Introduction
miR-30a has been implicated to function as tumor
suppressor in various kinds of cancer (1), such as breast cancer (2), colon cancer (3), osteosarcoma (4), hepatocellular carcinoma (5), non-small cell lung cancer (NSCLC)
(6), glioma (7), ovarian carcinoma (8) and renal clear cell carcinoma (9). Numerous studies have suggested that
miR-30a could influence tumor progression through modulating cancer
cell proliferation (10),
migration, invasion (4),
epithelial-mesenchymal transition (EMT) (2), apoptosis (5), autophagy (11) and other ways (12). A study on NSCLC tissues using miRNA
microarray demonstrated that miR-30a was downregulated in both
adenocarcinomas (14/20) and squamous cell carcinomas (20/20)
(P=0.448) (13). Other studies
(14) arrived to the same
conclusion and revealed that its low expression was associated with
cancer risk and indicated poor prognosis (15). In vitro experiment (16) also suggested miR-30a was
downregulated in NSCLC A549 cells compared with BEAS-2B normal lung
epithelial cells, overexpression of miR-30a could inhibit A549 lung
cancer cell malignancy (6,16). However, the exact role and
underlying mechanism whereby miR-30a regulates the development and
progression of NSCLC remains elusive.
MicroRNAs have been found to modulate tumor
radiosensitivity in modulating a variety of pathways and molecules
(17,18). The primary ways that miRNAs modulate
radiosensitivity were DNA damage repair, apoptosis, cell cycle
checkpoint and tumor microenvironment (19). miR-124, miR-200c, miR-302 and
miR-142 were found to affect the radiosensitivity of colorectal
cancer (20), NSCLC (21), breast cancer (22) and malignant pediatric brain tumors
(23), respectively. Moreover, a
recent study firstly found that miR-30a could increase the
radiosensitivity of prostate cancer cells (24). We did not find other studies
concerning miR-30a and radiosensitivity. So, we investigated
whether miR-30a could function as a radiosensitizer in NSCLC and
its mechanism.
In this study, the effects of miR-30a on the
radiosensitivity of NSCLC was studied in vitro and in
vivo. Bioinformatic analysis and luciferase reporter assay
illustrated that activating transcription factor 1 (ATF1) was a
predicted target of miR-30a on its 3′ untranslated region (3′UTR).
Overexpression of miR-30a in NSCLC cell lines enhances the
radiosensitivity of NSCLC, especially in A549 cells. Further
investigation found ionizing radiation (IR)-induced G2/M cell cycle
arrest was blocked by miR-30a and it may also affect the IR-induced
apoptosis. These changes may be partly through binding to the 3′UTR
of ATF1 mRNA, thereby affected the function of ATF1 in
ataxia-telangiectasia mutated (ATM) phosphorylation process.
Overall, our study indicated that miR-30a may be an important
factor in influencing the radiosensitivity of NSCLC. More studies
are needed to explore the precise molecular mechanism of miR-30a on
regulating radiosensitivity in NSCLC.
Materials and methods
Cells and animal culture
A549 and H460 cells were obtained from the Center
for Translational Medicine, Department of Xi'an Jiaotong University
(Shaanxi, China). Cells were maintained in RPMI-1640 medium (Gibco
Life Technologies, Carlsbad, CA, USA) containing 10% fetal bovine
serum (FBS; ExCell), cultured at 37°C, humidified thermostat with
5% CO2 atmosphere.
Five-week-old nude mice were used for tumor
xenograft model and housed in the Center of Laboratory Animals of
Xi'an Jiaotong University. The mice were all caged in specific
pathogen free condition with constant temperature and humidity.
Animals were randomly grouped to accept subcutaneous injection of
A549 cells with lenti-GFP or lenti-miR-30a-5p or lenti-inhibitor
stable expression. Animal experiments were authorized by the
Institutional Animal Care and Use Committee of Xi'an Jiaotong
University. Animal care abided by the rules of the Institutional
Animal Care and Use Committee of Xi'an Jiaotong University.
MicroRNA transient transfection and
lentiviral infection
miR-30a-5p agomir (50 nM), miR-30a-5p antagomir (100
nM) and their negative control (50 or 100 nM) (GenePharma,
Shanghai, China) (Table I) were
transfected into cells, respectively, with Lipofectamine 3000
(Invitrogen Life Technologies, Carlsbad, CA, USA) transfection
reagent following the manufacturer's instructions for 24 to 48 h.
Transfection efficiency were detected and cells were used in the
assays descried below.
 | Table I.Sequences of hsa-miR-30a agomir,
antagomir and their negative control. |
Table I.
Sequences of hsa-miR-30a agomir,
antagomir and their negative control.
| Names | Sequences |
|---|
| hsa-miR-30a |
5′-UGUAAACAUCCUCGACUGGAAG-3′ |
| agomir |
5′-UCCAGUCGAGGAUGUUUACAUU-3′ |
| Agomir NC |
5′-UUCUCCGAACGUGUCACGUTT-3′ |
|
|
5′-ACGUGACACGUUCGGAGAATT-3′ |
| hsa-miR-30a |
5′-CUUCCAGUCGAGGAUGUUUACA-3′ |
| antagomir |
|
| Antagomir NC |
5′-CAGUACUUUUGUGUAGUACAA-3′ |
Lentiviral transduction vector pGMLV-MA2 or
pGMLV-MI7 with miR-30a-5p overexpression or miR-30a-5p
downregulation were co-transfected into HEK293T cells with
packaging mix. Lentivirus particles were harvested 48 h after
transfection. A549 cells (Genomeditech Co., Ltd., Shanghai, China)
were infected using the recombinant lentivirus with 5 µg/ml
polybrene.
RNA extraction and qRT-PCR
analysis
RNA extraction kit (Takara Bio, Inc., Shiga, China)
was used to isolate total RNA and TRIzol (Invitrogen Life
Technologies) to extract microRNA, following the manufacturer's
instructions. PrimeScript RT Master Mix and Mir-X miRNA
First-Strand Synthesis kit (both from Takara Bio, Inc.) were used
to synthesise reverse transcribed complementary DNA, respectively.
SYBR Premix Ex Taq II and Mir-X miRNA qRT-PCR SYBR kit were used to
perform qRT-PCR. U6 was the internal control. Primer sequences
(5′-3′) were as follows: hsa-miR-30a-5p, GTGTAAA CATCCTCGACTGGAAG;
hsa-ATF1 forward, TTCTGGAG TTTCTGCTGCTGT and reverse,
CCATCTGTGCCTGGAC TTG. All of the primers were synthesized by Sangon
Biotech Co., Ltd. (Shanghai, China).
Dual-luciferase reporter assay
Fragments of ATF1 mRNA 3′UTR with either the
sequence of miR-30a-5p binding site or its complementary bases were
cloned in dual-luciferase report vector, obtaining
pmirGLO-ATF1-wild and pmirGLO-ATF1-mutant recombinant plasmids
(GenePharma). The two recombinant plasmids and pmirGLO-negative
control were then transfected into A549 cells with miR-30a-5p
agomir (50 nM). Thirty-six hours after transfection,
Dual-Luciferase Reporter assay system (Promega, Madison, WI, USA)
was used to measure the activity of luciferase.
IR
Linear accelerator (Siemens, Munich, Germany) was
used for irradiation. Cells were treated with 200 cGy/min dose rate
in room temperature to reach a required total dose using. IR group
contain fifteen mice, consist of five randomly selected nude mice
in each different miR-30a-5p expression group (10/group).
Tumor-bearing mice in the IR group were treated with 2.0 Gy
irradiation for 5 consecutive days from day 21 to 25, and achieved
a total dose of 10.0 Gy irradiation.
Colony formation assays
After 0, 2, 4, 6 and 8 Gy irradiation cells were
incubation for 10 to 14 days. Formaldehyde of 4% was used to fix
the cell clones and then stained with 1% crystal violet. Colonies
of ≥50 cells were counted and fitted to single target model using
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA).
Cell cycle and apoptosis analysis
For cell cycle assay, 70% ethanol was used to fix
the harvested cells and placed at −20°C overnight. Then incubated
for 10 min in 50 µg/ml propidium iodide (PI) for analysis. Annexin
V-PE/7-AAD apoptosis detection kit was used to test cell apoptosis,
based on to the manufacturer's instructions. Cell cycle and
apoptosis of the prepared cells were detected by flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA).
Western blotting
Cells were lysed in RIPA lysis buffer (BD
Pharmingen, San Diego, CA, USA), protein concentration was measured
by BCA reagent kit (Beyotime Institute of Biotechnology, Haimen,
China). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) was used to separate cellular lysates, then transferred
to polyvinylidene difluoride (PVDF) membranes (Millipore, Boston,
MA, USA). The transfected membranes were blocked in 5% skim milk
and incubated in 4°C overnight using the following primary
antibodies: anti-ATF1 (1:1,000), anti-ATM (1:1,000), anti-ATM
(phospho-1981; 1:5,000) (all from Abcam, Cambridge, MA, USA),
anti-p53 (1:800), anti-p21 (1:500), anti-Bax (1:1,000) and
anti-Bcl-2 (1:500) (all from Wanleibio, Shenyang, China),
anti-GAPDH (1:3,000; Proteintech Group, Inc., Chicago, IL, USA).
Then incubated with horseradish peroxidase-conjugated secondary
antibody, goat anti-rabbit (1:5,000) or goat anti-mouse (1:5,000)
(both from Cell Signaling Technology, Inc., Danvers, MA, USA). All
bands were visualized with enhanced chemiluminescence (ECL) kit
(Millipore).
Statistical analysis
Statistics of at least three different experiments
were analyzed by GraphPad Prism 5. Results are presented as means ±
SEM. Statistical significance of two groups were tested by
Student's t-test. P-value <0.05 was considered to have
statistical significance.
Results
miR-30a enhances radiosensitivity of
A549 and H460 cells
Colony survival assays were assessed to estimate the
radiosensitivity of A549 and H460 cells. The two cell lines were
treated with 0, 2, 4, 6 and 8 Gy radiation after transfected with
miR-30a agomir (50 nM) or miR-30a antagomir (100 nM) or their
negative controls (50 and 100 nM) for 36 h, respectively. Besides,
cell proliferation was evaluated using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay after A549 cells were transfected with miR-30a agomir or
miR-30a antagomir or their negative control. No statistical
differences were found between the groups in 24, 48 and 72 h (data
not shown). miR-30a expression were examined by qRT-PCR and
confirmed that the agomir and antagomir were transfected
successfully (P<0.01) (Fig. 1A and
B).
The miR-30a agomir groups of A549 cells showed a
decrease of colony formation rate after radiation exposure compared
to the controls, especially after 6 Gy (P=0.0408) or 8 Gy
(P=0.0258) irradiation (Fig. 1C and
E). Conversely, the colony formation rate was increased in the
miR-30a antagomir A549 cell groups than in the antagomir NC groups,
6 Gy (P=0.0103) and 8 Gy (P=0.0451) also showed statistical
significance (Fig. 1C and E).
Results of the four groups in H460 cell line were in accordance
with A549 cell line, but no statistical significance was found
(Fig. 1D and F).
ATF1 expression is a target of
miR-30a
In order to investigate the underlying mechanism of
miR-30a affecting the radiosensitivity of NSCLC, we conducted
bioinformatic analysis to predict the potential targets for miR-30a
through searching PicTar, TargetScan and miRDB. We found that ATF1,
which may also be associated with tumor radiosensitivity (25), was a predicted target of miR-30a
(Fig. 2A). Schematic diagram of
miR-30a targeting the 3′UTR of ATF1 is shown in Fig. 2B.
Dual luciferase reporter assay was performed to
further confirm that miR-30a directly target the 3′UTR of ATF1. The
luciferase activity of pmirGLO-ATF1-wild was significantly
decreased (P=0.0131), but pmirGLO-ATF1-mutant was not (P=0.2561),
compared to pmirGLO-negative control group (Fig. 2C). Confirming that ATF1 could
directly bind to the 3′UTR of miR-30a.
Furthermore, qRT-PCR and western blotting were
assessed to examine if miR-30a could regulate the expression of
ATF1 in A549 cell line. We found that ATF1 mRNA and protein were
decreased in the miR-30a agomir group compared to the control group
(Fig. 2D-F). Conversely, the ATF1
expression increased in the miR-30a antagomir group (Fig. 2D-F). These results further
demonstrated that ATF1 was inversely regulated by miR-30a in the
A549 cells.
miR-30a may enhance radiosensitivity
of A549 cells through ATM pathway
Lentivirus systems were used to further explore the
mechanism of miR-30a sensitizing radiation. A549 cells with stable
overexpression and downexpression of miR-30a were designated as
lenti-miR-30a and lenti-inhibitor, respectively. A549 cells with
stable expression of GFP was used as a control and named lenti-GFP.
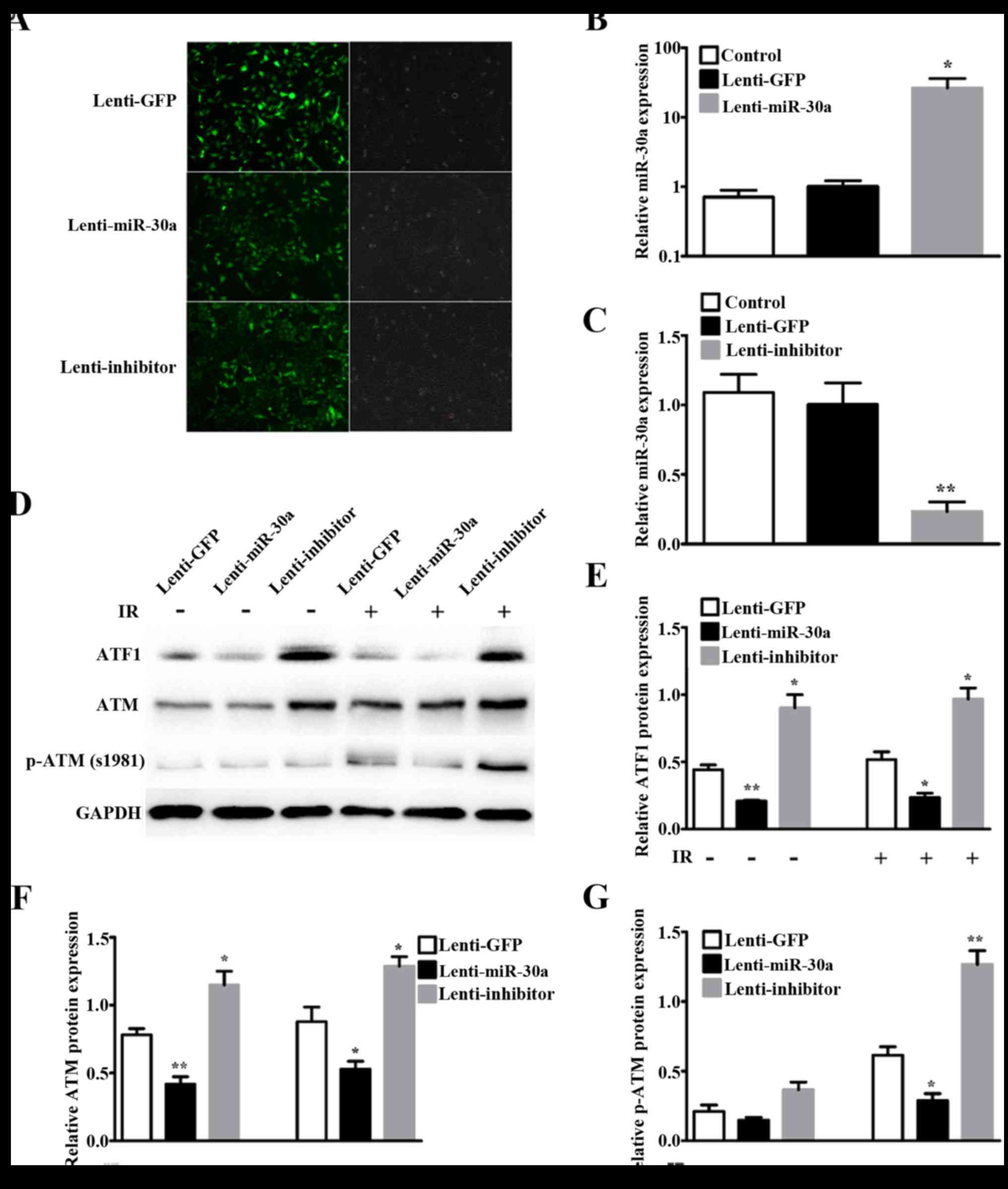
Infection efficiency at 48 h by fluorescence microscopy showed
bright GFP tag in lentiviruses (Fig.
3A). Relative miR-30a expression by qRT-PCR showed miR-30a was
significantly increased by lenti-miR-30a (P=0.0108) (Fig. 3B) and decreased by lenti-inhibitor
(P=0.0014) (Fig. 3C).
Western blotting results showed that ATF1 expression
was downregulated in lenti-miR-30a cells and upregulated in
lenti-inhibitor cells, compared with lenti-GFP cells (Fig. 3D and E). Given that ATM was an
important and the first responder to DNA double-strand breaks
(DSBs) (26) and by phosphorylation
it involves in many IR-induced cell processes (27), we then detected ATM and p-ATM
(S1981) expression. The results indicated that ATM protein
expression and phosphorylation of ATM at S1981 corresponded with
ATF1 (Fig. 3D, F and G). The
expression of ATF1 and ATM showed no difference with 8 Gy
irradiation or without irradiation. Phosphorylation of ATM at S1981
was very low without irradiation, and significantly increased after
8 Gy irradiation (Fig. 3G).
miR-30a enhances radiosensitivity by
blocking the radiation induced G2/M checkpoint arrest in A549 cell
line
To examine the impact of miR-30a on cell cycle
progression and cell cycle distribution of A549 cells were measured
by flow cytometry in lenti-miR-30a or lenti-inhibitor or lenti-GFP
cells as contrast. Cells after 0 or 8 Gy irradiation were collected
to assess the cell cycle. Results revealed that in non-irritated
group, cell cycle was not affected by miR-30a expression. However,
after 8 Gy irradiation lenti-miR-30a decreased the proportion in
G2/M phase (23.21±1.85% vs. 28.02±3.06%, P=0.0251) and
lenti-inhibitor contrarily showed increase in the proportion
(37.05±2.80% vs. 28.02±3.06%, P=0.0075) (Fig. 4A and B).
In addition, western blotting results showed that
miR-30a negatively influence IR-induced p53 expression, and the
expression of p21 was suppressed (Fig.
4C). Taken together, the above results demonstrated that
miR-30a may sensitize radiation by blocking the radiation induced
G2/M checkpoint arrest in A549 cells.
miR-30a may enhance
irradiation-induced apoptosis of A549 cells
Furthermore, we detected the effects of miR-30a on
apoptosis of A549 cells after irradiation by using flow cytometry.
We found that miR-30a could not induce apoptosis without IR. The
apoptosis rate was significantly increased after 8 Gy irradiation
in all the three groups (Fig. 5A and
B). Moreover, percentage of apoptosis in lenti-miR-30a cells
was higher than the rate in lenti-GFP cells after 8 Gy irradiation
(27.93±2.00 vs. 18.63±1.59%, P=0.0026) (Fig. 5B). On the contrary, the apoptosis
rate in lenti-inhibitor cells was modestly decreased, but not
statistically significant (14.1±1.73 vs. 18.63±1.59%, P=0.1409)
(Fig. 5B).
Since cell apoptosis after 8 Gy irradiation showed
inconformity with p53 expression, we detected Bcl-2 and Bax protein
expression by western blotting. After IR, Bcl-2/Bax ratio in
lenti-miR-30a group was decreased compared with lenti-GFP group,
but had no statistical significance and lenti-inhibitor group did
not exhibit the desired result (Fig. 5C
and D). Indicating that the apoptotic rate increase in the
miR-30a upregulated group after IR may be partly involved in
mitochondrial apoptotic pathway, but not in p53 apoptotic
pathway.
These data illustrate that increasing radio-induced
apoptosis may be another way in which miR-30a sensitizes the
radiosensitivity of A549 cells. The precise regulatory mechanism is
complicated and need further research.
miR-30a may enhance the sensitivity of
A549 cells murine xenograft model to irradiation
To explore the radiosensitization potential of
miR-30a in vivo, lenti-miR-30a and lenti-GFP cells were
injected subcutaneously on the back of nude mice. Tumor growth was
evaluated from the 7th day after injection until the mice were
sacrificed.
Our results illustrated that after IR tumor growth
trend slowed down (Fig. 6A and B).
Tumor volume in the lenti-miR-30a group were smaller than those
derived from lenti-GFP group (Fig.
6C), and tumors in lenti-inhibitor group showed counter trends,
but no statistical significance. Treatment dose and schedule may be
the two main influencing factors. Moreover, when irradiating the
tumor, the nude mice were completely exposed to X-ray. Nude mice in
the IR group gradually showed a series of symptoms, the most
obvious was weight loss, this may also influence the tumor
size.
Discussion
MicroRNAs are important radiosensitivity regulators
by interacting with the key molecules involved in radiosensitivity
(19) and miR-30a has been found to
act as a radiosensitizor in prostate cancer cells by targeting
TP53INP1 and modulating autophagy (24). Thus, the potential therapeutic value
of miR-30a attracted our interests in determine its function on
NSCLC radiosensitization. Here, we found miR-30a functions as a
sensitizer to irradiation in NSCLC cells, especially in A549 cells
and may enhances the effect of radiation on tumors in nude mice.
Furthermore, our data provide evidence for the potential role of
miR-30a in suppressing the IR-induced G2/M cell cycle arrest and
increasing the IR-induced cell apoptosis.
The main target of IR is cellular DNA, ATM has a key
role in the study of IR caused DNA damage (28). In response to DNA damage, by
phosphorylation of ATM S1981, a series of downstream molecules can
be actived to mediate cell cycle arrest, apoptosis (29) and initiate DNA repair (26). Shanware et al (25) announced that the downregulation of
ATF1 could inhibit ATM expression synergistically. Interestingly,
by using three public prediction databases we identified ATF1 as a
potential target gene of miR-30a. The dual luciferase reporter
assay, qRT-PCR and western blotting also proved that ATF1 is a
direct target of miR-30a in the 3′UTR. Consistent with a previous
study (25), we found that IR
exposure neither affect the expression of ATM nor ATF1, but
downregulation of ATF1 could reduce ATM expression and suppress IR
induced ATM S1981 phosphorylation. These data suggested that by
targeting ATF1, miR-30a could enhance the radiosensitivity of A549
cells through inhibiting the effect of ATF1 in IR induced ATM S1981
phosphorylation.
Since cell cycle arrest, DNA repair and apoptosis
are the main ways that cancer cells react to IR through ATM
(30), we further investigated the
effect of miR-30a on these aspects after IR. Our results indicated
that miR-30a could not alter cell cycle and apoptosis rate in
non-irradiated A549 cells. While, miR-30a expression can increase
IR-induced apoptosis and decrease IR-induced G2/M cell cycle arrest
after 8 Gy IR. In response to IR induced DNA damage,
phosphorylation of ATM can increase p53, either inducing DNA
repair, cell cycle arrest (31), or
apoptosis, thereby, maintain genomic stability (32) and this may also reduce the
therapeutic effectiveness (33).
p53 wild-type cell lines, when irradiating with ATM were
downregulated, p53 cannot be retarded and lead to cell cycle
checkpoint deficiency (1). In line
with these documented studies, we noted in p53 wild-type A549
cells, p53 expression was consistent with the activation of ATM
after IR. With p53 downregulation, cell cycle checkpoint was
shortened, damaged cells cannot be eliminated in time, in this way,
DNA repair ability can be decreased, thus radiosensitivity was
enhanced. Moreover, with the accumulation of unrepaired,
misrepaired and mutated DNA, the apoptosis can be subsequently
increased, this may also partly cause the enhancing of
radiosensitivity.
However, in human cancer, one individual miRNA could
participate in the whole cancer procedure from initiation,
progression to terminal by targeting hundreds of genes (34). They are involved in multiple
pathways and could not only restrain but also accelerate cancer
development (35). In our study, we
surprisingly found that unlike A549, when combined with miR-30a,
the colony survival of H460 showed a modest decrease, but no
statistical difference with its control group. This may be
associated with the modest miR-30a expression fold-change compared
with A549 cells after miR-30a transfection (Fig. 1A and B). The in vivo study
showed miR-30a can result in tumor volume regression, but still no
statistical differences. Possibly this is due to the IR starting
too late or the IR ceased too early or the IR dose was
insufficient. The relationship between miR-30a expression and the
time and dose of IR need further investigation to reveal the
accurate role and profound underlying mechanism of miR-30a.
In conclusion, our study indicated the importance of
miR-30a in enhancing the radiosensitivity of A549 cell line by
targeting ATF1, and association with the downregulation of ATM
pathway, which may be a potential therapeutic factor of
radiosensitization.
Acknowledgements
This study was supported by National Youth Science
Fund Project (no. 81301937) from the National Natural Science
Foundation of China.
Glossary
Abbreviations
Abbreviations:
|
ATF1
|
activating transcription factor 1
|
|
ATM
|
ataxia-telangiectasia mutated
|
|
3′UTR
|
3′ untranslated region
|
|
EMT
|
epithelial-mesenchymal transition
|
|
SDS-PAGE
|
sodium dodecyl sulfate-polyacrylamide
gel electrophoresis
|
|
PVDF
|
polyvinylidene difluoride
|
|
IR
|
ionizing radiation
|
|
DSBs
|
DNA double-strand breaks
|
References
|
1
|
Zhao JJ, Lin J, Zhu D, Wang X, Brooks D,
Chen M, Chu ZB, Takada K, Ciccarelli B, Admin S, et al: miR-30-5p
functions as a tumor suppressor and novel therapeutic tool by
targeting the oncogenic Wnt/β-catenin/BCL9 pathway. Cancer Res.
74:1801–1813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang CW, Yu JC, Hsieh YH, Yao CC, Chao
JI, Chen PM, Hsieh HY, Hsiung CN, Chu HW, Shen CY, et al:
MicroRNA-30a increases tight junction protein expression to
suppress the epithelial-mesenchymal transition and metastasis by
targeting Slug in breast cancer. Oncotarget. 7:16462–16478.
2016.PubMed/NCBI
|
|
3
|
Baraniskin A, Birkenkamp-Demtroder K,
Maghnouj A, Zöllner H, Munding J, Klein-Scory S, Reinacher-Schick
A, Schwarte-Waldhoff I, Schmiegel W and Hahn SA: miR-30a-5p
suppresses tumor growth in colon carcinoma by targeting DTL.
Carcinogenesis. 33:732–739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang R, Yan S, Wang J, Deng F, Guo Y, Li
Y, Fan M, Song Q, Liu H, Weng Y, et al: miR-30a regulates the
proliferation, migration, and invasion of human osteosarcoma by
targeting Runx2. Tumour Biol. 37:3479–3488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li WF, Dai H, Ou Q, Zuo GQ and Liu CA:
Overexpression of microRNA-30a-5p inhibits liver cancer cell
proliferation and induces apoptosis by targeting MTDH/PTEN/AKT
pathway. Tumor Biol. 37:5885–5895. 2016. View Article : Google Scholar
|
|
6
|
Liu K, Guo L, Guo Y, Zhou B, Li T, Yang H,
Yin R and Xi T: AEG-1 3′-untranslated region functions as a ceRNA
in inducing epithelial-mesenchymal transition of human non-small
cell lung cancer by regulating miR-30a activity. Eur J Cell Biol.
94:22–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Wang K, Han L, Zhang A, Shi Z,
Zhang K, Zhang H, Yang S, Pu P, Shen C, et al: PRDM1 is directly
targeted by miR-30a-5p and modulates the Wnt/β-catenin pathway in a
Dkk1-dependent manner during glioma growth. Cancer Lett.
331:211–219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sestito R, Cianfrocca R, Rosanò L, Tocci
P, Semprucci E, Di Castro V, Caprara V, Ferrandina G, Sacconi A,
Blandino G, et al: miR-30a inhibits endothelin A receptor and
chemoresistance in ovarian carcinoma. Oncotarget. 7:4009–4023.
2016.PubMed/NCBI
|
|
9
|
Huang QB, Ma X, Zhang X, Liu SW, Ai Q, Shi
TP, Zhang Y, Gao Y, Fan Y, Ni D, et al: Down-regulated miR-30a in
clear cell renal cell carcinoma correlated with tumor hematogenous
metastasis by targeting angiogenesis-specific DLL4. PLoS One.
8:e672942013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou Z, Ni M, Zhang J, Chen Y, Ma H, Qian
S, Tang L, Tang J, Yao H, Zhao C, et al: miR-30a can inhibit DNA
replication by targeting RPA1 thus slowing cancer cell
proliferation. Biochem J. 473:2131–2139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu Y, Cao L, Yang L, Kang R, Lotze M and
Tang D: microRNA 30A promotes autophagy in response to cancer
therapy. Autophagy. 8:853–855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ouzounova M, Vuong T, Ancey PB, Ferrand M,
Durand G, Le-Calvez Kelm F, Croce C, Matar C, Herceg Z and
Hernandez-Vargas H: MicroRNA miR-30 family regulates non-attachment
growth of breast cancer cells. BMC Genomics. 14:1392013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu J, Zeng Y, Xu C, Qin H, Lei Z, Shen D,
Liu Z and Huang JA: Expression profile analysis of microRNAs and
downregulated miR-486-5p and miR-30a-5p in non-small cell lung
cancer. Oncol Rep. 34:1779–1786. 2015.PubMed/NCBI
|
|
14
|
Xie K, Wang C, Qin N, Yang J, Zhu M, Dai
J, Jin G, Shen H, Ma H and Hu Z: Genetic variants in regulatory
regions of microRNAs are associated with lung cancer risk.
Oncotarget. 7:47966–47974. 2016.PubMed/NCBI
|
|
15
|
Tang R, Liang L, Luo D, Feng Z, Huang Q,
He R, Gan T, Yang L and Chen G: Downregulation of miR-30a is
associated with poor prognosis in lung cancer. Med Sci Monit.
21:2514–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan Y, Zheng S, Li Q, Xiang X, Gao T, Ran
P, Sun L, Huang Q, Xie F, Du J, et al: Overexpression of miR-30a in
lung adenocarcinoma A549 cell line inhibits migration and invasion
via targeting EYA2. Acta Biochim Biophys Sin (Shanghai).
48:220–228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Metheetrairut C and Slack FJ: MicroRNAs in
the ionizing radiation response and in radiotherapy. Curr Opin
Genet Dev. 23:12–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao L, Lu X and Cao Y: MicroRNA and
signal transduction pathways in tumor radiation response. Cell
Signal. 25:1625–1634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao L, Bode AM, Cao Y and Dong Z:
Regulatory mechanisms and clinical perspectives of miRNA in tumor
radiosensitivity. Carcinogenesis. 33:2220–2227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Zheng L, Huang J, Gao F, Lin X,
He L, Li D, Li Z, Ding Y and Chen L: miR-124 radiosensitizes human
colorectal cancer cells by targeting PRRX1. PLoS One. 9:e939172014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi L, Zhang S, Wu H, Zhang L, Dai X, Hu
J, Xue J, Liu T, Liang Y and Wu G: miR-200c increases the
radiosensitivity of non-small-cell lung cancer cell line A549 by
targeting VEGF-VEGFR2 pathway. PLoS One. 8:e783442013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang Z, Ahn J, Guo D, Votaw JR and Shim
H: MicroRNA-302 replacement therapy sensitizes breast cancer cells
to ionizing radiation. Pharm Res. 30:1008–1016. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee YY, Yang YP, Huang MC, Wang ML, Yen
SH, Huang PI, Chen YW, Chiou SH, Lan YT, Ma HI, et al:
MicroRNA142-3p promotes tumor-initiating and radioresistant
properties in malignant pediatric brain tumors. Cell Transplant.
23:669–690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu CG, Yang MF, Fan JX and Wang W: miR-30a
and miR-205 are downregulated in hypoxia and modulate
radiosensitivity of prostate cancer cells by inhibiting autophagy
via TP53INP1. Eur Rev Med Pharmacol Sci. 20:1501–1508.
2016.PubMed/NCBI
|
|
25
|
Shanware NP, Zhan L, Hutchinson JA, Kim
SH, Williams LM and Tibbetts RS: Conserved and distinct modes of
CREB/ATF transcription factor regulation by PP2A/B56gamma and
genotoxic stress. PLoS One. 5:e121732010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khoronenkova SV and Dianov GL: ATM
prevents DSB formation by coordinating SSB repair and cell cycle
progression. Proc Natl Acad Sci USA. 112:3997–4002. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chou WC, Hu LY, Hsiung CN and Shen CY:
Initiation of the ATM-Chk2 DNA damage response through the base
excision repair pathway. Carcinogenesis. 36:832–840. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rondeau S, Vacher S, De Koning L, Briaux
A, Schnitzler A, Chemlali W, Callens C, Lidereau R and Bièche I:
ATM has a major role in the double-strand break repair pathway
dysregulation in sporadic breast carcinomas and is an independent
prognostic marker at both mRNA and protein levels. Br J Cancer.
112:1059–1066. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Neumann J, Yang Y, Köhler R, Giaisi M,
Witzens-Harig M, Liu D, Krammer PH, Lin W and Li-Weber M: Mangrove
dolabrane-type of diterpenes tagalsins suppresses tumor growth via
ROS-mediated apoptosis and ATM/ATR-Chk1/Chk2-regulated cell cycle
arrest. Int J Cancer. 137:2739–2748. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang H, Reinhardt HC, Bartkova J,
Tommiska J, Blomqvist C, Nevanlinna H, Bartek J, Yaffe MB and
Hemann MT: The combined status of ATM and p53 link tumor
development with therapeutic response. Genes Dev. 23:1895–1909.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeng YC, Xing R, Zeng J, Xue M, Chi F, Xin
Y, Fan GL, Wang HM, Duan QY, Sun YN, et al: Sodium glycididazole
enhances the radiosensitivity of laryngeal cancer cells through
downregulation of ATM signaling pathway. Tumour Biol. 37:5869–5878.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gudkov AV and Komarova EA: The role of p53
in determining sensitivity to radiotherapy. Nat Rev Cancer.
3:117–129. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bruno T, De Nicola F, Iezzi S, Lecis D,
D'Angelo C, Di Padova M, Corbi N, Dimiziani L, Zannini L, Jekimovs
C, et al: Che-1 phosphorylation by ATM/ATR and Chk2 kinases
activates p53 transcription and the G2/M checkpoint. Cancer Cell.
10:473–486. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Berindan-Neagoe I, Monroig PC, Pasculli B
and Calin GA: MicroRNAome genome: a treasure for cancer diagnosis
and therapy. CA Cancer J Clin. 64:311–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|