Introduction
Transferrin receptor (TfR) is a type II
transmembrane glycoprotein found as a homodimer (180 kDa) on the
surface of cells, involved in iron uptake through interaction with
transferrin and in regulation of cell growth (1,2). TfR
is expressed at low levels on most normal cells, whereas it is
expressed at higher levels on cells with high proliferation rates
such as cancer cells (3–8). TfR is therefore an attractive molecule
for targeted therapy of cancer. Many laboratories have developed
various TfR-targeted therapeutic agents such as transferrin- and
anti-TfR antibodies conjugated with anticancer agents (7,9). An
antibody having high specificity and affinity to its target antigen
is one of the most suitable agents to prepare drug-conjugates.
Antibody-drug conjugate is initially tested in mice xenografted
with human cancers to verify the selective delivery of the
conjugate to tumor site and to validate non-specific tissue
distribution. However, if the antibody reacts only with human
antigen, it is not possible to verify the uptake to normal organs
and tissues reflecting physiological antigen expression. Whether
antigen expressed in normal tissues may or may not be accessible to
blood-borne antibody-drug conjugates is not fully predictable by
ex vivo immunohistochemical analysis. To predict the
distribution in humans, especially the uptake in normal organs and
tissues which is closely related to the toxicity of the
antibody-drug conjugate, the use of an antibody that cross-reacts
with murine antigen is essential. As far as we know, there are no
publications to date regarding such anti-human TfR antibodies
(7,9).
In this study, to provide the biodistribution data
of anti-TfR antibody in mice for predicting the biodistribution in
human, we isolated a new fully human monoclonal antibody that can
recognize both human and murine TfR from a large-scale human
antibody library. Designated as TSP-A18, the cross-reactivity was
evaluated using human and murine cell lines. It was radiolabeled
with In-111 and the in vitro binding characteristics using a
highly TfR-expressing cell line MIAPaCa-2, as well as the
biodistribution in two murine strains C57BL/6J and BALB/c-nu/nu,
were evaluated. Furthermore, the correlation of the uptake of
radiolabeled antibody with TfR expression in major organs and
tissues was analyzed, which was compared with the tissue
distribution of [67Ga]citrate.
Materials and methods
Antibody
The AIMS5 phage antibody library was used as a
source of human monoclonal antibodies (10,11).
The antigen used in the screenings of the library was prepared as
follows: cDNA encoding a myc-His tagged extracellular portion of
human TfR was inserted into an expression vector pCMV-Script
(Clontech, Mountain View, CA, USA). The resultant plasmid DNA was
transfected into 293T cells (American Type Culture Collection,
Manassas, VA, USA) with Lipofectamine (Invitrogen, Carlsbad, CA,
USA), and the transformants were grown in D-MEM medium (Sigma, St.
Louis, MO, USA) supplemented with 10% fetal bovine serum. The
extracellular portion of human TfR protein was purified from the
supernatant of cell culture using Ni-NTA chromatography. We also
prepared the murine antigen (extracellular portion of murine TfR)
following the same procedure. The phage library selection process
was described previously (12). In
this study, the phage library was incubated alternately with
immobilized human or murine antigen. Phages from individual
colonies obtained after four rounds of panning were tested for
binding to the extracellular portions of human and murine TfR by
ELISA [the procedure was described previously (13)]. The nucleotide sequence of the
positive clone was determined and the clone was designated as
TSP-A18. It was subsequently converted to human IgG1 and the
antibody was produced using Expi293 transient expression system
(Thermo Fisher Scientific, Waltham, MA, USA). The antibody was
purified with protein-A sepharose (ProteNova, Kagawa, Japan). An
isotype control HR1-007 (11) was
constructed in the same way.
Flow cytometric analysis
A human pancreatic cancer cell line MIAPaCa-2 and a
mouse fibroblast cell line NIH/3T3 were purchased from American
Type Culture Collection. The mouse B lymphocyte cell line Sp2/O,
the human chronic myelogenous leukemia cell line K562, the mouse
erythroleukemia cell line B8/3, the cynomolgus kidney derived
fibroblast-like cell MK.P3(F) and the cynomolgus fetal
spleen-derived T-cell like lymphocyte cell line HSC-F were
purchased from Japanese Collection of Research Bioresources Cell
Bank (Osaka, Japan). An insect cell line Sf9 derived from
Spodoptera frugiperda was obtained from Thermo Fisher
Scientific. Cells in suspension culture were harvested by
centrifugation at 1,000 µg for 5 min. Adherent cells were removed
from the dishes with EDTA-PBS (Thermo Fisher Scientific), then
washed with PBS (Thermo Fisher Scientific). Cell density was
adjusted at a concentration of 1×106 cells/ml. TSP-A18
was serially diluted at concentrations of 0.002, 0.02, 0.2 and 2
µg/ml. An equal volume of the serially diluted antibodies was added
to the cell suspensions and incubated for 1 h at 4°C, followed by
two washes with FACS buffer (1% BSA, 0.1% sodium azide, 2 mM EDTA
in Dulbecco's PBS). The washed cells were incubated with mouse
anti-human IgG (H+L) antibody labeled with Alexa 488 (Life
Technologies Japan, Tokyo, Japan) as a second antibody for 1 h at
4°C. The reacted cells were washed twice with FACS buffer and then
measured by FACSCalibur (Becton-Dickinson, San Jose, CA, USA).
Radiolabeling
The antibody was conjugated with
p-SCN-Bn-DOTA (DOTA) (Macrocyclics, Dallas, TX, USA)
according to the procedure as described previously (14) with slight modifications. Briefly,
during gentle shaking, a five molar excess of chelate in 13.7 µl
DMSO was added to the antibody (10 mg in 1 ml 0.05 M bicine-NaOH,
150 mM NaCl, pH 8.5), and incubated for 17 h at 25°C.
Non-conjugated chelate was removed by size exclusion chromatography
using a PD10 column (GE Healthcare, Little Chalfont, UK) and 0.1 M
acetate buffer (pH 6.0) as eluent. The conjugation ratio of chelate
to TSP-A18 was estimated to be ~3.5 by MALDI-TOF mass spectrometry.
For In-111 labeling, 50 µg of DOTA-conjugated antibodies were mixed
with 740 kBq of [111In]Cl3 (Nihon Medi-Physics, Tokyo,
Japan) in 0.5 M acetate buffer (pH 6.0) and the mixture was
incubated for 30 min at room temperature. Radiolabeled antibodies
were separated from free In-111 by Sephadex G-50 column (700 µg for
2 min once or twice). The labeling yields of
[111In]TSP-A18 ranged from 62.9 to 83.0%. The
radiochemical purity of [111In]TSP-A18 exceeded 97.5%.
The specific activities of [111In]TSP-A18 were 9.3–12.3
kBq/µg.
Cell binding and competitive
inhibition assays
MIAPaCa-2 cells were maintained in D-MEM medium
(Sigma) supplemented with 10% fetal bovine serum (Sigma). Cell
binding and competitive inhibition assays were conducted as
previously described (9). Briefly,
cells were detached 3–4 days after seeding and cell suspensions
were prepared. For cell binding assays, MIAPaCa-2 cells
(5.0×106, 2.6×106, 1.3×106,
6.3×105, 3.1×105, 1.6×105,
7.8×104 and 3.9×104) in PBS with 1% BSA
(Sigma) were incubated with [111In]TSP-A18 on ice for 60
min. After washing, the amount of cell-bound radioactivity was
measured using a gamma counter (ARC-370M; Aloka, Tokyo, Japan).
Immunoreactivity of [111In]TSP-A18 was estimated
according to the method of Lindmo et al (15). For competitive inhibition assays,
[111In]TSP-A18 were incubated with MIAPaCa-2 cells
(2.0×106) in the presence of varying concentrations of
the unlabeled intact TSP-A18, DOTA-conjugated TSP-A18, or HR1-007
as an isotype control (0, 0.3, 0.6, 3.0, 6.1, 30.3 and 60.6 nmol/l)
on ice for 60 min. After washing, the amount of cell-bound
radioactivity was measured. The dissociation constant
(Kd) was estimated by applying data to a one-site
competitive binding model using GraphPad Prism software version 6.0
for Mac (GraphPad Software, La Jolla, CA, USA).
Biodistribution of
[111In]TSP-A18 and [67Ga]citrate
The animal experimental protocol was approved by the
Animal Care and Use Committee of the National Institute of
Radiological Sciences, and all animal experiments were conducted in
accordance with the institutional guidelines regarding animal care
and handling. Male C57BL/6J (19.3–23.3 g body weight) and
BALB/c-nu/nu (15.4–19.4 g body weight) mice (Japan SLC, Hamamatsu,
Japan) were intravenously injected with 37 kBq of
[111In]TSP-A18. The total injected protein dose was
adjusted to 5 µg per mouse by adding the intact antibody. C57BL/6J
(19.4–22.4 g body weight) and BALB/c-nu/nu (18.5–22.4 g body
weight) mice were intravenously injected with 370 kBq of
[67Ga]citrate (Nihon Medi-Physics). At 1, 2, 4 and 7
days after injection of [111In]TSP-A18, and at 2 h, and
1, 2 and 3 days after injection of [67Ga]citrate, mice
(n=5 at each time-point) were euthanized by excess isoflurane
inhalation, and blood was obtained from the heart. Organs and
tissues of interest (blood, lung, liver, spleen, pancreas,
intestine, kidney, muscle and bone including marrow component) were
removed and weighed. The radioactivity was measured using a gamma
counter. The data were expressed as the percentage of injected dose
per gram of tissue (% ID/g) normalized to a 20-g body weight
mouse.
[111In]TSP-A18 uptake in
bone and bone marrow
On day 1 after i.v. injection of 37 kBq of
[111In]TSP-A18 into C57BL/6J and BALB/c-nu/nu mice (n=5
at each time-point), femur was removed, and then bone and bone
marrow were separated and weighed. The radioactivity were measured
using a gamma counter (ARC-370M; Aloka). The data were expressed as
the percentage of injected dose per gram of tissue (% ID/g)
normalized to a 20-g body weight mouse.
Western blotting for TfR protein
expression
Western blotting was conducted as previously
described (16). Briefly, blood,
lung, liver, spleen, pancreas, intestine, kidney, muscle and bone
marrow were removed from C57BL/6J and BALB/c-nu/nu mice (n=2), and
the lysates were prepared using cell lysate buffer (Cell Signaling
Technology, Danvers, MA, USA). The lysates were resolved by sodium
dodecyl sulfate polyacrylamide gel electrophoresis, transferred to
a polyvinylidene fluoride membrane (Hybond-P, GE Healthcare) and
incubated with a rabbit anti-murine TfR antibody (ab84036, Abcam,
Cambridge, UK) for 60 min at room temperature. After washing, the
membrane was reacted with a horseradish peroxidase-linked
anti-rabbit antibody (GE Healthcare) and visualized using an ECL
Plus kit (GE Healthcare). Images were captured using a LAS-3000
imaging system (Fuji Film, Tokyo, Japan) and analyzed by ImageJ
(National Institutes of Health, Bethesda, MD, USA). Two or three
independent experiments for each C57BL/6J mouse and three for each
BALB/c-nu/nu mouse were conducted.
Statistical analysis
The data of [111In]TSP-A18 and
[67Ga]citrate in C57BL/6J and BALB/c-nu/nu mice were
analyzed by the Student's t-test. The correlation between TfR
protein expression and the uptakes of [111In]TSP-A18 and
[67Ga]citrate, and between TfR protein expression and
the area under the curve (AUC) of the uptakes was examined by
simple regression analysis. AUC was calculated from the
biodistribution data using the trapezoidal rule. A value of
P<0.05 was considered statistically significant.
Results
Flow cytometric analysis for
cross-reactivity of TSP-A18
We selected a clone using extracellular portions of
human and murine TfR from the AIMS5 phage library as a source of
human antibodies (10,11). The clone was confirmed to bind to
the extracellular portions of human and murine TfR as determined by
ELISA (data not shown) and was designated as TSP-A18. The clone was
converted to human IgG1 and the antibody reacted with
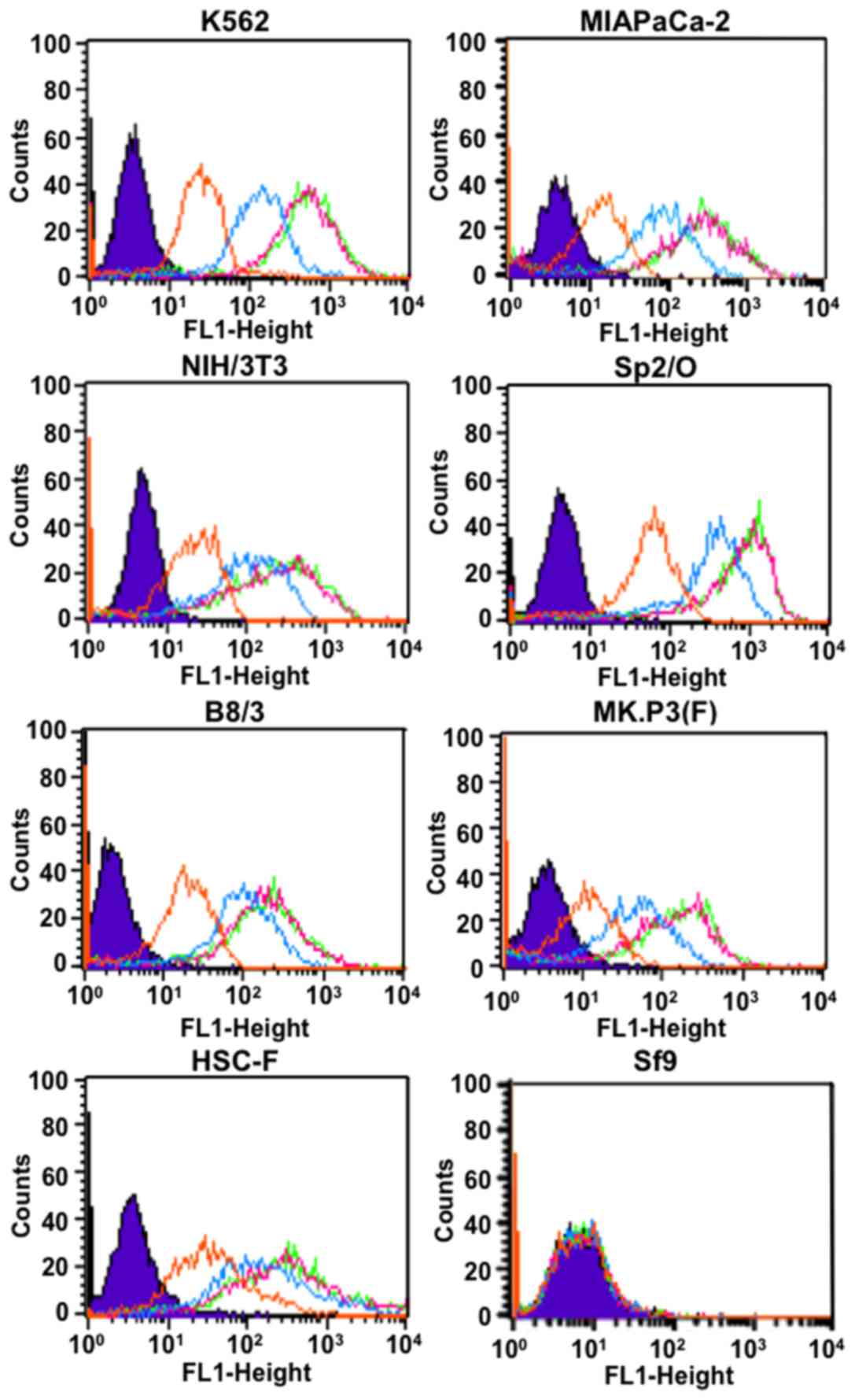
human cells (K562 and MIAPaCa-2) and murine cells (NIH/3T3, Sp2/O
and B8/3), but not insect cells (Sf9) as determined by flow
cytometry (Fig. 1). The intensity
increased depending on TSP-A18 concentration and reached a maximum
with TSP-A18 dose of 0.1 µg/ml or more (Fig. 1). The antibody TSP-A18 also reacted
with simian cells [MK.P3(F) and HSC-F] as shown in Fig. 1.
Cell binding and competitive
inhibition assays for TSP-A18
In the cell binding assay, [111In]TSP-A18
bound highly to MIAPaCa-2 cells and the maximum value was >50%
(Fig. 2A). From the competitive
inhibition assay, Kd of intact TSP-A18 and DOTA-TSP-A18
were estimated to be 0.22 and 0.49 nM, respectively (Fig. 2B), suggesting that the loss of
immunoreactivity by chelate conjugation was limited. In addition,
HR1-007 as an isotype control did not inhibit TSP-A18 binding to
TfR on the cell surface (Fig.
2B).
Biodistribution of
[111In]TSP-A18 in C57BL/6J and BALB/−nu/nu mice
Biodistribution experiments for
[111In]TSP-A18 were conducted in C57BL/6J and
BALB/−nu/nu mice from days 1 to 7 after injection (Table I). Although
[111In]TSP-A18 highly accumulated in the spleen and bone
containing marrow component of both C57BL/6J and BALB/−nu/nu mice,
there were significant differences in the uptakes between the mice
(Table I).
[111In]TSP-A18 uptake in the spleen of C57BL/6J and
BALB/c-nu/nu mice at day 1 was 59.8±3.7% and 109.7±6.5% ID/g,
respectively (Table I). The peak
value of 70.9±5.0% ID/g in C57BL/6J mice was observed at day 4,
whereas that of 109.7±6.5% ID/g in BALB/c-nu/nu mice was observed
at day 1 (Table I).
[111In]TSP-A18 uptake in bone containing marrow
component at day 1 was 50.5±8.0% ID/g for C57BL/6J mice and
24.8±3.7% ID/g for BALB/c-nu/nu mice (Table I). In another experiment, when
[111In]TSP-A18 uptake in bone and bone marrow was
determined separately, most radioactivity was observed in the bone
marrow of C57BL/6J and BALB/c-nu/nu mice (Table II).
 | Table I.Biodistribution of
[111In]TSP-A18 antibody in mice. |
Table I.
Biodistribution of
[111In]TSP-A18 antibody in mice.
| Strain | Day 1 | Day 2 | Day 4 | Day 7 |
|---|
| C57BL/6J |
|
|
|
|
|
Blood |
5.4±0.7a |
4.5±0.2a |
2.2±0.3 |
0.7±0.1 |
|
Lung |
2.7±0.2b |
2.7±0.4b |
2.6±0.1b |
1.1±0.1a |
|
Liver |
3.6±1.4 |
2.9±0.8 |
4.7±0.6b |
5.3±0.8b |
|
Spleen |
59.8±3.7b |
64.3±5.0b |
70.9±5.0b |
65.7±7.9b |
|
Pancreas |
2.0±0.3a |
2.0±0.4 |
2.1±0.3b |
1.6±0.2b |
|
Intestine |
3.6±0.3b |
2.2±0.4 |
1.9±0.2b |
1.6±0.2b |
|
Kidney |
4.0±0.2b |
3.2±0.2a |
2.7±0.0b |
2.2±0.1b |
|
Muscle |
1.2±0.4a |
0.6±0.1 |
0.4±0.0b |
0.3±0.1 |
|
Bonec |
50.5±8.0b |
34.5±4.1b |
25.1±4.3b |
16.2±2.7b |
| BALB/c-nu/nu |
|
|
|
|
|
Blood |
4.4±0.5 |
3.8±0.6 |
2.0±0.1 |
0.6±0.0 |
|
Lung |
2.0±0.2 |
1.8±0.26 |
2.1±0.2 |
1.0±0.1 |
|
Liver |
2.5±0.7 |
3.0±1.6 |
3.0±0.4 |
3.4±0.5 |
|
Spleen | 109.7±6.5 | 79.3±10.0 | 61.1±5.2 | 48.4±7.1 |
|
Pancreas |
1.6±0.2 |
1.6±0.4 |
1.3±0.2 |
0.9±0.1 |
|
Intestine |
1.9±0.3 |
1.9±0.5 |
1.5±0.1 |
0.9±0.2 |
|
Kidney |
3.4±0.2 |
3.0±0.2 |
2.3±0.2 |
1.5±0.1 |
|
Muscle |
0.7±0.1 |
0.7±0.2 |
0.5±0.0 |
0.3±0.0 |
|
Bonec |
24.8±3.7 | 18.6±3.9 | 16.6±3.3 |
8.1±1.6 |
 | Table II.Uptake of [111In]TSP-A18
antibody on day 1. |
Table II.
Uptake of [111In]TSP-A18
antibody on day 1.
| Tissue | C57BL/6J | BALB/c-nu/nu |
|---|
| Bone containing
marrow component | 8.8±4.8 | 11.0±3.2 |
| Bone marrow | 188.0±27.5 | 104.8±24.5 |
TfR protein expression in organs and
tissues
TfR protein expression in organs and tissues of
C57BL/6J and BALB/c-nu/nu mice was determined using western
blotting (Fig. 3). The spleen
showed the highest expression compared with other organs in both
C57BL/6J and BALB/c-nu/nu mice (Fig.
3). Although TfR protein was strongly expressed in the bone
marrow of C57BL/6J mice, only a faint expression was observed in
BALB/c-nu/nu (Fig. 3).
Biodistribution of
[67Ga]citrate in C57BL/6J and BALB/−nu/nu mice
Biodistribution experiments of
[67Ga]citrate were conducted in C57BL/6J and BALB/−nu/nu
mice from 2 h to 3 days after injection (Table III). [67Ga]citrate
uptake in blood at 2 h was 17.0±1.3% ID/g for C57BL/6J mice and
15.4±0.9% ID/g for BALB/c-nu/nu mice and decreased with time
(Table III).
[67Ga]citrate uptake in containing marrow component at 2
h was 12.2±1.1% ID/g for C57BL/6J mice and 9.4±1.2% ID/g for
BALB/c-nu/nu, and the peak values were 24.0±2.2% ID/g at day 2 for
C57BL/6J and 20.5±2.3% ID/g at day 1 for BALB/c-nu/nu (Table III).
 | Table III.Biodistribution of
[67Ga]citrate in mice. |
Table III.
Biodistribution of
[67Ga]citrate in mice.
| Strain | 2h | Day 1 | Day 2 | Day 3 |
|---|
| C57BL/6J |
|
|
|
|
|
Blood |
17.0±1.3a |
2.1±0.4b |
0.7±0.1b |
0.5±0.0b |
|
Lung |
6.8±0.6a |
2.6±0.2b |
1.9±0.1b |
1.8±0.1b |
|
Liver |
4.0±0.4 |
6.0±1.4a |
4.7±0.3 |
6.1±0.9b |
|
Spleen |
3.7±0.4 |
4.2±0.5b |
4.4±0.2b |
4.3±0.3b |
|
Pancreas |
4.7±0.5 | 4.9±1.0 |
4.9±0.3b |
4.4±1.0a |
|
Intestine |
2.8±0.7b |
2.3±0.4b |
1.3±0.2a |
1.0±0.1b |
|
Kidney |
6.5±0.7b |
6.3±0.7b |
5.6±0.4b |
5.3±0.5b |
|
Muscle |
2.1±0.2 |
1.3±0.3b |
1.0±0.1b |
0.9±0.4 |
|
Bonec |
12.2±1.1b | 20.2±2.4 |
24.0±2.2b |
20.5±2.0a |
| BALB/c-nu/nu |
|
|
|
|
|
Blood | 15.4±0.9 | 1.0±0.4 |
0.4±0.1 |
0.2±0.1 |
|
Lung |
6.0±0.6 | 1.6±0.4 |
1.4±0.1 |
1.3±0.1 |
|
Liver |
4.4±0.4 | 4.8±0.5 |
5.1±0.7 |
4.8±0.5 |
|
Spleen |
3.7±0.2 | 2.5±0.5 |
2.9±0.3 |
2.8±0.5 |
|
Pancreas |
4.4±0.3 | 3.9±0.6 |
3.9±0.4 |
3.4±0.2 |
|
Intestine |
1.7±0.3 | 1.0±0.3 |
0.9±0.2 |
0.5±0.1 |
|
Kidney |
5.4±0.3 | 4.1±0.5 |
3.4±0.3 |
2.8±0.3 |
|
Muscle |
2.0±0.2 | 1.2±1.1 |
0.6±0.1 |
0.6±0.1 |
|
Bonec |
9.4±1.2 | 20.5±2.3 | 18.7±1.4 | 18.1±1.5 |
Correlation analysis
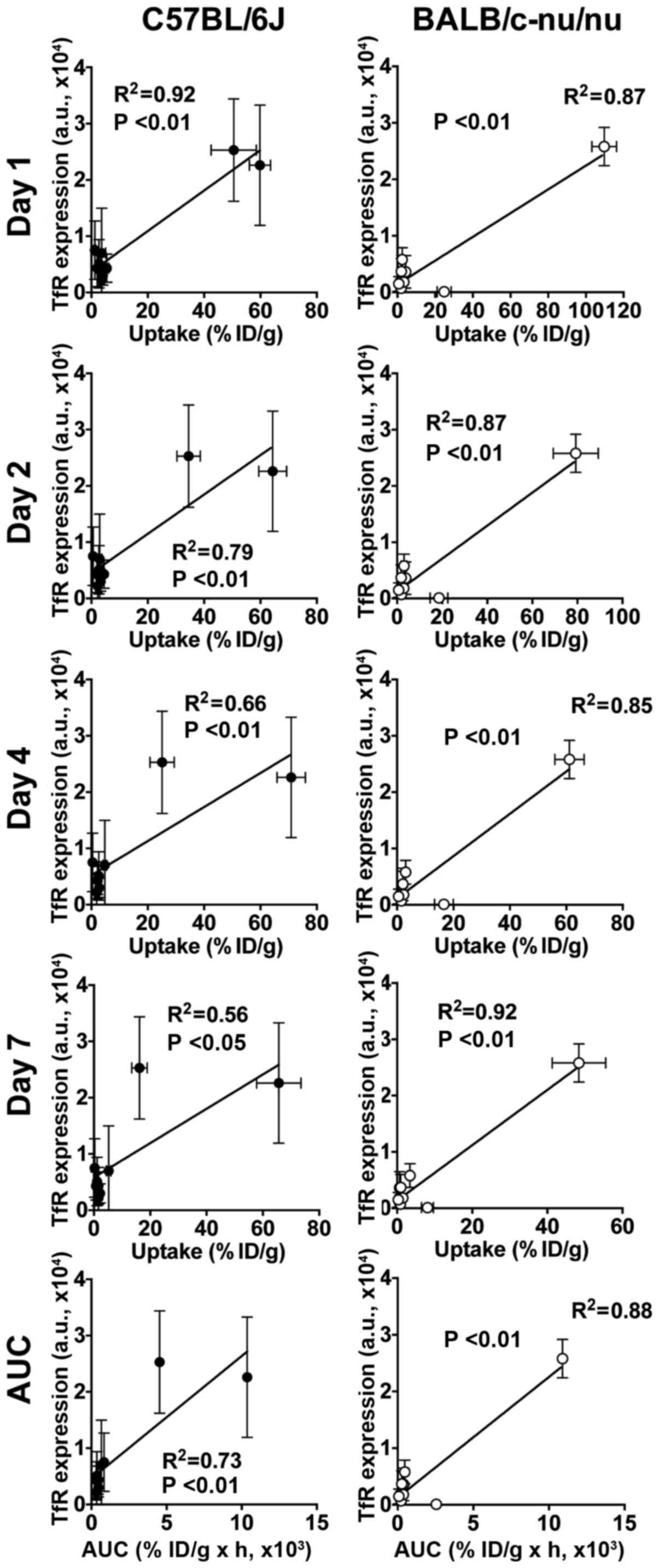
There were significant correlations between the TfR
protein expressions and [111In]TSP-A18 uptakes (all
time-points and AUC) in both C57BL/6J and BALB/c-nu/nu mice
(Fig. 4). There were significant
correlations between the TfR protein expressions and
[67Ga]citrate uptakes (days 1, 2 and 3, and AUC) in
C57BL/6J mice, whereas there was no correlation in BALB/c-nu/nu
(Fig. 5).
Discussion
In this study, we isolated a new fully human
antibody that recognizes the extracellular portions of human and
murine TfR from a human antibody library and it was designated as
TSP-A18. Flow cytometry confirmed that TSP-A18 recognizes TfR
expressed on the surface of human and murine cell lines. Cell
binding and competitive inhibition assays using
[111In]TSP-A18 showed high binding and affinity with TfR
expressed on human cancer cells.
To our knowledge, there is no report to date on the
distribution of anti-TfR antibody correlating with TfR expression
on normal murine organs, although there are several reports on
TfR-targeting via transferrin using [67Ga]citrate or
radiolabeled transferrin, in which biodistribution of these probes
was evaluated in mice (BALB/c, BALB/c-nu/nu and FVB) and rats
(Sprague-Dawley) (17–20). According to these reports, their
uptakes in the liver and bone marrow were relatively high and those
in the other organs and tissues including the spleen were
relatively low (17–20). Interestingly, our biodistribution
experiments with [111In]TSP-A18 in C57BL/6J and
BALB/c-nu/nu mice showed that the highest uptake was observed in
the spleen for both strains of mice, followed by that in bone
containing marrow component. As mentioned above, splenic uptakes of
[67Ga]citrate and radiolabeled transferrin were not high
(2.4–11.0% ID/g for mice and 0.3–1.2% ID/g for rats) (17,19,20).
To confirm the reported biodistribution results on
[67Ga]citrate, biodistribution experiments of
[67Ga]citrate in C57BL/6J and BALB/c-nu/nu mice were
conducted in this study. The biodistributions were similar to those
in the previous studies (17–20),
and the splenic uptakes were not high. In addition to the
unexpectedly high uptake of [111In]TSP-A18 in the
spleen, there were marked differences in the uptake patterns in the
spleen and bone containing marrow component between C57BL/6J and
BALB/c-nu/nu mice. These findings raised the possibility that TfR
expression levels in the murine tissues, especially the spleen and
bone marrow are different between C57BL/6J and BALB/c-nu/nu mice.
To test the possibility, TfR protein expression in major organs and
tissues of C57BL/6J and BALB/c-nu/nu mice was determined by western
blotting using a commercially available anti-TfR antibody and
analyzed the correlation with the uptakes of
[111In]TSP-A18 and [67Ga]citrate. The TfR
protein expression levels in the spleen were markedly high for both
strains, but the expression level in the bone marrow was high only
for C57BL/6J mice. The differences in TfR expression may be a
reason for the differences in the uptakes of
[111In]TSP-A18 in the two murine strains. There are
significant correlations of TfR expression and uptakes of
[111In]TSP-A18 in both strains, whereas there are
significant correlations of TfR expression with uptakes of
[67Ga]citrate only in C57BL/6J, but not in BALB/c-nu/nu.
[67Ga]citrate is also reported to accumulate in some
transferrin-independent manner (21–23).
These findings suggest that direct TfR-targeted imaging using
radiolabeled anti-TfR antibody is more suitable for evaluating TfR
expression in normal organs and tissues. TSP-A18 recognizes both
human and murine TfR, therefore, radiolabeled TSP-A18 has the
potential for imaging studies in human to assess the difference
between directly TfR-targeted imaging and transferrin-mediated
imaging such as [67Ga]citrate.
In humans, [67Ga]citrate accumulates
mainly in the liver, moderately in bone marrow, and at lower levels
in the spleen and other organs (24). However, this does not necessarily
indicate low TfR expression in the human spleen, considering the
results of this study as mentioned above. There are many reports of
TfR protein expression in major human organs, tissues and tumors,
and they showed high TfR expression in cells with high
proliferation rate including some lymphocytes and monocytes in the
bone marrow (5). However, to our
knowledge, there are two studies on TfR expression in the spleen:
one reported that frozen spleen sections were negatively stained as
determined by immunohistochemical staining (4), and the other reported that only 2.5%
of cells in the spleen were bound by anti-TfR antibody, whereas 40%
of bone marrow cells were positive as determined by visual
fluorescence assay (25). Taken
together the TfR expression in humans described in the literature
and the expression in mice that we determined in this study, there
could be a marked difference in TfR expression in the spleen of
humans and mice. This possible difference in TfR expression should
be carefully considered when testing for the toxicity of anti-TfR
antibody in mice.
TfR expression changes depending on the
physiological status such as tumorigenesis (5). TfR is directly upregulated by MYC
(26), which is an important
mediator of cancer initiation and maintenance, and thought to be
the cause of more than half of all human cancers (27,28).
There is no MYC-targeted non-invasive imaging applicable to humans,
whereas TfR is upregulated by MYC from the early stages of
tumorigenesis (5,27,28).
There is thus a potential role for TfR-targeted imaging to enable
early detection of tumors. In addition, since increased expression
of TfR correlates with tumor stage (7), TfR-targeted imaging could be a
promising imaging method for staging. To demonstrate the concept,
while it is not realistic to monitor TfR expression change with
disease progression in each cancer patient, this should be feasible
in carcinogenesis models such as genetically engineered and
carcinogen-induced models (29).
TfR is also reported to be upregulated by other factors such as
phosphoinositide 3-kinase and hypoxia-inducible factor-1 (30,31).
Temporal imaging for TfR expression change with disease progression
in carcinogenesis models could be useful for clarifying role(s) of
TfR in developing cancer and may provide clues for more efficient
prevention, diagnosis and therapy of cancer. TSP-A18 labeled with
gamma-ray or positron emitter could be used for monitoring in mouse
models.
In conclusion, we developed a new fully human
monoclonal antibody TSP-A18, which recognizes both human and murine
TfR. [111In]TSP-A18 accumulated highly in the spleen and
bone marrow of C57BL/6J and BALB/c-nu/nu mice. This biodistribution
pattern differed from that of [67Ga]citrate, which
accumulated highly in bone marrow, but not in the spleen. There was
significant correlation between [111In]TSP-A18 uptake
and TfR protein expression in both strains, whereas there was
significant correlation of [67Ga]citrate uptake with TfR
expression only in C57BL/6J. Radiolabeled TSP-A18 could be more
suitable for evaluating TfR expression in normal organs and tissues
of mice compared with [67Ga]citrate.
Acknowledgements
This study was supported in part by KAKENHI
25861140. The authors appreciate the technical assistance of Yuriko
Ogawa and Naoko Kuroda, and the staff in the Laboratory Animal
Sciences section for animal management.
References
|
1
|
Neckers LM and Trepel JB: Transferrin
receptor expression and the control of cell growth. Cancer Invest.
4:461–470. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lesley JF and Schulte RJ: Inhibition of
cell growth by monoclonal anti-transferrin receptor antibodies. Mol
Cell Biol. 5:1814–1821. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gatter KC, Brown G, Trowbridge IS,
Woolston RE and Mason DY: Transferrin receptors in human tissues:
Their distribution and possible clinical relevance. J Clin Pathol.
36:539–545. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Panaccio M, Zalcberg JR, Thompson CH,
Leyden MJ, Sullivan JR, Lichtenstein M and McKenzie IF:
Heterogeneity of the human transferrin receptor and use of
anti-transferrin receptor antibodies to detect tumours in vivo.
Immunol Cell Biol. 65:461–472. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Daniels TR, Delgado T, Rodríguez JA,
Helguera G and Penichet ML: The transferrin receptor part I:
Biology and targeting with cytotoxic antibodies for the treatment
of cancer. Clin Immunol. 121:144–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ryschich E, Huszty G, Knaebel HP, Hartel
M, Büchler MW and Schmidt J: Transferrin receptor is a marker of
malignant phenotype in human pancreatic cancer and in
neuroendocrine carcinoma of the pancreas. Eur J Cancer.
40:1418–1422. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daniels TR, Delgado T, Helguera G and
Penichet ML: The transferrin receptor part II: Targeted delivery of
therapeutic agents into cancer cells. Clin Immunol. 121:159–176.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Daniels TR, Bernabeu E, Rodríguez JA,
Patel S, Kozman M, Chiappetta DA, Holler E, Ljubimova JY, Helguera
G and Penichet ML: The transferrin receptor and the targeted
delivery of therapeutic agents against cancer. Biochim Biophys
Acta. 1820:291–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugyo A, Tsuji AB, Sudo H, Okada M,
Koizumi M, Satoh H, Kurosawa G, Kurosawa Y and Saga T: Evaluation
of efficacy of radioimmunotherapy with 90Y-labeled fully human
anti-transferrin receptor monoclonal antibody in pancreatic cancer
mouse models. PLoS One. 10:e0123761–e17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akahori Y, Kurosawa G, Sumitomo M, Morita
M, Muramatsu C, Eguchi K, Tanaka M, Suzuki K, Sugiura M, Iba Y, et
al: Isolation of antigen/antibody complexes through organic solvent
(ICOS) method. Biochem Biophys Res Commun. 378:832–835. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kurosawa G, Akahori Y, Morita M, Sumitomo
M, Sato N, Muramatsu C, Eguchi K, Matsuda K, Takasaki A, Tanaka M,
et al: Comprehensive screening for antigens overexpressed on
carcinomas via isolation of human mAbs that may be therapeutic.
Proc Natl Acad Sci USA. 105:7287–7292. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morino K, Katsumi H, Akahori Y, Iba Y,
Shinohara M, Ukai Y, Kohara Y and Kurosawa Y: Antibody fusions with
fluorescent proteins: A versatile reagent for profiling protein
expression. J Immunol Methods. 257:175–184. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurosawa G, Sumitomo M, Akahori Y, Matsuda
K, Muramatsu C, Takasaki A, Iba Y, Eguchi K, Tanaka M, Suzuki K, et
al: Methods for comprehensive identification of membrane proteins
recognized by a large number of monoclonal antibodies. J Immunol
Methods. 351:1–12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meares CF, Moi MK, Diril H, Kukis DL,
McCall MJ, Deshpande SV, DeNardo SJ, Snook D and Epenetos AA:
Macrocyclic chelates of radiometals for diagnosis and therapy. Br J
Cancer (Suppl). 10:21–26. 1990.PubMed/NCBI
|
|
15
|
Lindmo T, Boven E, Cuttitta F, Fedorko J
and Bunn PA Jr.: Determination of the immunoreactive fraction of
radiolabeled monoclonal antibodies by linear extrapolation to
binding at infinite antigen excess. J Immunol Methods. 72:77–89.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sugyo A, Tsuji AB, Sudo H, Nagatsu K,
Koizumi M, Ukai Y, Kurosawa G, Zhang MR, Kurosawa Y and Saga T:
Preclinical evaluation of 89Zr-labeled human antitransferrin
receptor monoclonal antibody as a PET probe using a pancreatic
cancer mouse model. Nucl Med Commun. 36:286–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chan SM, Hoffer PB, Maric N and Duray P:
Inhibition of gallium-67 uptake in melanoma by an anti-human
transferrin receptor monoclonal antibody. J Nucl Med. 28:1303–1307.
1987.PubMed/NCBI
|
|
18
|
Vavere AL and Welch MJ: Preparation,
biodistribution, and small animal PET of 45Ti-transferrin. J Nucl
Med. 46:683–690. 2005.PubMed/NCBI
|
|
19
|
Holland JP, Evans MJ, Rice SL, Wongvipat
J, Sawyers CL and Lewis JS: Annotating MYC status with
89Zr-transferrin imaging. Nat Med. 18:1586–1591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aloj L, Jogoda E, Lang L, Caracò C,
Neumann RD, Sung C and Eckelman WC: Targeting of transferrin
receptors in nude mice bearing A431 and LS174T xenografts with
[18F]holo-transferrin: Permeability and receptor dependence. J Nucl
Med. 40:1547–1555. 1999.PubMed/NCBI
|
|
21
|
Chen DC, Newman B, Turkall RM and Tsan MF:
Transferrin receptors and gallium-67 uptake in vitro. Eur J Nucl
Med. 7:536–540. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chitambar CR and Zivkovic Z: Uptake of
gallium-67 by human leukemic cells: Demonstration of transferrin
receptor-dependent and transferrin-independent mechanisms. Cancer
Res. 47:3929–3934. 1987.PubMed/NCBI
|
|
23
|
Sohn MH, Jones BJ, Whiting JH Jr, Datz FL,
Lynch RE and Morton KA: Distribution of gallium-67 in normal and
hypotransferrinemic tumor-bearing mice. J Nucl Med. 34:2135–2143.
1993.PubMed/NCBI
|
|
24
|
Jonkhoff AR, Plaizier MA, Ossenkoppele GJ,
Teule GJ and Huijgens PC: High-dose gallium-67 therapy in patients
with relapsed acute leukaemia: A feasibility study. Br J Cancer.
72:1541–1546. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haynes BF, Hemler M, Cotner T, Mann DL,
Eisenbarth GS, Strominger JL and Fauci AS: Characterization of a
monoclonal antibody (5E9) that defines a human cell surface antigen
of cell activation. J Immunol. 127:347–351. 1981.PubMed/NCBI
|
|
26
|
O'Donnell KA, Yu D, Zeller KI, Kim J-W,
Racke F, Thomas-Tikhonenko A and Dang CV: Activation of transferrin
receptor 1 by c-Myc enhances cellular proliferation and
tumorigenesis. Mol Cell Biol. 26:2373–2386. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dang CV: MYC on the path to cancer. Cell.
149:22–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Casey SC and Felsher DW:
Inactivation of MYC reverses tumorigenesis. J Intern Med.
276:52–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Day C-P, Merlino G and Van Dyke T:
Preclinical mouse cancer models: A maze of opportunities and
challenges. Cell. 163:39–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abe N, Inoue T, Galvez T, Klein L and
Meyer T: Dissecting the role of PtdIns(4,5)P2 in endocytosis and
recycling of the transferrin receptor. J Cell Sci. 121:1488–1494.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tacchini L, Bianchi L, Bernelli-Zazzera A
and Cairo G: Transferrin receptor induction by hypoxia.
HIF-1-mediated transcriptional activation and cell-specific
post-transcriptional regulation. J Biol Chem. 274:24142–24146.
1999. View Article : Google Scholar : PubMed/NCBI
|