Introduction
The incidence of cutaneous melanoma is increasing
(1,2). Although much progress has been made in
terms of immuno- and chemotherapy, the mortality rate for patients
with melanoma remains very high, as it is a very aggressive disease
(3,4). Cutaneous melanoma is influenced by
genetic and environmental factors.
The dysregulation of the RAS/RAF/MEK/extracellular
signal regulated kinase (ERK) pathway plays a key role in the
pathogenesis of several human cancers (5–8);
mutations at upstream membrane receptors, NRAS and BRAF, as well as
genes in other pathways [e.g., phosphatidylinositol
4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA),
phosphatase and tensin homolog (PTEN) and AKT], which serve to
regulate Raf activity, promote constitutive ERK signalling,
stimulating proliferation and survival and providing essential
tumour growth and maintenance functions (9). In melanoma, both the RAS-RAF-MEK-ERK
(MAPK) and the PI3K-AKT (AKT) signalling pathways are
constitutively activated through multiple mechanisms (9). Mutations of the BRAF gene have been
proposed to contribute to the development of melanoma (10–12).
The increased activity of the MAPK pathway prevents apoptosis and
induces cell cycle progression (13). The most frequent BRAF mutation,
which accounts for <60% of melanoma tumours with BRAF
activation, is a glutamic acid for valine substitution at codon 600
in exon 15, (Val600Glu; BRAFV600E) (9).
The melanoma microenvironment is a dynamic system,
largely orchestrated, not only by malignant cells, but also from
the stroma, that includes inflammatory cells, immune cells,
fibroblasts, soluble molecules and the surrounding extracellular
matrix (ECM) (14,15). The perturbations of this network may
interfere directly or indirectly with melanocyte-keratinocyte
and/or melanocyte-ECM relationships and may impact on melanoma
diagnosis, prognosis and treatment. Melanomagenesis requires the
cooperation of several molecules playing a role in the tumour
microenvironment (16–18).
In this context, the ECM plays an active role during
melanoma progression, promoting the epigenetic transdifferentiation
of melanocytes toward an invasive melanoma cell-like phenotype
(19,20). The invasion of tumour cells is a
complex multistage process that includes proteolysis in the
pericellular and stromal compartments, and has been shown to
largely contribute to altering the tumour microenvironment,
essential in promoting melanoma invasion, the degradation of
basement membranes and remodeling of the ECM by proteolytic
enzymes, such as matrix metalloproteinases (MMPs). MMPs,
particularly MMP-2 and MMP-9 play a major role in the regulation of
cancer cell migration, ECM invasion and metastasis through
autocrine or paracrine pathways (21,22).
The expression of MMP-9 has been shown to be associated withthe
intragenic hypermethylation of the MMP-9 gene in melanoma
(23). Furthermore, its expression
is regulated by lymphocytes and monocytes activated mostly at the
transcriptional level by a variety of growth factors, cytokines and
chemokines (24–26).
Numerous studies have suggested that the
overexpression of MMP-2 and MMP-9 is associated with an aggressive
behaviour, dissemination, advanced stages and poor prognosis in
various types of cancer, including melanoma (22,26–35).
Their activation in human cancer may be associated with the release
of osteopontin (OPN), a secreted multifunctional phosphoprotein
that has been implicated as an important mediator of tumour
metastasis (36–39). Several reports have indicated that
OPN, a matricellular protein found in the tumour microenvironment
and expressed by host and cancer cells, may regulate
tumourigenesis, cancer progression and metastasis (40–42).
In detail, OPN expression has been linked to
tumourigenesis and metastasis in a wide range of cancers, including
melanoma (43–48). Previous studies have suggested a
role for increased OPN tissue expression in the malignant
transformation of melanocytes, and as an important determinant of
melanoma progression (49,50). The aggressiveness of malignant
melanoma has been associated with a high OPN expression and its
overexpression correlates with advanced tumour stages (50,51).
OPN enhances the migration and invasion of malignant tumour cells
possibly through both the inhibition of apoptosis and by regulating
the activities of MMP-2 and MMP-9, which degrade the ECM (36,38,39).
Previous experimental data have suggested that in
melanoma cell lines, OPN upregulates MMP-9 activity, modulating
multiple signalling pathways via focal adhesion kinase (FAK), ERK
and nuclear factor-κB (NF-κB) (52–56)
that regulate cytoskeletal organization, cell motility, cell
growth, and ultimately control cell migration, ECM invasion and
tumour growth (36).
In this context, it has been indicated that NF-κB
may be a key player in OPN and MMP-9 activation. However, whether
NF-κB plays a role in the development and progression melanoma of
in association with the OPN/MMP-9 axis according to the
BRAFV600E mutation status has not been investigated in
detail to date. Thus, in the present study, OPN/MMP-9 pathway
activation was examined in patients with melanoma, particularly
those harbouring BRAFV600E mutations. Furthermore,
OPN/MMP-9 inhibition was observed in peripheral blood mononuclear
cells (PBMCs) isolated from patients with melanoma following
treatment with NF-κB inhibitor.
Materials and methods
Plasma samples from 148 patients with melanoma at
different stages of the disease were collected for use in the
present study. Samples were obtained prior to surgery, and were
collected from the Unit of Dermatology, University of Messina
(Messina, Italy) and stored until analyses at the Department of
Biomedical and Biotechnological Sciences, University of Catania
(Catania, Italy). Tumour samples from all patients were grouped
according to the mutation status of BRAFV600E. The
number of patients with melanoma harbouring the
BRAFV600E mutation was 86, and the remaining 62 patients
were BRAFWT. PBMCs were obtained from 29 patients with
melanoma harbouring the BRAFV600E mutation. As the
control group, PBMCs and plasma samples from 53 healthy subjects
were obtained and stored. Another set of plasma samples from 18 out
of the 148 patients with melanoma was obtained at 3 months
following surgery. The scientific Ethics Committee of the
Policlinic University of Messina (Messina, Italy) approved all the
procedures. All participants provided written consent prior to
blood collection. Blood was centrifuged (300 × g for 10 min at 4°C)
and the separated plasma was placed in aliquots and stored at −80°C
until analysis.
Plasma assays of OPN and MMP-9
The OPN and MMP-9 plasma concentrations were
measured using ELISA kits (R&D Systems Europe, Ltd., Abingdon,
UK). The MMP-9 assays recognised both pro- and active forms. Plasma
samples were diluted and the immunoassay was performed according to
the instructions of the manufacturer. All assays were carried out
in triplicate. The minimum detectable dose of OPN and MMP-9 was
<0.024 and 0,156 ng/ml, respectively. The optical density was
measured at 450 nm using a microplate reader (Thermo Labsystems,
Santa Rosa, CA, USA). The activities of MMP-9 were measured using
specific Biotrak MMP-9 activity assay kits [Amersham Pharmacia
Biotech (UK) Ltd., Little Chalfont, UK] according to the
manufacturer's instructions. The appropriate standards were
included in each assay. In order to measure the total content of
the MMPs, the activation of the pro-form of the MMPs was performed
using p-aminophenylmercuric acetate (APMA; Sigma-Aldrich Co. LLC.
St. Louis, MO, USA).
Chemicals
Dehydroxymethylepoxyquinomycin (DHMEQ), a new NF-κB
inhibitor, kindly provided by Professor Kazuo Umezawa (Keio
University, Kanagawa, Japan), was synthesised as previously
described (57). All other
chemicals were purchased from Sigma-Aldrich Co. (Milan, Italy).
Isolation of PBMCs and analysis of
NF-κB p65 activity and MMP-9 production
PBMCs were obtained using a Ficoll gradient.
Following isolation, PBMC pellets were collected and stored
immediately at −80°C until analysis. PBMCs (1×106
cells/ml), from patients with melanoma and healthy controls, were
cultured in RPMI-1640 medium supplemented with 1% fetal calf serum
(FCS), 2 mM L-glutamine, 100 IU/ml penicillin and 100 µg/ml
streptomycin (Gibco Life Technologies, Carlsbad, CA, USA). MMP-9
and NF-κB p65 activity was analysed in the supernatants and nuclear
extract of PBMCs isolated from 29 patients with melanoma harbouring
the BRAFV600E mutation and 53 control patients. The
PBMCs (following overnight incubation in serum-free medium) were
stimulated with or without 100 ng/ml of recombinant human OPN
(R&D Systems, Inc., Minneapolis, MN, USA) for 24 h (each
experimental condition was carried out in triplicate), with or
without pre-incubation (1 h) with DHMEQ (10 µg/ml). The
concentration of MMP-9 was determined using the human MMP-9 ELISA
kit (R&D Systems, Inc.) following the short-term culture of the
isolated PBMCs.
For the measurement of NF-κB activation, nuclear
fractions were prepared from the PBMCs (5–7×106 cells
per extraction) during batch processing using a Nuclear Extract kit
(Active Motif, Rixensart, Belgium). The binding of NF-κB to its
consensus DNA sequence was measured by ELISA using a Trans-AM™
NF-κB p65 Transcription Factor Assay kit (Active Motif), according
to the instructions of the manufacturer. Briefly, nuclear extract
protein (5 µg/well) was incubated in 96-well plates coated with
immobilised oligonucleotide (5′-AGTTGAGGGGACTTTCCCAGGC-3′)
containing a consensus (5′-GGGACTTTCC-3′) binding site for the p65
subunit of NF-κB. NF-κB binding to the target oligonucleotide was
detected by incubation with primary antibody specific for the
activated form of p65 (Active Motif; Cat. no. 40096), visualised by
anti-IgG horseradish peroxidase-conjugated secondary antibody
(Active Motif; Cat. no. 15015). At the end of the incubation
period, the developing and stop solution were added, and an optical
density of 450 nm (OD 450) was read on a Wallac Victor 1420
multilabel counter (PerkinElmer, Inc., Shelton, CT, USA).
Statistical analysis
The statistical methods have been previously
reported in detail in the study by Polesel et al (57). Briefly, the values of plasma OPN,
MMP-9 and MMP-9 activity were described as the median, minimum and
maximum values. Differences in the distribution of OPN, MMP-9 and
MMP-9 activity between the healthy controls and the melanoma cases
were examined using a non-parametric Wilcoxon test or
non-parametric χ2 tests. Correlations between markers
were evaluated by means of Pearson's correlation scores. Odds
ratios (ORs) and the corresponding 95% confidence intervals (CIs)
were calculated using the multiple logistic regression models
adjusted for gender and age. A two-tailed value of P<0.05 was
considered to indicate a statistically significant difference.
One-way ANOVA was also used to compare the OPN, MMP-9 and MMP-9
levels in the patients with melanoma who were BRAFWT and
BRAFMUT according to the clinicopathological
characteristics.
Results
The socio-demographic characteristics of the cases
and the controls, and the distribution of melanoma cases according
to clinical characteristics at diagnosis, are presented in Table I. The majority of cases were nodular
melanoma (54%), without ulcerations (55.4%), and with negative
sentinel lymph nodes (62.8%). Breslow thickness was >4 mm for 33
cases (22.3%) and the invasion of the reticular dermis or
subcutaneous fat had occurred in 17.6% of cases.
 | Table I.Distribution of 148 melanoma cases 53
healthy controls according to sociodemografic variables and main
clinical characteristics. |
Table I.
Distribution of 148 melanoma cases 53
healthy controls according to sociodemografic variables and main
clinical characteristics.
|
| Melanoma cases | Controls |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | No. (%) | No. (%) | χ2 |
|---|
| Gender |
|
|
|
|
Male | 90 (60.8) | 25 (47.2) |
|
|
Female | 58 (39.2) | 28 (52.8) | P=0.09 |
| Age, years |
|
|
|
|
<50 | 32 (21.6) | 27 (50.9) |
|
|
50–59 | 52 (35.1) | 18 (34.0) |
|
|
≥60 | 64 (43.2) | 8
(15.1) | P<0.01 |
| Hystological
subtype |
|
|
|
|
Suderficially spreading | 68 (46.0) |
|
|
|
Nodular | 80 (54.0) |
|
|
| Breslow thickness,
mm |
|
|
|
|
≤2.0 | 73 (49.3) |
|
|
|
2.1–4.0 | 42 (28.4) |
|
|
|
>4.0 | 33 (22.3) |
|
|
| Clark's level |
|
|
|
| 2.
Invasion of basal layer epidermis | 64 (43.2) |
|
|
| 3.
Invasion of papillary dermis | 58 (39.2) |
|
|
| 4.
Invasion of reticular dermis | 14 (9.5) |
|
|
| 5.
Invasion of subcutaneous fat | 12 (8.1) |
|
|
| Ulcerated
lesion |
|
|
|
| No | 82 (55.4) |
|
|
|
Yes | 61 (41.2) |
|
|
| Not
evaluated | 5 (3.4) |
|
|
| Sentinel
lymphonode |
|
|
|
|
Negative | 93 (62.8) |
|
|
|
Positive | 55 (37.2) |
|
|
| BRAF |
|
|
|
|
Wild-type | 62 (41.9) |
|
|
|
Mutated | 86 (58.1) |
|
|
Plasma levels of OPN and MMP-9
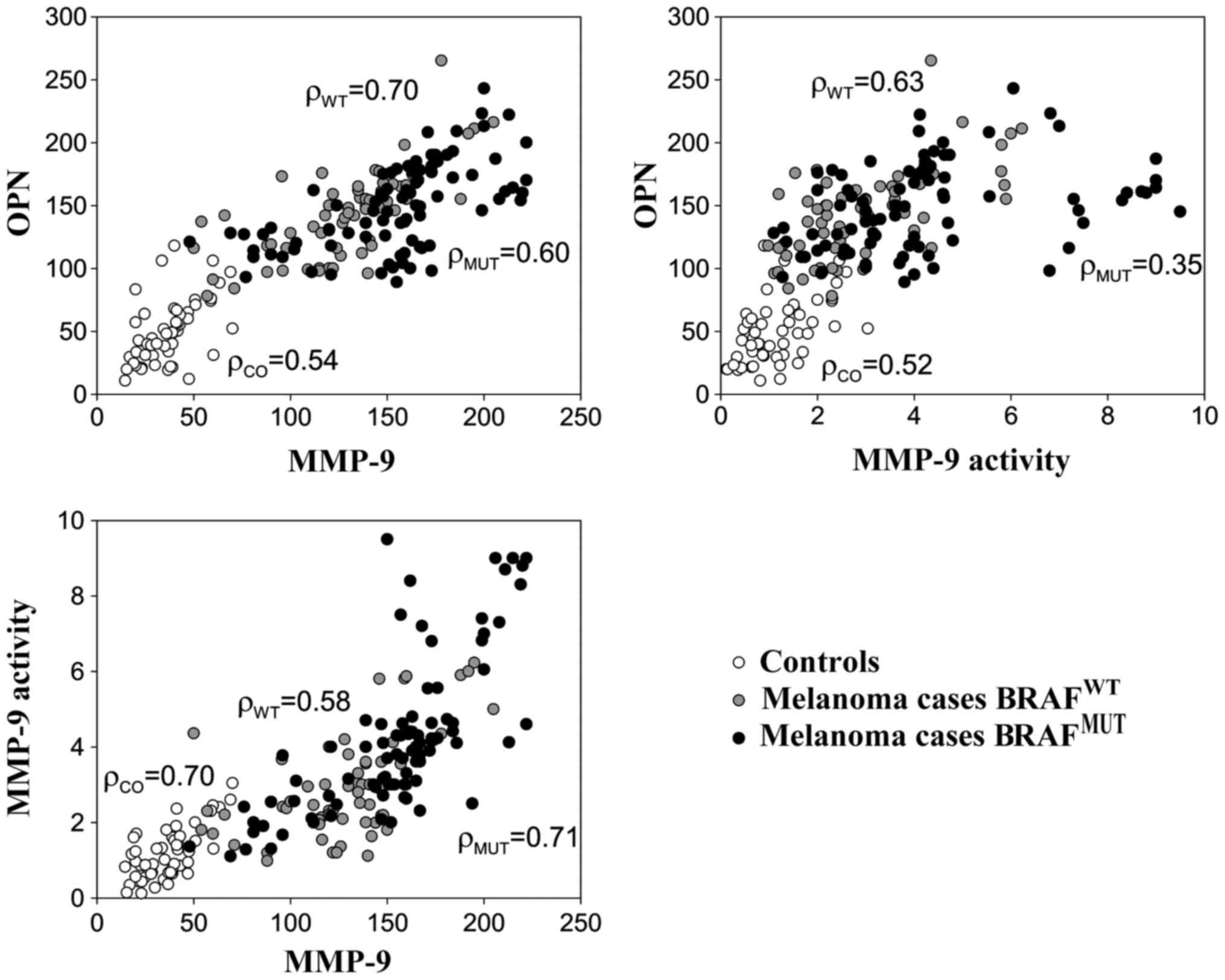
The plasma levels of OPN, MMP-9 and MMP-9 activity
are shown in Fig. 1. For all
considered markers, the levels were considerably higher among the
melanoma cases than the healthy controls, particularly for those
who had a positive sentinel lymph node and nodular histotype
(Fig. 1). Table II shows the mean concentrations of
these proteins according to the BRAFV600E status. Higher
levels of MMP-9 and MMP-9 activity were detected among the melanoma
samples harbouring the BRAFV600E mutation compared to
those which were BRAFWT; however, no significant
differences were observed in the OPN levels between these two
groups of patients (Table II). As
expected, higher MMP-9 levels were also associated with other
negative prognostic factors, such as sentinel lymph node
involvement, Breslow thickness, Clark's level and ulceration
(Table II). Linear correlations
between OPN, MMP-9 and MMP-9 activity were high (r>0.50) among
both melanoma cases and healthy subjects (Fig. 2).
 | Table II.Mean concentration of OPN and MMP-9,
and MMP-9a anctivity
in 148 cases of menaloma according to clinopathological
characteristics. |
Table II.
Mean concentration of OPN and MMP-9,
and MMP-9a anctivity
in 148 cases of menaloma according to clinopathological
characteristics.
|
| OPN | MMP-9 | MMP-9 activity |
|---|
|
|
|
|
|
|---|
|
|
BRAFWT |
BRAFMUT |
BRAFWT |
BRAFMUT |
BRAFWT |
BRAFMUT |
|---|
| Overall | 142.7 | 148.5 | 128.0 | 154.1d | 2.93 | 4.21d |
| Hystological
type |
|
|
|
|
|
|
Superficial spreading | 134.1 | 131.4 | 121.0 | 133.3 | 2.39 | 3.33c |
|
Nodular | 150.7 | 162.1 | 134.5 | 170.6d | 3.44 | 4.91c |
| Sentinel lymph
node |
|
|
|
|
|
|
Negative | 131.8 | 132.8 | 118.3 | 133.1b | 2.56 | 3.12c |
|
Positive | 171.5 | 168.4 | 153.6 | 180.6c | 3.90 | 5.59b |
| Breslow
thickness |
|
|
|
|
|
| ≤2
mm | 124.9 | 116.8 | 114.6 | 119.8 | 2.42 | 2.81 |
| >2
mm | 177.5 | 167.3b | 154.1 | 174.4d | 3.93 | 5.05b |
| Clark's level |
|
|
|
|
|
|
2–3 | 137.2 | 135.6 | 124.1 | 142.1c | 2.77 | 3.69c |
|
4–5 | 221.5 | 186.2b | 183.5 | 189.0 | 5.30 | 5.74 |
| Ulceration |
|
|
|
|
|
| No | 129.5 | 135.4 | 116.4 | 136.8b | 2.60 | 3.74c |
|
Yes | 161.9 | 163.3 | 144.8 | 174.8d | 3.40 | 4.87c |
The OPN and MMP-9 plasma levels, and MMP-9 activity
were monitored in 18 melanoma cases who were followed-up actively
(Table III). At cancer diagnosis,
the levels of the three markers were 3- to −6-fold higher in the
patients with melanoma than in the healthy controls. However, at 3
months post-surgery, the levels of OPN and MMP-9, and MMP-9
activity were significantly decreased and were comparable to those
of the healthy controls. Of note, the melanoma cases with a
positive sentinel lymph node maintained a higher expression of OPN
than the negative ones (45.6 and 34.3, respectively) (Table III). Furthermore, in 3 patients
with melanoma with the documented progression of disease after 3
months from the first surgery, the mean values of all markers
increased (data not shown).
 | Table III.Mean values and standrd deviation
(SD) of OPN and MMP-9 levels, and MMP-9 activity in 53 controls and
18 melanoma cases at diagnosis and at 3 months post-surgery. |
Table III.
Mean values and standrd deviation
(SD) of OPN and MMP-9 levels, and MMP-9 activity in 53 controls and
18 melanoma cases at diagnosis and at 3 months post-surgery.
|
|
| Time of markes
evaluation (mean ± SD) |
|---|
|
|
|
|
|---|
| Marker | No. | At enrolment | At 3 months
post-surgery |
|---|
| OPN |
|
|
|
|
Controls | 53 | 42.3
(25.3) |
|
|
Melanoma, SL Neg | 4 | 189.0 (14.6) | 34.3 (14.5) |
|
Melanoma, SL Pos | 14 | 208.1 (25.5) | 45.6 (15.8) |
| MMP-9 |
|
|
|
|
Controls | 53 | 36.6
(14.2) |
|
|
Melanoma, SL Neg | 4 | 162.6 (31.3) | 30.0 (9.0) |
|
Melanoma, SL Pos | 14 | 186.6 (17.7) | 31.4 (10.9) |
| MMP-9 activity |
|
|
|
|
Controls | 53 | 1.18 (0.69) |
|
|
Melanoma, SL Neg | 4 | 3.52 (1.32) | 1.14 (0.48) |
|
Melanoma, SL Pos | 14 | 5.65 (1.33) | 1.40 (0.57) |
Production of OPN and MMP-9 and NF-κB
p65 activity from PBMCs
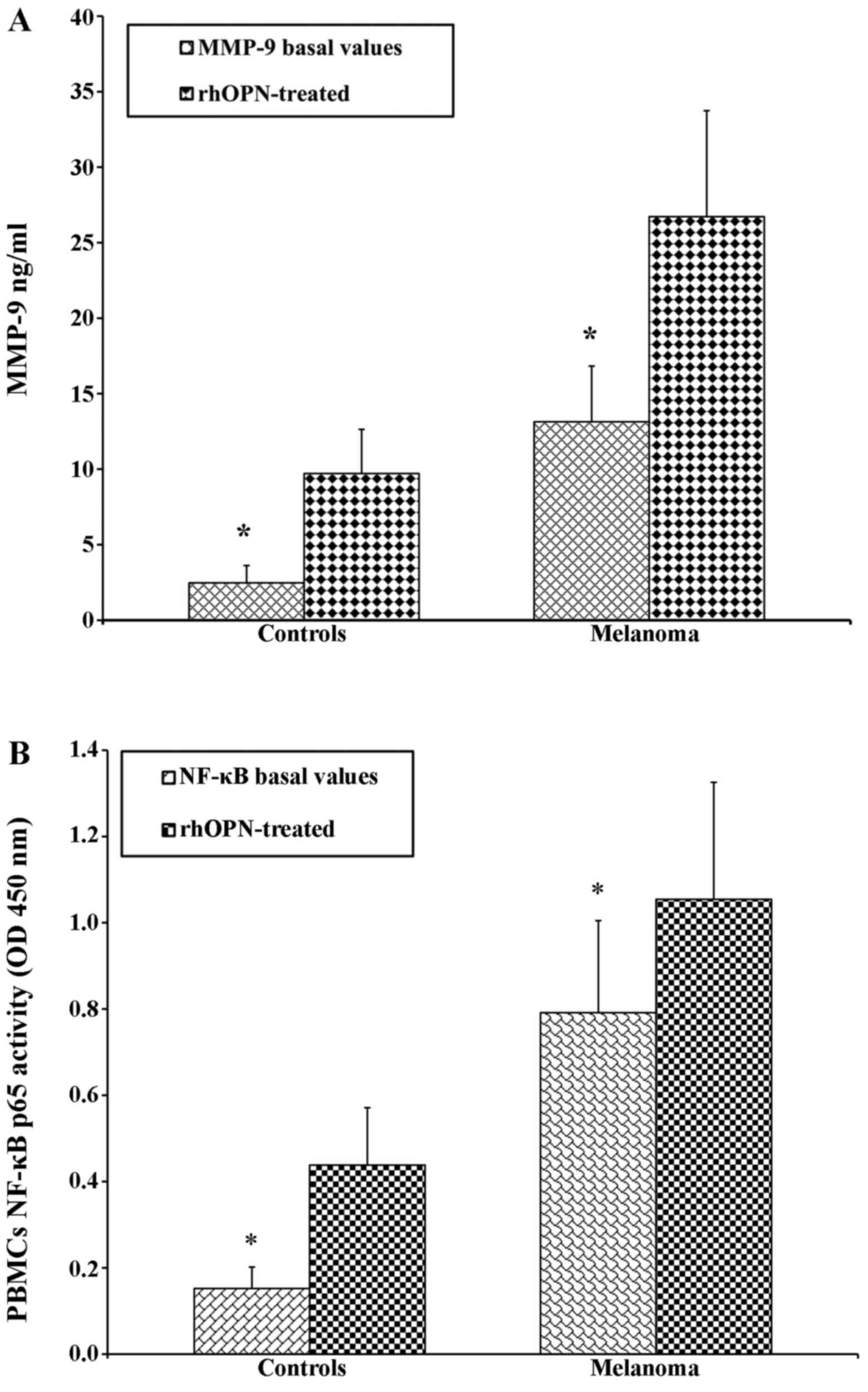
As PBMCs are the main source of the production of
both OPN and MMP-9 (39,59,60),
we then determined the production of OPN and MMP-9 in PBMCs from 53
healthy donors and 29 patients with melanoma that were cultured for
24 h with or without OPN. The secretion levels of OPN and MMP-9 in
the supernatants of the PBMCs from the patients with melanoma and
healthy donors showed the same trend to those obtained in plasma
(Table IV).
 | Table IV.OPN, MMP-9, NF-κB basal values in
PBMCs from the controls and patients with melanoma. |
Table IV.
OPN, MMP-9, NF-κB basal values in
PBMCs from the controls and patients with melanoma.
| PBMCs | Controls | Melanoma |
|---|
| OPN ng/ml | 0.93±0.48 | 8.4±3.5 |
| MMP-9 ng/ml |
2.5±1.1 | 13.1±3.7 |
| NF-κB p65
activity | 0.15±0.05 |
0.8±0.2 |
PBMCs from both the patients with melanoma and the
controls were treated with OPN in order to determine the direct
association with the increase in the MMP-9 levels in the
supernatant. As expected, following treatment with OPN, the levels
of MMP-9 were higher in the patients with melanoma than in the
controls (Fig. 3A). A similar trend
was observed by analysing the activity of NF-κB p65 (Fig. 3B). The PBMCs from the patients with
melanoma and the controls were treated with the inhibitor of NF-κB
(DHMQ) to demonstrate its effect on OPN, MMP-9 and NF-κB (Fig. 4). The results revealed that
treatment with DHMQ did not affect the release of OPN, MMP-9 and
NF-κB p65 activity in the PBMCs from healthy donors, whereas a
significant decrease in the levels each protein was observed in the
PBMCs from the patients with melanoma (Fig. 4). The PBMCs from the patients with
melanoma and the healthy controls were also treated with both OPN
and DHMQ in order to examine their effects on MMP-9 and NF-κB. In
this case, the MMP-9 release and NF-κB p65 activity were markedly
decreased in the PBMCs from both the controls and patients with
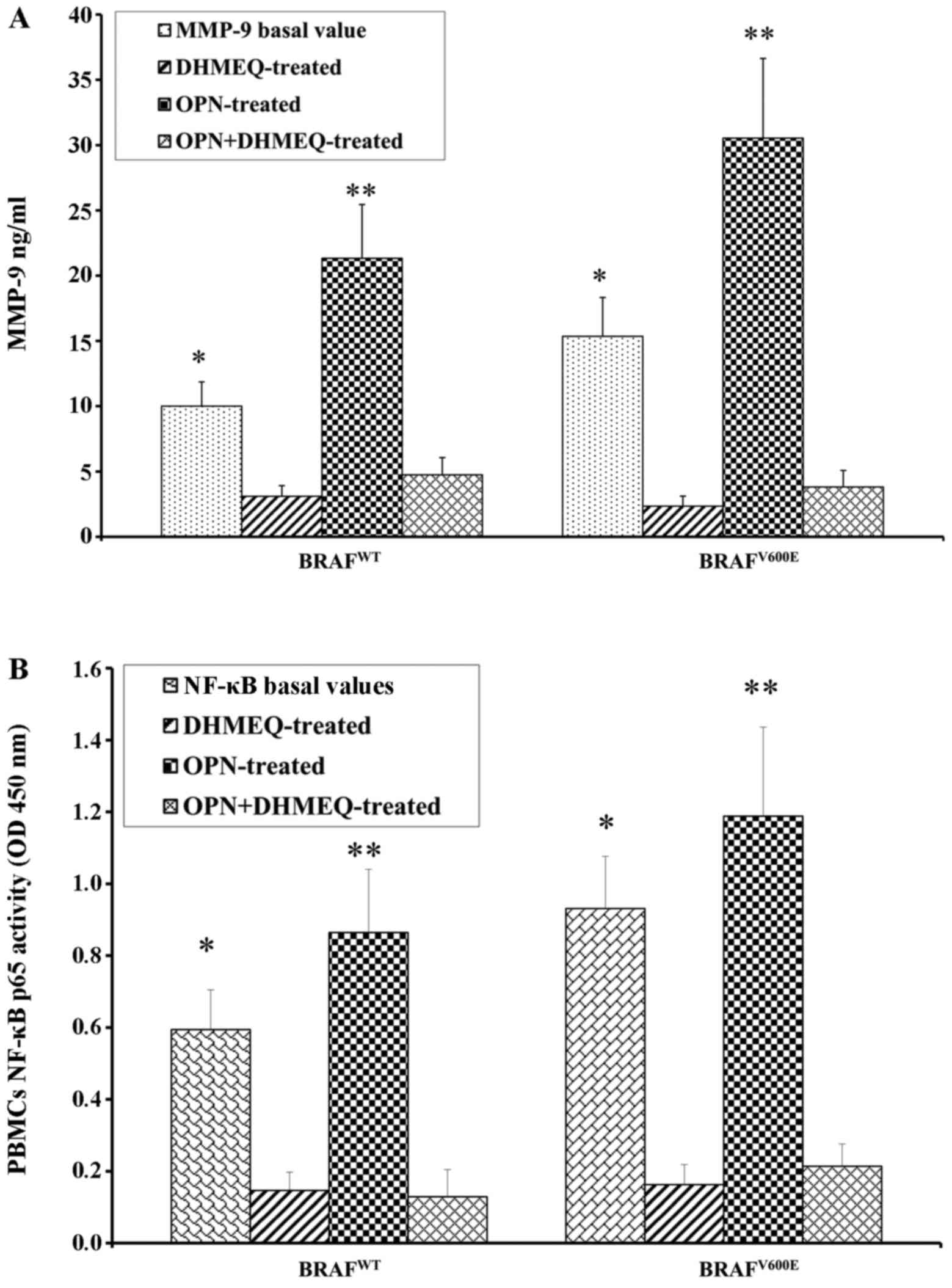
melanoma (Fig. 4). In parallel,
MMP-9 and NF-κB p65 activity were measured in PBMCs from patients
with melanoma with or without BRAFV600E mutation. The
results revealed that both NF-κB p65 activity and MMP-9 release
were higher in the PBMCs from patients with melanoma harbournig the
BRAFV600E mutation. Treatment with OPN further increased
MMP-9 release and NF-κB p65 activity in both the controls and
melanoma cases. The MMP-9 release and NF-κB p65 activity were both
decreased following treatment with DHMEQ (Fig. 5).
Discussion
Melanoma is a complex process that involves the
deregulation of interacting proteins and genes, which in turn are
responsible for the proliferation, invasion, angiogenesis of tumour
cells, and finally the evasion of host immune systems (61). A prerequisite for melanoma
progression, in addition to the ability of the cells to leave the
cellular bond and to migrate, is the degradation of components of
the ECM (62). Tumour cells and
their microenvironment mutually influence each other during tumour
development and progression (63).
Uncontrolled proliferation is a cancer hallmark, a result of the
activation various oncogenic and angiogenic molecules, and of the
crosstalk between many signalling pathways. Previous studies have
strongly supported the crucial role of OPN in tumour progression
through the regulation of multiple signalling events, including
those critically dependent on MMP bioavailability (36–40,52–55).
OPN contributes to aggressiveness both through the inhibition of
apoptosis and the activation of various matrix-degrading proteases,
such as the activation of MMP-2 and MMP-9 (37,64).
Although several studies have demonstrated that OPN and MMP-9 are
implicated in the development and progression of several types of
cancer (22,34,35,38,43,46,49–51,65),
it is important to investigate how these proteins may be involved
in the molecular mechanism linked with melanoma development.
Understanding such mechanisms may provide further insight into the
discovery of novel therapeutic approaches.
The results of the present study, comprising the
analysis of well-defined clinical material, including >100 blood
samples from patients with melanoma, revealed a significant
increase in OPN levels in the patients with melanoma compared to
the heathy subjects. Furthermore, the highest levels of OPN were
directly associated with ulceration, tumour thickness and nodular
type. In melanoma, OPN expression is significantly associated with
reduced survival and with a poor clinical outcome (48,66).
In agreement with other reports demonstrating that
MMP-9 is implicated in the regulation of tumour cell migration and
in the development and metastasis (22,26,27),
our data indicated that higher MMP-9 levels, as well as OPN, are
strongly associated with positive sentinel lymph-node and tumour
thickness, markers of malignancy. A positive correlation between
OPN and MMP-9, including MMP-9 activity, was found in both melanoma
cases and healthy subjects.
Intriguingly, the levels of OPN and MMP-9 were
monitored in 18 melanoma cases in a follow-up period of 3 months.
Prior to surgery, these 18 cases expressed both markers at 3- to
−6-fold higher levels than the healthy controls. At 3 months
psot-surgery, these levels were significantly decreased. These
results indicate the strong association of these molecules with the
clinical manifestation of the disease. These data were confirmed by
further experiments conducted on PBMCs from 53 healthy donors and
29 patients with melanoma that were cultured for 24 h with or
without OPN. Stimulation of the PBMCs with OPN increased the
production of MMP-9, particularly in the patients with melanoma.
These data suggest that the OPN/MMP-9 pathway is associated with
the transformation and progression of melanoma. The impact of OPN
regulating MMP-9 is cell type-dependent and involves a variety of
overlapping intracellular signalling pathways.
Therefore, the mechanism through which OPN mediates
MMP-9 activation in melanoma and consequently enhances tumour
aggressiveness may be associated with NF-κB activation. OPN
regulates MMP activity in various ways. It can bind MMP-9 and
promote its activation through an NF-κB/IκBa/IKK signalling pathway
(54). Accordingly, the treatment
of PBMCs from healthy donors and patients with melanoma with OPN
confirmed the production of NF-κB activity in patients with
melanoma. NF-κB activation has been connected with multiple
processes of oncogenesis, including the control of apoptosis, cell
cycle, differentiation and cell migration (67,68).
We have observed that the activity of the NF-κB p65 subunit in
PBMCs from patients with melanoma was higher when compared with
that of the controls. We also evaluated the effect of DHMEQ, a
specific inhibitor of NF-κB (69–71),
to determine whether NF-κB inhibition was associated with the
decrease of OPN and MMP-9 and to demonstrate the direct role of the
NF-κB-regulated OPN and MMP-9 markers in patients with melanoma.
Additional experiments with PBMCs from the two groups revealed that
the MMP-9 levels decreased following treatment with both OPN and
DHMEQ, suggesting a strong association between OPN and MMP-9 and
their upregulation mediated by NF-κB. Intriguingly, such an
activation was associated with melanoma diagnosed in patients
harbouring the BRAFV600E mutation. In the present study,
through the analysis of each patient's medical file,
BRAFV600E mutation was detected in 58% of the patients
with melanoma. The MMP-9 plasma levels were higher in the patients
with such a mutation compared with those who were
BRAFWT; furthermore, the association of higher plasma
levels of MMP-9 with several negative prognostic factors supports
the notion that MMP-9 may be implicated in the aggressiveness of
melanoma.
Overall, the findings of the present study indicate
that OPN and MMP-9 expression and their upstream regulator, NF-κB,
may be considered promising markers for melanoma development and
may thus be useful targets for therapeutic interventions.
Acknowledgements
This study was in part supported by Lega Italiana
per la Lotta contro i Tumori (LILT). We would like to thank
Professor Kazuo Umezawa and the Kanagawa Keio University of Japan
for providing the NF-κB inhibitor.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fava P, Astrua C, Chiarugi A, Crocetti E,
Pimpinelli N, Fargnoli MC, Maurichi A, Rubegni P, Manganoni AM,
Bottoni U, et al: Differences in clinicopathological features and
distribution of risk factors in Italian melanoma patients.
Dermatology. 230:256–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Russo A, Ficili B, Candido S, Pezzino FM,
Guarneri C, Biondi A, Travali S, McCubrey JA, Spandidos DA and
Libra M: Emerging targeted therapies for melanoma treatment
(Review). Int J Oncol. 45:516–524. 2014.PubMed/NCBI
|
|
4
|
Eggermont AM and Schadendorf D: Melanoma
and immunotherapy. Hematol Oncol Clin North Am. 23:547–564, ix-x.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Caporali S, Alvino E, Lacal PM, Levati L,
Giurato G, Memoli D, Caprini E, Cappellini GC Antonini and D'Atri
S: Targeting the PI3K/AKT/mTOR pathway overcomes the stimulating
effect of dabrafenib on the invasive behavior of melanoma cells
with acquired resistance to the BRAF inhibitor. Int J Oncol.
49:1164–1174. 2016.PubMed/NCBI
|
|
6
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Montalto G, Cervello M, Nicoletti F, Fagone P, Malaponte
G, Mazzarino MC, et al: Mutations and deregulation of
Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascades which alter therapy
response. Oncotarget. 3:954–987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grazia G, Penna I, Perotti V, Anichini A
and Tassi E: Towards combinatorial targeted therapy in melanoma:
From pre-clinical evidence to clinical application (Review). Int J
Oncol. 45:929–949. 2014.PubMed/NCBI
|
|
8
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Solus JF and Kraft S: Ras, Raf, and MAP
kinase in melanoma. Adv Anat Pathol. 20:217–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Libra M, Malaponte G, Navolanic PM,
Gangemi P, Bevelacqua V, Proietti L, Bruni B, Stivala F, Mazzarino
MC, Travali S, et al: Analysis of BRAF mutation in primary and
metastatic melanoma. Cell Cycle. 4:1382–1384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Candido S, Rapisarda V, Marconi A,
Malaponte G, Bevelacqua V, Gangemi P, Scalisi A, McCubrey JA,
Maestro R, Spandidos DA, et al: Analysis of the
B-RafV600E mutation in cutaneous melanoma patients with
occupational sun exposure. Oncol Rep. 31:1079–1082. 2014.PubMed/NCBI
|
|
12
|
Lin K, Baritaki S, Militello L, Malaponte
G, Bevelacqua Y and Bonavida B: The Role of B-RAF Mutations in
Melanoma and the Induction of EMT via Dysregulation of the
NF-κB/Snail/RKIP/PTEN Circuit. Genes Cancer. 1:409–420. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cantwell-Dorris ER, O'Leary JJ and Sheils
OM: BRAFV600E: Implications for carcinogenesis and molecular
therapy. Mol Cancer Ther. 10:385–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mbeunkui F and Johann DJ Jr: Cancer and
the tumor microenvironment: A review of an essential relationship.
Cancer Chemother Pharmacol. 63:571–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J and Liu J: Tumor stroma as targets
for cancer therapy. Pharmacol Ther. 137:200–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bevelacqua V, Bevelacqua Y, Candido S,
Skarmoutsou E, Amoroso A, Guarneri C, Strazzanti A, Gangemi P,
Mazzarino MC, D'Amico F, et al: Nectin like-5 overexpression
correlates with the malignant phenotype in cutaneous melanoma.
Oncotarget. 3:882–892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Whipple CA: Tumor talk: Understanding the
conversation between the tumor and its microenvironment. Cancer
Cell Microenviron. 2:e7732015.PubMed/NCBI
|
|
18
|
Villanueva J and Herlyn M: Melanoma and
the tumor microenvironment. Curr Oncol Rep. 10:439–446. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seftor EA, Brown KM, Chin L, Kirschmann
DA, Wheaton WW, Protopopov A, Feng B, Balagurunathan Y, Trent JM,
Nickoloff BJ, et al: Epigenetic transdifferentiation of normal
melanocytes by a metastatic melanoma microenvironment. Cancer Res.
65:10164–10169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Postovit LM, Seftor EA, Seftor RE and
Hendrix MJ: Influence of the microenvironment on melanoma cell fate
determination and phenotype. Cancer Res. 66:7833–7836. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
Outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.PubMed/NCBI
|
|
22
|
Stetler-Stevenson WG: The role of matrix
metalloproteinases in tumor invasion, metastasis, and angiogenesis.
Surg Oncol Clin N Am. 10:383–392, x. 2001.PubMed/NCBI
|
|
23
|
Falzone L, Salemi R, Travali S, Scalisi A,
McCubrey JA, Candido S and Libra M: MMP-9 overexpression is
associated with intragenic hypermethylation of MMP9 gene in
melanoma. Aging (Albany NY). 8:933–944. 2016.PubMed/NCBI
|
|
24
|
Candido S, Abrams SL, Steelman LS,
Lertpiriyapong K, Fitzgerald TL, Martelli AM, Cocco L, Montalto G,
Cervello M, Polesel J, et al: Roles of NGAL and MMP-9 in the tumor
microenvironment and sensitivity to targeted therapy. Biochim
Biophys Acta. 1863:438–448. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shellman YG, Makela M and Norris DA:
Induction of secreted matrix metalloproteinase-9 activity in human
melanoma cells by extracellular matrix proteins and cytokines.
Melanoma Res. 16:207–211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roomi MW, Kalinovsky T, Rath M and
Niedzwiecki A: In vitro modulation of MMP-2 and MMP-9 in pediatric
human sarcoma cell lines by cytokines, inducers and inhibitors. Int
J Oncol. 44:27–34. 2014.PubMed/NCBI
|
|
27
|
Heo DS, Choi H, Yeom MY, Song BJ and Oh
SJ: Serum levels of matrix metalloproteinase-9 predict lymph node
metastasis in breast cancer patients. Oncol Rep. 31:1567–1572.
2014.PubMed/NCBI
|
|
28
|
Aalinkeel R, Nair BB, Reynolds JL, Sykes
DE, Mahajan SD, Chadha KC and Schwartz SA: Overexpression of MMP-9
contributes to invasiveness of prostate cancer cell line LNCaP.
Immunol Invest. 40:447–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chou CH, Teng CM, Tzen KY, Chang YC, Chen
JH and Cheng JC: MMP-9 from sublethally irradiated tumor promotes
Lewis lung carcinoma cell invasiveness and pulmonary metastasis.
Oncogene. 31:458–468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Malaponte G, Polesel J, Candido S, et al:
IL-6-174 G > C and MMP-9-1562 C > T polymorphisms are
associated with increased risk of deep vein thrombosis in cancer
patients. Cytokine. 62:64–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Di Carlo A, Terracciano D, Mariano A and
Macchia V: Matrix metalloproteinase-2 and matrix
metalloproteinase-9 type IV collagenases in serum of patients with
pleural effusions. Int J Oncol. 26:1363–1368. 2005.PubMed/NCBI
|
|
32
|
Kato Y, Yamashita T and Ishikawa M:
Relationship between expression of matrix metalloproteinase-2 and
matrix metalloproteinase-9 and invasion ability of cervical cancer
cells. Oncol Rep. 9:565–569. 2002.PubMed/NCBI
|
|
33
|
Mook OR, Frederiks WM and Van Noorden CJ:
The role of gelatinases in colorectal cancer progression and
metastasis. Biochim Biophys Acta. 1705:69–89. 2004.PubMed/NCBI
|
|
34
|
Malaponte G, Zacchia A, Bevelacqua Y,
Marconi A, Perrotta R, Mazzarino MC, Cardile V and Stivala F:
Co-regulated expression of matrix metalloproteinase-2 and
transforming growth factor-beta in melanoma development and
progression. Oncol Rep. 24:81–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nikkola J, Vihinen P, Vuoristo MS,
Kellokumpu-Lehtinen P, Kähäri VM and Pyrhönen S: High serum levels
of matrix metalloproteinase-9 and matrix metalloproteinase-1 are
associated with rapid progression in patients with metastatic
melanoma. Clin Cancer Res. 11:5158–5166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen YJ, Wei YY, Chen HT, Fong YC, Hsu CJ,
Tsai CH, Hsu HC, Liu SH and Tang CH: Osteopontin increases
migration and MMP-9 up-regulation via alphavbeta3 integrin, FAK,
ERK, and NF-kappaB-dependent pathway in human chondrosarcoma cells.
J Cell Physiol. 221:98–108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Philip S, Bulbule A and Kundu GC:
Osteopontin stimulates tumor growth and activation of promatrix
metalloproteinase-2 through nuclear factor-kappa B-mediated
induction of membrane type 1 matrix metalloproteinase in murine
melanoma cells. J Biol Chem. 276:44926–44935. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Castellano G, Malaponte G, Mazzarino MC,
Figini M, Marchese F, Gangemi P, Travali S, Stivala F, Canevari S
and Libra M: Activation of the osteopontin/matrix
metalloproteinase-9 pathway correlates with prostate cancer
progression. Clin Cancer Res. 14:7470–7480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Malaponte G, Hafsi S, Polesel J,
Castellano G, Spessotto P, Guarneri C, Canevari S, Signorelli SS,
McCubrey JA and Libra M: Tumor microenvironment in diffuse large
B-cell lymphoma: Matrixmetalloproteinases activation is mediated by
osteopontin overexpression. Biochim Biophys Acta. 1863:483–489.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wai PY and Kuo PC: Osteopontin: Regulation
in tumor metastasis. Cancer Metastasis Rev. 27:103–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Weber GF: The metastasis gene osteopontin:
A candidate target for cancer therapy. Biochim Biophys Acta.
1552:61–85. 2001.PubMed/NCBI
|
|
42
|
El-Tanani MK, Campbell FC, Kurisetty V,
Jin D, McCann M and Rudland PS: The regulation and role of
osteopontin in malignant transformation and cancer. Cytokine Growth
Factor Rev. 17:463–474. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Libra M, Indelicato M, De Re V, Zignego
AL, Chiocchetti A, Malaponte G, Dianzani U, Nicoletti F, Stivala F,
McCubrey JA, et al: Elevated Serum Levels of Osteopontin in
HCV-Associated Lymphoproliferative Disorders. Cancer Biol Ther.
4:1192–1194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Poruk KE, Firpo MA, Scaife CL, Adler DG,
Emerson LL, Boucher KM and Mulvihill SJ: Serum osteopontin and
tissue inhibitor of metalloproteinase 1 as diagnostic and
prognostic biomarkers for pancreatic adenocarcinoma. Pancreas.
42:193–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang YD, Chen H, Liu HQ and Hao M:
Correlation between ovarian neoplasm and serum levels of
osteopontin: A meta-analysis. Tumour Biol. 35:11799–11808. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cao DX, Li ZJ, Jiang XO, Lum YL, Khin E,
Lee NP, Wu GH and Luk JM: Osteopontin as potential biomarker and
therapeutic target in gastric and liver cancers. World J
Gastroenterol. 18:3923–3930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin F, Li Y, Cao J, Fan S, Wen J, Zhu G,
Du H and Liang Y: Overexpression of osteopontin in hepatocellular
carcinoma and its relationships with metastasis, invasion of tumor
cells. Mol Biol Rep. 38:5205–5210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rangel J, Nosrati M, Torabian S, Shaikh L,
Leong SP, Haqq C, Miller JR III, Sagebiel RW and Kashani-Sabet M:
Osteopontin as a molecular prognostic marker for melanoma. Cancer.
112:144–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sturm RA: Osteopontin in melanocytic
lesions - a first step towards invasion? J Invest Dermatol.
124:xiv–xv. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kiss T, Ecsedi S, Vizkeleti L, Koroknai V,
Emri G, Kovács N, Adany R and Balazs M: The role of osteopontin
expression in melanoma progression. Tumour Biol. 36:7841–7847.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou Y, Dai DL, Martinka M, Su M, Zhang Y,
Campos EI, Dorocicz I, Tang L, Huntsman D, Nelson C, et al:
Osteopontin expression correlates with melanoma invasion. J Invest
Dermatol. 124:1044–1052. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Matušan-Ilijaš K, Damante G, Fabbro D,
Dorđević G, Hadžisejdić I, Grahovac M, Marić I, Spanjol J, Grahovac
B, Jonjić N, et al: Osteopontin expression correlates with nuclear
factor-κB activation and apoptosis downregulation in clear cell
renal cell carcinoma. Pathol Res Pract. 207:104–110. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Das R, Mahabeleshwar GH and Kundu GC:
Osteopontin stimulates cell motility and nuclear factor
kappaB-mediated secretion of urokinase type plasminogen activator
through phosphatidylinositol 3-kinase/Akt signaling pathways in
breast cancer cells. J Biol Chem. 278:28593–28606. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rangaswami H and Kundu GC: Osteopontin
stimulates melanoma growth and lung metastasis through
NIK/MEKK1-dependent MMP-9 activation pathways. Oncol Rep.
18:909–915. 2007.PubMed/NCBI
|
|
55
|
Rangaswami H, Bulbule A and Kundu GC:
Nuclear factor-inducing kinase plays a crucial role in
osteopontin-induced MAPK/IkappaBalpha kinase-dependent nuclear
factor kappaB-mediated promatrix metalloproteinase-9 activation. J
Biol Chem. 279:38921–38935. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu J, Liu Q, Wan Y, Zhao Z, Yu H, Luo H
and Tang Z: Osteopontin promotes the progression of gastric cancer
through the NF-κB pathway regulated by the MAPK and PI3K. Int J
Oncol. 45:282–290. 2014.PubMed/NCBI
|
|
57
|
Polesel J, Franceschi S, Talamini R, Negri
E, Barzan L, Montella M, Libra M, Vaccher E, Franchin G, La Vecchia
C and Serraino D: Tobacco smoking, alcohol drinking, and the risk
of different histological types of nasopharyngeal cancer in a
low-risk population. Oral Oncol. 47:541–545. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Matsumoto N, Ariga A, To-e S, Nakamura H,
Agata N, Hirano S, Inoue J and Umezawa K: Synthesis of NF-kappaB
activation inhibitors derived from epoxyquinomicin C. Bioorg Med
Chem Lett. 10:865–869. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Catalán V, Gómez-Ambrosi J, Rodríguez A,
Ramírez B, Valentí V, Moncada R, Silva C, Salvador J and Frühbeck
G: Peripheral mononuclear blood cells contribute to the
obesity-associated inflammatory state independently of glycemic
status: involvement of the novel proinflammatory adipokines
chemerin, chitinase-3-like protein 1, lipocalin-2 and osteopontin.
Genes Nutr. 10:4602015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Buommino E, De Filippis A, Gaudiello F,
Balato A, Balato N, Tufano MA and Ayala F: Modification of
osteopontin and MMP-9 levels in patients with psoriasis on
anti-TNF-α therapy. Arch Dermatol Res. 304:481–485. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tímár J, Gyorffy B and Rásó E: Gene
signature of the metastatic potential of cutaneous melanoma: Too
much for too little? Clin Exp Metastasis. 27:371–387. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Brooks SA, Lomax-Browne HJ, Carter TM,
Kinch CE and Hall DM: Molecular interactions in cancer cell
metastasis. Acta Histochem. 112:3–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hu M and Polyak K: Microenvironmental
regulation of cancer development. Curr Opin Genet Dev. 18:27–34.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Rangaswami H, Bulbule A and Kundu GC:
Nuclear factor inducing kinase: A key regulator in osteopontin-
induced MAPK/IkappaB kinase dependent NF-kappaB-mediated promatrix
metalloproteinase-9 activation. Glycoconj J. 23:221–232. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rangaswami H, Bulbule A and Kundu GC:
Osteopontin: Role in cell signaling and cancer progression. Trends
Cell Biol. 16:79–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mitra A, Conway C, Walker C, Cook M,
Powell B, Lobo S, Chan M, Kissin M, Layer G, Smallwood J, et al:
Melanoma sentinel node biopsy and prediction models for relapse and
overall survival. Br J Cancer. 103:1229–1236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Vlahopoulos SA, Cen O, Hengen N, Agan J,
Moschovi M, Critselis E, Adamaki M, Bacopoulou F, Copland JA,
Boldogh I, et al: Dynamic aberrant NF-κB spurs tumorigenesis: A new
model encompassing the microenvironment. Cytokine Growth Factor
Rev. 26:389–403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xia Y, Shen S and Verma IM: NF-κB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
McCubrey JA, Milella M, Tafuri A, Martelli
AM, Lunghi P, Bonati A, Cervello M, Lee JT and Steelman LS:
Targeting the Raf/MEK/ERK pathway with small-molecule inhibitors.
Curr Opin Investig Drugs. 9:614–630. 2008.PubMed/NCBI
|
|
70
|
Gupta SC, Sundaram C, Reuter S and
Aggarwal BB: Inhibiting NF-κB activation by small molecules as a
therapeutic strategy. Biochim Biophys Acta. 1799:775–787. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Malaponte G, Signorelli SS, Bevelacqua V,
Polesel J, Taborelli M, Guarneri C, Fenga C, Umezawa K and Libra M:
Increased Levels of NF-κB-Dependent Markers in Cancer-Associated
Deep Venous Thrombosis. PLoS One. 10:e01324962015. View Article : Google Scholar : PubMed/NCBI
|