Introduction
Hepatocellular carcinoma (HCC), one of the most
common malignancies, represents the third leading cause of
cancer-related deaths worldwide (1). Although screening in high-risk
populations has increased the detection of early-stage HCC and
consequently improved survival, most cases still present with
advanced and unresectable stages due to intrahepatic metastasis and
vascular invasion (2,3). Seeking alternative therapies to
improve the survival of HCC patients has been an urgent task for
oncologists.
A proliferation-inducing ligand (APRIL), also known
as TALL-2 and TNFSF13, was identified via database mining in 1998
by Hahne et al (4). APRIL is
a member of the TNF family located on human chromosome 17p13. APRIL
expression is low in normal cells, including monocytes, dendritic
and T cells (5,6). However, studies have demonstrated that
it is overexpressed in many tumors, which suggest that APRIL plays
a crucial role in the occurrence and development of these tumors
(7–9).
Heat-clearing and detoxifying Chinese herbs, which
play an important role in antitumor therapy in China, involve the
use of extracts from these herbs for the treatment of different
types of cancer (10–12). Jiedu Xiaozheng Yin (JXY), a
polyherbal Traditional Chinese Medicine (TCM) recipe, is composed
of Hedyotis diffusa Willd (HDW), Sophora flavescens
(SF), Prunella and Pseudobulbus Cremastrae (PC) and
is used as a heat-clearing and detoxicating adjuvant therapy for
HCC. Our previous study demonstrated that JXY can inhibit the
angiogenesis of tumors via the downregulation of vascular
endothelial growth factor-A (VEGF-A) and vascular endothelial
growth factor receptor-2 (VEGFR-2) expression (13). Moreover, an ethyl acetate extract
from JXY inhibits the cell proliferation of HCC by suppressing
polycomb gene product Bmi1 and Wnt/β-catenin signaling (14,15). A
randomized control trial showed that the addition of JXY to the
standard treatment of stage III HCC patients could improve the
immune function of patients, decrease recurrence and increase
overall survival (16).
In the present study, we evaluated the synergistic
effect of APRIL knockdown and JXY treatment on the proliferation of
HCC cells and elucidated the underlying mechanism.
Materials and methods
Preparation of the herbal
medicine
JXY is composed of HDW (30 g), Prunella (15
g), PC (15 g) and SF (15 g). These four herbs of JXY were purchased
from the Guo Yi Tang Hospital of Fujian University of Traditional
Chinese Medicine (Fuzhou, China). The quality of the 4 medicinal
plants met the criterion of the Pharmacopoeia of the People's
Republic of China. To prepare the crude water extract, 300, 150,
150 and 150 g of the 4 medicinal plants, HDW, Prunella, PC
and SF, respectively, were crushed into powders. Then, they were
mixed together and immersed in 10 l of distilled water. The mixture
was simmered for 2 h and filtered. Subsequently, the solution was
concentrated by a rotary evaporator and stored at 4°C until
use.
Animals
The clean grade level male Sprague-Dawley (SD) rats
(licence no. SCXK (Hu) 2007–0005), with a body weight of 180–220 g,
were purchased from Shanghai SLAC Experimental Animal Co., Ltd.
(Shanghai, China). All rats were maintained under a regulated
temperature (18–22°C) and a relative constant humidity (50–60%).
All animal handling and experimental procedures were approved by
the Animal Care and Use Committee of Fujian University of TCM.
Preparation of JXY-containing
serum
The 20 SD rats were divided into 4 groups using a
random digit table method (n=10/group) and were administered JXY
solution orally at a dose of 9 g/kg/day or saline respectively
twice a day for 7 days. One hour after the last treatment, blood
was collected from the main ventral artery under aseptic
conditions, and centrifuged at 3,000 rpm for 15 min. Serum from the
2 groups was referred to as JXY and control serums, respectively.
All sera were filtered through a 0.22-µm filter membrane and
inactivated at 56°C water for 30 min and stored at −20°C until its
use in pharmacological studies.
Cell culture
The HepG2 HCC cell line was purchased from the Cell
Bank of the Shanghai Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences (Shanghai, China), and cultured in
high-glucose Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) and
incubated in 5% CO2 at 37°C.
Real-time-quantitative polymerase
chain reaction (RT-qPCR)
Total RNA was prepared using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer's
instructions. Total RNA (2 µg) was used to synthesize the first
strand of cDNA. qPCR was performed using an Applied Biosystems 7500
Real-Time PCR system and SYBR® Premix Ex Taq™kit
(Perfect Real-Time) (Takara Bio Inc., Shiga, Japan). Primers used
were as follows: Tumor necrosis factor receptor-associated factor 6
(TRAF6) sense, 5′-CCCGCGCACTAGAACGAGCAA-3′ and antisense,
5′-GCCATGGCCACACAGCAGTCA-3′; NF-κB sense,
5′-CGCGCCGCTTAGGAGGGAGA-3′ and antisense,
5′-GGGCCATCTGCTGTTGGCAGT-3′; β-actin sense,
5′-CAATGAAGATCAAGATCATTGCTCCTCC-3′ and antisense,
5′-TCAAGAAAGGGTGTAACGCAACTAAGTC-3′. The expression of β-actin
served as the input reference. The relative mRNA expression of
TRAF6 and NF-κB were calculated with the comparative threshold
cycle (Ct) (2−ΔΔCt) method.
Protein extraction and western
blotting
Western blotting was performed as previously
described (17). In brief, cell
pellets were lysed in a radioimmunoprecipitation assay (RIPA)
buffer with 1 mM phenylmethanesulfonyl fluoride (both from Beyotime
Institute of Biotechnology, Haimen, China). The cell lysate was
centrifuged at 10,000 × g for 10 min at 4°C and the supernatant was
transferred to a microcentrifuge tube. The protein concentration
was determined with a Bicinchoninic Acid Protein Assay kit
(Beyotime Institute of Biotechnology) and 20 µg proteins were
loaded/lane. The protein samples were separated on SDS-PAGE at
10–15%, and electrotransferred onto nitrocellulose membranes
(Amersham Pharmacia Biotech, Zurich, Switzerland). The membranes
were blocked by 5% non-fat milk in Tris-buffered saline Tween-20
(TBST; pH 7.6) for 60 min at room temperature, followed by
incubation in primary antibodies overnight at 4°C and secondary
antibodies (Thermo Scientific Pierce, Rockford, IL, USA) for 90 min
at room temperature. After the membranes had been washed 3 times,
the proteins were detected using electrochemiluminescence (ECL;
Amersham Pharmacia Biotech). The antibodies used for western blot
analyses were as follows: APRIL, NF-κB and β-actin (Cell Signaling
Technology, Beverly, MA, USA).
Knockdown of APRIL
The short hairpin RNA targeting APRIL mRNA was
constructed by GeneChem Biotechnology (Shanghai, China). The shRNA
sequences were:
5′-GATCCGCAACCTTCTTCCCTTCTGCTTCAAGAGAGCAGAAGGGAAGAAGGTTGTTTTTGGAAA-3′
and
5′-AGCTTTTCCAAAAAACAACCTTCTTCCCTTCTGCTCTCTTGAAGCAGAAGGGAAGAAGGTTGCG-3′;
the negative control sequences were:
5′-GATCCGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTTGGAAA-3′
and
5′-AGCTTTTCCAAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAACG-3′.
Transfection with shRNA was carried out using
Lipofectamine™ 2000 according to the manufacturer's instructions
(Invitrogen). Cells transfected with APRIL-shRNA were seeded into
6-well culture plates at a density of 1×105 cells/well.
Cells were allowed to grow for 24, 36 and 72 h and were then
harvested for analysis. Insignificant control siRNA was used as a
negative control under similar conditions.
Cell viability assay
To analyze the viability of cells treated with JXY
and/or APRIL-shRNA, 1×104 cells/well were seeded in
96-well plates containing 0.2 ml of medium. After treatment, MTT (5
mg/ml; Sigma, St. Louis, MO, USA) was added to each well (including
the control well) and the mixture was incubated at 37°C for 4 h.
The culture medium was then replaced with an equal volume of
dimethyl sulfoxide (Sigma). After shaking at room temperature for
15 min, the 490-nm absorbance (A490) of each well was determined on
a microplate reader (Bio-Rad, Hercules, CA, USA). The cell
viability was calculated according to the following formula: Cell
viability (%) = A490 (sample)/A490 (control) × 100.
TUNEL assay of cell apoptosis
Cells were seeded onto slides and cultured before
being treated with JXY and/or APRIL-shRNA. The cells were then
fixed in 4% paraformaldehyde for 60 min and permeabilized with 0.1%
Triton X-100 on ice for 2 min. Cell apoptosis was determined using
a TUNEL Apoptosis Assay kit (Roche Applied Science, Mannheim,
Germany) according to the manufacturer's instructions 48 h
post-transfection. Briefly, TdT-mediated dUTP nick end labeling
(TUNEL) reaction mixture was added to the cells for 15 min and the
slides were then rinsed in phosphate-buffered saline (PBS) before
being incubated in a humidified chamber at 37°C for 60 min in the
dark. Anti-fluorescence quenching solution was added before
examination of the cells under a confocal laser-scanning microscope
(TCS SP5) at an excitation wavelength of 450–500 nm and an emission
wavelength of 515–565 nm (green fluorescence) in order to evaluate
the proportion of apoptotic cells.
Reporter gene transfection and
luciferase activity assay
Cells at 80–90% confluence growing on a 35-mm dish
were co-transfected with the firefly luciferase reporter of NF-κB
containing a TA promoter (1 µg, pNF-κB-TA-luc; Beyotime
Biotechnology, Shanghai, China) along with the Renilla
luciferase reporter (0.1 µg; Promega Co., Madison, WI, USA) for 6 h
using Lipofectamine™ 2000 according to the manufacturer's
instructions (Invitrogen). Some cells were further treated with JXY
and/or APRIL shRNA. The luciferase activity was assessed in the
cellular extracts using a dual-luciferase reporter gene assay kit
(Beyotime Biotechnology. Briefly, the relative fluorescence light
unit (RLU) at 560 nm of the mixture consisting of 50 µl total cell
lysate and 100 µl of the firefly luciferase assay reagent was
evaluated using a multimode microplate reader (Tecan Infinite M200;
Tecan, Männedorf, Switzerland) for a total period of 10 sec. Then,
100 µl of Renilla luciferase assay reagent was added into
the aforementioned mixture and its fluorescence at 465 nm was
measured. The relative activity of the reporter gene was calculated
by dividing the RLU at 560 nm by that at 465 nm.
Statistical analysis
Data are the results from at least 3 independent
experiments. All data are presented as the means ± SD. Significance
was assessed using one-way ANOVA among groups or unpaired t-test
for 2 groups. All P-values were two-sided, and the differences were
considered significant at a value of P<0.05. All statistical
analyses were carried out using SPSS 18.0.
Results
Synergistic inhibitory effect of APRIL
knockdown and JXY-containing serum on HCC cell proliferation
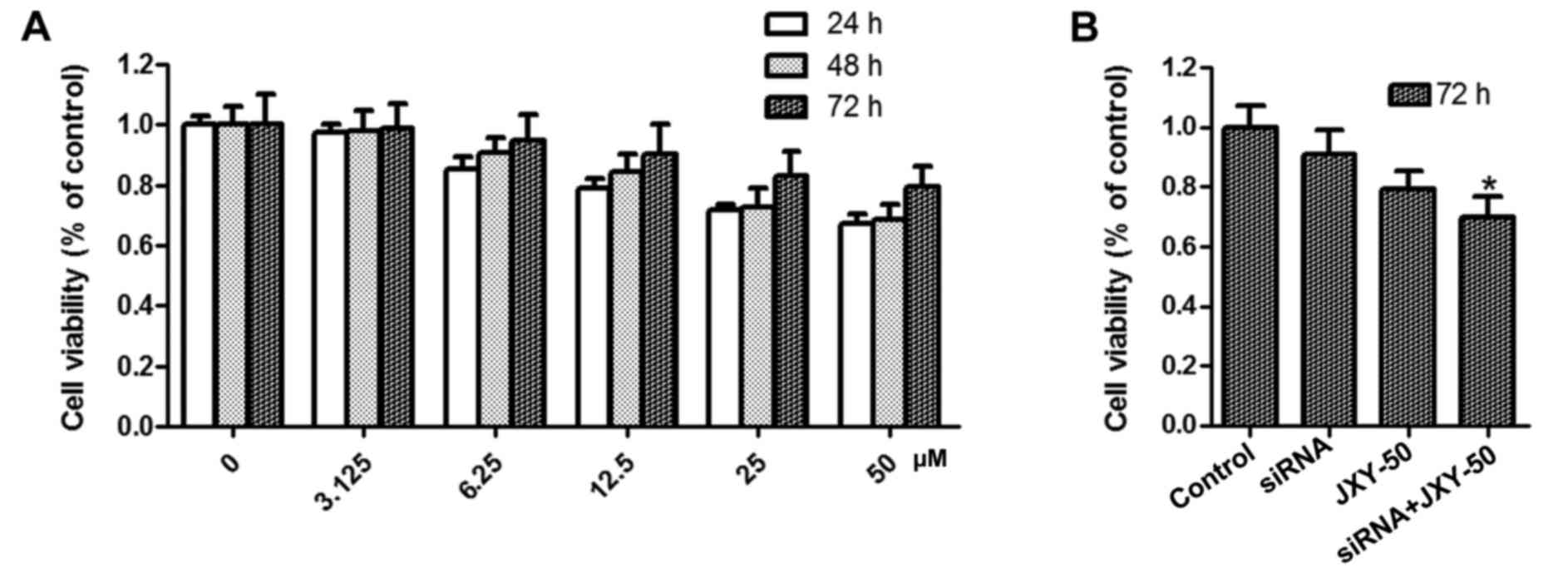
To investigate the effect of APRIL knockdown and/or
JXY treatment on HCC cell proliferation, an MTT assay was performed
in HCC cells following treatment with JXY-containing serum at
different concentrations. As shown in Fig. 1, in comparison with the control
groups, APRIL knockdown had no effect on HCC cell proliferation
while APRIL knockdown significantly enhanced the inhibitory effect
of JXY on cell proliferation (P<0.05). Collectively, these
results indicated that the synergistic effect of APRIL knockdown
and JXY suppressed HCC cell proliferation.
Synergistic effect of APRIL knockdown
and JXY on cell cycle arrest
A DNA cell cycle analysis was performed, in order to
assess the effects of APRIL knockdown and/or JXY treatment on cell
cycle distribution. As shown in Table
I, treatment with JXY or APRIL knockdown resulted in an
accumulation of cells in the G0/G1 phase of the cell cycle.
Notably, the combination of APRIL knockdown and JXY treatment
significantly induced G0/G1 cell cycle arrest (shRNA + Herb
59.01±5.01% vs. Herb 44.69±3.89%; shRNA + Herb 59.01±5.01% vs.
shRNA 49.68±4.76%) (P<0.05).
 | Table I.Synergistic effect of APRIL knockdown
or/and JXY treatment on cell cycle distribution in HCC cells. |
Table I.
Synergistic effect of APRIL knockdown
or/and JXY treatment on cell cycle distribution in HCC cells.
| Treatment | G1 (%) | G2/M (%) | S (%) |
|---|
| Blank | 42.46±2.35 | 38.93±2.03 | 18.16±1.97 |
| Control | 43.39±4.18 | 33.06±3.17 | 23.55±2.52 |
| shRNA | 49.68±4.76 | 37.09±3.63 |
13.23±2.98a |
| Herb | 44.69±3.89 |
22.06±3.53a,b | 33.25±3.43 |
| shRNA + Herb |
59.01±5.01a,b |
20.6±2.99a,b | 20.39±3.05 |
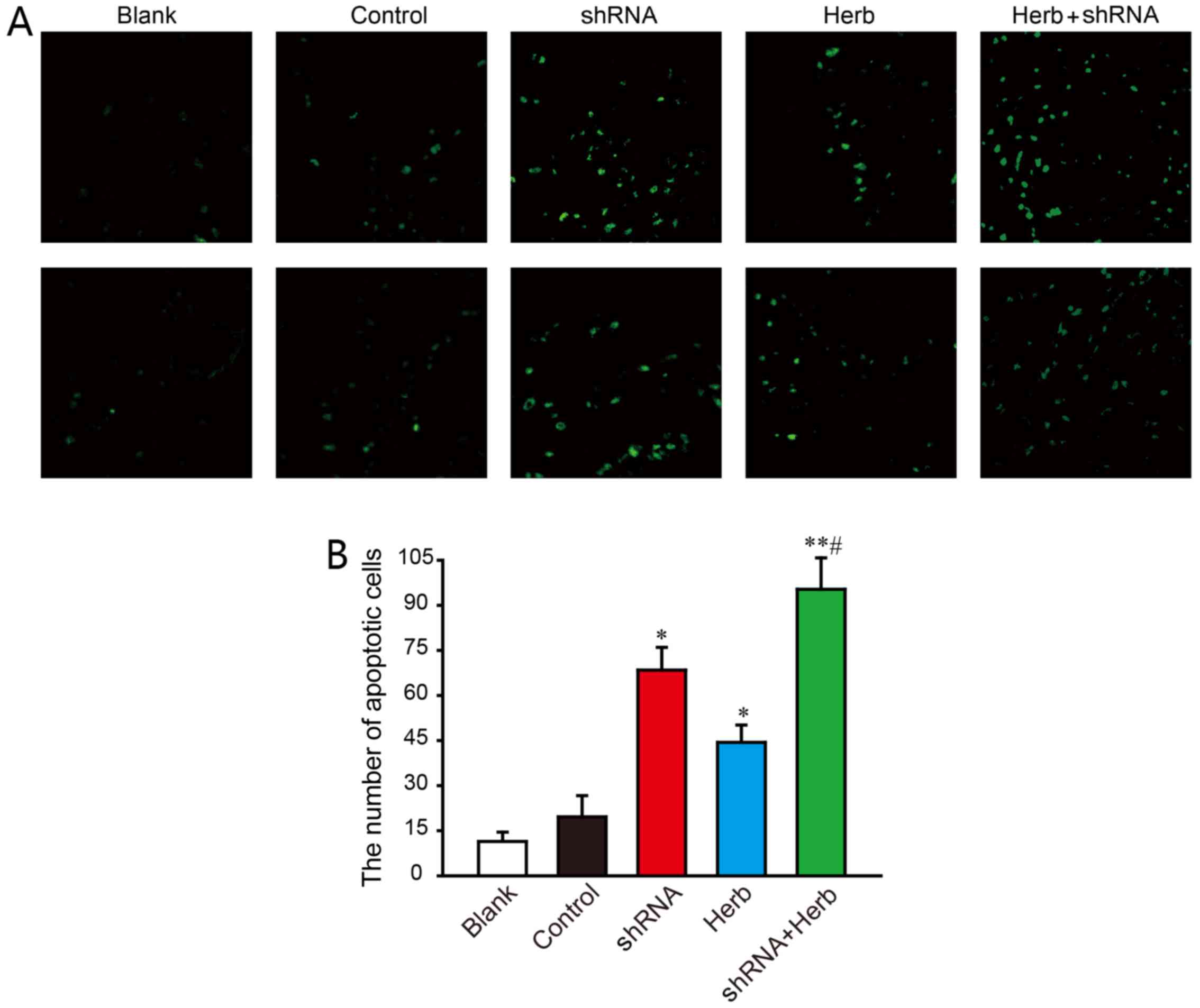
Next, we detected the percentage of cell apoptosis
using TUNEL staining. The results showed that both JXY treatment
and APRIL knockdown increases the percentage of cell apoptosis
(P<0.05; Fig. 2). In addition,
combination of APRIL knockdown and JXY treatment significantly
enhanced the number of apoptotic cells (P<0.05; Fig. 2). Collectively, these results
indicated the synergistic effect of APRIL knockdown and JXY
treatment on inhibition of HCC cell growth possibly through the
induction of G0/G1 cell cycle arrest and the promotion of cell
apoptosis.
Synergistic effect of APRIL knockdown
and JXY treatment inhibits NF-κB expression
TRAF6 is a unique adaptor protein of the tumor
necrosis factor receptor-associated factor family that mediates
both tumor necrosis factor receptors and interleukin-1
receptor/Toll-like receptor signaling. TRAF6 plays a very important
role in NF-κB activation (18,19).
Recent studies have shown that TRAF6/NF-κB signaling pathways play
an important role in tumorigenesis and invasion (20,21).
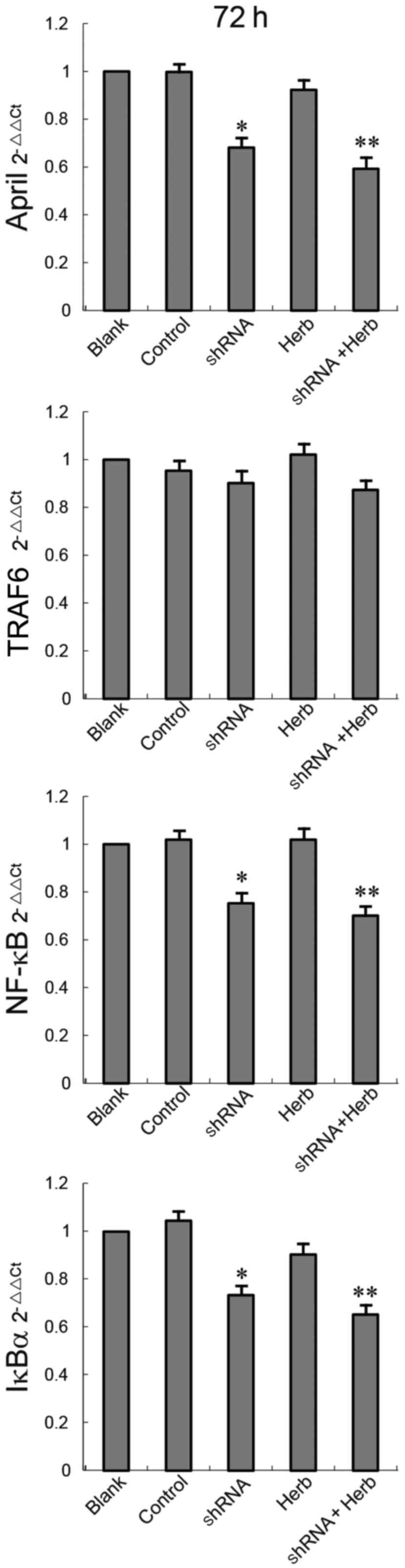
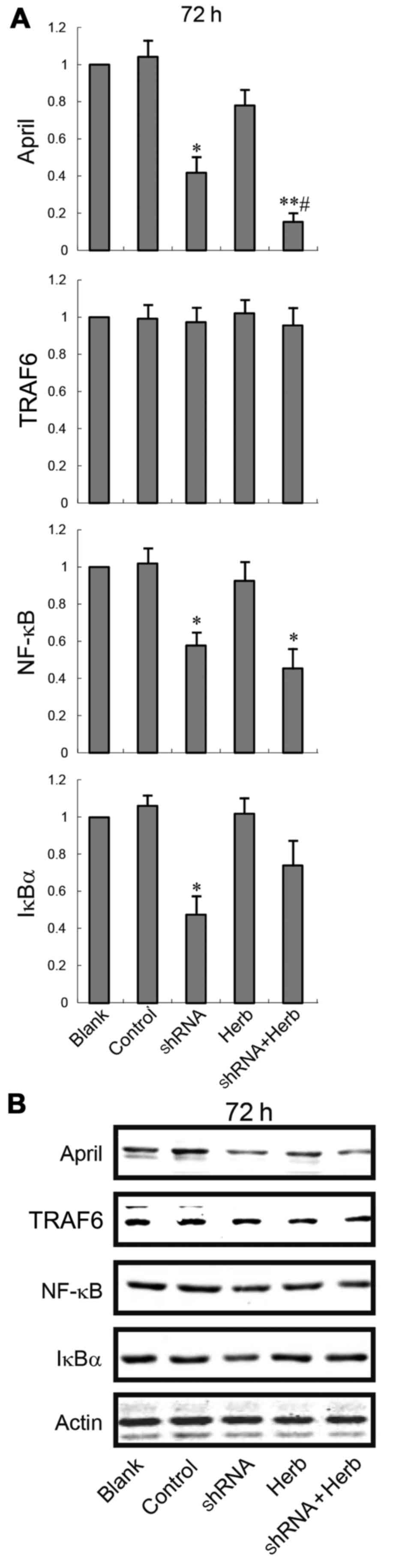
Next, we explored whether APRIL knockdown and/or JXY treatment
could affect TRAF6 and NF-κB expression. RT-qPCR (Fig. 3) and western blotting (Fig. 4) results showed that APRIL knockdown
decreased NF-κB mRNA and protein expression levels (P<0.05), but
no difference was found in the expression level of TRAF6
(P>0.05). In addition, the expression levels of both TRAF6 and
NF-κB were not altered after JXY treatment. However, the
combination of APRIL knockdown and JXY treatment significantly
reduced the expression of NF-κB (P<0.05; Figs. 3 and 4). These results suggest that the
synergistic effect of APRIL knockdown and JXY treatment in the
inhibition of NF-κB expression was dependent on TRAF6
signaling.
Synergistic effect of APRIL knockdown
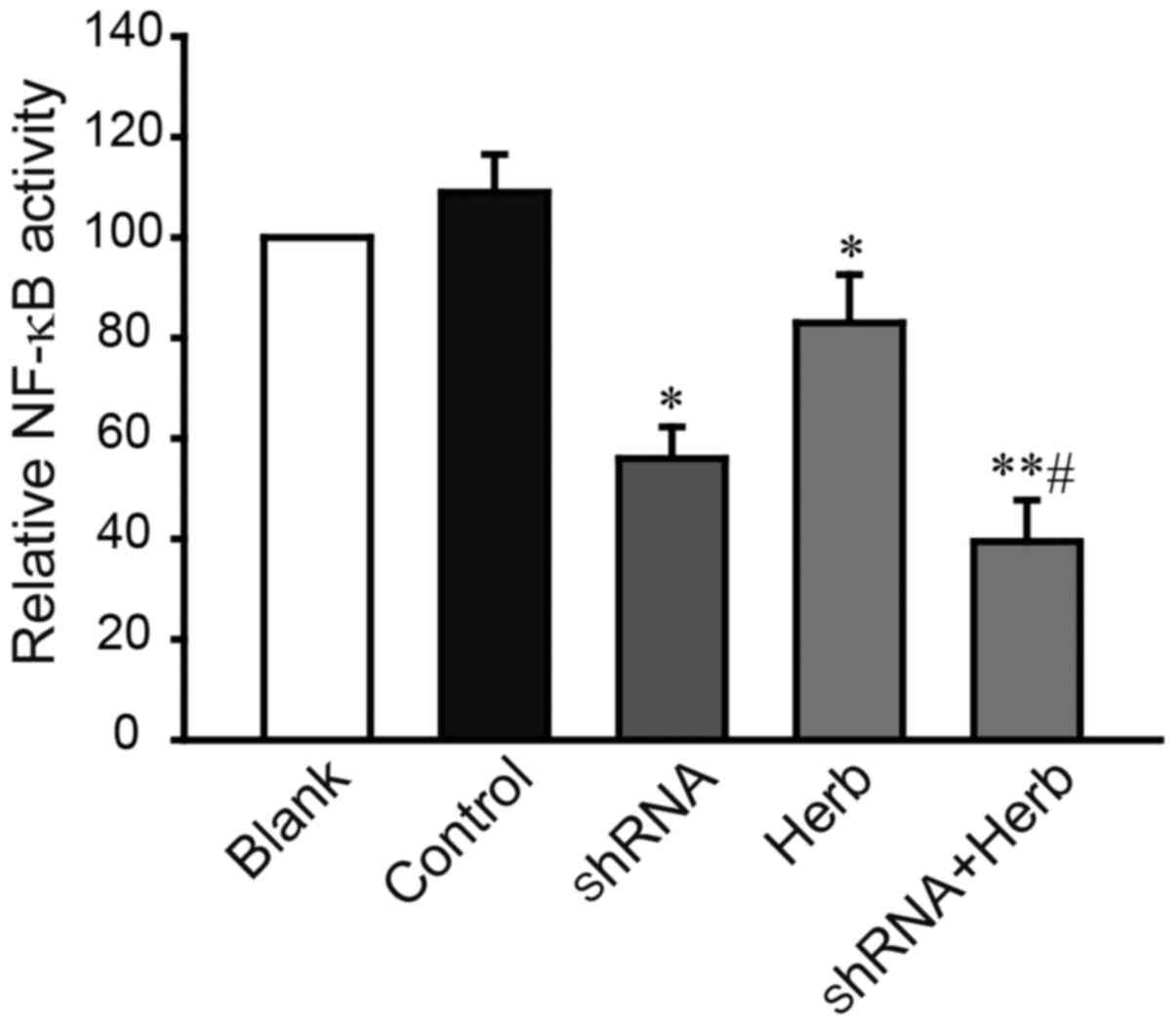
and JXY treatment decreases the activity of NF-κB
A previous study revealed that APRIL could
upregulate the activity of nuclear factor NF-κB (22). Therefore, we speculated that APRIL
knockdown and/or JXY treatment may downregulate the activity of
nuclear factor NF-κB directly. Luciferase activity of pNF-κB-TA-Luc
that contained multiple copies of the NF-κB responsive element was
used for monitoring NF-κB activity (23). As shown in Fig. 5, the result of NF-κB-controlled
luciferase reporter assay revealed that after the knockdown of
APRIL, the activity of NF-κB was significantly decreased
(P<0.05). In addition, the activity of NF-κB in the JXY-treated
cells was also lower than that in the non-treated cells
(P<0.05). Among the groups, the activity of NF-κB was the lowest
in the combination group (shRNA + Herb; P<0.05). Therefore, the
synergistic effect of APRIL knockdown and JXY treatment can trigger
cell cycle arrest and cell apoptosis and subsequent suppression of
HCC cell proliferation through an NF-κB-related pathway.
Discussion
JXY is a Chinese Traditional Medicinal recipe from
Chinese herbs exhibiting heat-clearing and detoxification activity.
Hedyotis diffusa Willd and Sophora flavescens have
been demonstrated to exhibit antiproliferative properties, promote
apoptosis and inhibit cell invasion through an NF-κB-related
pathway in a number of cancer cell lines (24,25).
In the present study, we demonstrated that APRIL knockdown and JXY
treatment synergistically triggered cell cycle arrest and cell
apoptosis and subsequent suppression of HCC cell proliferation
through an NF-κB-related pathway. Due to its safety profile, JXY is
a valuable adjuvant therapy for HCC patients.
APRIL has been implicated in the development of
tumors (26). Exogenous APRIL
confers a survival advantage to tumor cells from apoptosis
(27,28). Thus, APRIL plays a key role in human
tumor growth (29). RNA
interference (RNAi) has been widely used in the current research of
gene therapy for tumors. Recent progress has demonstrated that the
siRNA technique is an efficient method for silencing specific genes
(30,31). For siRNA to be applied
therapeutically, however, several problems need to be solved, such
as the short half-life and delivery method of siRNA-mediated
silencing. Despite these problems, siRNA remains a potentially
promising technique for the treatment of human cancer, particularly
given that it can be more selective and less toxic than traditional
approaches (32). Furthermore,
combination of the siRNA technique with other medications may be a
more effective treatment for HCC.
JXY, a TCM polyherbal decoction, is used to treat
HCC. In the present study, we evaluated the synergistic effect of
APRIL knockdown and JXY treatment on the proliferation of HCC
cells. The results showed that transfection with siRNA-APRIL
resulted in significant inhibition of APRIL mRNA and protein levels
in HepG2 cells. Moreover, APRIL depletion also inhibited cell
proliferation and induced G0/G1 cell cycle arrest and apoptosis in
HepG2 cells, whereas no inhibitory effect was observed in
non-transfected and siRNA control transfected cells. Although the
inhibitory effect of cell growth in HepG2 cells was not obvious
using JXY treatment alone, the combination of siRNA-APRIL and JXY
application had a synergistic inhibitory effect.
Next, we explored the underlying mechanisms.
Previous studies revealed that constitutive activation of NF-κB
could play an important role in the regulation of genes involved in
tumorigenesis, migration and invasion. In contrast, inhibition of
NF-κB activation suppressed migration and invasion (33,34).
TRAF6 has been found to play an important role in tumorigenesis,
metastasis and invasion by NF-κB activation (35,36).
Herein, we detected TRAF6 and NF-κB expression after APRIL
knockdown and/or JXY treatment. The results revealed that APRIL
knockdown, not JXY treatment, reduced NF-κB expression. In
addition, the combination of APRIL knockdown and JXY treatment
significantly reduced NF-κB expression. However, all of the
treatments had no effect on TRAF6 expression. These results suggest
that the synergistic effect of APRIL knockdown and JXY treatment on
the inhibition of NF-κB expression are not TRAF6-dependent.
Furthermore, we found that APRIL knockdown and/or JXY treatment
could downregulate the activity of nuclear factor NF-κB directly.
Collectively, we propose that the synergistic effect of APRIL
knockdown and JXY treatment triggers cell cycle arrest and cell
apoptosis and subsequent suppression of HCC cell proliferation
through the inhibition of the NF-κB signaling pathway.
In conclusion, the present study suggests that APRIL
is a feasible RNAi target gene for HCC and the combination of
siRNA-APRIL and JXY holds great promise as a novel therapeutic
approach for APRIL-positive HCC.
Acknowledgements
The present study was supported by the University
Distinguished Young Research Talent Training Program of Fujian
Province, the Fujian Province Natural Science Foundation (nos.
2010J01197 and 2015J01689), the National Natural Science Foundation
of China (81202856) and the International Science Joint Project of
the Ministry of Science and Technology of China (2008DFA32200).
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
APRIL
|
A proliferation-inducing ligand
|
|
JXY
|
Jiedu Xiaozheng Yin
|
|
NF-κB
|
nuclear factor-κB
|
|
VEGF
|
vascular endothelial growth factor
|
|
HDW
|
Hedyotis diffusa Willd
|
|
SF
|
Sophora flavescens
|
|
PC
|
Pseudobulbus cremastrae
|
|
TNF
|
tumor necrosis factor
|
|
TNFSF13
|
tumor necrosis factor superfamily
13
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
RIPA
|
radioimmunoprecipitation
|
|
MTT
|
methyl thiazolyl tetrazolium
|
|
ECL
|
electrochemiluminescence
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
dUTP nick end labeling
|
|
ANOVA
|
analysis of variance
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hahne M, Kataoka T, Schröter M, Hofmann K,
Irmler M, Bodmer JL, Schneider P, Bornand T, Holler N, French LE,
et al: APRIL, a new ligand of the tumor necrosis factor family,
stimulates tumor cell growth. J Exp Med. 188:1185–1190. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohr E, Serre K, Manz RA, Cunningham AF,
Khan M, Hardie DL, Bird R and MacLennan IC: Dendritic cells and
monocyte/macrophages that create the IL-6/APRIL-rich lymph node
microenvironments where plasmablasts mature. J Immunol.
182:2113–2123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Varfolomeev E, Kischkel F, Martin F,
Seshasayee D, Wang H, Lawrence D, Olsson C, Tom L, Erickson S,
French D, et al: APRIL-deficient mice have normal immune system
development. Mol Cell Biol. 24:997–1006. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Planelles L, Medema JP, Hahne M and
Hardenberg G: The expanding role of APRIL in cancer and immunity.
Curr Mol Med. 8:829–844. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dillon SR, Gross JA, Ansell SM and Novak
AJ: An APRIL to remember: Novel TNF ligands as therapeutic targets.
Nat Rev Drug Discov. 5:235–246. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ware CF: APRIL and BAFF connect
autoimmunity and cancer. J Exp Med. 192:F35–F38. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu M, Zhao M, An C, Yang M, Li Q, Zhang Y,
Suetsugu A, Tome Y, Yano S, Fu Y, et al: Real-time imaging of
apoptosis induction of human breast cancer cells by the traditional
Chinese medicinal herb tubeimu. Anticancer Res. 32:2509–2514.
2012.PubMed/NCBI
|
|
11
|
Park BH, Jung KH, Son MK, Seo JH, Lee HS,
Lee JH and Hong SS: Antitumor activity of Pulsatilla koreana
extract in anaplastic thyroid cancer via apoptosis and
anti-angiogenesis. Mol Med Rep. 7:26–30. 2013.PubMed/NCBI
|
|
12
|
Cao Z, Liao L, Chen X, Lan L, Hu H, Liu Z,
Chen L, Huang S and Du J: Enhancement of antitumor activity of
low-dose 5-fluorouracil by combination with Fuzheng-Yiliu granules
in hepatoma 22 tumor-bearing mice. Integr Cancer Ther. 12:174–181.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao Z, Lin W, Huang Z, Chen X, Zhao J,
Zheng L, Ye H, Liu Z, Liao L and Du J: Jiedu Xiaozheng Yin, a
Chinese herbal formula, inhibits tumor angiogenesis via
downregulation of VEGF-A and VEGFR-2 expression in vivo and in
vitro. Oncol Rep. 29:1080–1086. 2013.PubMed/NCBI
|
|
14
|
Chen XZ, Cao ZY, Li JN, Hu HX, Zhang YQ,
Huang YM, Liu ZZ, Hu D, Liao LM and Du J: Ethyl acetate extract
from Jiedu Xiaozheng Yin inhibits the proliferation of human
hepatocellular carcinoma cells by suppressing polycomb gene product
Bmi1 and Wnt/β-catenin signaling. Oncol Rep. 32:2710–2718.
2014.PubMed/NCBI
|
|
15
|
Cao Z, Lin W, Huang Z, Chen X, Zhao J,
Zheng L, Ye H, Liu Z, Liao L and Du J: Ethyl acetate extraction
from a Chinese herbal formula, Jiedu Xiaozheng Yin, inhibits the
proliferation of hepatocellular carcinoma cells via induction of
G0/G1 phase arrest in vivo and in vitro. Int J Oncol. 42:202–210.
2013.PubMed/NCBI
|
|
16
|
Chen LW, Lin J, Chen W and Zhang W: Effect
of Chinese herbal medicine on patients with primary hepatic
carcinoma in III stage during perioperational period: A report of
42 cases. Zhongguo Zhong Xi Yi Jie He Za Zhi. 25:832–834. 2005.(In
Chinese). PubMed/NCBI
|
|
17
|
Han T, Jiao F, Hu H, Yuan C, Wang L, Jin
ZL, Song WF and Wang LW: EZH2 promotes cell migration and invasion
but not alters cell proliferation by suppressing E-cadherin, partly
through association with MALAT-1 in pancreatic cancer. Oncotarget.
7:11194–11207. 2016.PubMed/NCBI
|
|
18
|
Zhang X, Zhang J, Zhang L, van Dam H and
ten Dijke P: UBE2O negatively regulates TRAF6-mediated NF-κB
activation by inhibiting TRAF6 polyubiquitination. Cell Res.
23:366–377. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saito S, Murata T, Kanda T, Isomura H,
Narita Y, Sugimoto A, Kawashima D and Tsurumi T: Epstein-Barr virus
deubiquitinase downregulates TRAF6-mediated NF-κB signaling during
productive replication. J Virol. 87:4060–4070. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong L, Cao F and You Q: Effect of TRAF6
on the biological behavior of human lung adenocarcinoma cell.
Tumour Biol. 34:231–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inoue J, Gohda J, Akiyama T and Semba K:
NF-kappaB activation in development and progression of cancer.
Cancer Sci. 98:268–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhi X, Tao J, Xiang G, Cao H, Liu Z, Yang
K, Lv C and Ni S: APRIL induces cisplatin resistance in gastric
cancer cells via activation of the NF-κB pathway. Cell Physiol
Biochem. 35:571–585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan D, Pan Y, Zhang J and Shao C: Role of
nuclear factor-kappaB and P53 in radioadaptive response in Chang
live cells. Mutat Res. 688:66–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Q, Lai Y, Wang C, Xu G, He Z, Shang X,
Sun Y, Zhang F, Liu L and Huang H: Matrine inhibits the
proliferation, invasion and migration of castration-resistant
prostate cancer cells through regulation of the NF-κB signaling
pathway. Oncol Rep. 35:375–381. 2016.PubMed/NCBI
|
|
25
|
Chen Y, Lin Y, Li Y and Li C: Total
flavonoids of Hedyotis diffusa Willd inhibit inflammatory responses
in LPS-activated macrophages via suppression of the NF-κB and MAPK
signaling pathways. Exp Ther Med. 11:1116–1122. 2016.PubMed/NCBI
|
|
26
|
Mackay F and Tangye SG: The role of the
BAFF/APRIL system in B cell homeostasis and lymphoid cancers. Curr
Opin Pharmacol. 4:347–354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klein B, Tarte K, Jourdan M, Mathouk K,
Moreaux J, Jourdan E, Legouffe E, De Vos J and Rossi JF: Survival
and proliferation factors of normal and malignant plasma cells. Int
J Hematol. 78:106–113. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moreaux J, Legouffe E, Jourdan E, Quittet
P, Rème T, Lugagne C, Moine P, Rossi JF, Klein B and Tarte K: BAFF
and APRIL protect myeloma cells from apoptosis induced by
interleukin 6 deprivation and dexamethasone. Blood. 103:3148–3157.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moreaux J, Veyrune JL, De Vos J and Klein
B: APRIL is overexpressed in cancer: Link with tumor progression.
BMC Cancer. 9:832009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Ding W, Sun B, Jing R, Huang H,
Shi G and Wang H: Targeting of colorectal cancer growth,
metastasis, and anti-apoptosis in BALB/c nude mice via APRIL siRNA.
Mol Cell Biochem. 363:1–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiao F, Hu H, Yuan C and Wang L, Jiang W,
Jin Z, Guo Z and Wang L: Elevated expression level of long
noncoding RNA MALAT-1 facilitates cell growth, migration and
invasion in pancreatic cancer. Oncol Rep. 32:2485–2492.
2014.PubMed/NCBI
|
|
32
|
Wang F, Chen L, Ni H, Wang G, Ding W, Cong
H, Ju S, Yang S and Wang H: APRIL depletion induces cell cycle
arrest and apoptosis through blocking TGF-β1/ERK signaling pathway
in human colorectal cancer cells. Mol Cell Biochem. 383:179–189.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tao T, Cheng C, Ji Y, Xu G, Zhang J, Zhang
L and Shen A: Numbl inhibits glioma cell migration and invasion by
suppressing TRAF5-mediated NF-κB activation. Mol Biol Cell.
23:2635–2644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sarkar D, Park ES, Emdad L, Lee SG, Su ZZ
and Fisher PB: Molecular basis of nuclear factor-kappaB activation
by astrocyte elevated gene-1. Cancer Res. 68:1478–1484. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Starczynowski DT, Lockwood WW, Deléhouzée
S, Chari R, Wegrzyn J, Fuller M, Tsao MS, Lam S, Gazdar AF, Lam WL,
et al: TRAF6 is an amplified oncogene bridging the RAS and NF-κB
pathways in human lung cancer. J Clin Invest. 121:4095–4105. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ramachandran C, Rodriguez S, Ramachandran
R, Nair PK Raveendran, Fonseca H, Khatib Z, Escalon E and Melnick
SJ: Expression profiles of apoptotic genes induced by curcumin in
human breast cancer and mammary epithelial cell lines. Anticancer
Res. 25:3293–3302. 2005.PubMed/NCBI
|