Introduction
Colon cancer is the third most commonly diagnosed
cancer and the fourth leading cause of cancer-related deaths
worldwide, accounting for over 1.2 million new cases and 608,700
deaths per year (1). Despite
advances in diagnosis and treatment strategies, cancer metastasis
contributes to the majority of cancer-related deaths in colon
cancer (2). Cancer metastasis is a
complex event that involves sequential, interlinked and selective
steps. Increasing evidence indicates that epithelial-mesenchymal
transition (EMT) plays a critical and intricate role in promoting
tumor invasion and metastasis in epithelium-driven malignancies
(3). In the event of EMT,
epithelial cells undergo multiple biochemical changes that enable
them to disrupt cell-cell adherence and acquire mesenchymal
characteristics such as enhanced migratory capacity, invasiveness
and elevated resistance to apoptosis.
Chemokine (C-X-C motif) ligand 8 (CXCL8 or
interleukin 8) and its receptor CXCR2 are two important regulators
of metastatic and advanced cancers. CXCL8 serves as an autocrine
growth factor that promotes tumor growth, invasion, angiogenesis,
metastases and resistance (4,5). CXCL8
expression in human colon cancer cells has been linked to
metastatic potential and high invasiveness (6). Our previous study showed that CXCL8
and chemokine (C-C motif) ligand 20 (CCL20) synergistically
promoted metastasis by coordinated induction of EMT in human
colorectal cancer cell lines SW480 and Caco-2 and patients with
co-expression of CXCL8 and CCL20 were more likely to develop liver
metastasis and had a poor prognosis (7). However, the exact role of CXCL8 in the
progressive growth of colon cancer remains unclear.
The aim of the present study was to investigate the
role of CXCL8 in proliferation, invasiveness and metastasis of
colon cancer. We hypothesized that overexpression of CXCL8 would
induce EMT-like phenotype in LoVo colon cancer cells. Our results
may provide a basis for the development of new therapies for colon
cancer to decrease proliferation and migration.
Materials and methods
Cell culture and transfection
The LoVo cell line purchased from the Cell Bank,
Chinese Academy of Science (Shanghai, China) was grown in RPMI-1640
medium, supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin in a 5% CO2 incubator at 37°C.
The empty plasmid vector and the plasmid vector containing CXCL8
cDNA were purchased from GeneChem Co. (Shanghai, China) and were
transfected into LoVo cells using Lipofectamine 2000 reagent and
Opti-MEM® reduced serum media (Invitrogen, Carlsbad, CA,
USA). Multiple clones were selected in the presence of 2 µg/ml
puromycin.
Growth inhibition assay
The LoVo cell suspension was seeded into a 96-well
plate (200 µl/well) with the adjusted concentration of
10×103 cells/ml, and immediately incubated overnight at
37°C with 5% CO2 and saturated humidity. Groups included
the control, empty vector and CXCL8 groups, in four duplications.
Cell growth was assessed 12, 24, 48, 72 and 96 h later. Thirty
microliters of 5 mg/ml MTT solution (Sigma, St. Louis, MO, USA) was
added to each well, and the plate was further incubated for 4 h.
After this incubation period the bulk of the medium was removed
using a Pasteur pipette fitted to a vacuum line, taking care to
leave the formazan crystals behind. After removing the medium,
dimethyl sulfoxide (DMSO) was supplemented with oscillation for 10
min. The optical density (OD) was measured with a spectrophotometer
(Olympus, Tokyo, Japan) at 450 nm. The graph of the OD value of the
cells was drawn with time as the x-axis and absorbance as the
y-axis. Proliferation inhibition rate = (1 - OD of experiment
group/OD of control group) × 100%.
In vitro scratch assay
The LoVo cell suspension (control, empty vector and
CXCL8-transfected groups) was seeded into a 6-well plate with the
adjusted concentration of 4×105 cells/ml and incubated
overnight until cells reached 90% confluency. A 20-µl pipette tip
was used to create a scratch in the cell monolayer, and the plate
was washed with phosphate-buffered saline (PBS). The cells were
cultured in RPMI-1640 medium with 5% FBS for 48 h before being
observed under a microscope.
Cell invasion assay. Transwell invasion experiments
were performed with 6-well Matrigel-coated chambers from BD
Biosciences (Bedford, MA, USA). Briefly, the LoVo cells (control,
empty vector and CXCL8 transfected groups) were allowed to grow to
subconfluency and were serum-starved for 24 h. After detachment
with trypsin, the cells were washed with PBS, resuspended in
serum-free medium and 6×104 cells were added to the
upper chamber. Complete medium was added to the bottom wells of the
chambers. After 24 h, the cells that had not migrated were removed
from the upper face of the filters using cotton swabs, and the
cells that had migrated were fixed and stained with Giemsa stain.
Images of three different ×10 fields of view were captured from
each membrane, and the number of migratory cells was counted using
Fujifilm v1.1 software.
Real-time PCR
CXCL8-transfected clones were screened for CXCL8
expression. The RNA was collected with TRIzol® reagent
(Roche, Mannheim, Germany) and the mRNA was reverse transcribed to
cDNA using the PrimeScript RT reagent kit using the gDNA Eraser
kit. Cycling conditions were 16°C for 30 min, 42°C for 30 min and
85°C for 10 min. Approximately 3 µl of total cDNA of each sample
was amplified using real-time PCR in a final volume of 20 µl of
reaction mixture using the reagent kit purchased from Toyobo Co.,
Ltd. (Osaka, Japan). Cycling conditions consisted of denaturation
at 95°C for 3 min, 40 cycles at 95°C for 12 sec, and 62°C for 40
sec. CXCL8 mRNA expression level was determined by normalizing
against GAPDH gene expression using the 2−ΔΔCt
method.
Western blotting
Total cellular proteins (40 µg/lane) prepared from
the cultured cells were electrophoresed on sodium dodecyl
sulfate-polyacrylamide gels and transferred to polyvinylidene
difluoride membranes. Blots were blocked in 5% skim milk and
sequentially immunostained with primary and secondary horseradish
peroxidase-conjugated antibodies at 4°C overnight and at room
temperature for 1 h, respectively. All antibodies were purchased
from Cell Signaling Technology (Danvers, MA, USA). Immunoreactive
proteins were visualized using an enhanced chemiluminescence
detection system with exposure to X-ray film. The images were
analyzed by Quantity One (Bio-Rad).
siRNA analysis
CXCL8 siRNAs (Santa Cruz Biotechnology, Inc., TX,
USA) and Lipofectamine 2000 (Invitrogen) were separately diluted
according to the manufacturer's protocol, mixed gently and
incubated for 20 min at room temperature. The siRNA-Lipofectamine
2000 mixture was added to LoVo cells grown in 6-well plates to
80–90% confluence. The transfection reagent was replaced after 6 h,
and the cells were then treated with chemokines as described above.
Scrambled control siRNA was used as the blank control.
In vivo studies in nude mice
BALB/c nude mice (Guangdong Medical Laboratory
Animal Center, Guangdong, China) aged 6–8 weeks were housed in
laminar flow cabinets under specific pathogen-free conditions in
our animal laboratory at Kunming Medical University. All animal
experiments were conducted with the approval from the Ethics
Committee for Animal Research of the Kunming Medical University.
Subcutaneous tumors were generated by subcutaneous injection of
1×106/0.2 ml of LoVo parental cells or CXCL8-transfected
LoVo cells in the right flank of the mice (three mice/group). The
subcutaneous tumors were measured using Vernier gauge, and tumor
volumes were calculated according to the equation: Tumor volume =
(short diameter)2 × (long diameter) × 0.5. After one
week of inoculation, the tumor volume was measured every four days
up to 28 days. The growth rate of the tumors was compared between
the parental cells and CXCL8-transfected cell groups.
Statistical analyses
All statistical analyses were performed using
GraphPad Prism 5.0. Results are expressed as mean ± SD.
Between-group comparisons were performed using analyses of
variance. All analyses were two-tailed and P<0.05 was considered
to indicate a statistically significant result.
Results
CXCL8 stable transfection
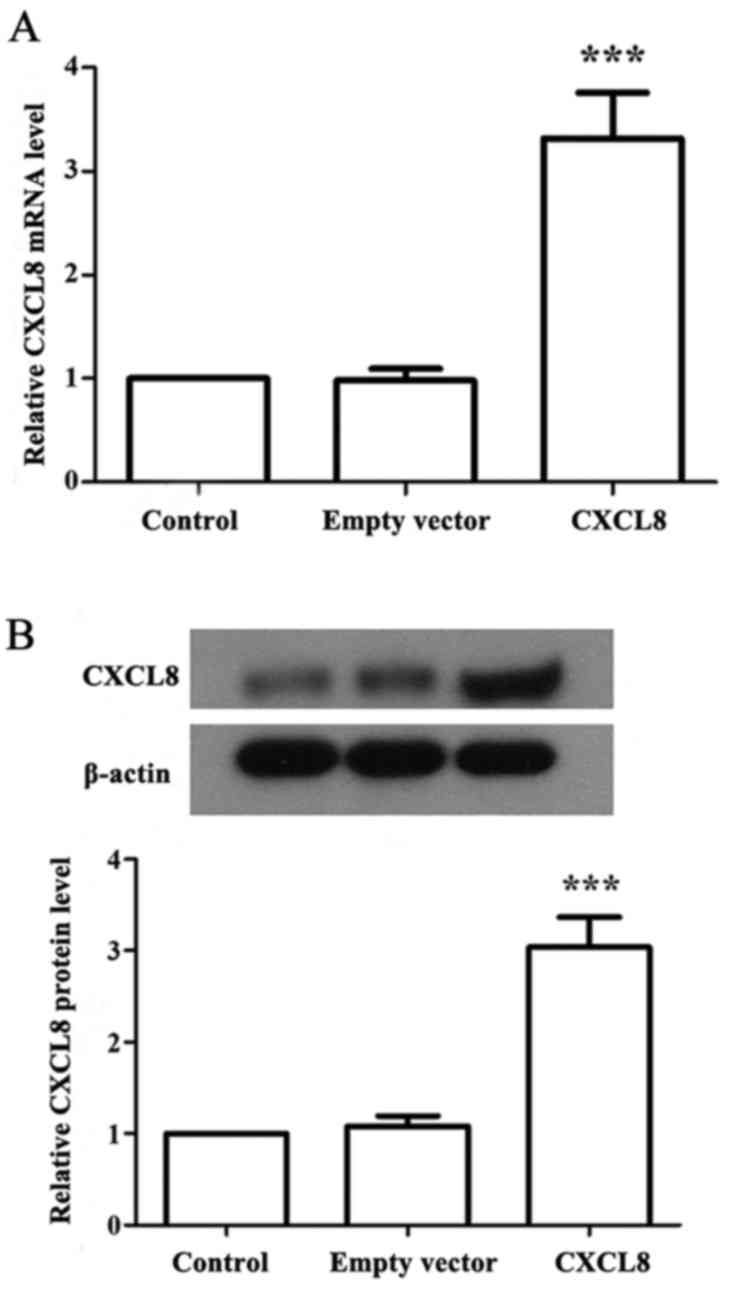
We quantified the expression of CXCL8 in the
transfected LoVo cancer cell lines (Fig. 1). Real-time PCR showed that the
CXCL8-transfected LoVo cells had significantly elevated CXCL8 mRNA
expression when compared with that noted in the control and
empty-vector cells (P<0.05). Western blotting showed a
significantly increased level of CXCL8 protein in the
CXCL8-transfected cells than the level noted in the control and
empty-vector cells (P<0.05).
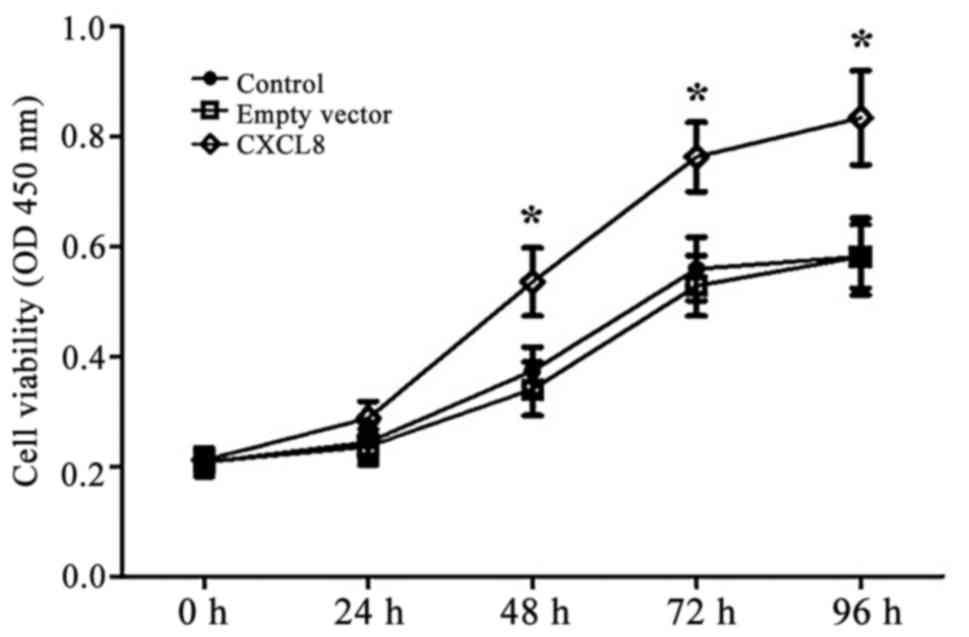
CXCL8 increases proliferation of the
LoVo cells
Fig. 2 shows the
changes in OD values for the control, empty-vector and
CXCL8-transfected LoVo cells using MTT assay. Overexpression of
CXCL8 increased proliferation of the LoVo cells and its growth
promotion effect was positively related to time. Significant
differences in cell viability were observed 48 h after transfection
(P<0.05) and remained significant at 72 and 96 h.
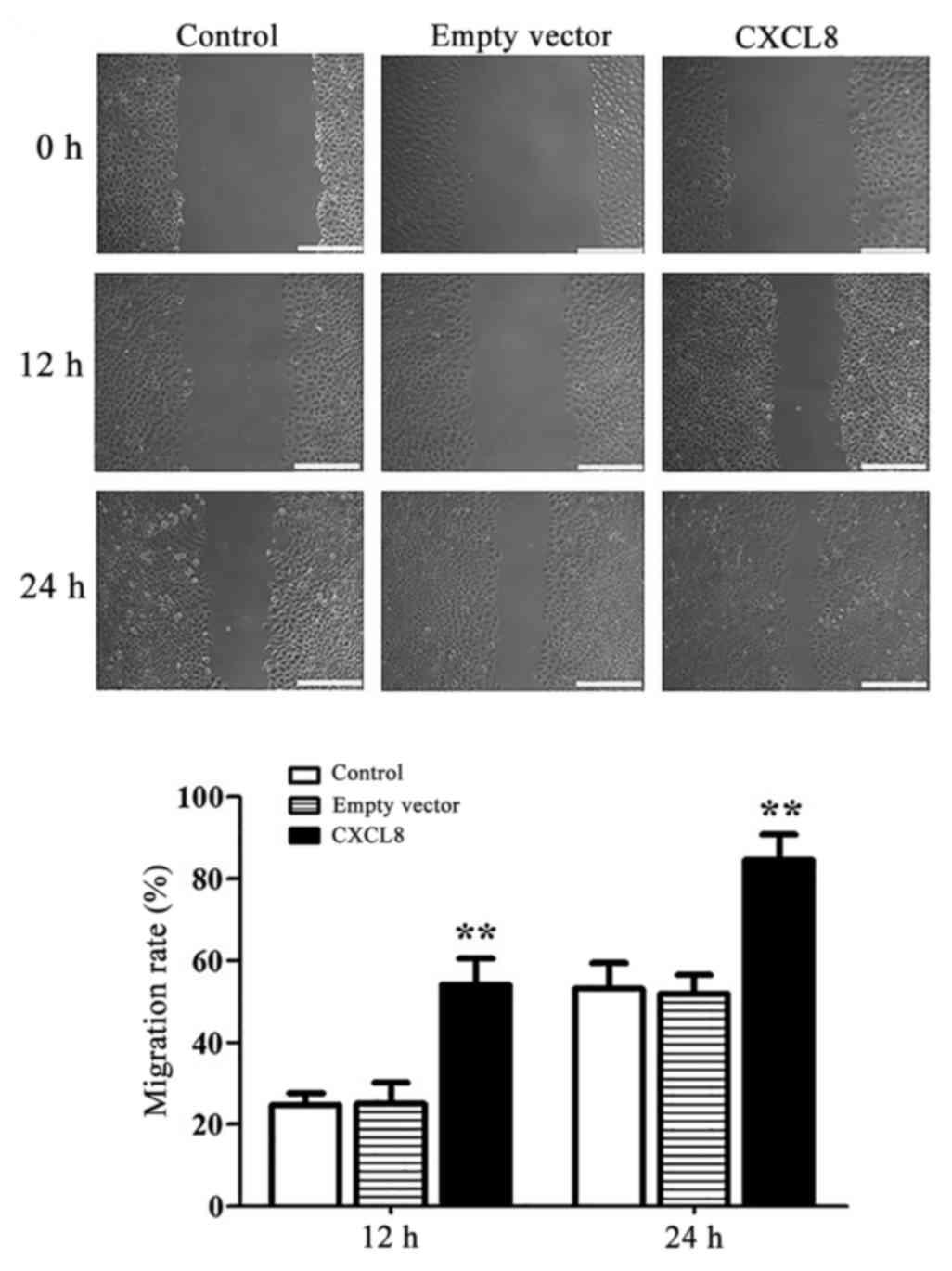
CXCL8 increases migration and invasion
of LoVo cells
The role of CXCL8 expression in the migratory
potential of LoVo cells was investigated by scratch test. Fig. 3 shows that 12 and 24 h after
transfection, the CXCL8-transfected cells had a significantly
(P<0.01) higher migration rate than that observed in the control
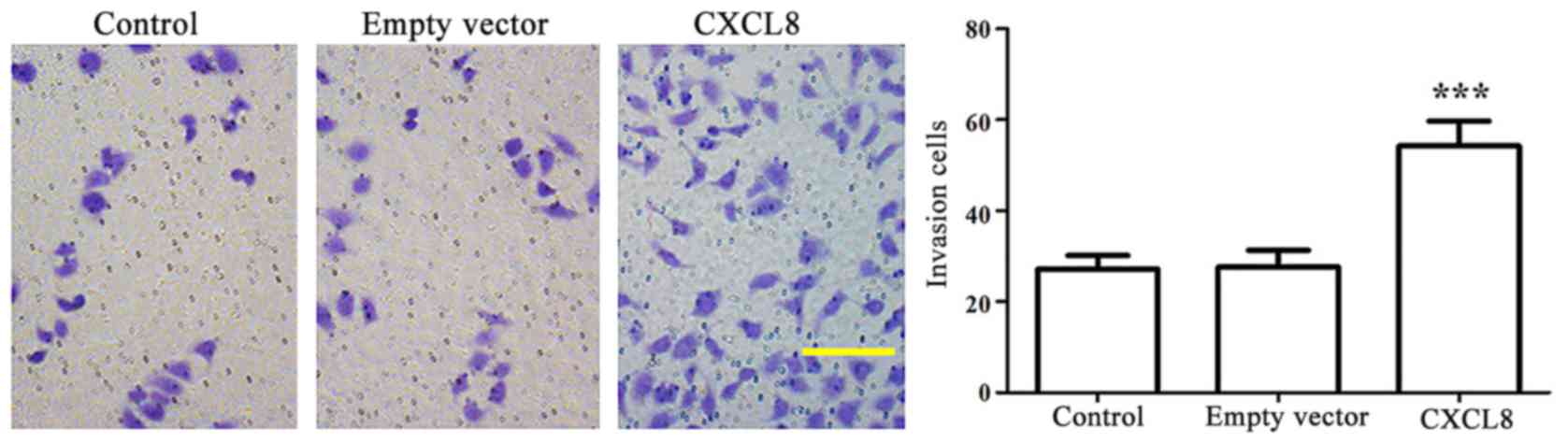
and empty-vector cells. Invasive potential was measured using
Transwell invasion assay (Fig. 4).
The CXCL8-transfected LoVo cells exhibited a 2-fold increased
invasion than that observed in the control and empty-vector cells
(P<0.001).
CXCL8 induces EMT-like phenotype in
LoVo cells
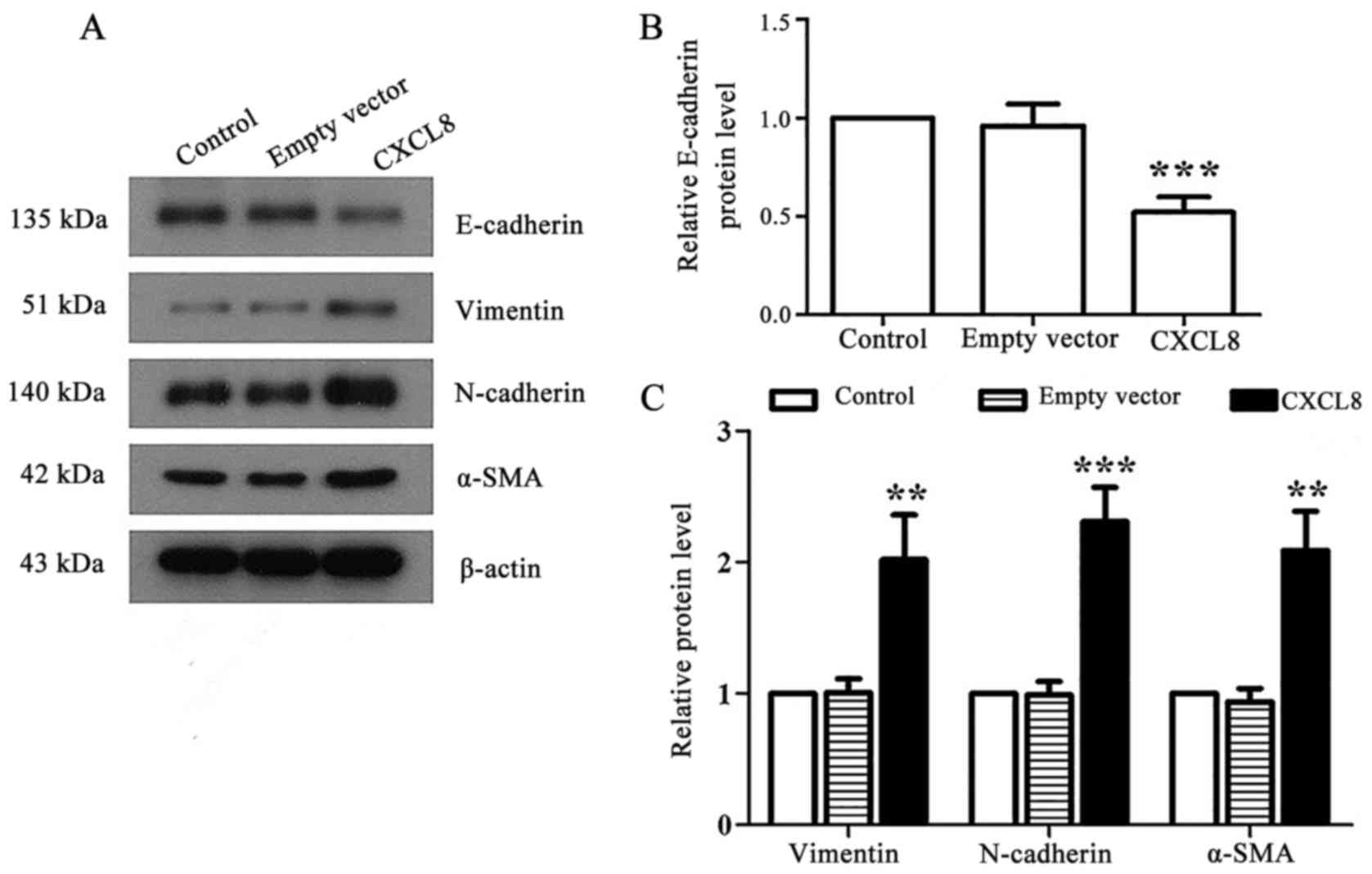
Western blotting showed that CXCL8-transfected LoVo
cells exhibited EMT-like phenotype compared with control and
empty-vector cells, with a decreased expression of E-cadherin
accompanied by increased expression of N-cadherin, vimentin and
α-SMA (Fig. 5).
CXCL8 activates the
phosphatidylinositol 3-kinase (PI3K)/ protein kinase B
(PKB/Akt)/nuclear factor-κB (NF-κB) pathway
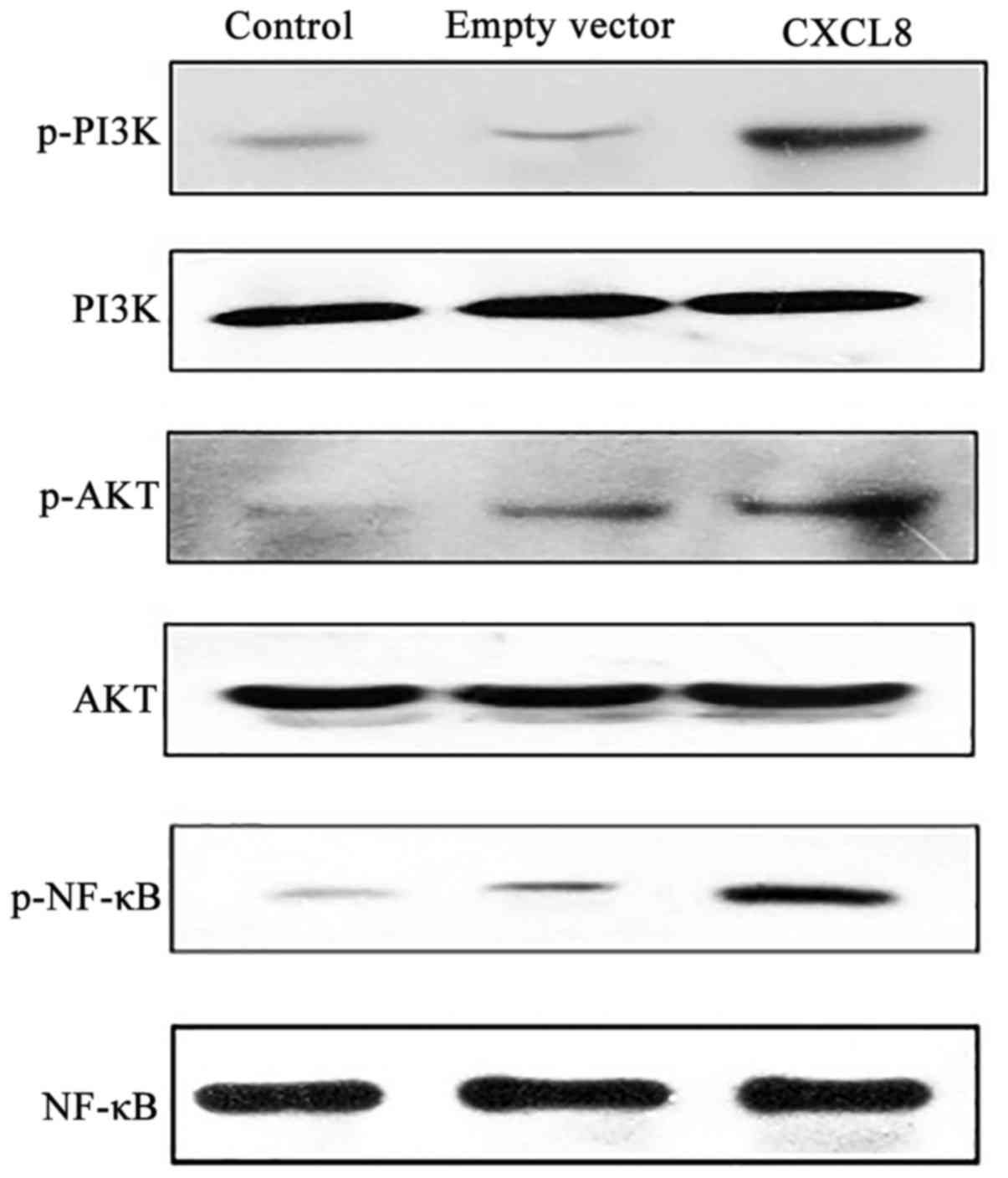
Compared with the control and empty-vector cells,
overexpression of CXCL8 activated the PI3K/Akt/NF-κB pathway by
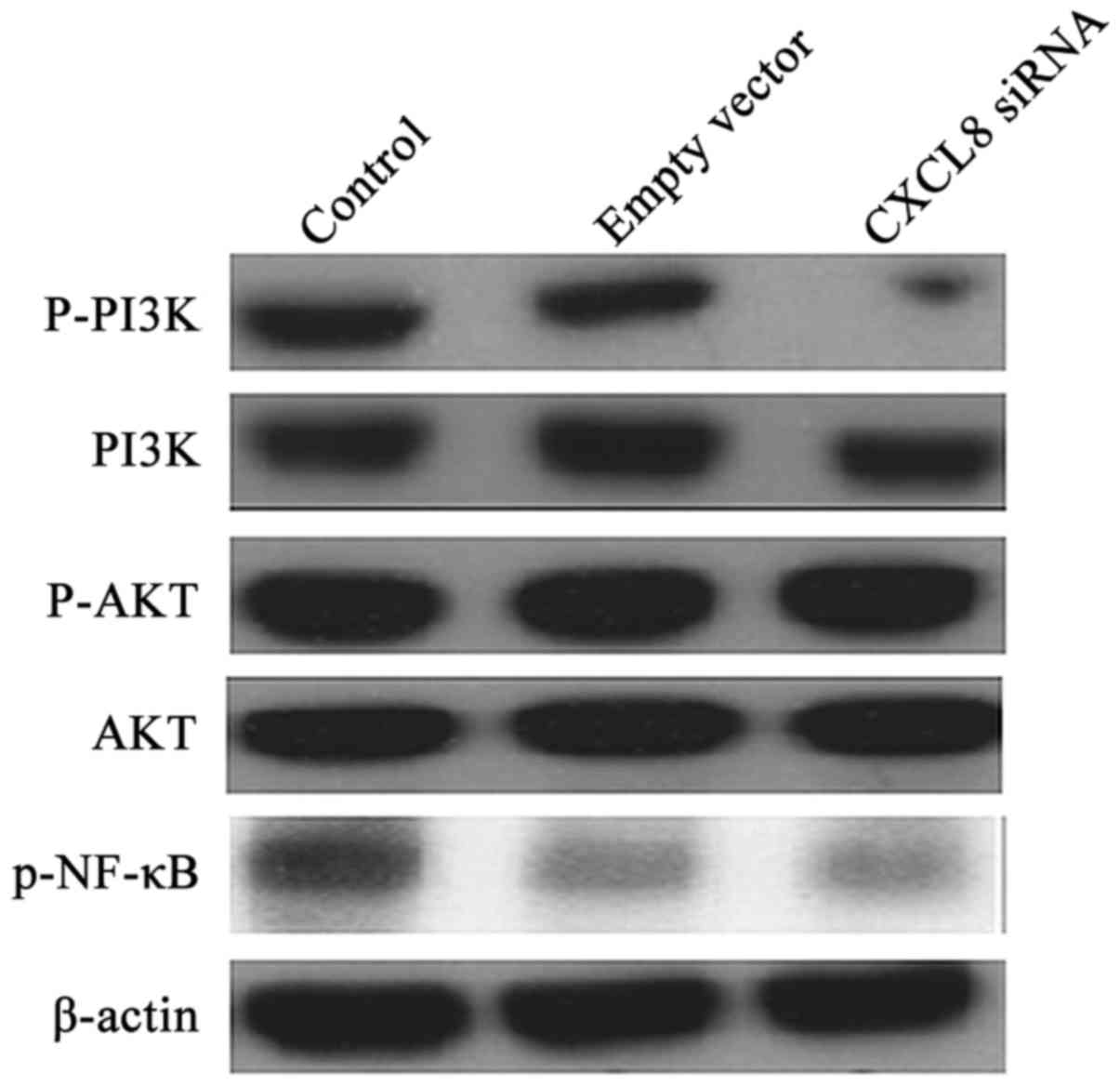
promoting phosphorylation of PI3K, Akt and NF-κB (Fig. 6). The efficacy of the silencing of
CXCL8 on the PI3K/Akt/NF-κB pathway was determined by western blot
analysis (Fig. 7). Compared to the
cells transfected with non-specific siRNA, silencing of CXCL8
lowered the phosphorylation of PI3K and NF-κB. Phosphorylation of
Akt appeared to be unaffected.
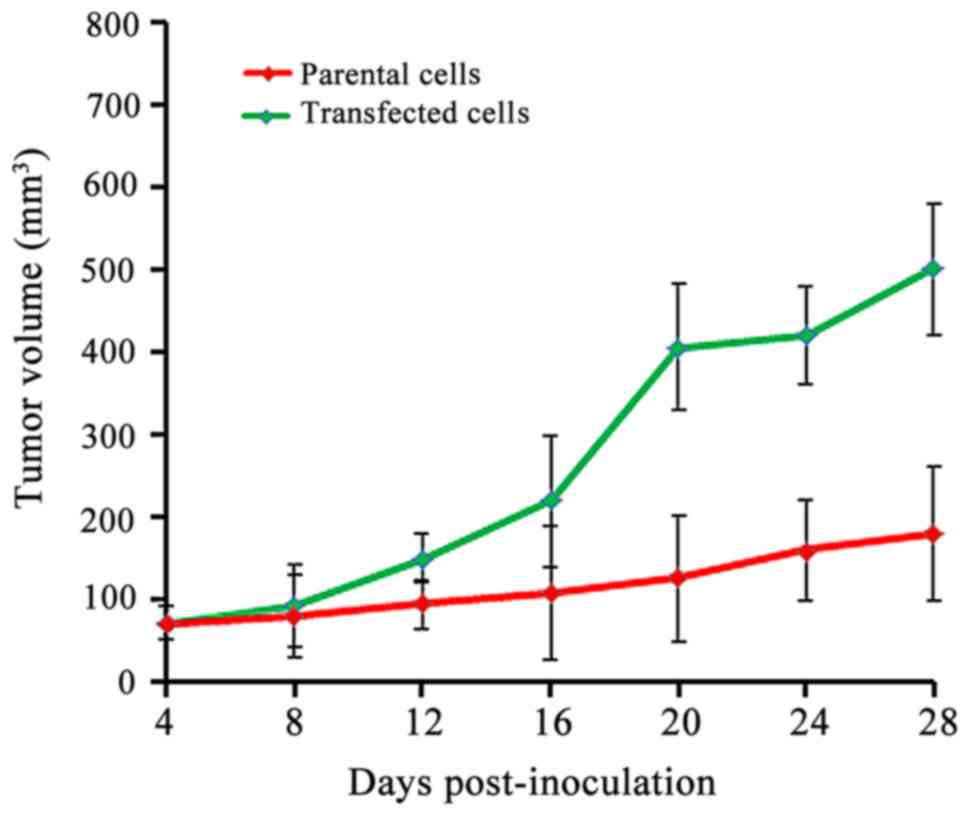
In vivo studies in nude mice
Subcutaneous tumors were generated by subcutaneous
injection of LoVo parental cells or CXCL8-transfected LoVo cells in
BALB/c nude mice. Fig. 8 shows the
growth of tumor in the two groups. Tumor growth was more rapid in
the CXCL8-transfected group than the growth observed in the
parental cell group. At day 28, the tumor volume was significantly
larger in the transfected cell group (P<0.05).
Discussion
In the present study, we examined the role of CXCL8
in the proliferation, migration and invasiveness in vitro in
LoVo colon cancer cell line models and its ability to promote EMT.
Our results showed that CXCL8-transfected LoVo cells exhibited
increased proliferation and enhanced migratory and invasive
ability. In vivo studies in nude mice confirmed a faster
tumor growth rate by the CXCL8-transfected LoVo cells. CXCL8 may
act through induction of EMT via the PI3K/AKT/NF-κB signaling axis.
Silencing of CXCL8 downregulated the phosphorylation of PI3K and
NF-κB.
Cancer metastasis is a complex process of multiple
intricate and sequential steps. Over the past decade, there is
accumulating evidence suggesting that EMT is a pathological process
that plays a crucial role in cancer progression, particularly in
cancer invasion, migration and metastasis (8). Recent studies have also shown that
chemokines and their receptors act as key regulators of metastatic
cancer, including colon cancer (9).
They are involved in various neoplastic processes including growth
signaling, acquisition of invasive properties, and emergence of a
vascular network.
CXCL8 is a chemokine that stimulates the migratory
capacity of a distinct set of leukocytes, and recent evidence
suggests that it plays an important role in the metastasis of
various types of cancers, including colon cancer (10,11).
Studies have shown significantly increased expression of CXCL8 in
the tumor microenvironment and serum of patients with colon cancer
(4,10,12).
Levels of CXCL8 were found to be correlated with tumor size, tumor
grade, depth of infiltration and survival in colon cancer and they
were decreased with successful treatment (6,10,13,14).
In the present study, stable CXCL8 transfection was successfully
generated in colon cancer LoVo cell lines. Using MTT growth
inhibition assay, we showed that constitutive expression of CXCL8
significantly stimulated the proliferation of LoVo cells.
Furthermore, scratch and Transwell invasion assays showed that
constitutive expression of CXCL8 significantly increased cell
migration and invasion. In vivo study showed a faster tumor
growth in the CXCL8-transfected cell group. Our results add to the
evidence that expression of CXCL8 plays an important role in
modulating cell proliferation, migration and invasion of colon
cancer cells.
Activation of the PI3K/Akt signaling pathway is
common in various types of cancers and it is involved in multiple
events, including metastasis, invasion and immunologic escape
(15–19). The PI3K/Akt signaling pathway has
also been reported to be hyperactivated in several models of EMT
(20,21). A study by Malinowsky et al
showed that activation of the PI3K/Akt pathway evidenced at the
protein level was significantly correlated with prognosis in
patients with stage II colon cancer (22). PI3K/Akt can be activated by a
variety of chemokines via phosphorylation and consequently
downregulates E-cadherin/β-catenin complex expression, promoting
cell migration and invasion in colon cancer cells (21,23,24).
Moreover, studies have shown that activation of the PI3K/Akt
pathway is also involved in activation of NF-κB via phosphorylation
of inhibitory inhibitor of κBα (IκBα) protein (25). This phosphorylation results in the
dissociation of IκBα from NF-κB, allowing NF-κB to freely migrate
into the nucleus and activate the transcription of several genes
involved in the suppression of cell death (26–28).
NF-κB also regulates proliferation and invasiveness by regulating
the expression of several genes involved in cell cycle machinery
(29–31). Our results are concurrent with
previous studies that demonstrated that overexpression of CXCL8
increased phosphorylation of the PI3K/Akt/NF-κB pathway,
accompanied by an EMT phenotype evidence by a decreased expression
of E-cadherin and an increased expression of N-cadherin, vimentin
and α-SMA. These results suggest that CXCL8 induces EMT in colon
cancer via activation of PI3K/Akt/NF-κB and CXCL8 has the potential
as a therapeutic target for the treatment of colon cancer.
Findings from the present study need to be confirmed
by future studies using human colon cancer tissue. In a previous
study by Cheng et al, patients with co-expression of CCL20
and CXCL8 were more likely to develop liver metastases and had a
poorer prognosis (7). Furthermore,
although we performed knockdown experiment using siRNA of CXCL8 to
investigate whether CXCL8 acts through the PI3K/Akt/NF-κB pathway,
the knockdown experiment was not performed to confirm the effect of
CXCL8 on colon cell proliferation, migration and invasion. A
previous study by Ning et al found that siRNA-mediated
knockdown of CXCL8 abrogated the increased cell migration and cell
invasion noted in CXCL8-transfected HCT116 cells (13).
In conclusion, the present study provides
significant evidence that overexpression of CXCL8 promotes colon
cancer cell proliferation, migration and invasion, and promotes
tumor growth in vivo. It may act via activation of the
PI3K/Akt/NF-κB signaling pathway. Our results could serve as a
basis for the development of novel anti-CXCL8-targeted therapies
for colon cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Ref.: 81560472).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leporrier J, Maurel J, Chiche L, Bara S,
Segol P and Launoy G: A population-based study of the incidence,
management and prognosis of hepatic metastases from colorectal
cancer. Br J Surg. 93:465–474. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brat DJ, Bellail AC and Van Meir EG: The
role of interleukin-8 and its receptors in gliomagenesis and
tumoral angiogenesis. Neuro Oncol. 7:122–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshimura T, Matsushima K, Oppenheim JJ
and Leonard EJ: Neutrophil chemotactic factor produced by
lipopolysaccharide (LPS)-stimulated human blood mononuclear
leukocytes: Partial characterization and separation from
interleukin 1 (IL 1). J Immunol. 175:5569–5574. 2005.PubMed/NCBI
|
|
6
|
Li A, Varney ML and Singh RK: Expression
of interleukin 8 and its receptors in human colon carcinoma cells
with different metastatic potentials. Clin Cancer Res. 7:3298–3304.
2001.PubMed/NCBI
|
|
7
|
Cheng XS, Li YF, Tan J, Sun B, Xiao YC,
Fang XB, Zhang XF, Li Q, Dong JH, Li M, et al: CCL20 and CXCL8
synergize to promote progression and poor survival outcome in
patients with colorectal cancer by collaborative induction of the
epithelial-mesenchymal transition. Cancer Lett. 348:77–87. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leroy P and Mostov KE: Slug is required
for cell survival during partial epithelial-mesenchymal transition
of HGF-induced tubulogenesis. Mol Biol Cell. 18:1943–1952. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanaka T, Bai Z, Srinoulprasert Y, Yang
BG, Hayasaka H and Miyasaka M: Chemokines in tumor progression and
metastasis. Cancer Sci. 96:317–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee YS, Choi I, Ning Y, Kim NY,
Khatchadourian V, Yang D, Chung HK, Choi D, LaBonte MJ, Ladner RD,
et al: Interleukin-8 and its receptor CXCR2 in the tumour
microenvironment promote colon cancer growth, progression and
metastasis. Br J Cancer. 106:1833–1841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McClelland MR, Carskadon SL, Zhao L, White
ES, Beer DG, Orringer MB, Pickens A, Chang AC and Arenberg DA:
Diversity of the angiogenic phenotype in non-small cell lung
cancer. Am J Respir Cell Mol Biol. 36:343–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doll D, Keller L, Maak M, Boulesteix AL,
Siewert JR, Holzmann B and Janssen KP: Differential expression of
the chemokines GRO-2, GRO-3, and interleukin-8 in colon cancer and
their impact on metastatic disease and survival. Int J Colorectal
Dis. 25:573–581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ning Y, Manegold PC, Hong YK, Zhang W,
Pohl A, Lurje G, Winder T, Yang D, LaBonte MJ, Wilson PM, et al:
Interleukin-8 is associated with proliferation, migration,
angiogenesis and chemosensitivity in vitro and in vivo in colon
cancer cell line models. Int J Cancer. 128:2038–2049. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rubie C, Frick VO, Pfeil S, Wagner M,
Kollmar O, Kopp B, Graber S, Rau BM and Schilling MK: Correlation
of IL-8 with induction, progression and metastatic potential of
colorectal cancer. World J Gastroenterol. 13:4996–5002. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dozmorov MG, Azzarello JT, Wren JD, Fung
KM, Yang Q, Davis JS, Hurst RE, Culkin DJ, Penning TM and Lin HK:
Elevated AKR1C3 expression promotes prostate cancer cell survival
and prostate cell-mediated endothelial cell tube formation:
Implications for prostate cancer progression. BMC Cancer.
10:6722010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiao M and Nan KJ: Activation of PI3
kinase/Akt/HIF-1α pathway contributes to hypoxia-induced
epithelial-mesenchymal transition and chemoresistance in
hepatocellular carcinoma. Int J Oncol. 40:461–468. 2012.PubMed/NCBI
|
|
17
|
Song L, Xiong H, Li J, Liao W, Wang L, Wu
J and Li M: Sphingosine kinase-1 enhances resistance to apoptosis
through activation of PI3K/Akt/NF-κB pathway in human non-small
cell lung cancer. Clin Cancer Res. 17:1839–1849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vredeveld LC, Possik PA, Smit MA, Meissl
K, Michaloglou C, Horlings HM, Ajouaou A, Kortman PC, Dankort D,
McMahon M, et al: Abrogation of BRAFV600E-induced
senescence by PI3K pathway activation contributes to
melanomagenesis. Genes Dev. 26:1055–1069. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang D, Chen J, Chen H, Duan Z, Xu Q, Wei
M, Wang L and Zhong M: Leptin regulates proliferation and apoptosis
of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J
Biosci. 37:91–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu G, Li X, Guo B, Ke Q, Dong M and Li F:
PAK5-mediated E47 phosphorylation promotes epithelial-mesenchymal
transition and metastasis of colon cancer. Oncogene. 35:1943–1954.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Li CL, Wang L, Yu WB, Yin HP,
Zhang GY, Zhang LF, Li S and Hu SY: Influence of CXCR4/SDF-1 axis
on E-cadherin/β-catenin complex expression in HT29 colon cancer
cells. World J Gastroenterol. 17:625–632. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Malinowsky K, Nitsche U, Janssen KP, Bader
FG, Späth C, Drecoll E, Keller G, Höfler H, Slotta-Huspenina J and
Becker KF: Activation of the PI3K/AKT pathway correlates with
prognosis in stage II colon cancer. Br J Cancer. 110:2081–2089.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen G, Chen SM, Wang X, Ding XF, Ding J
and Meng LH: Inhibition of chemokine (CXC motif) ligand
12/chemokine (CXC motif) receptor 4 axis (CXCL12/CXCR4)-mediated
cell migration by targeting mammalian target of rapamycin (mTOR)
pathway in human gastric carcinoma cells. J Biol Chem.
287:12132–12141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang CY, Fong YC, Lee CY, Chen MY, Tsai
HC, Hsu HC and Tang CH: CCL5 increases lung cancer migration via
PI3K, Akt and NF-kappaB pathways. Biochem Pharmacol. 77:794–803.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-κB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jacobs MD and Harrison SC: Structure of an
IkappaBalpha/NF-kappaB complex. Cell. 95:749–758. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kreuz S, Siegmund D, Scheurich P and
Wajant H: NF-kappaB inducers upregulate cFLIP, a
cycloheximide-sensitive inhibitor of death receptor signaling. Mol
Cell Biol. 21:3964–3973. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Micheau O, Lens S, Gaide O, Alevizopoulos
K and Tschopp J: NF-kappaB signals induce the expression of c-FLIP.
Mol Cell Biol. 21:5299–5305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guttridge DC, Albanese C, Reuther JY,
Pestell RG and Baldwin AS Jr: NF-kappaB controls cell growth and
differentiation through transcriptional regulation of cyclin D1.
Mol Cell Biol. 19:5785–5799. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hinz M, Krappmann D, Eichten A, Heder A,
Scheidereit C and Strauss M: NF-κB function in growth control:
Regulation of cyclin D1 expression and
G0/G1-to-S-phase transition. Mol Cell Biol.
19:2690–2698. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hinz M, Löser P, Mathas S, Krappmann D,
Dörken B and Scheidereit C: Constitutive NF-κB maintains high
expression of a characteristic gene network, including CD40, CD86,
and a set of antiapoptotic genes in Hodgkin/Reed-Sternberg cells.
Blood. 97:2798–2807. 2001. View Article : Google Scholar : PubMed/NCBI
|