Introduction
BRCA1 and BRCA2 heterozygous mutation
carriers have a strongly increased risk to develop breast cancer
(BC) and ovarian cancer (OC). The lifetime risk to develop BC is
70–80% for BRCA1 mutation carriers and 50–60% for
BRCA2 mutation carriers (1).
For relatives who did not inherit the germline BRCA1/2
mutation segregating in the family (non-carrier relatives), the
risk of BC occurrence is generally estimated to be as low as the
risk assessed in the general population. This may imply that
intensified BC detection screening, using, amongst others,
mammography screening and MRI, as applied in individuals at
high-risk is unnecessary in non-carriers (2–7).
However, one study reported a 2–5-fold increase in BC occurrence in
non-carriers of families with either BRCA1 or BRCA2
mutations (8). Another study
reported a younger than expected age at diagnosis of BC for
non-carriers, that was most evident in BRCA1 families
(9). Moreover, one study by Evans
et al detected a possible higher relative risk for BC in
non-carrier relatives of BRCA2 families, compared to
non-carriers in BRCA1 families (10). In summary, these studies suggest
that DNA alterations (for example SNPs) in other genes may modify
the relative risk for the development of BC in non-carriers,
compared to the general population. Moreover, the authors of these
studies recommend targeted BC detection screening using for example
mammography in non-carriers at a frequency comparable to the
intensive BC screening performed in individuals at high-risk.
Both BRCA1 and BRCA2 are caretaker
genes playing different roles in the repair of DNA double-strand
breaks (DSB), induced by exposure to genotoxic agents such as
ionizing radiation (IR). While BRCA1 has a more general
function in the detection and signaling of a DSB and in the
activation of the G2/M cell cycle checkpoint, BRCA2 exerts a
specific function in the recruitment of RAD51 recombinase to the
DSB site. This latter event is essential for the activation of the
homologous recombination (HR) pathway, that relies on the undamaged
sister chromatid as a template for resynthesis of the damaged
strand. This occurs in the late S and G2 phase of the cell cycle
and leads to error-free repair of DSB (1).
Knowing that both BRCA1 and BRCA2 are
important in the repair of DSB, exposure of mutation carriers to
IR, a potent inducer of DSB, for either diagnostic or therapeutic
purposes appears to be counterintuitive, as mutation carriers may
be more prone to develop radiation-induced BC (11).
Radiosensitivity of BRCA1 mutation carriers
has previously been reported in the literature and was investigated
and confirmed by our research group by means of the G2 micronucleus
(MN) assay in combination with an evaluation of the G2/M checkpoint
efficiency in peripheral blood lymphocytes of healthy BRCA1
mutation carriers compared to healthy volunteers (12). However, the impact of IR on
heterozygous cells of healthy BRCA2 mutation carriers
remains to be elucidated.
To date, several cohort studies were able to prove a
positive correlation between exposure to diagnostic X-rays and BC
risk in BRCA2 mutation carriers (11,13,14).
Others however, could not detect a similar correlation (15–18).
Furthermore, Bernstein et al detected no increased induction
of contralateral BC upon exposure to radiotherapy in BRCA2
mutation carriers (19). Such
discrepancies are likely due to differences in inclusion criteria,
data acquisition and other issues of the studies. It is however
difficult and unethical to design long-term unbiased studies to
evaluate the relationship between BRCA2 mutations, the
exposure to diagnostic or therapeutic radiation and BC risk. In
vitro chromosomal assays are effective tools to investigate
radiosensitivity. Chromosomal radiosensitivity testing on
lymphocytes from BRCA2 mutation carriers has been performed
with techniques such as the G0 MN and the G2 assays for chromatid
breaks, occasionally enhanced with a whole-chromosome painting FISH
(20–25). However, for several of these
studies, it was unclear whether the BRCA2 heterozygotes were
healthy individuals or BC patients, which was previously broached
by Baeyens et al (20).
Furthermore, differences in the experimental setup make comparisons
between studies difficult (26).
Despite these differences, all but one study was able to detect an
elevated chromosomal radiosensitivity in BC patients with a
BRCA2 mutation. However, no comparison was made with
sporadic BC patients. The study of Baeyens et al previously
demonstrated enhanced radiosensitivity in both BC patients with a
BRCA1/2 mutation and sporadic BC patients, suggesting that
the enhanced sensitivity may not be the result of the mutation
(20). No univocal results were
achieved for healthy BRCA2 mutation carriers.
Radiosensitivity in non-carrier relatives has not been studied
extensively, only one study reported no increased radiosensitivity
measured with the G0 MN and G2 chromatid break assay in a small
cohort (n=10) of relatives of both BRCA1 and BRCA2
families without the familial mutation when compared to a
population cohort (20).
In the present study, we aimed to investigate
chromosomal radiosensitivity in healthy BRCA2 mutation
carriers by means of the G2 MN assay. We previously used this assay
and confirmed radiosensitivity in healthy BRCA1 mutation
carriers (n=18) compared to healthy controls without a family
history of BC or OC (n=20) (12),
and in an ataxia-telangiectasia patient and family members
(27). In addition, we also
included healthy relatives not carrying the familial germline
BRCA1 or BRCA2 mutation in the present study. This
cohort of non-carriers was included to evaluate radiosensitivity in
individuals with a comparable genetic background, but without the
familial BRCA1 or BRCA2 mutation.
Materials and methods
Sample collection
Blood samples were collected from individuals
consulting the Centre for Medical Genetics of the Ghent University
Hospital (CMG; Ghent, Belgium), in the context of predictive
testing for hereditary BC. Heparin blood samples were collected for
the G2 MN assay. In addition, EDTA samples were collected for
mutation analysis. We collected blood samples from 18 BRCA2
mutation carriers and 17 subjects from both BRCA1 (n=9) and
BRCA2 (n=8) families not showing the familial mutation
(non-carriers). None of the individuals selected for the present
study had developed cancer at the time of the blood sample
collection. We also selected 18 blood samples from a historical
cohort of healthy volunteers without a personal or familial history
of BC or OC for optimal age and gender match, to determine the
normal distribution of MN yields in unaffected individuals from the
general population (12).
The present study was approved by the Ethics
Committee of Ghent University Hospital (B67020111641 d.d.
20/09/2011) and all participants signed an informed consent.
Molecular analysis
All healthy individuals selected for the present
study had a family history of BC or OC and a mutation in either
BRCA1 or BRCA2 was identified in each proband. All
BRCA2 mutation carriers are heterozygous for an unequivocal
deleterious mutation. This was confirmed by Sanger sequencing of
the relevant amplicon. Sanger sequencing was performed on the
ABI3730XL instrument using the BigDye® Terminator Cycle
Sequencing kit (Life Technologies, Carlsbad, CA, USA) according to
the manufacturer's instructions; sequences were analyzed using the
SeqPilot software (JSI Medical Systems GmbH, Ettenheim,
Germany).
Molecular analyses were not performed in healthy
volunteers due to the absence of a personal or familial anamnesis
for BC or OC.
The G2 MN assay
The G2 MN assay was performed as previously
described (12). In brief,
heparinized blood was cultured in the presence of
phytohaemagglutinin (PHA; 2% v/v; Gibco, Grand Island, NY, USA) to
stimulate T-lymphocyte division. After 3 days, a population of
cycling lymphocytes was obtained and the culture was irradiated
with a 2 Gy dose of 60Co γ-rays. We opted to use a dose
of 2 Gy as this is a well-accepted dose for chromosomal
radiosensitivity testing in lymphocytes (20–22,24).
Immediately after irradiation, cytochalasin B (cyto B; 6 µg/ml;
Sigma-Aldrich, St. Louis, MO, USA) was added to all cultures,
including a non-irradiated culture. Cyto B blocks the cytokinesis
and allows the identification of first-division cells as a
binucleated (BN) cell. After an incubation period of 8 h, all
cultures were fixed with the sequential addition of KCl (75 mM), a
solution of methanol, acetic acid and Ringer (4:1:5), and a
combination of methanol and acetic acid (4:1) to pelleted cells.
Finally, the cell suspension was concentrated and spread on slides.
Slides were stained with 4′,6-diamidino-2-phenlylindole (DAPI) and
scanned with a Metafer 4 platform and MN search software
(MetaSystems GmbH, Altlussheim, Germany). The automated image
analysis system selects BN cells and determines the number of MN/BN
cells. BN cells and MN selection are manually checked for false
positives or false negatives. For each condition, 2 cultures were
prepared and 2 slides/culture were analyzed. A minimum of 600 BN
cells were scored/coded slide. To assess individual
radiosensitivity, a radiosensitivity score (RS score) was
determined. The mean and SD of the MN yield of the group of healthy
volunteers (HV) was set as the cut-off value to determine the RS
score of HV, BRCA2 mutation carriers and non-carrier
relatives. An MN yield higher than the meanHV +
1SDHV was scored as 1, indicating a milder
radiosensitive phenotype, whereas a result higher than the
meanHV + 2SDHV was scored as 2, and indicated
a more severe radiosensitive phenotype. When the individual value
was lower than the meanHV + 1SDHV, a score of
0 was attributed to the tested subject.
Statistical analysis
Age and gender differences among the 3 groups were
judged by means of a one-way ANOVA and Chi-square test,
respectively. The median, interquartile range, average and standard
deviation of micronuclei yields (number of MN/1,000 BN cells) were
assessed in each group of subjects. Intergroup differences of MN
yields between HV, BRCA2 mutation carriers and non-carrier
relatives of BRCA1 and BRCA2 pedigrees were analyzed
by the Mann-Whitney-Wilcoxon test. A one-tailed Fishers exact test
was performed to compare the unpaired and independent proportion of
patients showing a radiosensitive phenotype, evaluated by RS
scoring. For both assays a 5% α error was set as the limit for
statistical significance. The odds ratio (OR) was calculated, based
on the RS scores in healthy individuals and BRCA2 mutation
carriers, to assess the association between the presence of a
BRCA2 mutation and radiosensitivity according to the
following formula:
OR=#BRCA2mutationcarrierswithanRS>0x#healthyvolunteerswithanRS=0#BRCA2mutationcarrierswithanRS=0x#healthyvolunteerswithanRS>0
The 95% confidence interval (CI) was used as a proxy
for significance. The VassarStats platform and the SPSS software
(IBM, version 23) were used to perform statistical analysis.
Results
The mean age did not significantly differ for the HV
(35.3 years), the BRCA2 mutation carriers (40.9 years) and
the non-carrier relatives (40.0 years) (P=0.56; one-way ANOVA). In
addition, no significant difference in gender distribution was
observed for these 3 groups (68, 61 and 71% of the individuals were
female, respectively) (P=0.84; Chi-square test). The number of
spontaneously occurring micronuclei (MN yields in non-irradiated
samples) was not significantly different among the 3 groups of
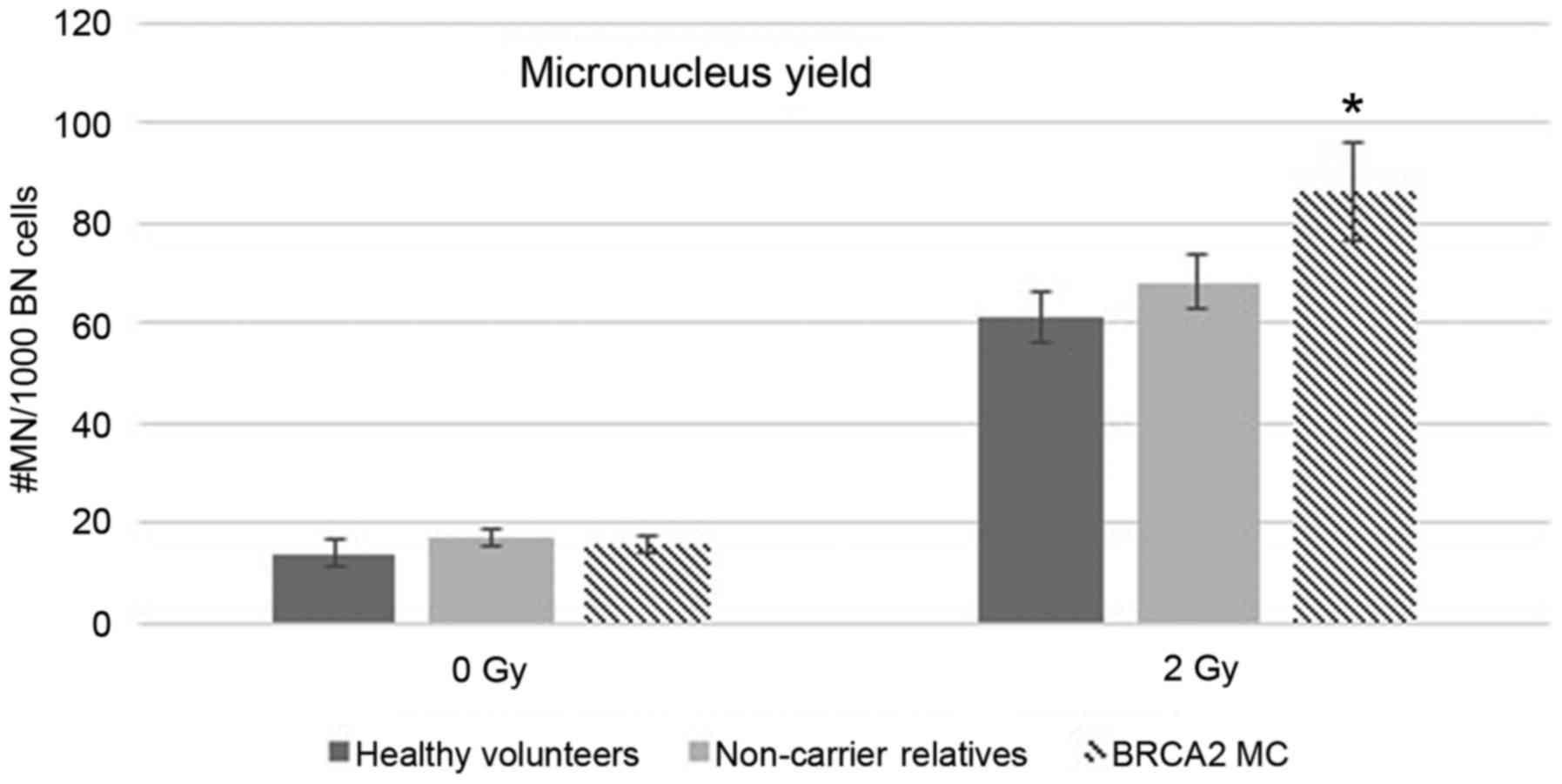
enrolled subjects (Table I and
Fig. 1).
 | Table I.Overview of the median, interquartile
range, mean and SD of the micronucleus yield (#MN/1,000 BN). |
Table I.
Overview of the median, interquartile
range, mean and SD of the micronucleus yield (#MN/1,000 BN).
| Group data | 0 Gy | 2 Gy |
|---|
| Healthy volunteers
(HV) |
|
Median | 12 | 56 |
|
Interquartile range | 9.75 | 27.5 |
|
Mean | 14.33 | 61.22 |
| SD | 8.85 | 21.73 |
| BRCA2
mutation carriers (MC) |
|
|
|
Median | 14 | 74 |
|
Interquartile range | 7.75 | 54.75 |
|
Mean | 16.11 | 86.11 |
| SD | 6.91 | 41.87 |
| P-value
vs. healthy volunteers (Mann-Whitney) | 0.177 | 0.046 |
| Relatives who did
not inherit the familial BRCA1/2 mutation |
|
|
|
Median | 16 | 69 |
|
Interquartile range | 8 | 26 |
|
Mean | 17.23 | 68.11 |
| SD | 7.74 | 22.30 |
| P-value
vs. healthy volunteers (Mann-Whitney) | 0.116 | 0.400 |
Compared to HV without a family history of BC/OC,
BRCA2 mutation carriers showed a significant increase in
mean MN yields after exposure to 2 Gy IR (P=0.046; Mann-Whitney).
Conversely, the radiation-induced MN yields were similar in
relatives who did not inherit the familial BRCA1/2 mutation
and HV without a family history of BC/OC. The mean MN yield in
BRCA2 mutation carriers was higher compared to the mean
yield in non-carriers (86.11 vs. 68.11 MN/1,000 BN cells,
respectively). This difference, however, was not significant
(P=0.298; Mann-Whitney), probably due to the small cohort and the
high SD (Table I and Fig. 1). Furthermore, MN yields did not
differ between non-carrier relatives from BRCA1 or
BRCA2 pedigrees (Table
II).
 | Table II.Overview of median, interquartile
range, mean and SD of the micronucleus yield (#MN/1,000 BN) for
healthy relatives who did not inherit the familial germline
BRCA1/2 mutation. |
Table II.
Overview of median, interquartile
range, mean and SD of the micronucleus yield (#MN/1,000 BN) for
healthy relatives who did not inherit the familial germline
BRCA1/2 mutation.
| Goup data | 0 Gy | 2 Gy |
|---|
| Relatives who did
not inherit the familial BRCA1 mutation (n=9) |
|
|
|
Median | 14 | 66 |
|
Interquartile range | 12 | 57 |
|
Mean | 16.44 | 69.04 |
| SD | 6.88 | 27.45 |
| Relatives who did
not inherit the familial BRCA2 mutation (n=8) |
|
|
|
Median | 16 | 70 |
|
Interquartile range | 12.75 | 51.71 |
|
Mean | 18.11 | 66.98 |
| SD | 9.18 | 16.59 |
| P-value
vs. BRCA1 non-carriers (Mann-Whitney) | 0.7339 | 0.9601 |
The individual MN yields after exposure to 2 Gy and
the RS score for each BRCA2 mutation carrier, non-carrier
relative and healthy volunteer group are listed in Table III. Furthermore, Table III shows mutational data (both
nucleotide and protein nomenclature) and individuals with the same
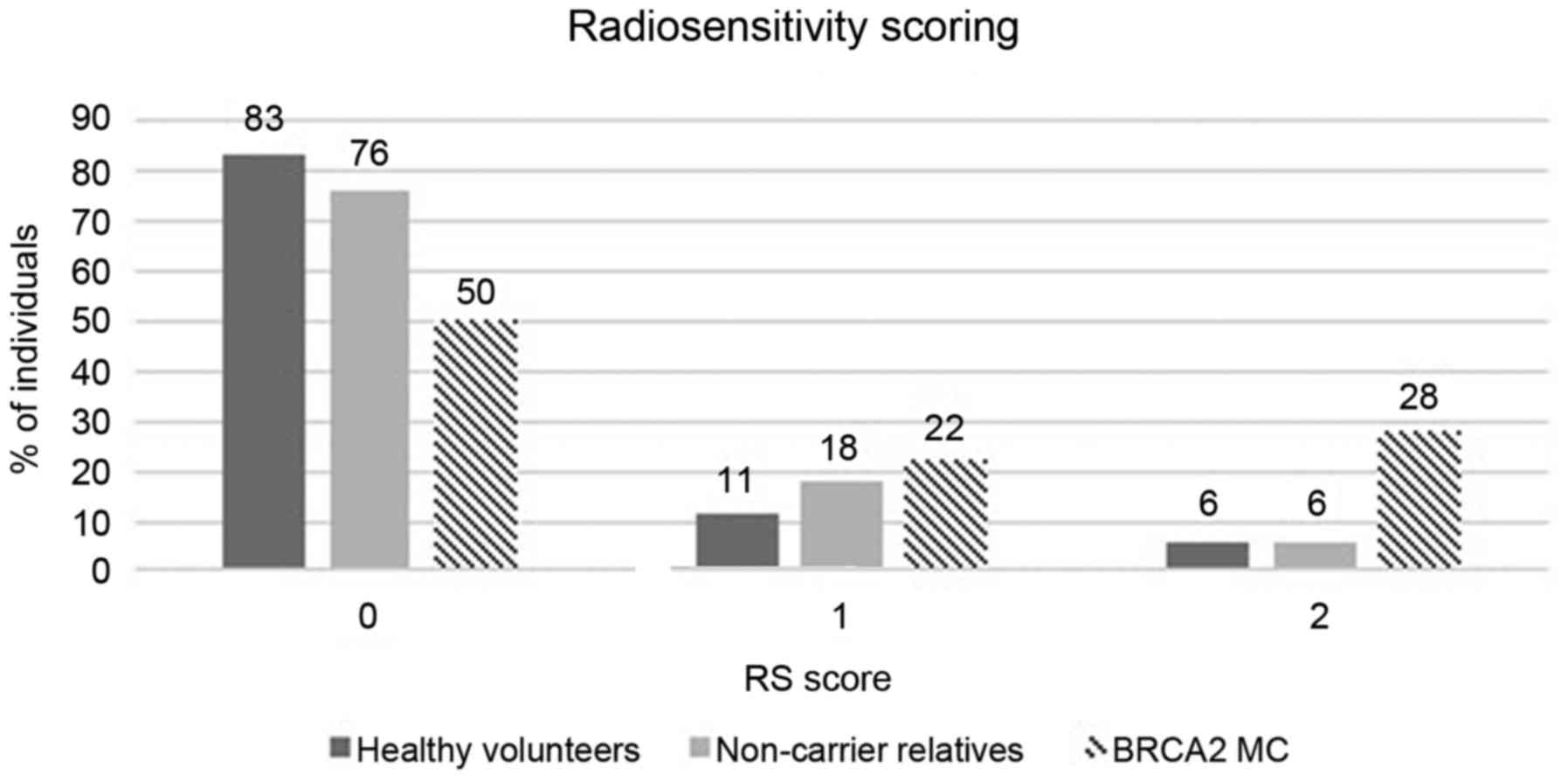
family ID are related. Fig. 2 shows
the distribution of the 3 groups for the different RS scores. A
significantly higher number of BRCA2 mutation carriers
(n=9/18; 50%) showed increased RS scores (score 1 or 2) compared to
HV (n=3/18; 17%) (P=0.038; one-tailed Fishers exact test). For the
relatives who did not inherit the familial germline mutation only
24% (n=4/17) showed an elevated radiosensitivity at the individual
level. RS scoring in related individuals (see family ID in Table III) however shows some variation.
An OR of 5 (95% CI, 1.07–23.46) for BRCA2 mutation carriers
vs. HV, indicates a significant association between the presence of
a BRCA2 mutation and radiosensitivity according to our
criteria.
 | Table III.Germline mutation, family ID,
micronucleus yields (#MN/1,000 BN) and RS score for BRCA2
mutation carriers, relatives who did not inherit the familial
mutation (non-carrier relatives) and healthy volunteers (numbering
of the nucleotides according to RefSeq nr. NM_000059.3; A of ATG
start codon=nucleotide +1). |
Table III.
Germline mutation, family ID,
micronucleus yields (#MN/1,000 BN) and RS score for BRCA2
mutation carriers, relatives who did not inherit the familial
mutation (non-carrier relatives) and healthy volunteers (numbering
of the nucleotides according to RefSeq nr. NM_000059.3; A of ATG
start codon=nucleotide +1).
|
| BRCA2 mutation
carriers | Non-carrier
relatives | Healthy
volunteers |
|---|
|
|
|
|
|
|---|
| ID | Family ID | Mutation:
nucleotide | Mutation:
protein | 0 Gy | 2 Gy | RS score | ID | Family ID | Family gene | 0 Gy | 2 Gy | RS score | ID | 0 Gy | 2 Gy | RS score |
|---|
| M2.01 | BR-32-2170 | c.658_659delGT | p.(Val220fs*4) | 14 | 119 | 2 | NM.06 | BR-32-0156 | BRCA2 | 17 | 51 | 0 | D01 | 19 | 83 | 1 |
| M2.02 | BR-32-1748 | c.1389_1390del | p.(Val464fs*3) | 15 | 91 | 1 | NM.17 | BR-32-0342 | BRCA1 | 8 | 29 | 0 | D12 | 10 | 52 | 0 |
| M2.03 | BR-32-1748 | c.1389_1390del | p.(Val464fs*3) | 19 | 83 | 1 | NM.01 | BR-32-0645 | BRCA1 | 20 | 63 | 0 | D13 | 7 | 47 | 0 |
| M2.04 | BR-32-1748 | c.1389_1390del | p.(Val464fs*3) | 12 | 58 | 0 | NM.10 | BR-32-1134 | BRCA1 | 14 | 74 | 0 | D15 | 12 | 55 | 0 |
| M2.05 | BR-32-1748 | c.1389_1390del | p.(Val464fs*3) | 16 | 56 | 0 | NM.13 | BR-32-1225 | BRCA1 | 18 | 43 | 0 | D16 | 17 | 44 | 0 |
| M2.06 | BR-32-1758 | c.1989del | p.(Phe663fs*5) | 12 | 163 | 2 | NM.12 | BR-32-1225 | BRCA1 | 11 | 57 | 0 | D17 | 7 | 58 | 0 |
| M2.07 | BR-32-0884 | c.4171del |
p.(Glu1391fs*19) | 37 | 65 | 0 | NM.07 | BR-32-1444 | BRCA1 | 12 | 66 | 0 | D21 | 13 | 48 | 0 |
| M2.08 | BR-32-0884 | c.4171del |
p.(Glu1391fs*19) | 20 | 90 | 1 | NM.08 | BR-32-1444 | BRCA1 | 12 | 78 | 0 | D04 | 12 | 40 | 0 |
| M2.09 | BR-32-1759 | c.4936_4939del |
p.(Glu1646fs*23) | 20 | 65 | 0 | NM.02 | BR-32-1494 | BRCA1 | 24 | 87 | 1 | D05 | 6 | 30 | 0 |
| M2.10 | BR-32-1759 | c.4936_4939del |
p.(Glu1646fs*23) | 18 | 53 | 0 | NM.16 | BR-32-1967 | BRCA1 | 29 | 125 | 2 | D06 | 15 | 74 | 0 |
| M2.11 | BR-32-0156 | c.6275_6276del |
p.(Leu2092Profs*7) | 12 | 63 | 0 | NM.03 | BR-32-0884 | BRCA2 | 20 | 91 | 1 | D29 | 9 | 29 | 0 |
| M2.12 | BR-32-1565 | c.6275_6276del |
p.(Leu2092Profs*7) | 8 | 44 | 0 | NM.04 | BR-32-0884 | BRCA2 | 16 | 73 | 0 | D30 | 30 | 109 | 2 |
| M2.13 | BR-32-1930 | c.6275_6276del |
p.(Leu2092Profs*7) | 23 | 183 | 2 | NM.09 | BR-32-1748 | BRCA2 | 21 | 70 | 0 | D32 | 7 | 96 | 1 |
| M2.14 | BR-32-1930 | c.6275_6276del |
p.(Leu2092Profs*7) | 12 | 86 | 1 | NM.11 | BR-32-1758 | BRCA2 | 8 | 45 | 0 | D31 | 26 | 73 | 0 |
| M2.15 | BR-32-1920 | c.8167G>C | p.(Asp2723His) | 10 | 118 | 2 | NM.05 | BR-32-1759 | BRCA2 | 38 | 85 | 1 | D35 | 37 | 76 | 0 |
| M2.16 | BR-32-1628 |
c.8332-?_8487-?del |
p.(Ile2778Lysfs*40) | 22 | 29 | 0 | NM.14 | BR-32-1759 | BRCA2 | 12 | 69 | 0 | D37 | 6 | 75 | 0 |
| M2.17 | BR-32-0937 | c.8904delC |
p.(Val2969fs*7) | 10 | 131 | 2 | NM.15 | BR-32-2170 | BRCA2 | 13 | 52 | 0 | D38 | 17 | 52 | 0 |
| M2.18 | BR-32-0082 | c.9256+1G>C |
r.9118_9256del; | 10 | 53 | 0 |
|
|
|
|
|
| D39 | 8 | 61 | 0 |
|
|
|
|
p.(Val3040Aspfs*18) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Median |
|
|
| 14.00 | 74.00 |
|
|
|
| 16.00 | 69.00 |
|
| 12.00 | 56.00 |
|
| Interquartile
range |
|
| 7.75 | 54.75 |
|
|
|
| 8.00 | 26.00 |
|
| 9.75 | 27.50 |
|
| Mean |
|
|
| 16.11 | 86.11 |
|
|
|
| 17.23 | 68.11 |
|
| 14.33 | 61.22 |
|
| SD |
|
|
| 6.91 | 41.87 |
|
|
|
| 7.74 | 22.30 |
|
| 8.85 | 21.73 |
|
All but one of the 18 mutation carriers enrolled in
the present study were heterozygous for a mutation predicted to
result in a premature termination codon (PTC). The patient with the
deleterious missense mutation [BRCA2 c.8167G>C;
p.(Asp2723His)] obtained an RS score of 2.
Discussion
Results of the G2 micronucleus (MN) assay performed
after exposure to 2 Gy γ-rays showed a significantly increased
radiosensitivity in healthy BRCA2 mutation carriers compared
to healthy controls. Previous studies with a large number of
different techniques were able to demonstrate enhanced
radiosensitivity in BC patients with a BRCA2 mutation,
however, no univocal results were achieved for healthy BRCA2
mutation carriers (20–25). Non-carrier relatives of either
BRCA1 or BRCA2 families did not show an increased
radiosensitive phenotype compared to the cohort of healthy
volunteers, which is in agreement with the study of Baeyens et
al (20). We previously
performed the G2 MN assay in a group of 18 healthy BRCA1
mutation carriers, and found a significantly increased MN yield
after exposure to 2 Gy γ-rays (12). These findings are analogous to the
results of the present study, performed in healthy carriers of
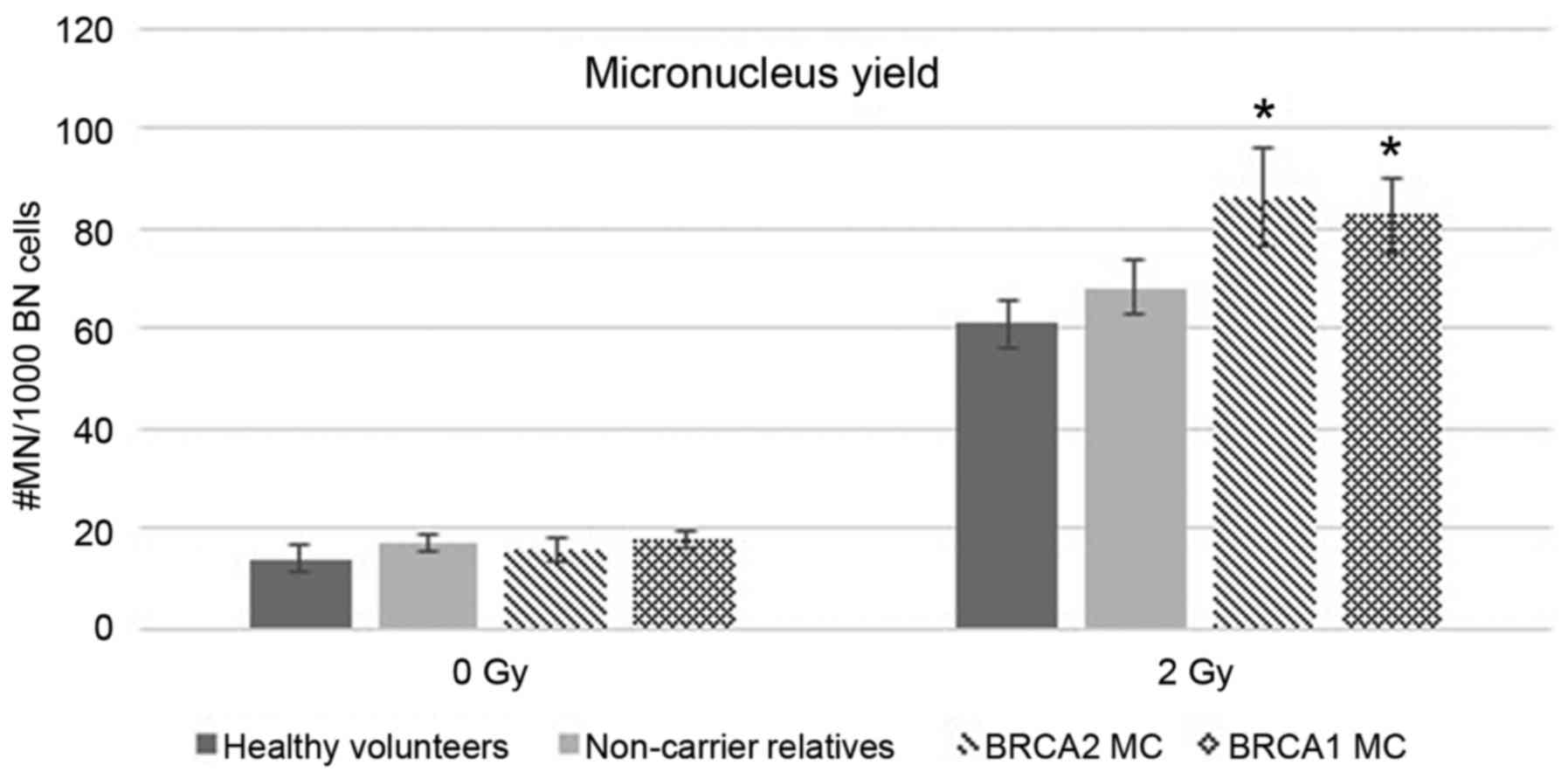
pathogenic BRCA2 mutations. Fig.
3 shows the integration of the data from healthy BRCA1
mutation carriers in the present study. The detection of an
increased mean MN yield in both BRCA1 and BRCA2
mutation carriers after exposure to ionizing radiation can be
explained by their mutual role in DNA double-strand break repair
reviewed by Roy et al (1).
In our previous study we also analyzed the G2/M
checkpoint activity by the addition of caffeine, an agent
abrogating the G2/M checkpoint, to the irradiated cultures and
demonstrated a significantly impaired checkpoint activation in
BRCA1 mutation carriers compared to healthy volunteers
(12). Analysis of the G2/M
checkpoint activation in the current BRCA2 cohort did not
reveal a significant difference (data not shown). This result is in
agreement with the fact that BRCA2 is not activated in this
particular checkpoint pathway as reviewed by Roy et al
(1), but does not support the data
obtained by Menzel et al (28), suggesting a role for BRCA2 as a
regulator of G2 checkpoint maintenance following DNA damage
introduced in a human osteosarcoma cell line (U2OS) expressing
dominant-negative p53 by a high-dose of ionizing radiation (6
Gy).
The role of BRCA2 in the HR pathway, a DNA repair
pathway active in the S and G2 phase of the cell cycle, is
extensively reported in literature (1). The present study, focusing on
radiosensitivity testing of lymphocytes in these phases of the cell
cycle, showed an OR of 5 (95% CI, 1.07–23.47) for healthy
individuals with a heterozygous BRCA2 mutation compared to
healthy controls. This indicates a positive association between the
presence of a BRCA2 mutation and radiosensitivity that could
be attributed to deficient HR capacity in heterozygous cells.
Two independent research groups have reported a
decreased DSB repair capacity in BRCA2 heterozygous cells.
Keimling et al used an enhanced green fluorescent protein
(EGFP)-based assay to report impaired HR capacity in lymphoblastoid
cells with a BRCA2 monoallelic truncating frameshift
mutation. They confirmed this decrease in HR capacity in a
BRCA2-knockdown HeLa cell line (29). Arnold et al demonstrated
distinct defects in DNA DSB repair in lymphoblastoid cell lines
(LCLs) from heterozygous BRCA2 mutation carriers through
analysis of γ-H2AX repair kinetics (30). Although, the latter study did not
focus on DNA repair by HR, it indicates a malfunction of DSB repair
in LCLs from BRCA2 mutation carriers that could be
attributed to diminished HR activity.
Most mutation carriers enrolled in the present study
(n=17/18, 94%) had a mutation resulting in a premature termination
codon (PTC). The presence of a PTC mutation is expected to activate
nonsense-mediated decay of the gene transcript. Previous research
from various groups including ours, demonstrated a reduction in
mutant mRNA to approximately half of the WT mRNA levels in
lymphocytes of individuals with a PTC mutation in BRCA1
(12,31,32).
Arnold et al (30) detected
a similar mutant mRNA reduction for BRCA2 mutations leading
to a PTC. Furthermore, Arnold et al (30) and Keimling et al (29) report distinct reduced protein levels
in LCLs from heterozygous BRCA2 mutation carriers, although
quantitative analysis of this variation was not performed.
Previously, haploinsufficiency has been suggested as the mechanism
for hereditary BC development in BRCA1 and BRCA2
mutation carriers (33). In the
present study, a higher than expected number of radiosensitive
individuals in the BRCA2 mutation carriers indicates that
haploinsufficiency may also be responsible for the radiosensitive
phenotype in carriers of a mutation generating a PTC. In the
present study, only one individual with a deleterious missense
mutation was included. This substitution results in an amino acid
change at position p.2723 and impairs protein functionality as
shown by a homology-directed DNA break-repair functional assay
(34). For this individual we
obtained a high RS score of 2. Further research in larger patient
cohorts with different types of mutations is needed to evaluate
whether the type of mutation influences the radiosensitive
phenotype or whether there are additional parameters determining
this phenotype.
Results of the G2 MN assay showed no increased
radiosensitivity in the group of non-carrier relatives of both
BRCA1 and BRCA2 families compared to a group of
healthy volunteers. Furthermore, only 24% of non-carriers showed an
elevated radiosensitivity at the individual level (RS score 1 or
2). This was not significantly different from the fraction of
healthy volunteers (17%) that was found to have an increased RS
score. In addition, no difference was observed between non-carriers
from BRCA1 (RS score, 0 in 7/9 investigated relatives) or
BRCA2 families (RS score, 0 in 6/8 investigated relatives).
However, we observed some variation within the different groups. We
hypothesize that modifiers may play a role: indeed, selected SNPs
in DNA-damage repair genes and other common variants have been
associated with increased radiosensitivity (35–37)
and increased BC risk (35,38). Further and larger studies are needed
to evaluate the subtle influence of possible modifying factors on
BC risk and radiosensitivity.
In conclusion, the present study demonstrated higher
radiosensitivity in healthy BRCA2 mutation carriers compared
to healthy volunteers by means of the G2 MN assay after exposure of
peripheral blood lymphocytes to a dose of 2 Gγ-rays. No increased
radiosensitivity was observed in non-carrier relatives of
BRCA1 and BRCA2 families. When evaluating
radiosensitivity at the individual level, a significantly higher
proportion of BRCA2 mutation carriers (50%) showed a mild or
more severe radiosensitivity compared to healthy volunteers (17%)
and non-carriers (24%). Furthermore, an OR of 5 indicated a
positive association between the BRCA2 mutation and an
increased radiosensitivity in healthy mutation carriers. These
results indicate that care should be taken when applying ionizing
radiation for either diagnostic or therapeutic purposes in
BRCA2 mutation carriers. However, a study including a larger
population of subjects carrying different types of BRCA2
mutations and non-carriers, must be performed to further elucidate
the effect of each single mutation on the radiosensitive phenotype
and the influence of possible underlying factors.
Acknowledgements
The authors would like to thank all participants who
donated a blood sample for the present study. They thank Céline De
Brock, Brecht Crombez, Ilse Coene, Johanna Aernoudt, Toke Thiron,
Greet De Smet and Leen Pieters for their technical assistance.
Professor Thierens is thanked for the use of the irradiation
facility. The present study was funded by the Belgian Foundation
against Cancer (project 2012–216).
References
|
1
|
Roy R, Chun J and Powell SN: BRCA1 and
BRCA2: Different roles in a common pathway of genome protection.
Nat Rev Cancer. 12:68–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bernholtz S, Laitman Y, Kaufman B,
Shimon-Paluch S and Friedman E: Phenocopy breast cancer rates in
Israeli BRCA1 BRCA2 mutation carrier families: Is the risk
increased in non-carriers? Breast Cancer Res Treat. 132:669–673.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Domchek SM, Gaudet MM, Stopfer JE,
Fleischaut MH, Powers J, Kauff N, Offit K, Nathanson KL and Robson
M: Breast cancer risks in individuals testing negative for a known
family mutation in BRCA1 or BRCA2. Breast Cancer Res Treat.
119:409–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harvey SL, Milne RL, McLachlan SA,
Friedlander ML, Birch KE, Weideman P, Goldgar D, Hopper JL and
Phillips KA: kConFab Investigators: Prospective study of breast
cancer risk for mutation negative women from BRCA1 or BRCA2
mutation positive families. Breast Cancer Res Treat. 130:1057–1061.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Korde LA, Mueller CM, Loud JT, Struewing
JP, Nichols K, Greene MH and Mai PL: No evidence of excess breast
cancer risk among mutation-negative women from BRCA
mutation-positive families. Breast Cancer Res Treat. 125:169–173.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kurian AW, Gong GD, John EM, Johnston DA,
Felberg A, West DW, Miron A, Andrulis IL, Hopper JL, Knight JA, et
al: Breast cancer risk for noncarriers of family-specific BRCA1 and
BRCA2 mutations: Findings from the Breast Cancer Family Registry. J
Clin Oncol. 29:4505–4509. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nielsen HR, Petersen J, Krogh L, Nilbert M
and Skytte AB: No evidence of increased breast cancer risk for
proven noncarriers from BRCA1 and BRCA2 families. Fam Cancer.
15:523–528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith A, Moran A, Boyd MC, Bulman M,
Shenton A, Smith L, Iddenden R, Woodward ER, Lalloo F, Maher ER, et
al: Phenocopies in BRCA1 and BRCA2 families: Evidence for modifier
genes and implications for screening. J Med Genet. 44:10–15. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vos JR, De Bock GH, Teixeira N, van der
Kolk DM, Jansen L, Mourits MJE and Oosterwijk JC: Proven
non-carriers in BRCA families have an earlier age of onset of
breast cancer. Eur J Cancer. 49:2101–2106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Evans DGR, Ingham SL, Buchan I, Woodward
ER, Byers H, Howell A, Maher ER, Newman WG and Lalloo F: Increased
rate of phenocopies in all age groups in BRCA1/BRCA2 mutation
kindred, but increased prospective breast cancer risk is confined
to BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev.
22:2269–2276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pijpe A, Andrieu N, Easton DF, Kesminiene
A, Cardis E, Noguès C, Gauthier-Villars M, Lasset C, Fricker JP,
Peock S, et al: GENEPSO; EMBRACE; HEBON: Exposure to diagnostic
radiation and risk of breast cancer among carriers of BRCA1/2
mutations: Retrospective cohort study (GENE-RAD-RISK). BMJ.
345:e56602012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baert A, Depuydt J, Van Maerken T, Poppe
B, Malfait F, Storm K, van den Ende J, Van Damme T, De Nobele S,
Perletti G, et al: Increased chromosomal radiosensitivity in
asymptomatic carriers of a heterozygous BRCA1 mutation. Breast
Cancer Res. 18:522016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Andrieu N, Easton DF, Chang-Claude J,
Rookus MA, Brohet R, Cardis E, Antoniou AC, Wagner T, Simard J,
Evans G, et al: Effect of chest X-rays on the risk of breast cancer
among BRCA1/2 mutation carriers in the international BRCA1/2
carrier cohort study: A report from the EMBRACE, GENEPSO,
GEO-HEBON, and IBCCS Collaborators' Group. J Clin Oncol.
24:3361–3366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lecarpentier J, Noguès C, Mouret-Fourme E,
Stoppa-Lyonnet D, Lasset C, Caron O, Fricker JP, Gladieff L, Faivre
L, Sobol H, et al: GENEPSO: Variation in breast cancer risk with
mutation position, smoking, alcohol, and chest X-ray history, in
the French National BRCA1/2 carrier cohort (GENEPSO). Breast Cancer
Res Treat. 130:927–938. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Narod SA, Lubinski J, Ghadirian P, Lynch
HT, Moller P, Foulkes WD, Rosen B, Kim-Sing C, Isaacs C, Domchek S,
et al: Hereditary Breast Cancer Clinical Study Group: Screening
mammography and risk of breast cancer in BRCA1 and BRCA2 mutation
carriers: A case-control study. Lancet Oncol. 7:402–406. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giannakeas V, Lubinski J, Gronwald J,
Moller P, Armel S, Lynch HT, Foulkes WD, Kim-Sing C, Singer C,
Neuhausen SL, et al: Mammography screening and the risk of breast
cancer in BRCA1 and BRCA2 mutation carriers: A prospective study.
Breast Cancer Res Treat. 147:113–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goldfrank D, Chuai S, Bernstein JL, Ramon
Y, Cajal T, Lee JB, Alonso MC, Diez O, Baiget M, Kauff ND, Offit K,
et al: Effect of mammography on breast cancer risk in women with
mutations in BRCA1 or BRCA2. Cancer Epidemiol Biomarkers Prev.
15:2311–2313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
John EM, McGuire V, Thomas D, Haile R,
Ozcelik H, Milne RL, Felberg A, West DW, Miron A, Knight JA, et al:
Kathleen Cuningham Foundation Consortium for Research into Familial
Breast Cancer (kConFab); Whittemore AS: Diagnostic chest X-rays and
breast cancer risk before age 50 years for BRCA1 and BRCA2 mutation
carriers. Cancer Epidemiol Biomarkers Prev. 22:1547–1556. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bernstein JL, Thomas DC, Shore RE, Robson
M, Boice JD Jr, Stovall M, Andersson M, Bernstein L, Malone KE,
Reiner AS, et al: WECARE Study Collaborative Group: Contralateral
breast cancer after radiotherapy among BRCA1 and BRCA2 mutation
carriers: A WECARE study report. Eur J Cancer. 49:2979–2985. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baeyens A, Thierens H, Claes K, Poppe B,
de Ridder L and Vral A: Chromosomal radiosensitivity in BRCA1 and
BRCA2 mutation carriers. Int J Radiat Biol. 80:745–756. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gutiérrez-Enríquez S, Ramón Y, Cajal T,
Alonso C, Corral A, Carrasco P, Cornet M, Sanz J, Ribas M, Baiget M
and Diez O: Ionizing radiation or mitomycin-induced micronuclei in
lymphocytes of BRCA1 or BRCA2 mutation carriers. Breast Cancer Res
Treat. 127:611–622. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Trenz K, Rothfuss A, Schütz P and Speit G:
Mutagen sensitivity of peripheral blood from women carrying a BRCA1
or BRCA2 mutation. Mutat Res. 500:89–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beroukas E, Pandis N, Giannoukakos K,
Rizou E, Beroukas K, Giatromanolaki A and Koukourakis M: Increased
chromosomal radiosensitivity in women carrying BRCA1/BRCA2
mutations assessed with the G2 assay. Int J Radiat Oncol Biol Phys.
76:1199–1205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Becker AA, Graeser MK, Landwehr C, Hilger
T, Baus W, Wappenschmidt B, Meindl A, Weber RG and Schmutzler RK: A
24-color metaphase-based radiation assay discriminates heterozygous
BRCA2 mutation carriers from controls by chromosomal
radiosensitivity. Breast Cancer Res Treat. 135:167–175. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bolognesi C, Bruzzi P, Gismondi V, Volpi
S, Viassolo V, Pedemonte S and Varesco L: Clinical application of
micronucleus test: A case-control study on the prediction of breast
cancer risk/susceptibility. PLoS One. 9:e1123542014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cardinale F, Bruzzi P and Bolognesi C:
Role of micronucleus test in predicting breast cancer
susceptibility: A systematic review and meta-analysis. Br J Cancer.
106:780–790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Claes K, Depuydt J, Taylor AMR, Last JI,
Baert A, Schietecatte P, Vandersickel V, Poppe B, De Leeneer K,
D'Hooghe M, et al: Variant ataxia telangiectasia: Clinical and
molecular findings and evaluation of radiosensitive phenotypes in a
patient and relatives. Neuromolecular Med. 15:447–457. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Menzel T, Nähse-Kumpf V, Kousholt AN,
Klein DK, Lund-Andersen C, Lees M, Johansen JV, Syljuåsen RG and
Sørensen CS: A genetic screen identifies BRCA2 and PALB2 as key
regulators of G2 checkpoint maintenance. EMBO Rep. 12:705–712.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Keimling M, Volcic M, Csernok A, Wieland
B, Dörk T and Wiesmüller L: Functional characterization connects
individual patient mutations in ataxia telangiectasia mutated (ATM)
with dysfunction of specific DNA double-strand break-repair
signaling pathways. FASEB J. 25:3849–3860. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arnold K, Kim MK, Frerk K, Edler L,
Savelyeva L, Schmezer P and Wiedemeyer R: Lower level of BRCA2
protein in heterozygous mutation carriers is correlated with an
increase in DNA double strand breaks and an impaired DSB repair.
Cancer Lett. 243:90–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perrin-Vidoz L, Sinilnikova OM,
Stoppa-Lyonnet D, Lenoir GM and Mazoyer S: The nonsense-mediated
mRNA decay pathway triggers degradation of most BRCA1 mRNAs bearing
premature termination codons. Hum Mol Genet. 11:2805–2814. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Anczuków O, Ware MD, Buisson M, Zetoune
AB, Stoppa-Lyonnet D, Sinilnikova OM and Mazoyer S: Does the
nonsense-mediated mRNA decay mechanism prevent the synthesis of
truncated BRCA1, CHK2, and p53 proteins? Hum Mutat. 29:65–73. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Berger AH, Knudson AG and Pandolfi PP: A
continuum model for tumour suppression. Nature. 476:163–169. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guidugli L, Pankratz VS, Singh N, Thompson
J, Erding CA, Engel C, Schmutzler R, Domchek S, Nathanson K, Radice
P, et al: A classification model for BRCA2 DNA binding domain
missense variants based on homology-directed repair activity.
Cancer Res. 73:265–275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Willems P, Claes K, Baeyens A,
Vandersickel V, Werbrouck J, De Ruyck K, Poppe B, van den Broecke
R, Makar A, Marras E, et al: Polymorphisms in nonhomologous
end-joining genes associated with breast cancer risk and
chromosomal radiosensitivity. Genes Chromosomes Cancer. 47:137–148.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo Z, Shu Y, Zhou H, Zhang W and Wang H:
Radiogenomics helps to achieve personalized therapy by evaluating
patient responses to radiation treatment. Carcinogenesis.
36:307–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Popanda O, Marquardt JU, Chang-Claude J
and Schmezer P: Genetic variation in normal tissue toxicity induced
by ionizing radiation. Mutat Res. 667:58–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Antoniou AC, Kuchenbaecker KB, Soucy P,
Beesley J, Chen X, McGuffog L, Lee A, Barrowdale D, Healey S,
Sinilnikova OM and et al; CIMBA: SWE-BRCA; HEBON; EMBRACE; GEMO
Collaborators Study; kConFab Investigators: Common variants at
12p11, 12q24, 9p21, 9q31.2 and in ZNF365 are associated with breast
cancer risk for BRCA1 and/or BRCA2 mutation carriers. Breast Cancer
Res. 14:R332012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Claes K, Poppe B, Machackova E, Coene I,
Foretova L, DePaepe A and Messiaen L: Differentiating pathogenic
mutations from polymorphic alterations in the splice sites of BRCA1
and BRCA2. Genes Chromosomes Cancer. 37:314–320. 2003. View Article : Google Scholar : PubMed/NCBI
|