Introduction
Glioma is one of the most prevalent brain tumors,
with a high yearly mortality rate worldwide (1,2). In
spite of the current advances in cancer treatments, glioma is still
an intractable disease due to the rapid growth, angiogenesis and
metastasis of glioma cells (3,4). The
overall survival has not been significantly improved during the
past decades (4,5). Therefore, there is an urgent need to
develop new therapies for glioma. However, the mechanism of glioma
tumorigenesis is still not fully understood that hampers the
development of new treatments for glioma.
In recent years, microRNAs (miRNAs), a group of
small and non-coding RNA, have emerged as potential and promising
tools for cancer diagnosis and treatments (6,7).
miRNAs can regulate target gene expression by interacting with the
3-untranslated region (UTR) of the mRNA that inhibits translation
or induces mRNA degradation (8,9). Thus,
miRNAs function as native regulators of gene expression. miRNAs are
capable of modulating cancer cell proliferation, apoptosis,
differentiation, migration and invasion by targeting oncogenes or
tumor suppressors (10). There is
increasing evidence suggesting that various miRNAs are dysregulated
in glioma, which function as oncogenes or tumor suppressors
(11,12), highlighting their potential as novel
agents for the treatment of glioma. However, the precise role of
miRNAs in glioma remains largely unknown.
The B cell-specific moloney murine leukemia virus
insertion site 1 (Bmi1) is a self-renewal gene that is
overexpressed in multiple human cancers (13). Bmi1 is a key component of the
polycomb regulatory complex-1 regulating the transcription of a
variety of important genes (14,15).
The inhibition of Bmi1 induces tumor cell apoptosis and increases
susceptibility to chemotherapy and radiation therapy, indicating an
oncogenic role of Bmi1 (16,17).
Bmi1 can induce cell transformation and promote cancer
proliferation in vivo and in vitro through
transcriptional repression of tumor suppressor genes including
p16INK4A and p14ARF (18–20).
The epithelial-to-mesenchymal transition (EMT) is an essential
process for cancer invasion and metastasis (21). Notably, a recent study has shown
that Bmi1 plays an important role in inducing EMT (22–24).
Bmi1 plays an important role in glioma tumorigenesis through
promoting the invasion, migration, angiogenesis and proliferation
of glioma cells (25–28). Therefore, targeted therapy on Bmi1
may have promising and potential therapeutic values on glioma
treatment.
Emerging evidence has suggested that miR-194
functions as a tumor suppressor in many types of human cancers
including lung cancer (29),
hepatocellular carcinoma (30) and
colorectal cancer (31). However,
the role of miR-194 in glioma remains unclear. In the present
study, we aimed to explore the biological role of miR-194 and the
underlying molecular mechanism in regulating glioma procession. We
found that miR-194 expression was significantly decreased in glioma
tissues and cell lines. Overexpression of miR-194 inhibited while
the suppression of miR-194 promoted cell migration, invasion and
EMT of glioma cells. Bioinformatic analysis indicated that Bmi1 was
the direct target of miR-194 in glioma cells by which miR-194
regulated the glioma EMT process. Our findings provide a better
understanding of the mechanism of glioma development and suggest
that targeting Bmi1 by miR-194 may serve as an attractive treatment
method for glioma.
Materials and methods
Tissue specimens and cell line
A total of 20 glioma specimens were
histopathologically diagnosed at the Department of Neurosurgery,
the Second Affiliated Hospital of Xi'an Jiaotong University (Xi'an,
China). The specimens were resected prior to any therapeutic
treatment. Normal brain tissues obtained from internal
decompression patients undergoing surgical operation were used as a
control. Prior written informed consent of the patients and
approval were obtained from the Institutional Human Experiment and
Ethic Committee of the Second Affiliated Hospital of Xi'an Jiaotong
University. This study was performed in accordance with the
Helsinki Declaration. U87, U251, SHG44 and A172 glioma cell lines
and normal human astrocytes (NHA) were purchased from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). All cells were routinely cultured in Dulbeccos modified
Eagles medium (DMEM; Gibco, Rockville, MD, USA) containing 10%
fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin mix
(Sigma-Aldrich, St. Louis, MO, USA) in a humidified atmosphere
containing 5% CO2 at 37°C.
Real-time quantitative polymerase
chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) as per the manufacturers
instructions. For the analysis of Bmi1 expression, RNA was
reverse-transcribed into cDNA by Moloney murine leukemia virus
(M-MLV) reverse transcriptase (Takara Bio, Dalian, China).
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an
internal control for normalization. For the analysis of miR-194
expression, RNA was reverse-transcribed into cDNA by TaqMan
MicroRNA reverse transcription kit (Applied Biosystems, Inc.,
Carlsbad, CA, USA). Small nuclear RNA U6 was used for
normalization. The PCR amplification was performed by using the
SYBR-Green Master Mix kit (Bio-Rad Laboratories, Hercules, CA, USA)
on an Applied Biosystems AB 7500 real-time PCR system (Applied
Biosystems). The primers used were as follows: miR-194, forward,
5-atggacctggggccacgaag-3 and reverse, 5-tctggcct gggagcgtcg-3; U6,
forward, 5-cgcttcggcagcacatatactaa-3 and reverse,
5-tatggaacgcttcacgaatttgc-3; Bmi1, forward, 5-atgca
tcgaacaacgagaatcaagatcact-3 and reverse, 5-tcaaccagaagaa
gttgctgatgaccc-3; and GAPDH, forward, 5-ccatgttcgtcatggtg tg-3 and
reverse, 5-ggtgctaagcagttggtggtg-3. Gene expression was calculated
using the 2−ΔΔCt method, normalized against U6 or GAPDH
and then compared with the control group.
Oligonucleotides, plasmids and
transfection
miR-194 mimics, miR-194 inhibitor and their negative
controls were purchased from Shanghai GenePharma, Co., Ltd.
(Shanghai, China). The open reading frame of Bmi1 was inserted into
the pcDNA3.0 vector (Invitrogen). All the transfections were
performed using Lipofectamine 2000 (Invitrogen) according to the
manufacturers instructions.
Migration and invasion assay
For cell migration, 1×105 cells
transfected with miR-194 mimics or miR-194 inhibitors were placed
in the upper chambers of a Transwell (Costar, Cambridge, MA, USA)
with serum-free medium and the lower chambers were filled with
medium containing 10% FBS. For cell invasion, the upper chambers
were coated with Matrigel (BD Biosciences, San Jose, CA, USA).
After being cultured for 24 h at 37°C, the non-migrated or
non-invaded cells on the top of the well were gently removed and
the cells on the lower surface of the membrane were fixed with 70%
ethanol and stained with 0.1% violet (Sigma-Aldrich). The cells
were observed and counted under a microscope (Olympus Corp., Tokyo,
Japan).
Western blot analysis
Proteins were extracted by cell lysis buffer and the
protein concentration was detected by a BCA kit (Beyotime Institute
of Biotechnology, Haimen, China). A total of 50 µg protein was
separated by SDS-PAGE and electrotransferred to a polyvinylidene
fluoride membrane (Bio-Rad Laboratories). The membrane was blocked
by 5% non-fat milk and then incubated with primary antibodies at
4°C overnight. The membrane was then incubated with horseradish
peroxidase-conjugated secondary antibodies (Bosis, Beijing, China)
for 1 h at 37°C. Ultimately, the membrane was detected by an ECL
western blotting kit (Pierce, Rockford, IL, USA) according to the
manufacturers instructions. The image was analyzed by Image-Pro
Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
The values were normalized against GAPDH and then were compared
with the control group. Primary antibodies used in this experiment
including anti-E-cadherin, anti-vimentin, anti-Bmi1 and anti-GAPDH
were purchased from Santa Cruz Biotechnology (Santa Cruz, CA,
USA).
Dual-Luciferase reporter assay
The Bmi1 3-UTR harboring the seed-matched sequences
of miR-194 with or without mutation was inserted into pmirGLO
Dual-Luciferase vector (Promega Corp., Madison, WI, USA). Glioma
cells were seeded into 24-well plates and co-transfected with
miR-194 mimics or miR-194 inhibitor and the constructed wild-type
or mutant pmirGLO-Bmi1 3-UTR into glioma cells using Lipofectamine
2000. After 48 h, the luciferase activity was analyzed by a
Dual-luciferase reporter assay system kit (Promega).
Data analysis
Data are reported as means ± standard deviation
(SD). Statistical analyses were performed using SPSS version 11.5
software (SPSS, Inc., Chicago, IL, USA). Differences were
calculated by the Students t-test or one-way analysis of variance
(ANOVA) followed by Bonferroni post hoc. When the P-value was
<0.05, the difference was considered to be statistically
significant.
Results
miR-194 is frequently lower in glioma
tissues and cell lines
To determine whether miR-194 is involved in glioma
development, we first analyzed the expression patterns of miR-194
in glioma specimens by RT-qPCR. The results showed that miR-194 was
frequently downregulated in glioma tissues compared with normal
brain tissue (Fig. 1A). Moreover,
the expression levels of miR-194 were also decreased in the four
glioma cell lines examined, particularly in A172 and U87, when
compared with normal human astrocytes (Fig. 1B). These results indicate that
miR-194 may play an important role in glioma.
miR-194 suppresses glioma cell
migration and invasion
To investigate the biological role of miR-194 in
glioma, we performed gain- and loss-of-function experiments by
transiently transfecting miR-194 mimics or miR-194 inhibitors in
A172 and U87 cells. We then detected the functional effect of
miR-194 on glioma cell migration and invasion by Transwell assay.
The results showed that miR-194 overexpression significantly
inhibited glioma cell migration (Fig.
2A and C) and invasion (Fig. 2B and
D) in A172 and U87 cells. Conversely, miR-194 suppression
markedly increased glioma cell migration (Fig. 2A and C) and invasion (Fig. 2B and D). These data imply a
suppressive role of miR-194 on glioma metastasis.
miR-194 suppresses EMT of glioma
cells
To further investigate the contribution of miR-194
on glioma metastasis, we examined the biological role of miR-194 in
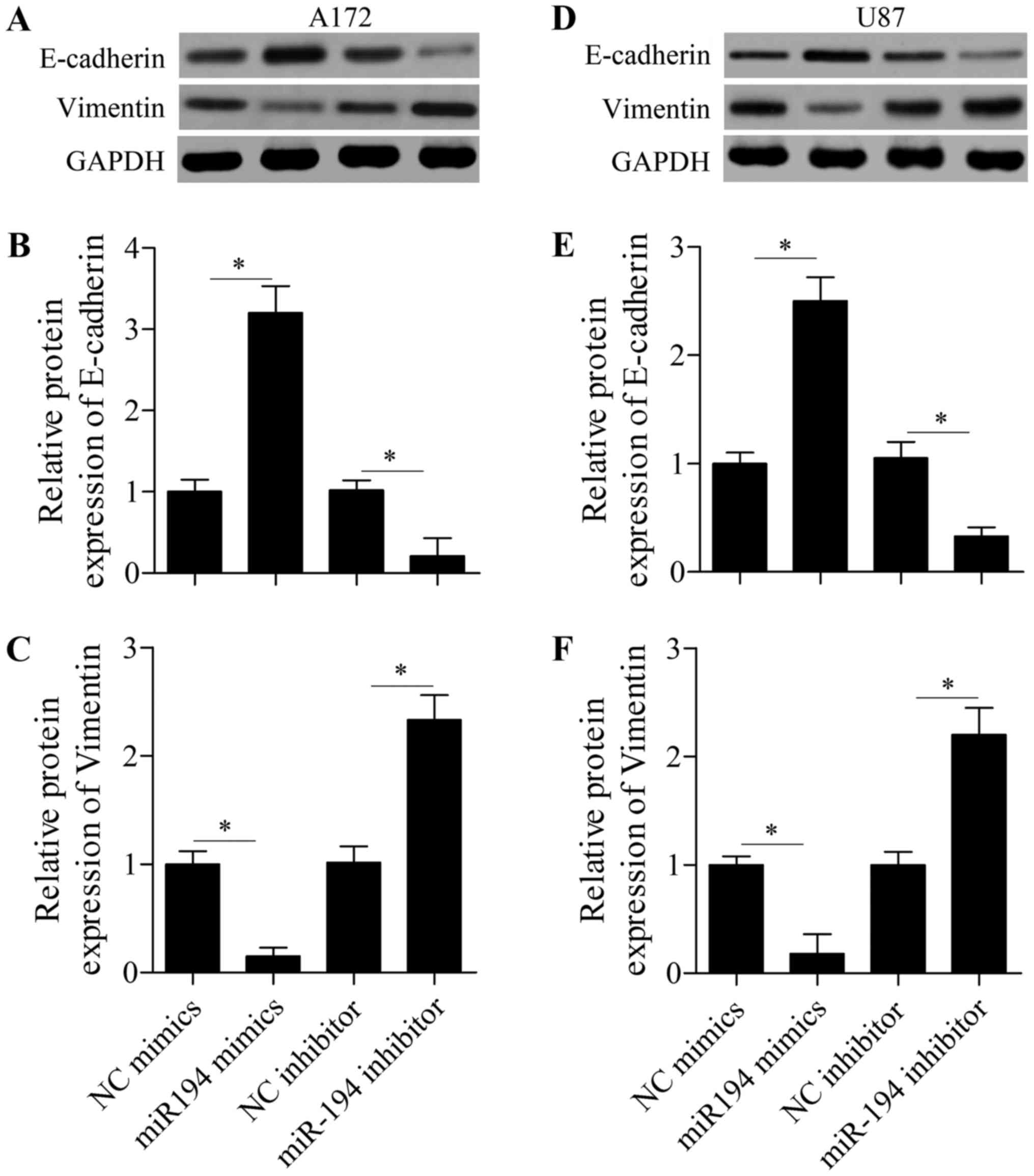
regulating glioma cell EMT. Western blot analysis showed that the
expression of epithelial marker E-cadherin was significantly
increased, whereas expression of the mesenchymal marker vimentin
was markedly decreased by miR-194 overexpression in A172 cells
(Fig. 3A-C). In contrast, miR-194
suppression showed the opposite effects (Fig. 3A-C). Similar results were also
observed in U87 cells (Fig. 3D-F),
suggesting a suppressive role of miR-194 in glioma cell EMT.
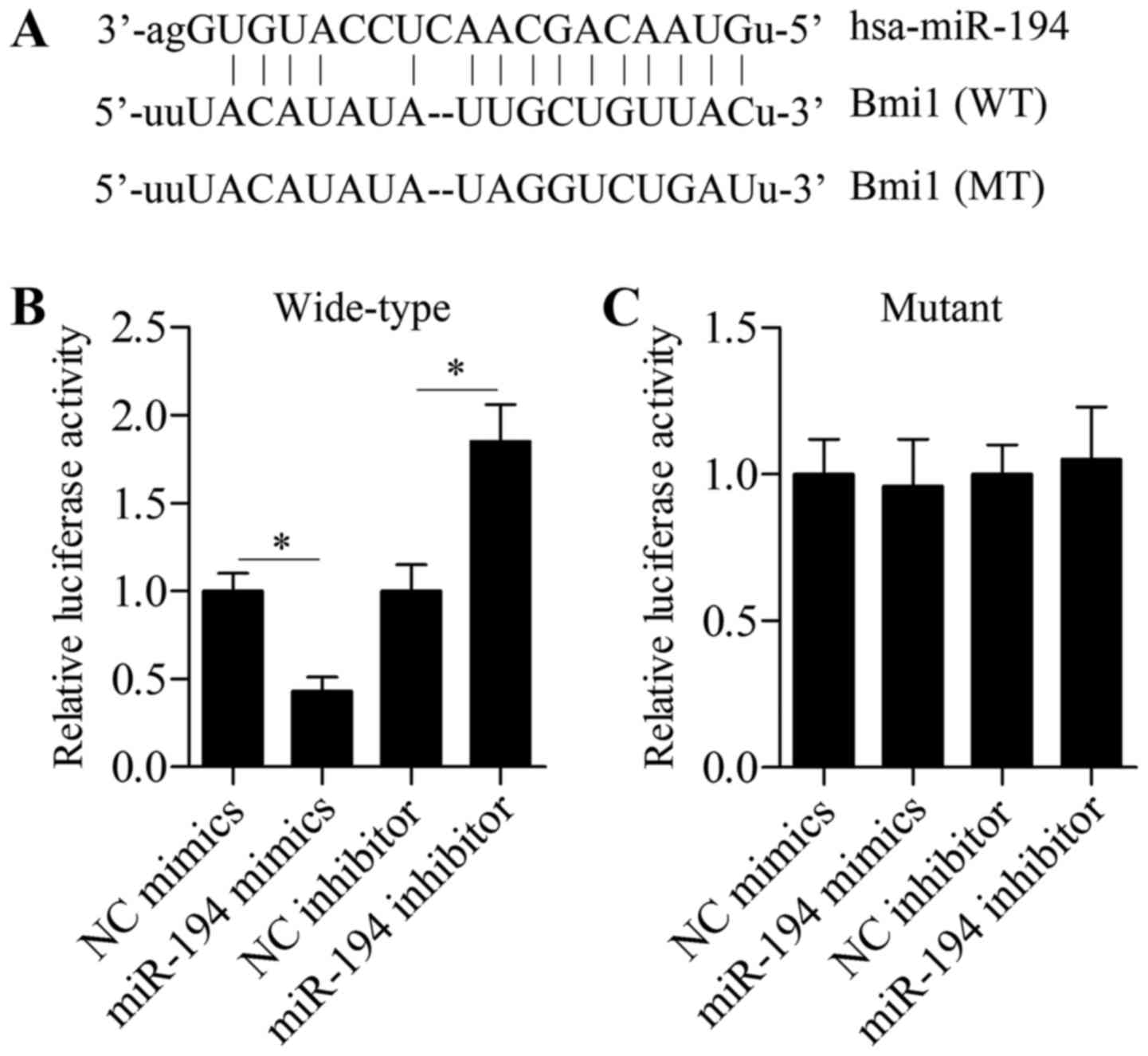
miR-194 directly targets Bmi1
To investigate the underlying molecular mechanism by
which miR-194 regulates glioma cell EMT, we used bioinformatic
analysis to identify the potential target of miR-194. Among these
predicted target genes, Bmi1, an important regulator for cancer
metastasis (22–24), caught our attention. The predicted
binding site between miR-194 and Bmi1 3-UTR is illustrated in
Fig. 4A. To verify that Bim1 is a
direct target gene of miR-194, we performed a Dual-Luciferase
reporter assay. The 3-UTR fragments of Bmi1, which contain a
wild-type or mutant binding site of miR-194, were cloned into the
luciferase reporter vector. Overexpression of miR-194 significantly
restrained the luciferase activity in U87 cells transfected with
luciferase reporter vector containing wild-type Bmi1 3-UTR, whereas
miR-194 suppression promoted the luciferase activity (Fig. 4B). However, neither miR-194
overexpression nor suppression showed an obvious effect on
luciferase activity of the luciferase reporter vector containing a
mutant 3-UTR (Fig. 4C). Next, we
further determined the direct effect of miR-194 on regulating Bmi1
expression. RT-qPCR analysis showed that the mRNA expression of
Bmi1 was significantly decreased or increased by miR-194
overexpression or suppression, respectively (Fig. 5A and B). Consistently, western blot
analysis identified that Bmi1 protein expression was regulated by
miR-194 (Fig. 5C and D). Taken
together, these results suggest that miR-194 regulates Bmi1
expression by directly targeting the 3-UTR of Bmi1.
miR-194 inhibits glioma EMT through
downregulating Bmi1
To verify whether the tumor suppressor role of
miR-194 in glioma is mediated by suppressing the expression of
Bmi1, we performed a rescue assay. Glioma cells were co-transfected
with miR-194 mimics and Bmi1-overexpressing vector. The decreased
protein expression of Bmi1 induced by miR-194 overexpression was
significantly restored by transfection with a Bmi1-overexpressing
vector (Fig. 6A and E). As
expected, the inhibitory role of miR-194 on glioma EMT was rescued
by Bmi1 overexpression (Fig. 6A-H).
These results suggest that the suppressive role of miR-194 on
glioma EMT is mediated by inhibiting Bmi1.
Discussion
It has now been clearly established that miRNAs play
an important role in regulating the tumorigenesis and metastasis of
glioma (32–34). Therefore, the identification of
glioma-associated miRNAs as biomarkers for glioma diagnosis,
prognosis and treatment is of great importance. In the present
study, we found that miR-194 was a novel glioma-associated miRNA
that was decreased in glioma specimens and cell lines. The
functional experiments indicated that miR-194 inhibited glioma cell
migration, invasion and EMT, possibly through targeting and
regulating Bmi1. Collectively, our data suggest that miR-194 plays
an important role in glioma procession, indicating a novel
therapeutic target for glioma.
Growing evidence has demonstrated that miR-194 is a
tumor-associated miRNA that is frequently dysregulated in many
types of human cancers. miR-194 expression was markedly decreased
in hepatocellular carcinoma and miR-194 overexpression inhibited
cell proliferation by targeting mitogen-activated protein kinase 4
(30). Other studies reported that
miR-194 was frequently downregulated in colorectal cancer (35,36).
miR-194 can inhibit colorectal carcinogenesis by targeting
mitogen-activated protein kinase 4 (37) or Akt2 (31). Several studies have reported that
miR-194 represses the progression and metastasis of non-small lung
cancer through targeting bone morphogenetic protein 1 and
p27kip1 (38), forkhead
box A1 protein (39) and human
nuclear distribution protein C (29). miR-194 can suppress gastric cancer
cell proliferation and EMT through targeting RING box protein 1
(40) and forkhead box protein M1
(41). The tumor suppressive role
of miR-194 is also observed in oral squamous cell carcinoma
(42), bladder cancer (43) and clear cell renal cell carcinoma
(44). However, the role of miR-194
in glioma remains unknown. In line with these findings, this study
demonstrated that miR-194 functioned as a tumor suppressor in
glioma. We found that miR-194 was decreased in glioma tissues and
cell lines. Overexpression of miR-194 inhibited glioma cell
migration, invasion and EMT through inhibiting Bmi1. However, an
oncogenic role of miR-194 is observed in pancreatic ductal
adenocarcinoma that promotes tumor growth and progression through
inhibiting the tumor suppressor DACH1 (45). Therefore, the precise role of
miR-194 in cancer progression requires further investigation.
Bmi1 has been suggested as an oncogene that is
frequently elevated in various cancer types and is correlated with
a worse prognosis (13). Bmi1 has
been reported to inhibit tumor suppressor genes including
p14ARF, p16INK4a, KIF1Bβ and TSLC1 (46). Bmi1 is a critical regulator for EMT
and cancer metastasis (47).
Knockdown of Bmi1 abolishes the ionizing irradiation-induced EMT of
breast cancer cells (48). Twist1
and Bmi-1 can act cooperatively to inhibit E-cadherin and
p16INK4a, leading to EMT induction (49). Bmi1 plays an important role in
development of the central nervous system; however, the
dysregulation of Bmi1 contributes to the development of brain
tumors (50,51). Overexpression of Bmi1 was found in
human medulloblastomas, correlated with activation of the sonic
hedgehog pathway (50). Bmi1 is
highly elevated in glioma tissues and patients with high levels of
Bmi1 show a poor survival rate (52). Bmi1 promotes glioma cell migration
and invasion via nuclear factor kappa B and p16 (25,28,53).
Consistently, our results showed that the inhibition of Bmi1 by
miR-194 significantly suppressed the glioma cell migration,
invasion and EMT, further implying that Bmi1 can serve as a
therapeutic target for preventing glioma metastasis.
In the present study, we demonstrated that miR-194
functions as an EMT regulator in glioma via targeting Bmi1. We
showed that miR-194 could directly target the 3-UTR of Bmi1 and
inhibit Bmi1 expression. Our findings are consistent with a
previous study which revealed that miR-194 suppresses the EMT of
endometrial cancer cells by targeting Bmi1 (54). The present study further confirms
that Bmi1 is a functional target of miR-194. A recent study
(55) suggested that targeting Bmi1
by miRNAs is a promising strategy for cancer prevention. Deng et
al (55) reported that miR-376c
suppresses cervical cancer cell proliferation and invasion by
targeting Bmi1. miR-494-3p enhances the radiosensitivity of oral
squamous carcinoma cells through targeting Bmi1 (56). miR-452 and miR-403 can inhibit the
metastasis of non-small cell lung cancer through inhibiting Bmi1
(57,58). miR-218, functioning as a negative
regulator of Bmi1, is also found in bladder cancer (59) and esophageal squamous cell carcinoma
(60). Besides these miRNAs,
miR-429, miR-200c, mIR-221 and miR-320a have been demonstrated to
directly target Bmi1 in various cancer cells (22,61–63).
These studies indicate that Bim1 undergoes epigenetic regulation by
various miRNAs that may contribute to the development of cancer. A
better understanding of Bmi1 by miRNAs will provide a new insight
into understanding glioma pathogenesis.
EMT is considered as major modulator of metastasis
formation in epithelial solid tumors, and the EMT of glioma has
also been widely studied (64).
Twist1, ZEB1/ZEB2 and SNAI1/SNAI2 have been suggested as the major
regulators for EMT in glioma (65–67).
Various miRNAs such as miR-200b, miR-590-3p and miR-181c are
reported to be involved in regulating EMT of glioma (68–70).
Our present study showed for the first time that miR-194 was
decreased in glioma which could inhibit glioma cell migration,
invasion and EMT. Importantly, we found that Bmi1 was a direct
target of miR-194 in glioma cells by which miR-194 inhibited glioma
cell EMT. miR-194 may serve as a novel biomarker as well as a
therapeutic target in glioma. However, further in vivo
studies are required to fully elucidate the exact role and
molecular mechanism of miR-194 in the regulation of glioma.
Acknowledgements
The present study was supported by the Social
Research and Development Program of Shaanxi Province (no.
2016SF-110).
Glossary
Abbreviations
Abbreviations:
|
Bmi1
|
B cell-specific moloney murine
leukemia virus insertion site
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
FBS
|
fetal bovine serum
|
|
miRNAs
|
microRNAs
|
|
RT-qPCR
|
real- time quantitative polymerase
chain reaction
|
|
UTR
|
3-untranslated region
|
References
|
1
|
Westphal M and Lamszus K: The neurobiology
of gliomas: From cell biology to the development of therapeutic
approaches. Nat Rev Neurosci. 12:495–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Furnari FB, Fenton T, Bachoo RM, Mukasa A,
Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, et al:
Malignant astrocytic glioma: Genetics, biology, and paths to
treatment. Genes Dev. 21:2683–2710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pollack IF: Neuro-oncology: Therapeutic
benefits of reirradiation for recurrent brain tumors. Nat Rev
Neurol. 6:533–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khasraw M and Lassman AB: Neuro-oncology:
Late neurocognitive decline after radiotherapy for low-grade
glioma. Nat Rev Neurol. 5:646–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pang JC, Kwok WK, Chen Z and Ng HK:
Oncogenic role of microRNAs in brain tumors. Acta Neuropathol.
117:599–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Silber J, James CD and Hodgson JG:
microRNAs in gliomas: Small regulators of a big problem.
Neuromolecular Med. 11:208–222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manikandan J, Aarthi JJ, Kumar SD and
Pushparaj PN: Oncomirs: The potential role of non-coding microRNAs
in understanding cancer. Bioinformation. 2:330–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Dutta A and Abounader R: The role
of microRNAs in glioma initiation and progression. Front Biosci
(Landmark Ed). 17:700–712. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Besse A, Sana J, Fadrus P and Slaby O:
MicroRNAs involved in chemo- and radioresistance of high-grade
gliomas. Tumour Biol. 34:1969–1978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao L, Bombard J, Cintron K, Sheedy J,
Weetall ML and Davis TW: BMI1 as a novel target for drug discovery
in cancer. J Cell Biochem. 112:2729–2741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guney I, Wu S and Sedivy JM: Reduced c-Myc
signaling triggers telomere-independent senescence by regulating
Bmi-1 and p16INK4a. Proc Natl Acad Sci USA. 103:3645–3650. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Silva J, García JM, Peña C, García V,
Domínguez G, Suárez D, Camacho FI, Espinosa R, Provencio M, España
P, et al: Implication of polycomb members Bmi-1, Mel-18, and Hpc-2
in the regulation of p16INK4a, p14ARF, h-TERT, and c-Myc expression
in primary breast carcinomas. Clin Cancer Res. 12:6929–6936. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu J, Hu D, Yang G, Zhou J, Yang C, Gao Y
and Zhu Z: Down-regulation of BMI-1 cooperates with artemisinin on
growth inhibition of nasopharyngeal carcinoma cells. J Cell
Biochem. 112:1938–1948. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu Z, Min L, Chen D, Hao D, Duan Y, Qiu G
and Wang Y: Overexpression of BMI-1 promotes cell growth and
resistance to cisplatin treatment in osteosarcoma. PLoS One.
6:e146482011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jacobs JJ, Scheijen B, Voncken JW, Kieboom
K, Berns A and van Lohuizen M: Bmi-1 collaborates with c-Myc in
tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF.
Genes Dev. 13:2678–2690. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang L, Li J and Song L: Bmi-1, stem
cells and cancer. Acta Biochim Biophys Sin (Shanghai). 41:527–534.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Itahana K, Zou Y, Itahana Y, Martinez JL,
Beausejour C, Jacobs JJ, Van Lohuizen M, Band V, Campisi J and
Dimri GP: Control of the replicative life span of human fibroblasts
by p16 and the polycomb protein Bmi-1. Mol Cell Biol. 23:389–401.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiu M, Liang Z, Chen L, Tan G, Wang K, Liu
L, Liu J and Chen H: MicroRNA-429 suppresses cell proliferation,
epithelial-mesenchymal transition, and metastasis by direct
targeting of BMI1 and E2F3 in renal cell carcinoma. Urol Oncol.
33:332.e9–18. 2015. View Article : Google Scholar
|
|
23
|
Wei XL, Dou XW, Bai JW, Luo XR, Qiu SQ, Xi
DD, Huang WH, Du CW, Man K and Zhang GJ: ERα inhibits
epithelial-mesenchymal transition by suppressing Bmi1 in breast
cancer. Oncotarget. 6:21704–21717. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paranjape AN, Balaji SA, Mandal T, Krushik
EV, Nagaraj P, Mukherjee G and Rangarajan A: Bmi1 regulates
self-renewal and epithelial to mesenchymal transition in breast
cancer cells through Nanog. BMC Cancer. 14:7852014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang J, Wang P, Xie S, Wang W, Zhou X, Hu
J, Shi Q, Zhang X and Yu R: Bmi-1 regulates the migration and
invasion of glioma cells through p16. Cell Biol Int. 39:283–290.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tu Y, Gao X, Li G, Fu H, Cui D, Liu H, Jin
W and Zhang Y: MicroRNA-218 inhibits glioma invasion, migration,
proliferation, and cancer stem-like cell self-renewal by targeting
the polycomb group gene Bmi1. Cancer Res. 73:6046–6055. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang L, Song L, Wu J, Yang Y, Zhu X, Hu
B, Cheng SY and Li M: Bmi-1 promotes glioma angiogenesis by
activating NF-κB signaling. PLoS One. 8:e555272013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang L, Wu J, Yang Y, Liu L, Song L, Li J
and Li M: Bmi-1 promotes the aggressiveness of glioma via
activating the NF-kappaB/MMP-9 signaling pathway. BMC Cancer.
12:4062012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou L, Di Q, Sun B, Wang X, Li M and Shi
J: MicroRNA-194 restrains the cell progression of non-small cell
lung cancer by targeting human nuclear distribution protein C.
Oncol Rep. 35:3435–3444. 2016.PubMed/NCBI
|
|
30
|
Zhao Y, Li F, Zhang X, Liu A, Qi J, Cui H
and Zhao P: MicroRNA-194 acts as a prognostic marker and inhibits
proliferation in hepatocellular carcinoma by targeting MAP4K4. Int
J Clin Exp Pathol. 8:12446–12454. 2015.PubMed/NCBI
|
|
31
|
Zhao HJ, Ren LL, Wang ZH, Sun TT, Yu YN,
Wang YC, Yan TT, Zou W, He J, Zhang Y, et al: MiR-194 deregulation
contributes to colorectal carcinogenesis via targeting AKT2
pathway. Theranostics. 4:1193–1208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwak PB, Iwasaki S and Tomari Y: The
microRNA pathway and cancer. Cancer Sci. 101:2309–2315. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karsy M, Arslan E and Moy F: Current
progress on understanding MicroRNAs in glioblastoma multiforme.
Genes Cancer. 3:3–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tivnan A and McDonald KL: Current progress
for the use of miRNAs in glioblastoma treatment. Mol Neurobiol.
48:757–768. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chiang Y, Song Y, Wang Z, Liu Z, Gao P,
Liang J, Zhu J, Xing C and Xu H: microRNA-192, −194 and −215 are
frequently downregulated in colorectal cancer. Exp Ther Med.
3:560–566. 2012.PubMed/NCBI
|
|
36
|
Basati G, Razavi AE, Pakzad I and Malayeri
FA: Circulating levels of the miRNAs, miR-194, and miR-29b, as
clinically useful biomarkers for colorectal cancer. Tumour Biol.
37:1781–1788. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang B, Shen ZL, Gao ZD, Zhao G, Wang CY,
Yang Y, Zhang JZ, Yan YC, Shen C, Jiang KW, et al: MiR-194,
commonly repressed in colorectal cancer, suppresses tumor growth by
regulating the MAP4K4/c-Jun/MDM2 signaling pathway. Cell Cycle.
14:1046–1058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu X, Liu T, Fang O, Leach LJ, Hu X and
Luo Z: miR-194 suppresses metastasis of non-small cell lung cancer
through regulating expression of BMP1 and p27kip1. Oncogene.
33:1506–1514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu X, Li D, Yu F, Jia C, Xie J, Ma Y, Fan
S, Cai H, Luo Q, Lv Z, et al: miR-194 inhibits the proliferation,
invasion, migration, and enhances the chemosensitivity of non-small
cell lung cancer cells by targeting forkhead box A1 protein.
Oncotarget. 7:13139–13152. 2016.PubMed/NCBI
|
|
40
|
Chen X, Wang Y, Zang W, Du Y, Li M and
Zhao G: miR-194 targets RBX1 gene to modulate proliferation and
migration of gastric cancer cells. Tumour Biol. 36:2393–2401. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Z, Ying X, Chen H, Ye P, Shen Y, Pan W
and Zhang L: MicroRNA-194 inhibits the epithelial-mesenchymal
transition in gastric cancer cells by targeting FoxM1. Dig Dis Sci.
59:2145–2152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chi H: miR-194 regulated AGK and inhibited
cell proliferation of oral squamous cell carcinoma by reducing
PI3K-Akt-FoxO3a signaling. Biomed Pharmacother. 71:53–57. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang M, Zhuang Q and Cui L: MiR-194
inhibits cell proliferation and invasion via repression of RAP2B in
bladder cancer. Biomed Pharmacother. 80:268–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nofech-Mozes R, Khella HW, Scorilas A,
Youssef L, Krylov SN, Lianidou E, Sidiropoulos KG, Gabril M, Evans
A and Yousef GM: MicroRNA-194 is a marker for good prognosis in
clear cell renal cell carcinoma. Cancer Med. 5:656–664. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang J, Zhao CY, Zhang SH, Yu DH, Chen Y,
Liu QH, Shi M, Ni CR and Zhu MH: Upregulation of miR-194
contributes to tumor growth and progression in pancreatic ductal
adenocarcinoma. Oncol Rep. 31:1157–1164. 2014.PubMed/NCBI
|
|
46
|
Kamijo T: Role of stemness-related
molecules in neuroblastoma. Pediatr Res. 71:511–515. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ren H, Du P, Ge Z, Jin Y, Ding D, Liu X
and Zou Q: TWIST1 and BMI1 in cancer metastasis and
chemoresistance. J Cancer. 7:1074–1080. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yuan W, Yuan Y, Zhang T and Wu S: Role of
Bmi-1 in regulation of ionizing irradiation-induced
epithelial-mesenchymal transition and migration of breast cancer
cells. PLoS One. 10:e01187992015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY,
Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, et al: Bmi1 is
essential in Twist1-induced epithelial-mesenchymal transition. Nat
Cell Biol. 12:982–992. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Leung C, Lingbeek M, Shakhova O, Liu J,
Tanger E, Saremaslani P, Van Lohuizen M and Marino S: Bmi1 is
essential for cerebellar development and is overexpressed in human
medulloblastomas. Nature. 428:337–341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Molofsky AV, Pardal R, Iwashita T, Park
IK, Clarke MF and Morrison SJ: Bmi-1 dependence distinguishes
neural stem cell self-renewal from progenitor proliferation.
Nature. 425:962–967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu Z, Wang Q, Wang L, Li G, Liu H, Fan F,
Li Z, Li Y and Tu Y: Combined aberrant expression of Bmi1 and EZH2
is predictive of poor prognosis in glioma patients. J Neurol Sci.
335:191–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sun P, Mu Y and Zhang S: A novel
NF-κB/MMP-3 signal pathway involves in the aggressivity of glioma
promoted by Bmi-1. Tumour Biol. 35:12721–12727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dong P, Kaneuchi M, Watari H, Hamada J,
Sudo S, Ju J and Sakuragi N: MicroRNA-194 inhibits epithelial to
mesenchymal transition of endometrial cancer cells by targeting
oncogene BMI-1. Mol Cancer. 10:992011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Deng Y, Xiong Y and Liu Y: miR-376c
inhibits cervical cancer cell proliferation and invasion by
targeting BMI1. Int J Exp Pathol. 97:257–265. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Weng JH, Yu CC, Lee YC, Lin CW, Chang WW
and Kuo YL: miR-494-3p induces cellular senescence and enhances
radiosensitivity in human oral squamous carcinoma cells. Int J Mol
Sci. 17:172016. View Article : Google Scholar
|
|
57
|
He Z, Xia Y, Pan C, Ma T, Liu B, Wang J,
Chen L and Chen Y: Up-regulation of miR-452 inhibits metastasis of
non-small cell lung cancer by regulating BMI1. Cell Physiol
Biochem. 37:387–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen T, Xu C, Chen J, Ding C, Xu Z, Li C
and Zhao J: MicroRNA-203 inhibits cellular proliferation and
invasion by targeting Bmi1 in non-small cell lung cancer. Oncol
Lett. 9:2639–2646. 2015.PubMed/NCBI
|
|
59
|
Cheng Y, Yang X, Deng X, Zhang X, Li P,
Tao J and Lu Q: MicroRNA-218 inhibits bladder cancer cell
proliferation, migration, and invasion by targeting BMI-1. Tumour
Biol. 36:8015–8023. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang T, Chen T, Niu H, Li C, Xu C, Li Y,
Huang R, Zhao J and Wu S: MicroRNA-218 inhibits the proliferation
and metastasis of esophageal squamous cell carcinoma cells by
targeting BMI1. Int J Mol Med. 36:93–102. 2015.PubMed/NCBI
|
|
61
|
Xuan H, Xue W, Pan J, Sha J, Dong B and
Huang Y: Downregulation of miR-221, −30d, and −15a contributes to
pathogenesis of prostate cancer by targeting Bmi-1. Biochemistry
(Mosc). 80:276–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu L, Qiu M, Tan G, Liang Z, Qin Y, Chen
L, Chen H and Liu J: miR-200c inhibits invasion, migration and
proliferation of bladder cancer cells through down-regulation of
BMI-1 and E2F3. J Transl Med. 12:3052014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Qi X, Li J, Zhou C, Lv C and Tian M:
MicroRNA-320a inhibits cell proliferation, migration and invasion
by targeting BMI-1 in nasopharyngeal carcinoma. FEBS Lett.
588:3732–3738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kahlert UD, Nikkhah G and Maciaczyk J:
Epithelial-to-mesenchymal(−like) transition as a relevant molecular
event in malignant gliomas. Cancer Lett. 331:131–138. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Elias MC, Tozer KR, Silber JR, Mikheeva S,
Deng M, Morrison RS, Manning TC, Silbergeld DL, Glackin CA, Reh TA,
et al: TWIST is expressed in human gliomas and promotes invasion.
Neoplasia. 7:824–837. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Han SP, Kim JH, Han ME, Sim HE, Kim KS,
Yoon S, Baek SY, Kim BS and Oh SO: SNAI1 is involved in the
proliferation and migration of glioblastoma cells. Cell Mol
Neurobiol. 31:489–496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mikheeva SA, Mikheev AM, Petit A, Beyer R,
Oxford RG, Khorasani L, Maxwell JP, Glackin CA, Wakimoto H,
González-Herrero I, et al: TWIST1 promotes invasion through
mesenchymal change in human glioblastoma. Mol Cancer. 9:1942010.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Pang H, Zheng Y, Zhao Y, Xiu X and Wang J:
miR-590-3p suppresses cancer cell migration, invasion and
epithelial-mesenchymal transition in glioblastoma multiforme by
targeting ZEB1 and ZEB2. Biochem Biophys Res Commun. 468:739–745.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li J, Yuan J, Yuan X, Zhao J, Zhang Z,
Weng L and Liu J: MicroRNA-200b inhibits the growth and metastasis
of glioma cells via targeting ZEB2. Int J Oncol. 48:541–550.
2016.PubMed/NCBI
|
|
70
|
He X, Liu Z, Peng Y and Yu C:
MicroRNA-181c inhibits glioblastoma cell invasion, migration and
mesenchymal transition by targeting TGF-β pathway. Biochem Biophys
Res Commun. 469:1041–1048. 2016. View Article : Google Scholar : PubMed/NCBI
|