Introduction
Combination chemotherapy has long been adopted as
the standard of care against various cancer types. Compared to the
single-drug chemotherapy, the proper drug combination, especially
the combination of cytotoxic and chemosensitizing agents exhibited
better synergistic actions and target selectivity, more effective
for tumor localization, thus overcoming multi-drug resistance (MDR)
(1–3). For instance, the combination of
anticancer agents such doxorubicin (DOX) and curcumin (CUR) has
been shown to modulate different signaling pathways in breast
cancer cells, which are beneficial to reverse multidrug resistance
(MDR) (4,5).
Curcumin (CUR), a naturally occurring polyphenol
extracted from the rhizome Curcuma longa, is widely used in
China and India as a traditional herb (6). Recently, more and more studies have
shown that CUR, either alone or in combination with other
anticancer agents, has pleiothropic antineoplastic effects, due to
modulation of nuclear factor-kappa B (NF-κB) and other cell
signaling pathways (7–9). Furthermore, CUR is known to inhibit
the ATP-binding cassette (ABC) drug transporters implicated in
cancer MDR, such as P-glycoprotein (P-gp), breast cancer resistance
protein (ABCG2/BCRP), and multidrug resistance-associated proteins
1 and 5 (MRP1 and MRP5) (10–12).
Therefore, CUR may be the optimum small-molecule chemosensitizer
and brings new hope for the combination therapy of breast cancer.
However, the clinical application of CUR is still hindered due to
its low aqueous solubility, extreme degradation and metabolization
which are responsible for poor bioavalability and pharmacological
activity (13). Hence, it is highly
desirable to develop a novel drug delivery system to solve these
obstacles.
Stimuli-responsive and prodrug-based nanoassemblies
have many advantages as a potent platform for co-delivery of
anticancer drugs, such as improved drug availability, and high drug
loading efficiency, to realize responsive release of large amount
of drugs (14). In this study, a
novel pH-sensitive CUR prodrug was designed. It was composed of
poly(ethylene glycol)-aldehyde (PEG-CHO), adipodihydrazide (ADH),
and CUR by the pH-sensitive biodegradable chemical linkers
(hydrazone bonds). Furthermore, dual targeted nanoparticles (NPs),
pH-sensitive and transferrin (Tf) functionalized NPs, were
engineered based on the prodrug technology.
Among the various types of ligands used for active
targeting, transferrin (Tf) or folate (FA) stands out to be a
desirable choice for malignant tumors, since most solid tumor cells
express a high level of Tf receptor (TfR) or folate receptor (FR)
on their surfaces while the level of TfR or FR is much lower in
non-epithelial tumors and normal tissues (15–18).
Tf and FA, both have been extensively used to decorate PEG-based
amphiphilic nanomaterials for the development of drug delivery
system (18). Our group has
constructed folate (FA) decorated nanostructured lipid carriers
(NLCs) for targeted delivery of curcumin (CUR). The results
demonstrated that FA-CUR-NLCs were efficient in selective delivery
to MCF-7 human breast cancer cells overexpressing FA receptors
(FRs) (19). In this study, we
designed Tf decorated nanoparticles (NPs) to co-deliver CUR and DOX
for breast cancer treatment.
The pH-sensitive prodrug, Tf-PEG-CUR, was
synthesized. Tf-PEG-CUR was capable of self-assembling into
nanoparticles (Tf-PEG-CUR NPs). Because of the hydrophobic nature
of DOX, it could be entrapped within the Tf-PEG-CUR NPs in the core
of the NPs to obtain Tf-PEG-CUR/DOX NPs. In vitro
cytotoxicity studies and in vivo antitumor activity were
carried out using MCF-7 cells and mice bearing MCF-7 cells,
respectively. This system was anticipated to achieve stable, dual
targeting NPs of CUR and DOX, to improve synergistic anticancer
effects, and reduce toxicity.
Materials and methods
Materials
Doxorubicin (DOX), curcumin (CUR), human Tf
(iron-free), maleic anhydride (MA),
1-ethyl-3-(3-dimethylamino-propyl) carbodiimide (EDC) and adipic
acid dihydrazide (ADH) were purchased from Sigma-Aldrich (St.
Louis, MO, USA). CHO-PEG-CHO (average molecular weight, 3.4 kDa)
was purchased from Taiyuan Pegchem Technology Co., Ltd. (Shanxi,
China). N,N'-dicyclohexyl-carbodimide (DCC) and
4-dimethylaminopyridine (DMAP) were obtained from GL Biochem Co.,
Ltd. (Shanghai, China). Doxorubicin hydrochloride liposome
injection (DOX Lip) and doxorubicin hydrochloride injection (DOX
Inj) were purchased from Cardinal Health China (Shanghai, China).
All other chemicals were of analytical grade or higher.
Cells and animals
The MCF-7 human breast cancer cell lines (MCF-7
cells) were obtained from American Type Culture Collection
(Manassas, VA, USA) and cultured in Dulbecco's Modified Eagle's
medium (DMEM) (Sigma, St. Louis, MO, USA) supplemented with 10%
fetal bovine serum (FBS) (Fisher Chemicals, Fairlawn, NJ, USA) and
100 U/ml penicillin and 100 mg/ml streptomycin (Sigma) in a 5%
CO2 fully humidified atmosphere. All experiments were
performed on cells in the logarithmic phase of growth.
BALB/c nude mice (6–8 weeks) were purchased from the
Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China).
Mice were acclimated at 25°C and 55% of humidity under natural
light/dark conditions for 7 days before the experiment. All the
animal experiments were performed in accordance with the Animal
Management Rules of the Ministry of Health of the People's Republic
of China.
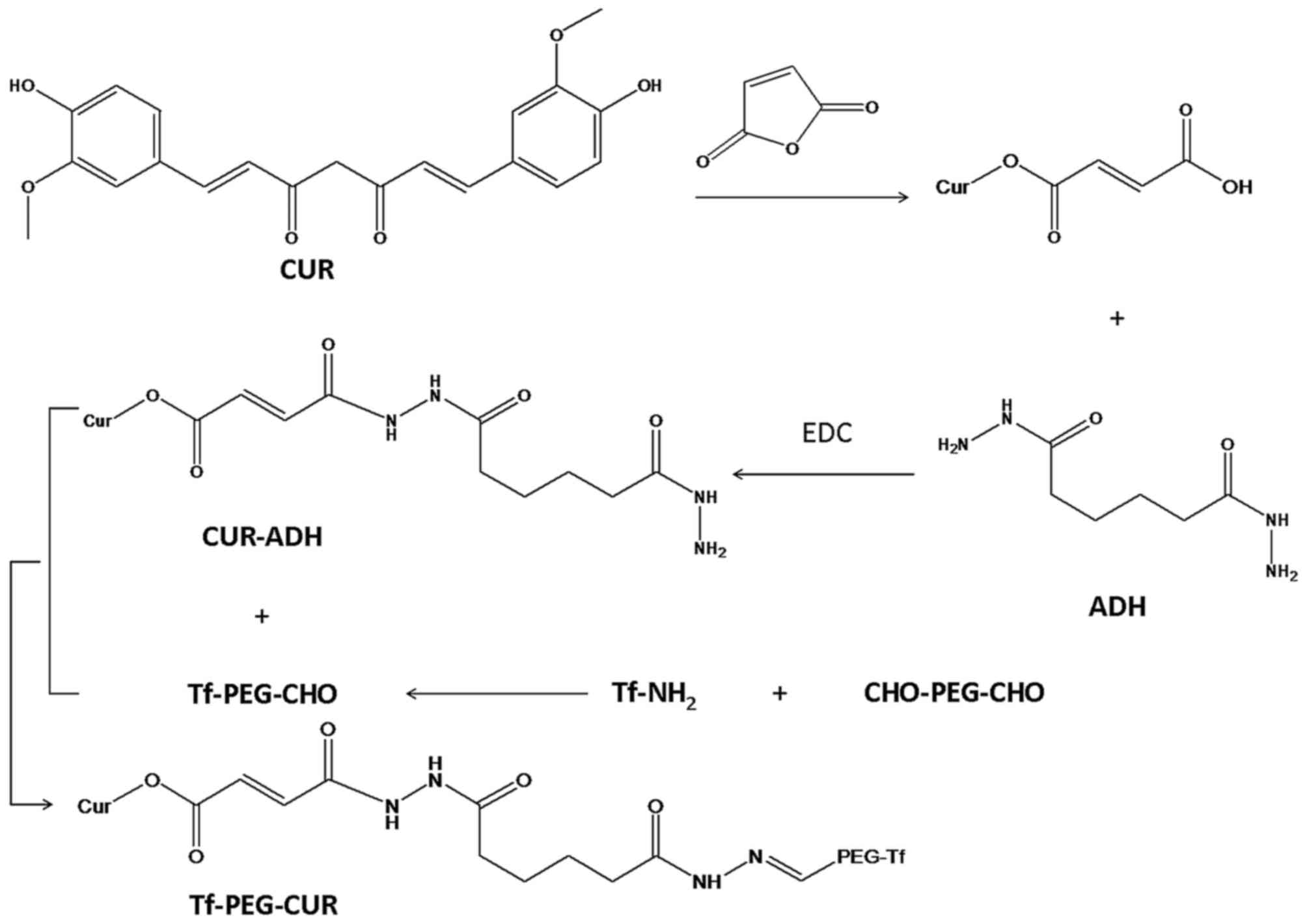
Synthesis and characterization of
Tf-PEG-CUR
Tf-PEG-CUR was achieved by four-step reaction
(Fig. 1). Firstly, CUR (0.2 mmol),
MA (0.15 mmol), DCC (0.2 mmol), DMAP (0.02 mmol) were dissolved in
anhydrous dimethyl sulfoxide (DMSO). The reaction mixture was
stirred at 400 rpm for 24 h under the protection of nitrogen, and
filtered to remove byproducts. The solution was poured into 10 ml
cold ethyl acetate. The solvent was removed by a rotary evaporator
to obtain CUR-MA.
Secondly, CUR-MA (0.1 mmol), ADH (0.2 mmol) and EDC
(0.2 mmol) were dissolved in DMSO. The reaction mixture was stirred
at 400 rpm for 24 h under the protection of nitrogen. CUR-ADH was
obtained by vacuum drying.
Thirdly, Tf-PEG-CHO was synthesized. CHO-PEG-CHO and
Tf were dissolved in acetate buffer (pH 5.0), then 20 mM sodium
cyanoborohydride (NaCNBH3) was added under stirring for
24 h (20). The reaction mixture
was separated by gel-filtration chromatography.
Finally, CUR-ADH was conjugated to Tf-PEG-CHO via a
hydrazone linkage formed between the ketone group of Tf-PEG-CHO and
the hydrazide end group of CUR-ADH. Tf-PEG-CHO (0.05 mmol) and
CUR-ADH (0.05 mmol) were dissolved in DMSO, then 3 µl triethylamine
was added and reacted under stirring for 24 h at room temperature.
The sample was purified by dialysis with a dialysis bag and
freeze-dried to obtain Tf-PEG-CUR. Chemical structure of Tf-PEG-CUR
was analyzed by 1H-NMR spectroscopy.
Preparation of Tf-PEG-CUR NPs and
Tf-PEG-CUR/DOX NPs
Tf-PEG-CUR (100 mg) was dissolved in DMSO (5 ml) at
25°C, and then the solution was dropwise added to 10 ml of PBS
under gentle stirring (21,22). The solutions were dialyzed against
excess PBS with a dialysis bag (MWCO: 10 kDa) for 48 h, and then
filtered through a 0.45-µm pore-sized microporous membrane to
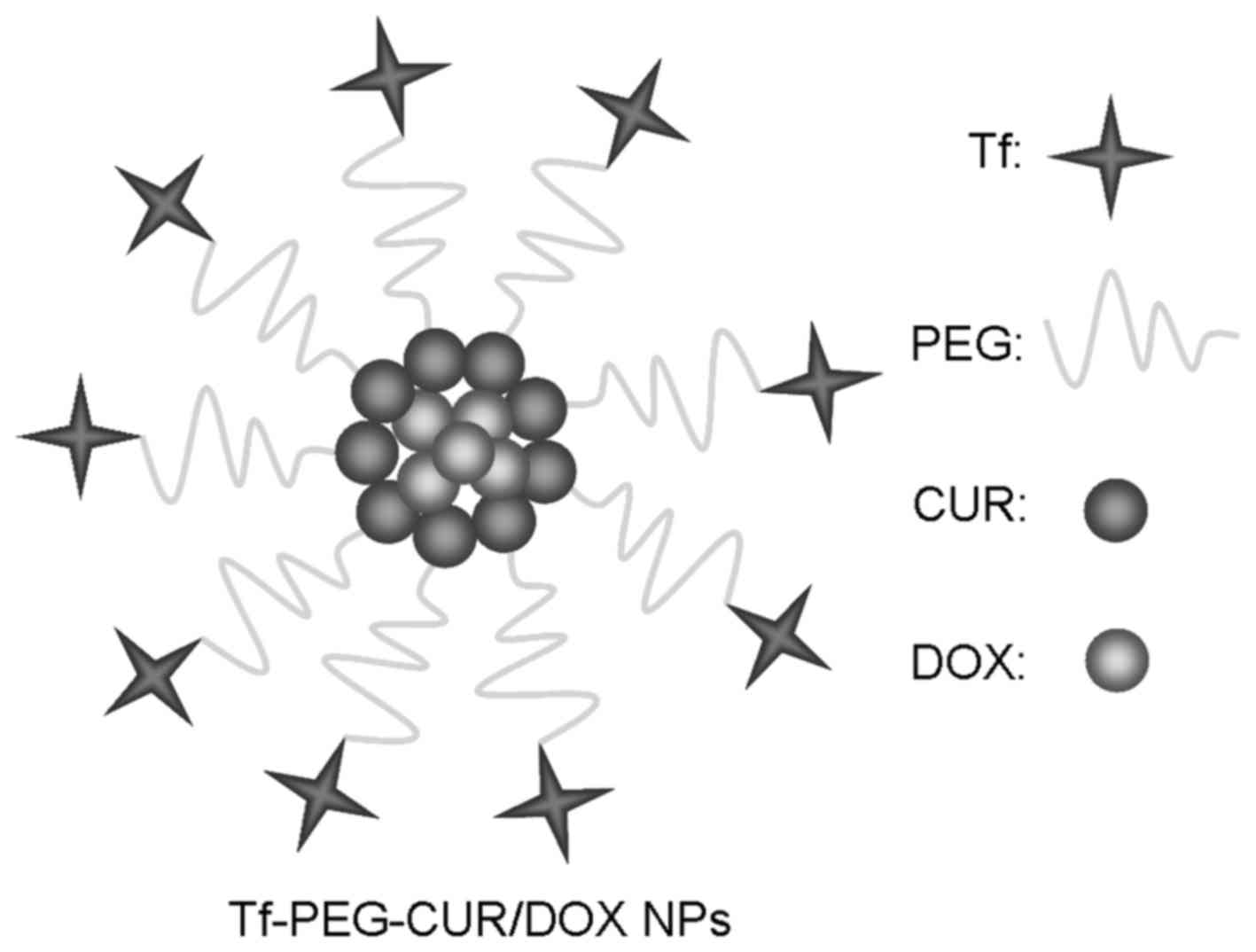
obtain Tf-PEG-CUR NPs. Tf-PEG-CUR/DOX NPs (Fig. 2) were prepared with the same
procedure as Tf-PEG-CUR NPs by adding DOX into the DMSO solution:
the Tf-PEG-CUR (100 mg) and DOX (10 mg) were dissolved in DMSO (5
ml) at 25°C. CUR and DOX mixed injection (CUR/DOX Inj) was prepared
by adding 10 mg of CUR into 5 ml of DOX Inj (5 ml: 10 mg). The
obtained NPs and Inj were stored at 2–8°C.
Particle morphology, size and zeta
potential
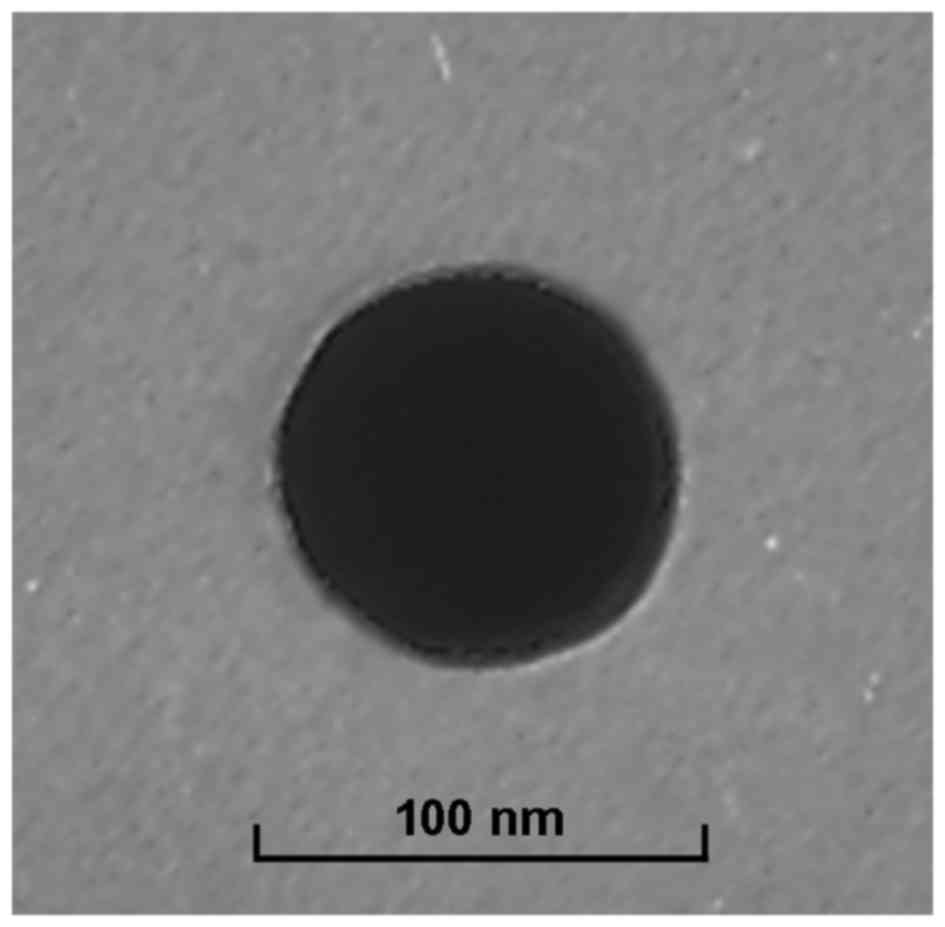
Particle morphology of Tf-PEG-CUR/DOX NPs was
observed by a transmission electron microscopy (TEM, Hitachi,
Tokyo, Japan) (23). Diluted NPs
were placed on a carbon-coated copper grid, negatively stained with
2% phosphotungstic acid, and then observed with TEM. The size,
polydispersity index, and zeta potential of Tf-PEG-CUR NPs and
Tf-PEG-CUR/DOX NPs were measured using a Zetasizer (Nano ZS 90,
Malvern, Worcestershire, UK).
Drug encapsulation efficiency and drug
loading capacity
The encapsulation efficacy (EE) and drug loading
capacity (DL) of DOX and CUR was determined using ultrafiltration
method. The amount of DOX was determined with F-4500 fluorescence
spectrophotometer (emission wavelength: 480 nm, excitation
wavelength: 556 nm, Hitachi). The amount of CUR was measured with
an HPLC method at 420 nm using Agilent 1260 Infinity LC (Agilent
Technologies, Santa Clara, CA, USA). HPLC analyses were performed
on a Hypersil ODS2 C18 column (250 mm × 4.6 mm, 5 µm). The mobile
phase used was acetonitrile: 4% glacial acetic acid in water (v/v,
45:55). The EE and DL were calculated using the following equations
(6): EE (%) = the amount of loaded
DOX or CUR / total amount of DOX or CUR used for NP preparation ×
100. DL (%) = the amount of loaded DOX or CUR / total amount of the
DOX or CUR and NPs × 100.
In vitro stability in plasma
Plasma stability of Tf-PEG-CUR/DOX NPs was evaluated
(24). Tf-PEG-CUR/DOX NPs were
incubated in phosphate buffer (PBS) solution containing 10% fetal
bovine serum (FBS, v/v) at 37°C for 24 h, respectively. At
scheduled times (0, 1, 2, 4, 8, 12 and 24 h), 1 ml of each sample
was diluted with 2 ml THF and the mixture was bath sonicated for 5
min, followed by centrifugation at 10,000 rpm for 5 min. The
variation trends of the size and EE were calculated by the method
described in the above two sections.
In vitro drug release study
CUR and DOX released from Tf-PEG-CUR/DOX NPs in
vitro was investigated by the dialysis method against the
release medium at pH 7.4, or 5.0, respectively (25). Briefly, 2 ml of NPs suspension was
added into a dialysis tube with MWCO 3 kDa, and incubated in 40 ml
of release medium with then centrifuging at 100 rpm, at 37°C. At
different time-points, release medium (2 ml) was collected and the
same volume of fresh release medium was added into the system. CUR
contents in the release medium were determined by the same method
as described in ‘Drug encapsulation efficiency and drug loading
capacity’.
In vitro cytotoxicity assay
The cytotoxicity of Tf-PEG-CUR NPs and
Tf-PEG-CUR/DOX NPs was determined using the standard MTT cell
proliferation kit (ATCC) according to the manufacturer's protocol
(26). DOX Lip, CUR/DOX Inj, and
DOX Inj were used as contrast. Briefly, MCF-7 cells were seeded
onto 96-well plates with a density of 1×104 cells per
well and incubated at 37°C in a humidified atmosphere of 95% air
and 5% CO2 for 16 h. The medium in each well was
replaced with 200 ml of culture medium containing the samples and
cultured for 72 h. The medium in each well was then replaced with
fresh medium and the cells were incubated for another 24 h. The
incubation medium was then replaced with 100 ml of fresh medium and
10 ml of MTT reagent. After 6 h, 100 ml of detergent reagent was
added to each well and incubated for 18 h at room temperature in
the dark until all the crystals were dissolved. The absorbance
intensity at 570 nm was recorded on a Bio-Rad Microplate Reader
Model 550 (Bio-Rad Laboratories, Hercules, CA, USA). Cell viability
was defined as the percent live cells compared with untreated
controls. The half maximal inhibitory concentration
(IC50) values were calculated accordingly.
In vivo tissue distribution study
BALB/c mice were inoculated subcutaneously with
1×106 MCF-7 cells. MCF-7 tumor xenografts were grown in
Balb/C mice and estrogen was provided as a β-estradiol pellet 1
week prior to the injection of the cells. The tumors were allowed
to develop on the posterolateral side of the mice for one week
prior to treatment to obtain the breast cancer-bearing animal model
(27). Tf-PEG-CUR/DOX NPs and
CUR/DOX Inj were injected into the mice by tail vein, respectively.
In the in vivo organ distribution study each sample was
investigated at 10 min, 1, 8, 24 and 48 h after intravenous
injection. At predetermined time intervals, mice were sacrificed
and the tumor, heart, liver, spleen, lung and kidney were
collected. Tissues were initially weighed and homogenized with
physiological saline to determine the amount of DOX or CUR in each
tissue. The concentrations of released DOX or CUR were determined
as described in ‘Drug encapsulation efficiency and drug loading
capacity’.
In vivo antitumor evaluation
After the breast cancer-bearing animal model was
built, the mice were randomized into 6 groups and intravenously
injected once a week for 7 weeks with 0.9% normal saline (NS),
Tf-PEG-CUR/DOX NPs, Tf-PEG-CUR NPs, DOX Lip, CUR/DOX Inj, and DOX
Inj contained 50 mg/kg CUR and/or DOX (4,28).
Tumor growth was determined by measuring the major (L) and minor
(W) diameter with a caliper. The tumor volume was calculated
according to the formula: Tumor volume = 0.5 × L × W2.
The body weight was measured. The inhibition rate of tumor growth
(IRT) was calculated according to the following formula: IRT (%) =
(mean tumor weight of a control group - mean tumor weight of a
treatment group) / mean tumor weight of a control group × 100.
Statistical analysis
Assignment to treatments were made at random.
Treatment comparisons were made by analysis of variance and
protected least significant difference or Student's t-test.
Differences were considered statistically significant at P<0.05.
Data are presented as means ± standard errors.
Results
Characterization of Tf-PEG-CUR
As showed in Fig. 1,
during the synthesis of Tf-PEG-CUR, hydrazone bond (−NH-N=CH-),
amido linkage (−NH-C=O), and ester bond (−O-C=O) was formed.
1H-NMR analyses were performed to confirm the synthesis
(δ, ppm): 8.13 (−NH-NH-); 7.59 (−N-N=CH-); 7.08 (−NH-N=CH-); 6.89
(−O-C=O-CH=); 6.57–6.79 (H of benzene ring); 5.41
(−CH2-O-C=O). The proton signals in the
1H-NMR suggest the successful synthesis of
Tf-PEG-CUR.
Characterization of Tf-PEG-CUR/DOX
NPs
Tf-PEG-CUR/DOX NPs display a spherical morphology
with a smooth exterior (Fig. 3).
The size of NPs measured was ~90 nm and characterized by a
relatively narrow size distribution (Table I). The zeta potential of Tf-PEG-CUR
NPs and Tf-PEG-CUR/DOX NPs was −11.3 and −15.6 mV, respectively.
CUR and DOX were efficiently loaded in Tf-PEG-CUR/DOX NPs, reaching
the DL of 4.6 and 5.9% with the EE of 85.3 and 82.7%,
respectively.
 | Table I.Physicochemical characterization. |
Table I.
Physicochemical characterization.
| Formulation | Particle size
(nm) | Polydispersity
index | Zeta potential
(mV) | CUR EE (%) | DOX EE (%) | CUR DL (%) | DOX DL (%) |
|---|
| Tf-PEG-CUR NPs | 89.5±3.4 | 0.12±0.02 | −11.3±1.1 | 84.6±2.8 | N/A | 5.4±0.7 | N/A |
| Tf-PEG-CUR/DOX
NPs | 88.7±3.9 | 0.14±0.03 | −15.6±1.6 | 85.3±3.2 | 82.7±4.1 | 4.6±0.8 | N/A |
In vitro plasma stability
Changes in size and EE in the presence of serum are
described in Table II.
Tf-PEG-CUR/DOX NPs were stable up to 24 h without any significant
size or EE changes. Thus, Tf-PEG-CUR/DOX NPs were considered very
stable after incubation with FBS and suggest that this formulation
will not aggregate or disassemble after intravenous
administration.
 | Table II.Changes in size and EE in the
presence of serum. |
Table II.
Changes in size and EE in the
presence of serum.
| Time (h) | Particle size
(nm) | CUR EE (%) | DOX EE (%) |
|---|
| 0 | 88.9±4.1 | 85.7±3.4 | 82.9±3.8 |
| 1 | 89.1±3.6 | 85.1±3.7 | 82.3±3.4 |
| 2 | 89.8±4.3 | 85.3±3.9 | 82.8±3.7 |
| 4 | 90.6±3.9 | 84.8±3.8 | 82.1±3.5 |
| 8 | 89.4±4.6 | 83.9±4.1 | 81.6±4.0 |
| 12 | 91.1±4.5 | 83.5±4.6 | 80.9±4.4 |
| 24 | 90.7±3.8 | 84.1±4.4 | 81.3±4.2 |
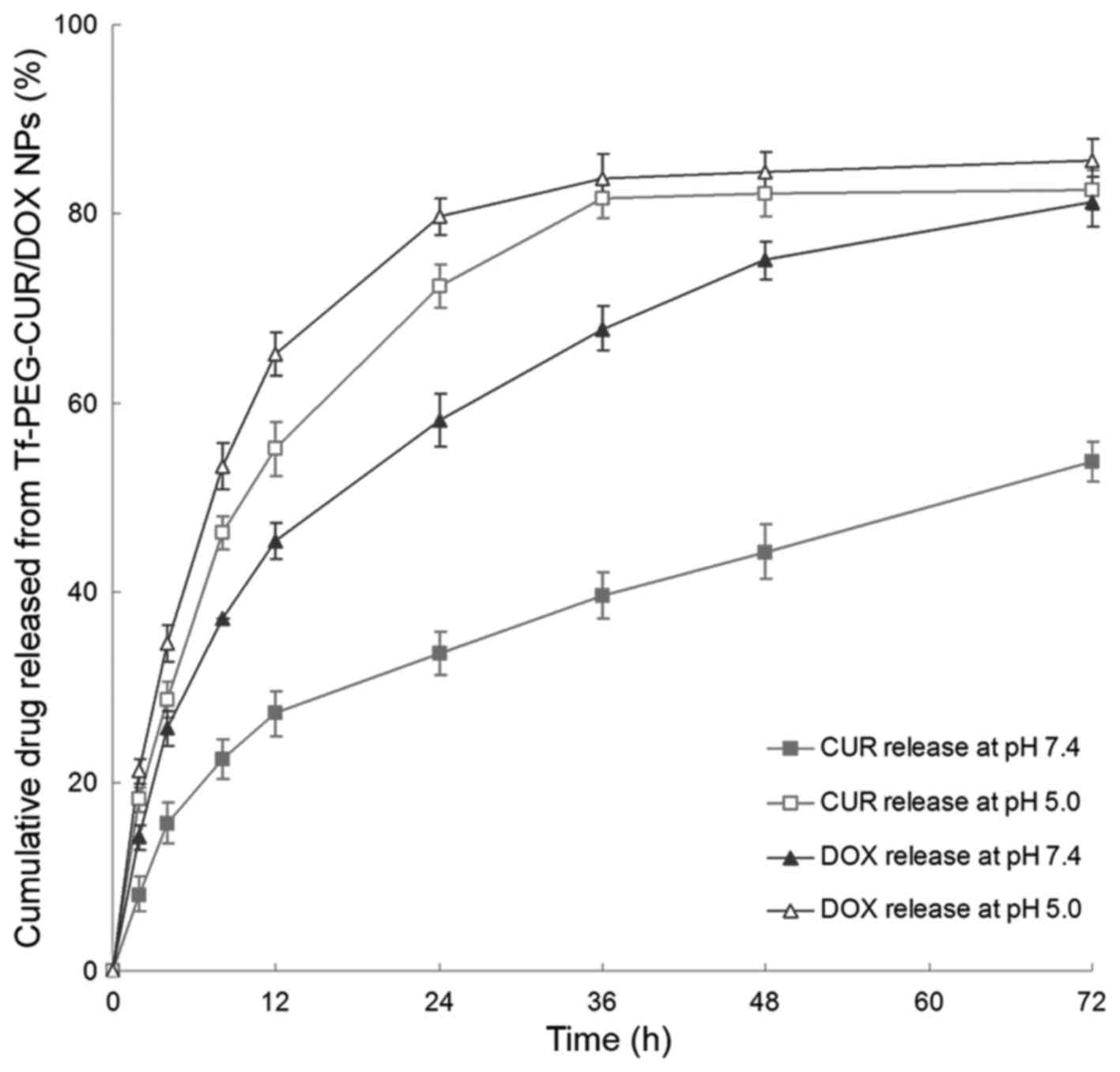
In vitro drug release
The in vitro drug release from Tf-PEG-CUR/DOX
NPs was studied at 37°C under pH 7.4 or 5.0. The results showed
that CUR release was significantly accelerated under mildly acidic
environments (Fig. 4). For example,
72.4 and 33.6% of CUR was released from NPs in 24 h at pH 5.0 and
7.4, respectively. Moreover, DOX release was also observed to be
faster at pH 5.0.
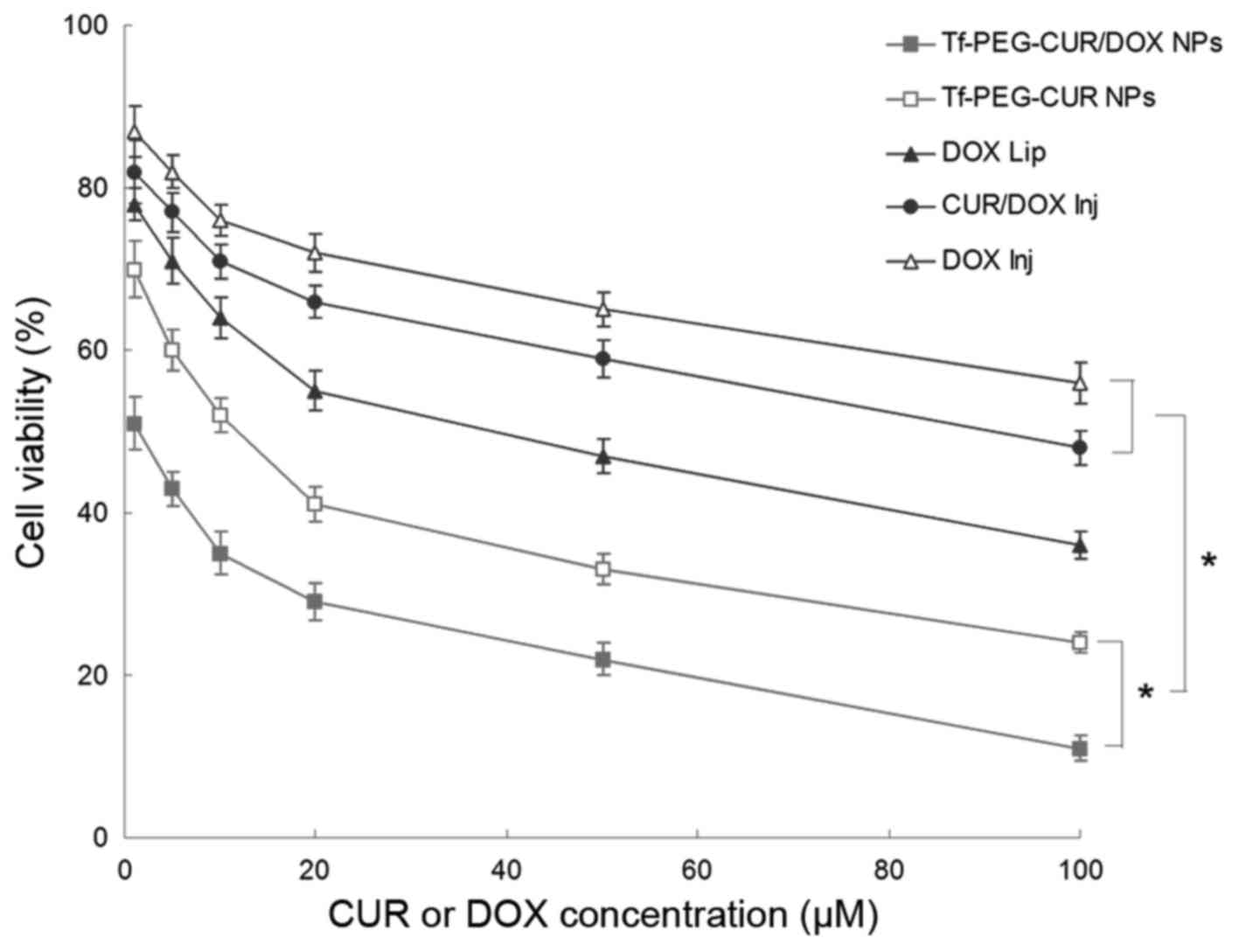
In vitro cytotoxicity
As shown in Fig. 5,
Tf-PEG-CUR/DOX NPs exhibited relatively higher toxicity in
comparison with Tf-PEG-CUR NPs in MCF-7 cells (P<0.05). NP
formulations showed slightly higher toxicity than liposomes and
injections (P<0.05). Table III
summarizes the IC50 of different samples tested. The
IC50 of Tf-PEG-CUR/DOX NPs was the lowest (2.5 µM),
which is many-fold dose advantage over the liposome and free drug
injections.
 | Table III.IC50 values of in
vitro cytotoxicity assay. |
Table III.
IC50 values of in
vitro cytotoxicity assay.
| Formulation | Tf-PEG-CUR/DOX
NPs | Tf-PEG-CUR NPs | DOX Lip | CUR/DOX Inj | DOX Inj |
|---|
| IC50 of
CUR (µM) | 2.6±0.3 | 11.3±1.1 | N/A | 83.5±5.6 | N/A |
| IC50 of
DOX (µM) | 2.6±0.3 | N/A | 39.4±2.7 | 83.5±5.6 | 163.5±12.7 |
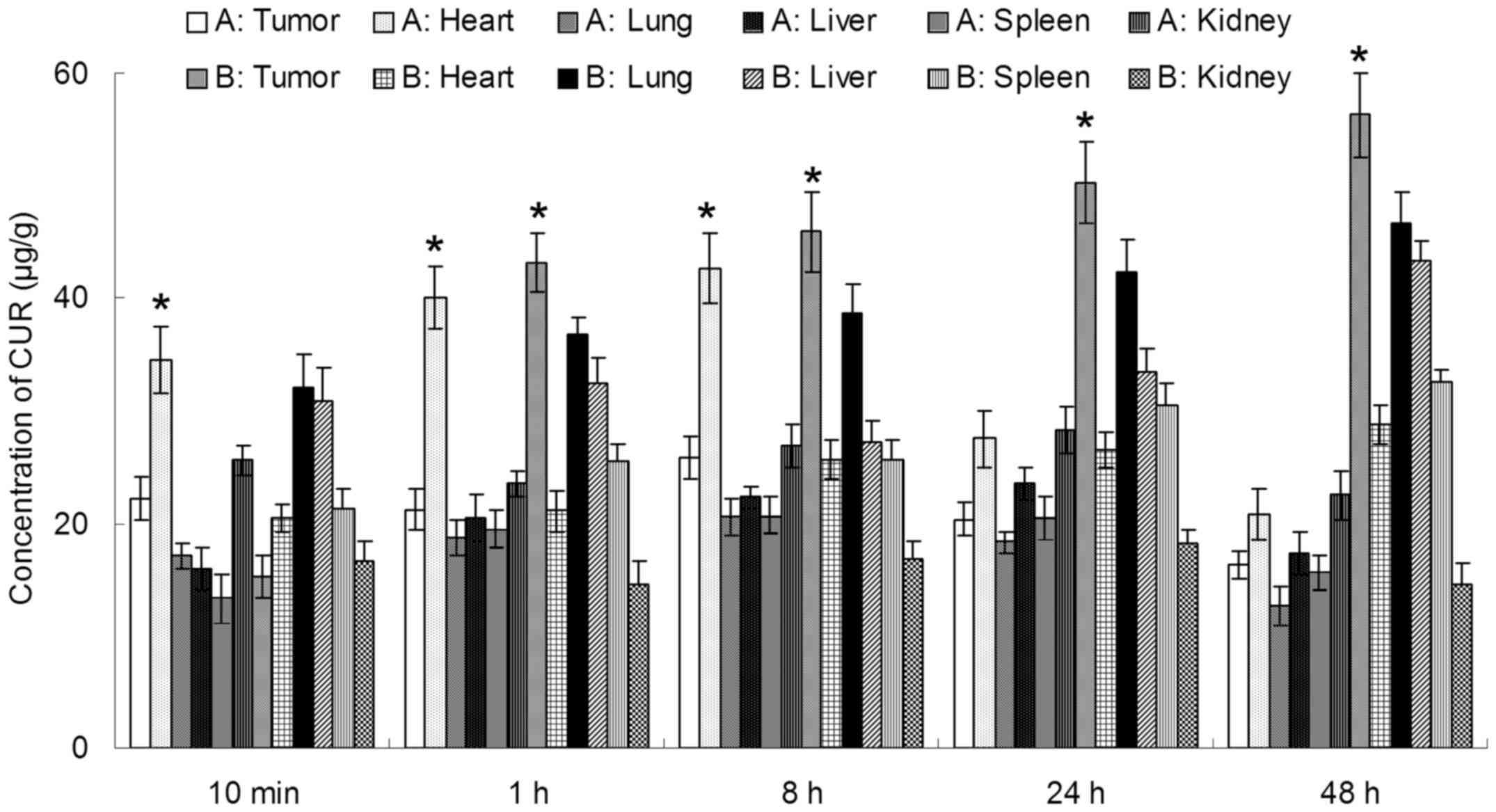
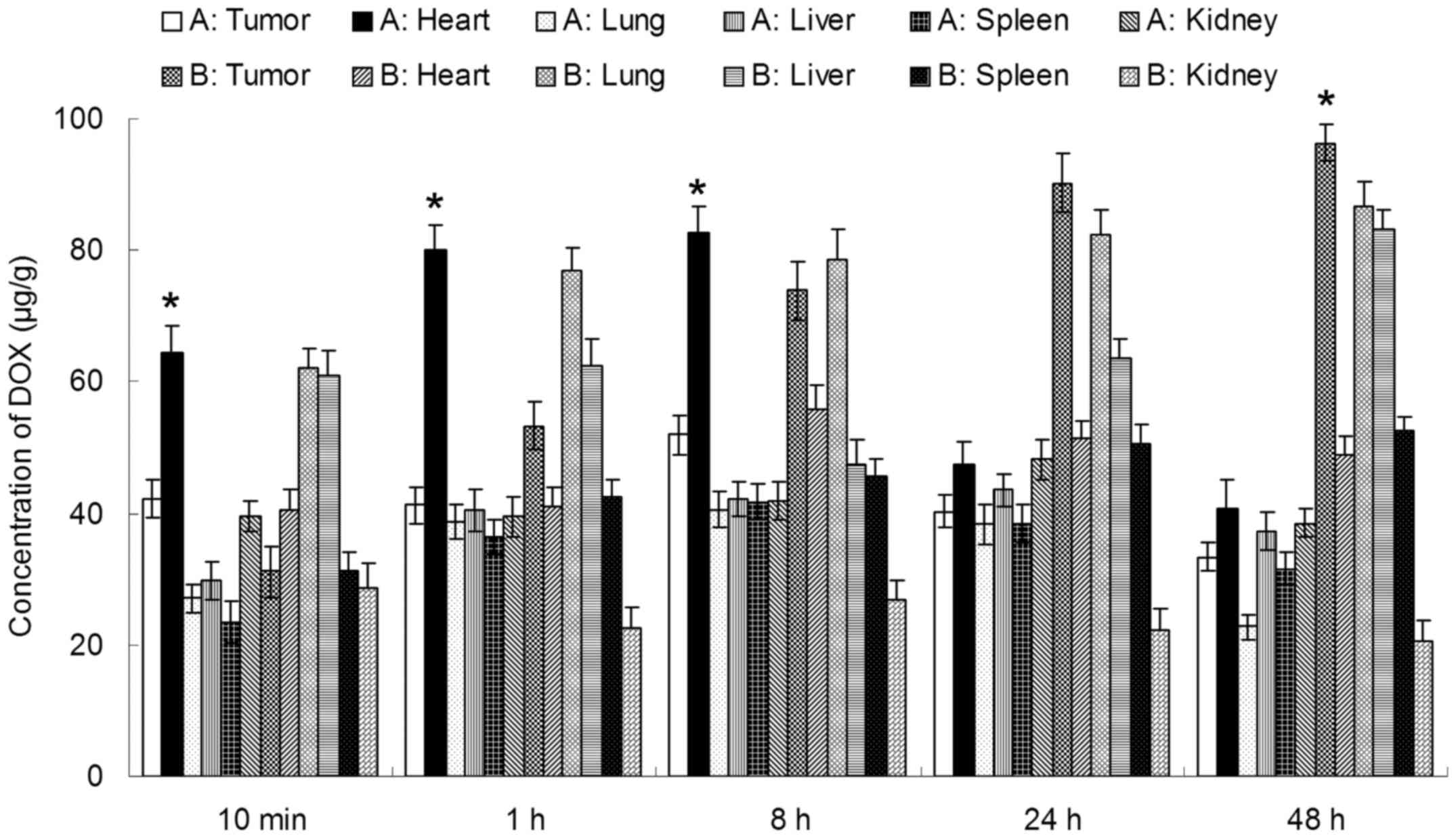
In vivo tissue distribution
In vivo CUR and DOX tissue distribution
outcomes of Tf-PEG-CUR/DOX NPs and CUR/DOX Inj are shown in
Figs. 6 and 7, respectively. The CUR and DOX
concentration in tumor, lung and liver following injection of
Tf-PEG-CUR/DOX NPs was higher than the injection of CUR/DOX Inj,
whereas, the drug concentration of Tf-PEG-CUR/DOX NP group in heart
and kidney was lower than CUR/DOX Inj group. Drug concentrations of
Tf-PEG-CUR/DOX NPs group in the tumor tissue remained relatively
stable at all time-points until 48 h after injection.
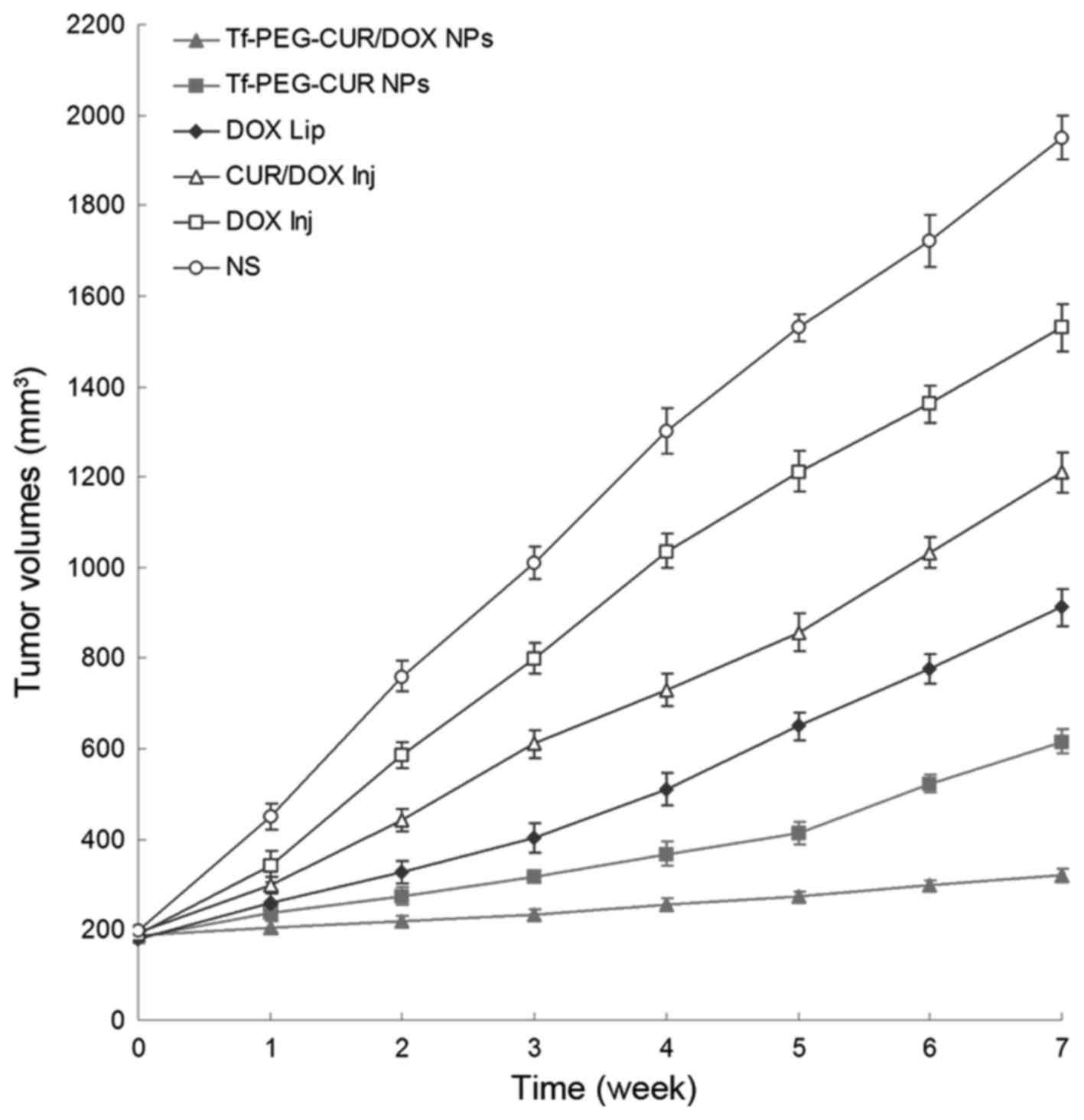
In vivo antitumor efficiency
In vivo antitumor activity of Tf-PEG-CUR/DOX
NPs was performed in MCF-7/ADR tumor bearing mice. As shown in
Fig. 8, tumor volumes of saline
group increased markedly over time, while tumor volume remained
almost unchanged in Tf-PEG-CUR/DOX NP treated group. In contrast,
other treatment groups showed a relatively weaker inhibitory effect
on the tumor growth. Seven weeks after treatment, the tumors from
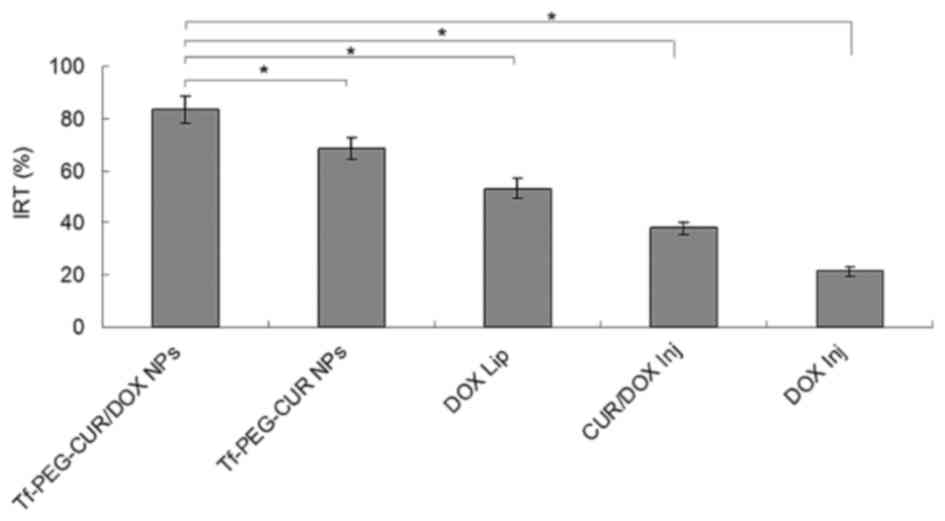
each treatment group were weighed and IRT was calculated. The data
of IRT in Fig. 9 show similar
results to tumor volume. Tf-PEG-CUR/DOX NPs had the highest IRT
(83.5%), while IRT of Tf-PEG-CUR NPs, DOX Lip, and CUR/DOX Inj were
68.4, 53.2 and 37.9%, respectively. The obvious body weight loss
could be observed in the free drug solution groups. However, NP
groups did not cause significant body weight loss.
Discussion
This study developed and characterized the
pH-sensitive prodrug loaded self-assembled particles in order to
evaluate them as a potential drug delivery system for the treatment
of breast cancer. The novel system allows for effective
simultaneous loading of lipophilic drugs. Such a strategy could be
beneficial in treating many types of cancer that develops
resistance to the same chemotherapeutic agent administered over
time (29).
Tf-PEG-CUR/DOX NPs displayed beneficial
physicochemical characteristics, including uniform particle size,
narrow particle size distribution, high encapsulation efficacy and
sustained drug release. Under TEM observation, Tf-PEG-CUR/DOX NPs
exhibited a spherical morphology. The NPs showed a negatively
charged surface and an average hydrodynamic diameter of ~90 nm
(Table I). The most likely origin
of this negative charge is the presence of the anionic materials
used in the preparation process. The PDI of each formulation was
lower than 0.2, indicating the homogeneous nature of the
formulation (23). A successful NP
system should have a good loading capacity and high encapsulation
efficacy to reduce the quantity of the carrier required for
administration (30).
Tf-PEG-CUR/DOX NPs had EE of >80% for both CUR and DOX,
suggesting the fine loading efficiency of the NPs.
In vitro drug release study of Tf-PEG-CUR/DOX
NPs was investigated at pH 7.4, and 5.0. In acidic media, the
release of CUR was more rapid than that of the neutral environment.
This may be because of the pH-sensitive hydrazone bonds could
cleave much more easily in low pH and release the drug faster
(31). Besides, DOX release rate of
also increased as pH value decreased from pH 7.4 to 5.0. It could
be explained by the degradation of the NPs that occurs
predominantly via hydrazone bond cleavage at lower pH medium
releasing the DOX loaded in the NPs (32). The pH value of the bloodstream is
~7.4, while the existing tumoral pH and that of endocytic
compartments of the cells generally range from 4 to 6 (33,34).
This difference in pH value makes pH-triggered drug release
possible. Thus, the use of pH-sensitive biodegradable polymeric
materials as drug delivery carries has been considered as a common
procedure. These properties can make the multifunctional drug
loading NPs to internalize into tumor cells easier, and then
release antitumor drug and kill the tumor cells.
The cytotoxicity data unambiguously showed that the
pH-sensitive Tf-PEG-CUR/DOX NPs proved to significantly outclass
the free drug in terms of relative potency (25). It inhibited the viability and
proliferation of the breast cancer cell lines at low
concentrations, with IC50 value many times lower than
other contrast formula tested. Higher toxicity of Tf-PEG-CUR/DOX
NPs in comparison with Tf-PEG-CUR NPs may be evidence of the
co-delivery of the two drugs has a synergistic effect to inhibit
cancer cells. IC50 values of NP formulations were much
lower than liposome and free drug injections. This may because the
pH-sensitive prodrug containing NPs would disassemble after
internalized by cancer cells, and the CUR and DOX released from NPs
continuously accumulated within the tumor cells, and then killed
cancer cells (22).
In vivo drug distribution in heart and kidney
may cause systemic toxicity; on the contrary, distribution mainly
in tumor tissue compared with the other tissues could decrease the
side effects and lead to better antitumor therapeutic efficiency
(35). Solid tumors have leakage in
the micro-vasculature and the nano-sized particles could target the
tumor owing to the enhanced permeability and retention (EPR)
effects (36). EPR effects
prevented the entry of nanoformulation in the normal cell at the
same time favoring selective entry into tumors, which resulted in
efficient drug accumulation in tumor tissue (37). Drug concentrations of Tf-PEG-CUR/DOX
NP group in the tumor tissue remained high until 48 h after
injection, indicating the sustained-release behavior of the DOX+CUR
LPNs. The long circulating effect of NPs was due to the presence of
PEG chain on the surface of particles, which provided stealth
effect to the NPs (38).
In vivo antitumor effect of the
Tf-PEG-CUR/DOX NPs was evaluated in MCF-7/ADR tumor xenograft
model. Compared with CUR/DOX Inj, Tf-PEG-CUR/DOX NPs presented a
remarkably higher inhibition effect towards tumor growth, which is
consistent with their in vitro efficiency (4). After Tf-PEG-CUR/DOX NP was
intravenously injected, they would accumulate into tumor because
NPs preferentially extravasated from pores in tumor vessel walls
(39). Moreover, Tf-PEG-CUR/DOX NP
was able to release drugs more quickly upon their arrival on the
acidic tumor area, resulting in higher CUR and DOX concentration in
the local region of the tumor. This corresponded with the results
of drug concentration in the tumor. Higher IRT (83.5%) than that of
DOX Lip (53.2%) suggests better anticancer ability of NPs than
their liposome counterparts. In order to evaluate the systemic
toxicity of different systems, the body weight variation was
calculated. No obvious body weight loss of the NP groups
illustrated the low systemic toxicity of the systems.
In conclusion, we developed self-assembled NPs
containing pH-sensitive prodrug, which can be used as a nanocarrier
for the co-delivery of hydrophobic anticancer drugs. The results
demonstrate that co-encapsulation of CUR and DOX in NPs can promote
the cytotoxicity by both drugs in vitro in breast cancer
cells and in vivo in breast cancer-bearing mouse model. The
synergistic effect is important and may provide combinatorial
strategies in cancer therapy. This study suggests that simultaneous
delivery of CUR and DOX by Tf-PEG-CUR/DOX NPs might be a promising
treatment for breast cancer.
References
|
1
|
Hu CM and Zhang L: Nanoparticle-based
combination therapy toward overcoming drug resistance in cancer.
Biochem Pharmacol. 83:1104–1111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang P, Wang D, Su Y, Huang W, Zhou Y,
Cui D, Zhu X and Yan D: Combination of small molecule prodrug and
nanodrug delivery: Amphiphilic drug-drug conjugate for cancer
therapy. J Am Chem Soc. 136:11748–11756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saraswathy M and Gong S: Different
strategies to overcome multidrug resistance in cancer. Biotechnol
Adv. 31:1397–1407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo S, Lv L, Shen Y, Hu Z, He Q and Chen
X: A nanoparticulate pre-chemosensitizer for efficacious
chemotherapy of multidrug resistant breast cancer. Sci Rep.
6:214592016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li WM, Chiang CS, Huang WC, Su CW, Chiang
MY, Chen JY and Chen SY: Amifostine-conjugated pH-sensitive calcium
phosphate-covered magnetic-amphiphilic gelatin nanoparticles for
controlled intracellular dual drug release for dual-targeting in
HER-2-overexpressing breast cancer. J Control Release. 220:107–118.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duan J, Mansour HM, Zhang Y, Deng X, Chen
Y, Wang J, Pan Y and Zhao J: Reversion of multidrug resistance by
co-encapsulation of doxorubicin and curcumin in chitosan/poly(butyl
cyanoacrylate) nanoparticles. Int J Pharm. 426:193–201. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sinha D, Biswas J, Sung B, Aggarwal BB and
Bishayee A: Chemopreventive and chemotherapeutic potential of
curcumin in breast cancer. Curr Drug Targets. 13:1799–1819. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mezzanotte L, An N, Mol IM, Löwik CW and
Kaijzel EL: A new multicolor bioluminescence imaging platform to
investigate NF-κB activity and apoptosis in human breast cancer
cells. PLoS One. 9:e855502014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aggarwal BB, Shishodia S, Takada Y,
Banerjee S, Newman RA, Bueso-Ramos CE and Price JE: Curcumin
suppresses the paclitaxel-induced nuclear factor-kappaB pathway in
breast cancer cells and inhibits lung metastasis of human breast
cancer in nude mice. Clin Cancer Res. 11:7490–7498. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Angelini A, Iezzi M, Di Febbo C, Di Ilio
C, Cuccurullo F and Porreca E: Reversal of P-glycoprotein-mediated
multidrug resistance in human sarcoma MES-SA/Dx-5 cells by
nonsteroidal anti-inflammatory drugs. Oncol Rep. 20:731–735.
2008.PubMed/NCBI
|
|
11
|
Revalde JL, Li Y, Hawkins BC, Rosengren RJ
and Paxton JW: Heterocyclic cyclohexanone monocarbonyl analogs of
curcumin can inhibit the activity of ATP-binding cassette
transporters in cancer multidrug resistance. Biochem Pharmacol.
93:305–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Q, Ye M, Lu Y, Zhang H, Chen Q, Huang
S and Su S: Curcumin improves the tumoricidal effect of mitomycin C
by suppressing ABCG2 expression in stem cell-like breast cancer
cells. PLoS One. 10:e01366942015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burgos-Morón E, Calderón-Montaño JM,
Salvador J, Robles A and López-Lázaro M: The dark side of curcumin.
Int J Cancer. 126:1771–1775. 2010.PubMed/NCBI
|
|
14
|
Cheng R, Meng F, Deng C, Klok HA and Zhong
Z: Dual and multi-stimuli responsive polymeric nanoparticles for
programmed site-specific drug delivery. Biomaterials. 34:3647–3657.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qian ZM, Li H, Sun H and Ho K: Targeted
drug delivery via the transferrin receptor-mediated endocytosis
pathway. Pharmacol Rev. 54:561–587. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Widera A, Norouziyan F and Shen WC:
Mechanisms of TfR-mediated transcytosis and sorting in epithelial
cells and applications toward drug delivery. Adv Drug Deliv Rev.
55:1439–1466. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mulik RS, Mönkkönen J, Juvonen RO, Mahadik
KR and Paradkar AR: Transferrin mediated solid lipid nanoparticles
containing curcumin: Enhanced in vitro anticancer activity by
induction of apoptosis. Int J Pharm. 398:190–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen S, Yang K, Tuguntaev RG, Mozhi A,
Zhang J, Wang PC and Liang XJ: Targeting tumor microenvironment
with PEG-based amphiphilic nanoparticles to overcome
chemoresistance. Nanomedicine. 12:269–286. 2016.PubMed/NCBI
|
|
19
|
Lin M, Teng L, Wang Y, Zhang J and Sun X:
Curcumin-guided nanotherapy: A lipid-based nanomedicine for
targeted drug delivery in breast cancer therapy. Drug Deliv.
23:1420–1425. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shao Z, Shao J, Tan B, Guan S, Liu Z, Zhao
Z, He F and Zhao J: Targeted lung cancer therapy: Preparation and
optimization of transferrin-decorated nanostructured lipid carriers
as novel nanomedicine for co-delivery of anticancer drugs and DNA.
Int J Nanomed. 10:1223–1233. 2015. View Article : Google Scholar
|
|
21
|
Carlson LJ, Cote B, Alani AW and Rao DA:
Polymeric micellar co-delivery of resveratrol and curcumin to
mitigate in vitro doxorubicin-induced cardiotoxicity. J Pharm Sci.
103:2315–2322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Yang C, Wang W, Liu J, Liu Q,
Huang F, Chu L, Gao H, Li C, Kong D, et al: Co-delivery of
doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for
combination therapy of cancer. Sci Rep. 6:212252016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao X, Chen Q, Li Y, Tang H, Liu W and
Yang X: Doxorubicin and curcumin co-delivery by lipid nanoparticles
for enhanced treatment of diethylnitrosamine-induced hepatocellular
carcinoma in mice. Eur J Pharm Biopharm. 93:27–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qu J, Zhang L, Chen Z, Mao G, Gao Z, Lai
X, Zhu X and Zhu J: Nanostructured lipid carriers, solid lipid
nanoparticles, and polymeric nanoparticles: Which kind of drug
delivery system is better for glioblastoma chemotherapy? Drug
Deliv. 08–Jun;2016.(Epub ahead of print). View Article : Google Scholar
|
|
25
|
Jelezova I, Drakalska E, Momekova D,
Shalimova N, Momekov G, Konstantinov S, Rangelov S and Pispas S:
Curcumin loaded pH-sensitive hybrid lipid/block copolymer nanosized
drug delivery systems. Eur J Pharm Sci. 78:67–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang H, Murphy CJ, Zhang B, Shen Y, Van
Kirk EA, Murdoch WJ and Radosz M: Curcumin polymers as anticancer
conjugates. Biomaterials. 31:7139–7149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ling G, Zhang T, Zhang P, Sun J and He Z:
Nanostructured lipid-carrageenan hybrid carriers (NLCCs) for
controlled delivery of mitoxantrone hydrochloride to enhance
anticancer activity bypassing the BCRP-mediated efflux. Drug Dev
Ind Pharm. 42:1351–1359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin L, Hutzen B, Zuo M, Ball S, Deangelis
S, Foust E, Pandit B, Ihnat MA, Shenoy SS, Kulp S, et al: Novel
STAT3 phosphorylation inhibitors exhibit potent growth-suppressive
activity in pancreatic and breast cancer cells. Cancer Res.
70:2445–2454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garbuzenko OB, Winkler J, Tomassone MS and
Minko T: Biodegradable Janus nanoparticles for local pulmonary
delivery of hydrophilic and hydrophobic molecules to the lungs.
Langmuir. 30:12941–12949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Misra R and Sahoo SK: Coformulation of
doxorubicin and curcumin in poly(D,L-lactide-co-glycolide)
nanoparticles suppresses the development of multidrug resistance in
K562 cells. Mol Pharm. 8:852–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gu Y, Zhong Y, Meng F, Cheng R, Deng C and
Zhong Z: Acetal-linked paclitaxel prodrug micellar nanoparticles as
a versatile and potent platform for cancer therapy.
Biomacromolecules. 14:2772–2780. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou L, Liang D, He X, Li J, Tan H, Li J,
Fu Q and Gu Q: The degradation and biocompatibility of pH-sensitive
biodegradable polyurethanes for intracellular multifunctional
antitumor drug delivery. Biomaterials. 33:2734–2745. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bae Y, Jang WD, Nishiyama N, Fukushima S
and Kataoka K: Multifunctional polymeric micelles with
folate-mediated cancer cell targeting and pH-triggered drug
releasing properties for active intracellular drug delivery. Mol
Biosyst. 1:242–250. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee ES, Gao Z, Kim D, Park K, Kwon IC and
Bae YH: Super pH-sensitive multifunctional polymeric micelle for
tumor pHe specific TAT exposure and multidrug resistance. J Control
Release. 129:228–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang Y, Li N, Nie Y, Sheng M, Yue D, Wang
G, Tang JZ and Gu Z: Folate-modified poly(malic acid) graft
polymeric nanoparticles for targeted delivery of doxorubicin:
Synthesis, characterization and folate receptor expressed cell
specificity. J Biomed Nanotechnol. 11:1628–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kobayashi H, Watanabe R and Choyke PL:
Improving conventional enhanced permeability and retention (EPR)
effects; what is the appropriate target? Theranostics. 4:81–89.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mei L, Fu L, Shi K, Zhang Q, Liu Y, Tang
J, Gao H, Zhang Z and He Q: Increased tumor targeted delivery using
a multistage liposome system functionalized with RGD, TAT and
cleavable PEG. Int J Pharm. 468:26–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nilsson C, Østergaard J, Larsen SW, Larsen
C, Urtti A and Yaghmur A: PEGylation of phytantriol-based lyotropic
liquid crystalline particles - the effect of lipid composition, PEG
chain length, and temperature on the internal nanostructure.
Langmuir. 30:6398–6407. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang J, Zhang L, Gao H, Liu Y, Zhang Q,
Ran R, Zhang Z and He Q: Co-delivery of doxorubicin and P-gp
inhibitor by a reduction-sensitive liposome to overcome multidrug
resistance, enhance anti-tumor efficiency and reduce toxicity. Drug
Deliv. 23:1130–1143. 2016.PubMed/NCBI
|