each colony was smaller than
that of the H460/pc-DNA3-Axl cells (Fig. 3B). Consistent with the cell
viability and colony formation assays, the Axl protein level in the
H460/pcDNA3-Axl cells was found to be higher than that in
the control cells even after luteolin treatment (Fig. 3C). These results point to the fact
that overexpression of Axl attenuates the antiproliferative effect
of luteolin.
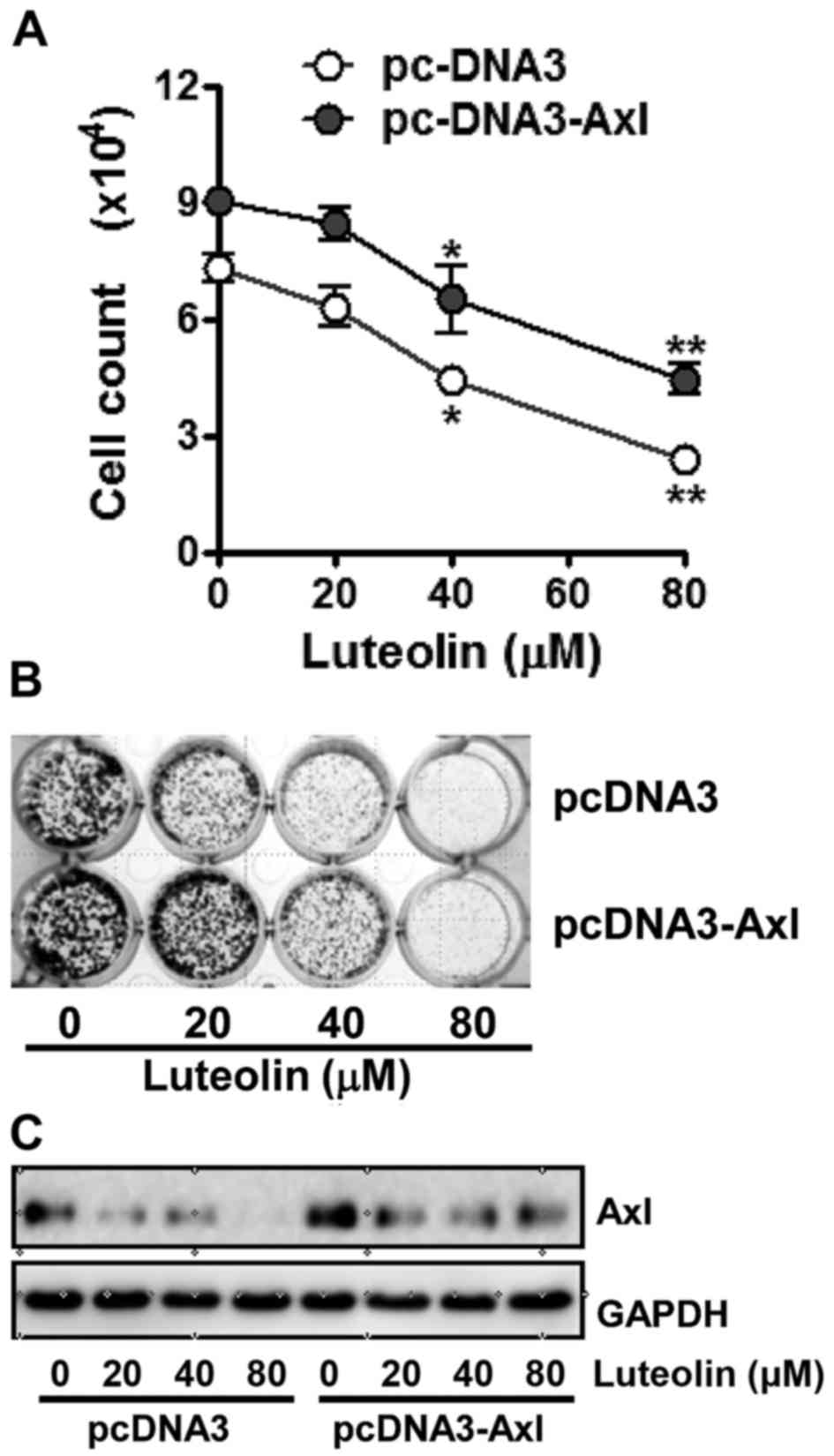 | Figure 3.Antiproliferative effect of luteolin
is decreased by Axl overexpression in non-small lung cancer cells.
H460 cells were transfected with pcDNA3 or pcDNA3-Axl plasmid using
Lipofectamine 2000. (A) The transfected cells (3×105
cells/dish) were treated with 20, 40 and 80 µM of luteolin for 24
h, then harvested, and stained with trypan blue in order to count
the viable cells. Data are expressed as the means ± SD from three
independent experiments. The asterisks indicate a significant
difference compared to the control value (*P<0.05, **P<0.01,
vs. the untreated group). (B) Cells (2×103 cells/well)
transfected with pcDNA3 or pcDNA3-Axl plasmid were seeded into
24-well culture plates, grown overnight, exposed to 20, 40 and 80
µM of luteolin, and allowed to grow for the next 7–10 days. The
colonies were visualized by crystal violet staining. The data shown
are representative of at least three independent experiments. (C)
The transfected cells (3×105 cells/dish) were treated
with 20, 40 and 80 µM of luteolin for 24 h, then harvested, and Axl
protein levels were determined by western blot analysis. GAPDH was
used as a loading control and results shown are representative of
at least three independent experiments. |
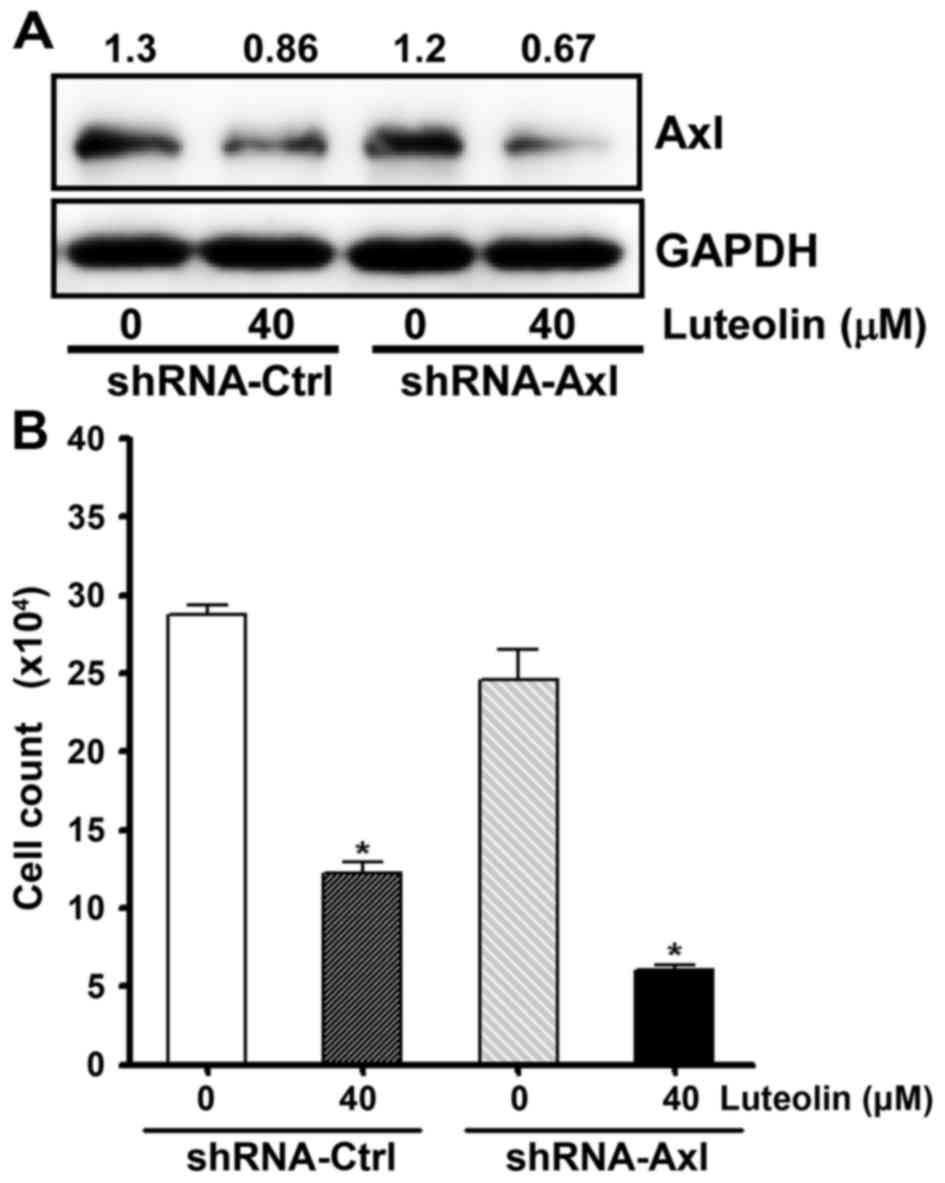
Next, we examined whether knockdown of Axl
increases the antiproliferative effect of luteolin. To decrease Axl
expression, we used gold nanoparticle (AuNP)-assisted gene delivery
systems (GDS) (44,45). H460 cells were incubated with AuNP
GDS conjugates which were annealed with Axl-specific shRNA, or
control shRNA. As shown in Fig. 4A,
AuNP-GDS-Axl conjugates resulted in the additional decrease
in Axl expression following luteolin treatment. The cell viability
assay also showed that the AuNP-GDS-Axl conjugates increased
cytotoxicity of luteolin (Fig. 4B).
These results indicate that the amount of Axl protein was tightly
correlated with cell viability and imply that luteolin exerts an
antiproliferative effect via the downregulation of Axl expression.
Collectively, these results indicate that luteolin inhibits Axl
expression, concomitantly induces the protein level of p21, and
subsequently abrogates cell proliferation.
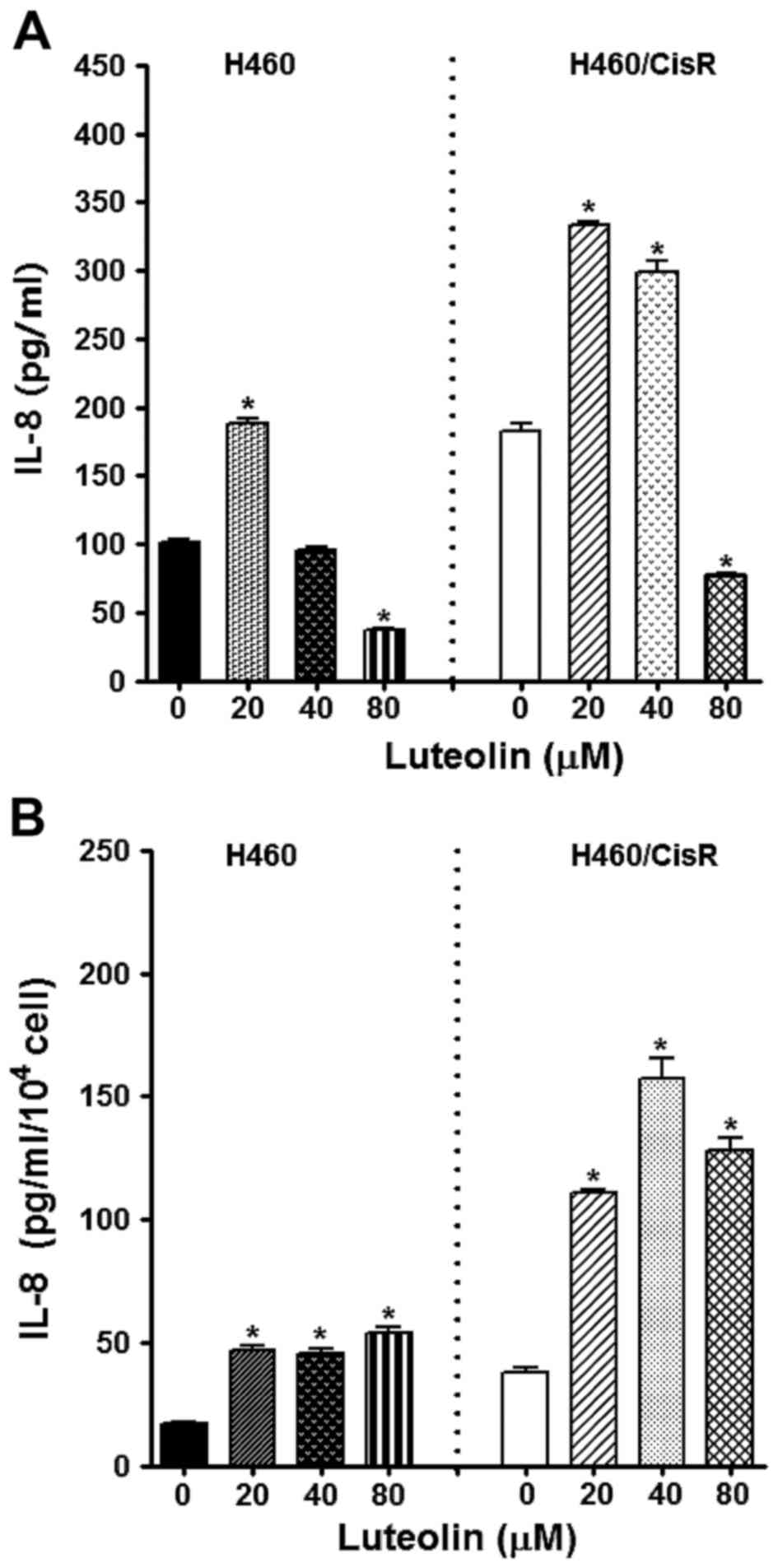
Since IL-8, a multifunctional inflammatory cytokine,
has been reported to be associated with cell survival, growth,
angiogenesis and metastasis in various types of cancers such as
melanoma, lung cancer, nasopharyngeal, hepatocellular, ovarian,
colorectal and prostate cancer (33,46–49),
we observed the effect of luteolin on IL-8 expression. ELISA
results showed that the IL-8 production in cells treated with 80 µM
luteolin was decreased, whereas 20 and 40 µM luteolin rather
increased IL-8 level (Fig. 5A).
Notably, IL-8 expression/cell was found to be fairly increased by
luteolin treatment (Fig. 5B),
indicating that the antiproliferative effect of luteolin was not
associated with dysregulation of IL-8 expression.
Discussion
A series of recent studies have shown the positive
correlation between the expression levels of Axl and the resistance
to anticancer drugs, invasiveness and poor outcome in several types
of cancer. Moreover, Axl silencing was found to significantly
reduce the cell proliferation in NSCLC cells. Therefore, Axl has
received more and more attention as a promising therapeutic target
in the restriction of disease (50).
In the present study, luteolin was found to suppress
expression of all three TAM receptor tyrosine kinases (RTKs)
(Fig. 2A). We also observed the
inhibitory effect of luteolin on Axl expression (Fig. 2B and C) and Gas6-induced activation
of Axl (Fig. 2D). In addition, our
data demonstrated that overexpression of Axl by ectopic expression
of the Axl gene or knockdown of the Axl protein levels by
gold nanoparticle-assisted gene delivery system led to the
attenuation or increase of luteolin-induced cytotoxicity,
respectively (Figs. 3A and B, and
4B). These results strongly
indicate that luteolin targets TAM RTKs, particularly Axl, to
function as an anticancer drug. Notably, some increase in the
protein level of Axl was found in cisplatin-resistant A549/CisR and
H460/CisR cells (Fig. 2A), which
appears to be a strategy in order to survive in the presence of
cisplatin and/or a mechanism for the acquisition of
cisplatin-resistance. However, cisplatin-resistant cells were still
sensitive enough to luteolin in spite of the elevated Axl protein
level of these cells, suggesting that luteolin can potentially
overcome chemoresistance and that there must be other molecules
including Tyro3 and MerTK which are affected by luteolin and
contribute to its cytotoxicity.
Increasing evidence indicates that cancer-related
inflammation of which cytokines are key constituents promotes
survival and proliferation of malignant cells (47), supports angiogenesis and metastasis
(51–53), and evokes epithelial-to-mesenchymal
transition (EMT) (54,55) and chemoresistance against anticancer
agents (33,56,57).
Interleukin-8 (IL-8) has been reported to be overexpressed in tumor
tissue and associated with advanced stage disease and poor
prognosis (48). Consistent with
previous studies (48,58), we found that the level of IL-8 was
increased in the cisplatin-resistant H460/CisR cells compared to
the parental H460 cells. However, luteolin did not appear to be
useful in modulating the levels of IL-8, since only a high
concentration of luteolin (80 µM) was found to decrease the
expression IL-8 and relatively low concentrations of luteolin (20
and 40 µM) were observed to elevate the levels of IL-8. In
parallel, we also found that IL-8 production from a single cell was
increased by luteolin. Our results indicate that an adjuvant
therapy which decreases the levels of IL-8 or blocks IL-8-mediated
signaling pathways may result in a synergistic outcome in cancer
treatment. Therefore, IL-8 is a noteworthy target for improved
cancer treatment.
In summary, we demonstrated that luteolin suppressed
the expression of TAM RTKs, but not IL-8, and activation of Axl
upon Gas6 binding, facilitated the inhibitory effect of luteolin on
cell proliferation in both the parental and cisplatin-resistant
NSCLC cells. Thus, our data imply that TAM RTKs may be potent
therapeutic targets of luteolin by which it exerts its anticancer
activity, particularly to circumvent chemoresistance in NSCLC
cells.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (2014R1A1A2056565),
Korea.
Glossary
Abbreviations
Abbreviations:
|
AuNP
|
gold nanoparticle
|
|
IL-8
|
interleukin-8
|
|
Gas6
|
growth arrest-specific 6
|
|
GDS
|
gene delivery system
|
|
NSCLC
|
non-small cell lung cancer
|
|
RTK
|
receptor tyrosine kinase
|
References
|
1
|
Stinchcombe TE and Socinski MA: Treatment
paradigms for advanced stage non-small cell lung cancer in the era
of multiple lines of therapy. J Thorac Oncol. 4:243–250. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lemke G: Biology of the TAM receptors.
Cold Spring Harb Perspect Biol. 5:a0090762013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lemke G and Rothlin CV: Immunobiology of
the TAM receptors. Nat Rev Immunol. 8:327–336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lemke G and Burstyn-Cohen T: TAM receptors
and the clearance of apoptotic cells. Ann NY Acad Sci. 1209:23–29.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagata K, Ohashi K, Nakano T, Arita H,
Zong C, Hanafusa H and Mizuno K: Identification of the product of
growth arrest-specific gene 6 as a common ligand for Axl, Sky, and
Mer receptor tyrosine kinases. J Biol Chem. 271:30022–30027. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Braunger J, Schleithoff L, Schulz AS,
Kessler H, Lammers R, Ullrich A, Bartram CR and Janssen JW:
Intracellular signaling of the Ufo/Axl receptor tyrosine kinase is
mediated mainly by a multi-substrate docking-site. Oncogene.
14:2619–2631. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sasaki T, Knyazev PG, Clout NJ, Cheburkin
Y, Göhring W, Ullrich A, Timpl R and Hohenester E: Structural basis
for Gas6-Axl signalling. EMBO J. 25:80–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Bryan JP, Frye RA, Cogswell PC, Neubauer
A, Kitch B, Prokop C, Espinosa R III, Le Beau MM, Earp HS and Liu
ET: axl, a transforming gene isolated from primary human myeloid
leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell
Biol. 11:5016–5031. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rochlitz C, Lohri A, Bacchi M, Schmidt M,
Nagel S, Fopp M, Fey MF, Herrmann R and Neubauer A: Axl expression
is associated with adverse prognosis and with expression of Bcl-2
and CD34 in de novo acute myeloid leukemia (AML): Results from a
multicenter trial of the Swiss Group for Clinical Cancer Research
(SAKK). Leukemia. 13:1352–1358. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berclaz G, Altermatt HJ, Rohrbach V,
Kieffer I, Dreher E and Andres AC: Estrogen dependent expression of
the receptor tyrosine kinase axl in normal and malignant human
breast. Ann Oncol. 12:819–824. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Craven RJ, Xu LH, Weiner TM, Fridell YW,
Dent GA, Srivastava S, Varnum B, Liu ET and Cance WG: Receptor
tyrosine kinases expressed in metastatic colon cancer. Int J
Cancer. 60:791–797. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nemoto T, Ohashi K, Akashi T, Johnson JD
and Hirokawa K: Overexpression of protein tyrosine kinases in human
esophageal cancer. Pathobiology. 65:195–203. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rankin EB, Fuh KC, Taylor TE, Krieg AJ,
Musser M, Yuan J, Wei K, Kuo CJ, Longacre TA and Giaccia AJ: AXL is
an essential factor and therapeutic target for metastatic ovarian
cancer. Cancer Res. 70:7570–7579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sainaghi PP, Castello L, Bergamasco L,
Galletti M, Bellosta P and Avanzi GC: Gas6 induces proliferation in
prostate carcinoma cell lines expressing the Axl receptor. J Cell
Physiol. 204:36–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ito T, Ito M, Naito S, Ohtsuru A, Nagayama
Y, Kanematsu T, Yamashita S and Sekine I: Expression of the Axl
receptor tyrosine kinase in human thyroid carcinoma. Thyroid.
9:563–567. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Byers LA, Diao L, Wang J, Saintigny P,
Girard L, Peyton M, Shen L, Fan Y, Giri U, Tumula PK, et al: An
epithelial-mesenchymal transition gene signature predicts
resistance to EGFR and PI3K inhibitors and identifies Axl as a
therapeutic target for overcoming EGFR inhibitor resistance. Clin
Cancer Res. 19:279–290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Asiedu MK, Beauchamp-Perez FD, Ingle JN,
Behrens MD, Radisky DC and Knutson KL: AXL induces
epithelial-to-mesenchymal transition and regulates the function of
breast cancer stem cells. Oncogene. 33:1316–1324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu F, Li J, Jang C, Wang J and Xiong J:
The role of Axl in drug resistance and epithelial-to-mesenchymal
transition of non-small cell lung carcinoma. Int J Clin Exp Pathol.
7:6653–6661. 2014.PubMed/NCBI
|
|
19
|
Brand TM, Iida M, Stein AP, Corrigan KL,
Braverman CM, Coan JP, Pearson HE, Bahrar H, Fowler TL, Bednarz BP,
et al: AXL is a logical molecular target in head and neck squamous
cell carcinoma. Clin Cancer Res. 21:2601–2612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wimmel A, Glitz D, Kraus A, Roeder J and
Schuermann M: Axl receptor tyrosine kinase expression in human lung
cancer cell lines correlates with cellular adhesion. Eur J Cancer.
37:2264–2274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shieh YS, Lai CY, Kao YR, Shiah SG, Chu
YW, Lee HS and Wu CW: Expression of axl in lung adenocarcinoma and
correlation with tumor progression. Neoplasia. 7:1058–1064. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Lee JC, Lin L, Olivas V, Au V,
LaFramboise T, Abdel-Rahman M, Wang X, Levine AD, Rho JK, et al:
Activation of the AXL kinase causes resistance to EGFR-targeted
therapy in lung cancer. Nat Genet. 44:852–860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rho JK, Choi YJ, Kim SY, Kim TW, Choi EK,
Yoon SJ, Park BM, Park E, Bae JH, Choi CM, et al: MET and AXL
inhibitor NPS-1034 exerts efficacy against lung cancer cells
resistant to EGFR kinase inhibitors because of MET or AXL
activation. Cancer Res. 74:253–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ye X, Li Y, Stawicki S, Couto S,
Eastham-Anderson J, Kallop D, Weimer R, Wu Y and Pei L: An anti-Axl
monoclonal antibody attenuates xenograft tumor growth and enhances
the effect of multiple anticancer therapies. Oncogene.
29:5254–5264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J,
Kallop D, Ludlam MJ and Pei L: Axl as a potential therapeutic
target in cancer: Role of Axl in tumor growth, metastasis and
angiogenesis. Oncogene. 28:3442–3455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen QY, Xu LQ, Jiao DM, Yao QH, Wang YY,
Hu HZ, Wu YQ, Song J, Yan J and Wu LJ: Silencing of Rac1 modifies
lung cancer cell migration, invasion and actin cytoskeleton
rearrangements and enhances chemosensitivity to antitumor drugs.
Int J Mol Med. 28:769–776. 2011.PubMed/NCBI
|
|
27
|
Gastonguay A, Berg T, Hauser AD, Schuld N,
Lorimer E and Williams CL: The role of Rac1 in the regulation of
NF-κB activity, cell proliferation, and cell migration in non-small
cell lung carcinoma. Cancer Biol Ther. 13:647–656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scheibenbogen C, Möhler T, Haefele J,
Hunstein W and Keilholz U: Serum interleukin-8 (IL-8) is elevated
in patients with metastatic melanoma and correlates with tumour
load. Melanoma Res. 5:179–181. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Browne A, Sriraksa R, Guney T, Rama N, Van
Noorden S, Curry E, Gabra H, Stronach E and El-Bahrawy M:
Differential expression of IL-8 and IL-8 receptors in benign,
borderline and malignant ovarian epithelial tumours. Cytokine.
64:413–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim SJ, Uehara H, Karashima T, Mccarty M,
Shih N and Fidler IJ: Expression of interleukin-8 correlates with
angiogenesis, tumorigenicity, and metastasis of human prostate
cancer cells implanted orthotopically in nude mice. Neoplasia.
3:33–42. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Qu Y, Niu XL, Sun WJ, Zhang XL and
Li LZ: Autocrine production of interleukin-8 confers cisplatin and
paclitaxel resistance in ovarian cancer cells. Cytokine.
56:365–375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stronach EA, Cunnea P, Turner C, Guney T,
Aiyappa R, Jeyapalan S, de Sousa CH, Browne A, Magdy N, Studd JB,
et al: The role of interleukin-8 (IL-8) and IL-8 receptors in
platinum response in high grade serous ovarian carcinoma.
Oncotarget. 6:31593–31603. 2015.PubMed/NCBI
|
|
33
|
Duan Z, Feller AJ, Penson RT, Chabner BA
and Seiden MV: Discovery of differentially expressed genes
associated with paclitaxel resistance using cDNA array technology:
Analysis of interleukin (IL) 6, IL-8, and monocyte chemotactic
protein 1 in the paclitaxel-resistant phenotype. Clin Cancer Res.
5:3445–3453. 1999.PubMed/NCBI
|
|
34
|
Fernando RI, Hamilton DH, Dominguez C,
David JM, McCampbell KK and Palena C: IL-8 signaling is involved in
resistance of lung carcinoma cells to erlotinib. Oncotarget.
7:42031–4204. 2016.PubMed/NCBI
|
|
35
|
Liu YN, Chang TH, Tsai MF, Wu SG, Tsai TH,
Chen HY, Yu SL, Yang JC and Shih JY: IL-8 confers resistance to
EGFR inhibitors by inducing stem cell properties in lung cancer.
Oncotarget. 6:10415–10431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Giles KM, Kalinowski FC, Candy PA, Epis
MR, Zhang PM, Redfern AD, Stuart LM, Goodall GJ and Leedman PJ: Axl
mediates acquired resistance of head and neck cancer cells to the
epidermal growth factor receptor inhibitor erlotinib. Mol Cancer
Ther. 12:2541–2558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gong J, Morishita A, Kurokohchi K, Tani J,
Kato K, Miyoshi H, Inoue H, Kobayashi M, Liu S, Murota M, et al:
Use of protein array to investigate receptor tyrosine kinases
activated in gastric cancer. Int J Oncol. 36:101–106.
2010.PubMed/NCBI
|
|
38
|
Tu SH, Ho CT, Liu MF, Huang CS, Chang HW,
Chang CH, Wu CH and Ho YS: Luteolin sensitises drug-resistant human
breast cancer cells to tamoxifen via the inhibition of cyclin E2
expression. Food Chem. 141:1553–1561. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chian S, Li YY, Wang XJ and Tang XW:
Luteolin sensitizes two oxaliplatin-resistant colorectal cancer
cell lines to chemotherapeutic drugs via inhibition of the Nrf2
pathway. Asian Pac J Cancer Prev. 15:2911–2916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qu Q, Qu J, Guo Y, Zhou BT and Zhou HH:
Luteolin potentiates the sensitivity of colorectal cancer cell
lines to oxaliplatin through the PPARγ/OCTN2 pathway. Anticancer
Drugs. 25:1016–1027. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin Y, Shi R, Wang X and Shen HM:
Luteolin, a flavonoid with potential for cancer prevention and
therapy. Curr Cancer Drug Targets. 8:634–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hutterer M, Knyazev P, Abate A, Reschke M,
Maier H, Stefanova N, Knyazeva T, Barbieri V, Reindl M, Muigg A, et
al: Axl and growth arrest-specific gene 6 are frequently
overexpressed in human gliomas and predict poor prognosis in
patients with glioblastoma multiforme. Clin Cancer Res. 14:130–138.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gustafsson A, Martuszewska D, Johansson M,
Ekman C, Hafizi S, Ljungberg B and Dahlbäck B: Differential
expression of Axl and Gas6 in renal cell carcinoma reflecting tumor
advancement and survival. Clin Cancer Res. 15:4742–4749. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ryou SM, Kim JM, Yeom JH, Hyun S, Kim S,
Han MS, Kim SW, Bae J, Rhee S and Lee K: Gold nanoparticle-assisted
delivery of small, highly structured RNA into the nuclei of human
cells. Biochem Biophys Res Commun. 416:178–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rosi NL, Giljohann DA, Thaxton CS,
Lytton-Jean AK, Han MS and Mirkin CA: Oligonucleotide-modified gold
nanoparticles for intracellular gene regulation. Science.
312:1027–1030. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Araki S, Omori Y, Lyn D, Singh RK,
Meinbach DM, Sandman Y, Lokeshwar VB and Lokeshwar BL:
Interleukin-8 is a molecular determinant of androgen independence
and progression in prostate cancer. Cancer Res. 67:6854–6862. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Luppi F, Longo AM, de Boer WI, Rabe KF and
Hiemstra PS: Interleukin-8 stimulates cell proliferation in
non-small cell lung cancer through epidermal growth factor receptor
transactivation. Lung Cancer. 56:25–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cheng D, Kong H and Li Y: Prognostic value
of interleukin-8 and MMP-9 in nasopharyngeal carcinoma. Eur Arch
Otorhinolaryngol. 271:503–509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Levin PA, Brekken RA, Byers LA, Heymach JV
and Gerber DE: Axl receptor axis: A new therapeutic target in lung
cancer. J Thorac Oncol. 11:1357–1362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shi J and Wei PK: Interleukin-8: A potent
promoter of angiogenesis in gastric cancer. Oncol Lett.
11:1043–1050. 2016.PubMed/NCBI
|
|
52
|
Lin Y, Huang R, Chen L, Li S, Shi Q,
Jordan C and Huang RP: Identification of interleukin-8 as estrogen
receptor-regulated factor involved in breast cancer invasion and
angiogenesis by protein arrays. Int J Cancer. 109:507–515. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kubo F, Ueno S, Hiwatashi K, Sakoda M,
Kawaida K, Nuruki K and Aikou T: Interleukin 8 in human
hepatocellular carcinoma correlates with cancer cell invasion of
vessels but not with tumor angiogenesis. Ann Surg Oncol.
12:800–807. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Long X, Ye Y, Zhang L, Liu P, Yu W, Wei F,
Ren X and Yu J: IL-8, a novel messenger to cross-link inflammation
and tumor EMT via autocrine and paracrine pathways (Review). Int J
Oncol. 48:5–12. 2016.PubMed/NCBI
|
|
55
|
Fu XT, Dai Z, Song K, Zhang ZJ, Zhou ZJ,
Zhou SL, Zhao YM, Xiao YS, Sun QM, Ding ZB, et al:
Macrophage-secreted IL-8 induces epithelial-mesenchymal transition
in hepatocellular carcinoma cells by activating the
JAK2/STAT3/Snail pathway. Int J Oncol. 46:587–596. 2015.PubMed/NCBI
|
|
56
|
Duan Z, Lamendola DE, Penson RT, Kronish
KM and Seiden MV: Overexpression of IL-6 but not IL-8 increases
paclitaxel resistance of U-2OS human osteosarcoma cells. Cytokine.
17:234–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Maheshwari A, Lu W, Guida WC, Christensen
RD and Calhoun DA: IL-8/CXC ligand 8 survives neonatal gastric
digestion as a result of intrinsic aspartyl proteinase resistance.
Pediatr Res. 57:438–444. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zuco V, Cassinelli G, Cossa G, Gatti L,
Favini E, Tortoreto M, Cominetti D, Scanziani E, Castiglioni V,
Cincinelli R, et al: Targeting the invasive phenotype of
cisplatin-resistant non-small cell lung cancer cells by a novel
histone deacetylase inhibitor. Biochem Pharmacol. 94:79–90. 2015.
View Article : Google Scholar : PubMed/NCBI
|