Introduction
Although the immune system generally recognizes
abnormal proteins on tumor cells as tumor antigens, spontaneous
immune responses are too weak to suppress tumor growth. To overcome
this problem, a variety of adjuvants, including toll-like receptor
(TLR) ligands, are investigated to potentiate antitumor immunity.
The bacillus Calmette-Guérin (BCG), historically known for its
effective adjuvant properties, is often used in the treatment of
cancer patients (1).
The innate immune system recognizes
pathogen-associated molecular patterns expressed on microorganisms
through corresponding TLRs, and the activation of innate immunity
by TLRs produces proinflammatory cytokines such as interleukin
(IL)-6 and IL-12, leading to the subsequent induction of adaptive
immune responses (2,3). IL-12 is produced by macrophages (MΦ)
and dendritic cells (DC) and dictates the differentiation of CD4
Th1 cells, which produce interferon (IFN)-γ and activate natural
killer (NK) cells and cytotoxic CD8 T cells (4). IFN-γ plays an important role in the
prevention of primary tumor development and intracellular pathogen
invasion (5–7). Among the different TLR ligands,
lipopolysaccharide (LPS) from gram-negative bacteria exhibits
antitumor activity in addition to marked toxicity (8). The study of the bioactivity of LPS
from various species revealed that LPS prepared from Bordetella
pertussis and a synthetic analog of the LPS lipid A subunit are
less toxic than E. coli LPS, and display antitumor effects
(9,10).
Recent studies have revealed that IL-23/IL-17
signaling plays an important role in tumorigenesis and metastasis
in humans and in mice (11–16). IL-17 is primarily produced by T
cells and acts on tumor cells and tumor-associated stromal cells to
induce angiogenesis and the production of IL-6, IL-8, and matrix
metalloproteinases. IL-23 is produced by Mϕ/DC and facilitates the
expansion and survival of IL-17-producing CD4 T (Th17) cells and
therefore, the production of IL-17 (17,18).
Moreover, it has become evident that a combination of IL-6 and
transforming growth factor (TGF)-β induces Th17 differentiation
from naive T cells (19,20). Since Mϕ/DC produces both antitumor
(IL-12) and tumor-promoting (IL-6/IL-23) cytokines upon stimulation
with TLR ligands (21,22), the regulation of this balance is
critical for TLR-based cancer immunotherapy (17,18).
Moreover, IL-17 stimulates tumor cells and tumor-surrounding cells
to induce IL-6 expression, which in turn leads to the activation of
signal transducer and activator of transcription 3 (STAT3)
(16,23). STAT3 is linked to numerous oncogenic
signaling pathways and is constitutively activated both in tumor
cells and in immune cells under tumor microenvironment-like
conditions. Thus, ideal candidate molecules for tumor immunotherapy
are TLR-based immunomodulators that do not induce or partially
induce IL-6/IL-23.
While exploring TLR4 responsiveness of the material
extracted from algae and cyanobacteria (24), we found that LPS phenol-water
extracts from Spirulina (Arthrospira) were able to induce
IL-12. Noteworthy, Spirulina LPS showed a much lower in
vitro induction of IL-6 and IL-23 by Mϕ/DC than E. coli
LPS. Spirulina is a gram-negative, oxygenic, photosynthetic,
filamentous cyanobacterium (blue-green alga), and since the Aztec
civilization in Mexico, it has been widely used as a nutritional
and therapeutic supplement (25).
Spirulina LPS is reported to be less toxic compared to LPS
from Salmonella abortus (26), but its effects on cytokine
production or antitumor activities have not been studied
extensively. Thus, it would be very interesting to study how
Spirulina LPS affects tumor growth and in vivo
production of inflammatory cytokines.
Although most experiments examined the in
vitro production of IL-17-associated inflammatory cytokines
thus far, the expression patterns of these cytokines in tumor host
tissue remain to be established. We report here that
Spirulina LPS did not induce or only partially induced IL-6
and IL-23 and efficiently suppressed the growth of hepatocellular
carcinoma MH134 in a TLR4-dependent manner, by reducing the serum
levels of IL-17 and IL-23, while increasing those of IFN-γ.
Interestingly, anti-IL-17 monoclonal antibodies (mAb) clearly
suppressed tumor growth as efficiently as Spirulina LPS.
Furthermore, Spirulina LPS was quite effective in inhibiting
spontaneous development of mammary tumors in an oncogene transgenic
mouse model.
Materials and methods
Mice and tumor cells
Female C3H/HeN and C3H/HeJ mice were purchased from
CLEA Japan Inc. (Tokyo, Japan). DO11.10 transgenic mice for αβ
T-cell receptor (TCR) recognizing ovalbumin (OVA) in the context of
I-Ad and transgenic mice carrying an activated rat
HER-2/neu oncogene driven by a mouse mammary tumor virus
promoter (HER-2/neu mice) were obtained from Jackson
Laboratory (Bar Harbor, ME, USA) and Charles River Laboratories
(Cambridge, MA, USA), respectively. All mice were maintained in a
pathogen-free environment, and experiments were performed following
the ethical guidelines of Kochi Medical School and Osaka Ohtani
University. The mouse tumor MH134 (hepatocellular carcinoma; kindly
provided by Dr T. Kudo, Tohoku University, Sendai, Japan) and YAC-1
(T-cell lymphoma) cell lines were maintained in RPMI-1640 medium
(Sigma-Aldrich, St. Louis, MO, USA) with 10% heat-inactivated fetal
calf serum (FCS, HyClone Laboratories, Logan, UT, USA),
5×10−5 M 2-mercaptoethanol (2-ME), and 50 µg/ml
gentamicin (Sigma-Aldrich).
Reagents
LPS from Escherichia coli (E. coli) 0111:B4
was purchased from Difco (Detroit, MI, USA). Anti-IFN-γ mAb
(R4-6A2, rat IgG1; no. MM701), anti-IL-17 mAb (50104, rat IgG2a;
no. MAB421), anti-CD8 mAb (53–6.7, rat IgG2a; no. 100735), and rat
IgG were obtained from Endogen (Rockford, IL, USA), R&D Systems
(Minneapolis, MN, USA), BioLegend (San Diego, CA, USA), and
Sigma-Aldrich, respectively. Anti-CD4 mAb (GK1.5, rat IgG2b) was
kindly provided by Dr F.W. Fitch (University of Chicago, Chicago,
IL, USA).
Preparation of LPS from Spirulina
pacifica
S. pacifica was a generous gift from Dr
Genrald Cysewski (Cyanotech Corporation, Kailua-Kona, Hawaii, USA)
and Mr. Nobuyuki Miyaji (Toyo Koso Kagaku Co., Ltd., Chiba, Japan).
S. pacifica has been selected from a strain of edible S.
platensis in 1984 and its enzyme expression profile differs
from that of the parental strain. LPS was prepared from S.
pacifica freeze-dried cells as previously described (27). Briefly, cells were washed with
acetone, suspended in distilled water, and then extracted by
addition of 90% phenol-water and vigorous agitation at 68°C. The
crude preparation was dialyzed to remove phenol and residual
freeze-dried cells. The sample was dissolved in water, and the
insoluble material was eliminated by centrifugation, followed by
ultracentrifugation at 100,000 × g. The molecular mass of the LPS
sample was estimated between approximately 1,000 and 20,000 Da by
electrophoresis and mass spectral analysis.
Tumor growth in vivo
Tumor growth was measured 3 times per week after
intradermal (i.d.) injection of 1×106 MH134 cells in the
back of C3H/HeN or C3H/HeJ mice. The tumor volume was calculated
using the following formula: Volume (mm3) =
width2 × length/2. In some experimental settings,
Spirulina LPS or E. coli LPS in saline solution was
injected intraperitoneally (i.p.) every week starting 6 days after
tumor inoculation. To deplete the T-cell subsets, mice injected
with MH134 tumor cells on day 0 were injected i.p. with rat IgG,
anti-CD4, or anti-CD8 mAb (150 µg/mouse on days, −1, 0, +3) as
previously described (28). T-cell
depletion was confirmed to be >95% by fluorescence-activated
cell sorting (FACS).
Surgical tumor resection and
rechallenge
MH134 tumors were surgically removed 3 weeks after
tumor inoculation. Mice were re-challenged i.d. with
1.5×106 MH134 cells of the same tumor as previously
described (29).
Immunohistochemistry
MH134 tumors taken from C3H/HeN mice treated with
saline solution or Spirulina LPS 22 days after tumor
implantation were embedded in O.C.T. compound (Sakura Finetec USA,
Inc., Torrance, CA, USA) and frozen. Frozen tumors were sectioned
and stained with anti-CD4 (RM4-5, rat IgG2a, no. 100520, BioLegend)
or anti-CD8 (53–6.7) antibodies using simple stain mouse MAX-PO
[F(ab)'2 goat anti-rat Ig and peroxidase coupled to the
amino acid polymer] and 3,3′-diaminobenzidine according to the
manufacturer's protocol (Nichirei-Biosciences Inc., Tokyo,
Japan).
Preparation of lymphoid cells
Mϕ/DC or CD4 T cell fractions were prepared from
whole spleen cells by positive selection using a MACS cell
separation system (Miltenyi Biotec, Auburn, CA, USA) according to
manufacturer's instructions. Anti-CD4 and a mixture of anti-CD11b
and anti-CD11c microbeads were used for the fractionation of CD4 T
cells and Mϕ/DC, respectively. The purity was usually demonstrated
to be >90% by FACS.
Culture of splenocytes from
tumor-bearing mice
Whole splenocytes (5×106/well) depleted
of red blood cells were cultured in 10% FCS RPMI-1640 medium in
24-well culture plates (Becton Dickinson Labware, Franklin Lakes,
NJ, USA) at 37°C in a 5% CO2 humidified atmosphere.
After 4 days of culture, supernatants were collected to assess
cytokine levels by ELISA.
In vitro IL-17 production
Whole spleen cells (8×105/well) from
OVA-specific TCR transgenic (DO11.10) mice were cultured in
flat-bottomed 96-well plates (Costar Corning, NY, USA) in 10% FCS
RPMI-1640 medium for 5 days. In some experiments,
2.8×105 CD4 T cells, prepared from C3H/HeJ mice that had
been immunized i.p. with 150 µg OVA and 5 mg Alum approximately 2
months earlier, were cultured with 1.2×105 splenic Mϕ/DC
cells from C3H/HeN or C3H/HeJ mice in the presence of 20 µg/ml OVA
for 5 days. Spirulina or E. coli LPS was added at the
initiation of the culture to evaluate its effect on IL-17
production. The levels of IL-17 in the culture supernatants were
evaluated by ELISA.
B cell proliferation
Whole spleen cells (8×105/well) were
cultured in flat-bottomed 96-well plates in 10% FCS RPMI-1640
medium in the presence or absence of graded doses of
Spirulina LPS or E. coli LPS. Five days later, the
cultured cells were collected and stained with FITC-conjugated
anti-B220 mAb (RA3-6B2, rat IgG2a, no. 553088, BD Biosciences, San
Jose, CA, USA). B220+ cells were counted as B cells
using a FACSCalibur instrument (Becton Dickinson, San Jose, CA,
USA).
NK assay
Briefly, 2.5×106 target cells were
labeled with 25 µCi of 51Cr sodium chromate for 60 min
at 37°C in 10% FCS RPMI-1640 medium. After washing,
51Cr-labeled target cells (1×104) and
effector cells were mixed in flat-bottomed 96-well plates at the
indicated effector/target (E/T) ratio. After 4 h of incubation, the
radioactivity in the cell-free supernatants was measured using a
1470 Automatic Gamma Counter (PerkinElmer, Waltham, MA, USA).
Percentage-specific lysis for 51Cr release was
calculated according to the following formula: % specific lysis =
[(experimental - spontaneous) release] / [(maximal - spontaneous)
release] ×100.
ELISA for cytokine measurement
Cytokine levels in serum or culture supernatants
were quantified by sandwich ELISA. The following pairs of capture
and biotinylated detection rat anti-mouse mAbs were used: R4-6A2
(no. 551216) and XMG1.2 (no. 554410) for IFN-γ, TC11-18H10 (no.
555068) and TC11-8H4 (no. 555067) for IL-17, 9A5 (no. 554658) and
C17.8 (no. 554476) for IL-12(p35/40), MP5-20F3 (no. 554400) and
MP5-32C11 (no. 554402) for IL-6, and A75-2 (no. 555052) and A75-3
(no. 555053) for TGF-β1 (all were purchased from BD Biosciences).
For IL-23(p19/40), rat anti-mouse IL-23p19 (G23-8, no. 14-7232-85,
eBioscience, San Diego, CA, USA) and biotinylated anti-IL-12p40
(C17.8, BD Biosciences) were used. The ELISA assays were performed
according to the manufacturer's instructions.
Statistical analysis
Differences in mean values between groups were
calculated using an unpaired two-tailed Student's t-test,
Mann-Whitney U test, or Fischer's exact test. P-values of the
Student's t tests are shown unless otherwise indicated.
Results
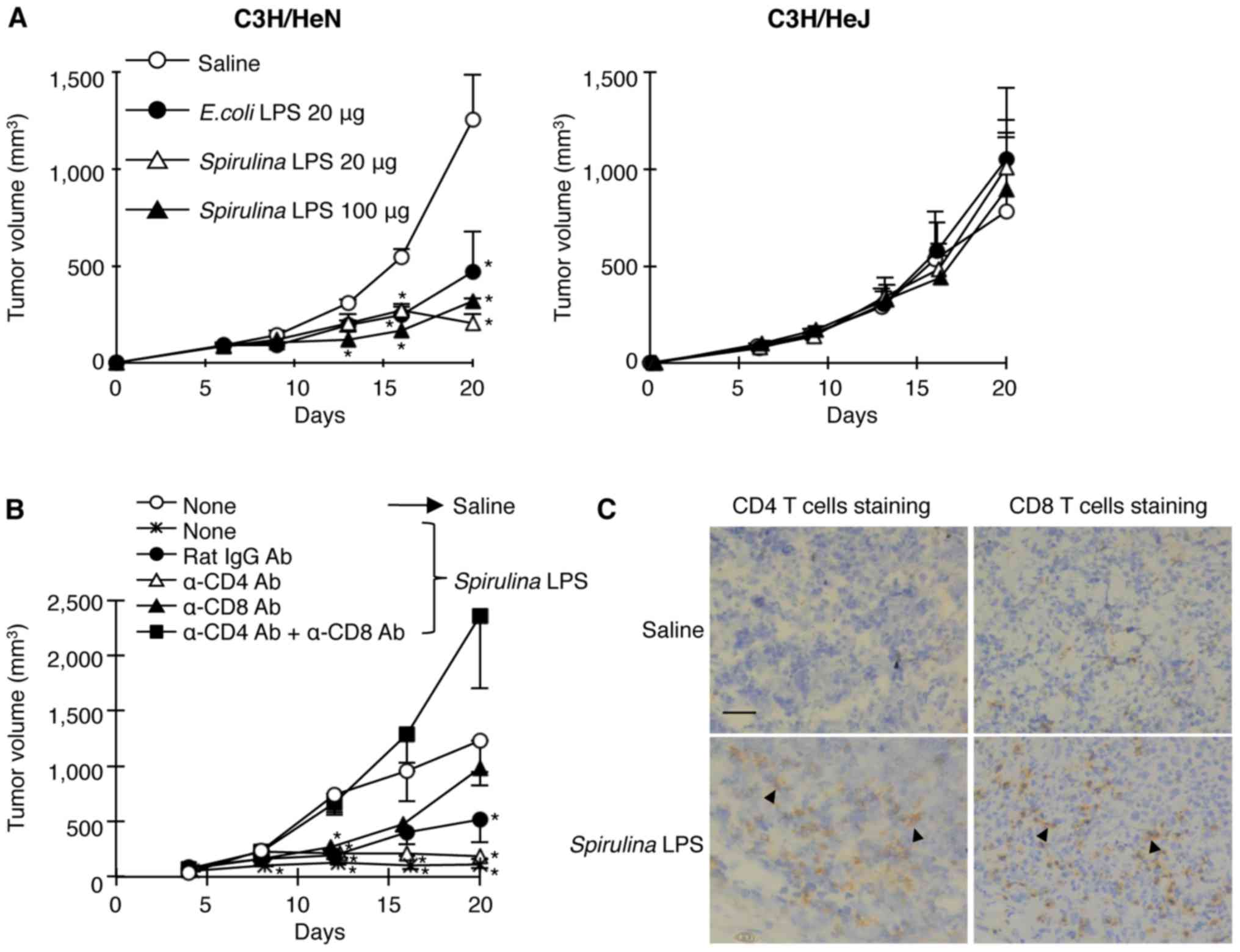
TLR4-dependent suppression of tumor
growth by Spirulina LPS is mediated by CD4 and CD8 T cells
To examine the antitumor activities of
Spirulina LPS in comparison with that of E. coli LPS,
we inoculated hepatocellular carcinoma MH134 cells i.d. into
syngeneic C3H/HeN and TLR4 mutant C3H/HeJ mice (30), followed by i.p. administration of
different doses of Spirulina LPS or E. coli LPS 6
days later. The injection with different doses of Spirulina
LPS suppressed the tumor growth in C3H/HeN but not in C3H/HeJ mice,
to the same degree as E. coli LPS treatment (Fig. 1A). This suggests that
Spirulina LPS as well as E. coli LPS reduced tumor
growth in a TLR4-dependent manner.
In order to assess the involvement of T cells in the
Spirulina LPS-induced antitumor effect, we administered
anti-CD4 and/or anti-CD8 mAbs to deplete the T-cell subsets before
Spirulina LPS injection. While injection of both anti-CD4
and anti-CD8 mAbs completely abolished the antitumor activity of
Spirulina LPS, anti-CD4 or anti-CD8 mAb alone did not result
in a strong effect (Fig. 1B). In
accordance with the results of the in vivo T-cell depletion,
immunohistochemical examinations revealed the enhancement of
infiltration of both CD4 and CD8 T cells in the tumor masses upon
administration of Spirulina LPS (Fig. 1C). These results suggest that CD4
and CD8 T cells are both involved in the antitumor effect induced
by Spirulina LPS.
Spirulina and E. coli LPS differ in
their ability to activate NK cells and induce secondary immune
responses
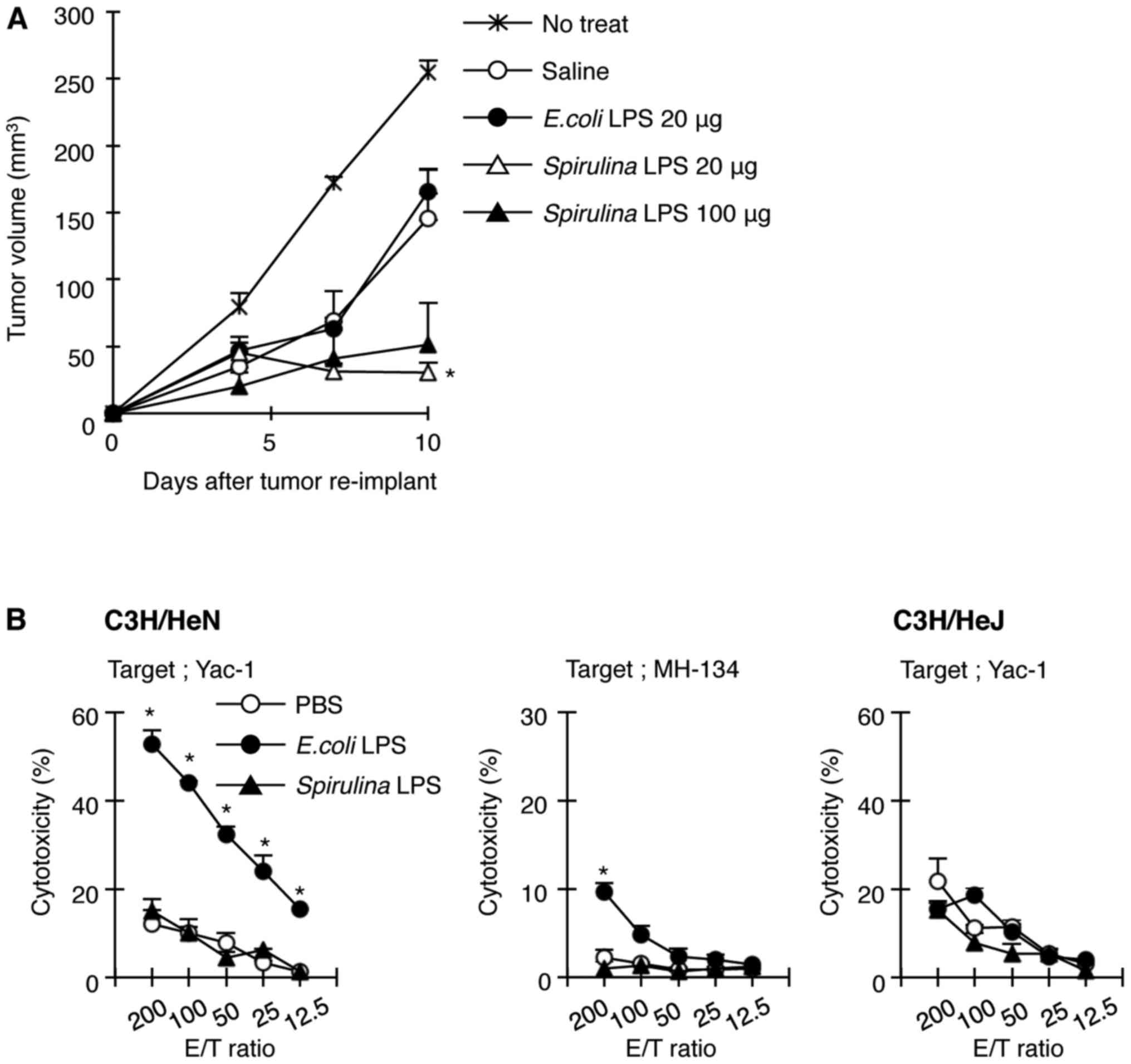
To examine whether Spirulina LPS induces
immunity against MH134 tumors, we reinoculated MH134 cells into
C3H/HeN mice that had been implanted with an MH134 tumor and were
treated with saline, E. coli, or Spirulina LPS,
followed by surgical resection of primary tumors 5 days before the
re-challenge. The growth rate of the reimplanted tumors reduced
even in saline-treated mice, compared with that in untreated mice
implanted with only new tumor cells without the first inoculum of
MH134 tumor cells (Fig. 2A),
implicating the induction of antitumor immunity without LPS
administration when the primary tumor was removed. Spirulina
LPS induced a stronger resistance to reimplanted MH134 tumors than
saline (Fig. 2A). In contrast, the
tumor growth rate in E. coli LPS-treated mice was comparable
to that in saline-treated mice (Fig.
2A). These results suggest that while Spirulina LPS
facilitated the generation of immunity against MH134 tumors, E.
coli LPS did not enhance secondary antitumor immune response.
Since E. coli LPS was effective in the prevention of primary
tumor growth as shown in Fig. 1A
and is known to exhibit antitumor activity partly through
activation of NK cells, we evaluated the ability of E. coli
and Spirulina LPS to activate NK cells. Indeed, spleen cells
from C3H/HeN mice injected with E. coli LPS showed
remarkable toxicity towards NK-sensitive YAC-1 cells and a weak but
significant toxicity towards MH134 cells, whereas Spirulina
LPS failed to activate NK cells (Fig.
2B). NK activation was not induced in C3H/HeJ mice, not even
when E. coli LPS was administered (Fig. 2B). Thus, this result may explain why
E. coli LPS elicited antitumor effects against the primary
tumor, regardless of its failure to enhance adaptive immunity to
tumors.
Administration of Spirulina LPS to a
tumor-bearing host downregulates serum levels of IL-17 and IL-23
but increases IFN-γ production by T cells through the TLR4
pathway
IFN-γ plays a crucial role in the prevention of
tumor development (6), whereas
IL-17 and IL-23 are considered to promote tumor growth by inducing
inflammation and by regulating the expansion/survival of Th17
cells, respectively (11,14–16).
We measured serum levels of IFN-γ, IL-17, and IL-23 in
tumor-bearing C3H/HeN and C3H/HeJ mice treated with saline, E.
coli, or Spirulina LPS. E. coli and
Spirulina LPS (100 µg) markedly increased serum IFN-γ levels
in C3H/HeN mice with a peak response at days 7 and 14 after tumor
inoculation, respectively (Fig.
3A). A low dose of Spirulina LPS (20 µg) induced slight
but significant IFN-γ production from days 7 to 14. However, the
serum levels of IFN-γ were not elevated in C3H/HeJ mice at any
time, not even after injection of either LPS. The treatment with
anti-CD4 mAb or a combination of anti-CD4 and anti-CD8 mAbs
abrogated the increase in serum IFN-γ in C3H/HeN mice receiving
Spirulina LPS, whereas anti-CD8 mAb alone slightly
diminished the activity of Spirulina LPS to induce IFN-γ
(Fig. 3D). On the other hand, serum
levels of IL-17 and IL-23 in saline-treated C3H/HeN mice gradually
increased during tumor progression and reached a maximum on day 21
(Fig. 3B and C). However, serum
levels of IL-6 only showed an approximately 2-fold increase in
saline-treated tumor-bearing mice even on day 21 as compared to
non-treated mice (data not shown). Noteworthy, Spirulina LPS
significantly reduced IL-17 and IL-23 levels, while E. coli
LPS hardly suppressed IL-17 production on day 21 (Fig. 3B and C). On the contrary, E.
coli LPS enhanced serum levels of IL-23 in tumor-bearing
C3H/HeN mice on day 7 and 14. Taken together, these results support
the notion that Spirulina LPS induces antitumor immune
responses through the induction of IFN-γ mostly by CD4 T cells and
suppressed serum levels of IL-17 and IL-23, whereas E. coli
LPS exerts its antitumor effect primarily through activation of NK
cells.
We have previously shown that antigen-presenting
cells (APCs)-expressing tumor Ag and tumor-reactive T cells are
both stimulated in vivo in tumor-bearing mice, and that
in vitro culture of spleen cells from tumor-bearing mice at
early stages leads to cytokine production without exogenous
addition of tumor Ag as a result of the collaboration between
antitumor T cells and APCs (29,31).
We tested whether in vivo treatment with E. coli or
Spirulina LPS affects the in vitro production of
IFN-γ by culturing spleen cells from MH134 tumor-bearing mice.
Spirulina LPS clearly enhanced IFN-γ production in spleen
cells of tumor-bearing C3H/HeN mice, whereas E. coli LPS was
unable to upregulate IFN-γ production (Fig. 3D). These results implicate that
Spirulina LPS enhances IFN-γ production through the
generation of memory T cells.
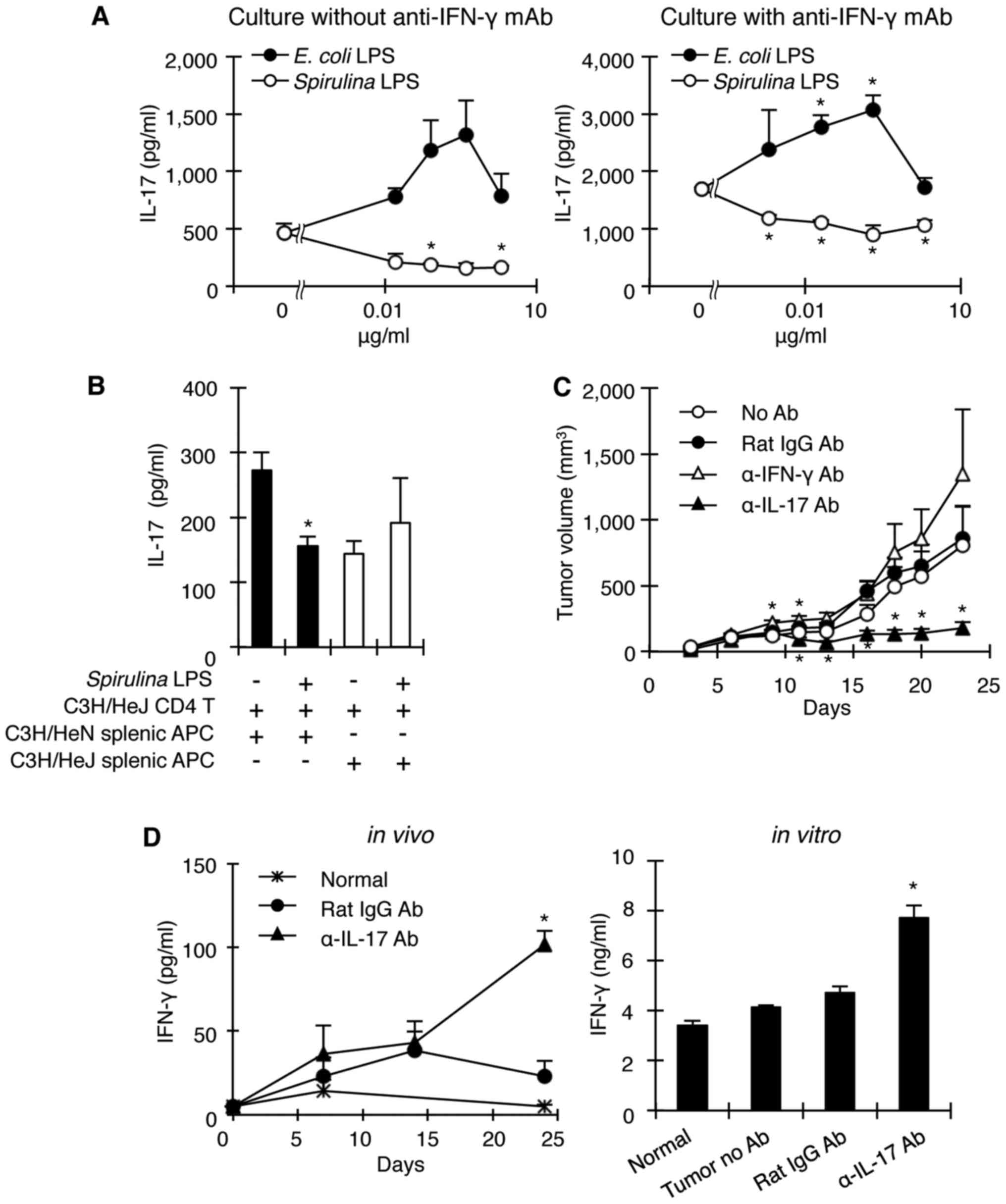
Spirulina LPS inhibits IL-17
production in an IFN-γ-independent manner through interaction with
APCs expressing TLR4
Since Spirulina LPS reduced serum IL-17
levels in tumor-bearing mice while increasing IFN-γ levels and
because IFN-γ negatively regulates the generation of Th17 cells
(32,33), we investigated whether IL-17
downregulation by Spirulina LPS occurs via inhibition of the
induction of IL-17-producing cells by facilitating IFN-γ
production, or alternatively by directly downregulating
IL-17-producing cells. To address this question, spleen cells from
OVA-specific TCR transgenic DO11.10 mice were stimulated by OVA
with or without E. coli or Spirulina LPS and in the
presence or absence of anti-IFN-γ mAb. Spirulina LPS
significantly suppressed IL-17 production by DO11.10 spleen cells
in a dose-dependent manner, regardless of the presence of
anti-IFN-γ mAb. However, E. coli LPS augmented IL-17
production (Fig. 4A), possibly
because of its ability to induce IL-6 (Fig. 5A). In addition, it is noteworthy
that anti-IFN-γ mAb enhanced IL-17 production by DO11.10 spleen
cells in response to OVA without the addition of either LPS
(Fig. 4A), indicating that IFN-γ
regulates IL-17-producing cells. Thus, Spirulina LPS
suppresses the in vivo production of IL-17 with or without
involvement of IFN-γ. Moreover, although Spirulina LPS
inhibited IL-17 production when CD4 T cells from OVA-primed C3H/HeJ
mice were co-cultured with C3H/HeN APCs in the presence of OVA, it
failed to suppress IL-17 production when OVA-primed C3H/HeJ CD4 T
cells were stimulated with OVA in the context of C3H/HeJ APCs
(Fig. 4B). These results suggest
that Spirulina LPS acted on APCs to inhibit the generation
of Th17 cells in a TLR4-dependent manner.
Anti-IL-17 mAb administration inhibits
tumor development upon elevated IFN-γ production
Thus far, our results are consistent with the notion
that the antitumor effect of Spirulina LPS is caused by the
downregulation of IL-17 production. To examine whether
neutralization of IL-17 by anti-IL-17 mAb would result in a
reduction of tumor growth in mice in the absence of
Spirulina LPS, we injected anti-IL-17 or anti-IFN-γ mAb into
C3H/HeN mice 1 day before and 4 days after MH134 tumor
implantation, and monitored tumor development. As expected,
anti-IL-17 mAb markedly suppressed tumor growth compared to control
rat IgG antibodies, whereas anti-IFN-γ mAb slightly enhanced tumor
development (Fig. 4C). Importantly,
mice receiving anti-IL-17 mAb exhibited high levels of serum IFN-γ
(Fig. 4D). In addition, in
vitro culture of spleen cells from tumor-bearing mice treated
with anti-IL-17 mAb resulted in the production of large amounts of
IFN-γ (Fig. 4D). These results
indicate that IL-17 provides an environment suitable for tumor
growth partly by inhibiting the generation of IFN-γ-producing T
cells.
Spirulina LPS reduces or abrogates
IL-6 and IL-23 production in vivo, but augments T cell-dependent
IL-12 induction
It was recently reported that IL-17 is mainly
produced by Th17 cells, and that both IL-6 and TGF-β are
indispensable for the generation of Th17 cells (20). To test the ability of
Spirulina LPS to induce IL-6 and TGF-β, normal C3H/HeN and
C3H/HeJ mice were injected i.p. with E. coli or
Spirulina LPS and serum cytokine levels were measured 4 h
later. C3H/HeN mice produced high levels of IL-6 in response to
E. coli LPS, but showed only a small response upon
stimulation with Spirulina LPS (Fig. 5A). Contrary to IL-6, the substantial
TGF-β levels present in serum of untreated groups of both strains
were not significantly elevated when stimulated with E. coli
LPS (Fig. 5A). However,
Spirulina LPS slightly reduced TGF-β levels, but only in
C3H/HeN mice (Fig. 5A). Noteworthy,
E. coli LPS elicited a considerable increase in IL-23
levels, only in C3H/HeN mice, while Spirulina LPS showed
almost no induction of IL-23 even in C3H/HeN mice (Fig. 5A). Thus, Spirulina LPS seems
to be inferior to E. coli LPS in terms of stimulating immune
cells. The possibility that Spirulina LPS has a general
defect in the stimulation of immune system was excluded, because
both E. coli and Spirulina LPS induced B cell
proliferation in a dose-dependent manner (Fig. 5B).
Since IL-12 and IFN-γ are known to play a crucial
role in the differentiation of IFN-γ-producing Th1 cells (4), we measured the serum levels of IL-12
in tumor-bearing mice that were treated with saline, E.
coli, or Spirulina LPS. Spirulina LPS augmented
IL-12 production more than E. coli LPS. In contrast to a
transient increase of IL-12 by E. coli LPS, the enhanced
IL-12 production by Spirulina LPS was still observed after
14 days (Fig. 5C). Of note, these
high IL-12 serum levels on day 14 decreased when C3H/HeN mice were
injected with anti-CD4, but not anti-CD8 mAb (Fig. 5D), implicating CD4 T cell-dependent
IL-12 production. Taken together, these findings support the notion
that Spirulina LPS facilitates the priming of CD4 T cells
with tumor cells and the subsequent CD4 T cell-dependent activation
of APCs, leading to IL-12 production, which in turn induces
IFN-γ-producing T cells.
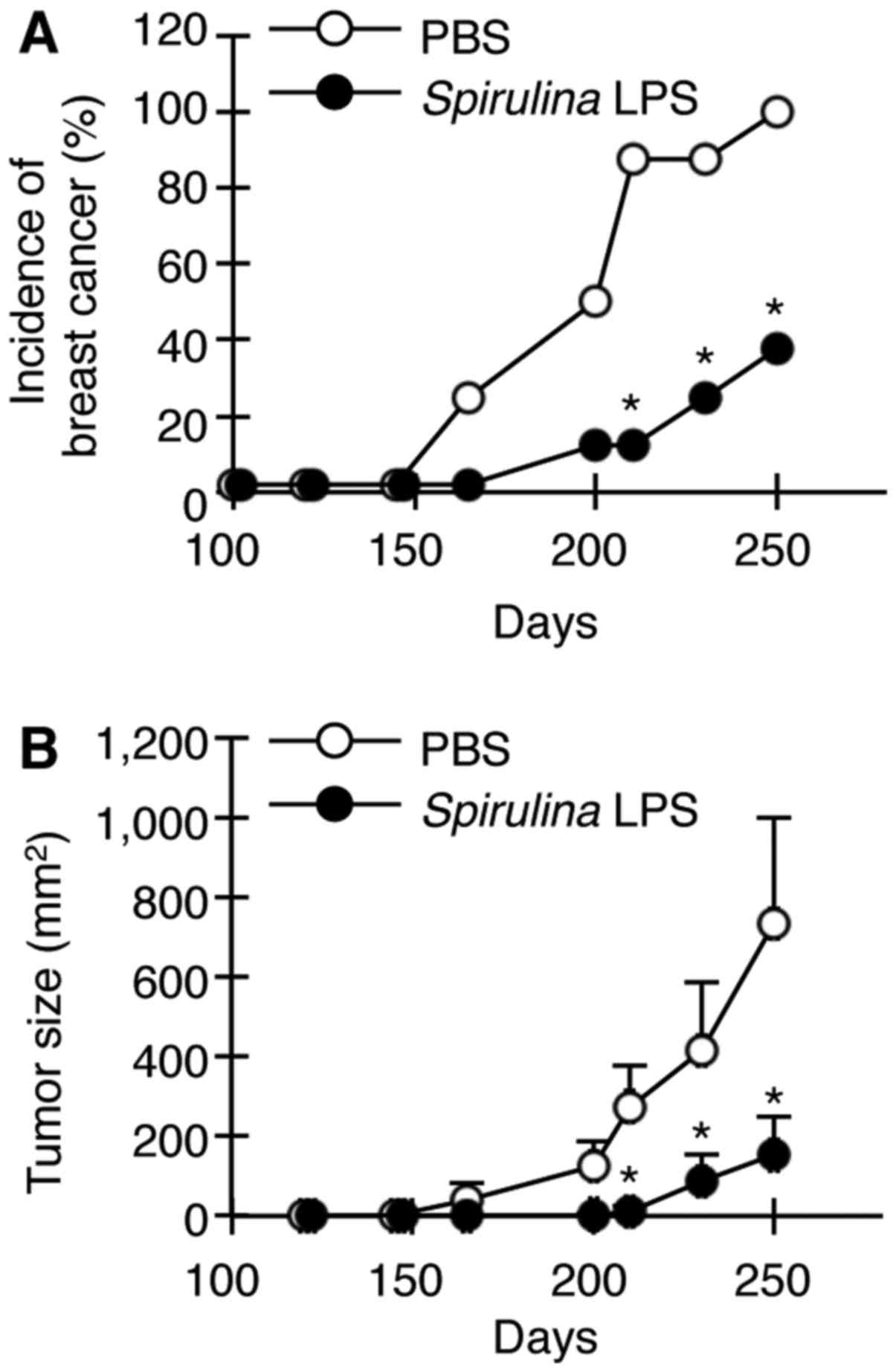
Spirulina LPS attenuates the
spontaneous development of mammary tumors
We finally examined whether Spirulina LPS is
also effective in suppressing the spontaneous development of
mammary tumors in female transgenic mice carrying the activated
HER-2/neu oncogene. HER-2/neu transgenic mice display
apparent hyperplasia in the mammary glands at 10 weeks of age and
develop palpable mammary tumors around 24 weeks (28). Female HER-2/neu mice were
injected with Spirulina LPS or PBS once per week between 120
and 240 days after birth. Spirulina LPS delayed the
appearance of tumors and significantly reduced both tumor incidence
and growth (Fig. 6).
Discussion
The IL-12/IFN-γ pathway is crucial for antitumor
immunity by inducing IFN-γ-producing CD4 and CD8 T cells (4,6,7,34).
In this context, various adjuvants including microbial products are
explored to potentiate antitumor immunity. On the other hand,
IL-23/IL-17 signaling plays an important role in tumorigenesis and
metastasis in humans and mice by inducing angiogenesis and IL-6,
IL-8, and matrix metalloproteinase expression (11–17).
Since most adjuvants induce IL-6, which is indispensable for Th17
differentiation, adjuvants that do not induce or slightly induce
IL-6 are desired for tumor immunotherapy. The present study
demonstrates that Spirulina LPS is a very poor inducer of
IL-6 and IL-23 and that it elicits strong antitumor immunity by
suppressing IL-17 induction while enhancing IFN-γ production
through the TLR4 pathway.
In a tumor-based mouse model, Spirulina LPS
treatment enhanced serum levels of IFN-γ at early stages of tumor
development and decreased serum IL-17 and IL-23 levels at later
stages (Fig. 3). Spirulina
LPS also induced IL-12 production in a CD4 T cell-dependent manner,
probably through CD4 T cell-APC interaction (Fig. 5C and D), and induced IFN-γ
production primarily by CD4 T cells. Based on previous reports on
the inhibition of Th17 differentiation by IFN-γ (32,33),
these results can be interpreted as follows: Spirulina LPS
facilitates the differentiation of tumor-primed CD4 T cells to Th1
cells. Subsequently, Th1-derived IFN-γ prevents Th17
differentiation and promotes CD8 T-cell activation. In addition,
Spirulina LPS might also suppress IL-17 production through
IFN-γ-independent pathways as shown in our in vitro
experiments (Fig. 4A).
Alternatively, it is possible that although IL-17
elevation during tumor progression could prevent the generation of
IFN-γ-producing T cells, Spirulina LPS might restore the
generation of Th1 cells by reducing IL-17 production (Fig. 3). Recent experiments have revealed
IL-17-mediated inhibition of Th1 differentiation (35). It is also conceivable that
Spirulina LPS inhibits tumor growth by reducing
IL-17-induced angiogenesis, as previously reported (11–13,36).
Of note, administration of anti-IL-17 mAb into tumor-bearing mice
induced marked tumor regression accompanying IFN-γ production
(Fig. 4C and D), consistent with a
recent report showing tumor growth inhibition by IL-17
neutralization (15). Regardless of
the pathway, Spirulina LPS inhibits tumor growth by skewing
the balance between IFN-γ and IL-17 responses toward IFN-γ
production.
In this study, Spirulina LPS was shown to
inhibit the in vitro generation of IL-17-producing cells
through its action on APCs in a TLR4-dependent manner (Fig. 4A and B). There are reports
demonstrating that CD86, but not CD80, on APCs plays an important
role in the regulation of IL-17 production by T cells (37), and that IL-27 produced by Mϕ/DC
negatively regulates the development of Th17 cells (38). Thus, Spirulina LPS-mediated
TLR4 signaling may induce a change in APC status by modulating the
expression patterns of co-stimulatory molecules and cytokines,
leading to the attenuation of Th17 cell development. However, the
precise mechanisms by which Spirulina LPS suppresses IL-17
and IL-23 remain to be elucidated.
In contrast to the tumor-promoting effects of the
IL-17/IL-23 signaling axis, demonstrated in IL-17- or
IL-23-deficient mice, several groups have described that Th17 cells
or IL-17 promote tumor inhibition by increasing the generation of
tumor-specific cytotoxic CD8 T cells (39,40).
Thus, whether Th17 cells or IL-17 induce tumor progression or
antitumor immunity might be dependent on the varied main effector
cells in the distinct tumor-host relationship (41). Namely, Th17 cells/IL-17 could
activate CD8 T cells, while inhibiting Th1 type of CD4 T cells.
Otherwise, the strength of tumor immunogenicity or frequency of
tumor-specific effector T cells might cause a discrepancy in the
function of Th17 cells/IL-17, because adoptive transfer of
tumor-reactive Th17 cells obtained from TCR transgenic mice or from
in vitro stimulation with tumor inhibits tumor development
(39,40). In either case, IL-17-induced
angiogenesis seems to be required to some extent for effector T
cells to migrate into tumor tissue. Even in an IL-17-deficient
host, IL-17 secreted by tumor cells may contribute to T-cell
infiltration. Despite these possible explanations, the paradox in
the function of Th17 cells/IL-17 is still unresolved.
IL-17 is also produced by certain tumors and IL-17
receptors have been detected in virtually all cells (12). It has been reported that IL-17
stimulates tumor cells to proliferate and upregulates the
expression of various cytokines, chemokines, and their receptors,
leading to angiogenesis (16,42–44).
However, we did not detect expression of IL-17 and IL-17 receptor
in MH134 tumor cells (data not shown), indicating the importance of
IL-17 responses in host-derived cells in MH134 tumor growth.
Moreover, although TLR4 was expressed on MH134 tumor cells, E.
coli and Spirulina LPS had virtually no effect on the
in vitro growth of tumor cells (data not shown).
Interestingly, we found that MH134 tumor cells expressed IFN-γ
receptors and that cellular in vitro growth was inhibited by
exogenous addition of IFN-γ (data not shown). Thus, in vivo
induction of IFN-γ by E. coli and Spirulina LPS may
partly contribute to the antitumor effects by virtue of the IFN-γ
direct action on the tumor.
E. coli LPS induced antitumor effects
against a primary tumor, but failed to enhance secondary immune
responses in the same tumor after reimplantation (Fig. 2A). E. coli LPS induced high
levels of IL-6 and IL-23, favoring the development of Th17 cells,
and it considerably activated NK function (Fig. 2B). Thus, it is conceivable that
E. coli LPS inhibits primary tumor growth via activation of
NK cells, but prevents the generation of T cell-mediated antitumor
immunity through IL-17 induction.
The effects of E. coli and Spirulina
LPS involve TLR4 pathways, but Spirulina LPS was different
from E. coli LPS in terms of cytokine induction. The
structure of LPS of all gram-negative bacteria consists of a
polysaccharide attached to a lipid component, lipid A, which is
assumed to be responsible for the induction of cytokines. Although
lipid A molecules from different bacteria were initially thought to
be similar, recent evidence suggests structural and functional
differences among LPS from different species (25,45).
Differences in the three-dimensional conformation of lipid A have
been proposed to determine the strength of fitness to TLR4-CD14-MD2
complex, leading to the activation of intracellular signaling for
cytokines (46,47). LPS fractions from E. coli and
Salmonella spp. are more potent cytokine inducers than those
from Bordetella pertussis and Bacteroides fragilis
(48,49), while lipid A analog and LPS from
Rhodobacter spp. have antagonistic properties against
cytokine stimulation (47,50). Although the structure of
Spirulina LPS remains to be analyzed, the molecular
conformation of E. coli LPS seems to be different from that
of Spirulina LPS based on its ability to downregulate IL-17
with minimum induction of IL-6 and IL-23. Moreover,
Spirulina LPS failed to induce endotoxin shock in contrast
to E. coli LPS (20% and 100% survival in mice given 25 µg of
E. coli and Spirulina LPS, respectively, 36 h after
administration).
Experiments using different Spirulina
preparations free of LPS have demonstrated antitumor activity. A
calcium-chelating, sulfated polysaccharide from S. platensis
suppressed metastasis of murine melanoma (51). Oral administration of hot water
extract of Spirulina is reported to suppress tumor growth
through IFN-γ-mediated activation of NK cells but not CD8 T cells
(52). However, it was not clear
whether IL-17 and IL-23 production were involved in the
experimental system of that specific study. In contrast to those
Spirulina preparations, we used a Spirulina LPS
fraction extracted with phenol-water. Differences in the components
of the Spirulina extracts may cause distinct
bioactivities.
In conclusion, Spirulina LPS suppressed
tumor growth by downregulating serum IL-17/IL-23 with concomitant
induction of IFN-γ through TLR4. Furthermore, Spirulina LPS
showed limited or no induction of IL-6 and IL-23 and altered the
cytokine milieu in the tumor-bearing host from the Th17 to the Th1
type. Thus, we confirmed the importance of a balance between IFN-γ
and IL-17/IL-23 levels in the regulation of tumor growth. It is
noteworthy that Spirulina LPS was able to suppress
spontaneous development of mammary tumors. Our results provide
novel insights into the exploitation of TLR-based immunomodulators
for cancer immunotherapy.
Acknowledgements
This study was supported by The Osaka Foundation of
Promotion for Clinical Immunology (S.O. and Y K.) and JSPS
Grant-in-Aid for Scientific Research #21550168 (S.F.). We would
like to thank Dr G. Cysewski (Cyanotech Corporation, Hawaii) and
Mr. N. Miyaji (Toyo Koso Kagaku Co., Ltd., Japan) for providing
Spirulina pacifica.
References
|
1
|
Sylvester RJ, van der Meijden AP, Witjes
JA and Kurth K: Bacillus calmette-guerin versus chemotherapy for
the intravesical treatment of patients with carcinoma in situ of
the bladder: A meta-analysis of the published results of randomized
clinical trials. J Urol. 174:86–91; discussion 91–92. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Medzhitov R and Janeway CA Jr: Innate
immunity: The virtues of a nonclonal system of recognition. Cell.
91:295–298. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akira S, Takeda K and Kaisho T: Toll-like
receptors: Critical proteins linking innate and acquired immunity.
Nat Immunol. 2:675–680. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trinchieri G: Interleukin-12 and the
regulation of innate resistance and adaptive immunity. Nat Rev
Immunol. 3:133–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dalton DK, Pitts-Meek S, Keshav S, Figari
IS, Bradley A and Stewart TA: Multiple defects of immune cell
function in mice with disrupted interferon-γ genes. Science.
259:1739–1742. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shankaran V, Ikeda H, Bruce AT, White JM,
Swanson PE, Old LJ and Schreiber RD: IFNgamma and lymphocytes
prevent primary tumour development and shape tumour immunogenicity.
Nature. 410:1107–1111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dighe AS, Richards E, Old LJ and Schreiber
RD: Enhanced in vivo growth and resistance to rejection of tumor
cells expressing dominant negative IFN γ receptors. Immunity.
1:447–456. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mizuno D, Yoshioka O, Akamatu M and
Kataoka T: Antitumor effect of intracutaneous injection of
bacterial lipopolysaccharide. Cancer Res. 28:1531–1537.
1968.PubMed/NCBI
|
|
9
|
Ohnishi M, Kimura S, Yamazaki M, Oshima H,
Mizuno DI, Abe S and Yamaguchi H: Anti-tumour activity of
low-toxicity lipopolysaccharide of Bordetella pertussis. Br J
Cancer. 69:1038–1042. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang D, Satoh M, Ueda H, Tsukagoshi S and
Yamazaki M: Activation of tumor-infiltrating macrophages by a
synthetic lipid A analog (ONO-4007) and its implication in
antitumor effects. Cancer Immunol Immunother. 38:287–293. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tartour E, Fossiez F, Joyeux I, Galinha A,
Gey A, Claret E, Sastre-Garau X, Couturier J, Mosseri V, Vives V,
et al: Interleukin 17, a T-cell-derived cytokine, promotes
tumorigenicity of human cervical tumors in nude mice. Cancer Res.
59:3698–3704. 1999.PubMed/NCBI
|
|
12
|
Kato T, Furumoto H, Ogura T, Onishi Y,
Irahara M, Yamano S, Kamada M and Aono T: Expression of IL-17 mRNA
in ovarian cancer. Biochem Biophys Res Commun. 282:735–738. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Numasaki M, Watanabe M, Suzuki T,
Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze
MT, Kolls JK, et al: IL-17 enhances the net angiogenic activity and
in vivo growth of human non-small cell lung cancer in SCID mice
through promoting CXCR-2-dependent angiogenesis. J Immunol.
175:6177–6189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Langowski JL, Zhang X, Wu L, Mattson JD,
Chen T, Smith K, Basham B, McClanahan T, Kastelein RA and Oft M:
IL-23 promotes tumour incidence and growth. Nature. 442:461–465.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He D, Li H, Yusuf N, Elmets CA, Li J,
Mountz JD and Xu H: IL-17 promotes tumor development through the
induction of tumor promoting microenvironments at tumor sites and
myeloid-derived suppressor cells. J Immunol. 184:2281–2288. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Yi T, Kortylewski M, Pardoll DM,
Zeng D and Yu H: IL-17 can promote tumor growth through an
IL-6-Stat3 signaling pathway. J Exp Med. 206:1457–1464. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kortylewski M, Xin H, Kujawski M, Lee H,
Liu Y, Harris T, Drake C, Pardoll D and Yu H: Regulation of the
IL-23 and IL-12 balance by Stat3 signaling in the tumor
microenvironment. Cancer Cell. 15:114–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kastelein RA, Hunter CA and Cua DJ:
Discovery and biology of IL-23 and IL-27: Related but functionally
distinct regulators of inflammation. Annu Rev Immunol. 25:221–242.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Korn T, Bettelli E, Oukka M and Kuchroo
VK: IL-17 and Th17 Cells. Annu Rev Immunol. 27:485–517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bettelli E, Carrier Y, Gao W, Korn T,
Strom TB, Oukka M, Weiner HL and Kuchroo VK: Reciprocal
developmental pathways for the generation of pathogenic effector
TH17 and regulatory T cells. Nature. 441:235–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goriely S, Neurath MF and Goldman M: How
microorganisms tip the balance between interleukin-12 family
members. Nat Rev Immunol. 8:81–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kelly MG, Alvero AB, Chen R, Silasi DA,
Abrahams VM, Chan S, Visintin I, Rutherford T and Mor G: TLR-4
signaling promotes tumor growth and paclitaxel chemoresistance in
ovarian cancer. Cancer Res. 66:3859–3868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu H, Kortylewski M and Pardoll D:
Crosstalk between cancer and immune cells: Role of STAT3 in the
tumour microenvironment. Nat Rev Immunol. 7:41–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tominaga A, Okuyama H, Fukuoka S, Taguchi
T, Kusumoto Y, Shimizu K and Ono S: Effects of edible algae
polysaccharides on allergic, inflammatory, and anti-tumor responses
through toll-like receptor 4. Antiinflamm Antiallergy Agents Med
Chem. 9:238–250. 2010. View Article : Google Scholar
|
|
25
|
Stewart I, Schluter PJ and Shaw GR:
Cyanobacterial lipopolysaccharides and human health - a review.
Environ Health. 5:7–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tornabene TG, Bourne TF, Raziuddin S and
Ben-Amotz A: Lipid and lipopolysaccharide constituents of
cyanobacterium Spirulina platensis (Cyanophyceae, Nostocales). Mar
Ecol Prog Ser. 22:121–125. 1985. View Article : Google Scholar
|
|
27
|
Westphal O and Jann K: Bacterial
lipopolysaccharides. Extraction with phenol-water and further
applications of the procedureMethods in Carbohydrate Chemistry.
Whistler RL and Wolfan ML: Academic Press Inc.; New York: pp.
83–91. 1965
|
|
28
|
Takeuchi N, Hiraoka S, Zhou XY, Nagafuku
M, Ono S, Tsujimura T, Nakazawa M, Yura Y, Hamaoka T and Fujiwara
H: Anti-HER-2/neu immune responses are induced before the
development of clinical tumors but declined following tumorigenesis
in HER-2/neu transgenic mice. Cancer Res. 64:7588–7595. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zou JP, Shimizu J, Ikegame K, Yamamoto N,
Ono S, Fujiwara H and Hamaoka T: Tumor-bearing mice exhibit a
progressive increase in tumor antigen-presenting cell function and
a reciprocal decrease in tumor antigen-responsive CD4+ T
cell activity. J Immunol. 148:648–655. 1992.PubMed/NCBI
|
|
30
|
Poltorak A, He X, Smirnova I, Liu MY, Van
Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al:
Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations
in Tlr4 gene. Science. 282:2085–2088. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamamoto N, Zou JP, Li XF, Takenaka H,
Noda S, Fujii T, Ono S, Kobayashi Y, Mukaida N, Matsushima K, et
al: Regulatory mechanisms for production of IFN-γ and TNF by
antitumor T cells or macrophages in the tumor-bearing state. J
Immunol. 154:2281–2290. 1995.PubMed/NCBI
|
|
32
|
Park H, Li Z, Yang XO, Chang SH, Nurieva
R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harrington LE, Hatton RD, Mangan PR,
Turner H, Murphy TL, Murphy KM and Weaver CT: Interleukin
17-producing CD4+ effector T cells develop via a lineage
distinct from the T helper type 1 and 2 lineages. Nat Immunol.
6:1123–1132. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakajima C, Uekusa Y, Iwasaki M, Yamaguchi
N, Mukai T, Gao P, Tomura M, Ono S, Tsujimura T, Fujiwara H, et al:
A role of interferon-γ (IFN-γ) in tumor immunity: T cells with the
capacity to reject tumor cells are generated but fail to migrate to
tumor sites in IFN-γ-deficient mice. Cancer Res. 61:3399–3405.
2001.PubMed/NCBI
|
|
35
|
O'Connor W Jr, Kamanaka M, Booth CJ, Town
T, Nakae S, Iwakura Y, Kolls JK and Flavell RA: A protective
function for interleukin 17A in T cell-mediated intestinal
inflammation. Nat Immunol. 10:603–609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kawanishi Y, Tominaga A, Okuyama H,
Fukuoka S, Taguchi T, Kusumoto Y, Yawata T, Fujimoto Y, Ono S and
Shimizu K: Regulatory effects of Spirulina complex polysaccharides
on growth of murine RSV-M glioma cells through Toll-like receptor
4. Microbiol Immunol. 57:63–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Odobasic D, Leech MT, Xue JR and
Holdsworth SR: Distinct in vivo roles of CD80 and CD86 in the
effector T-cell responses inducing antigen-induced arthritis.
Immunology. 124:503–513. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stumhofer JS, Laurence A, Wilson EH, Huang
E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D,
et al: Interleukin 27 negatively regulates the development of
interleukin 17-producing T helper cells during chronic inflammation
of the central nervous system. Nat Immunol. 7:937–945. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martin-Orozco N, Muranski P, Chung Y, Yang
XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW and Dong C: T
helper 17 cells promote cytotoxic T cell activation in tumor
immunity. Immunity. 31:787–798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Muranski P, Boni A, Antony PA, Cassard L,
Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K,
et al: Tumor-specific Th17-polarized cells eradicate large
established melanoma. Blood. 112:362–373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Murugaiyan G and Saha B: Protumor vs.
antitumor functions of IL-17. J Immunol. 183:4169–4175. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yao Z, Fanslow WC, Seldin MF, Rousseau AM,
Painter SL, Comeau MR, Cohen JI and Spriggs MK: Herpesvirus Saimiri
encodes a new cytokine, IL-17, which binds to a novel cytokine
receptor. Immunity. 3:811–821. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kolls JK and Lindén A: Interleukin-17
family members and inflammation. Immunity. 21:467–476. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cirée A, Michel L, Camilleri-Bröet S,
Louis F Jean, Oster M, Flageul B, Senet P, Fossiez F, Fridman WH,
Bachelez H, et al: Expression and activity of IL-17 in cutaneous
T-cell lymphomas (mycosis fungoides and Sezary syndrome). Int J
Cancer. 112:113–120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Netea MG, van Deuren M, Kullberg BJ,
Cavaillon JM and Van der Meer JW: Does the shape of lipid A
determine the interaction of LPS with Toll-like receptors? Trends
Immunol. 23:135–139. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Miller SI, Ernst RK and Bader MW: LPS,
TLR4 and infectious disease diversity. Nat Rev Microbiol. 3:36–46.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Schromm AB, Brandenburg K, Loppnow H,
Moran AP, Koch MH, Rietschel ET and Seydel U: Biological activities
of lipopolysaccharides are determined by the shape of their lipid A
portion. Eur J Biochem. 267:2008–2013. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lebbar S, Cavaillon JM, Caroff M, Ledur A,
Brade H, Sarfati R and Haeffner-Cavaillon N: Molecular requirement
for interleukin 1 induction by lipopolysaccharide-stimulated human
monocytes: Involvement of the heptosyl-2-keto-3-deoxyoctulosonate
region. Eur J Immunol. 16:87–91. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gangloff SC, Hijiya N, Haziot A and Goyert
SM: Lipopolysaccharide structure influences the macrophage response
via CD14-independent and CD14-dependent pathways. Clin Infect Dis.
28:491–496. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
50
|
Loppnow H, Libby P, Freudenberg M, Krauss
JH, Weckesser J and Mayer H: Cytokine induction by
lipopolysaccharide (LPS) corresponds to lethal toxicity and is
inhibited by nontoxic Rhodobacter capsulatus LPS. Infect Immun.
58:3743–3750. 1990.PubMed/NCBI
|
|
51
|
Mishima T, Murata J, Toyoshima M, Fujii H,
Nakajima M, Hayashi T, Kato T and Saiki I: Inhibition of tumor
invasion and metastasis by calcium spirulan (Ca-SP), a novel
sulfated polysaccharide derived from a blue-green alga, Spirulina
platensis. Clin Exp Metastasis. 16:541–550. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Akao Y, Ebihara T, Masuda H, Saeki Y,
Akazawa T, Hazeki K, Hazeki O, Matsumoto M and Seya T: Enhancement
of antitumor natural killer cell activation by orally administered
Spirulina extract in mice. Cancer Sci. 100:1494–1501. 2009.
View Article : Google Scholar : PubMed/NCBI
|