Introduction
Gastric cancer (GC), with approximately 1 million
new diagnosed patients every year (1), is the fourth most common human cancer
and ranks second as leading cause of cancer-related mortality
worldwide (2). For patients in
advanced stage of GC, the prognosis is very poor, with a 5-year
overall survival less than 30% (3).
Malignant growth and systemic metastasis are the major reasons for
the unsatisfactory survival of GC patients in advanced stage.
Therefore, clarifying the molecular mechanisms for the development
and progression of GC will be critical for identifying novel
therapeutic targets for GC patients.
MicroRNAs (miRNAs), a class of short non-coding RNA
sequences, play versatile roles in cellular processes including
cell proliferation, apoptosis and movement (4). In addition, miRNAs have been confirmed
to be aberrantly expressed and play fundamental roles in
tumorigenesis and progression of human cancers (5) including GC (6). Several miRNAs were suggested as novel
biomarkers and therapeutic targets in GC, including let7 (7), miR-196a (8), miR-101 (9) and miR-130b (10).
Recently, miR-187, a novel cancer-related microRNA,
has been reported to play important roles in non-small cell lung
(11), colorectal (12), prostate (13) and breast cancer (14). In non-small cell lung cancer,
miR-187 was found to promote the cancer development by inhibiting
Bcl-6 (11). Besides, the study of
breast cancer showed that miR-187 is a prognostic marker and could
potentiate the invasive ability of cancer cells (14). However, miR-187 was found to be
downregulated in prostate cancer and was correlated with adverse
clinicopathological features of patients (13). In addition, in colorectal cancer,
miR-187 was found to be the downstream target of TGFβ pathway and
could suppress Smad-mediated epithelial-mesenchymal transition in
colorectal cancer cells (12).
Therefore, the expression and function of miR-187 vary between
different human types of cancers. However, the expression and
function of miR-187 in GC remain uninvestigated.
The present study found that miR-187 was
significantly upregulated in GC tissues. Increased expression of
miR-187 was associated with poor clinicopathological features and
worse survival of GC patients. Functionally, miR-187 enhanced
proliferation, migration and invasion of GC cells both in
vitro and in vivo. Moreover, we found that FOXA2 was a
direct downstream target of miR-187 and mediated the biological
functions of miR-187 in GC cells.
Materials and methods
Cell cultures
Gastric cancer cell lines SGC-7901, MKN-45, MGC-803
and normal gastric epithelial GES-1 cells were purchased from the
American Type Culture Collection (ATCC; Rockville, MD, USA) and the
Cell Bank of Chinese Academy of Sciences (Shanghai, China). All
cells were cultured in RPMI-1640 medium (Life Technologies, Inc.,
Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco, Grand Island, NY, USA), penicillin (100 U/ml) and
streptomycin (100 mg/ml). All cell cultures were kept at 37°C in a
humidified incubator with 5% CO2.
Clinical tissues
The approval to conduct the experiments involving
human tissue samples was obtained from the Institutional Research
Ethics Committee of Tongren Hospital, Shanghai Jiao Tong University
School of Medicine. One hundred pairs of gastric cancer tissues and
adjacent non-tumor tissues were collected from Tongren Hospital,
Shanghai Jiao Tong University School of Medicine. All these
clinical tissues were stored at −80°C before the RNA extraction.
The informed consents were obtained from all enrolled patients in
this study. Demographic and clinicopathological information of the
included patients are presented in Table I.
 | Table I.Clinical association analysis of
miR-187 expression in gastric cancer. |
Table I.
Clinical association analysis of
miR-187 expression in gastric cancer.
|
|
| No. of patients |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | Total no. of patients
(n=100) | miR-187low
group |
miR-187high group | P-value |
|---|
| Age (years) |
|
<65 | 54 | 25 | 29 | 0.547 |
| ≥65 | 46 | 25 | 21 |
| Gender |
| Male | 68 | 31 | 37 | 0.284 |
|
Female | 32 | 19 | 13 |
| Size (cm) |
|
<5 | 33 | 24 | 9 | <0.001 |
| ≥5 | 47 | 6 | 41 |
| Tumor depth |
| T1 | 26 | 11 | 15 | 0.494 |
|
T2-T4 | 74 | 39 | 35 |
| Lymph node
metastasis |
|
Absent | 34 | 27 | 7 | <0.001 |
|
Present | 66 | 23 | 43 |
| Venous
infiltration |
|
Absent | 70 | 39 | 31 | 0.126 |
|
Present | 30 | 11 | 19 |
| TNM stage |
| I,
II | 56 | 41 | 15 |
<0.001 |
| III,
IV | 44 | 9 | 35 |
Cell transfection
The mimic and the inhibitor of miR-187 were obtained
from Ruibobio, Guangzhou, China. FOXA2 siRNA and FOXA2 expression
vector was purchased from Sigma-Aldrich (St. Louis, MO, USA). The
day before the transfection, GC cells were seeded in 6-well plates.
Then, miR-187 mimic or inhibitor/FOXA2 siRNA or plasmid were
transfected into GC cells with Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) following the manusfacturers protocol.
Real-time quantitative reverse
transcription-PCR (qRT-PCR)
Total RNA from GC tissues and cells were extracted
with TRIzol reagent (Invitrogen). Reverse transcription reactions
and real-time PCR were performed with Transcriptor First Strand
cDNA Synthesis kit (Roche, Indianapolis, IN, USA) and SYBR-Green
PCR Master Mix (Applied Biosystems, Foster City, CA, USA). Primers
for miR-187 and U6 were purchased from GeneCopoeia (Guangzhou,
China). U6 was used as the control gene for the relative expression
level of miR-187.
Western blot analysis
Western blot analysis was performed following
standard protocols. Generally, cellular protein was extracted with
the RIPA lysis buffer, and protein concentration was measured using
the BCA kit (Pierce, Rockford, IL, USA). A total of 20–40 µg
cellular proteins were separated by SDS-PAGE and transferred to
PVDF membrane. The following primary antibodies including FOXA2
(1:1,000; Cell Signaling Technology, Danvers, MA, USA) and GAPDH
(1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) were
incubated with the membrane overnight at 4°C. The blots were then
incubated with secondary antibodies (1:3,000; Santa Cruz
Biotechnology) at room temperature for 2 h. The signals were
visualized with ECL reagents (Amersham Biosciences Corp.,
Piscataway, NJ, USA).
MTT
For MTT assay, 5,000 GC cells transfected with
miR-187 mimic or inhibitor were plated into 96-well plates. At 24,
28 and 72 h after cell seeding, the cells were stained with MTT
(Sigma-Aldrich) for 4 h at 37°C, and then the absorbance at 490 nm
was examined.
Wound healing assay
GC cells were cultured to confluence in 6-well
plates. A 100-µl pipette tip was used to make the wounds on the
confluent cells. The width of wounds was measured at 0 and 24 h
after scratching and then the percentage of wound healing was
calculated.
Transwell assays
The invasive ability of GC cells was evaluated by
Transwell assays. Generally, upper chamber of Transwell device was
coated with 80 µl mixture of RPMI-1640 and Matrigel with a ratio of
1:8. Then, GC cells suspended in serum-free RPMI-1640 medium were
plated in the upper chamber while 700 µl serum-containing medium
RPMI-1640 were placed in the lower chamber. Twenty-four hours
later, GC cells invaded into the lower surface were stained with
crystal violet. Cell number for the invaded cells was counted under
a microscope.
Luciferase assay
Wild-type 3-UTR sequence of FOXA2 containing the
miR-187 predicted target site or the mutated sequence within the
predicted target sites was cloned into the pGL3 control vector
(Promega, Madison, WI, USA), designated as the wild-type
FOXA2-3-UTR or mutant FOXA2-3-UTR, respectively. Then, GC cells
seeded into 12-well plates were maintained in Opti-MEM reduced
serum media (Life Technologies) the day before the transfection,
and were cotransfected with wild-type or mutant 3′-UTR of FOXA2
along with miR-187 mimic or inhibitor. Forty-eight hours after the
transfection, luciferase activity was measured by a single
luciferase reporter assay (Promega).
In vivo tumor growth and metastasis
assay
All animal experiments were approved by the Animal
Care Committee of Tongren Hospital, Shanghai Jiao Tong University
School of Medicine. For tumor growth studies, nude mice were
injected subcutaneously with 1×106 cancer cells
transfected with control vector or miR-187 mimic. Tumor sizes were
measured every three days after cancer cell injection. Three weeks
later, tumors were removed and the volumes were measured. For
metastasis assay, GC cells were injected through tail vein into
nude mice. Eight weeks later, the lungs of nude mice were subjected
to H&E staining for potential lung metastasis of GC cells.
Statistical analysis
All quantatively data are presented as mean ±
standard error of the mean (SEM). Statistical analysis including
Student's t-test, Chi-square, correlation analysis and Kaplan-Meier
analysis was performed with SPSS software (SPSS, Inc., Chicago, IL,
USA) and GraphPad software. P<0.05 was considered statistically
significant.
Results
miR-187 expression is increased in GC
tissues and cells
We performed qRT-PCR to determine the expression
status of miR-187 in GC tissues. Compared with adjacent normal
tissues, the expression of miR-187 in GC tissues was increased
significantly (Fig. 1A; P<0.01).
In addition, compared to those of small size, tumors of large size
had significantly increased level of miR-187 (Fig. 1B; P<0.05). Furthermore, compared
to those without metastasis, the expression level of miR-187 in
patients with metastasis was significantly elevated (Fig. 1C; P<0.05). Moreover, we examined
the expression of miR-187 in GC cells and the normal gastric
epithelial GES-1 cells. Compared to GES-1 cells, miR-187 in GC
cells lines was significantly increased (Fig. 1D; P<0.05). Besides, among the
four GC cell lines, miR-187 level was lowest in SGC-7901 cells
while was highest in AGS cells (Fig.
1D), indicating that miR-187, which is elevated in GC tissues
and cells, probably plays an oncogenic role in GC.
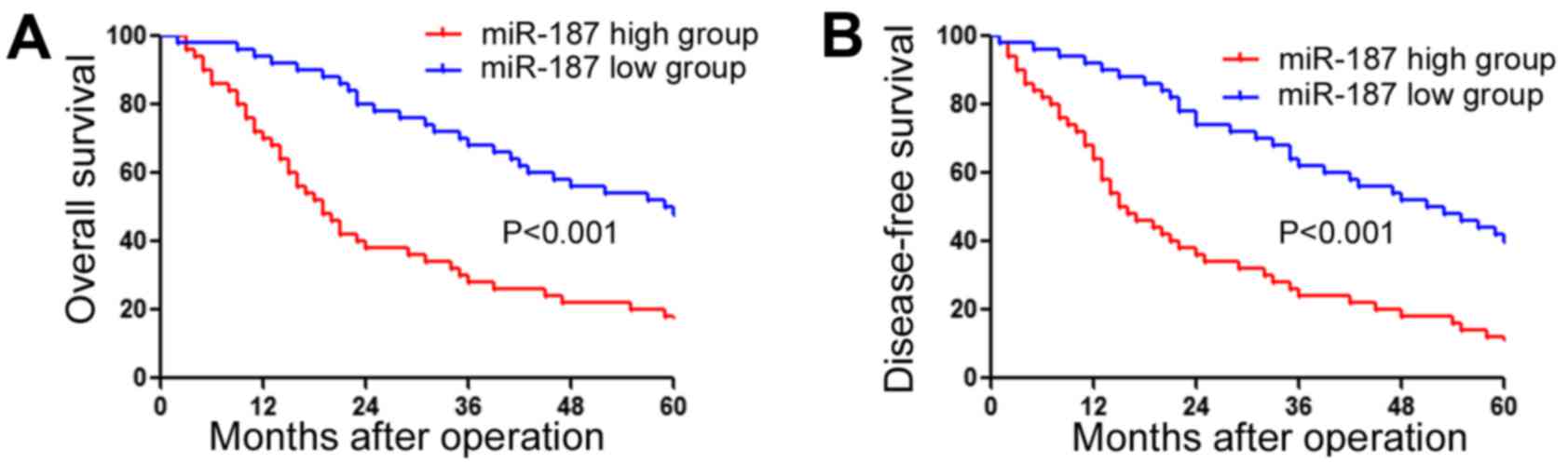
Increased miR-187 expression is
associated with poor clinicopathological features and prognosis of
GC patients
After confirming the increased expression of miR-187
in GC, we further determined whether miR-187 expression level was
associated with clinical features and the survival of GC patients.
Association analysis (Table II)
showed that high expression level was associated with large tumor
size (P<0.001), lymphatic metastasis (P<0.001) and advanced
TNM stage (P<0.001). Furthermore, survival analysis showed the
patients with high level of miR-187 had significantly decreased
overall survival (Fig. 2A;
P<0.001) and disease-free survival (Fig. 2B; P<0.001). These data indicate
that miR-187 is actively involved in the pathogenesis of GC, and
can potentially serve as a novel biomarker for the prognosis of GC
patients.
 | Table II.Multivariate Cox regression analysis
of 5-year overall and disease-free survival of 80 gastric cancer
patients. |
Table II.
Multivariate Cox regression analysis
of 5-year overall and disease-free survival of 80 gastric cancer
patients.
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Histology | 0.557 | 0.319–0.971 | 0.039a | 0.686 | 0.390–1.205 | 0.190 |
| Size (cm) | 2.344 | 1.482–3.706 |
<0.001a | 1.355 | 0.825–2.225 | 0.229 |
| Stage | 3.188 | 1.964–5.175 |
<0.001a | 2.660 | 1.588–4.457 |
<0.001a |
| miR-340
expression | 2.269 | 1.472–3.498 | 0.001a | 1.604 | 1.011–2.543 | 0.045a |
miR-187 promotes the proliferation,
migration and invasion of GC cells
Aberrantly increased expression of miR-187 in GC
prompted us to explore the biological function of miR-187 in GC
cells. Transfection of miR-187 mimic in SGC-7901 cells
significantly increased miR-187 expression in SGC-7901 cells
(Fig. 3A; P<0.01). Functionally,
overexpression of miR-187 obviously increased cell proliferation of
SGC-7901 cells (Fig. 3B; P<0.05)
as suggested by MTT assay. Moreover, the wound healing assay and
Transwell assay showed that the migration and invasion of SGC-7901
was obviously increased after overexpression of miR-187 (Fig. 3C and D; P<0.05 for wound healing
assay, P<0.01 for invasion assay). On the other hand, miR-187
inhibitor significantly decreased the expression of miR-187 in AGS
cells (Fig. 4A; P<0.01).
Subsequently, inhibition of miR-187 resulted in decreased cell
proliferation (Fig. 4B; P<0.05),
migration (Fig. 4C; P<0.05) and
invasion (Fig. 4D; P<0.01) of
AGS cells. These data indicate that miR-187 promotes the
proliferation, migration and invasion of GC cells in
vitro.
miR-187 potentiates the growth and
metastasis of GC cells in vivo
To further confirm the in vitro influence of
miR-187 on GC cells, we further carried out nude mouse experiments
to determine whether miR-187 could promote the in vivo
growth and metastasis of GC cells. The result of subcutaneous tumor
formation assay showed that the growth of SGC-7901 cells was
significantly increased after overexpression of miR-187 (Fig. 5A and B; P<0.01). Moreover, the
tail vein injection experiments in nude mice showed that
overexpression of miR-187 significantly increased the lung
metastasis of SGC-7901 cells (Fig. 5C
and D; P<0.01). These data suggest that miR-187 potentiate
the growth and metastasis of GC cells in vivo.
FOXA2 is a downstream target of
miR-187 in GC cells
After confirming the functional influence of miR-187
in GC cells, we further investigated the underlying mechanism
mediating the function of miR-187. The data from the database of
TargetScan and miRNA showed that FOXA2 contained the potential
binding sites for miR-187 (Fig.
6A), suggesting FOXA2 is a potential downstream target of
miR-187. Then, we performed luciferase assay and found that
overexpression of miR-187 significantly inhibited the luciferase
activity of wild-type 3-UTR of FOXA2 (Fig. 6B; P<0.01) while inhibiting
miR-187 significantly increased the luciferase activity of
wild-type FOXA2 3-UTR (Fig. 6B;
P<0.05). The influence of miR-187 overexpression and inhibition
on the luciferase activity of wild-type 3-UTR of FOXA2 was absent
in the mutant 3-UTR of FOXA2 (Fig.
6B). Furthermore, we performed qRT-PCR and western blot
analysis to confirm that miR-187 could affect the expression of
FOXA2 in GC cells. As suggested by Fig.
6C and D, overexpression of miR-187 significantly decreased the
mRNA (P<0.01) and protein (P<0.01) level of FOXA2 in SGC-7901
cells. On the other hand, inhibition of miR-187 significantly
increased the expression of FOXA2 mRNA (Fig. 6E; P<0.05) and protein (Fig. 6F; P<0.01) in AGS cells.
FOXA2 mediates the biological
functions of miR-187 in GC cells
Lastly, we examined whether FOXA2 could affect the
biological function of miR-187 in gastric cancer cells. FOXA2
expression vector significantly increased the protein level of
FOXA2 in SGC-7901 cells overexpressing miR-187 (Fig. 7A; P<0.01). Functionally,
overexpression of FOXA2 abrogated the promoting effects of miR-187
mimic on the proliferation (Fig.
7B; P<0.05), migration (Fig.
7C; P<0.05) and invasion (Fig.
7D; P<0.01) of SGC-7901 cells. On the contrary, FOXA2 siRNA
significantly decreased the expression of FOXA2 in AGS cells with
miR-187 inhibition (Fig. 8A;
P<0.05). Inhibition of FOXA2 prevented the inhibitory effects of
miR-187 inhibitor on the proliferation (Fig. 8B; P<0.01 for MTT), migration
(Fig. 8C; P<0.05) and invasion
(Fig. 8D; P<0.01) of AGS cells.
These data indicate that FOXA2 is a functional mediator for miR-187
in GC cells.
Discussion
Biological functions of microRNAs in human diseases
have been extensively investigated during the past two decades
(15). In addition, increasing
amount of studies showed that miRNAs were active players in the
pathogenic process of human cancers (16). miRNAs have been found to be
therapeutic targets and biomarkers for human cancers including GC
(17,18).
Among numerous miRNAs, miR-187 was recently found to
be a novel cancer-associated microRNA. It could promote the
development of non-small cell lung cancer by inhibiting Bcl-6
(11). The study involving breast
cancer showed that miR-187 increased the invasive behavior of
cancer cells (14). On the
contrary, miR-187 expression was found to be decreased in the
tissues of prostate cancer and was correlated with poor
clinicopathological features of patients (13,19).
In colorectal cancer, miR-187 was found to prevent Smad-mediated
epithelial-mesenchymal transition in colorectal cancer cells
(12). Therefore, the expression
and biological function of miR-187 in human cancers vary between
different cancer types.
The present study found that miR-187 expression was
significantly increased in GC tissues and cell lines. In addition,
association analysis showed that miR-187 was associated with poor
clinical features and poor prognosis of GC patients. The in
vitro functional assays showed that overexpression of miR-187
could promote the proliferation and metastatic ability of SGC-7901
cells while inhibition of miR-187 decreased the proliferation and
invasive ability of AGC cells. Furthermore, the in vivo
experiments confirmed that miR-187 not only promoted the
proliferation and invasive ability of GC cell in vitro, but
also potentiated the growth and lung metastasis of GC in nude mice.
Taken together, these data strongly demonstrated that miR-187 plays
an oncogenic function in GC by promoting the growth and metastasis
of GC cells.
After elucidating the biological function of miR-187
in GC, we further explored the underlying mechanisms mediating the
function of miR-187 in GC. The data from public database showed
that FOXA2 was a potential downstream target of miR-187.
Interestingly, previous study showed that FOXA2 expression was
decreased in GC tissues and could suppress the development and
progression of GC both in vitro and in vivo (20,21).
Our luciferase assay data showed that altering miR-187 level could
significantly affect the luciferase activity of wild-type 3-UTR of
FOXA2 while had no effect on that of mutant 3-UTR of FOXA2.
Moreover, overexpression of miR-187 significantly decreased while
inhibition of miR-187 significantly increased the mRNA and protein
expression of FOXA2 in GC cells. These data strongly showed that
FOXA2 was a downstream target of miR-187 in GC cells. Lastly, we
determined whether FOXA2 mediated the biological functions of
miR-187 in GC. Our data showed that overexpression of FOXA2
abrogated the promoting effects of miR-187 mimic on the
proliferation and metastasis of GC cells while knockdown of FOXA2
reversed the inhibitory effects of miR-187 inhibitor on the
proliferation and metastasis of GC cells. These data confirmed that
FOXA2 was the functional mediator of miR-187 in GC cells. However,
it is important that microRNAs usually have more than one
downstream target in the cells. Therefore, it is possible that
miR-187 has other downstream targets in gastric cancer cells which
is worth investigation in the future.
In summary, we demonstrated for the first time that
miR-187 was increased in GC tissues and cells. Increased expression
level of miR-187 was associated with poor clinical features and
prognosis of GC patients. Moreover, miR-187 was able to promote the
growth and metastasis of GC cells. Mechanically, FOXA2 was
confirmed to be the downstream target of miR-187 in GC and to
mediate the functional influence of miR-187 on GC cells. Therefore,
the present study indicates that miR-187 is potentially a biomarker
and therapeutic target for GC patients.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Orditura M, Galizia G, Sforza V,
Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J,
Savastano B, Mabilia A, et al: Treatment of gastric cancer. World J
Gastroenterol. 20:1635–1649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM
and Zhang GZ: Biological functions of microRNAs: A review. J
Physiol Biochem. 67:129–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iorio MV and Croce CM: microRNA
involvement in human cancer. Carcinogenesis. 33:1126–1133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song S and Ajani JA: The role of microRNAs
in cancers of the upper gastrointestinal tract. Nat Rev
Gastroenterol Hepatol. 10:109–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohshima K, Inoue K, Fujiwara A, Hatakeyama
K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi
K, et al: Let-7 microRNA family is selectively secreted into the
extracellular environment via exosomes in a metastatic gastric
cancer cell line. PLoS One. 5:e132472010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng S, Kuang Z, Sheng C, Zhang Y, Xu H
and Cheng Q: Association of microRNA-196a-2 gene polymorphism with
gastric cancer risk in a Chinese population. Dig Dis Sci.
55:2288–2293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang HJ, Ruan HJ, He XJ, Ma YY, Jiang XT,
Xia YJ, Ye ZY and Tao HQ: MicroRNA-101 is down-regulated in gastric
cancer and involved in cell migration and invasion. Eur J Cancer.
46:2295–2303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai KW, Koh KX, Loh M, Tada K, Subramaniam
MM, Lim XY, Vaithilingam A, Salto-Tellez M, Iacopetta B, Ito Y, et
al: Singapore Gastric Cancer Consortium: MicroRNA-130b regulates
the tumour suppressor RUNX3 in gastric cancer. Eur J Cancer.
46:1456–1463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun C, Li S, Yang C, Xi Y, Wang L, Zhang F
and Li D: MicroRNA-187-3p mitigates non-small cell lung cancer
(NSCLC) development through down-regulation of BCL6. Biochem
Biophys Res Commun. 471:82–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang F, Luo Y, Shao Z, Xu L, Liu X, Niu
Y, Shi J, Sun X, Liu Y, Ding Y, et al: MicroRNA-187, a downstream
effector of TGFβ pathway, suppresses Smad-mediated
epithelial-mesenchymal transition in colorectal cancer. Cancer
Lett. 373:203–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Casanova-Salas I, Masiá E, Armiñán A,
Calatrava A, Mancarella C, Rubio-Briones J, Scotlandi K, Vicent MJ
and López-Guerrero JA: MiR-187 targets the androgen-regulated gene
ALDH1A3 in prostate cancer. PLoS One. 10:e01255762015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mulrane L, Madden SF, Brennan DJ, Gremel
G, McGee SF, McNally S, Martin F, Crown JP, Jirström K, Higgins DG,
et al: miR-187 is an independent prognostic factor in breast cancer
and confers increased invasive potential in vitro. Clin Cancer Res.
18:6702–6713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davis-Dusenbery BN and Hata A: MicroRNA in
cancer: The Involvement of aberrant MicroRNA biogenesis regulatory
pathways. Genes Cancer. 1:1100–1114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu R, Zhang C, Hu Z, Li G, Wang C, Yang
C, Huang D, Chen X, Zhang H, Zhuang R, et al: A five-microRNA
signature identified from genome-wide serum microRNA expression
profiling serves as a fingerprint for gastric cancer diagnosis. Eur
J Cancer. 47:784–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Slack FJ and Weidhaas JB: MicroRNA in
cancer prognosis. N Engl J Med. 359:2720–2722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Casanova-Salas I, Rubio-Briones J,
Calatrava A, Mancarella C, Masiá E, Casanova J, Fernández-Serra A,
Rubio L, Ramírez-Backhaus M, Armiñán A, et al: Identification of
miR-187 and miR-182 as biomarkers of early diagnosis and prognosis
in patients with prostate cancer treated with radical
prostatectomy. J Urol. 192:252–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Katoh M and Katoh M: Integrative genomic
analyses of CXCR4: Transcriptional regulation of CXCR4 based on
TGFbeta, Nodal, Activin signaling and POU5F1, FOXA2, FOXC2, FOXH1,
SOX17, and GFI1 transcription factors. Int J Oncol. 36:415–420.
2010.PubMed/NCBI
|
|
21
|
Zhu C-P, Wang J, Shi B, Hu PF, Ning BF,
Zhang Q, Chen F, Chen WS, Zhang X and Xie WF: The transcription
factor FOXA2 suppresses gastric tumorigenesis in vitro and in vivo.
Dig Dis Sci. 60:109–117. 2015. View Article : Google Scholar : PubMed/NCBI
|