Introduction
Globally, gastric cancer (GC) is one of the most
common malignancies of the digestive tract with a 5-year patient
survival rate of less than 25%, and represents the second leading
cause of cancer-related deaths worldwide (1,2). Tumor
growth and metastasis of GC are major causes of increased
mortality. Unfortunately, due to the absence of biomarkers for
early detection, most patients are often diagnosed in advanced
stages of metastasis (3). Despite
identification of the molecular mechanisms, the pathophysiology of
GC remains largely obscure. Therefore, investigation of the
regulatory mechanisms underlying the suppression of proliferation
and metastasis of GC cells holds the key to effective diagnosis and
treatment of GC.
MicroRNAs (miRNAs) regulate multiple genes and
target pathways in several human diseases, including cancer, and
therefore, represent potential therapeutic targets (4–6).
miRNAs are endogenous small non-coding RNAs consisting of 19–24
nucleotides, each. They regulate gene expression by targeting the
3′-untranslated region of cancer-related mRNAs and function as a
‘fine-tuner’ in the most important biological processes, including
proliferation, apoptosis, invasion and metastasis (7–9). These
miRNAs have been shown to modulate pathways involved in GC cell
proliferation and metastasis (10,11).
Nevertheless, further investigation into the molecular mechanisms
of miRNAs in GC is essential for early diagnosis or effective
therapeutic intervention. Noteworthy, Rawlings-Goss et al
found that miR-647 is associated with the most extensive cancers
and may serve as a biomarker for GC (12). A recent study also reported that
overexpression of miR-647 was associated with a better prognosis of
Taxol-resistant ovarian cancer (13). However, the underlying role of
miR-647 in GC remains indeterminate.
In the present study, miR-647 was found to play a
tumor-suppressor role since inhibition of miR-647 expression in GC
was strongly associated with tumor size and metastasis.
Overexpression of miR-647 in GC cell lines in vitro and
in vivo, inhibited proliferation and metastasis of GC via
transcriptional regulation of genes including ANK2,
FAK, MMP2, MMP12, CD44 and
SNAIL1.
Materials and methods
Cell lines and clinical samples
Human GC samples and their corresponding adjacent
non-tumor gastric tissues (located ~5 cm away from the tumor) were
collected during surgical resection at The First Affiliated
Hospital of Guangxi Medical University (Nanning, China) from 2013
to 2015. All samples were immediately frozen on dry ice and
preserved in liquid nitrogen. All the patients provided written
informed consent. The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Guangxi Medical
University. Four human GC cell lines (HGC-27, AGS, SGC-7901 and
MGC-803) as well as immortalized gastric epithelial GES-1 cells
were obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in RPMI-1640 medium (GE
Healthcare Life Sciences, South Logan, UT, USA) supplemented with
50 mg/ml penicillin, 100 mg/ml streptomycin, and 10% fetal bovine
serum (FBS) at 37.8°C and 5.0% CO2.
RNA extraction and quantitative
reverse transcription real-time polymerase chain reaction
(qRT-PCR)
RNAs were extracted from tissue samples and cells
using TRIzol (Invitrogen Corporation, Carlsbad, CA, USA). The cDNA
was synthesized from 1,000 ng RNA samples using PrimeScript™ RT
reagent kit (Takara Bio, Inc., Tokyo, Japan). SYBR®
Premix Ex Taq™ II (Tli RNaseH Plus) and a ROX Plus reagent kit
(Takara Bio, Inc.) were used to amplify mRNA or miRNA expression
with GAPDH (mRNA) or U6 (miRNA) as internal standards. The mRNA and
miRNA expression was analyzed using the 2−ΔΔCt method.
All primer sequences are listed in Table I.
 | Table I.Sequences of theprimers for
quantitative reverse transcriptase real-time polymerase chain
reaction. |
Table I.
Sequences of theprimers for
quantitative reverse transcriptase real-time polymerase chain
reaction.
| Gene |
| Primer
sequences |
|---|
| miR-647 | (F) |
5′-GUGGCUGCACUCACUUCCUUC-3′ |
| miR-647 | (R) |
5′-CTCAACTGGTGTCGTGGA-3′ |
| U6 | (F) |
5-TTATGGGTCCTAGCCTGAC-3 |
| U6 | (R) |
5′-CACTATTGCGGGTCTGC-3′ |
| ANK2 | (F) |
5′-CAGCTACATTGTGTGGCATTCTA-3′ |
| ANK2 | (R) |
5′-CTACAGTGCAGTGGCCAGAAG-3′ |
| ZNF609 | (F) |
5′-TGCCTTTGTACATTCAGGCAAGA-3′ |
| ZNF609 | (R) |
5′-AGGCAGCTCAGCAGCAAACTC-3′ |
| KNDC1 | (F) |
5′-CTCTCACGGTGCCAATCACAA-3′ |
| KNDC1 | (R) |
5′-TGCCACTAGCTGGCTGCTCTAA-3′ |
| TRIM4 | (F) |
5′-CAGTGTGCCAGATACCATTGATGA-3′ |
| TRIM4 | (R) |
5′-CCTCCATCCAGCAAAGTAGTTCC-3′ |
| FAK | (F) |
5′-CAACCACCTGGGCCAGTATTATC-3′ |
| FAK | (R) |
5′-CCATAGCAGGCCACATGCTTTA-3′ |
| MMP2 | (F) |
5′-CCGTGTTTGCCATCTGTTTTAG-3′ |
| MMP2 | (R) |
5′-AGGTTCTCTTGCTGTTTACTTTGGA-3′ |
| MMP12 | (F) |
5′-ACGTGGCATTCAGTCCCTGT-3′ |
| MMP12 | (R) |
5′-AACACTGGTCTTTGGTCTCTCAGAA-3′ |
| CD44 | (F) |
5′-CAGGGCTGGGCTTAGACAGA-3′ |
| CD44 | (R) |
5′-CTGGCCAATGATGTTCACAGA-3′ |
| SNAIL1 | (F) |
5′-CAGACCCACTCAGATGTCAAGAA-3′ |
| SNAIL1 | (R) |
5′-GGGCAGGTATGGAGAGGAAGA-3′ |
| GAPDH | (F) |
5′-GCACCGTCAAGGCTGAGAAC-3′ |
| GAPDH | (R) |
5′-TGGTGAAGACGCCAGTGGA-3′ |
Cell invasion assay
The Transwell insert was coated with 75 µl (1 µg/ml)
Matrigel (BD Biosciences, Mountain View, CA, USA) and
5.0×104 cells in 200 µl serum-free media were
transferred to the upper chamber (6.5 mm; Corning, New York, NY,
USA). The lower compartment was filled with 700 µl medium
containing 5% FBS as a chemoattractant. After culturing for 24 h,
the cells in the lower chamber were stained with Giemsa and the
migrating cells were counted under a microscope from 8 randomly
selected fields (magnification, ×200).
Transfection
The miR-647 mimic and the null vector LV-GFP were
purchased from GeneChem (Shanghai, China). Cells were infected with
the lentiviral vector at 25 PFU/cell (SGC-7901) or 60 PFU/cell
(MGC-803) [multiplicity of infection (MOI), 25 or 60] at a cell
confluency of 35%. Six groups of cells were identified as follows.
Cells in the 7901-Ctrl (or 803-Ctrl) represent SGC-7901 (or
MGC-803) cells without any treatment. Cells in the 7901-NC (or
803-NC) group were SGC-7901 (or MGC-803) cells transfected with the
negative control lentiviral vector LV-GFP, and cells in the
7901–647 (or 803–647) group were SGC-7901 (or MGC-803) cells
transfected with the recombinant lentivirus vector miR-647 mimic.
After 24 h of transfection, the cells were cultured under normal
conditions. The cells were then harvested and used.
Cell proliferation assay
Cell proliferation was assessed using the Cell
Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan). Cells in each
group were transferred into 96-well plates at a density of
1.5×103/well and incubated at 37°C and 5% CO2
for 24, 48, 72 or 96 h followed by the addition of 10 µl of CCK-8
reagent into each well and incubation at 37°C for 4 h. The
absorbance was read at 450 nm using a microplate reader.
Cell cycle assay
Flow cytometry was performed to analyze the cell
cycle in 1×106 trypsinized cells, fixed with 70% ethanol
and stored at 4°C for 12 h. The cells were incubated in a 100 µl
solution containing 200 ng/ml RNase at 37°C for 30 min. Propidium
iodide (PI) (400 µl) was added. The cells were incubated at ambient
temperature for 30 min in the dark. The results were analyzed using
flow cytometry (BD Biosciences).
Cell apoptosis assay
An apoptosis detection kit (BD Biosciences) was used
to determine the cellular apoptosis by incubating 2×106
cells with 100 ml 1X binding buffer containing 50 µl/ml Annexin
V-PE and 50 µl/ml 7-amino-actinomycin D. After incubation at an
ambient temperature in the dark for 30 min, an additional 400 µl 1X
binding buffer was added. The results were analyzed by flow
cytometry (BD Biosciences).
Transmission electron microscopy
(TEM)
The ultrastructure of the cells was observed using
TEM. The cells were fixed with 2.5% glutaraldehyde at 4°C overnight
and post-fixed in 1% osmium tetroxide for 30 min, dehydrated in a
graded series of ethanol baths, and embedded in Epon. Ultrathin
sections were stained with uranyl acetate and lead citrate, and
observed under a JEM-2000EX transmission electron microscope (Jeol,
Tokyo, Japan).
Wound-healing assay
Cell migration was measured by culturing the GC
cells in 6-well plates, and a straight line scratch wound was
created with a 200-µl sterile pipette tip once cells attained 100%
confluency. The wound conditions were monitored at 0, 24 and 48 h
microscopically and the relative motility was calculated using the
following formula: Relative motility = (initial distance - a time
point distance)/initial distance × 100%.
Animal studies
All animals were provided by the Guangxi Animal
Center (Nanning, China), and the animal studies were approved by
the Ethics Committee of The First Affiliated Hospital of Guangxi
Medical University. In vivo tumor growth was conducted with
2×106 GC cells in each group re-suspended in 100 µl of
phosphate-buffered saline (PBS; Beyotime Institute of
Biotechnology, Shanghai, China) and the cells were subcutaneously
inoculated in the armpit region, respectively, of 5- to 6-week-old
BALB/c nude mice (8 mice/group). After 3 days, the tumors increased
in size to ~5.0 mm in diameter. The tumor size was measured every 3
days using a Vernier caliper and calculated by the formula: Tumor
volume = a × b2/2 (a, length diameter; b, width
diameter). The relative tumor volume (RTV) was estimated as
follows: RTV = Vt/V0 (V0, initial
tumor volume; Vt, current tumor volume at the time of
measurement). Mice were euthanized 21 days after inoculation of the
cells. The xenografts were excised and embedded in paraffin for
hematoxylin and eosin staining.
To study metastasis in vivo, 8×104
cells of each group were re-suspended in 100 µl PBS, and
intravenously injected into the tail vein of 5- to 6-week-old
BALB/c nude mice (10 mice/group). After 36 days, lungs and livers
were excised and embedded in paraffin for further histopathological
analysis. The number of mice with metastasis was counted.
Microarray analysis and miR-647 target
prediction
The microarray data obtained in the present study
from cells in the 803-NC and 803–647 groups were deposited in the
UniGene database 219. RNA samples were analyzed by microarray
expression profiling using the GeneChip Hybridization Wash and
Stain kit (Affymetrix, Santa Clara, CA, USA) according to the
manufacturer's instructions. All the raw data were normalized by
quantile method and analyzed. Genes upregulated and downregulated
>1.5-fold in the 803–647 group compared with those in the 803-NC
were selected. The target genes of miR-647 were analyzed by
TargetScan (http://www.targetscan.org),
microRNA.org (http://www.microrna.org/microrna/home.do) and MiRanda
(http://www.miranda.com/).
Western blotting
Cell protein lysates were separated by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
to polyvinylidene fluoride (PVDF) membranes. After blocking with 5%
non-fat milk, the membranes were incubated with the primary
antibody overnight at 4°C and washed with Tris-buffered saline and
Tween-20 (TBST). The membranes were incubated with a dilution of
infrared-labeled secondary antibody (1:10,000) for 1 h. After
washing twice with TBST, the optical density was quantified using
software provided with the Odyssey scanner (LI-COR Biosciences,
Lincoln, NE, USA).
Statistical analysis
Data are presented as mean ± SE as analyzed by SPSS
13.0 (SPSS, Inc., Chicago, IL, USA), and deemed to have statistical
significance at P<0.05 using the Student's t-test, one-way
analysis of variance (ANOVA) or χ2 test.
Results
Downregulation of miR-647 in GC
correlates with proliferation and metastasis
To determine the role of miR-647 in the pathogenesis
of GC, we investigated the miR-647 levels in GC tissue specimens
and the corresponding non-tumor samples of 70 GC patients and 4 GC
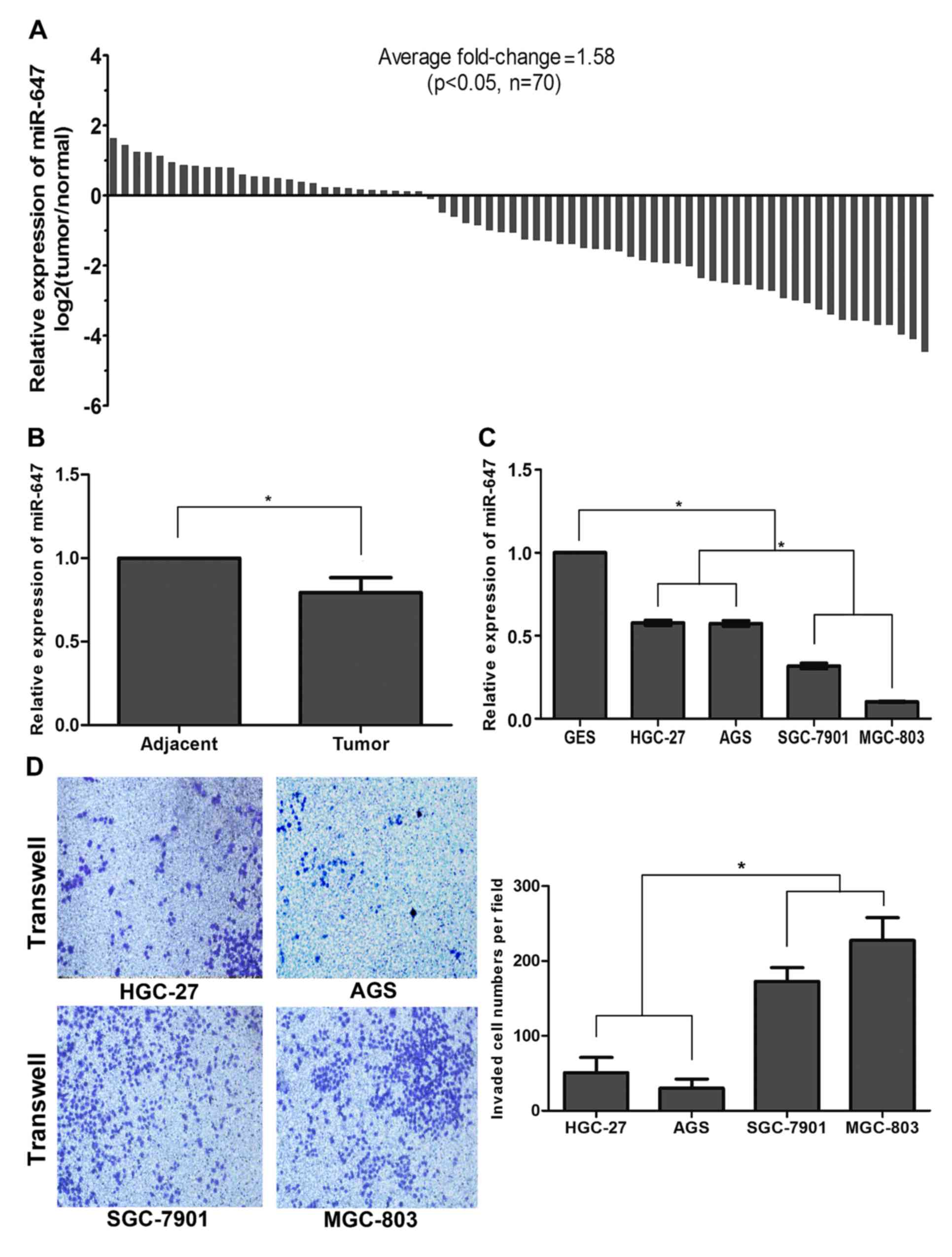
cell lines using qRT-PCR. As shown in Fig. 1A, compared with the corresponding
non-tumor samples, the miR-647 level in the GC tissue specimens was
significantly decreased in 43 of 70 patients (P<0.05). The
histogram also demonstrated an average 0.79±0.09-fold
downregulation of miR-647 in the GC tissue specimens compared with
that noted in the non-tumor samples (P<0.05; Fig. 1B). To address the clinical
significance of the downregulation of miR-647 in GC, the
relationship between miR-647 expression and clinical pathology was
determined (Table II). The GC
samples were assigned a cut-off point to distinguish tumors with
low-level expression of miR-647 from those with high-level
expression of miR-647. Correlation analysis showed that the
low-level expression of miR-647 in GC was significantly associated
with tumor size, tumor-node-metastasis (TNM) staging and metastasis
including lymph node metastasis and distant metastasis that
characterize more aggressive tumor phenotypes (P<0.05; Table II).
 | Table II.Correlations between miR-647
expression and clinical characteristics of the gastric cancer
patients (n=70). |
Table II.
Correlations between miR-647
expression and clinical characteristics of the gastric cancer
patients (n=70).
|
|
| miR-647 |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Total cases | High (n) | Low (n) | P-value |
|---|
| Age, years |
|
|
| 0.478 |
|
|
<60 | 40 | 14 | 26 |
|
|
≥60 | 30 | 13 | 17 |
|
| Gender |
|
|
| 0.698 |
|
Male | 50 | 20 | 30 |
|
|
Female | 20 | 7 | 13 |
|
| Tumor size
(cm) |
|
|
| 0.012a |
|
>3 | 57 | 18 | 39 |
|
| ≤3 | 13 | 9 | 4 |
|
| TNM staging |
|
|
| 0.007a |
|
I/II | 30 | 17 | 13 |
|
|
III/IV | 40 | 10 | 30 |
|
| Node
metastasis |
|
|
| 0.007a |
|
None | 23 | 14 | 9 |
|
|
Metastasis | 47 | 13 | 34 |
|
| Distant
metastasis |
|
|
| 0.028a |
|
None | 49 | 23 | 26 |
|
|
Metastasis | 21 | 4 | 17 |
|
|
Differentiation |
|
|
| 0.797 |
| Poor or
undifferentiated | 48 | 19 | 29 |
|
|
Moderate or well | 22 | 8 | 14 |
|
Since miR-647 was downregulated in GC and associated
with tumor growth and metastasis, we speculated that it may play a
similar role in GC cells. The expression of miR-647 was also
investigated. Consistent with the results in GC patients, the
expression of miR-647 in the HGC-27, AGS, SGC-7901 and MGC-803 cell
lines was significantly downregulated with a fold-change of
0.58±0.03, 0.57±0.03, 0.32±0.03 and 0.10±0.00, respectively,
compared with that noted in the GES-1 cells (P<0.05; Fig. 1C). We next investigated the invasive
ability of the GC cell lines using the Transwell assay. The data
demonstrated that the GC cell lines MGC-803 and SGC-7901 displayed
a stronger invasive ability with lower levels of miR-647 compared
with HGC-27 and AGS cell lines (P<0.05; Fig. 1D). Overall, these results suggest
that miR-647 expression is frequently downregulated in GC, and is
correlated with proliferation and metastasis, suggesting that
miR-647 acts as a tumor-suppressor in GC development.
miR-647 suppresses GC cell growth
To explore the potential growth-suppressive role of
miR-647 in GC, we first established GC cells stably overexpressing
miR-647. The miR-647 mimic and LV-GFP were transfected into
SGC-7901 or MGC-803 cells that displayed a stronger invasive
ability, respectively. The expression of miR-647 in the stably
transfected cells was confirmed by qRT-PCR (P<0.05; Fig. 2A). Cell proliferation assays
revealed that miR-647 overexpression significantly reduced the
growth rates of the SGC-7901 and MGC-803 cells (P<0.05; Fig. 2B). As shown in Fig. 2C, cell cycle analysis also supported
the results, as overexpression of miR-647 was related to G0/G1
phase arrest. Cell counts in the G0/G1 phase apparently increased
while those in the S phase decreased (P<0.05). Furthermore, we
found that miR-647 increased the basal rate of cells undergoing
apoptosis (P<0.05, Fig. 2D). As
shown in Fig. 2E, typical features
of apoptosis such as formation of apoptotic bodies, chromatin
fragmentation or concentration and mitochondrial disappearance or
crest fracture were observed in the 7901–647 and 803–647 groups
under TEM. Consistent with the results obtained in the in
vitro growth assays, miR-647 significantly inhibited the
xenograft tumor growth in nude mice (P<0.05, Fig. 2F and G). Collectively, these data
clearly demonstrate that miR-647 plays a growth-suppressive
function in GC.
miR-647 suppresses GC metastasis
We confirmed that the ectopic expression of miR-647
is closely associated with GC metastasis including lymph node and
distant metastasis; that is to say, miR-647 mediates the processes
of metastasis in the development of GC. To determine the role of
miR-647 in metastasis, the effect of miR-647 overexpression on cell
migration and invasion was investigated using wound-healing and
Transwell assays with Matrigel. The results demonstrated that
overexpression of miR-647 in the SGC7901 and MGC803 cells
substantially impaired cell migration (P<0.05; Fig. 3A). Similarly, miR-647 significantly
decreased GC cell invasion in the Matrigel-coated Transwell assay
(P<0.05; Fig. 3B). To probe the
effect of abnormal expression of miR-647 on tumor metastasis in
vivo, controls and miR-647-overexpressing cells were injected
into nude mice via the lateral tail vein. As shown in Fig. 3C and Table III, the result was confirmed by
histological staining and statistical analysis, demonstrating that
miR-647 overexpression strongly inhibited the ability of GC
metastases in the liver. These results clearly indicate that
miR-647 suppresses GC migration and invasion.
 | Table III.Cases of metastasis in lung and liver
tissue in a model of metastasis in nude mice for each group
(aP<0.05). |
Table III.
Cases of metastasis in lung and liver
tissue in a model of metastasis in nude mice for each group
(aP<0.05).
|
|
| Lung
metastasis |
| Liver
metastasis |
|
|---|
|
|
|
|
|
|
|
|---|
| Groups | Total cases | Cases | Ratio (%) | P-value | Cases | Ratio (%) | P-value |
|---|
| 7901-Ctrl | 10 | 4 | 40 | 0.451 | 7 | 70 |
0.035a |
| 7901-NC | 10 | 3 | 30 |
| 5 | 50 |
|
| 7901-647 | 10 | 1 | 10 |
| 1 | 10 |
|
| 803-Ctrl | 10 | 4 | 40 | 0.293 | 6 | 60 | 0.061 |
| 803-NC | 10 | 4 | 40 |
| 7 | 70 |
|
| 803–647 | 10 | 1 | 10 |
| 2 | 20 |
|
miR-647 modulates proliferation and
metastasis by reducing the expression of ANK2, FAK, MMP2, MMP12,
CD44 and SNAIL1
To investigate the molecular mechanisms of
miR-647-mediated tumor suppression, potential direct targets for
miR-647 were screened by microarray data analysis combined with
bioinformatic target prediction. The functional downstream genes
for miR-647 were selected via gene expression profiling of 803–647
and 803-NC cells. Of the 31,741 genes analyzed by UinGene Whole
Genome DNA array analysis, 113 and 110 were upregulated and
downregulated, respectively, which had an expression change
>1.5-fold in GC cells of the 803–647 group compared with the
803-NC group. Following suppression of most targets by miRNAs, the
downregulation of 110 genes in the 803–647 group was analyzed for
potential miR-647 direct targets predicted by at least two of the 3
computational target prediction algorithms (miRanda, TargetScan and
microRNA). Four potential miR-647 targets, ANK2, ZNF609, KNDC1 and
TRIM4 were identified (Fig. 4A). To
examine whether or not the 4 potential targets were regulated by
miR-647 in GC cell lines, the miR-647 overexpression of SGC7901 and
MGC803 cells was analyzed for transcriptional expression of
ANK2, ZNF609, KNDC1 and TRIM4 by
qRT-PCR. As shown in Fig. 4B, among
the 4 potential targets, ANK2 and KNDC1 mRNA expression was
suppressed by miR-647 in both cells. Since only ANK2 plays an
oncogenic function in tumorigenesis, it was selected for further
evaluation as the miR-647 potential target. Furthermore,
suppression of ANK2 expression by miR-647 overexpression was
confirmed in the SGC7901 and MGC803 cells (P<0.05; Fig. 4C). As shown in Fig. 4D, ANK2 was a potential target of
miR-647 as predicted by TargetScan and miRNA.
 | Figure 4.ANK2 is a potential target of
miR-647. miR-647 mediates proliferation and metastasis by reducing
the expression of ANK2, FAK, MMP2, MMP12, CD44 and SNAIL1. (A)
Potential target genes of miR-647 were screened by microarray gene
expression profiling combined with bioinformatic target prediction.
(B) Quantitative reverse transcription real-time polymerase chain
reaction (qRT-PCR) analysis of potential target genes. (C) Western
blotting of ANK2 protein. (D) Results of TargetScan and microRNA
predicted the target gene of miR-647. *P<0.05 for 7901–647
(803–647) group vs. the 7901-NC (803-NC) and 7901-Ctrl (803-Ctrl)
groups; #P>0.05 for 7901–647 group vs. the 7901-NC
and 7901-Ctrl groups. All values are expressed as mean ± SE. (E)
qRT-PCR of FAK, MMP2, MMP12, CD44 and SNAIL1. (F) Western blotting
of FAK, MMP2, MMP12, CD44 and SNAIL1 proteins; *P<0.05 for
7901–647 (803–647) group vs. the 7901-NC (803-NC) and 7901-Ctrl
(803-Ctrl) groups; #P>0.05 for 7901–647 group vs. the
7901-NC and 7901-Ctrl groups. All values are expressed as mean ±
SE. |
To investigate the mechanism by which miR-647
suppresses GC proliferation and metastasis, we utilized qRT-PCR and
western blotting to determine the expression of genes that regulate
proliferation and metastasis. Our data demonstrated that the
expression of FAK, MMP2, MMP12, CD44
and SNAIL1 was downregulated after miR-647 overexpression in
both SGC7901 and MGC803 cell lines (P<0.05, Fig. 4E and F).
Discussion
According to the 2012 statistics of the
International Agency for Research on Cancer (IARC), gastric cancer
is the fifth most common malignancy, and the third leading cause of
cancer-related deaths worldwide (14). Recently, tumor invasion, metastatic
dissemination, disease relapse, and drug resistance have been
identified as classical hallmarks of cancer malignancy, and
represent major factors contributing to poor clinical outcomes in
cancer patients (15,16). Unfortunately, due to lack of
effective diagnostic methods for early stage disease and
tumorigenesis in GC, patients are often diagnosed with metastatic
disease. Therefore, the discovery of novel molecular biomarkers and
targets for diagnosis and treatment of GC is imperative.
Emerging evidence indicates that miRNAs modulate the
development of GC. They are closely involved in the regulation of
various biological and pathological processes, including tumor
growth, cell invasion and tumor metastasis (16,17).
Notably, Yang et al (18)
reported that miR-647 expression is significantly altered during
lymphatic metastasis of GC. A recent study also showed that miR-647
is associated with malignant cancer phenotypes and is often used as
a biomarker for GC (12). However,
miR-647 is a newly identified molecule, with limited data
supporting its role in GC. The evidence suggests that miR-647 may
function as a tumor suppressor or tumorigenic miRNA. In the present
study, we found a significant decrease in the level of miR-647
expression in GC tumors compared with that in the corresponding
non-tumor tissues. We further analyzed the correlation of miR-647
expression with clinicopathological characteristics. A robust
association between low miR-647 expression and tumor size, lymph
node and distant metastases was confirmed in 70 patients with GC.
Furthermore, consistent with the results in GC patients, we showed
that the expression of miR-647 in SGC-7901, MGC-803, HGC-27 and AGS
cells was significantly downregulated compared with that in the
GES-1 cells. The SGC-7901 and MGC-803 cells, with lower levels of
miR-647, displayed higher invasive ability compared with the HGC-27
and AGS cell lines. These findings suggest that miR-647 plays a
tumor-suppressor role in GC and acts as a metastatic biomarker in
GC.
To investigate the effect of miR-647 on the
proliferation and metastasis of GC cell lines, we re-expressed
miR-647 in GC cell lines SGC7901 and MGC803, both of which express
a low basal level of miR-647. Re-expression of miR-647 effectively
slowed tumor cell growth, inhibited cell cycle entry into the S
phase and increased apoptosis as well as suppressed GC xenograft
tumor growth in nude mice. In addition, re-expression of miR-647
decreased tumor cell migration and invasion. Consistently, an
experimental animal model of metastasis also confirmed that miR-647
significantly reduced the colonization of metastatic tumors in
liver. These data point toward an important role of miR-647,
similar to a tumor suppressor, in GC cell growth and
metastasis.
To further elucidate the mechanism underlying the
role of miR-647, we used three open access programs (miRanda,
TargetScan and microRNA) and Microarray Data Analysis to predict
targets of miR-647. Among the 4 potential targets (ANK2,
ZNF609, KNDC1 and TRIM4), the mRNA expression
of KNDC1 and ANK2 was suppressed by miR-647 overexpression in the
SGC7901 and MGC803 cells. As ANK2 was previously reported to
play an oncogenic role, we focused on the oncogene ANK2 as
the potential target. Furthermore, we confirmed that miR-647
overexpression suppressed ANK2 protein expression.
Cytoskeletal proteins are positively associated with cell growth
and metastasis (19,20). As a member of the cytoskeletal
protein family, ANK2 encodes one of the 3 ankyrins: ankyrin-R
(ANK1), ankyrin-B (ANK2) and ankyrin-G (ANK3), mediating various
integral membrane proteins (21,22).
Overexpression of ankyrins has been observed in various cancer
cells, including prostate, breast and ovarian cancers (23–25).
They play a crucial role in cell growth, membrane transportation,
metastasis and migration of cancer cells (22,23).
Furthermore, consistent with the present study, a recent study
showed that ANK2 was overexpressed in pancreatic tumors and
downregulation of ANK2 attenuated the growth and invasion of
pancreatic cancers (26). However,
there is no sufficient evidence to support the role of ANK2 in GC.
Our findings suggest that miR-647 inhibits proliferation and
metastasis in GC by downregulating ANK2. To the best of our
knowledge, the present study provides initial evidence supporting
the role of miR-647 in the downregulation of ANK2 in GC.
We also demonstrated that FAK, MMP2, MMP12, CD44 and
SNAIL1 are downstream effectors of miR-647-ANK2 based on
interference studies demonstrating that FAK, MMP2, MMP12, CD44 and
SNAIL1 expressions were decreased by miR-647-ANK2 expression. The
ANK2/FAK/MMP2/MMP12 and ANK2/CD44/SNAIL1 signaling pathways
represent a rare integrated network mediating the proliferation and
metastasis of GC. Our results were consistent with previous data
indicating that ANK2 may affect signal transduction mediated by FAK
activity. FAK plays a pivotal role in the secretion of matrix
metalloproteinases (MMPs) (26,27).
As a potential prognostic indicator, decreased focal adhesion
kinase (FAK) expression mediates the inhibition of cell
proliferation and limits the induction of metastasis in GC
(28,29). Additionally, MMP2 and MMP12 belong
to the group of MMPs, which are known for their key role in tumor
metastasis and invasiveness (30,31).
Recent studies have shown that inhibition of MMP2 and MMP12
suppresses the invasion of gastric and lung cancers (32,33).
Ankyrins (including ANK1, ANK2 and ANK3) link
various transmembrane proteins to actin network, and bind to CD44
domains. CD44, an important cell membrane receptor, which consists
of a conserved intracellular ankyrin-binding region, links the
external environment to the cytoskeleton. It mediates a variety of
cellular behaviors including mitogenesis, adhesion and migration
and was found to interact with growth and metastasis in malignant
cells (34–36). Inhibition of CD44 was found to
decrease the expression of SNAIL1 and the potential for invasion in
pancreatic cancer cells (37,38).
Recent studies have shown that epithelial-to-mesenchymal transition
(EMT) is associated with the acquisition of metastasis in GC cells
(39). The transcription factor
SNAIL1 represses epithelial factors such as E-cadherin, and its
expression is sufficient for EMT (40). Consequently, inhibition of SNAIL1
expression prevents EMT resulting in suppression of cell
proliferation and metastasis (41).
In conclusion, in the present study, miR-647
expression was found to decrease the expression of
ANK2/FAK/MMP2/MMP12 and ANK2/CD44/SNAIL1 signaling pathways,
directly or indirectly, resulting in inhibition of the
proliferation and metastasis of GC cells in vitro and in
vivo. For the first time, the present study demonstrated a
relationship between miR-647 and ANK2, indicating the potential
role of miR-647 in the regulation of GC development. Furthermore,
it underscores the role of miR-647 as a potential biomarker in GC
and further investigation should be carried out to confirm that
targeting this gene may aid in the diagnosis and treatment of
gastric carcinoma.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81660511,
30860273 and 81060201), the Natural Science Foundation of Guangxi
(no. 2015GXNSFDA227001), the Key Health Science Foundation of
Guangxi (no. 14124004-1-9), and the Innovation Project of Guangxi
Graduate Education.
References
|
1
|
Hamashima C: Current issues and future
perspectives of gastric cancer screening. World J Gastroenterol.
20:13767–13774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shmulevich I: Large-scale molecular
characterization and analysis of gastric cancer. Chin J Cancer.
33:369–370. 2014.PubMed/NCBI
|
|
3
|
Piazuelo MB and Correa P: Gastric cáncer:
Overview. Colomb Med. 44:192–201. 2013.PubMed/NCBI
|
|
4
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alizadeh S, Azizi SG, Soleimani M, Farshi
Y and Khatib Z Kashani: The role of microRNAs in myeloproliferative
neoplasia. Int J Hematol Oncol Stem Cell Res. 10:172–185.
2016.PubMed/NCBI
|
|
6
|
Zhao Y and Srivastava D: A developmental
view of microRNA function. Trends Biochem Sci. 32:189–197. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bushati N and Cohen SM: microRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hata A and Lieberman J: Dysregulation of
microRNA biogenesis and gene silencing in cancer. Sci Signal.
8:re32015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng H, Zhang F and Lin X, Huang C, Zhang
Y, Li Y, Lin J, Chen W and Lin X: MicroRNA-1225-5p inhibits
proliferation and metastasis of gastric carcinoma through
repressing insulin receptor substrate-1 and activation of β-catenin
signaling. Oncotarget. 7:4647–4663. 2016.PubMed/NCBI
|
|
11
|
Zuo QF, Zhang R, Li BS, Zhao YL, Zhuang Y,
Yu T, Gong L, Li S, Xiao B and Zou QM: MicroRNA-141 inhibits tumor
growth and metastasis in gastric cancer by directly targeting
transcriptional co-activator with PDZ-binding motif, TAZ. Cell
Death Dis. 6:e16232015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rawlings-Goss RA, Campbell MC and Tishkoff
SA: Global population-specific variation in miRNA associated with
cancer risk and clinical biomarkers. BMC Med Genomics. 7:532014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim YW, Kim EY, Jeon D, Liu JL, Kim HS,
Choi JW and Ahn WS: Differential microRNA expression signatures and
cell type-specific association with Taxol resistance in ovarian
cancer cells. Drug Des Devel Ther. 8:293–314. 2014.PubMed/NCBI
|
|
14
|
Yuan DD, Zhu ZX, Zhang X and Liu J:
Targeted therapy for gastric cancer: Current status and future
directions (Review). Oncol Rep. 35:1245–1254. 2016.PubMed/NCBI
|
|
15
|
Nishida T, Egashira Y, Akutagawa H, Fujii
M, Uchiyama K, Shibayama Y and Hirose Y: Predictors of lymph node
metastasis in T1 colorectal carcinoma: An immunophenotypic analysis
of 265 patients. Dis Colon Rectum. 57:905–915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Díaz-López A, Moreno-Bueno G and Cano A:
Role of microRNA in epithelial to mesenchymal transition and
metastasis and clinical perspectives. Cancer Manag Res. 6:205–216.
2014.PubMed/NCBI
|
|
17
|
Santos JI, Teixeira AL, Dias F, Gomes M,
Nogueira A, Assis J and Medeiros R: Restoring TGFβ1 pathway-related
microRNAs: Possible impact in metastatic prostate cancer
development. Tumour Biol. 35:6245–6253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang B, Jing C, Wang J, Guo X, Chen Y, Xu
R, Peng L, Liu J and Li L: Identification of microRNAs associated
with lymphangiogenesis in human gastric cancer. Clin Transl Oncol.
16:374–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu L, Ito T, Nakahara T, Nagae K, Fuyuno
Y, Nakao M, Akahoshi M, Nakagawa R, Tu Y, Uchi H, et al:
Upregulation of S100P, receptor for advanced glycation end products
and ezrin in malignant melanoma. J Dermatol. 40:973–979. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujimoto D, Hirono Y, Goi T, Katayama K,
Matsukawa S and Yamaguchi A: The activation of proteinase-activated
receptor-1 (PAR1) promotes gastric cancer cell alteration of
cellular morphology related to cell motility and invasion. Int J
Oncol. 42:565–573. 2013.PubMed/NCBI
|
|
21
|
Lambert S and Bennett V: Postmitotic
expression of ankyrinR and beta R-spectrin in discrete neuronal
populations of the rat brain. J Neurosci. 13:3725–3735.
1993.PubMed/NCBI
|
|
22
|
De Matteis MA and Morrow JS: The role of
ankyrin and spectrin in membrane transport and domain formation.
Curr Opin Cell Biol. 10:542–549. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu D and Bourguignon LY: Interaction
between CD44 and the repeat domain of ankyrin promotes hyaluronic
acid-mediated ovarian tumor cell migration. J Cell Physiol.
183:182–195. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bourguignon LY, Zhu H, Shao L and Chen YW:
Ankyrin-Tiam1 interaction promotes Rac1 signaling and metastatic
breast tumor cell invasion and migration. J Cell Biol. 150:177–191.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu D and Bourguignon LY: The
ankyrin-binding domain of CD44s is involved in regulating
hyaluronic acid-mediated functions and prostate tumor cell
transformation. Cell Motil Cytoskeleton. 39:209–222. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Y, Löhr M and Jesnowski R: Inhibition
of ankyrin-B expression reduces growth and invasion of human
pancreatic ductal adenocarcinoma. Pancreatology. 10:586–596. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sein TT, Thant AA, Hiraiwa Y, Amin AR,
Sohara Y, Liu Y, Matsuda S, Yamamoto T and Hamaguchi M: A role for
FAK in the Concanavalin A-dependent secretion of matrix
metalloproteinase-2 and −9. Oncogene. 19:5539–5542. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pei YF, Tao R, Li JF, Su LP, Yu BQ, Wu XY,
Yan M, Gu QL, Zhu ZG and Liu BY: TET1 inhibits gastric cancer
growth and metastasis by PTEN demethylation and re-expression.
Oncotarget. 7:31322–31335. 2016.PubMed/NCBI
|
|
29
|
You T, Gao W, Wei J, Jin X, Zhao Z, Wang C
and Li Y: Overexpression of LIMK1 promotes tumor growth and
metastasis in gastric cancer. Biomed Pharmacother. 69:96–101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu L, Tanimoto A, Murata Y, Sasaguri T,
Fan J, Sasaguri Y and Watanabe T: Matrix metalloproteinase-12 gene
expression in human vascular smooth muscle cells. Genes Cells.
8:225–234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie S, Issa R, Sukkar MB, Oltmanns U,
Bhavsar PK, Papi A, Caramori G, Adcock I and Chung KF: Induction
and regulation of matrix metalloproteinase-12 in human airway
smooth muscle cells. Respir Res. 6:1482005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang B, Xu YF, He BS, Pan YQ, Zhang LR,
Zhu C, Qu LL and Wang SK: RNAi-mediated silencing of CD147 inhibits
tumor cell proliferation, invasion and increases chemosensitivity
to cisplatin in SGC7901 cells in vitro. J Exp Clin Cancer Res.
29:612010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lv FZ, Wang JL, Wu Y, Chen HF and Shen XY:
Knockdown of MMP12 inhibits the growth and invasion of lung
adenocarcinoma cells. Int J Immunopathol Pharmacol. 28:77–84. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Turley EA, Noble PW and Bourguignon LY:
Signaling properties of hyaluronan receptors. J Biol Chem.
277:4589–4592. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bourguignon LY, Singleton PA, Diedrich F,
Stern R and Gilad E: CD44 interaction with Na+-H+ exchanger (NHE1)
creates acidic microenvironments leading to hyaluronidase-2 and
cathepsin B activation and breast tumor cell invasion. J Biol Chem.
279:26991–27007. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bourguignon LY, Zhu H, Zhou B, Diedrich F,
Singleton PA and Hung MC: Hyaluronan promotes CD44v3-Vav2
interaction with Grb2-p185HER2 and induces Rac1 and Ras signaling
during ovarian tumor cell migration and growth. J Biol Chem.
276:48679–48692. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lokeshwar VB, Fregien N and Bourguignon
LY: Ankyrin-binding domain of CD44(GP85) is required for the
expression of hyaluronic acid-mediated adhesion function. J Cell
Biol. 126:1099–1109. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang W, Zhang Y, Kane KT, Collins MA,
Simeone DM, diMagliano MP and Nguyen KT: CD44 regulates pancreatic
cancer invasion through MT1-MMP. Mol Cancer Res. 13:9–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao J, Yang C, Guo S and Wu Y: GM130
regulates epithelial-to-mesenchymal transition and invasion of
gastric cancer cells via snail. Int J Clin Exp Pathol.
8:10784–10791. 2015.PubMed/NCBI
|
|
40
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kudo-Saito C, Shirako H, Takeuchi T and
Kawakami Y: Cancer metastasis is accelerated through
immunosuppression during Snail-induced EMT of cancer cells. Cancer
Cell. 15:195–206. 2009. View Article : Google Scholar : PubMed/NCBI
|