Introduction
Malignant melanoma is responsible for 4% of all skin
cancer cases, but accounts for 79% of skin cancer-related deaths
(1). First-line treatment includes
resection of the primary tumor. If all cancerous cells are not
removed, the risk of metastasis and poor survival is high (2).
In recent years, with the development of targeted
immune and individualized targeted therapies, treatment of melanoma
has made considerable progress (3).
The latest research results using programmed cell death protein
(PD)-1 have pushed the treatment of advanced melanoma to new
levels. Data show that the anti-PD-1 monoclonal antibodies MK-3475
and BMS-936558 have a mean efficiency of 40%, complete remission
rate of 17%, and that progression-free survival is ~2 years
(4). However, the relationship
between expression of programmed death-ligand-1 in tumor tissues
and the efficacy of single agent PD-1 inhibition is controversial
(5). Therefore, chemotherapy for
Chinese patients with melanoma remains an important means of
treatment.
The alkylating agent temozolomide (TMZ) is
recommended in several countries for the first-line treatment of
malignant melanoma (6). It is
thought that TMZ passes through the blood-brain barrier and has
high bioavailability, and could be used to prevent/treat brain
metastasis. TMZ causes DNA single-strand or double-strand breaks
and blocks DNA replication, leading to the death of tumor cells
through methylation of DNA chains (7). In recent years, chemotherapy for
malignant melanoma has not made significant progress. The poor
prognosis of malignant melanoma is related directly to its high
capacity for tissue infiltration and its resistance to
chemotherapeutic drugs (8).
Nanotechnology has revolutionized cancer treatment.
Attachment of a drug to a carrier enables the fast transfer of the
complex to the tumor. In addition, sustained release of the drug
achieves an effective concentration while reducing systemic
toxicity (9).
The polyamide-amine dendrimer (PAMAM) has a
hyperbranched, symmetrical and radiating structure (10,11).
PAMAM has three obvious structural advantages compared with other
carrier molecules (12,13). Firstly, PAMAM has a diameter of 1–15
nm with a wide cavity for embedding of a drug. Secondly, PAMAM is
susceptible to chemical modification to better protect the drug
from enzymatic degradation. Thirdly, the termini of PAMAM contain
several reactive functional groups, which enable ligand binding to
target cells. A PAMAM-based drug-carrier system enables gradual
accumulation of the drug in tumor tissue based on the enhanced
permeability and retention (EPR) effect. Compared with the free
drug, the drug concentration in tumor tissue is increased, thereby
aiding the passive targeting of tumor cells.
The unique role of the epidermal growth factor
receptor (EGFR) in the growth and metastasis of tumors has made it
a ‘hot topic’ in the development of targeted cancer drugs (14). GE11 is a small polypeptide
comprising 11 amino acids, and is a ligand of the EGFR. Song et
al (15) showed that GE11
modified with liposomes has high targeting efficiency for the
EGFR.
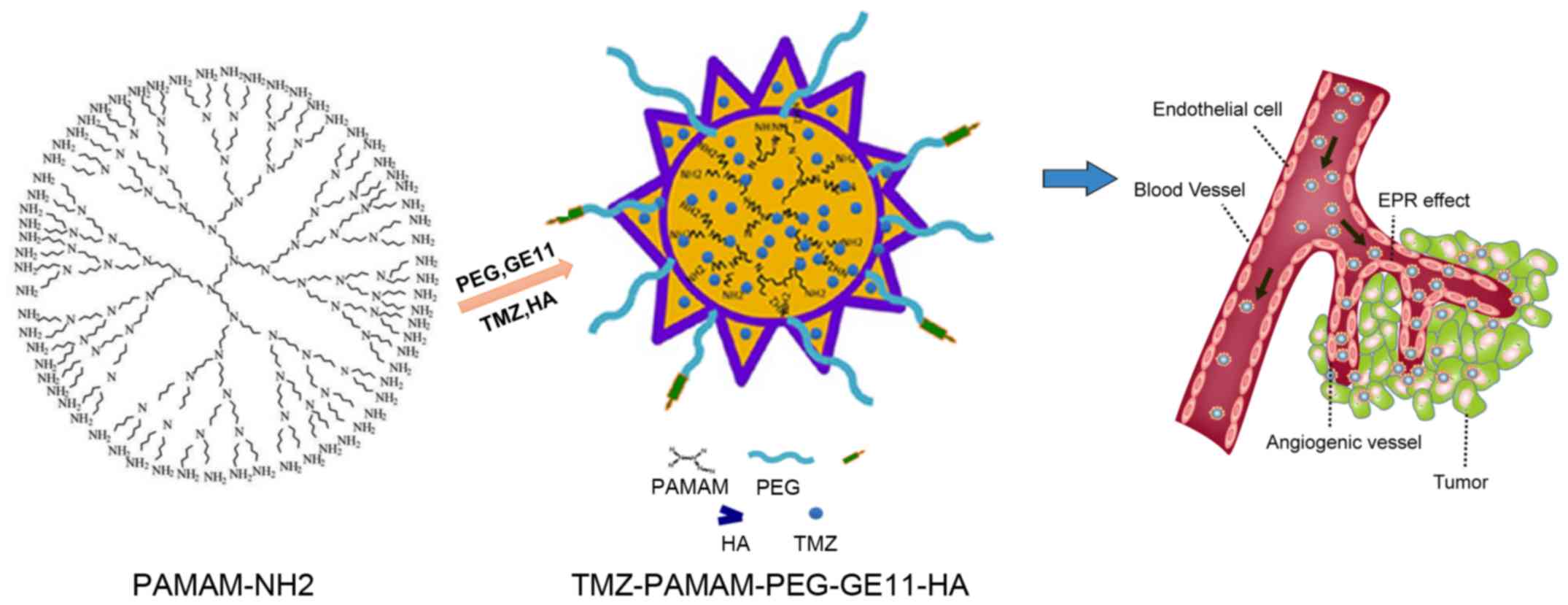
Based on previous studies, we used PAMAM as a
polymer framework material. We employed NHS-PEG-MAL as a polymer
modifier and connecting bridge. The active amino group of PAMAM is
bound to one end of the NHS-PEG active carboxyl group via an
acidamide reaction, whereas the other end of the PEG active group
(−MAL) is connected to the active group (−SH) of the targeting
agent (GE11 polypeptide). Then, using hyaluronic acid (HA) as an
emulsifier, TMZ is embedded by ‘phacoemulsification’ to form the
composite TMZ-PAMAM-PEG-GE11-HA (Fig.
1). Continued and sustained release of TMZ enables reduced
accumulation of TMZ in normal tissues, and thus reduces the
systemic side-effects of this drug.
Materials and methods
Chemicals
Generation-5 poly(amidoamine) dendrimer (G5 PAMAM
dendrimer) was purchased from Xian Ruixi Biological Technology
(Xian, China). MEO-PEG-NHS and MAL-PEG-NHS were obtained from
JenKem Technology (Beijing, China). GE11-SH was purchased from
ChinaPeptides (Beijing, China). Temozolomide (TMZ) was obtained
from TCI Shanghai (Shanghai, China). 4′,6-Diamidino-2-phenylindole
(DAPI) was purchased from Invitrogen (Carlsbad, CA, USA).
N,N-dimethylformamide (DMF), dimethyl sulfoxide
(DMSO), methanol, acetonitrile, fluorescein isothiocyanate (FITC)
and all other chemicals were purchased from Sigma-Aldrich (St.
Louis, MO, USA).
Synthesis of PAMAM-PEG
PAMAM and MEO-PEG-NHS (1:48) were added to
phosphate-buffered saline (PBS; pH 8.0) and stirred for 12 h at
room temperature. The reaction mixture was dialyzed using a
dialysis tube [molecular weight cut-off (MWCO), 15 kDa) for 24 h.
The aqueous solution was lyophilized to afford PAMAM-PEG as a white
solid.
Synthesis of PAMAM-PEG-GE11
PAMAM (10 mg) and MEO-PEG-NHS (83.26 mg) were added
to PBS (pH 8.0) to synthesize PAMAM-PEG. To a solution of PAMAM-PEG
in PBS, GE11-SH (9.04 mg) in acetonitrile was added. After stirring
for 3 h at room temperature, the pH of the reaction mixture was
regulated to 7.0 and β-mercaptoethanol (55.5 µmol) was added. After
stirring for 1 h, the reaction mixture was dialyzed using a
dialysis tube (MWCO, 15 kDa) for 24 h. The aqueous solution was
lyophilized to afford PAMAM-PEG-GE11 as a white solid.
FITC-labeled dendrimers were synthesized by coupling
FITC to the amino group of the proximal lysine group between PEG
and cholic acid after removal of the deprotecting group in methanol
solution.
Fourier transform-infrared (FTIR) and
nuclear magnetic resonance (NMR) spectroscopy
The chemical structures of the three polymers,
PAMAM, PAMAM-PEG and PAMAM-PEG-GE11, were identified by analyses of
FTIR and proton NMR (1H-NMR) spectra. FTIR spectra of
these polymers were recorded on a spectrophotometer (Olympus,
Tokyo, Japan) using KBr as a reference. 1H-NMR spectra
of these polymers were obtained using an MR 400 NMR system (Agilent
Technologies, Palo Alto, CA, USA), with samples dissolved in
deuterium oxide (D2O) or DMSO, respectively.
Characterization of
PAMAM-PEG-GE11
The mean size, polydispersity index and zeta
potential of PAMAM-PEG-GE11 were determined by dynamic light
scattering (DLS) using a ZetaSizer (Nano ZS90; Malvern Instruments,
Malvern, UK). Determinations were made when samples were diluted in
distilled water (~1 mg/ml). Morphology of the samples was
characterized by transmission electron microscopy (TEM) using
Tecnai™ G2 F20 U-TWIN (FEI, Eindhoven, The Netherlands).
Cell culture
Human melanoma cells (A375) and human skin
fibroblasts (HSFs) were purchased from Shanghai Cell Collection
(Shanghai, China). Cells were grown in an atmosphere of 5%
CO2 and relative humidity of 95% using DMEM (Gibco-BRL,
Grand Island, NY, USA) supplemented with 10% fetal bovine serum and
1% penicillin-streptomycin solution.
Cell uptake
Cell-uptake studies were undertaken to investigate
whether three nanomaterials, FITC-PAMAM, FITC-PAMAM-PEG and
FITC-PAMAM-PEG-GE11, were internalized by receptor-mediated
endocytosis. Briefly, A375 cells (4×104/well) were
seeded into 24-well plates overnight before experimentation. Cells
were incubated with 5 nM of these three nanomaterials at 37̊C for 4
h. The cells were washed thrice with PBS (pH 7.4), fixed with 4%
paraformaldehyde and stained with DAPI for 5 min. Then, the cells
were washed thrice with PBS. Fluorescence micrographs of cells were
captured using an inverted microscope (IX70; Olympus).
A375 cells (2×105/well) were seeded in
6-well plates overnight before experimentation. Cells were
incubated with 5 nM of these three nanomaterials at 37̊C for 4 h.
Cells were washed thrice with PBS (pH 7.4), trypsinized and
centrifuged at 0.4 × g for 5 min at room temperature. After washing
twice with PBS, the samples underwent flow cytometry (FACSCalibur™
flow cytometer; BD Biosciences, Franklin Lakes, NJ, USA).
Preparation and characterization of
TMZ-PAMAM-PEG-GE11-HA complexes
TMZ-PAMAM-PEG-GE11-HA complexes were prepared by
ultrasonic emulsification. Briefly, TMZ (1 mg) and PAMAM-PEG
copolymers (10 mg) were dissolved in methanol (1 ml). In addition,
TMZ (3 mg) and HA (10 mg) were dissolved in 4 ml aqueous solution.
The solution of TMZ and PAMAM-PEG copolymers was added drop-by-drop
to the aqueous solution with vigorous stirring at room temperature.
Then, the mixture was emulsified by sonication for 10 min at 40 W,
and evaporated under reduced pressure to remove the remaining
methanol. These complexes were transferred to an ultrafiltration
tube (MWCO, 10 kDa), centrifuged at 3.5 × g for 10 min at room
temperature, and washed with deionized water thrice. DLS and TEM
were undertaken to evaluate the particle size, zeta potential and
morphology of the TMZ-PAMAM-PEG-GE11-HA complexes.
Loading rate (LR) and encapsulation
efficiency (EE) of the TMZ-PAMAM-PEG-GE11-HA complexes
The LR and EE were assayed by ultraviolet
spectrophotometry. A certain amount of TMZ was dissolved in DMF (to
10 µg/ml) and its absorption peak was measured by an ultraviolet
spectrophotometer with DMF as a blank. Standard solutions of TMZ
(4, 6, 8, 10 and 16 µg/ml) were prepared. The absorbance of
different concentrations was measured with UV detection at 329 nm
and a linear regression equation was obtained. An ultrafiltration
tube (MWCO, 10 kDa) was used to separate free TMZ. LR and EE were
defined as follows:
LR=(W0–Wf)/W0x100%EE=(W0–Wf)/Wsx100%
where W0 and Wf are the weight
of initial TMZ and free TMZ detected in solution, respectively, and
Ws is the weight of the complex after
lyophilization.
Statistical analyses
Statistical analyses were conducted using the
Student's t-test for comparison of two groups and one-way ANOVA for
multiple groups. P<0.05 was considered to indicate a
statistically significant result.
Results
Synthesis of PAMAM-PEG and
PAMAM-PEG-GE11 compounds
G5 PAMAM dendrimer with 128 amino groups was used in
the present study. To reduce their cytotoxicity and increase their
circulation and targeting of cancer cells, PEG and GE11 polypeptide
were modified on the surface of the dendrimer (Fig. 1).
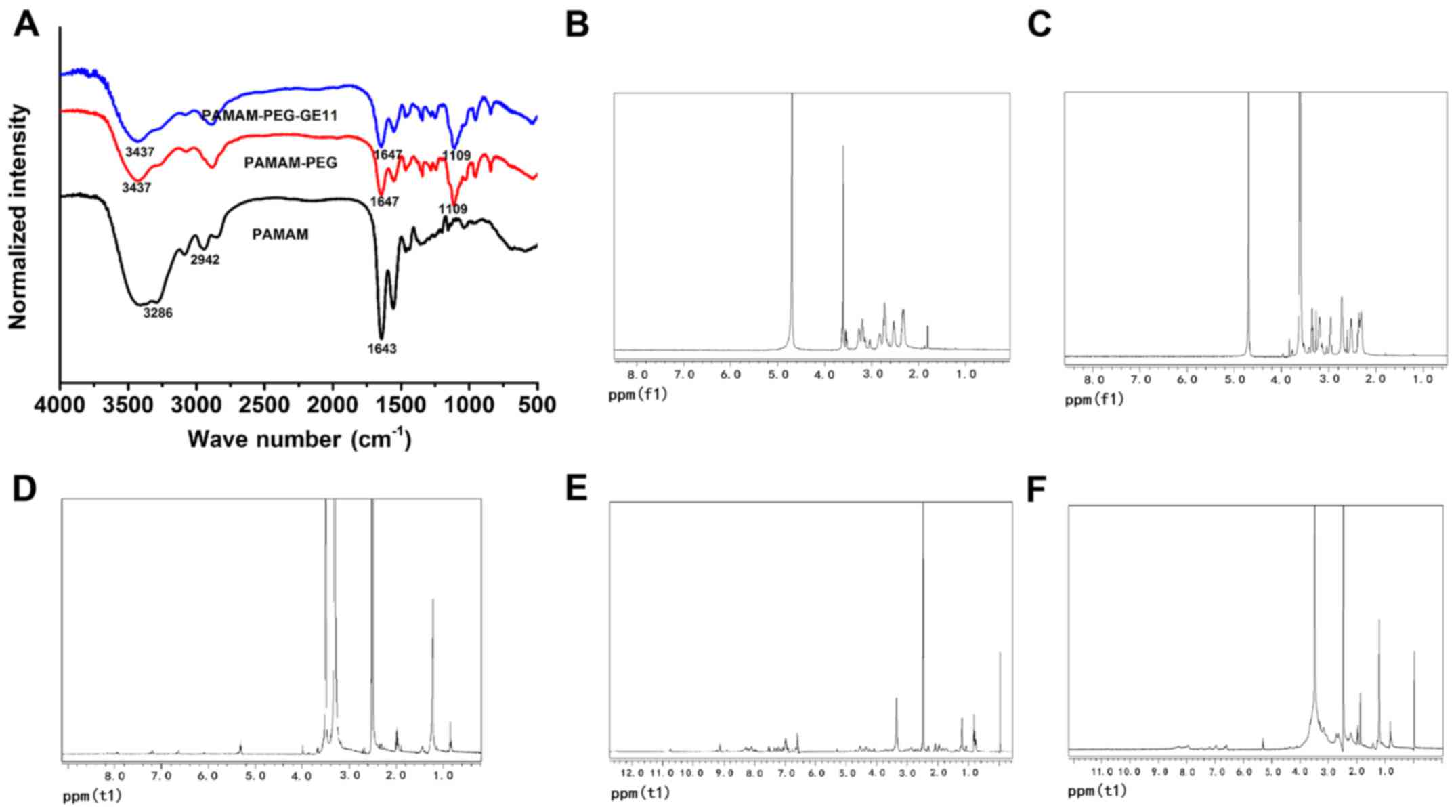
FTIR spectra showed characteristic peaks of the
hydroxyl and ether groups of PEG at 3,437 and 1,109
cm−1, and those of amide and carbonyl groups peak were
found at 3,286 and 1,647 cm−1. These results successful
confirmed coupling of PEG and PAMAM. We did not find the typical
peak of GE11 in the FTIR spectra of PAMAM-PEG-GE11, probably due to
duplication at 1,104.9 cm−1 of the stretching vibration
peak of C-O-C (δas) in PAMAM-PEG and hydroxyl group (νOH) in GE11
(Fig. 2A). NMR was used to confirm
the success of coupling of PEG and PAMAM. Protons of PAMAM are
derived from the -CH2-CH2-N- structure and
their chemical shift is 2.2–3.4 ppm. Protons in PEG have a chemical
shift at 3.4–3.8 ppm, and the characteristic methylene peak of PEG
was found at 3.60 ppm, and the characteristic peak of the methyl
groups at 3.42 ppm. The characteristic peak of PEG and PAMAM could
be found in the 1H-NMR spectrum of PEG-PAMAM in
D2O, suggesting that PEG-PAMAM was successfully
synthesized (Fig. 2B and C).
Protons in the GE11 polypeptide have a chemical shift at 6.5–9.3
ppm. The 1H-NMR spectrum of PAMAM-PEG-GE11 in DMSO
displayed characteristic peaks of the PAMAM-PEG and GE11
polypeptide (Fig. 2D-F). Taken
together, these results suggest that the nanodrug delivery system
was successfully created.
Characterization of the PAMAM-PEG-GE11
conjugates
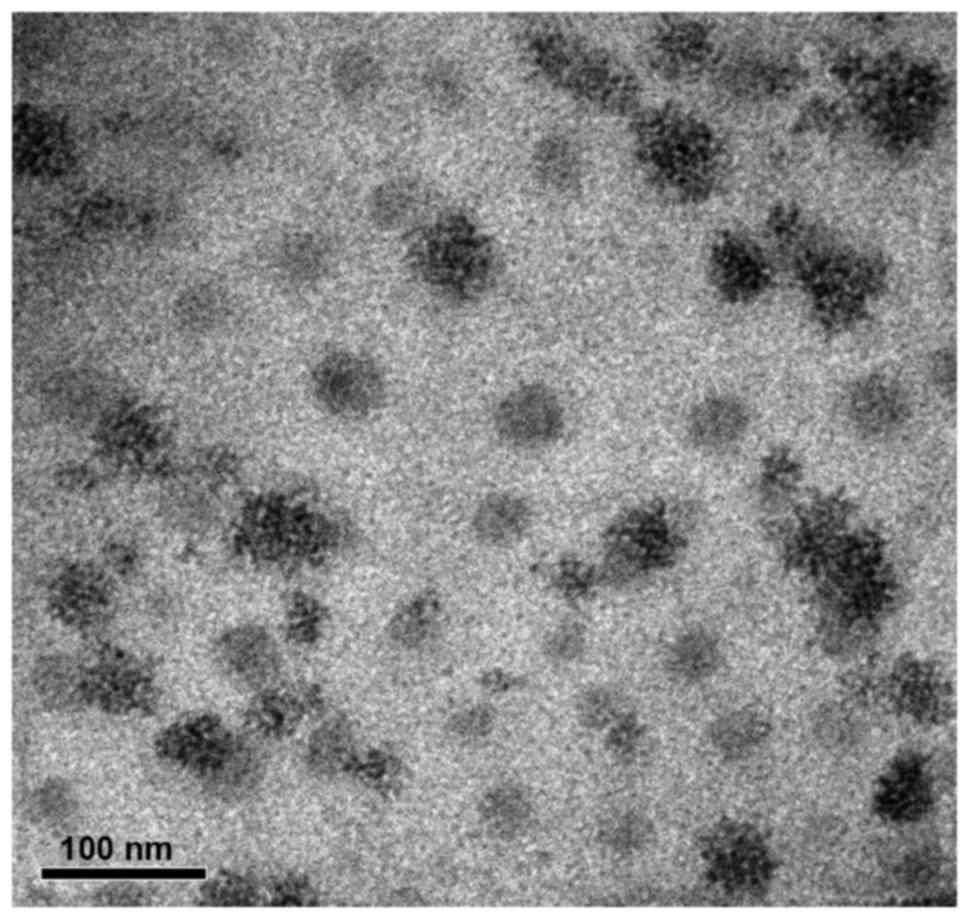
TEM and DLS were carried out to characterize the
PAMAM-PEG-GE11 conjugates. Transmission electronic micrographs
showed that the PAMAM-PEG-GE11 conjugates were close to spherical
and that the mean diameter was 40 nm (Fig. 3). The mean hydrodynamic diameter
measured by DLS was 249.2±18.4 nm, which may have been due to
hydration shells. The zeta potential of PAMAM-PEG-GE11 conjugates
was 20.2±0.8 mV. The reduced positive charge compared with that of
PAMAM (~40 mV) may have been due to the fact that the terminal
amino groups of PAMAM were partly neutralized.
Active targeting of PAMAM-PEG-GE11 to
A375 cells
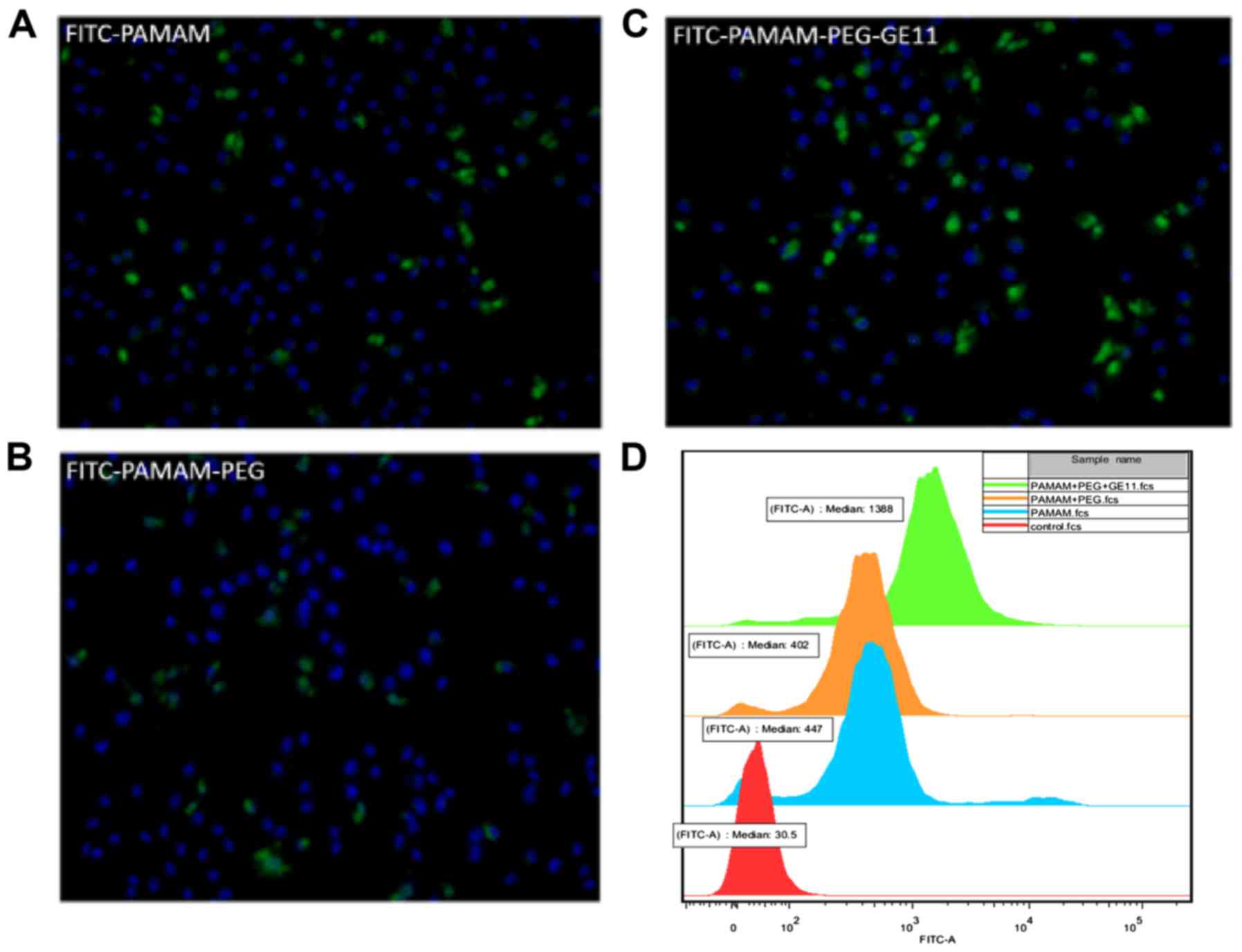
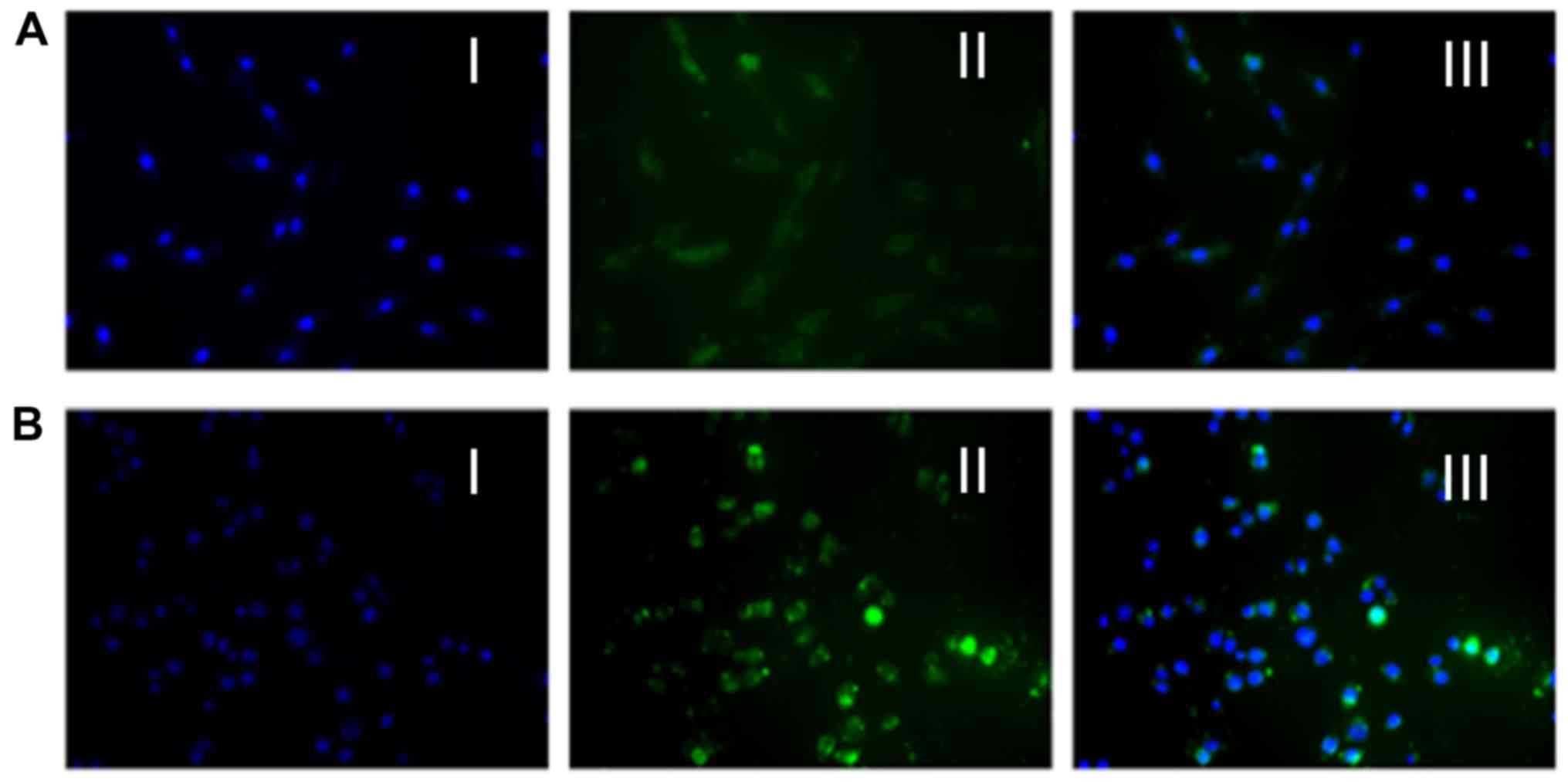
To assess active targeting of PAMAM-PEG-GE11
conjugates to A375 cells (which overexpress the EGFR), fluorescence
microscopy and flow cytometry were carried out. Three
nanomaterials, PAMAM, PAMAM-PEG and PAMAM-PEG-GE11, were modified
with FITC. Fluorescence microscopy images showed PAMAM (Fig. 4A), PAMAM-PEG (Fig. 4B) and PAMAM-PEG-GE11 (Fig. 4C) to be present in different amounts
in the A375 cells (blue, cell nucleus; green, FITC-nanomaterials).
This result showed that all three nanomaterials entered the A375
cells. In addition, coupling with GE11 was internalized
considerably more than with PAMAM and PAMAM-PEG. Notably, the
amount of nanocarriers that entered the cells was slightly reduced
whether PAMAM was modified with PEG. The reason for this phenomenon
may be neutralization of the positive charge on the PAMAM surface
by PEG, which may result in reduction in electrostatic forces
between PAMAM and cell membranes. Subsequently, we studied the
fluorescence intensity of PAMAM, PAMAM-PEG and PAMAM-PEG-GE11 in
the A375 cells by flow cytometry, which elicited results that were
in accordance with those of the fluorescence microscopy (Fig. 4D). Taken together, these results
showed that PAMAM-PEG-GE11 could be transported to A375 cells in
vitro.
Measurement of LR and EE of the
TMZ-PAMAM-PEG-GE11-HA conjugates
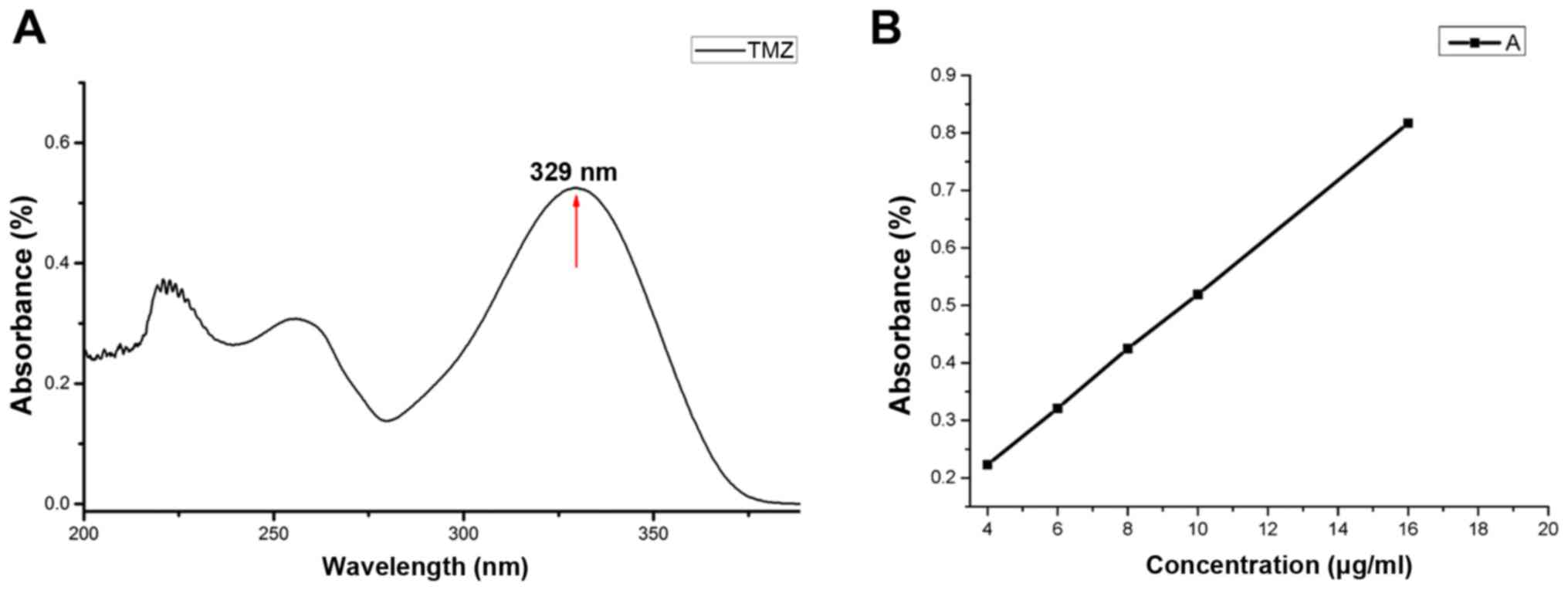
TMZ was dissolved in DMF, and a concentration of 10
µg/ml was measured by ultraviolet-visible spectrometry. The
absorption spectrum showed that the typical intense absorption peak
of TMZ was at ~329 nm (Fig. 5A),
which could be used to quantify the TMZ concentration. To measure
the EE and LR of the TMZ-PAMAM-PEG-GE11-HA conjugates, the
absorbance of different concentrations of TMZ was measured
(Fig. 5B), and a linear regression
equation is provided (absorbance = 0.0495 × concentration + 0.0256;
r=0.9999). The EE and LR of the synthesized TMZ-PAMAM-PEG-GE11-HA
conjugates were 50.63 and 10.40%, respectively.
Characterization of the
TMZ-PAMAM-PEG-GE11-HA conjugates
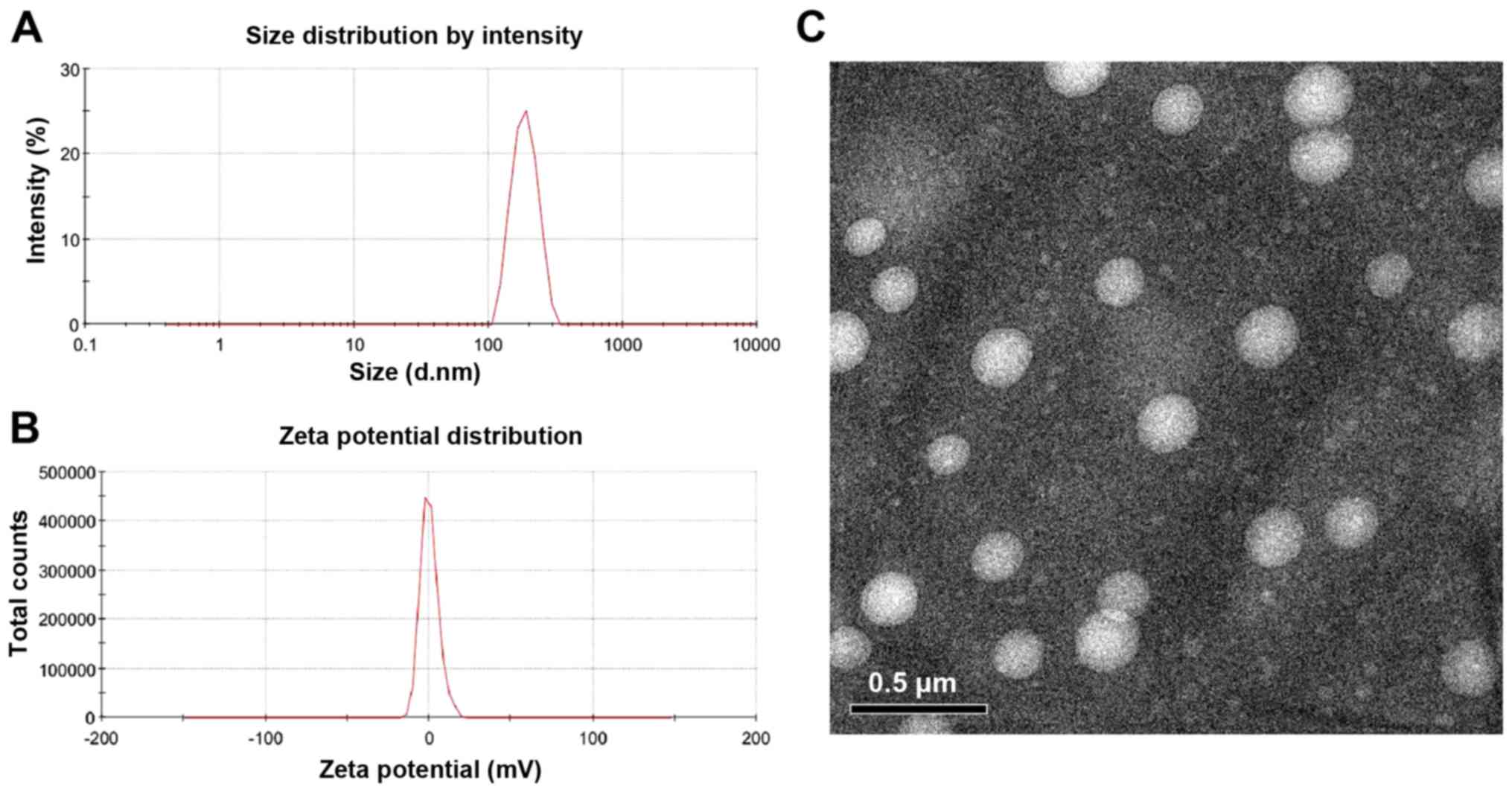
To characterize the TMZ-PAMAM-PEG-GE11-HA
conjugates, DLS was carried out to measure the hydrodynamic size
and zeta potential. The mean hydrodynamic diameter was 183.2 nm
(Fig. 6A). The zeta potential of
the TMZ-PAMAM-PEG-GE11-HA conjugates was −0.01 mV (approximate to
neutral), and the zeta deviation was 5.91 mV, suggesting good
dispersion in the dissolution and low toxicity (Fig. 6B). Transmission electron micrographs
showed that the TMZ-PAMAM-PEG-GE11-HA conjugates were close to
spherical and that their size was ~183.2 nm (Fig. 6C).
Uptake of the TMZ-PAMAM-PEG-GE11-HA
conjugates
Uptake of the TMZ-PAMAM-PEG-GE11-HA conjugates by
HSFs and A375 cells was analyzed by fluorescence microscopy. After
treatment with the TMZ-PAMAM-PEG-GE11-HA conjugates for 4 h,
samples were fixed and stained. The fluorescence intensity of the
conjugates in the A375 cells was stronger than that noted in the
HSFs (Fig. 7). This result
suggested that TMZ-loading nanocarriers modified by GE11 entered
malignant melanoma cells efficiently, and that only a negligible
amount were internalized by normal tissue cells.
Discussion
Melanoma is one of the most malignant forms of skin
cancer. Early-stage melanoma is curable by surgery, but if it
becomes widespread, the survival rate is extremely low owing to its
poor response to drugs (16).
Conventional treatments (e.g., surgery, chemotherapy and
radiotherapy) combined with immunotherapy and molecular-targeted
therapy, have improved the prognosis of patients with malignant
melanoma (17). However,
radiotherapy and chemotherapy can kill cancer cells, but also
normal cells, thus, development of more effective delivery systems
to target tumor cells is particularly urgent.
TMZ has poor solubility and stability in water.
Whether administered via the oral route, its half-life is 1.8 h,
and it is rapidly cleared from the circulation (18). Nanocarriers can increase the water
solubility of TMZ, aid sustained release of the drug, and thus,
improve the half-life of TMZ under physiologic conditions, which
protects it from degradation and loss of therapeutic effects
(19).
In the present study, PAMAM dendrimers were modified
with PEG by chemical synthesis. Due to the hydrophilicity and
flexibility of PEG, PEG-modified compounds lower the risk of being
recognized by macrophages after intravenous injection, thus, they
cannot be phagocytized readily by macrophages. Therefore, compared
with conventional nanodrug delivery systems and the original drug,
PEG-modified compounds can extend the plasma half-life of TMZ.
Hence, targeted tumor therapy through enhancement of the EPR effect
can be carried out.
Thomas et al (20) suggest that macromolecular drugs of
molecular weight >35 kDa and drug systems of particle size
<200 nm can take advantage of the EPR effect whether targeted at
tumor tissue. The TMZ-PAMAM-PEG-GE11-HA nanoparticles prepared in
the present study (molecular weight >35 kDa; mean particle
diameter=183.2 nm) suggest that the EPR effect can be enhanced.
However, a particle size for TMZ-PAMAM-PEG-GE11-HA conjugates of
183.2 nm also reduces cell uptake. Usually, different sizes of
nanoparticles can enter cells by different routes, including
directly through the membrane and by endocytosis (21,22),
and complexes with a particle size of 10–50 nm can enter cells more
efficiently (23). However, we
created a new active targeting complex: TMZ-PAMAM-PEG-GE11-HA. In
addition to undergoing non-specific uptake by melanoma cells, they
can also be taken up through ligand receptor-mediated endocytosis
in a cell-specific way.
The GE11 polypeptide is a ligand of the EGFR. The
latter is highly expressed on the surface of many types of tumor
cells (including melanoma cells) and is closely related with tumor
development and prognosis. Hence, by targeting the EGFR, a GE11
polypeptide-modified delivery system can identify and locate the
tumor site specifically, and be rapidly taken up by A375 cells. We
found that the GE11 polypeptide-modified nanocarrier PAMAM-PEG-GE11
was taken up by cells to a greater extent than that observed for
PAMAM and PAMAM-PEG. In addition, PAMAM-PEG-GE11 was covered with a
layer of HA to aid drug loading. As an emulsifier, HA enables PAMAM
to embed a greater amount of the drug and neutralize the positive
charge on the PAMAM surface, thereby reducing the cytotoxicity and
hemolytic toxicity of the cationic polymer. HA also exerts two-way
targeting with the GE11 polypeptide through binding to CD44
molecules (which are highly expressed on the surface of melanoma
cells) (24). Systemic side-effects
can be reduced by targeting drug delivery to the tumor site and
reducing drug accumulation in normal tissues.
Various studies have shown that HA-modified
liposomes bind to the melanoma cell line B16-F10 more readily (and
with higher expression of CD44 receptors) than the fibroblast cell
line CV-1 (which does not have CD44 receptors) (24,25).
Similarly, the presents study showed that binding of A375 cells to
nanomaterials was greater than that observed for HSFs. This
phenomenon can be explained by the fact that the membrane surface
of A375 cells expresses many CD44 molecules, whereas low expression
of CD44 molecules is observed in normal cells. Thus, the
HA-modified drug complex TMZ-PAMAM-PEG-GE11-HA can bind to A375
cells more readily. Therefore, we showed that the
TMZ-PAMAM-PEG-GE11-HA conjugates can target melanoma cells in
vitro. However, whether the TMZ-PAMAM-PEG-GE11-HA conjugates
can target melanoma cells in vivo may require animal
experiments. In addition, TMZ is unstable in phosphate buffer
(pH=6.8 and pH=5.5), and is broken down readily to the intermediate
3-methyl-(triazen-1-yl)imidazole-4-carboximide (MTIC). However, it
has been reported that MTIC is relatively stable (26); thus, the TMZ content is indirectly
measured. However, in the present study, MTIC was unstable in a
shaker at 37̊C, thus, detection of TMZ release was difficult.
Therefore, release of the drug TMZ in TMZ-PAMAM-PEG-GE11-HA
conjugates remains unclear.
In conclusion, the TMZ-PAMAM-PEG-GE11-HA nanodrug
delivery system was successfully prepared, and its potential for
targeting human melanoma (A375) cells in vitro was
demonstrated. This system enhanced the sensitivity of A375 cells to
TMZ, and provides a novel targeted strategy for the treatment of
metastatic melanoma.
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Das Thakur M, Salangsang F, Landman AS,
Sellers WR, Pryer NK, Levesque MP, Dummer R, McMahon M and Stuart
DD: Modelling vemurafenib resistance in melanoma reveals a strategy
to forestall drug resistance. Nature. 494:251–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luke JJ and Schwartz GK: Chemotherapy in
the management of advanced cutaneous malignant melanoma. Clin
Dermatol. 31:290–297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakagawa E, Aimi Y, Yasuhara O, Tooyama I,
Shimada M, McGeer PL and Kimura H: Enhancement of progenitor cell
division in the dentate gyrus triggered by initial limbic seizures
in rat models of epilepsy. Epilepsia. 41:10–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luke JJ and Ott PA: PD-1 pathway
inhibitors: The next generation of immunotherapy for advanced
melanoma. Oncotarget. 6:3479–3492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Biasco G, Pantaleo MA and Casadei S:
Treatment of brain metastases of malignant melanoma with
temozolomide. N Engl J Med. 345:621–622. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johannessen TC and Bjerkvig R: Molecular
mechanisms of temozolomide resistance in glioblastoma multiforme.
Expert Rev Anticancer Ther. 12:635–642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ott PA, Chang J, Madden K, Kannan R, Muren
C, Escano C, Cheng X, Shao Y, Mendoza S, Gandhi A, et al:
Oblimersen in combination with temozolomide and albumin-bound
paclitaxel in patients with advanced melanoma: A phase I trial.
Cancer Chemother Pharmacol. 71:183–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bourzac K: Nanotechnology: Carrying drugs.
Nature. 491:S58–S60. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dufès C, Uchegbu IF and Schätzlein AG:
Dendrimers in gene delivery. Adv Drug Deliv Rev. 57:2177–2202.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh I, Rehni AK, Kalra R, Joshi G and
Kumar M: Dendrimers and their pharmaceutical applications - a
review. Pharmazie. 63:491–496. 2008.PubMed/NCBI
|
|
12
|
Svenson S: Dendrimers as versatile
platform in drug delivery applications. Eur J Pharm Biopharm.
71:445–462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sadekar S and Ghandehari H:
Transepithelial transport and toxicity of PAMAM dendrimers:
Implications for oral drug delivery. Adv Drug Deliv Rev.
64:571–588. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vetter A, Virdi KS, Espenlaub S, Rödl W,
Wagner E, Holm PS, Scheu C, Kreppel F, Spitzweg C and Ogris M:
Adenoviral vectors coated with PAMAM dendrimer conjugates allow CAR
independent virus uptake and targeting to the EGF receptor. Mol
Pharm. 10:606–618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song S, Liu D, Peng J, Sun Y, Li Z, Gu JR
and Xu Y: Peptide ligand-mediated liposome distribution and
targeting to EGFR expressing tumor in vivo. Int J Pharm.
363:155–161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rubin KM: Management of primary cutaneous
and metastatic melanoma. Semin Oncol Nurs. 29:195–205. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maverakis E, Cornelius LA, Bowen GM, Phan
T, Patel FB, Fitzmaurice S, He Y, Burrall B, Duong C, Kloxin AM, et
al: Metastatic melanoma - a review of current and future treatment
options. Acta Derm Venereol. 95:516–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
del Burgo LS, Hernández RM, Orive G and
Pedraz JL: Nanotherapeutic approaches for brain cancer management.
Nanomedicine. 10:905–919. 2014.PubMed/NCBI
|
|
19
|
Patil R, Portilla-Arias J, Ding H, Inoue
S, Konda B, Hu J, Wawrowsky KA, Shin PK, Black KL, Holler E, et al:
Temozolomide delivery to tumor cells by a multifunctional nano
vehicle based on poly(β-L-malic acid). Pharm Res. 27:2317–2329.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thomas TP, Majoros IJ, Kotlyar A,
Kukowska-Latallo JF, Bielinska A, Myc A and Baker JR Jr: Targeting
and inhibition of cell growth by an engineered dendritic
nanodevice. J Med Chem. 48:3729–3735. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia T, Kovochich M, Liong M, Zink JI and
Nel AE: Cationic polystyrene nanosphere toxicity depends on
cell-specific endocytic and mitochondrial injury pathways. ACS
Nano. 2:85–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Conner SD and Schmid SL: Regulated portals
of entry into the cell. Nature. 422:37–44. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Albanese A, Tang PS and Chan WC: The
effect of nanoparticle size, shape, and surface chemistry on
biological systems. Annu Rev Biomed Eng. 14:1–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoon HY, Koo H, Choi KY, Lee SJ, Kim K,
Kwon IC, Leary JF, Park K, Yuk SH, Park JH, et al: Tumor-targeting
hyaluronic acid nanoparticles for photodynamic imaging and therapy.
Biomaterials. 33:3980–3989. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eliaz RE and Szoka FC Jr:
Liposome-encapsulated doxorubicin targeted to CD44: A strategy to
kill CD44-overexpressing tumor cells. Cancer Res. 61:2592–2601.
2001.PubMed/NCBI
|
|
26
|
Appel EA, Rowland MJ, Loh XJ, Heywood RM,
Watts C and Scherman OA: Enhanced stability and activity of
temozolomide in primary glioblastoma multiforme cells with
cucurbit[n]uril. Chem Commun. 48:9843–9845. 2012. View Article : Google Scholar
|