Introduction
Cervical cancer is one of malignant tumors which
seriously threaten the health of women worldwide (1). The occurrence and development of a
variety of cancer including cervical cancer is a multi-gene and
factor-involved, multi-stage pathological process (2). HPV is now widely accepted as the
essential infectious etiological agent to the development of
cervical cancer (3,4). It is well established that the two
viral oncoproteins E6 and E7 mediate the oncogenic activities of
high-risk human papillomavirus (hrHPV), especially HPV16 and HPV18
(HPV16/18), which have been demonstrated to play critical roles in
cervical cancer through different pathways (5,6). HrHPV
alone is necessary but insufficient for cervical carcinogenesis;
only a small proportion of hrHPV-infected patients develop invasive
cervical cancer, and the majority remain subclinical or exhibit
only precursor lesions (7,8). This can be accounted for in the
involvement of genetic and epigenetic factors either independently
or in conjunction with hrHPV infection, therefore, these factors
may be implicated in the development of cervical cancer (9). Research has indicated that epigenetic
abnormalities, particularly aberrant methylation changes, play
causative roles in tumorigenesis (10,11).
The Ras associated domain family gene 1A (RASSF1A)
located on 3p21.3, is an established TSG that can regulate cell
cycle, apoptosis, and microtubule stability, whose abnormal
transcription or expression are closely associated with tumor
occurrence and development (12–15).
It has been reported that silencing of RASSFIA via DNA methylation
rather than mutational events and other genetic changes is a common
phenomenon occurring in most epithelial tumors, including kidney,
bladder, breast, pancreatic, nasopharyngeal and cervical carcinomas
(16–22). The aberrant methylation of RASSF1A
gene plays important roles in the pathogenesis of cervical cancers,
which may be an early event (9,23).
Some studies revealed an inverse correlation between RASSF1A
methylation and HPV16/18 infection in cervical squamous cell
carcinoma (SCC) (23,24), while other studies found no
correlation between hrHPV infection and RASSF1A promoter
methylation. Thus, further studies are needed to formally establish
the relationship between RASSF1A methylation and hrHPV
infection.
Materials and methods
Cell lines and constructs
HPV16/18-positive cervical cancer cell lines HeLa,
CaSki, SiHa and HPV-negative cervical cancer cells C33A and HT-3
were obtained from Shanghai Institute for Biological Sciences,
Chinese Academy of Sciences Institute of Cell Resource Center
(Shanghai, China). In the present study, we established the
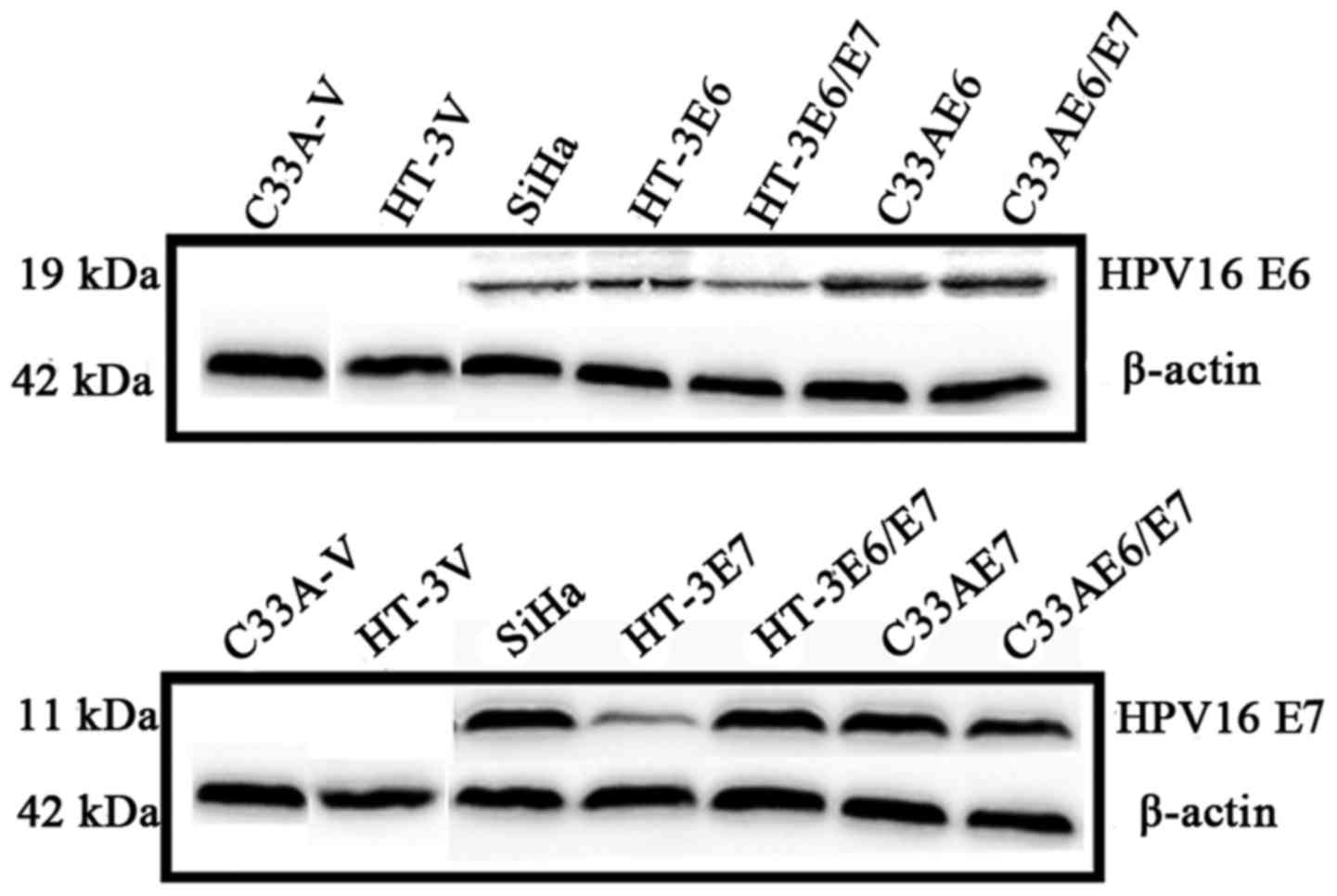
ectopically expressed HPV16 E6, E7 and E6/E7 cell models of HT-3E6,
HT-3E7, HT-3E6/E7, C33AE6, C33AE7 and C33AE6/E7 by transfecting
HPV16 E6, E7 and E6/E7 oncogenes with lentivirus vectors into the
HPV-negative cervical cancer cell line C33A and HT-3 cells. The
C33A-vector (C33A-V) and HT-3 vector (HT-3V) cells were established
by transfecting C33A and HT-3 cells with lentivirus vectors that
did not code for the HPV16 E6, E7, or E6/E7 proteins as controls.
Stable transfectants were selected with 10 µg/ml puromycin for 3
weeks. The transfection efficiency was tested by western blotting.
Western blotting showed that the transfected cells successfully
expressed the E6, E7, or E6/E7 proteins (Fig. 1).
Cell treatment with
5′-Aza-2-deoxycytidine (5-Aza-CdR)
For the demethylation experiments, demethylation was
induced with 5-Aza-CdR treatment at a concentration that was able
to induce the demethylation of the DNA without killing the cells.
CasKi, HT-3 and C33A cells were plated at a density of
8×104 cells/25 cm2 flask and treated with
various concentrations (5 and 10 µM) of 5-Aza-CdR for 96 h.
Methylation-specific polymerase chain
reaction (MSP)
bisulphate-modified DNA (2 µl) was used as template
to amplify in 15 µl total reaction mixture which contained 2 µl
DNA, 1.5 µl 10X PCR buffer, 0.75 µl each of the forward and reverse
primers, 1.2 µl dNTPs mixture, 0.15 µl Hot Start Taq polymerase
(Takara), and 9.4 µl PCR water. Primers for RASSF1A gene promoter
amplification was from the literature (25). The primers for the methylated form
were 5′-GAGAGCGCGTTTAGTTTCGTTTTC-3′ and
5′-ACCCGTACTTCGCTAACTTTAAAG-3′, and the primers for the
unmethylated form were 5′- GAGAGTGTGTTTGTTTTGTTTTTG-3′ and
5′-CCCATACTTCACTAACTTTAAACC-3′. The PCR involved an initial
denaturation at 94°C for 10 min, followed by 35 cycles consisting
of 94°C for 30 sec, optimal annealing temperature 56°C for 30 sec,
72°C for 30 sec, with a final extension at 72°C for 10 min.
double-distilled water as the blank control. PCR product (5.0 µl)
was directly loaded on 2% agarose gels, stained with ethidium
bromide, visualized and analysis under UV illumination. Each
experiment was repeated three times.
DNA extraction and bisulfite genomic
sequencing (BGS)
The DNA was extracted by using SDS-proteinase K and
purified with phenol:chloroform:isoamyl alcohol. A QIAampDNAFFPE
tissue kit (Qiagen GmbH, Hilden, Germany) was used for DNA
extraction from the paraffin-embedded tumor tissues. The bisulfite
conversion of the DNA was performed using an EpiTect Fast DNA
Bisulfite kit (Qiagen, Valencia, CA, USA) according to the
instruction of the manufacturer. The methylation status of the
promoter/exon 1 region of RASSF1A gene in the cervical cancer cells
and cervical specimens was analyzed by BGS. BGS strand-specific
primers BGSF (GGTTAAGTGTGTTGTTTTAGTAAT, from −271 to −246) and BGSR
(CTACCCCTTAACTACCCCTTCC, from +413 to +434) gene were used to
amplify a 704-bp region of RASSF1A gene. The purified PCR products
were cloned into the pUC18-T vector, and six clones from each
sample were randomly selected and sequenced.
RNA extraction and real-time
quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cervical cancer cell
lines and tissue samples using TRIzol (Invitrogen, Waltham, MA,
USA). The cDNAs were synthesized from the templates in presence of
reverse transcriptase and oligo(dT) 18 primers in accordance with
the manufacturer's instructions. Forward primer (RASSF1A-F:
GTGGGAGACACCTGACCTTT) and reverse primer (RASSF1A-R:
TGAAGCCTGTGTAAGACCG) were design to generate a 118 bp PCR product
at the appropriate annealing temperature. Double distilled water
was used as a negative control and the housekeeping gene
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified by
primers (5′-GATGACCTTGCCCACAGCCT-3′ and 5′-ATCTCTGCCCCCTCTGCTGA-3′)
to generate a 303 bp PCR product as the internal control. PCR
reaction (20 µl) mixture was pre-denatured at 95°C for 2 min, and
35 cycles of 94°C for 40 sec, 55°C for 30 sec, and 72°C for 60 sec
was performed with a final extension at 72°C for 10 min.
Western blotting
The harvested cells were lysed in RIPA lysis buffer
supplemented with protease inhibitor cocktail. Each protein lysate
(30 µg) was mixed with 5X SDS-PAGE sample loading buffer, and the
mixtures were boiled for 5 min. The boiled mixtures (50 µg) were
then fractionated on 12% SDS-PAGE gels and subsequently transferred
onto PVDF membranes (Merck Millipore, Billerica, MA, USA). The
primary antibodies used for the western blot analyses were
anti-RASSF1A and anti-β-actin. β-actin was used as a housekeeping
protein to normalize the protein loads.
Cervical tissue specimens
This study was approved by the Institutional Review
Board (IRB) of the Affiliated Hospital of Qingdao University. All
human cervical tissue samples, including 70 HPV16-positive squamous
cell carcinoma, 20 HPV-16-positive adenocarcinoma, 8 HPV-negative
cervical cancers, 30 HPV16-positive cervical cancers with PACT
(pre-operative adjuvant chemotherapy), 30 HPV16-positive normal
cervical tissue, were obtained with written informed consent from
the donors who underwent primary surgical treatment for cervical
tumors or other benign uterine lesions at the Affiliated Hospital
of Qingdao University between 2003 and 2015. All cases were
reviewed by at least two pathologists to confirm the primary
diagnoses.
HPV-DNA detection and genotyping
The detection of HPV DNA was performed in cervical
cancer samples by using MY09/11, GP5+/6+ and
SPF1/2 methods. Both MY09/11 and GP5+/6+ were
performed in a final reaction volume of 20 µl, containing 3 µl of
the isolated DNA, 2 µl of the 10X Buffer, 0.8 µl of each
deoxynucleoside triphosphate, 0.4 µl of forward and reverse
primers, and 1.0 units of Taq DNA polymerase (Genebase Bioscience,
Guangzhou, China). PCR conditions of MY09/11 were as follows:
preheating for 5 min at 94°C was followed by 40 cycles of 1 30 sec
at 94°C, 1 min at 45°C, and 1 min at 72°C and a final extension of
7 min at 72°C; PCR conditions of GP5+/6+ were
as follows: preheating for 10 min at 94°C was followed by 40 cycles
of 1 min at 94°C, 2 min at 40°C, and 1.5 min at 72°C and a final
extension of 7 min at 72°C. Each PCR experiment was performed with
positive and several negative PCR controls. The final PCR products
were separated by electrophoresis in 1.8% agarose gel. SPF was
performed in a final reaction volume of 40 µl, containing 4 µl of
the isolated DNA, 4 µl of the 10X Buffer, 3.2 µl of each
deoxynucleoside triphosphate, 0.5 µl of forward and reverse
primers, and 2.5 units of Taq DNA polymerase (Genebase Bioscience).
PCR conditions were as follows: preheating for 1 min at 94°C was
followed by 40 cycles of 1 min at 94°C, 1 min at 45°C, and 1 min at
72°C and a final extension of 5 min at 72°C. Each PCR experiment
was performed with positive and several negative PCR controls. The
products of PCR were then fractionated on 12% polyacrylamide gel
electrophoresis, PAGE gels. As for all methods of HPV-DNA detection
and genotyping, see Table I for the
detailed primer sequences and size of the product. If results of
the three methods were all consistent with each other and negative,
the samples were then demonstrated as HPV-negative samples;
conversely, if HPV detection of the sample was positive in at least
one of the three measures, the samples were defined as positive
samples and were further detected by HPV type specific PCR for
HPV16 and HPV18 (26,27). Details of PCR are available from the
corresponding author.
 | Table I.Primers and conditions of MY09/11),
GP5+/6+ and SPF. |
Table I.
Primers and conditions of MY09/11),
GP5+/6+ and SPF.
| Primer | Sequence | Size (bp) |
|---|
| MY09/11 | F:
5′-CGTCCMARRGGAWACTGATC-3′ | 450 |
|
| R:
5′-GCMCAGGGWCATAAYAATGG-3′ |
|
|
GP5+/6+ | F:
5′-TTTGTTACTGTGGTAGATACTAC-3′ | 150 |
|
| R:
5′-GAAAATAAACTGTAAATCATATTC-3′ |
|
| SPF1A | F:
5′-GCiCAGGGiCACAATAATGG-3′ | 65 |
| SPF1B | R:
5′-GCiCAGGGiCATAACAATGG-3′ |
|
| SPF1C | F:
5′-GCiCAGGGiCATAATAATGG-3′ |
|
| SPF1D | R:
5′-GCiCAAGGiCATAATAATGG-3′ |
|
| SPF2B-bio | F:
5′-GTiGTATCiACAACAGTAACAAA-3′ |
|
| SPF2D-bio | F:
5′-GTiGTATCiACTACAGTAACAAA-3′ |
|
Statistical analysis
Statistical tests were performed with SPSS version
17.0 (SPSS, Chicago, IL, USA). The correlation between the RASSF1A
mRNA expression and the cell lines was analyzed by the analysis of
variance (ANOVA), and multiple comparisons were analyzed by the
LSD. The correlations between the clinicopathological
characteristics and the methylation status of RASSF1A gene were
tested using the χ2 test. Differences were considered
significant at P<0.05.
Results
Analysis of RASSF1A methylation and
expression in HPV-positive and HPV-negative cervical cancer cell
lines
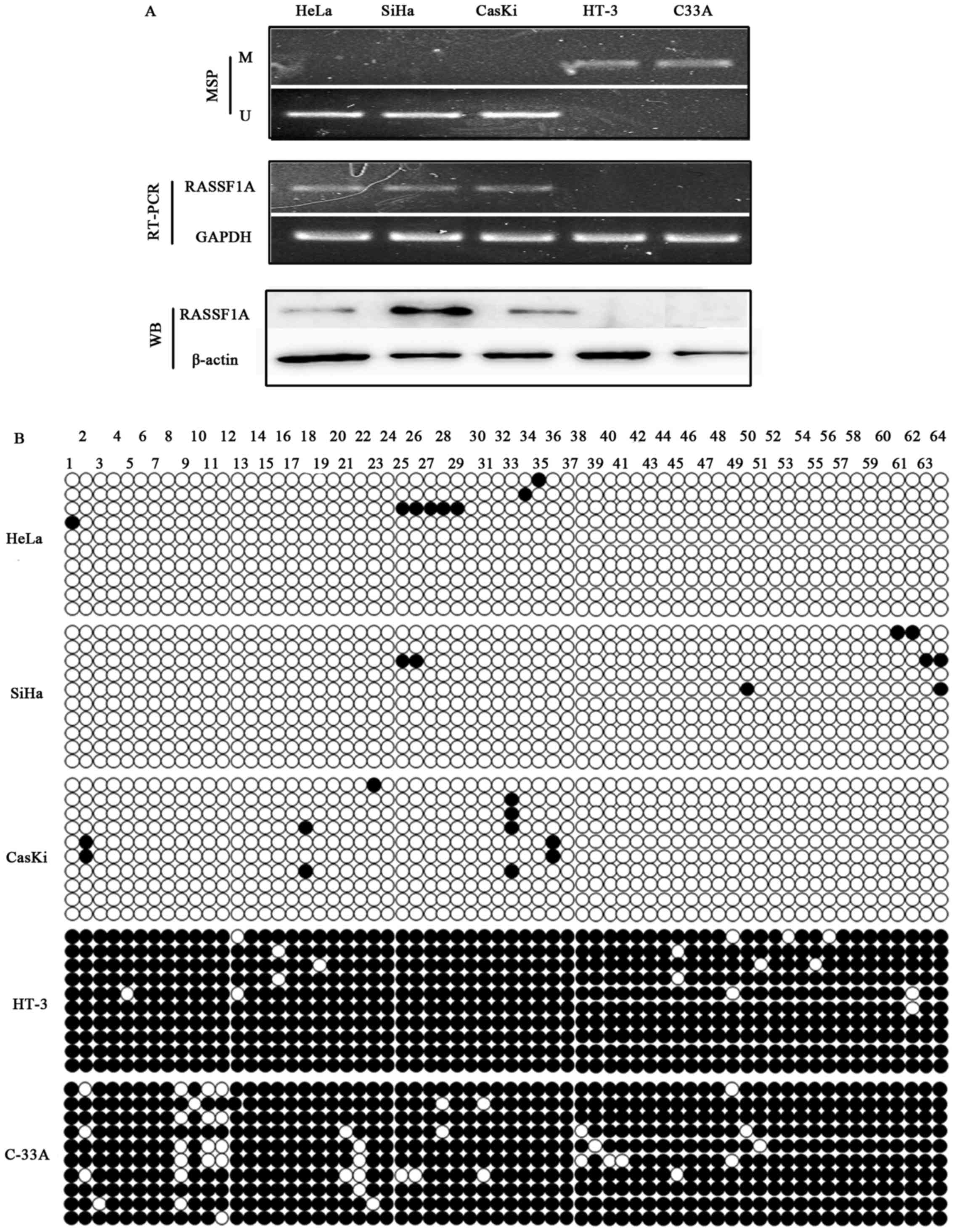
RASSF1A promoter methylation status was detected in
five cervical cancer cell lines by MSP. The results showed that
RASSF1A promoter was hypermethylated in two HPV-negative cell lines
(C-33A, HT-3), but not in the three HPV-positive cell lines (HeLa,
SiHa and Caski) (Fig. 2A). RASSF1A
mRNA and protein expression were detected in unmethylated cell
lines HeLa, SiHa and CasKi, whereas no RASSFIA expression was
detected in the two HPV-negative cell lines HT-3 and C33A with
hypermethylated promoters (Fig.
2A). TA clone and BGS results of RASSF1A in these five cell
lines showed densely methylated CpG sites C-33A and HT-3 cell
lines, while only scattered methylated CpG sites were detected in
HeLa, SiHa and Caski (Fig. 2B). BGS
analysis confirmed the MSP results.
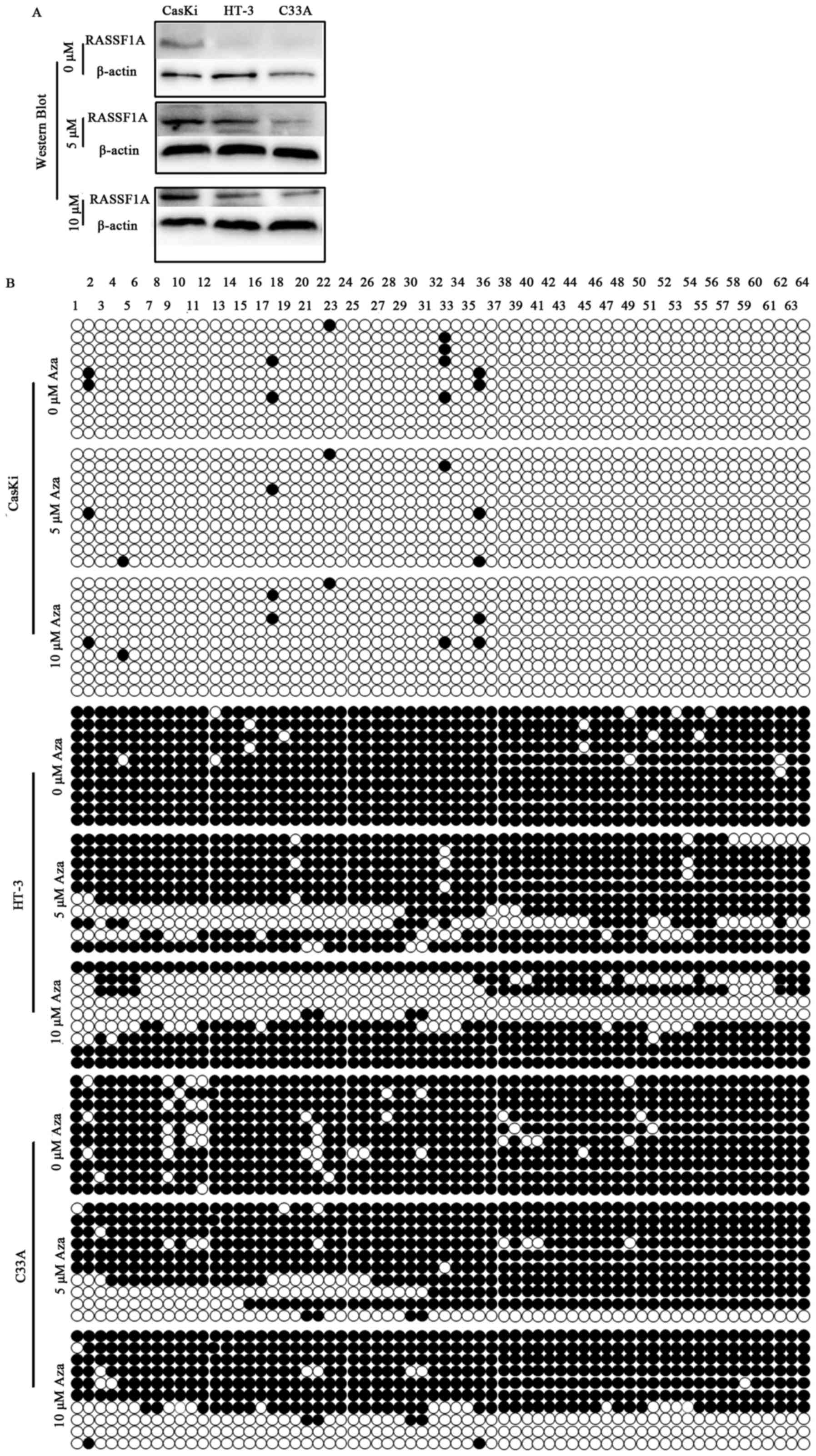
Effects of 5-Aza-dc on expression and
methylation status of RASSF1A in CasKi, HT-3 and C33A cells
To determine whether methylation status of the
RASSF1A gene promoter directly mediates its transcriptional
expression, the two cell lines HT-3 and C33A with a complete
methylation promoter and CasKi with a complete hypomethylation were
treated with 5-Aza-dc of various concentrations (5 and 10 µM) for
96 h. A gradual restoration of RASSF1A expression was detected in
HT-3 and C33A cells with two different concentrations (5 and 10 µM)
of 5-Aza-dc treatment by real-time quantitative PCR (Table II) and western blotting (Fig. 3A), concomitant with the
demethylation of its promoter detected by BGS (Fig. 3B). No significant changes in the
expression and promoter methylation of RASSF1A were detected in
CasKi cell before and after being treated with 5-Aza-dc of
different concentrations. As the results in Table II show, after being treated with
5-Aza-dc of various concentrations (5 and 10 µM), RASSF1A mRNA
expression was restored to different levels in HT-3 and C33A cell
lines. While the expression was not influenced by the demethylating
agent 5-aza-dC in CasKi cell line. Thus, in effective concentration
range, RASSF1A mRNA expression may be induced by 5-Aza-CdR
concentration dependently in HT-3 and C33A cell lines.
 | Table II.The comparison of RASSF1A mRNA
expression in three cervical cancer cell lines treated with
5-Aza-dc. |
Table II.
The comparison of RASSF1A mRNA
expression in three cervical cancer cell lines treated with
5-Aza-dc.
| Cell lines | 0 µM | 5 µM | 10 µM | F-value | P-value |
|---|
| CasKi | 0.0407±0.0056 | 0.0408±0.0081 | 0.0419±0.0302 |
1.183 | 0.324 |
| HT-3 | 0 | 0.0108±0.0036 | 0.0140±0.0017 | 110.792 | 0.000 |
| C-33A | 0 | 0.0115±0.0011 | 0.01380±0.0034 | 101.558 | 0.000 |
Impact of HPV16 oncogene E6/E7 on
methylation status and expression of RASSF1A in HT-3 and C33A
cells
BGS results (Fig.
4A) showed that among the HPV16 E6, E7 or E6/E7-transfected
cell lines, RASSF1A promoter methylation status was relatively
downregulated in the HT-3E6/E7 cells but not in the HT-3E6, HT-3E7,
HT-3V, C33A-V, C33AE6, C33AE7 and C33AE6/E7 cells compared with the
primary HT-3 and C33A cells. The RT-PCR and western blot results
(Fig. 4B) revealed that among the
HPV16 E6, E7 or E6/E7-transfected cell lines, RASSF1A re-expressed
in HT-3E6/E7 cells but not in the HT-3E6, HT-3E7, HT-3V, C33A-V,
C33AE6, C33AE7 and C33AE6/E7 cells, which is consistent with the
BGS result (Fig. 4A).
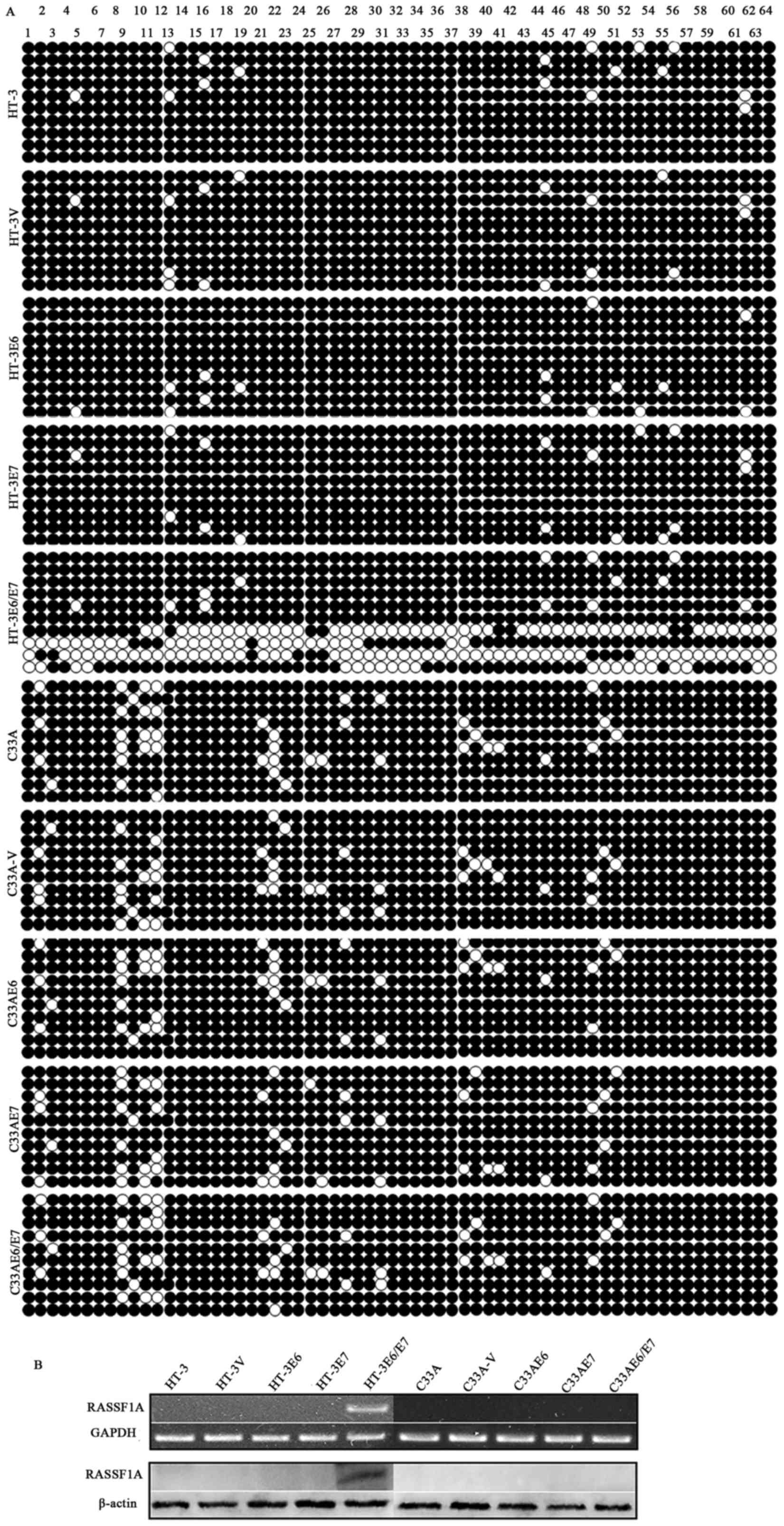 | Figure 4.The impact of HPV16 oncogenes E6, E7
and E6/E7 on methylation status and expression of RASSF1A in HT-3
and C33A cells. (A) BGS results showed that among the HPV16 E6, E7
or E6/E7-transfected cell lines, RASSF1A promoter methylation
status was relatively downregulated in the HT-3E6/E7 cells but not
in other HT-3E6, HT-3E7, HT-3V, C33A-V, C33AE6, C33AE7 and
C33AE6/E7 cells compared with the primary HT-3 and C33A cells. (B)
The RT-PCR and western blot results were consistent with BGS
analyses, showing that RASSF1A gene re-expressed in HT-3E6/E7 cells
but not in other HPV16 E6 or/and E7 ectopically expressed cells. ●,
Methylated cytosines; ○, unmethylated cytosines. |
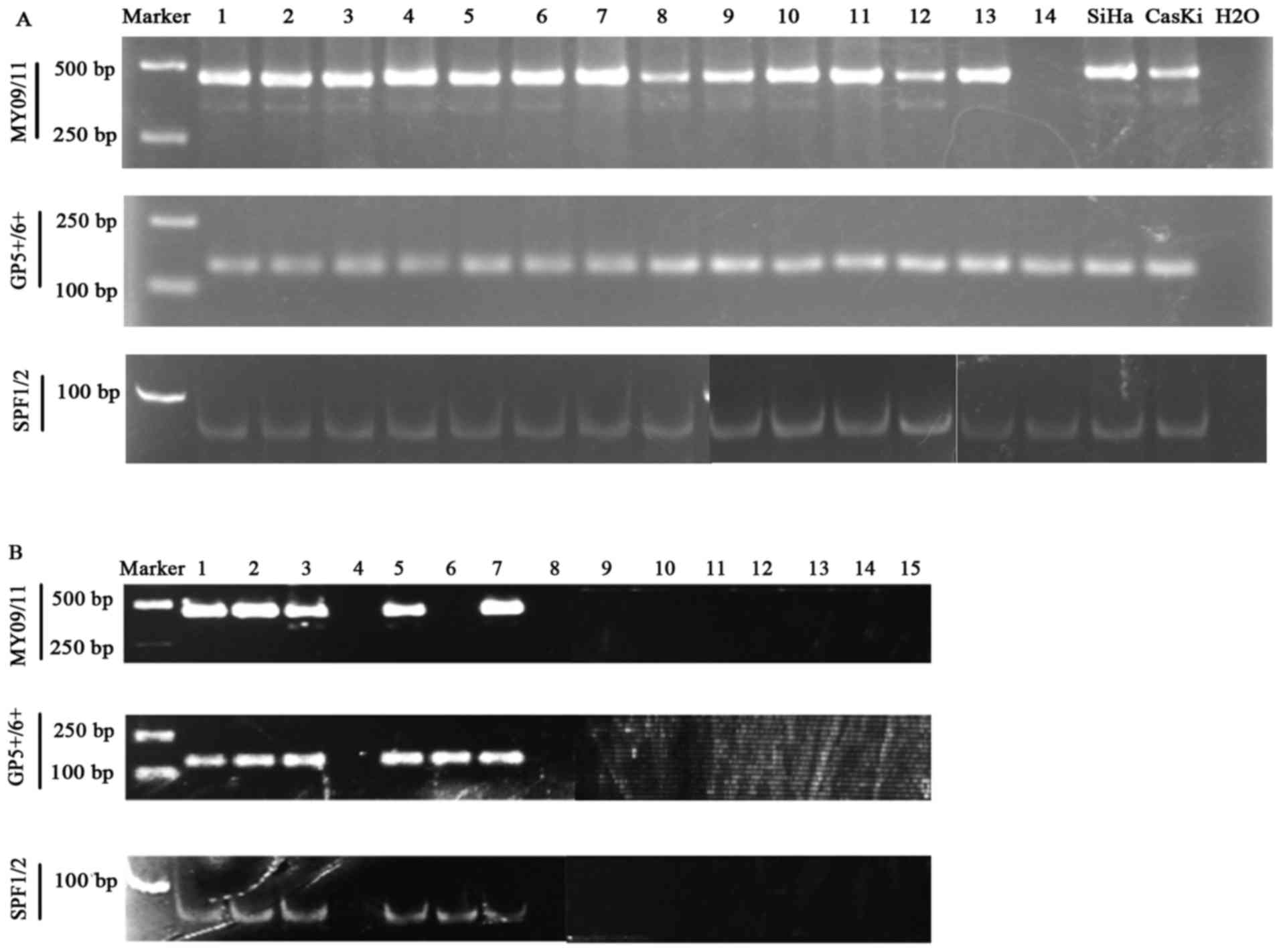
HPV-DNA detection results of cervical
cancer samples by MY09/11, GP5+/6+ and SPF1/2
methods
All 90 cases of cervical cancer patients were
positive in previous HC2 test. The HPV DNA was detected in at least
one of the MY09/11, GP5+/6+ and SPF1/2
methods (Fig. 5A) in all the tumor
tissues. Of the 14 HPV-negative cervical samples previously
identified by HC2 clinically, we retested the HPV DNA infection by
combining the MY09/11, GP5+/6+ and SPF1/2
methods. The results showed that only 8/14 (57.14%) was confirmed
all negative combining MY09/11, GP5+/6+ and
SPF1/2 methods, 9/14 (64.29%) was negative with MY09/11 method,
8/14 (57.14%) was confirmed negative with
GP5+/6+ and SPF1/2 method, respectively
(Fig. 5B). Thus, there was a false
negative rate (FNR) of 6.25% by HC2 method, when confirmed HPV
detection combining the MY09/11, GP5+/6+ and
SPF1/2 methods.
Clinicopathological significance of
RASSF1A promoter methylation status in cervical cancer tissues and
normal cervical tissues
MSP analysis indicated RASSF1A hypermethylation was
found in 16 of 98 (16%) cancer samples and none in 30 normal
cervical samples (χ2=4.20; P=0.04). The relations
between the methylation status of RASSF1A and the
clinicopathological characteristics in the cervical cancer patients
were analyzed. The methylation status of RASSF1A promoter was not
associated with neoadjuvant chemotherapy, tumor size, smoking or
hormone receptors (P>0.05; Table
III). Of the 70 SCC, the result showed a statistically reversed
relationship between RASSF1A methylation and HPV16 infection
(P<0.05). While of the 20 adenocarcinoma (AC), no significant
differences was found between RASSF1A methylation and HPV16
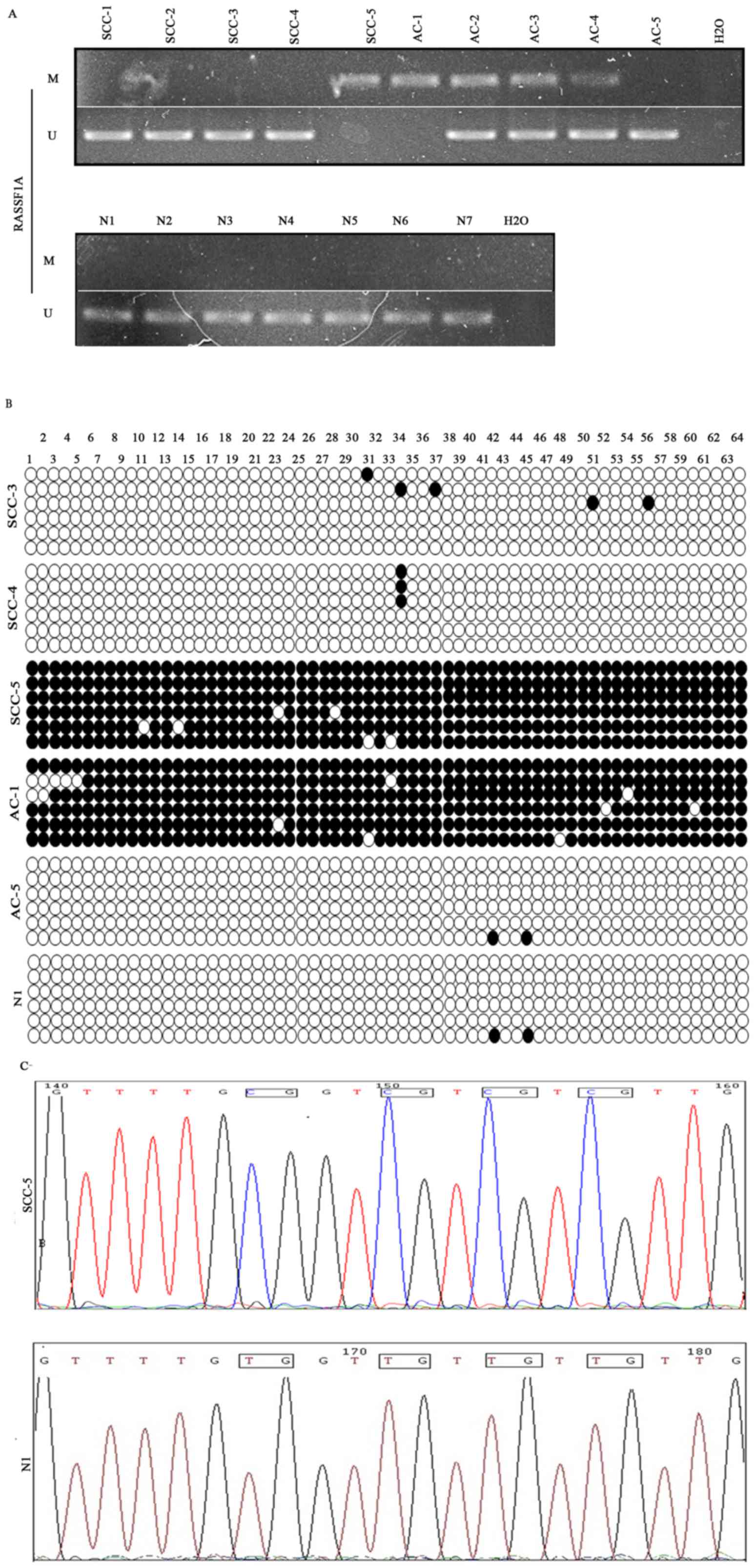
infection. Examples of MSP and BGS results are shown in Fig. 6A and B. DNA sequencing analyzed by
chromas software confirmed the results (Fig. 6C).
 | Table III.Correlations between RASSF1A promoter
methylation and the different clinicopathological features of human
cervical cancers. |
Table III.
Correlations between RASSF1A promoter
methylation and the different clinicopathological features of human
cervical cancers.
|
|
| RASSF1A |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | No. | + | − | P-value |
|---|
| Neoajuvant
chemotherapy | 90 |
|
|
|
| No |
| 12 | 48 | 0.595 |
|
Yes |
| 7 | 21 |
|
| Tumor size
(cm) | 60 |
|
|
|
|
<1 |
| 6 | 34 | 0.903 |
| ≥1 |
| 4 | 16 |
|
| Smoking | 90 |
|
|
|
| No |
| 13 | 71 | 0.938 |
|
Yes |
| 1 | 5 |
|
| Hormone
receptors | 60 |
|
|
|
|
Negative |
| 4 | 21 | 1.000 |
|
Positive |
| 6 | 29 |
|
| SCC | 70 |
|
|
|
|
HPV-positive |
| 9 | 56 | 0.032 |
|
HPV-negative |
| 3 | 2 |
|
| AC | 20 |
|
|
|
|
HPV-positive |
| 3 | 14 | 1.000 |
|
HPV-negative |
| 1 | 2 |
|
Discussion
Specific changes in gene expression in cancer cells
could result from epigenetic modifications, such as DNA
hypermethylation, which subsequently inactivate some tumor
suppressor genes (28). Together
with genetic changes, epigenetic modifications can be a driving
force behind the stepwise progress of cancer initiation, promotion
and progression (29). Despite
cervical cancer screening with Pap cytology and HPV testing,
cervical cancer remains one of the leading causes of death in women
worldwide. HPV is the known etiologic agent for cervical cancer,
which can induce epigenetic changes in host cells (30). After HPV infection, normal cervical
epithelia must accumulate some important genetic and epigenetic
changes which play pivotal roles in gene transcriptional regulation
to become an invasive cancer (31).
These results indicate that methylation and silence of TSGs induced
by HPV may be an important oncogenic mechanism for the development
of cervical cancer. It is scientifically significant to explore the
relationship between hrHPV infection and TSGs inactivation.
The RASSF1A gene has been demonstrated to be a
potential TSG in cervical cancers and its hypermethylation is
considered as one of the earliest events in tumorigenesis (9,23,32,33).
Both aberrant methylation of the RASSF1A promoter and hrHPV
infection are often observed in cervical cancers but their mutual
relationship has not been determined (23,24,34).
Our current study confirmed that two HPV-negative cervical cancer
cell lines had a methylated and silenced RASSF1A promoter (C-33A
and HT-3), whereas the other three HPV positive cervical cancer
cell lines expressed RASSF1A mRNA (HeLa, SiHa and CaSki), which is
consistent with the findings of previous research (24,35).
The silence and hypermethylation status of RASSF1A in the two
HPV-negative cervical cancer cell lines (C-33A and HT-3) could be
reversed by demethylating agent 5-Aza-dC treatment and the
influence was concentration dependent. This is the first
demonstration that the RASSF1A mRNA could be reactivated by
downregulating its promoter methylation status using demethylating
agent 5-Aza-dC, similar to the impact of 5-Aza-dC on RASSF1A gene
in esophageal squamous cell carcinoma, synovial sarcoma and
melanoma (36–38). Our results showed that the
methylation status and expression of RASSF1A gene between the two
HPV-negative cervical cancer cell lines (C-33A and HT-3) and three
HPV-positive cervical cancer cell lines (HeLa, SiHa and CaSki) were
significantly different. Besides, our result showed that the
methylation status and expression of RASSF1A gene could be
regulated by 5-Aza-dC. The above evidence may imply that there
exists a possible interaction between hrHPV infection and RASSF1A
methylation in tumorigenesis of cervical cancer.
The oncoproteins E6 and E7 of hrHPV, especially in
HPV16/18, have been proved to play critical roles in cervical
cancer through different pathways, including inactivating the
products of tumor suppressor genes p53 and Rb, respectively
(39,40). RASSF1A can induce cell cycle arrest
by engaging the Rb family cell cycle checkpoint and the
RASSF1A-induced cell cycle arrest can be relieved by the downstream
activators of the G1/S-phase transition (cyclin A and E7) (13,24,41),
revealing that E6, E7 may participate in the pathogenetic
mechanisms of RASSF1A. In order to explore whether the hrHPV E6 and
E7 could be correlated to RASSF1A promoter methylation and
expression, we detected the expression and methylation status of
RASSF1A in two HPV-negative cervical cancer cell lines HT-3 and
C33A before and after they were transfected into HPV16 oncogenic
genes E6, E7 and E6/E7 with lentivirus vectors.
Our results showed that re-expression and
downregulated promoter methylation status were found in the
HT-3E6/E7 cells, but not in the HT-3V, C33A-V cells, indicating the
ectopic expression of HPV16 E6/E7 may interact with aberrant
methylation and expression of the RASSF1A gene without a
disturbance of the transfected lentivirus vectors. However,
expression and downregulated promoter methylation status of the
RASSF1A gene were not found in HT-3E6, HT-3E7, C33AE6, C33AE7 and
C33AE6/E7 cells (Fig. 4A and B). In
our research, the re-expression and downregulated methylation
status of RASSF1A were not detected in HT-3E6, HT-3E7, HT-3V cells.
Possible reasons may be the changes of RASSF1A need the co-action
of E6 and E7. Likewise, reasons why the re-expression and
downregulated promoter methylation status of RASSF1A gene were not
found in the C33AE6, C33AE7 and C33AE6/E7 cells may arise from
different cell types. Nevertheless, the finding of the
downregulated methylation status and re-expression of RASSF1A in
HT-3E6/E7 was a phenomenon. This phenomenon is associated with the
interaction between HPV16 E6/E7 and RASSF1A. However, further
studies need to be focused on understanding the molecular
mechanism(s) by which HPV16 E6/E7 impacts RASSF1A methylation
status and expression.
Previous studies have showed an inconclusive result
of mutual interaction between hrHPV infection and aberrant RASSF1A
promoter methylation status. Some studies found an inverse
correlation between RASSF1A methylation and hrHPV (type 16/18)
infection in SCC (23,24), while other studies detected no
correlation between HPV infection and RASSF1A promoter methylation.
When exploring the potential relationship between hrHPV infection
and RASSF1A promoter methylation in cervical cancers, selection of
HPV-positive and HPV-negative cervical cancer samples are required.
However, HPV detection could also be false negative (42,43).
In our study, we used three PCR primer sets (MY09/11,
GP5+/6+ and SPF1/2) to confirm HPV infection
status. The statistical analysis indicated there was no false
positive in the HPV-positive cervical cancer samples, previously
identified by HC2. While a false negative existed in the HPV
detection by HC2 method, showing that only 8/14 (57.14%)
HPV-negative clinical cervical cancer samples (previously
identified by HC2) were confirmed all negative combining MY09/11,
GP5+/6+ and SPF1/2 methods. The poorly
prepared cervical samples for Her2 test may contribute to the
uncertain relationship between hrHPV infection and aberrant RASSF1A
promoter methylation status to some extent.
Clinicopathological significance of RASSF1A promoter
methylation status has been analyzed in various kind of human
cancers. Evidence showed that aberrant methylation of RASSF1A
promoter were associated with hormone receptor status in breast
cancer (44), correlated to
responsiveness to chemotherapy in hepatoblastoma patients (45), relevant to cigarette smoking in lung
cancer. In cervical cancer, RASSF1A promoter methylation status was
demonstrated to be not related to age, the type of cancer, lymph
node metastasis, cancer grade, the FIGO stage or HPV genotyping
(13,46). Our analysis showed that the
methylation status of RASSF1A was not associated with neoadjuvant
chemotherapy, tumor size, smoking or hormone receptors (HRs).
Consistent with the previous studies (23,24),
our result showed a statistically reversed relationship between
RASSF1A methylation and HPV infection in SCC (P<0.05), while no
significant differences was found in AC. Moreover, MSP analysis
showed a significant hypermethylation RASSF1A in cervical cancer
samples compared to normal cervical samples (P<0.05), revealing
RASSF1A may act as a potential TSG in cervical tissues.
Acknowledgements
This study was supported by the National Nature
Science Foundation of China (grant no. 81172480) and Science
Foundation for Postdoctoral application project 14 of Qingdao
City.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feinberg AP and Tycko B: The history of
cancer epigenetics. Nat Rev Cancer. 4:143–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
zur Hausen H: Papillomaviruses in
anogenital cancer as a model to understand the role of viruses in
human cancers. Cancer Res. 49:4677–4681. 1989.PubMed/NCBI
|
|
4
|
Kitchener HC, Denton K, Soldan K and
Crosbie EJ: Developing role of HPV in cervical cancer prevention.
BMJ. 347:f47812013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ajiro M and Zheng ZM: E6^E7, a novel
splice isoform protein of human papillomavirus 16, stabilizes viral
E6 and E7 oncoproteins via HSP90 and GRP78. MBio. 6:e02068–e14.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Narisawa-Saito M and Kiyono T: Basic
mechanisms of high-risk human papillomavirus-induced
carcinogenesis: Roles of E6 and E7 proteins. Cancer Sci.
98:1505–1511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Flatley JE, McNeir K, Balasubramani L,
Tidy J, Stuart EL, Young TA and Powers HJ: Folate status and
aberrant DNA methylation are associated with HPV infection and
cervical pathogenesis. Cancer Epidemiol Biomarkers Prev.
18:2782–2789. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ostör AG: Natural history of cervical
intraepithelial neoplasia: A critical review. Int J Gynecol Pathol.
12:186–192. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neyaz MK, Kumar RS, Hussain S, Naqvi SH,
Kohaar I, Thakur N, Kashyap V, Das BC, Husain SA and Bharadwaj M:
Effect of aberrant promoter methylation of FHIT and RASSF1A genes
on susceptibility to cervical cancer in a North Indian population.
Biomarkers. 13:597–606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baylin SB: The cancer epigenome: Its
origins, contributions to tumorigenesis, and translational
implications. Proc Am Thorac Soc. 9:64–65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiong J, Li Y, Huang K, Lu M, Shi H, Ma L,
Luo A, Yang S, Lu Z, Zhang J, et al: Association between DAPK1
promoter methylation and cervical cancer: A meta-analysis. PLoS
One. 9:e1072722014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song MS, Song SJ, Ayad NG, Chang JS, Lee
JH, Hong HK, Lee H, Choi N, Kim J, Kim H, et al: The tumour
suppressor RASSF1A regulates mitosis by inhibiting the APC-Cdc20
complex. Nat Cell Biol. 6:129–137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shivakumar L, Minna J, Sakamaki T, Pestell
R and White MA: The RASSF1A tumor suppressor blocks cell cycle
progression and inhibits cyclin D1 accumulation. Mol Cell Biol.
22:4309–4318. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agathanggelou A, Cooper WN and Latif F:
Role of the Ras-association domain family 1 tumor suppressor gene
in human cancers. Cancer Res. 65:3497–3508. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Whang YM, Kim YH, Kim JS and Yoo YD:
RASSF1A suppresses the c-Jun-NH2-kinase pathway and inhibits cell
cycle progression. Cancer Res. 65:3682–3690. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peters I, Rehmet K, Wilke N, Kuczyk MA,
Hennenlotter J, Eilers T, Machtens S, Jonas U and Serth J: RASSF1A
promoter methylation and expression analysis in normal and
neoplastic kidney indicates a role in early tumorigenesis. Mol
Cancer. 6:492007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao T, Wang S, He B, Pan Y, Song G, Gu L,
Chen L, Nie Z, Xu Y and Li R: The association of RAS association
domain family Protein1A (RASSF1A) methylation states and bladder
cancer risk: A systematic review and meta-analysis. PLoS One.
7:e483002012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hagrass HA, Pasha HF, Shaheen MA, Bary
Abdel EH and Kassem R: Methylation status and protein expression of
RASSF1A in breast cancer patients. Mol Biol Rep. 41:57–65. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malpeli G, Amato E, Dandrea M, Fumagalli
C, Debattisti V, Boninsegna L, Pelosi G, Falconi M and Scarpa A:
Methylation-associated down-regulation of RASSF1A and up-regulation
of RASSF1C in pancreatic endocrine tumors. BMC Cancer. 11:3512011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu K, Xu XN, Chen Y, Pu XL, Wang BY and
Tang XD: RASSF1A gene methylation is associated with nasopharyngeal
carcinoma risk in Chinese. Asian Pac J Cancer Prev. 16:2283–2287.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maliukova AV, Loginov VI, Khodyrev DS,
Kadyrova EL, Pronina IV, Ivanova TA, Kiselev FL, Zabarovskiĭ NP and
Braga EA: Methylation of the putative tumor suppressor gene,
RASSF1A, in primary cervical tumors. Mol Biol (Mosk). 38:1005–1013.
2004.(In Russian). PubMed/NCBI
|
|
22
|
Pfeifer GP and Dammann R: Methylation of
the tumor suppressor gene RASSF1A in human tumors. Biochemistry
(Mosc). 70:576–583. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li JY, Huang T, Zhang C, Jiang DJ, Hong
QX, Ji HH, Ye M and Duan SW: Association between RASSF1A promoter
hypermethylation and oncogenic HPV infection status in invasive
cervical cancer: A meta-analysis. Asian Pac J Cancer Prev.
16:5749–5754. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuzmin I, Liu L, Dammann R, Geil L,
Stanbridge EJ, Wilczynski SP, Lerman MI and Pfeifer GP:
Inactivation of RAS association domain family 1A gene in cervical
carcinomas and the role of human papillomavirus infection. Cancer
Res. 63:1888–1893. 2003.PubMed/NCBI
|
|
25
|
Muñoz J, Inda MM, Lázcoz P, Zazpe I, Fan
X, Alfaro J, Tuñón T, Rey JA and Castresana JS: Promoter
methylation of RASSF1A associates to adult secondary glioblastomas
and pediatric glioblastomas. ISRN Neurol.
2012:5765782012.PubMed/NCBI
|
|
26
|
Shukla S, Bharti AC, Mahata S, Hussain S,
Hedau S, Sharma R, Pillai MR, Krishna S, Chiplunkar S, Tongaonkar
H, et al: Application of a multiplex PCR to cervical cells
collected by a paper smear for the simultaneous detection of all
mucosal human papillomaviruses (HPVs) and typing of high-risk HPV
types 16 and 18. J Med Microbiol. 59:1303–1310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee SH, Vigliotti VS, Vigliotti JS and
Pappu S: Validation of human papillomavirus genotyping by signature
DNA sequence analysis. BMC Clin Pathol. 9:32009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gal-Yam EN, Saito Y, Egger G and Jones PA:
Cancer epigenetics: Modifications, screening, and therapy. Annu Rev
Med. 59:267–280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Esteller M: Epigenetics in cancer. N Engl
J Med. 358:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Paschos K and Allday MJ: Epigenetic
reprogramming of host genes in viral and microbial pathogenesis.
Trends Microbiol. 18:439–447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Woodman CB, Collins SI and Young LS: The
natural history of cervical HPV infection: Unresolved issues. Nat
Rev Cancer. 7:11–22. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meng W, Huebner A, Shabsigh A, Chakravarti
A and Lautenschlaeger T: Combined RASSF1A and RASSF2A promoter
methylation analysis as diagnostic biomarker for bladder cancer.
Mol Biol Int. 2012:7018142012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Senchenko VN, Kisseljova NP, Ivanova TA,
Dmitriev AA, Krasnov GS, Kudryavtseva AV, Panasenko GV, Tsitrin EB,
Lerman MI, Kisseljov FL, et al: Novel tumor suppressor candidates
on chromosome 3 revealed by NotI-microarrays in cervical cancer.
Epigenetics. 8:409–420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cohen Y, Singer G, Lavie O, Dong SM,
Beller U and Sidransky D: The RASSF1A tumor suppressor gene is
commonly inactivated in adenocarcinoma of the uterine cervix. Clin
Cancer Res. 9:2981–2984. 2003.PubMed/NCBI
|
|
35
|
Yu MY, Tong JH, Chan PK, Lee TL, Chan MW,
Chan AW, Lo KW and To KF: Hypermethylation of the tumor suppressor
gene RASSFIA and frequent concomitant loss of heterozygosity at
3p21 in cervical cancers. Int J Cancer. 105:204–209. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo W, Cui L, Wang C, Guo Y, Shen S, Kuang
G and Dong Z: Decreased expression of RASSF1A and up-regulation of
RASSF1C is associated with esophageal squamous cell carcinoma. Clin
Exp Metastasis. 31:521–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Numoto K, Yoshida A, Sugihara S, Kunisada
T, Morimoto Y, Yoneda Y, Fujita Y, Nishida K, Ouchida M and Ozaki
T: Frequent methylation of RASSF1A in synovial sarcoma and the
anti-tumor effects of 5-aza-2′-deoxycytidine against synovial
sarcoma cell lines. J Cancer Res Clin Oncol. 136:17–25. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Borden EC: Augmentation of effects of
interferon-stimulated genes by reversal of epigenetic silencing:
Potential application to melanoma. Cytokine Growth Factor Rev.
18:491–501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deshpande R, Mansara P and Kaul-Ghanekar
R: Alpha-linolenic acid regulates Cox2/VEGF/MAP kinase pathway and
decreases the expression of HPV oncoproteins E6/E7 through
restoration of p53 and Rb expression in human cervical cancer cell
lines. Tumour Biol. 37:3295–3305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shaikh F, Sanehi P and Rawal R: Molecular
screening of compounds to the predicted Protein-Protein Interaction
site of Rb1-E7 with p53- E6 in HPV. Bioinformation. 8:607–612.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Farthing AJ and Vousden KH: Functions of
human papillomavirus E6 and E7 oncoproteins. Trends Microbiol.
2:170–174. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Naryshkin S and Austin RM: Limitations of
widely used high-risk human papillomavirus laboratory-developed
testing in cervical cancer screening. Drug Healthc Patient Saf.
4:167–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nocon M, Roll S, Mittendorf T, von der
Schulenburg JM and Willich SN: Human papilloma virus (HPV) testing
for cervical carcinoma screening. Z Evid Fortbild Qual Gesundhwes.
104:138–142. 2010.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Feng W, Shen L, Wen S, Rosen DG, Jelinek
J, Hu X, Huan S, Huang M, Liu J, Sahin AA, et al: Correlation
between CpG methylation profiles and hormone receptor status in
breast cancers. Breast Cancer Res. 9:R572007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Honda S, Haruta M, Sugawara W, Sasaki F,
Ohira M, Matsunaga T, Yamaoka H, Horie H, Ohnuma N, Nakagawara A,
et al: The methylation status of RASSF1A promoter predicts
responsiveness to chemotherapy and eventual cure in hepatoblastoma
patients. Int J Cancer. 123:1117–1125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lai HC, Lin YW, Chang CC, Wang HC, Chu TW,
Yu MH and Chu TY: Hypermethylation of two consecutive tumor
suppressor genes, BLU and RASSF1A, located at 3p21.3 in cervical
neoplasias. Gynecol Oncol. 104:629–635. 2007. View Article : Google Scholar : PubMed/NCBI
|