Introduction
Bladder cancer is a major cause of morbidity and
mortality worldwide, and in the United States alone, 76,960 newly
diagnosed cases and 16,390 deaths are estimated in 2015 (1). Approximately 70% of patients with
bladder cancer are non-muscle-invasive (NMIBC) at diagnosis
(2). NMIBC is characterized by
significant rates of recurrence and progression. The range of
recurrence is 50–80%, and progression 10–45%, depending on disease
risks (based upon grade, stage, and tumor size) (3). Transurethral resection of bladder
tumor (TURBT) combined with intravesical chemotherapy is the
primary method for treatment of NMIBC (4). The aim of intravesical chemotherapy is
to decrease the possibility of tumor recurrence and progression. At
present, Epirubicin (EPI), a derivative of doxorubicin, is one of
the most used intravesical chemotherapy agents to treat NMIBC
(5). Comparing with TURBT alone,
EPI instillation after TURBT decreased nearly half of recurrence
and progression of NMIBC (6).
Although intravesical EPI chemotherapy has improved the clinical
outcome of patients with NMIBC, efforts to potentiate drug action
and enhance chemosensitivity should be investigated for further
improvement of patient outcomes (7).
Recepteur d'origine Nantais (RON) belongs to the MET
proto-oncogene family (8). The
expression of RON is highly altered in many primary cancer samples
including colon, breast and bladder cancer, and has prognostic
value in predicting patient survival and clinical outcome (9–11).
Aberrant RON activation, featured by overexpression of RON protein
(12–14), isoform generation (15–17),
and persistent activation of downstream signaling pathways
(18), has been found in various
types of cancers. Those aberrations contribute to tumorigenic
phenotype, malignant progression and chemoresistance (9,11,19).
Due to the importance of RON in cancer pathogenesis, targeting RON
signal pathway has therapeutic potential. Currently, various
approaches including therapeutic monoclonal antibodies (mAb), siRNA
and small molecule inhibitors (SMI) have been evaluated to inhibit
RON signaling (20–22). Results from these studies
demonstrate that inhibition of RON signaling contributes to reduced
cell growth, diminished cell invasiveness, and impaired tumor
metastasis. Combining RON signaling inhibition and chemotherapy
agents were also under investigation in treating with various
cancers. In colon cancer, 5-Fu in combination with RON specific mAb
Zt/f2 has been showed to markedly improve treatment effects
(20), suggesting that inhibiting
RON pathway may enhance chemosensitivity of chemotherapy drugs.
In bladder cancers, RON is overexpressed in more
than 35% of samples (11,14). RON expression has been documented in
RT4, TCCSUP, UB09 and other bladder cancer cell lines (11). Overexpression of RON was associated
with poor clinical outcome (11,23).
Furthermore, MSP, the only known ligand of RON, was also detected
in human urine samples (11). These
findings suggested that RON plays a role in bladder cancer
tumorigenesis and invasion. Evidence has indicated that
RON-specific mAbs such as Zt/g4 and Zt/f2 rapidly induce RON
internalization by cancer cells, which diminish RON signal
transduction and enhance cytotoxic drug delivery and sensitivity
(20,24–27).
Thus, RON-specific mAbs are potentially effective approach to RON
signal inhibition and enhancement of chemosensitivity.
In the present study, we selected a mouse mAb Zt/g4
highly specific to the RON extracellular domain to induce RON
internalization and subsequent RON signal pathway inhibition. EPI
was used as chemotherapeutical agent to determine the effects of
RON on chemosensitivity in bladder cancer cells. This study
provides new strategy to reduce NMIBC recurrence and
progression.
Materials and methods
Cell lines and cell culture
The human 5637, T24, RT4, J82, UMUC and BIU87
bladder cancer cell lines were purchased from the Shanghai
Institute of Cell Biology, Chinese Academy of Sciences (Shanghai,
China). The cells were cultured at 37°C in a humidified atmosphere
of 5% CO2 in RPMI-1640 medium supplemented with 10%
(v/v) fetal bovine serum (FBS), 2 mM L-glutamine and 100 U/ml
penicillin. The medium was replaced every 3 days.
Main reagents
EPI was purchased from the Hisun Pharmaceutical Co.,
Ltd. (Zhejiang, China). Cell Counting Kit-8 (CCK-8) was purchased
from Dojindo Molecular Technologies (Rockville, MD, USA). Mouse mAb
Zt/g4 specific to RON sema domain and rabbit antibody (R5029,
specific to the RON C-terminal peptide) were kindly supplied by
Professor Yao (Laboratory of Cancer Biology and Therapeutics, First
Affiliated Hospital, Zhejiang University School of Medicine).
Rabbit anti-Bcl-2, anti-Bax, anti-Erk1/2, anti-AKT, anti-caspase-3,
anti-cyclin D1, anti-CDK4, anti-CDK6 and anti-p27 antibodies were
from Cellular Signaling Technology (Danvers, MA, USA). FBS,
RPMI-1640, L-glutamine and penicillin were purchased from Life
Technologies Inc. (Carlsbad, CA, USA).
Western blotting
Western blot analysis was performed to measure the
expression levels of various proteins in cells. Each sample
equivalent of 100 µg total proteins were electrophoresed in 8%
SDS-PAGE and blotted on a nitrocellulose membrane (Millipore Inc.,
Billerica, MA, USA). Blots were blocked at room temperature for 2 h
in 1X Tris-buffered saline (TBS) buffer, and then incubated with
primary antibodies specific to Ron, Bcl-2, Bax and β-actin
overnight at 4°C, respectively. After three washes for 3×10 min in
TBST, the membrane was incubated with horseradish peroxidase
(HRP)-conjugated anti-mouse or anti-rabbit immunoglobulin G at room
temperature for 1 h. Immunoreactive proteins were visualized by
enhanced chemiluminescent reagents (Thermo Scientific, Rockford,
IL, USA). The optical density was quantified by VersaDoc Imaging
system (Bio-Rad, Hercules, CA, USA).
Transwell invasion assay
The cells were seeded at a concentration ratio of
5×104 cells/chamber in serum-free RPMI-1640 and placed
on the 8 µm pore-size upper chamber (Corning Incorporated, Corning,
NY, USA). The lower chambers contained RPMI-1640 culture media with
10% FBS. After 24 h of incubation, non-invading cells on the top
chamber were removed by using a cotton swab, and cells that
penetrated to the lower surface were fixed with 800 µl methanol for
30 min, stained with 0.5% crystal violet solution for 2 h, washed
with 1X PBS and counted under a microscope. Six fields were
randomly selected from each sample, with triplicates.
Cell viability assay
Sensitivity of cells to EPI was assayed with the
CCK-8 kit. Briefly, cells were seeded in 96-well plates with a
density of 1×104/well and incubated for 24 h at 37°C and
then treated with EPI (0.2, 1. 2, 3 and 6 µg/ml), Zt/g4 (8 µg/ml)
combined with EPI for 48 h. The proportion of live cells of the two
groups was analyzed by CCK-8 kit according to the manufacturer's
instructions. Finally, the absorbance (OD) value of each well was
measured by a microplate reader at the wavelength of 450 nm. The
experiment was carried out in triplicate. Dose-dependent response
curve was plotted and the half maximal inhibitory concentration
(IC50) was determined by fitting the concentration
response curves with the sigmaplot software.
Cell cycle analysis
Cell cycle status was detected by flow cytometry and
analyzed by Flowjo software. Briefly, after exposed to Zt/g4, EPI
or both for 24 h, cells (2×105-106) were
harvested, fixed with 75% ethanol overnight at −20°C. The fixed
cells were incubated in darkness at 37°C with 1 mg/ml RNase A
(Sigma-Aldrich, St. Louis, MO, USA) for 30 min and with 50 µg/ml
propidium iodide (PI) (Sigma-Aldrich) for 30 min. The cells were
analyzed by flow cytometry (FACScan, Becton-Dickinson, Franklin
Lakes, NJ, USA).
Apoptosis analysis
After Zt/g4, EPI or both for 48 h, the cells were
collected and washed twice with cold PBS, followed by resuspension
in binding buffer at the density of 1×106 cells/ml. 100
µl (1×105 cells) of the solution was removed and stained
with 5 µl Annexin V-FITC and PI (BD Biosciences) for 15 min in the
dark at room temperature. Then a total of 400 dilution buffer was
added to each tube and cell apoptosis was analysed by flow
cytometry (FACScan, Becton-Dickinson). The percentage of apoptotic
cells with Annexin V+/PI+ was evaluated. Each
group was measured three times.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 18; SPSS Inc., Chicago, IL, USA). Data are
presented as the means ± standard deviation (SD). Statistical
significance between two groups was evaluated by Student's t-test.
Differences between multiple groups were performed by one-way
analysis of variance. A difference was considered significant at
P<0.05.
Results
Expression of RON receptors in bladder
cancer cell lines
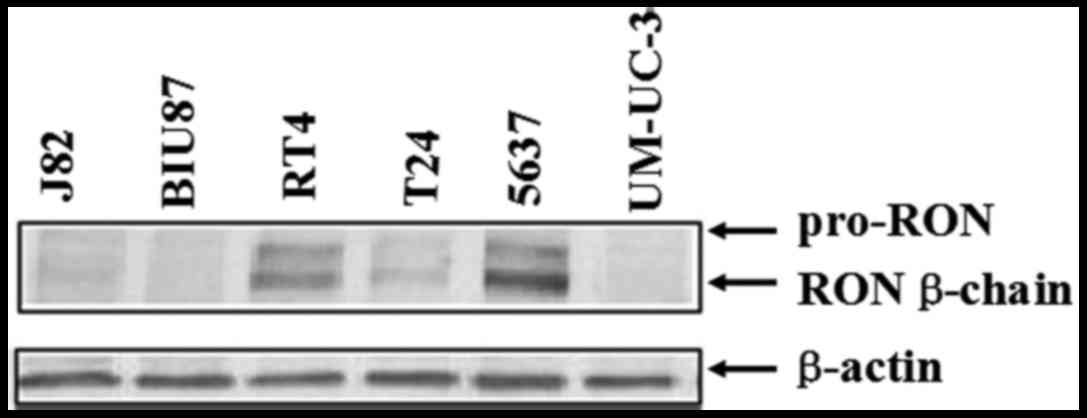
The expression of RON was detected by western
blotting in seven bladder cancer cell lines (Fig. 1). In this panel, 5637, T24 and RT4
cell lines had a high level of RON expression, while the expression
levels of RON in other three cancer cell lines were barely found.
As the 5637 cells expressed a relatively higher level of RON than
those in T24 and RT4 cells, we chose 5637 cells for future
studies.
Zt/g4 induces reduction of RON
expression and signal inhibition
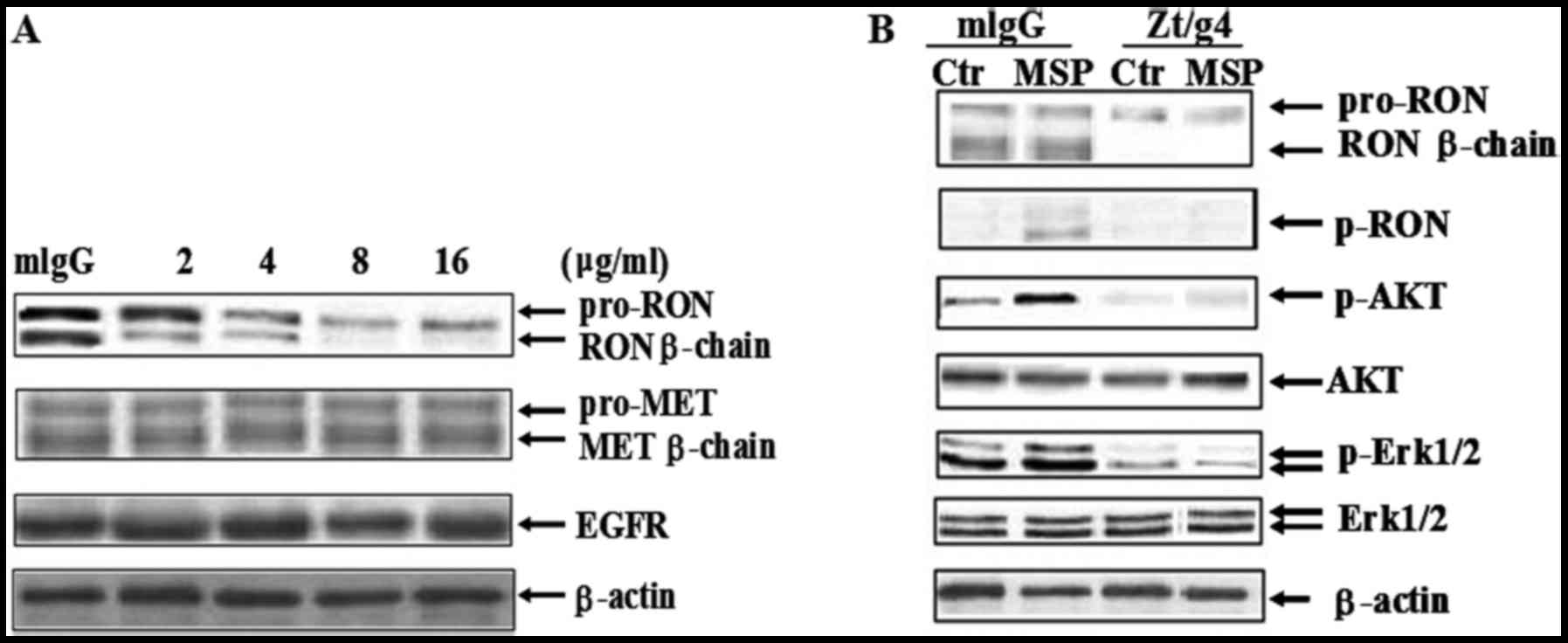
Western blot analysis confirmed that Zt/g4 treatment
for 24 h caused diminished RON expression in a dose-dependent
manner in 5637 cells (Fig. 2A).
When used with 2 µg/ml of Zt/g4, Zt/g4-induced reduction of RON
β-chain expression was significantly identified. However, the
concentration of Zt/g4 increased up to 8 µg/ml, but did not further
cause RON reduction. Thus, the maximal effect induced by Zt/g4 was
at the range of 8 µg/ml. We chose this concentration as the
standard for further experiments. Cross-talk between RON and MET or
EGFR has been found in various cancer cells (28,29).
We verified whether Zt/g4-induced RON reduction affected MET or
EGFR expression in 5637 cells. Result showed that there were no
significant differences in the total level of MET and EGFR
expression in 5637 cells after Zt/g4 treatment. Taken together,
these results demonstrated that Zt/g4-induced RON reduction was
specific to RON and had no effect on MET or EGFR.
As MSP was detected in human urine samples (11), MSP induced RON activation and
signaling transduction may be a key event in bladder tumorigenesis
and invasion. To test whether Zt/g4 blocks MSP induced RON
activation and downstream signaling transduction, 5 nM MSP was
added to induce RON activation with or without 8 µg/ml Zt/g4
treatment (Fig. 2B). Result showed
that after Zt/g4 treatment for 48 h, MSP induced RON activation was
completely inhibited. Phosphorylation of downstream signal
molecules such as Erk1/2 and AKT were also interrupted (Fig. 2B).
Zt/g4 enhances the chemosensitivity of
EPI on 5637 cells
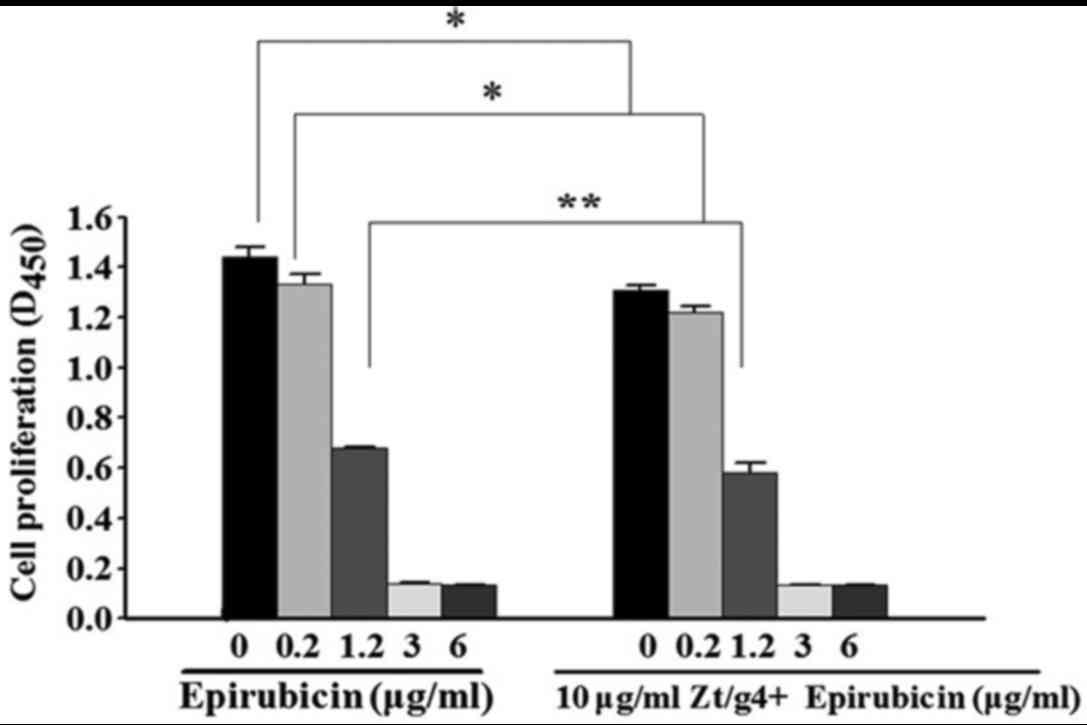
To determine whether inhibition of RON signaling by
Zt/g4 enhance chemosensitivity of EPI in 5637 cells, a CCK-8 assay
was carried out to measure the proliferation status of the cells.
5637 cells were treated with EPI or Zt/g4 combined with EPI at
different concentrations. After 48 h, the cell viability was
reduced with increasing concentrations of EPI (Fig. 3). The levels of cytotoxicity were
indicated as the concentration that inhibits the response by 50%
IC50 value. The IC50 values in Zt/g4 combined
with EPI and EPI were (0.9±0.13, 1.2±0.09), respectively. Data
showed that RON inhibition by Zt/g4 was able to enhance the
sensitivity of 5637 cells to EPI at the concentrations of 0.2 and
1.2 µg/ml (P<0.05). Since the IC50 value in EPI
treated 5637 cells was (1.2±0.09 µg/ml), the concentration of EPI
intervention in subsequent experiments was determined as 1.2
µg/ml.
Zt/g4 combining with EPI markedly
decreases 5637 cell invasion
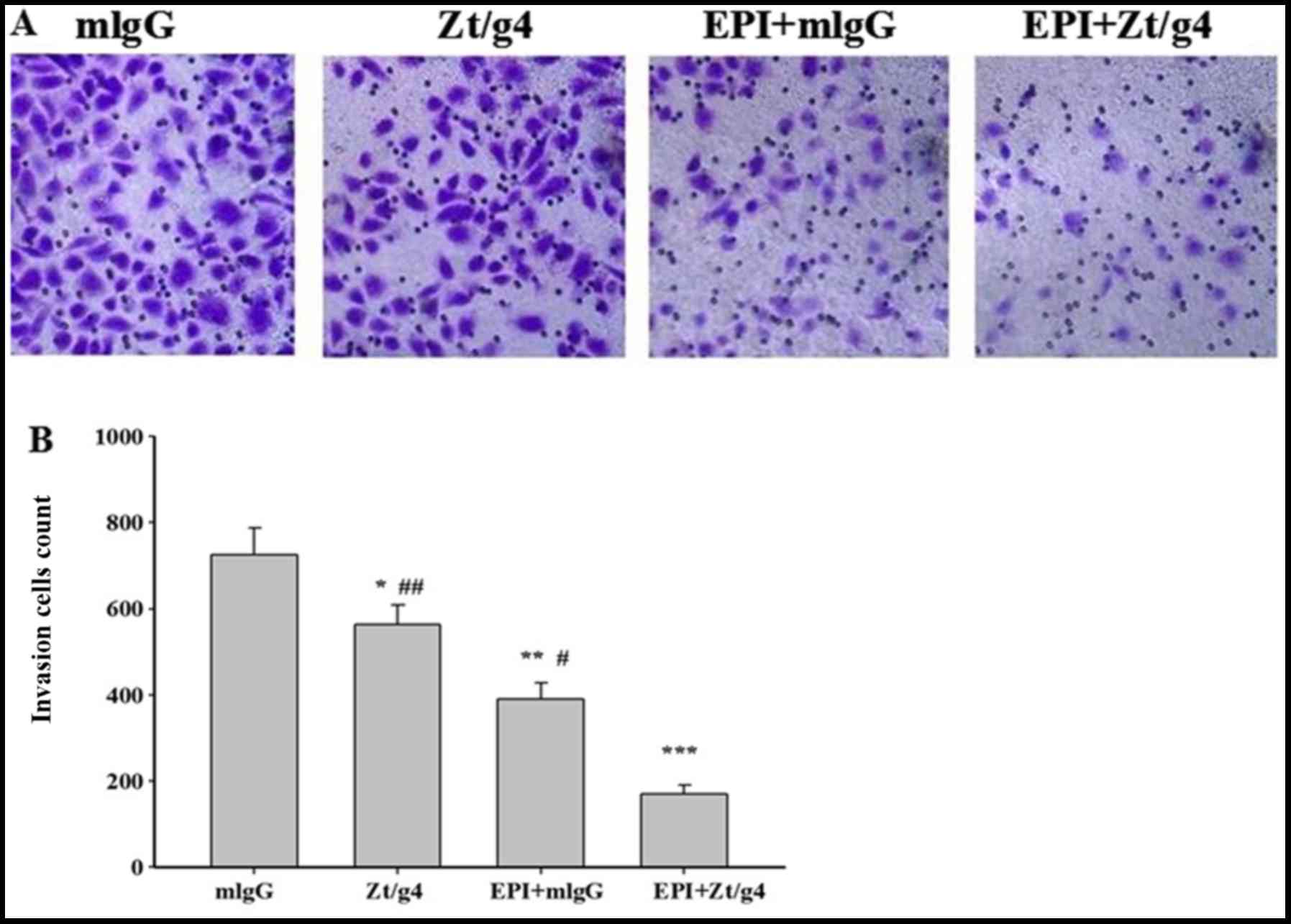
Tumor invasion and progression is a common event in
NMIBC even after TURBT and intravesical chemotherapy. To study
whether combining Zt/g4 and EPI affected cellular invasion, we
further carried out Transwell assay on the 5637 cells. The cells
were treated with 8 µg/ml Zt/g4 alone, 1.2 µg/ml EPI alone, EPI
combined with Zt/g4, or mouse IgG in transmembrane chambers for 24
h. The results showed that the invasive cell count of Zt/g4 in
combination with EPI was significantly lower than that of Zt/g4 or
EPI alone (170±21 vs. 564±49 or 390±37 individually) (Fig. 4A and B), and the cell invasive
capacity of the Zt/g4 treatment group (564±49) was significantly
lower than the mlgG group (726±62) (mlgG was used as the
control).
Cell cycle arrested at G1/S phase in
5637 cells by Zt/g4 combining with EPI treatment
EPI can often inhibit cell proliferation through
induction of cell cycle arrest. To determine whether Zt/g4
intracellular delivery of EPI resulted in cell cycle changes, we
incubated cells with Zt/g4, EPI or both for 24 h and then examined
the DNA content using propidium iodide (PI) staining. The
proportions of cells treated with Zt/g4 in each phase of the cell
cycle showed no significant difference comparing with those of
control group. The changes in cell-cycle profile were observed
after addition of EPI combined with Zt/g4, featuring a significant
reduction in S phase, an increase in G1 phase, compared to the EPI
treatment alone (P<0.05) (Fig. 5A
and B). These changes were present in all three 5637 cell lines
tested. Moreover, we checked several key factors including cyclin
D1, CDK4, CDK6 and p27 regulating the G1/S cell cycle transition.
The expression of cyclin D1, CDK4, CDK6 and p27was not changed by
Zt/g4 treatment in 5637 cells. However, cotreatment of cells with
EPI and Zt/g4 increased the expression of p27 while downregulated
cyclin D1, CDK4 and CDK6 comparing with Zt/g4 or EPI treatment
alone (Fig. 5C). These results
suggested that the G1/S cell cycle arrest induced by Zt/g4 combined
with EPI was related with upregulation of the cell cycle inhibitory
proteins and downregulation of the cyclin-dependent protein kinases
(CDKs) and cyclins.
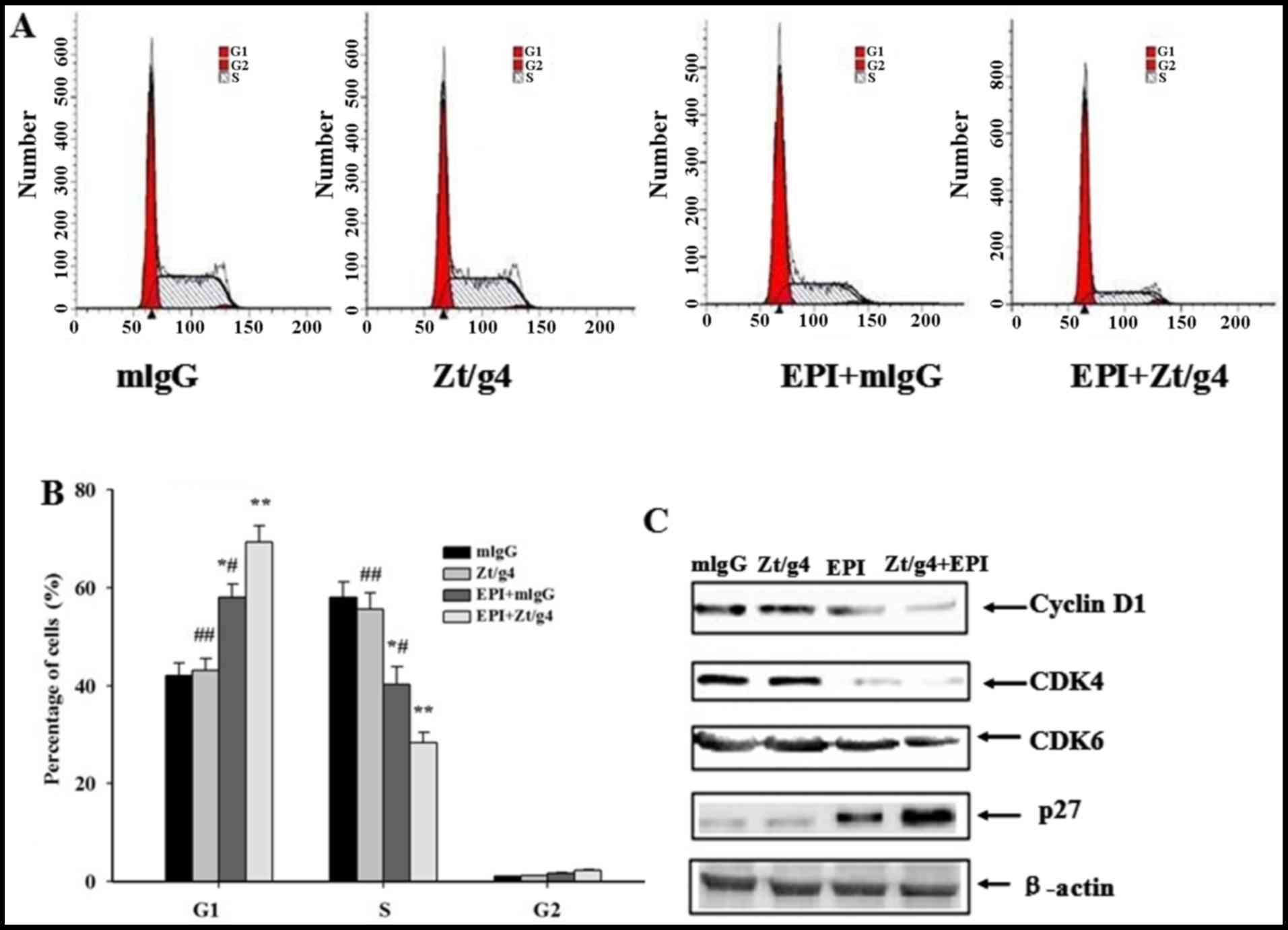 | Figure 5.Effects of Zt/g4 on EPI-induced cell
cycle in 5637 cells. (A) Changes in cell cycles: 5637 cells were
treated at 37C with Zt/g4 alone, EPI alone or both for 24 h,
collected, stained with propidium iodide, and then analyzed by flow
cytometer. (B) Quantitative analysis of cell cycle distribution of
5637 cells treated with Zt/g4, EPI or both. *P<0.05,
**P<0.01, ***P<0.001 compared to mlgG group;
#P<0.05, ##P<0.01, compared to
EPI+Zt/g4group.Values are presented as the means ± SD from three
independent experiments. (C) Representative western blot analysis
showed changes in the expression of cyclins (CDK4 and CDK6),
cyclin-dependent kinases (cyclin D1) and cyclin-dependent protein
kinase inhibitors (p27) following Zt/g4, EPI or combined treatment
in 5637 cells. Data represent the mean ± SD (n=3). |
Zt/g4 combining with EPI treatment
promotes apoptosis in 5637 cells
Flow cytometry (FACS) was used to further
investigate whether Zt/g4, EPI or both exerted anticancer effect on
5637 cells through inducing apoptosis. We exposed 5637 cells to
Zt/g4, EPI or both as previously mentioned, and then stained them
with Annexin V-PE and PI to measure apoptosis rates after
incubation for 48 h. FACS analysis showed that total apoptosis
rates of EPI combined with Zt/g4 were significantly increased when
comparing with EPI or Zt/g4 treatment alone (Fig. 6A and B). This result indicated that
Zt/g4 was able to promote the EPI-induced apoptosis in 5637 cells.
To further unveil the mechanisms by which Zt/g4 enhanced
EPI-induced apoptosis in 5637 cells, the cells were treated with
Zt/g4, EPI or both for 48 h and subjected to western blot analysis.
The bcl-2 protein expression in EPI plus Zt/g4 cells was markedly
lower while Bax and active caspase-3 protein expression levels were
higher than those in the Zt/g4 or EPI treatment cells (Fig. 6C). This result suggested that
downregulation of RON by Zt/g4 promoted EPI induced apoptosis of
5637 cells through mitochondria-mediated apoptotic pathway.
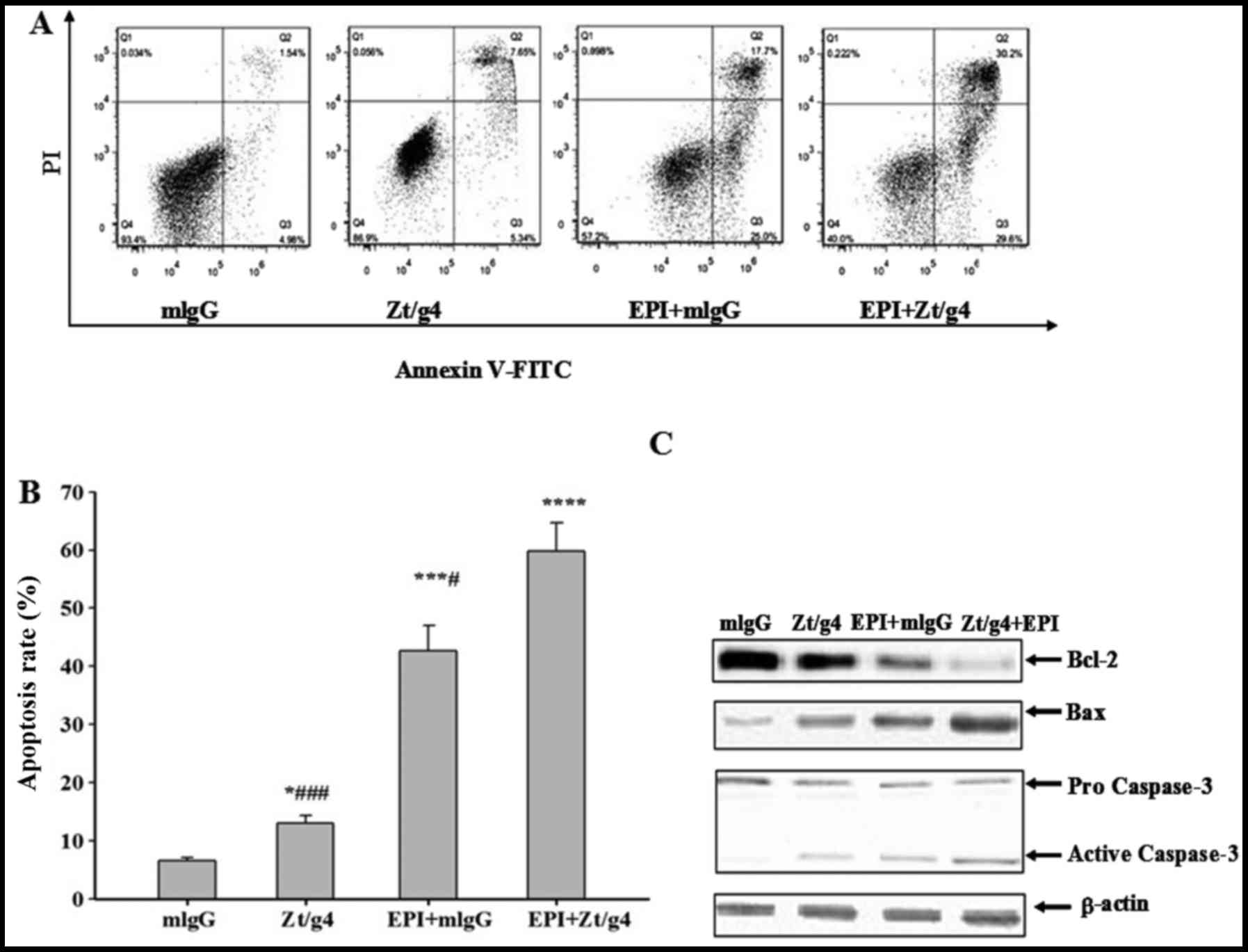 | Figure 6.Effects of Zt/g4 on EPI-induced cell
apoptosis in 5637 cells. Flow cytometric analysis demonstrated the
apoptotic effects of Zt/g4, EPI and both treatment on 5637 cells.
(A) Cells were treated with Zt/g4, EPI or both for 48 h, collected,
stained with Annexin V and PI and then analyzed by flow cytometer.
(B) Quantitative results obtained using Annexin V/PI staining.
*P<0.05, ***P<0.01, ****P<0.0001 compared to mlgG group;
#P<0.05, ###P<0.001, compared to
EPI+Zt/g4 group. (C) Representative western blot analysis showed
changes in the expression of Bcl-2, Bax and activity-caspase-3
following Zt/g4, EPI or combined treatment in 5637 cells. Data are
derived from three independent experiments and are expressed as the
mean ± SD. |
Discussion
Bladder cancer can be divided into three categories
based on its prognosis and management. The first category consists
of non-muscle-invasive tumors. The second category consists of
muscle invasive bladder cancer (MIBC). The third group is
metastasis bladder cancer. Therapeutic aim is different to each of
these categories (30). To NMIBC,
the main concern is to reduce recurrences and preventing
progression to a more advanced stage. TURBT followed by
chemotherapy agents and immunotherapy agents are now clinically
used to achieve this goal (31).
However, even treated with these current approaches, half of NIMBC
will recur and progress. More strategies should be investigated to
further decrease the rate of NIMBC recurrence and progression.
We and others have found that RON plays an important
role in the pathogenesis of bladder cancer (11,14).
Although RON is recently reported to associate with the
chemosensitivity in human malignancies such as breast cancer and
pancreatic cancer (19,32), its role in chemotherapy of bladder
cancer remains largely unknown. In this study, our results showed
that Ron signaling inhibition by Zt/g4 could remarkably enhance the
chemosensitivity of epirubicin (EPI) in human 5637 cells and
decrease cell invasion. Possible mechanisms include promotion of
cell cycle arrest and induction of Bcl-2 dependent apoptosis.
Zt/g4 has unique binding specificity to the RON
extracellular domains. Recent studies have shown that Zt/g4 is
highly effective in downregulation of RON expression by colon,
breast and pancreatic cancer cells (26). The binding of Zt/g4 to the epitopes
either on sema or IPT domains is sufficient to cause RON reduction
due to RON internalization and degradation by proteasome (33). In this study, we found the effect of
Zt/g4 is concentration-dependent. Significant reduction of RON was
seen when using 2 µg per ml of Zt/g4 and the maximal effect was at
the range of 8 µg per ml. However, further increase of Zt/g4 up to
16 µg per ml did not show additional effect (Fig. 2A). In a recent study, Li reported
that the maximal rate of Zt/g4 was 10 µg per ml in colon SW620
cells (26). The maximal effect is
related to the amounts of Zt/g4 that bind to RON extracellular
domain. Cross-talk between RON and MET or EGFR exist on the cell
membrane surface, and specifically blocking RON is under intensive
investigation (28,29). In the present study, the effect of
Zt/g4 was only specific to RON. It had no effect on the
structurally-related MET or unrelated EGFR in Fig. 2B. Moreover, after persistent Zt/g4
treatment, MSP induced RON activation was completely inhibited also
the downstream pMAPK and pAkt activation was inhibited in 5637
cells.
Cancer cell unlimited growth and invasion are the
most fatal features of malignant tumors, accounting for >90% of
tumor-related mortality (34).
Overexpression of RON contributes to increased cell growth and
invasion (35,36). The results of this study showed that
Zt/g4 or EPI alone could moderately inhibit cell proliferation and
invasion in 5637 cells, but when Zt/g4 was used in combination with
EPI it showed significant inhibition of cell proliferation
(Fig. 3)and invasion (Fig. 4). Without Zt/g4, IC50 of
EPI to 5637 cells was 1.2±0.09 µg/ml per 105 cells,
after combining with Zt/g4, IC50 of EPI to 5637 cells
was markedly decreased to 0.9±0.13 µg/ml per 105 cells.
Therefore, Zt/g4 was efficient in enhancing chemosensitivity of
EPI.
It was documented recently that Zt/g4 intracellular
delivery of maytansinoid could result in cell cycle changes,
suggesting that cell cycle arrest might contribute to enhancement
of chemosensitivity by Zt/g4 (37).
Therefore, we analyzed the cell cycle distribution of EPI-treated
5637 cells. EPI alone moderately affected cell cycle distribution
in the cells, but when it was combined with Zt/g4, an obvious G1/S
arrest was found (Fig. 5). Thus,
Zt/g4-targeted delivery of EPI affects the cell cycle in 5637
cells. The cyclin-dependent kinases (CDKs) and cyclins play a
crucial role in the regulation of cell cycle progression (38,39).
The G1 cyclin-CDK complex cyclin D-CDK4/6 is required for S phase
entry (40). CDK inhibitors (CKIs)
such as p21CIP1/WAF1 and p27KIP1 bind to cyclin-CDK complexes and
render them inactive, which inhibit cell cycle progression
(41). In our study, we found that
cotreatment of Zt/g4 and EPI leads to G1/S arrest into 5637 cells
(Fig. 5A and B), which is
accompanied by the downregulation of cyclin D1, CDK4 and CDK6,
whereas it increased the level of P27 (Fig. 5C).
Apoptosis is a defensive mechanism of the body
against the progression and development of tumor. Several studies
have suggested that RON is associated with apoptosis in various
cancer cells (42,43). However, the impact of RON on
apoptosis in human bladder cancer is not reported. Thus,
investigating the effect of Zt/g4 on EPI-induced cell apoptosis in
5637 cells is highly desirable. Our experiments showed that
EPI-treated 5637 cells exhibited an increase in Annexin V(+)/PI(−)
staining, and combined with Zt/g4 treatment strengthened this
effect, indicating that Zt/g4 promoted EPI induced apoptosis
(Fig. 6B). The mitochondrial
apoptotic pathway is mainly mediated by proteins of the Bcl-2
family such as Bcl-2 and Bax (44).
Bcl-2 is an anti-apoptotic protein, which negatively regulates the
activation of caspase-3 that acted as an effector of mammalian cell
death pathways (45). Bax is a
proapoptotic protein, and the activation of Bax can increase the
mitochondrial permeability and the release of pro-apoptotic
molecules such as cytochrome-c. Releasing of cytochrome c can
active caspase-3 and leads finally to apoptosis (46). In this study, combining EPI and
Zt/g4 significantly decreased the protein expression levels of
Bcl-2 while increased the protein expression levels of Bax and
cleaved caspase-3, suggesting Zt/g4 promoted EPI-induced apoptosis
via the mitochondrial-dependent pathway.
In summary, the results of this study demonstrated
that inhibition of RON signaling pathway by Zt/g4 markedly improved
chemosensitivity of EPI in bladder cancer cells. Both cell
proliferation and invasion were effectively inhibited by combining
Zt/g4 and EPI treatment. Possible mechanisms underlying this
combination include induction of cell cycle arrested at G1/S and
promotion of mitochondrial pathway of apoptosis. These data provide
new strategies to prevent recurrence and progression, which may
further improve clinical outcomes of current approaches in
treatment with NMIBC.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81272828 to Q.M., and no.
31501113 to R.Y.), Zhejiang Provincial Foundation for Medical and
Health Sciences (grant no. 2016KYB263 and 2014KYB355 to Q.M.), and
Ningbo Natural Science Foundation (grant no. 2015A610224 to J.-F.C.
and no. 2015A610177 to R.Y.).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parekh DJ, Bochner BH and Dalbagni G:
Superficial and muscle-invasive bladder cancer: Principles of
management for outcomes assessments. J Clin Oncol. 24:5519–5527.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Porten SP, Leapman MS and Greene KL:
Intravesical chemotherapy in non-muscle-invasive bladder cancer.
Indian J Urol. 31:297–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herr HW, Dotan Z, Donat SM and Bajorin DF:
Defining optimal therapy for muscle invasive bladder cancer. J
Urol. 177:437–443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Babjuk M, Oosterlinck W, Sylvester R,
Kaasinen E, Böhle A, Palou-Redorta J and Rouprêt M: European
Association of Urology (EAU): EAU guidelines on non-muscle-invasive
urothelial carcinoma of the bladder, the 2011 update. Eur Urol.
59:997–1008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oosterlinck W, Kurth KH, Schröder F,
Bultinck J, Hammond B and Sylvester R: A prospective European
Organization for Research and Treatment of Cancer Genitourinary
Group randomized trial comparing transurethral resection followed
by a single intravesical instillation of epirubicin or water in
single stage Ta, T1 papillary carcinoma of the bladder. J Urol.
149:749–752. 1993.PubMed/NCBI
|
|
7
|
Yu R, Yu BX, Chen JF, Lv XY, Yan ZJ, Cheng
Y and Ma Q: Anti-tumor effects of Atractylenolide I on bladder
cancer cells. J Exp Clin Cancer Res. 35:402016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ronsin C, Muscatelli F, Mattei MG and
Breathnach R: A novel putative receptor protein tyrosine kinase of
the met family. Oncogene. 8:1195–1202. 1993.PubMed/NCBI
|
|
9
|
Park YL, Lee GH, Kim KY, Myung E, Kim JS,
Myung DS, Park KJ, Cho SB, Lee WS, Jung YD, et al: Expression of
RON in colorectal cancer and its relationships with tumor cell
behavior and prognosis. Tumori. 98:652–662. 2012.PubMed/NCBI
|
|
10
|
Feres KJ, Ischenko I and Hayman MJ: The
RON receptor tyrosine kinase promotes MSP-independent cell
spreading and survival in breast epithelial cells. Oncogene.
28:279–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng HL, Liu HS, Lin YJ, Chen HH, Hsu PY,
Chang TY, Ho CL, Tzai TS and Chow NH: Co-expression of RON and MET
is a prognostic indicator for patients with transitional-cell
carcinoma of the bladder. Br J Cancer. 92:1906–1914. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Catenacci DV, Cervantes G, Yala S, Nelson
EA, El-Hashani E, Kanteti R, El Dinali M, Hasina R, Brägelmann J,
Seiwert T, et al: RON (MST1R) is a novel prognostic marker and
therapeutic target for gastroesophageal adenocarcinoma. Cancer Biol
Ther. 12:9–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren X, Daa T, Yada N, Kashima K, Fujitomi
Y and Yokoyama S: Expression and mutational status of RON in
neoplastic lesions of the breast: Analysis of MSP/RON signaling in
ductal carcinoma in situ and invasive ductal carcinoma. APMIS.
120:358–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang MH, Lee W, Luo YL, Weis MT and Yao
HP: Altered expression of the RON receptor tyrosine kinase in
various epithelial cancers and its contribution to tumourigenic
phenotypes in thyroid cancer cells. J Pathol. 213:402–411. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang MH, Kurtz AL and Chen Y:
Identification of a novel splicing product of the RON receptor
tyrosine kinase in human colorectal carcinoma cells.
Carcinogenesis. 21:1507–1512. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eckerich C, Schulte A, Martens T, Zapf S,
Westphal M and Lamszus K: RON receptor tyrosine kinase in human
gliomas: Expression, function, and identification of a novel
soluble splice variant. J Neurochem. 109:969–980. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma Q, Zhang K, Guin S, Zhou YQ and Wang
MH: Deletion or insertion in the first
immunoglobulin-plexin-transcription (IPT) domain differentially
regulates expression and tumorigenic activities of RON receptor
Tyrosine Kinase. Mol Cancer. 9:3072010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao HP, Zhou YQ, Zhang R and Wang MH:
MSP-RON signalling in cancer: Pathogenesis and therapeutic
potential. Nat Rev Cancer. 13:466–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prislei S, Mariani M, Raspaglio G,
Mozzetti S, Filippetti F, Ferrandina G, Scambia G and Ferlini C:
RON and cisplatin resistance in ovarian cancer cell lines. Oncol
Res. 19:13–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao HP, Zhou YQ, Ma Q, Guin S, Padhye SS,
Zhang RW and Wang MH: The monoclonal antibody Zt/f2 targeting RON
receptor tyrosine kinase as potential therapeutics against tumor
growth-mediated by colon cancer cells. Mol Cancer. 10:82–93. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu XM, Wang D, Shen Q, Chen YQ and Wang
MH: RNA-mediated gene silencing of the RON receptor tyrosine kinase
alters oncogenic phenotypes of human colorectal carcinoma cells.
Oncogene. 23:8464–8474. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yao HP, Zhuang CM, Zhou YQ, Zeng JY, Zhang
RW and Wang MH: Oncogenic variant RON160 expression in breast
cancer and its potential as a therapeutic target by small molecule
tyrosine kinase inhibitor. Curr Cancer Drug Targets. 13:686–697.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsu PY, Liu HS, Cheng HL, Tzai TS, Guo HR,
Ho CL and Chow NH: Collaboration of RON and epidermal growth factor
receptor in human bladder carcinogenesis. J Urol. 176:2262–2267.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guin S, Yao HP and Wang MH: RON receptor
tyrosine kinase as a target for delivery of chemodrugs by antibody
directed pathway for cancer cell cytotoxicity. Mol Pharm.
7:386–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guin S, Ma Q, Padhye S, Zhou YQ, Yao HP
and Wang MH: Targeting acute hypoxic cancer cells by
doxorubicin-immunoliposomes directed by monoclonal antibodies
specific to RON receptor tyrosine kinase. Cancer Chemother
Pharmacol. 67:1073–1083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Z, Yao H, Guin S, Padhye SS, Zhou YQ
and Wang MH: Monoclonal antibody (mAb)-induced down-regulation of
RON receptor tyrosine kinase diminishes tumorigenic activities of
colon cancer cells. Int J Oncol. 37:473–482. 2010.PubMed/NCBI
|
|
27
|
Padhye SS, Guin S, Yao HP, Zhou YQ, Zhang
R and Wang MH: Sustained expression of the RON receptor tyrosine
kinase by pancreatic cancer stem cells as a potential targeting
moiety for antibody-directed chemotherapeutics. Mol Pharm.
8:2310–2319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Follenzi A, Bakovic S, Gual P, Stella MC,
Longati P and Comoglio PM: Cross-talk between the proto-oncogenes
Met and Ron. Oncogene. 19:3041–3049. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peace BE, Hill KJ, Degen SJ and Waltz SE:
Cross-talk between the receptor tyrosine kinases Ron and epidermal
growth factor receptor. Exp Cell Res. 289:317–325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Clark PE, Agarwal N, Biagioli MC,
Eisenberger MA, Greenberg RE, Herr HW, Inman BA, Kuban DA, Kuzel
TM, Lele SM, et al: National Comprehensive Cancer Network (NCCN):
Bladder cancer. J Natl Compr Canc Netw. 11:446–475. 2013.PubMed/NCBI
|
|
31
|
Babjuk M, Burger M, Zigeuner R, Shariat
SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A,
Redorta J Palou, et al: European Association of Urology: EAU
guidelines on non-muscle-invasive urothelial carcinoma of the
bladder: Update 2013. Eur Urol. 64:639–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Logan-Collins J, Thomas RM, Yu P, Jaquish
D, Mose E, French R, Stuart W, McClaine R, Aronow B, Hoffman RM, et
al: Silencing of RON receptor signaling promotes apoptosis and
gemcitabine sensitivity in pancreatic cancers. Cancer Res.
70:1130–1140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yao HP, Luo YL, Feng L, Cheng LF, Lu Y, Li
W and Wang MH: Agonistic monoclonal antibodies potentiate
tumorigenic and invasive activities of splicing variant of the RON
receptor tyrosine kinase. Cancer Biol Ther. 5:1179–1186. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin X, Zhu Z and Shi Y: Metastasis
mechanism and gene/protein expression in gastric cancer with
distant organs metastasis. Bull Cancer. 101:E1–E12. 2014.PubMed/NCBI
|
|
35
|
Wagh PK, Peace BE and Waltz SE:
Met-related receptor tyrosine kinase Ron in tumor growth and
metastasis. Adv Cancer Res. 100:1–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Camp ER, Liu W, Fan F, Yang A, Somcio R
and Ellis LM: RON, a tyrosine kinase receptor involved in tumor
progression and metastasis. Ann Surg Oncol. 12:273–281. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Feng L, Yao HP, Wang W, Zhou YQ, Zhou J,
Zhang R and Wang MH: Efficacy of anti-RON antibody Zt/g4-drug
maytansinoid conjugation (Anti-RON ADC) as a novel therapeutics for
targeted colorectal cancer therapy. Clin Cancer Res. 20:6045–6058.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morgan DO: Cyclin-dependent kinases:
Engines, clocks, and microprocessors. Annu Rev Cell Dev Biol.
13:261–291. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Murray AW and Marks D: Can sequencing shed
light on cell cycling? Nature. 409:844–846. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Connell-Crowley L, Elledge SJ and Harper
JW: G1 cyclin-dependent kinases are sufficient to initiate DNA
synthesis in quiescent human fibroblasts. Curr Biol. 8:65–68. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chung CY, Park YL, Song YA, Myung E, Kim
KY, Lee GH, Ki HS, Park KJ, Cho SB, Lee WS, et al: Knockdown of RON
inhibits AP-1 activity and induces apoptosis and cell cycle arrest
through the modulation of Akt/FoxO signaling in human colorectal
cancer cells. Dig Dis Sci. 57:371–380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Song YA, Park YL, Kim KY, Myung E, Chung
CY, Cho SB, Lee WS, Jung YD, Kweon SS and Joo YE: RON is associated
with tumor progression via the inhibition of apoptosis and cell
cycle arrest in human gastric cancer. Pathol Int. 62:127–136. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nakashima T, Miura M and Hara M:
Tetrocarcin A inhibits mitochondrial functions of Bcl-2 and
suppresses its anti-apoptotic activity. Cancer Res. 60:1229–1235.
2000.PubMed/NCBI
|
|
46
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|