Introduction
The American Cancer Society estimated ~23,670 new
cases and 16,050 deaths from brain and nervous system tumors in
adults and children in 2015 (1).
The most common causes of cancer-related deaths in adolescents and
young adults aged 15–39 are malignant brain tumors (2). Gliomas, tumors arising from the
supportive tissue of the brain, represent 27% of all brain tumors
and 80% of all malignant tumors (2). Glioblastomas, which represent 15.1% of
all primary brain tumors and 55.1% of all gliomas, constitute the
highest number of cases of all malignant tumors, with an estimated
12,120 new cases predicted in 2016 (2).
Brain tumors are prone to recur locally or invade
other regions of the central nervous system (CNS). Tumor cell
invasion is dependent upon degradation of the extracellular matrix
(ECM), which, when intact, acts as a barrier to block cancer cell
invasion (3–5). Numerous clinical and experimental
studies have demonstrated that elevated levels of matrix
metalloproteinases (MMPs) are associated with the progression of
brain tumors. Elevated levels of several MMPs, such as MMP-1,
MMP-2, MMP-7, MMP-9, MMP-11, MMP-12, MMP-14, MMP-15, MMP-19, MMP-24
and MMP-25 have been reported in malignant glioma samples from
patients, suggesting that malignant progression is correlated to
MMP expression (6). Jäälinojä et
al found that mean survival in patients with MMP-2-negative
tumors was 36 months in contrast to 7–14 months in those patients
with MMP-2-positive tumors (7).
Smith et al reported that immunohistochemical examination of
urine, cerebrospinal fluid and tissue specimens from patients with
brain tumors, revealed a significant correlation between brain
tumor presence and elevated MMP-2 and MMP-9 levels and that
resection of tumors correlated with decreased levels of MMPs
(8).
MMP activity is regulated by and dependent upon
environmental influences from surrounding stroma cells, ECM
proteins, systemic hormones and other factors. Inflammation has
been reported to drive cancer progression (9–11).
Inflammatory cytokines such as interleukin (IL)-1β and tumor
necrosis (TNF)-α play significant roles in inflammation driven
tumor growth and progression (12,13).
These cytokines were found to be upregulated following radiation
therapy in glioblastoma patients (14,15).
Ilyin et al noted that IL-1β drives neuroinflammation by
upregulating expression of other pro-inflammatory cytokines
(16). Ryuto et al reported
the induction of vascular endothelial growth factor (VEGF)
expression by TNF-α in gliomas, which leads to the increased
angiogenesis observed in these tumors (17).
In the present study, we investigated the effects of
select cytokines, inducers and inhibitors affecting cancer cell
metabolism on the regulation of MMP-2 and MMP-9 activities in the
glioblastoma T-98G cell line.
Materials and methods
Materials
Human glioblastoma T-98G cells were obtained from
the American Type Culture Collection (ATCC; Rockville, MD, USA).
Antibiotics, penicillin and fetal bovine serum (FBS) were obtained
from Gibco-BRL (Long Island, NY, USA). Twenty-four well tissue
culture plates were obtained from Costar (Cambridge, MA, USA).
Gelatinase zymography was performed on 10% Novex pre-cast SDS
polyacrylamide gel (Invitrogen Inc., Carlsbad, CA, USA) with 0.1%
gelatin in non-reducing conditions. Interleukin-1β (IL-1β), tumor
necrosis factor-α (TNF-α), phorbol 12-myristate 13-acetate (PMA),
lipopolysaccharide (LPS), doxycycline, epigallocatechin gallate
(EGCG), actinomycin D, cyclohexamide, retinoic acid and
dexamethasone were purchased from Sigma (St. Louis, MO, USA). The
nutrient mixture (NM), prepared by VitaTech (Hayward, CA, USA), was
composed of the following ingredients in the relative amounts
indicated: vitamin C (as ascorbic acid and as Mg, Ca and palmitate
ascorbate) 700 mg; L-lysine 1,000 mg; L-proline 750 mg; L-arginine
500 mg; N-acetyl cysteine 200 mg; standardized green tea
extract (80% polyphenol) 1,000 mg; selenium 30 µg; copper 2 mg;
manganese 1 mg. All other reagents used were of high quality and
were obtained from Sigma, unless otherwise indicated.
Cell cultures
Glioblastoma cells were grown in DME, supplemented
with 15% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin in
24-well tissue culture plates. The cells were plated at a density
of 1×105 cells/ml and grown to confluency in a
humidified atmosphere at 5% CO2 at 37̊C.
Serum-supplemented media were removed and the cell monolayer was
washed once with phosphate-buffered saline (PBS) and with the
recommended serum-free media. The cells were then incubated in 0.5
ml of serum-free medium with various cytokines, mitogens, inducers
and inhibitors in triplicates, as indicated: PMA (10, 25, 50 and
100 ng/ml); TNF-α and IL-1β (0.1, 1, 10 and 25 ng/ml); LPS (10, 25,
50 and 100 µg/ml); EGCG (10, 25, 50 and 100 µM) without and with
PMA 100 ng/ml; doxycycline (10, 25, 50 and 100 µM) without and with
PMA 100 ng/ml; NM (10, 50, 100, 500 and 1,000 µg/ml) without and
with PMA 100 ng/ml, retinoic acid (50 µM); dexamethasone (50 µM);
actinomycin D and cyclohexamide (2 and 4 µg/ml). The plates were
then returned to the incubator. The conditioned medium from each
treatment was separately collected, pooled and centrifuged at 4̊C
for 10 min at 3,000 rpm to remove cells and cell debris. The clear
supernatant was collected and used for gelatinase zymography, as
described below.
Gelatinase zymography
Gelatinase zymography was utilized due to its high
sensitivity to gelatinolytic enzymatic activity and ability to
detect both pro and active forms of MMP-2 and MMP-9. Upon
renaturation of the enzyme, the gelatinases digest the gelatin in
the gel and reveal clear bands against an intensely stained
background. Gelatinase zymography was performed using 10% Novex
pre-cast SDS polyacrylamide gel in the presence of 0.1% gelatin
under non-reducing conditions. Culture media (20 µl) were mixed
with sample buffer and loaded for SDS-PAGE with Tris-glycine SDS
buffer, as suggested by the manufacturer (Novex). Samples were not
boiled before electrophoresis. Following electrophoresis, the gels
were washed twice in 2.5% Triton X-100 for 30 min at room
temperature to remove SDS. The gels were then incubated at 37̊C
overnight in substrate buffer containing 50 mM Tris-HCl and 10 mM
CaCl2 at pH 8.0 and stained with 0.5% Coomassie Blue
R250 in 50% methanol and 10% glacial acetic acid for 30 min and
destained. Protein standards were concurrently run and approximate
molecular weights were determined by plotting the relative
mobilities of known proteins. Gelatinase zymograms were scanned
using CanoScan 9950F Canon scanner at 300 dpi. The intensity of the
bands was evaluated using the pixel-based densitometer program
Un-Scan-It, version 5.1, 32-bit, by Silk Scientific Corporation
(Orem, UT, USA), at a resolution of 1 Scanner Unit (1/100 of an
inch for an image that was scanned at 100 dpi).
Statistical analysis
Microsoft Excel 2010 linear trend analysis was
utilized to determine the linear trend analyses of the densitometry
results.
Results
Effects of inducers and cytokines on
glioblastoma T-98G secretion of MMP-2 and MMP-9
Glioblastoma T-98G cells expressed a band
corresponding to MMP-2. Table I
shows the quantitative densitometry results from the effects of
PMA, TNF-α, IL-1β and LPS on MMP-2 and MMP-9 expression in the
T-98G cells. Upon gelatinase zymography, T-98G cells demonstrated
strong expression of MMP-2 and induction of MMP-9 with PMA
treatment. PMA treatment showed increased MMP-2 secretion from 0–25
ng/ml and decreased secretion at 50 and 100 ng/ml with no
significant overall effect on expression of MMP-2 (linear trend
R2=0.153). PMA showed increased MMP-9 secretion from 10
to 25 ng/ml followed by decreased secretion at 50 and 100, again
with no significant overall effect on secretion of MMP-9 (linear
trend R2=0.281). See Fig.
1 for PMA results. T-98G cells demonstrated strong expression
of MMP-2 and slight induction of MMP-9 with TNF-α treatment. TNF-α
treatment showed increased MMP-2 secretion from 0–1 ng/ml and
decreased secretion at 10 and 25 ng/ml with no significant overall
effect on expression of MMP-2 (linear trend R2=0.0.066).
TNF-α showed dose-dependent increased MMP-9 secretion (linear trend
R2=0.721). See Fig. 2
for TNF-α results. IL-1β demonstrated a slight dose-dependent
increase in T-98G secretion of MMP-2 (linear trend
R2=0.529), but no induction of MMP-9. See Fig. 3 for IL-1β results. LPS treatment
showed dose-dependent decreased inactive MMP-2 secretion (linear
trend R2=0.473) and increased active MMP-2 secretion
(linear trend R2=0.977) (Fig. 4).
 | Table I.Effect of inducers on glioblastoma
T-98G MMP-2 and MMP-9 secretion. |
Table I.
Effect of inducers on glioblastoma
T-98G MMP-2 and MMP-9 secretion.
|
| MMP-2 (%) | MMP-9 (%) |
|---|
| PMA (ng/ml) |
|
|
| Control | 16.1 | 0 |
| 10 | 16.2 | 1.67 |
| 25 | 22.5 | 7.37 |
| 50 | 15.35 | 6.42 |
| 100 | 11.7 | 2.85 |
| TNF-α (ng/ml) |
|
|
| Control | 12.9 | 0 |
|
0.1 | 20.6 | 0.7 |
|
1 | 23.6 | 1.4 |
| 10 | 22.1 | 1.6 |
| 25 | 15.8 | 1.3 |
| IL-1β (ng/ml) |
|
|
| Control | 15.8 | 0 |
|
0.1 | 13.5 | 0 |
|
1 | 24.4 | 0 |
| 10 | 24.3 | 0 |
| 25 | 22.0 | 0 |
|
| MMP-2 inactive
(%) | MMP-2 active
(%) |
| LPS (µg/ml) |
|
|
| Control | 11.7 | 0 |
| 10 | 17.4 | 3.9 |
| 25 | 15.1 | 9.4 |
| 50 | 9.7 | 10.8 |
| 100 | 5.3 | 16.7 |
Effects of chemical inhibitors on
glioblastoma T-98G cell secretion of MMP-2 and MMP-9
Table II shows the
quantitative densitometry results from the effects of chemical
inhibitors doxycycline, actinomycin D, cyclohexamide and
dexamethasone on MMP-2 and MMP-9 expression in the glioblastoma
T-98G cell line. Doxycycline inhibited T-98G cell MMP-2 secretion
in a dose-dependent manner with 73% blockage at 100 µM (linear
trend R2=0.899). Following treatment with PMA 100 ng/ml,
doxycycline downregulated the expression of T-98G cell MMP-2 in a
dose-dependent manner, with 73% blockage at 100 µM
(R2=0.898) and total blockage of MMP-9 at 10 µM (linear
trend R2=0.500). See Fig.
5 for doxycycline effects on untreated and PMA-treated T-98G
cells. Actinomycin D had moderate inhibitory effect on MMP-2
secretion (R2=0.725) with ~25% inhibition at 2 and 4 µM,
as shown in Fig. 6. Cyclohexamide
had a potent dose-dependent inhibitory effect on MMP-2 secretion by
T-98G cells with 93% inhibition at 4 µM (linear trend
R2=0.787), as shown in Fig.
7. Dexamethasone had a moderate inhibitory effect on MMP-2,
with inhibition of 23% at 50 µM compared to the control (Fig. 8).
 | Table II.Effect of inhibitors on glioblastoma
T-98G MMP-2 and MMP-9 secretion. |
Table II.
Effect of inhibitors on glioblastoma
T-98G MMP-2 and MMP-9 secretion.
|
| Untreated | PMA 100
ng/ml-treated |
|---|
|
|
|
|
|---|
|
| MMP-2 (%) | MMP-2 (%) | MMP-9 (%) |
|---|
| Doxycycline
(µM) |
|
|
|
|
Control | 36.9 | 33.0 | 10.6 |
| 10 | 22.2 | 19.8 | 0 |
| 25 | 19.1 | 17.1 | 0 |
| 50 | 11.8 | 10.5 | 0 |
|
100 | 10.0 | 8.9 | 0 |
| EGCG (µM) |
|
|
|
|
Control | 40.0 | 44.9 | 9.2 |
| 10 | 53.5 | 29.3 | 7.0 |
| 25 | 6.5 | 8.3 | 1.2 |
| 50 | 0.5 | 0 | 0 |
|
100 | 0 | 0 | 0 |
| NM (µg/ml) |
|
|
|
|
Control | 36.9 | 31.4 | 1.9 |
| 10 | 32.8 | 37.1 | 1.2 |
| 50 | 19.3 | 22.0 | 0.1 |
|
100 | 8.2 | 5.0 | 0 |
|
500 | 2.2 | 1.3 | 0 |
| 1,000 | 0.6 | 0 | 0 |
| Actinomycin D
(µg/ml) |
|
|
|
|
Control | 100.0 | 0 |
| 2 | 75.3 | 0 |
| 4 | 76.1 | 0 |
| Cyclohexamide
(µg/ml) |
|
|
|
|
Control | 100.0 | 0 |
| 2 | 11.1 | 0 |
| 4 | 6.5 | 0 |
| Dexamethasone
(µM) |
|
|
|
|
Control | 100.0 | 0 |
| 50 | 77.4 | 0 |
| Retinoic acid
(µM) |
|
|
|
|
Control | 100.0 | 0 |
| 50 | 45.7 | 0 |
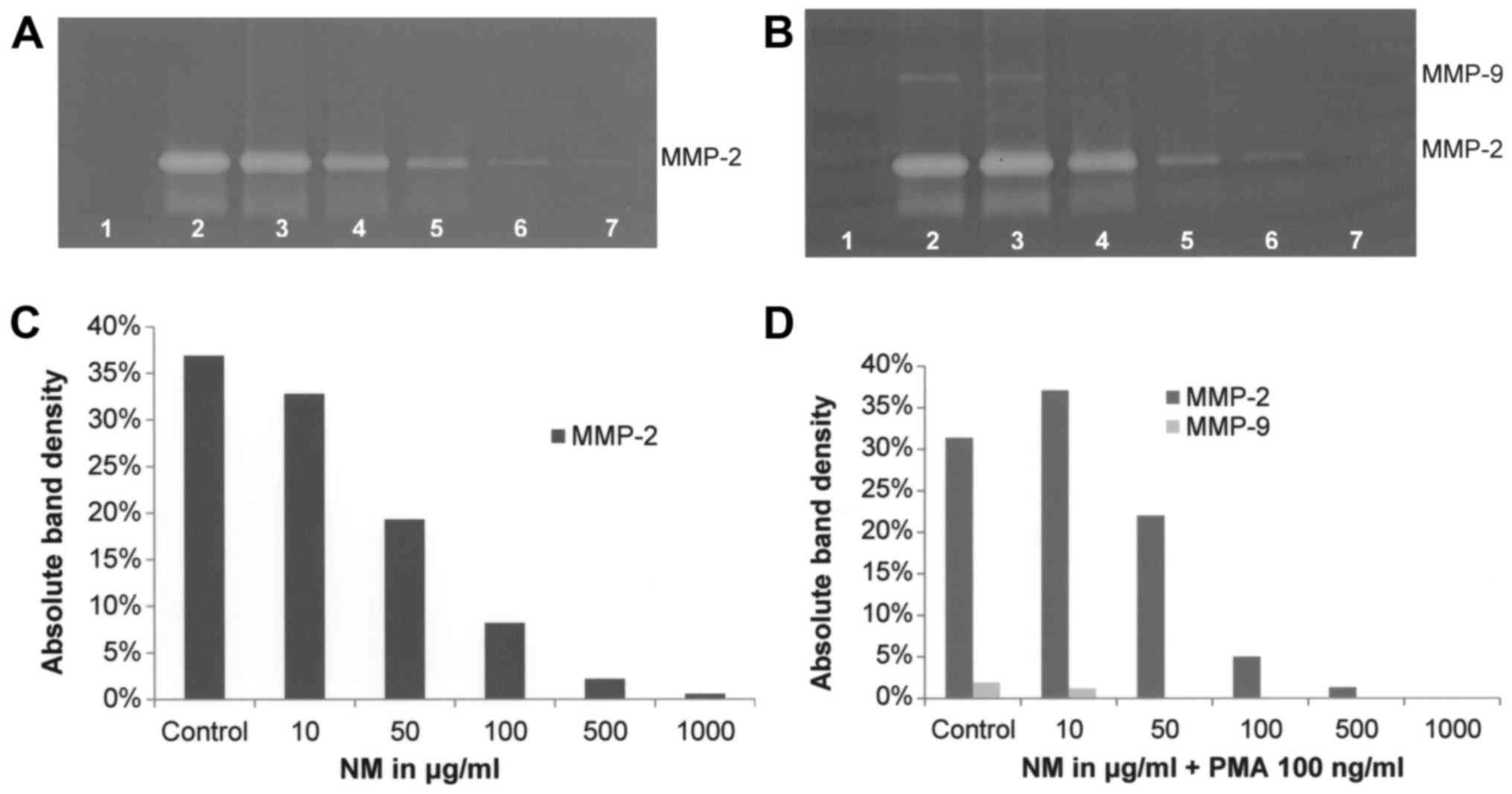
Effects of natural inhibitors on
glioblastoma T-98G secretion of MMP-2 and MMP-9
Table II shows the
quantitative densitometry results from the effects of natural
inhibitors EGCG, the NM and retinoic acid on MMP-2 and MMP-9
expression in glioblastoma T-98G cells. EGCG potently downregulated
T-98G expression of MMP-2 in a dose-dependent manner, with total
blockage at 50 µM (linear trend R2=0.712), as shown in
Fig. 9A and C. EGCG showed
dose-dependent inhibition of PMA (100 ng/ml)-induced MMP-9
secretion (linear trend R2=0.866) and of MMP-2 (linear
trend R2=0.877) with total blockage of both at 50 µM, as
shown in Fig. 9B and D. NM
inhibited secretion of MMP-2 by uninduced T-98G cells in a
dose-dependent manner (linear trend R2=0.8706) with
virtual total block at 1,000 µg/ml (Fig. 10A and C). NM showed dose-dependent
inhibition of MMP-2 and MMP-9 expression in PMA-treated T-98G cells
with total blockage of MMP-2 at 1,000 µg/ml and MMP-9 at 100 µg/ml
(linear trends R2=0.863 and 0.742, respectively), as
shown in Fig. 10B and D. Retinoic
acid inhibited T-98G MMP-2 secretion by 54% at 50 µM (Fig. 8).
Discussion
Elevated MMP levels correlate with glioblastoma
tumor progression, as documented in clinical studies (6–8). Thus,
knowledge of MMP regulation is of importance for developing
therapeutic strategies for glioblastoma. Extracellular factors,
such as the inflammatory cytokines IL-1β and TNF-α have been
implicated in inflammation-driven tumor growth and progression of
glioblastoma (12,13). Esteve et al reported that
production of MMP-9 in glioma cells is tightly regulated by IL-1,
TNFα and TGFβ2 (18).
In the present study, we compared MMP secretion
patterns by inducers, cytokines and mitogens, such as PMA, TNFα,
IL-1β and LPS in glioblastoma T-98G cells. Surprisingly, we found
that MMP-9 was not significantly enhanced by PMA, TNFα and IL-1β in
the present study. LPS increased active MMP-2 secretion and
decreased inactive MMP-2 secretion, but had no effect on MMP-9. In
addition, we investigated the effect of inhibitors, such as
doxycycline, EGCG, NM, dexamethasone, cyclohexamide, retinoic acid
and agents that affect transcription and translation levels, such
as actinomycin D. All inhibitors tested downregulated MMP secretion
by T-98G cells. Doxycycline, a chemical inhibitor, potently
downregulated T-98G secretion of MMP-2 and PMA-induced MMP-9.
Cyclohexamide demonstrated potent inhibitory action on T-98G MMP-2
secretion, while actinomycin D and dexamethasone had moderate
inhibitory effects on MMP-2. Among the natural inhibitors, NM and
its component EGCG potently downregulated T-98G cell secretion of
MMP-2 and MMP-9. Retinoic acid strongly inhibited MMP-2
secretion.
In addition to individual compounds, we tested the
effects of a specific nutrient mixture, NM, which has demonstrated
anticancer efficacy in various in vitro and in vivo
studies by affecting various mechanisms, including secretion of
MMPs (19). Optimal ECM structure
is dependent upon sufficient ascorbic acid, lysine and proline to
support proper collagen synthesis and hydroxylation of collagen
fibers. Lysine also contributes to ECM stability as a natural
inhibitor of plasmin-induced proteolysis (20,21).
Copper and manganese act as co-factors for hydroxylase enzymes and
galactosyl and glucosyl transferases, respectively, to form
collagen (22,23). Green tea extract has been shown to
be potent in modulating cancer cell growth, apoptosis, metastasis
and angiogenesis (23–29), and daily consumption has
demonstrated delayed cancer onset of breast cancer, as well as a
lower recurrence rate and longer remission (30). N-acetyl cysteine and selenium
have been documented to inhibit tumor cell MMP-9 and invasive
activities and migration of endothelial cells through the ECM
(31–33). Ascorbic acid has been documented to
modulate cancer cell and tumor growth as well as to prevent
metastasis (34–39) and low levels of ascorbic acid are
found in cancer patients (40,41).
Low levels of arginine limit NO production, an inducer of apoptosis
(42).
In conclusion, our results showed that inducers,
cytokines and mitogens showed variable effects on T-98G cell
secretion of MMP-2 and MMP-9. However, all inhibitors tested
downregulated glioblastoma T-98G cell MMP-2 and MMP-9 secretion,
suggesting the clinical use of MMP inhibitors, particularly potent
and non-toxic ones as the nutrient mixture and its component EGCG
in the management of glioblastomas.
Acknowledgements
The present study was funded by Dr. Rath Health
Foundation (Santa Clara, CA, USA), a non-profit organization.
References
|
1
|
ACS, . What are the key statistics about
brain and spinal cord tumors? 1 21–2016.Accessed 9/15/16.
http://www.cancer.org/cancer/braincnstumorsinadults/detailedguide/brain-and-spinal-cord-tumors-in-adults-key-statistics
|
|
2
|
ABTA (American Brain Tumor Association), .
Brain Tumor Facts. Last revised 12/2015. 9 15–16.http://www.abta.org/about-us/news/brain-tumor-statistics
|
|
3
|
Yurchenco PD and Schittny JC: Molecular
architecture of basement membranes. FASEB J. 4:1577–1590.
1990.PubMed/NCBI
|
|
4
|
Barsky SH, Siegal GP, Jannotta F and
Liotta LA: Loss of basement membrane components by invasive tumors
but not by their benign counterparts. Lab Invest. 49:140–147.
1983.PubMed/NCBI
|
|
5
|
Liotta LA, Tryggvason K, Garbisa S, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forsyth PA, Laing TD, Gibson AW, Rewcastle
NB, Brasher P, Sutherland G, Johnston RN and Edwards DR: High
levels of gelatinase-B and active gelatinase-A in metastatic
glioblastoma. J Neurooncol. 36:21–29. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jäälinojä J, Herva R, Korpela M, Höyhtyä M
and Turpeenniemi-Hujanen T: Matrix metalloproteinase 2 (MMP-2)
immunoreactive protein is associated with poor grade and survival
in brain neoplasms. J Neurooncol. 46:81–90. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith ER, Zurakowski D, Saad A, Scott RM
and Moses MA: Urinary biomarkers predict brain tumor presence and
response to therapy. Clin Cancer Res. 14:2378–2386. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Visser KE and Coussens LM: The
inflammatory tumor microenvironment and its impact on cancer
development. Contrib Microbiol. 13:118–137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Germano G, Allavena P and Mantovani A:
Cytokines as a key component of cancer-related inflammation.
Cytokine. 43:374–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allavena P, Garlanda C, Borrello MG, Sica
A and Mantovani A: Pathways connecting inflammation and cancer.
Curr Opin Genet Dev. 18:3–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gridley DS, Loredo LN, Slater JD,
Archambeau JO, Bedros AA, Andres ML and Slater JM: Pilot evaluation
of cytokine levels in patients undergoing radiotherapy for brain
tumor. Cancer Detect Prev. 22:20–29. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou W, Jiang Z, Li X, Xu Y and Shao Z:
Cytokines: Shifting the balance between glioma cells and tumor
microenvironment after irradiation. J Cancer Res Clin Oncol.
141:575–589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ilyin SE, González-Gómez I, Romanovicht A,
Gayle D, Gilles FH and Plata-Salamán CR: Autoregulation of the
interleukin-1 system and cytokine-cytokine interactions in primary
human astrocytoma cells. Brain Res Bull. 51:29–34. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ryuto M, Ono M, Izumi H, Yoshida S, Weich
HA, Kohno K and Kuwano M: Induction of vascular endothelial growth
factor by tumor necrosis factor alpha in human glioma cells.
Possible roles of SP-1. J Biol Chem. 271:28220–28228. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Esteve PO, Tremblay P, Houde M, St-Pierre
Y and Mandeville R: In vitro expression of MMP-2 and MMP-9 in
glioma cells following exposure to inflammatory mediators. Biochim
Biophys Acta. 1403:85–96. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Niedzwiecki A, Roomi MW, Kalinovsky T and
Rath M: Micronutrient synergy - a new tool in effective control of
metastasis and other key mechanisms of cancer. Cancer Metastasis
Rev. 29:529–542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rath M and Pauling L: Plasmin-induced
proteolysis and the role of apoprotein(a), lysine and synthetic
analogs. Orthomolecular Med. 7:17–23. 1992.
|
|
21
|
Sun Z, Chen YH, Wang P, Zhang J, Gurewich
V, Zhang P and Liu JN: The blockage of the high-affinity lysine
binding sites of plasminogen by EACA significantly inhibits
prourokinase-induced plasminogen activation. Biochim Biophys Acta.
1596:182–192. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mussini E, Hutton JJ Jr and Udenfriend S:
Collagen proline hydroxylase in wound healing, granuloma formation,
scurvy, and growth. Science. 157:927–929. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kivirikko KI and Myllylä R: Collagen
glycosyltransferases. Int Rev Connect Tissue Res. 8:23–72. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Valcic S, Timmermann BN, Alberts DS,
Wächter GA, Krutzsch M, Wymer J and Guillén JM: Inhibitory effect
of six green tea catechins and caffeine on the growth of four
selected human tumor cell lines. Anticancer Drugs. 7:461–468. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mukhtar H and Ahmad N: Tea polyphenols:
Prevention of cancer and optimizing health. Am J Clin Nutr. 71
Suppl 6:S1698S–S1704S. 2000.
|
|
26
|
Yang GY, Liao J, Kim K, Yurkow EJ and Yang
CS: Inhibition of growth and induction of apoptosis in human cancer
cell lines by tea polyphenols. Carcinogenesis. 19:611–616. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Taniguchi S, Fujiki H, Kobayashi H, Go H,
Miyado K, Sadano H and Shimokawa R: Effect of (−)-epigallocatechin
gallate, the main constituent of green tea, on lung metastasis with
mouse B16 melanoma cell lines. Cancer Lett. 65:51–54. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hara Y: Green Tea: Health Benefits and
Applications. Marcel Dekker; New York, Basel: 2001, http://dx.doi.org/10.1201/9780203907993
View Article : Google Scholar
|
|
29
|
Gupta S, Hastak K, Afaq F, Ahmad N and
Mukhtar H: Essential role of caspases in
epigallocatechin-3-gallate-mediated inhibition of nuclear factor
kappa B and induction of apoptosis. Oncogene. 23:2507–2522. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujiki H, Suganuma M, Okabe S, Sueoka E,
Suga K, Imai K, Nakachi K and Kimura S: Mechanistic findings of
green tea as cancer preventive for humans. Proc Soc Exp Biol Med.
220:225–228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kawakami S, Kageyama Y, Fujii Y, Kihara K
and Oshima H: Inhibitory effect of N-acetylcysteine on invasion and
MMP-9 production of T24 human bladder cancer cells. Anticancer Res.
21:213–219. 2001.PubMed/NCBI
|
|
32
|
Morini M, Cai T, Aluigi Mg, Noonan DM,
Masiello L, De Flora S, D'Agostini F, Albini A and Fassina G: The
role of the thiol N-acetylcysteine in the prevention of tumor
invasion and angiogenesis. Int J Biol Markers. 14:268–271.
1999.PubMed/NCBI
|
|
33
|
Yoon SO, Kim MM and Chung AS: Inhibitory
effect of selenite on invasion of HT1080 tumor cells. J Biol Chem.
276:20085–20092. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cha J, Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Ascorbate supplementation inhibits growth
and metastasis of B16FO melanoma and 4T1 breast cancer cells in
vitamin C-deficient mice. Int J Oncol. 42:55–64. 2013.PubMed/NCBI
|
|
35
|
Naidu KA, Karl RC, Naidu KA and Coppola D:
Antiproliferative and proapoptotic effect of ascorbyl stearate in
human pancreatic cancer cells: Association with decreased
expression of insulin-like growth factor 1 receptor. Dig Dis Sci.
48:230–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Anthony HM and Schorah CJ: Severe
hypovitaminosis C in lung-cancer patients: The utilization of
vitamin C in surgical repair and lymphocyte-related host
resistance. Br J Cancer. 46:354–367. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maramag C, Menon M, Balaji KC, Reddy PG
and Laxmanan S: Effect of vitamin C on prostate cancer cells in
vitro: Effect on cell number, viability, and DNA synthesis.
Prostate. 32:188–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Koh WS, Lee SJ, Lee H, Park C, Park MH,
Kim WS, Yoon SS, Park K, Hong SI, Chung MH, et al: Differential
effects and transport kinetics of ascorbate derivatives in leukemic
cell lines. Anticancer Res. 18:2487–2493. 1998.PubMed/NCBI
|
|
39
|
Chen Q, Espey Mg, Krishna MC, Mitchell JB,
Corpe CP, Buettner GR, Shacter E and Levine M: Pharmacologic
ascorbic acid concentrations selectively kill cancer cells: Action
as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl
Acad Sci USA. 102:13604–13609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Núñez M, artín C, de Apodaca y Ruiz A
Ortiz and Ruiz A: Ascorbic acid in the plasma and blood cells of
women with breast cancer. The effect of the consumption of food
with an elevated content of this vitamin. Nutr Hosp. 10:368–372.
1995.(In Spanish). PubMed/NCBI
|
|
41
|
Kurbacher CM, Wagner U, Kolster B,
Andreotti PE, Krebs D and Bruckner HW: Ascorbic acid (vitamin C)
improves the antineoplastic activity of doxorubicin, cisplatin, and
paclitaxel in human breast carcinoma cells in vitro. Cancer Lett.
103:183–189. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cooke JP and Dzau VJ: Nitric oxide
synthase: Role in the genesis of vascular disease. Annu Rev Med.
48:489–509. 1997. View Article : Google Scholar : PubMed/NCBI
|