Introduction
Colorectal cancer is the third most commonly
occurring cancer worldwide and accounts for the third highest
cancer-related mortality rate in the US (1,2).
Colorectal cancer development may take decades by transformation of
normal epithelial cells to invasive tumor cells and involves many
genetic alterations and is influenced by many environmental
factors. Dr Vogelstein illustrated decades ago a model of the
adenoma-carcinoma sequence with gene alterations (3–5).
Clinically, ~65% of colorectal cancer patients survive for an
average of 5 years (1,2). However, when diagnosed with early
stages and localized disease, the 5-year survival rate can reach
90.3%, while the survival rate may be reduced to only 12.5% when
patients are diagnosed with advanced stages with tumor distant
metastasis (6,7). The most common metastatic sites
include the liver and peritonea and ~20% of patients have liver
metastases at the first diagnosis of colorectal (8–10).
Moreover, tumor metastasis accounts for the majority of
cancer-related deaths, and only 40% of patients are implicated to
receive curable surgery. Thus, it is urgent to search for and
identify novel biomarkers for the early detection of colorectal
cancer, particularly for detection of tumor metastasis and
progression.
Glia maturation factor γ (GMFG) is a 17-kDa protein
and the GMF family of proteins includes GMFB and GMFG (11–14).
GMFG levels are detectable in sera of healthy individuals without
obvious difference between women and men and are enriched in
various human organs, such as the thymus, spleen and colon
(15). Structurally, GMFG protein
belongs to the ADF/cofilin family and can modulate actin
cytoskeleton reorganization in microvascular endothelial, human
airway smooth muscle and ovarian cancer cells (16,17).
Moreover, GMFG can affect Toll-like receptor 4 (TLR4) signaling in
macrophages, regulate chemotaxis of neutrophil and T lymphocytes
(18,19), and affect the angiogenic sprouting
in zebra fish (20). Taken
together, GMFG could regulate cell mobility and angiogenesis and
therefore, we speculated that GMFG could play a role in cancer
metastasis. Indeed, Zuo et al (14) showed that GMFG expression was able
to influence migration and invasion of epithelial ovarian cancer.
In addition, alteration and reduction in epithelial cell adhesion
ability enhance epithelial-mesenchymal transition (EMT), and
contribute to tumor cell migration, invasion and metastasis.
E-cadherin (E-Cad) has the ability to regulate cell adhesion,
whereas vimentin is usually overexpressed in carcinoma cells
(21–23) and the matrix metalloproteinase
(MMPs) family is critical in the modulation of tumor cell invasion
and metastasis, particularly MMP2 and MMP9 (24), since these MMPs are enzymes that can
digest collagen I and IV and laminin in the extracellular matrix
(25–27). A previous study demonstrated that
aberrant MMP2 gene-phenotype was associated with colorectal cancer
prognosis (28).
In the present study, we first assessed GMFG
expression in colorectal cancer cells and tissue specimens, and
then explored the role of GMFG in the regulation of colorectal
cancer cell migration and invasion. We aimed to provide insightful
information in order to emphasize that detection of GMFG expression
is a valuable biomarker with which to diagnose colorectal cancer
metastasis and predict tumor progression.
Materials and methods
Cancer cell lines and colorectal
cancer tissues
Different human cancer cell lines LoVo, HCT-116,
LS174T, SW-620, SW-480, U87, U251, KBV, A549, MDA-231, HeLa, CaSki,
SiHa, and MG-63 were obtained from the Research Center of Clinical
Medicine, Nanfang Hospital (Guangzhou, China) and were maintained
in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum (FBS) (both from HyClone, Logan, UT, USA) in a
humidified incubator with 5% CO2 at 37°C.
Colorectal cancer tissue specimens were obtained
from 68 patients who received surgery for tumor resection in
Nanfang Hospital between December 2011 and January 2013. These
patients did not receive any treatments before surgery and all
cases were histologically diagnosed and retrospectively reviewed by
two pathologists in the present study. The present study was
approved by the Human Ethics Committee of Nanfang Hospital. In the
present study, we collected paired normal and colorectal cancer
tissue specimens and clinicopathological data from each patient.
Tissue samples were stored in liquid nitrogen until use according
to our previous study (29). Among
these patients, 24 patients had rectal cancer and 44 had colon
cancer, while 40 were at early stages of disease without tumor
metastasis while 28 had different degrees of tumor metastasis
according to the standard of the National Comprehensive Cancer
Network (NCCN). The clinicopathological characteristics of the
patients are presented in Table
I.
 | Table I.GMFG expression and its association
with the clinicopathological data from the colorectal cancer
patients. |
Table I.
GMFG expression and its association
with the clinicopathological data from the colorectal cancer
patients.
|
|
| Level of GMFG
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinical
parameters | Cases | − | + | ++ | +++ | Positive rate
(%) | P-value |
|---|
| Age (years) |
|
|
|
|
|
| 0.270 |
|
≥60 | 39 | 10 | 7 | 16 | 6 | 74.4 |
|
|
<60 | 29 | 11 | 5 | 8 | 5 | 62.1 |
|
| Gender |
|
|
|
|
|
| 0.850 |
|
Male | 41 | 13 | 6 | 15 | 7 | 68.3 |
|
|
Female | 27 | 8 | 6 | 9 | 4 | 70.4 |
|
| Lymph node
metastasis |
|
|
|
|
|
| 0.013a |
|
Yes | 28 | 4 | 7 | 12 | 5 | 85.7 |
|
| No | 40 | 17 | 5 | 12 | 6 | 57.5 |
|
| Tumor
differentiation |
|
|
|
|
|
| 0.480 |
|
Well | 20 | 8 | 4 | 5 | 3 | 60.0 |
|
|
Moderate | 42 | 12 | 7 | 17 | 6 | 71.4 |
|
|
Poor | 6 | 1 | 1 | 2 | 2 | 83.3 |
|
| Total no. |
|
|
|
|
|
| 0.046a |
|
Normal | 68 | 40 | 13 | 8 | 7 | 41.2 |
|
|
Cancer | 68 | 21 | 12 | 24 | 11 | 69.1 |
|
Protein extraction and western
blotting
Different cancer cell lines were grown and then
collected from cell culture plates after being washed with
phosphate-buffered saline (PBS) 3 times and lysed in a lysis buffer
containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% sodium
deoxycholate, 0.1% sodium dodecyl sulfate and phosphorylase and
metalloproteinase inhibitor (Beyotime, Inc., Beijing, China).
Colorectal tumor tissues were grinded on ice and then lysed in the
same lysis buffer. Both types of cell lysis were then centrifuged
and the concentration of protein samples was measured using the BCA
protein assay kit (Beyotime). The protein samples were then treated
with 5X loading buffer and boiled at 100°C for 5 min, separated
using sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) on 12% SDS-PAGE gels and transferred onto polyvinylidene
fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The
membranes were then blocked in 5% skim milk solution in PBS and
then incubated with different primary anti-human antibodies at 4°C
overnight and the secondary antibody and enhanced chemiluminescence
(ECL) kit according to a standard protocol. The primary antibodies
were anti-GAPDH (1:1,000), anti-GMFG (1:1,000) (both from
ProteinTech Group, Wuhan, China), anti-MMP2 (1:1,000), anti-E-Cad
(1:1,000) and anti-vimentin (1:1,000) (all from Abcam, Cambridge,
MA, USA).
Immunohistochemistry
Paraffin-embedded tissue specimens were sectioned
into 4-µm thick sections and put onto 3-aminopropyltriethoxysilane
(APES)-coated glass slides. For immunohistochemistry, the sections
were deparaffinized in xylene and rehydrated in a series of ethanol
solution and submerged in tap-water. The antigen retrieval was then
performed using an antigen unmasking solution, which was preheated
in a microwave for 5 min and the sections in the buffer were heated
at highest energy levels for 8 min in a microwave and cooled down
to room temperature. Potential endogenous peroxidase activity was
blocked in 3% hydrogen peroxide for 20 min and the sections were
incubated in 5% casein in PBS for 1 h to block non-specific
antibody binding. The sections were then incubated at 4°C overnight
with a rabbit anti-GMFG antibody (ProteinTech) at a dilution of
1:100 and subjected to a post primary block using the polymer
penetration enhancer (Boshide, Wuhan, China) for 30 min. Sections
were then washed with PBS 5 min each for 3 times and further
incubated at room temperature for 30 min with an anti-rabbit
IgG-Poly-HRP (Merck, Kenilworth, NJ, USA). The sections were
visualized with 3,3′-diaminobenzidine (DAB) solution (Boshide) and
briefly counterstained with hematoxylin solution.
The immunostained sections were reviewed and scored
by two investigators under a light microscope for intensity and
percentage of staining. Protein localization was evaluated and
classified as nuclear, cytoplasmic and plasmalemma locations. The
percentage of positively stained tumor cells was graded as 0–10%
(1+), 11–50% (2++) and >50% stained cells (3+++), while the
staining intensity was graded as 1 (weakly positive), 2 (positive)
and 3 (strongly positive). Subsequently, an immunostaining index
was calculated by multiplying the staining intensity with the
percentage of positive cells to reach negative (0), weak (1+),
medium (2++) and strong (3+++) expression of GMFG (Table I).
Quantitative reverse
transcription-polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) and reversely transcribed
into cDNA using 5X PrimeScript RT Master Mix (Takara, Dalian,
China) according to the manufacturers protocols. After that, these
cDNA samples were subjected to qPCR amplification using 2X SYBR
Premix Ex Taq (Takara) in the 7500 ABI Real-Time PCR System
(Applied Biosystems, Foster City, CA, USA). The amplification
conditions were 95°C for 30 sec, 95°C for 5 sec, and 60°C for 34
sec for 40 cycles and the relative level of mRNA expression was
calculated using the 2−ΔΔCt method after normalization
to the level of GAPDH mRNA. The primers used were: GMFG, 5′-CGC GGG
AAG TAA AAA CAGG C-3′ and 5′-GGT CTC GTT GAG GTC GTC TG-3′; and
GAPDH, 5′-AGA AGG TGG GGC TCA TTT G-3 and 5-AGG GGC CAT CCA CAGT
CTT C-3′.
GMFG RNA interference
To knockdown GMFG expression in colorectal cancer
cells, we used GMFG siRNA purchased from Ruibo Co. (Guangzhou,
China) and transfected GMFG siRNA or negative control (NC) siRNA
into the colorectal cells for 24 h using Hylimax (Dojindo,
Kumamoto, Japan) in Opti-MEM (Thermo Fisher Scientific, Waltham,
MA, USA) according to the manufacturer's instructions and assayed.
The NC siRNA was random siRNA duplex sequences.
Tumor cell migration and invasion
assays
For the migration assay, 5×104 cells in a
serum-free medium were seeded onto an uncoated filter with 8.0-µm
pores (Corning, Corning, NY, USA), whereas 1×105 cells
were seeded onto a filter with 8.0 mm pores precoated the
extracellular matrix (BD Biosciences, Franklin Lakes, NJ, USA) for
the invasion assays. The bottom chambers were filled with 10% FBS
and cultured for 24 h. In separated experiments, different
concentrations of recombinant GMFG (0, 5, 10, 20 and 30 µg/ml) were
added into the bottom chambers. At the end of the experiments,
cells on the surface of the filters were removed using cotton swabs
and PBS, while cells that had migrated or invaded into the low
surface of the filters were fixed and stained with crystal violet
(Beyotime). The numbers of migrated and invaded cells were counted
under a microscope for 10 of 20x microscopic fields and
averaged.
ELISA detection of GMFG in cell
culture medium
The level of GMFG in cell culture medium was
measured using the GMFG ELISA kit (Cusabio Biotech Co., Ltd.,
Wuhan, China) according to the manufacturer's protocol. GMFG levels
were expressed as mean ± SD of triplicate experiments and repeated
at least 3 times.
Statistical analysis
Statistical analyses were performed using the SPSS
13.0 statistical software (SPSS, Chicago, IL, USA). The data are
expressed as mean ± SD and were statistically analyzed using
one-way ANOVA and χ2 test. P<0.05 indicates
statistically significant results.
Results
Expression of GMFG in different cancer
cell lines and colorectal cancer tissue specimens
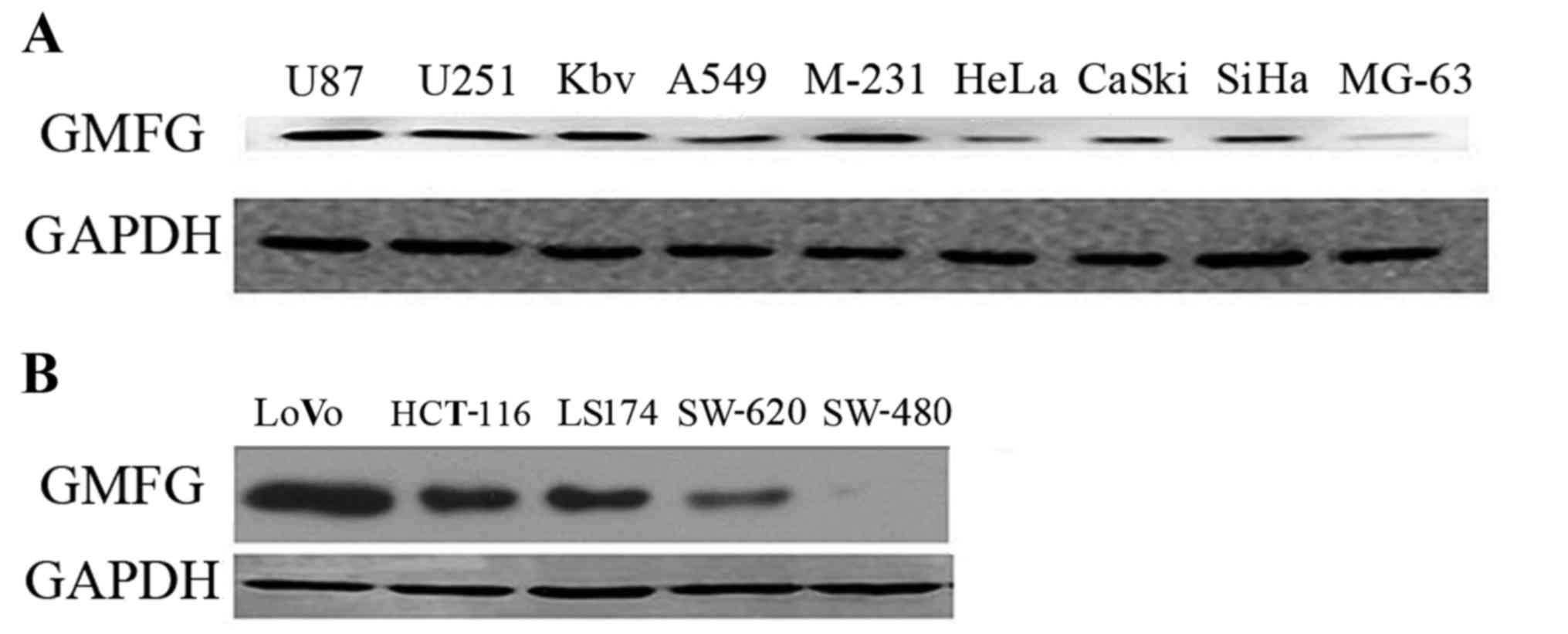
In the present study, we first detected GMFG
expression in 14 different cancer cell lines and found that GMFG
was expressed in the majority of the cell lines (Fig. 1A). GMFG protein was preferentially
expressed in U87, U251, KBV, MDA-231, SiHa and LoVo cells, while
colorectal cancer cell line LoVo expressed the highest level of
GMFG compared with the other cell lines (Fig. 1B).
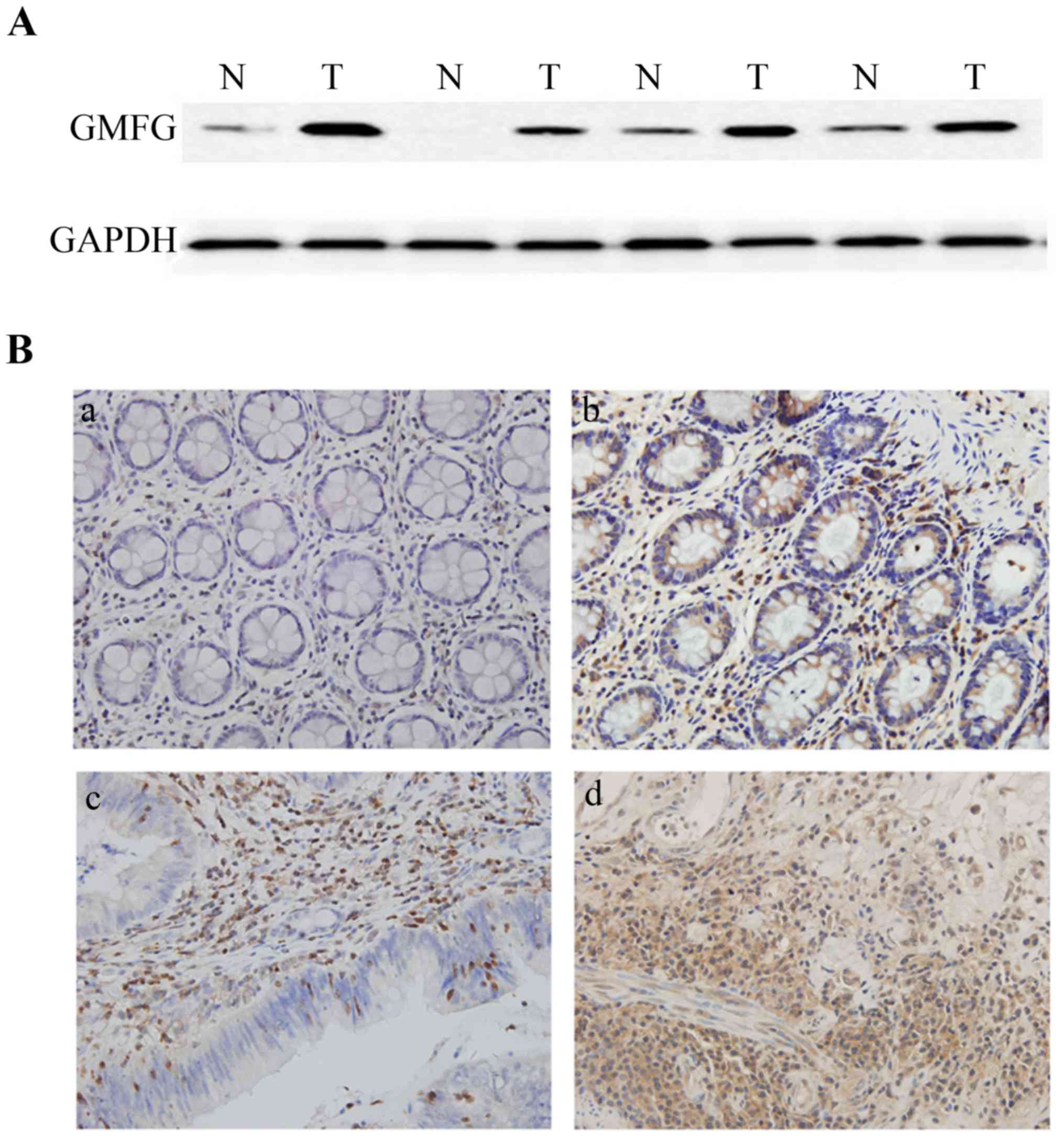
We then analyzed GMFG expression in 68 colorectal
cancer surgical samples and found that 42 samples (61.8%) expressed
a high level of GMFG protein using western blotting compared with
the adjacent non-tumor tissues, while 14 samples (20.6%) showed no
obvious difference, whereas 12 samples (17.6%) had lower levels of
GMFG compared with normal tissues (Fig.
2A).
Immunohistochemical data showed that GMFG was highly
expressed in colorectal tissues compared to normal tissues
(Fig. 2B). GMFG protein was mainly
localized in mesenchymal cells of the non-tumor colorectal tissues
and in the gland cells in the tumor tissues. We then associated
GMFG expression with clinicopathological parameters from these
patients and found that GMFG was associated with lymph node
metastasis (85.7 vs. 57.5% in non-metastatic cases; Table I), but there was no statistically
significant difference in GMFG expression associated with patient
gender and age and tumor differentiation (Table I).
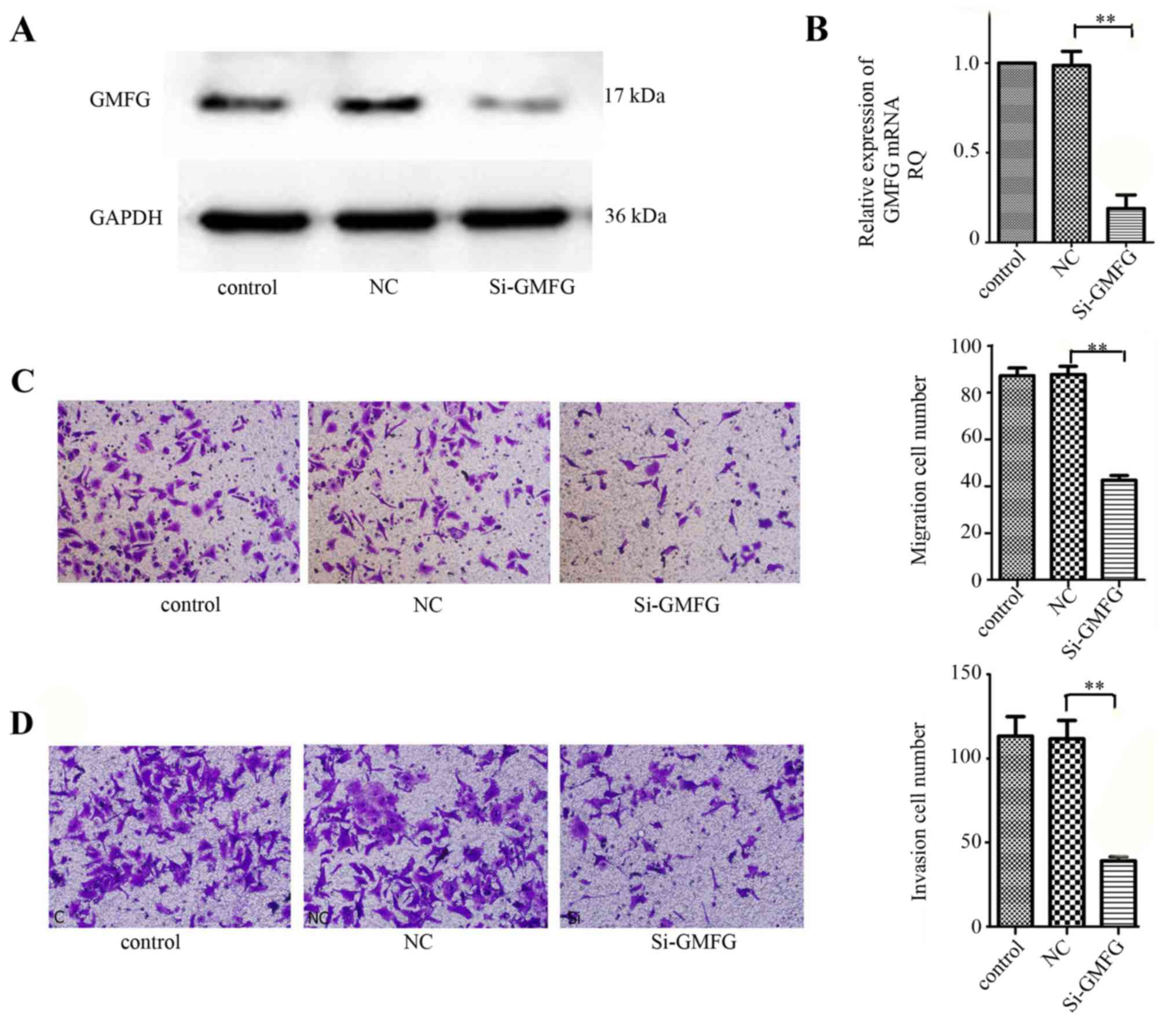
Suppression of LoVo cell migration and
invasion abilities after knockdown of GMFG expression
We then assessed the effects of GMFG knockdown in
colorectal cancer cells using GMFG siRNA. Our data showed that GMFG
siRNA significantly silenced GMFG expression in the LoVo cells
(Fig. 3A and B). We then performed
the Transwell assay to assay the altered ability of tumor cell
migration and invasion, and found that both tumor cell migration
and invasion capacities were reduced after knockdown of GMFG
expression (Fig. 3C and D).
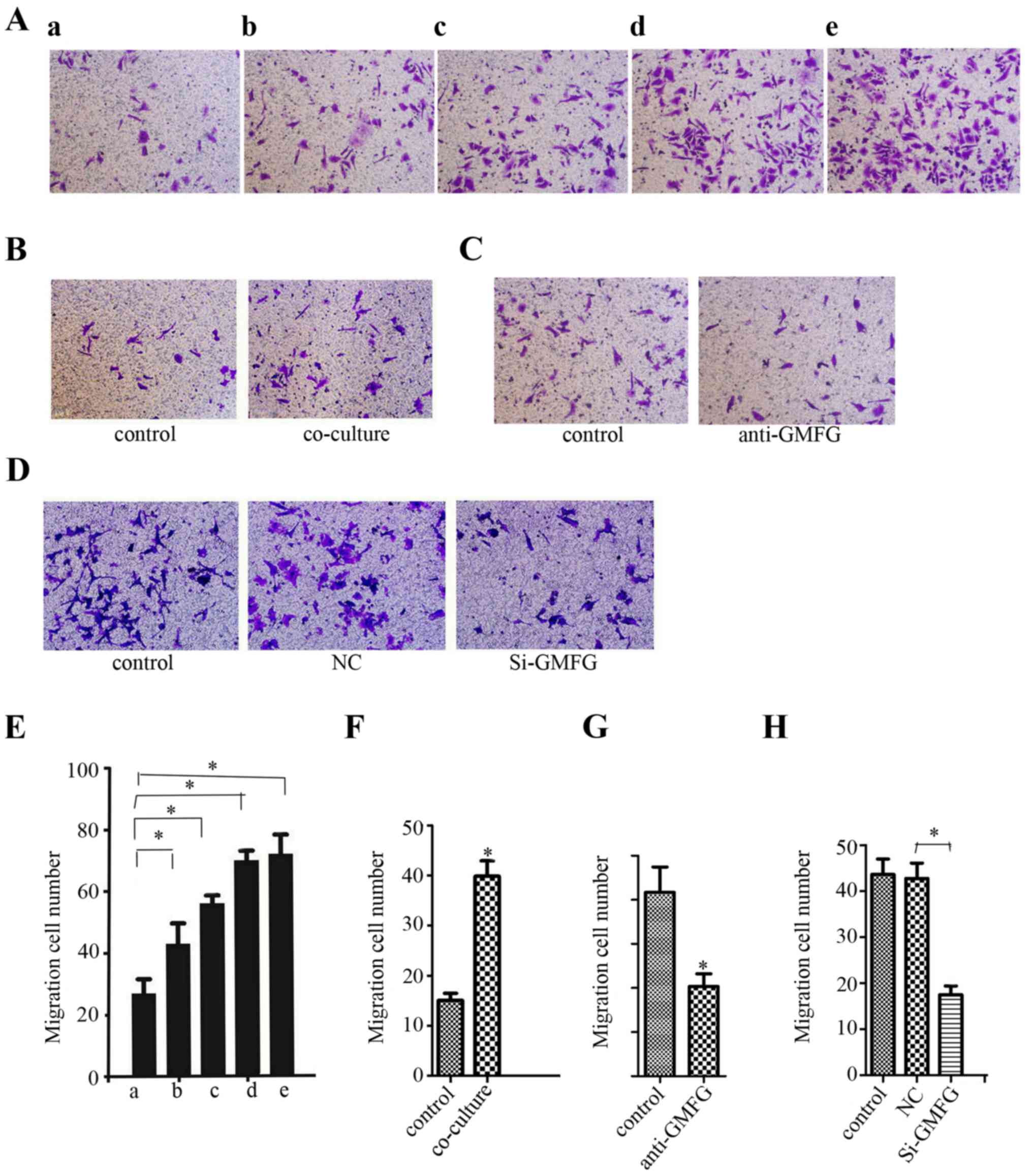
Induction of LoVo cell migration and
invasion by exogenous GMFG treatment
We further determined whether addition of exogenous
GMFG could affect LoVo cell migration. As shown in Fig. 4A, the number of LoVo cells was
increased after addition of exogenous GMFG and the maximal GMFG
concentration was 20 µg/ml, although the level of LoVo cell
migration did not obviously increase as GMFG concentration
continued to rise.
Induction of LoVo cell migration after
co-culture with HUVECs
As discussed in the Introduction section, GMFG can
promote angiogenesis. We, thus, co-cultured LoVo cells with human
umbilical vein endothelial cells (HUVECs). Our data showed that the
migration capacity of the LoVo cells was increased after being
co-cultured with the HUVECs (Fig.
4B). However, addition of the GMFG antibody into this
co-culture downregulated LoVo cell migration (Fig. 4C). Moreover, knockdown of GMFG
expression in HUVECs and then co-culture with LoVo cells showed a
decrease in LoVo cell migration compared with the NC group
(Fig. 4D). However, we failed to
detect GMFG levels in cell culture medium in our cancer cell lines
by using GMFG ELISA kit (data not shown).
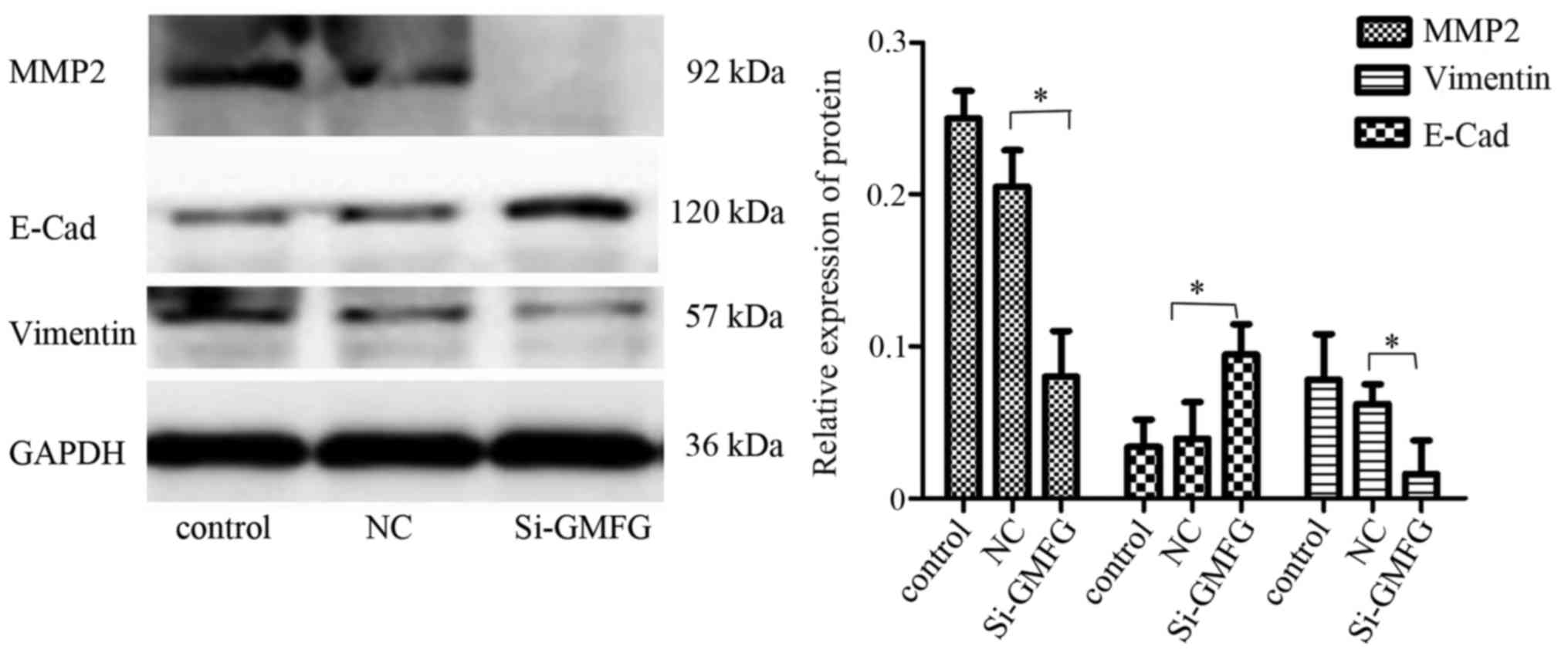
Inhibition of MMP2 expression and
reversal of EMT after knockdown of GMFG expression in LoVo
cells
Western blot analysis revealed that knockdown of
GMFG expression downregulated expression of E-Cad and MMP2, but
upregulated the vimentin level in the LoVo cells (Fig. 5), indicating that GMFG promoted
tumor cell EMT while silencing of GMFG reversed the EMT phenotype
in the LoVo cells.
Discussion
In the present study, we first assessed GMFG
expression in colorectal cancer cell lines and tissue specimens,
and then explored the role of GMFG in the regulation of colorectal
cancer cell migration and invasion in vitro. We found that
GMFG protein was expressed in 14 different common human cancer cell
lines and the highest GMFG level was noted in colorectal cancer
LoVo cell line. Furthermore, we also found that GMFG was highly
expressed in colorectal cancer tissue samples, particularly in
patients with lymph node metastasis (85.7 vs. 57.5% of
non-metastatic patients). However, knockdown of GMFG expression or
anti-GMFG antibody reduced the migration and invasion abilities of
the LoVo cells, whereas GMFG treatment induced LoVo cell migration
capacity, which is consistent with a recent study reported by Zuo
et al who demonstrated that GMFG expression promoted
migration and invasion of epithelial ovarian cancer cells (14). In addition, our current data also
showed that knockdown of GMFG expression altered expression of
tumor EMT-related proteins, such as MMP2, E-cadherin and vimentin.
Since GMFG protein functions to reorganize the actin cytoskeleton
and previous studies have shown that GMFG induced the migration of
human T lymphocytes (16,19), our current data suggest that
GMFG-induced colorectal cancer cell migration and invasion could be
through GMFG-altered tumor cell EMT, although future study is
needed to confirm this.
Furthermore, the LoVo cell line was derived from a
patient with metastatic colon cancer and has a high ability to
invade and metastasize, while the SW480 cell line was established
from a patient with primary colon adenocarcinoma. The present study
showed that expression of GMFG protein was higher in the LoVo cells
than that in the SW480 cell line. In tissue samples, we also found
a similar phenomenon, i.e., GMFG protein was higher in tissues with
metastatic colorectal cancer. These results suggest that detection
of GMFG protein could be used as a biomarker for detection of
colorectal cancer, particularly, metastatic colorectal cancer.
Previous studies have confirmed that EMT is an
important early step in the conversion of tumor cells into a
migratory population capable of systemic tumor metastasis (30–32).
At the molecular level, EMT is associated with a gain in
mesenchymal markers, including vimentin and N-cadherin but a loss
of adherent junction proteins, such as E-cadherin (33–36).
In the present study, we found that knockdown of GMFG expression
upregulated E-cadherin expression but downregulated vimentin and
MMP2 expression in LoVo cells. To the best of our knowledge, MMP2
is a key protein in the process of tumor cell migration and
invasion (37–39). Our current finding provides a more
plausible explanation for highly expressed GMFG protein in most
colorectal cancer tissues. However, it is true that we do not know
the mechanism of how GMFG mediated alteration of the expression of
these proteins. This mechanism could involve GMFG activation of
cell receptors or modulation of integrin expression or functions.
Regardless, the targeting of GMFG expression or function may be a
novel strategy by which to control colorectal cancer
progression.
In addition, the present study for the first time
confirmed that exogenous GMFG treatment promoted colorectal cancer
cell migration and that co-culture of LoVo cells with HUVECs also
promoted tumor cell migration, whereas knockdown of GMFG expression
or anti-GMFG antibody decreased colorectal cancer migration. We
then assessed whether HUVECs could secrete GMFG into the cell
culture medium to play its role in the promotion of colorectal
cancer cell migration. However, we could not detect expression of
GMFG in the culture media, although we cannot exclude the role of
secreted GMFG in the regulation of colorectal cancer migration and
invasion, which may be due to the fact that ELISA was unable to
detect the low GMFG level in the growth media. Thus, we will
continue to investigate the underlying molecular mechanism by which
GMFG modulates colorectal cancer invasion and metastasis.
In conclusion, the present study demonstrated that
GMFG expression is associated with colorectal cancer metastasis to
lymph nodes ex vivo and knockdown of GMFG expression or the
anti-GMFG antibody could reduce tumor cell migration and invasion
in vitro. Thus, GMFG should be further evaluated as a
diagnostic marker in the early detection of colorectal cancer
metastasis. The targeting of GMFG expression may suppress
colorectal cancer progression.
Glossary
Abbreviations
Abbreviations:
|
GMFG
|
glia maturation factor γ
|
|
MMP2
|
matrix metalloproteinase 2
|
|
EMT
|
epithelial-mesenchymal transition
|
|
E-Cad
|
E-cadherin
|
|
ADF
|
actin-depolymerizing factor
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shih IM, Zhou W, Goodman SN, Lengauer C,
Kinzler KW and Vogelstein B: Evidence that genetic instability
occurs at an early stage of colorectal tumorigenesis. Cancer Res.
61:818–822. 2001.PubMed/NCBI
|
|
4
|
Höglund M, Gisselsson D, Hansen GB, Säll
T, Mitelman F and Nilbert M: Dissecting karyotypic patterns in
colorectal tumors: Two distinct but overlapping pathways in the
adenoma-carcinoma transition. Cancer Res. 62:5939–5946.
2002.PubMed/NCBI
|
|
5
|
Hagland HR, Berg M, Jolma IW, Carlsen A
and Søreide K: Molecular pathways and cellular metabolism in
colorectal cancer. Dig Surg. 30:12–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Howlader N, Noone AM, Krapcho M, Garshell
J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,
et al: SEER Cancer Statistics Review, 1975–2010. National Cancer
Institute; Bethesda, MD: 2013, http://seer.cancer.gov/csr/1975_2010/
|
|
7
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: Metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Willett WC: Diet and cancer: An evolving
picture. JAMA. 293:233–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Jong AE, Morreau H, Nagengast FM,
Mathus-Vliegen EM, Kleibeuker JH, Griffioen G, Cats A and Vasen HF:
Prevalence of adenomas among young individuals at average risk for
colorectal cancer. Am J Gastroenterol. 100:139–143. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haggar FA and Boushey RP: Colorectal
cancer epidemiology: Incidence, mortality, survival, and risk
factors. Clin Colon Rectal Surg. 22:191–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mao M, Fu G, Wu JS, Zhang QH, Zhou J, Kan
LX, Huang QH, He KL, Gu BW, Han ZG, et al: Identification of genes
expressed in human CD34+ hematopoietic stem/progenitor
cells by expressed sequence tags and efficient full-length cDNA
cloning. Proc Natl Acad Sci USA. 95:8175–8180. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asai K, Fujita K, Yamamoto M, Hotta T,
Morikawa M, Kokubo M, Moriyama A and Kato T: Isolation of novel
human cDNA (hGMF-gamma) homologous to Glia Maturation Factor-beta
gene. Biochim Biophys Acta. 1396:242–244. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi Y, Chen L, Liotta LA, Wan HH and
Rodgers GP: Glia maturation factor gamma (GMFG): A
cytokine-responsive protein during hematopoietic lineage
development and its functional genomics analysis. Genomics
Proteomics Bioinformatics. 4:145–155. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zuo P, Ma Y, Huang Y, Ye F, Wang P, Wang
X, Zhou C, Lu W, Kong B and Xie X: High GMFG expression correlates
with poor prognosis and promotes cell migration and invasion in
epithelial ovarian cancer. Gynecol Oncol. 132:745–751. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inagaki M, Aoyama M, Sobue K, Yamamoto N,
Morishima T, Moriyama A, Katsuya H and Asai K: Sensitive
immunoassays for human and rat GMFB and GMFG, tissue distribution
and age-related changes. Biochim Biophys Acta. 1670:208–216. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ikeda K, Kundu RK, Ikeda S, Kobara M,
Matsubara H and Quertermous T: Glia maturation factor-gamma is
preferentially expressed in microvascular endothelial and
inflammatory cells and modulates actin cytoskeleton reorganization.
Circ Res. 99:424–433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang T, Cleary RA, Wang R and Tang DD:
Glia maturation factor-γ phosphorylation at Tyr-104 regulates actin
dynamics and contraction in human airway smooth muscle. Am J Respir
Cell Mol Biol. 51:652–659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aerbajinai W, Liu L, Chin K, Zhu J, Parent
CA and Rodgers GP: Glia maturation factor-γ mediates neutrophil
chemotaxis. J Leukoc Biol. 90:529–538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lippert DN and Wilkins JA: Glia maturation
factor gamma regulates the migration and adherence of human T
lymphocytes. BMC Immunol. 13:212012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zuo P, Fu Z, Tao T, Ye F, Chen L, Wang X,
Lu W and Xie X: The expression of glia maturation factors and the
effect of glia maturation factor-γ on angiogenic sprouting in
zebrafish. Exp Cell Res. 319:707–717. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arias AM: Epithelial mesenchymal
interactions in cancer and development. Cell. 105:425–431. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bates RC and Mercurio AM: The
epithelial-mesenchymal transition (EMT) and colorectal cancer
progression. Cancer Biol Ther. 4:365–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Turpeenniemi-Hujanen T: Gelatinases (MMP-2
and −9) and their natural inhibitors as prognostic indicators in
solid cancers. Biochimie. 87:287–297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi M, Yu B, Gao H, Mu J and Ji C: Matrix
metalloproteinase 2 overexpression and prognosis in colorectal
cancer: A meta-analysis. Mol Biol Rep. 40:617–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mook OR, Frederiks WM and Van Noorden CJ:
The role of gelatinases in colorectal cancer progression and
metastasis. Biochim Biophys Acta. 1705:69–89. 2004.PubMed/NCBI
|
|
27
|
Théret N, Lehti K, Musso O and Clément B:
MMP2 activation by collagen I and concanavalin A in cultured human
hepatic stellate cells. Hepatology. 30:462–468. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Langers AM, Sier CF, Hawinkels LJ, Kubben
FJ, van Duijn W, van der Reijden JJ, Lamers CB, Hommes DW and
Verspaget HW: MMP-2 geno-phenotype is prognostic for colorectal
cancer survival, whereas MMP-9 is not. Br J Cancer. 98:1820–1823.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Z, Wang L, Hu J, Fan R, Zhou J, Wang
L and Zhong J: RARRES3 suppressed metastasis through suppression of
MTDH to regulate epithelial-mesenchymal transition in colorectal
cancer. Am J Cancer Res. 5:1988–1999. 2015.PubMed/NCBI
|
|
30
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bravo-Cordero JJ, Hodgson L and Condeelis
J: Directed cell invasion and migration during metastasis. Curr
Opin Cell Biol. 24:277–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grünert S, Jechlinger M and Beug H:
Diverse cellular and molecular mechanisms contribute to epithelial
plasticity and metastasis. Nat Rev Mol Cell Biol. 4:657–665. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pohl M, Radacz Y, Pawlik N, Schoeneck A,
Baldus SE, Munding J, Schmiegel W, Schwarte-Waldhoff I and
Reinacher-Schick A: SMAD4 mediates mesenchymal-epithelial reversion
in SW480 colon carcinoma cells. Anticancer Res. 30:2603–2613.
2010.PubMed/NCBI
|
|
35
|
Micalizzi DS and Ford HL:
Epithelial-mesenchymal transition in development and cancer. Future
Oncol. 5:1129–1143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bae GY, Choi SJ, Lee JS, Jo J, Lee J, Kim
J and Cha HJ: Loss of E-cadherin activates EGFR-MEK/ERK signaling,
which promotes invasion via the ZEB1/MMP2 axis in non-small cell
lung cancer. Oncotarget. 4:2512–2522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu L, Xue X, Lan J, Gao Y, Xiong Z, Zhang
H, Jiang W, Song W and Zhi Q: MicroRNA-29a upregulates MMP2 in oral
squamous cell carcinoma to promote cancer invasion and
anti-apoptosis. Biomed Pharmacother. 68:13–19. 2014. View Article : Google Scholar : PubMed/NCBI
|