Introduction
Myeloproliferative neoplasms (MPNs) are a group of
clinically related diseases characterized by extensive
proliferation and expansion of one or more myeloid cell lineages in
the bone marrow and peripheral blood, with relatively preserved
differentiation (1,2). MPNs are divided into Philadelphia
chromosome (BCR-ABL) positive (Ph+) chronic myeloid
leukemia (CML) and Philadelphia negative (Ph−) disorders
including polycythemia vera (PV), essential thrombocythemia (ET),
and primary myelofibrosis (PMF) (3). From the clinical and histopathological
standpoints, the differentiation between the various types of MPN
often presents difficulties, especially in cases of fibrosis or
thrombocytosis. In addition, PV, ET and PMF can progress, usually
after several years, to secondary myelofibrosis or, in rare cases,
into acute leukemia (AML) (4–6). These
secondary AML are associated with an extremely poor prognosis
compared to de novo AML (4).
The genetic cause of MPNs was not known until 2005,
when four independent groups (7–10)
published that an identical, point mutation in Janus kinase 2 gene
(JAK2), G to T substitution, at position 1849, was present
in the majority of Ph-negative patients. This mutation occurs
within the JAK homology domain 2 (JH2) pseudokinase and leads to
substitution of valine by phenylalanine at codon 617 (V617F) of
JAK2 tyrosine kinase (7–9,11).
This mutation causes constitutive phosphorylation of JAK2 protein
and activation of substrate molecules such as STAT proteins and ERK
(12). JAK2V617F mutation has been
identified as a unifying event for typical MPNs, rarely occurring
in other myeloid malignancies (13–16).
The frequency of JAK2V617F version is approximately 65–97% in
patients with PV, approximately 23–57% with ET, and 35–57% with PMF
(8–11). The discovery of JAK2V617F allowed
WHO to formulate a new definition of classification of MPNs, until
2008 called myeloproliferative disorders (17). JAK2 is a cytoplasmic protein kinase,
a member of the signal transducers located downstream of cellular
receptors such as EPO, IL-6, and GM-CSF receptor. JAK2 tyrosine
kinase plays a critical role in the proliferation and
differentiation of hematopoietic cells (18). In normal cells, ligand binding
induces oligomerization of the receptor subunits, and subsequent
activation of the JAKs. The JAK2V617F mutation causes a
constitutive activation of JAK2 (19) and enhanced proliferation capacity
even in the absence of the ligand (8,9,11). The
pathogenic significance of JAK2V617F has been demonstrated in a
mouse model whereby transplanted bone marrow cells containing
JAK2V617F led to the development of MPN features in the recipient
mice (11,20). It is noteworthy that high levels of
retroviral expression of JAK2V617F in transgenic murine models have
been associated with the development of PV-like phenotype (11,20,21),
whereas lower levels of JAK2V617F expression has induced in mice
different MPN phenotypes, including ET and PMF-like disorders
(22,23).
Nevertheless, the identification of the
JAK2V617F mutation was a cardinal step in distinguishing
BCR/ABL negative disorders from other hematologic diseases, the
mechanisms of MPNs development are not thoroughly understood.
Hence, further cellular and genetic characterization is needed.
Cellular characterization is important especially for all
pathomorphological laboratories, which are not well equipped with
molecular biology assay tools, and immunohistochemistry is widely
available. However, the discovery of JAK2V617F mutation has
inspired an intensive search for novel molecular changes in MPNs,
because it is not yet clear how single mutation can generate at
least three different phenotypes (PV, ET and PMF) and also some
other myeloid neoplasms with different clinical features and
prognosis (15,16,24,25).
It is also unclear how it is possible that patients without V617F
mutation in JAK2 gene can develop MPNs. That is why we
searched for other factors in members of the JAK2 kinase signal
transduction pathways, which could be markers distinguishing PV, ET
and PMF entities; factors that may be involved in these
pathological processes.
In our studies we collected data from a series of
132 patients with PV, ET, PMF and also from 17 healthy individuals
as control group and then addressed the issue of whether i) the
presence, distribution and protein expression level of transducer
activator of transcription 5 (STAT5) and extracellular
signal-regulated kinases (ERK) 1 and 2, and ii) gene expression
level of STAT5a and ERK2 in bone marrow trephine biopsies could
clearly distinguish between each MPN. We selected these molecules,
because they are the elements of JAK2/STAT and JAK2/Raf/MEK/ERK
signalling pathways, which probably plays a critical role in the
promotion of hematopoietic cells growth, prevention of apoptosis
and consequently contribute to induction of MPNs. These pathways
are constructively phosphorylated by V617F form of JAK2 kinase
(9,11,26).
Materials and methods
Bone marrow study group
The trephine biopsies were independently diagnosed
at the Department of Clinical Pathology in Bydgoszcz by two
experienced pathologists (A.M. and G.D.) based on the
histopathological typing of the World Health Organization. Patient
samples were collected between 2008 and 2013 in the Department of
Clinical Pathomorphology of Dr Antoni Jurasz, University Hospital
in Bydgoszcz. From more than 1000 cases, we selected 132 patient
samples for this study. Out of these, 52 samples derived from
patients suffered from PV (37 with JAK2V617F mutation), 41 from ET
(22 with JAK2V617F mutation), and 22 from PMF (10 with JAK2V617F
mutation) and 17 healthy controls with no signs of neoplastic
proliferation.
Histopathological analysis
The bone marrow trephine biopsies were fixed in 5%
neutral buffered formalin (pH 7.6) for 24 h, at 4°C and then
decalcificated with 0.1 M ethylenediaminetetraacetic acid (EDTA) in
phosphate-buffered saline (PBS) for 24 h at 4°C. Then samples were
dehydrated, passing through several changes of xylene and finally
embedded in paraffin wax. This method has been found to offer
excellent morphology, good antigen for immunohistochemical (IHC)
reaction, satisfactory DNA and even RNA preservation for molecular
studies (27). Fixation in neutral
buffered formalin and decalcification with EDTA is also recommended
by International Council for Standardization in Hematology (ICSH).
Tissue samples for accurate pathological diagnosis were analyzed in
3–4 µm sections stained with H&E, Gomori silver impregnation,
periodic acid-Schiff (PAS), Masson trichrome and other histological
stains. Then slides were viewed under light microscopy (BX51,
Olympus Polska, Warszawa, Poland). The study was approved by the
ethics committee of the Collegium Medicum in Bydgoszcz Nicolaus
Copernicus University in Torun (approval no. 267/10).
Immunohistochemistry for pSTAT5, ERK2
and total ERK1/2 localization
The tissues were cut with an Accu-Cut SRM 200 rotary
microtome (Sakura Finetek Europe B.V., Olympus Polska) into 3–4 µm
sections, which were collected onto electrostatically charged
Superfrost Plus glass slides (Thermo Scientific, Life Technologies,
Polska, Warszawa, Poland) and dried on a warm plate. IHC reactions
were performed with the use of several monoclonal antibodies in
order to detect specific proteins within the cell components of
bone marrow tissues. These were anti-pSTAT5, anti-ERK2 and
anti-ERK1/2. The antibodies are characterized in detail in Table I.
 | Table I.Antibodies used to detect respective
elements of JAK2 kinase pathway during IHC and dot-blot
reactions. |
Table I.
Antibodies used to detect respective
elements of JAK2 kinase pathway during IHC and dot-blot
reactions.
| Antibody | Manufacturer
catalogue no. | Dilution for in
situ reaction | Dilution for
dot-blot reaction | Specificity |
|---|
| pSTAT5 monoclonal
rabbit anti-STAT5 (phospho Y694) | Abcam; ab32364 | 1:800 | 1:500 | The antibody only
detects Stat5 phosphorylated on tyrosine 694. |
| ERK2 polyclonal
rabbit anti-ERK2 | Abcam; ab72096 | 1:50 | 1:600 | Antibody detecting
the epitope corresponding to a site near C-terminal of these
molecule. |
| ERK1/2 polyclonal
rabbit anti-p44/42 MAPK (ERK1/2) | Cell Signalling
Technology; 9102 | 1:300 | 1:1500 | Antibody detects
endogenous levels of total p44 (ERK1) and p42 (ERK2) proteins. In
some cell types, antibody recognizes ERK1 more easily then ERK2
molecules. Antibody is produced by immunizing rabbit with a
synthetic peptide corresponding to a sequence in the C-terminus of
rat p44 MAPK. |
| GAPDH monoclonal
mouse anti-GAPDH | Abcam ab9484 | – | 1:4000 | Full length native
protein (purified) corresponding to human GAPDH |
Paraffin was removed from tissue sections by passing
the slides through several changes of xylene, followed by tissue
rehydratation in graded ethanols and finally in distilled water
solutions. Antigens retrieval was carried out in water bath using
Dako retrieval solution pH 9.0, at 95°C for 40 min. After cooling
and rinsing in 0.01 M PBS buffer (0.138 M NaCl, 0.03 M
Na2HPO4, 0.002 M
KH2PO4, pH 7.4), the slides were incubated
with 3% H2O2 for 10 min for block endogenous
peroxidases activity. Non-specific binding sites were blocked by
0.01 M PBS containing 5% bovine serum albumin (BSA; Sigma-Aldrich,
Poznań, Poland) for 10 min. Then the primary antibodies, diluted in
0.01 M PBS supplemented with 1% BSA (Table I) were applied for 16 h at 4°C. At
the next step, sections were incubated with secondary anti-rabbit
antibody coupled to peroxidase (EnVision system, Dako, Gdynia,
Poland), for 1 h. Between each step, the sections were rinsed in
pure PBS buffer. Reaction products were visualized with 3,3′-
diaminobenzidine chromogen (DAB; Dako) for 5 min. The reaction was
stopped by using distilled water. Next, the slides were
counterstained with Mayer's haematoxylin, dehydrated, cleared in
xylene and finally mounted in Shandon Consul-Mount (Thermo
Scientific, Life Technologies Polska).
All reactions took place at room temperature, except
for incubation with antigens retrieval solution and with the panel
of primary antibodies (Table I).
The incubation with primary and secondary antibodies as well as
with DAB was performed in a humid chamber. Negative control
reactions were performed by leaving out the incubation with primary
antibodies which were replaced by 1% BSA in 0.01 M PBS.
Morphometric analysis
Interpretation of IHC staining was performed as
described in detail earlier (28,29).
Remmele and Stegner (30) scoring
system was used. The slides were viewed under light microscope
Eclipse E800 (Nikon Instruments Europe, Amsterdam, The Netherlands)
at a magnification of ×400 and analyzed with the NIS-Elements 3.30
software (Nikon).
Preparation of protein extract and dot
blot reaction
Proteins isolated from trephine biopsies derived
from patients with ET, PV and PMF and from healthy control group
were analyzed by using dot-blot assay. This technique allowed for
immunodetection of pSTAT5, ERK2 and ERK1/2 antigens and their
quantitative estimate in total protein extract.
Dot blot analysis was performed as previously
described (29) with some
modifications. In brief, the 10 µm tissue sections were cut with an
Accu-Cut SRM 200 rotary microtome. Depending on the size of
patients trephine biopsy 5–7 sections to one protein extraction
were used. The proteins were isolated by using Qproteome FFPE
Tissue kit (Qiagen, Alab-Gen, Warszawa, Poland) dedicated to
formalin-fixed, paraffin-embedded tissues, in accordance with the
provided protocol. All proteins concentrations ranged between
25–120 µg/µl and were determined by using a Micro BCA Protein Assay
kit (no. 23235; Thermo Fisher Scientific, Life Technologies
Polska). It should be mentioned that the quality of obtained
proteins was lower than protein quality isolated from fresh, not
fixed in formalin and not embedded in paraffin tissue material.
Protein quality was checked by using SDS-PAGE electrophoresis and
staining protein gel with Coomassie Blue dye. Similar protein
quality is recommended by the kit manufacturer Qiagen (Qproteome
FFPE Tissue, Alab-Gen). Finally, for dot-blot assay only slightly
degraded protein was used. The reaction was carried out on the
protein isolated from 25 patients with PV (15 with JAKV617F
mutation), 20 with ET (10 with JAKV617F mutation), 19 with PMF (9
with JAKV617F mutation) and also from 12 healthy persons.
Proteins were diluted to 30 µg/µl. Two microliters
(60 µg) of each protein were slowly spotted onto membrane (Bio-Rad
Laboratories, Warszawa, Poland) and air-dried. Then membranes were
incubated with 5% BSA in TBST containing 0.1% Tween-20 for 40 min
at 25°C. After the blocking, the membranes were incubated overnight
at 4°C with the respective primary antibodies: anti-STAT5,
anti-ERK2, anti-ERK1/2 and anti-GAPDH antibodies (all described in
detail in Table I). Then the
membranes were washed 5 times in TBST buffer and incubated with
secondary alkaline phosphatase conjugated antibody purchased from
Sigma-Aldrich: mouse anti-rabbit (no. A2306) or rabbit anti-mouse
(no. A4312). Both antibodies were diluted 1:4000 in TBST buffer
with 0.2% BSA for 2 h at 22°C. After rinsing in TBST buffer the
dots were visualized by developing the membranes in substrate for
alkaline phosphatase (NCBI/BCIP, no. B3679, Sigma-Aldrich, for 5
min, at 22°C. The reaction was stopped by washing several times in
distilled water. The expression of pSTAT5, ERK2 and ERK1/2 proteins
was comparable to those seen for GAPDH and analyzed by using ImageJ
software.
RNA isolation/reverse transcription
reaction/relative quantification of STAT5a and ERK2 genes
RT-qPCR was performed to determine the transcription
level of STAT5a and ERK2 genes in bone marrow samples (obtained by
trephine biopsies) derived from patients with PV, ET, PMF and
healthy individuals. The two genes mentioned above were selected
for RT-qPCR analysis because after performing by the IHC and
dot-blot reactions, STAT5a and ERK proteins encoded by these STAT5a
and ERK2 genes showed differential expression levels in various
disease entities within MPN.
Total RNA was extracted from seven 10 µm
paraffin-wax sections using NucleoSpin FFPE RNA kit
(Marcherey-Nagel, Aqua Lab, Warszawa, Poland) according to the
instructions provided by manufacturer. Then RNA was treated with
DNase to remove any contaminating genomic DNA, by using RNasy Mini
kit (no. 74106, Qiagen, Alab-Gen) according to manufacturer's
instructions. The average yields of RNA isolation depending on the
size of the biopsy was 20 µg. For further analysis only RNA on the
quality of a specific absorbance ratio A260/A280 comprised in the
range 1.90–1.98 was used. These values were measured
spectrophotometrically using a NanoDrop ND-2000 spectrophotometer
(Thermo Fisher Scientific, Life Technologies Polska). Finally for
RT-qPCR the RNA isolated from 20 patients with PV (14 with JAKV617F
mutation), 17 with ET (10 with JAKV617F mutation), 14 with PMF (6
with JAKV617F mutation) and also from 11 healthy persons were
used.
For cDNA synthesis 200 ng purified RNA and First
Strand cDNA Synthesis kit (Thermo Scientific, Life Technologies,
Polska) was used according to the manufacturer's instructions.
Real-time PCR was performed in the final volume of 25 µl, in
LightCycler 480 detection system (Roche Polska, Warszawa, Poland).
The components of each reaction were: 100 ng cDNA as template, 300
nM forward and reverse primer (appropriate to the amplified
sequence, Table II) and 1X Master
mix (LightCycler 480 SYBR Green I Master; nr. 04 887 352 001; Roche
Polska) including FastStart Taq DNA polymerase, reaction buffer,
dNTP, MgCl2 and SYBR Green I Dye. Primer pairs to
STAT5a, ERK2 and housekeeping genes were designed on the 5′ border
of one exon and 3′ border of the neighbouring exon to minimize the
probability of DNA amplification instead of cDNA by using Primer3
software. Primer sequences are presented in Table II. A non-template control
(RNase-free water) was included on each 96-well plate.
Amplification was carried out for 45 cycles, and PCR cycle
parameters were: i) for STAT5a: denaturation at 95°C for 15 sec;
annealing at 56°C for 35 sec; extension at 72°C for 5 sec. ii) for
ERK2: denaturation at 95°C for 15 sec; annealing at 58°C for 45
sec; extension at 72°C for 10 sec.
 | Table II.Primer sequences used for real-time
PCR. |
Table II.
Primer sequences used for real-time
PCR.
| Gene/sequence
identification no. in GeneBank | Primer sequence
(5′-3′) | Size of qPCR
amplicon |
|---|
| GAPDH | Forward (F):
AGCCGAGCCACATCGCT | 125 bp |
| M17851.1 | Reverse (R):
TGGCAACAATATCCACTTTACCAGAGT |
|
| TBP | Forward (F):
GCACAGGAGCCAAGAGTGAA | 127 bp |
| M55654.1 | Reverse (R):
TCACAGCTCCCCACCATGTT |
|
| β-actin | Forward (F):
CCCCGCGAGCACAGA | 171 bp |
| NM_001101.3 | Reverse (R):
CCACGATGGAGGGGAAGAC |
|
| PRGK1 | Forward (F):
GGGAAAAGATGCTTCTGGGAA | 75 bp |
| NM_000291.3 | Reverse (R):
TTGGAAAGTGAAGCTCGGAAA |
|
| STAT5a | Forward (F):
GGCAAGGCCTGTAGAGAGTT | 76 bp |
| NM_003152.3 | Reverse (R):
TTGAGCCCCTTCAGAAAAGTCC |
|
| ERK2 | Forward (F):
CGGAACTTGCAATCCTCAGT | 76 bp |
| NM_003154.4 | Reverse (R):
TCGTGTGGGTCCTGAATTGG |
|
Additionally, in each qPCR reaction we carried out a
melting curve, according to the follow program: 95°C for 7 sec;
65°C for 1 min, to assess whether qPCR had generated specific and
single reaction product.
Housekeeping gene to all qPCR experiments was
selected from among four conventionally used genes to that purpose:
glyceraldehydes-3-phosphate dehydrogenase (GAPDH), TATA box-binding
gene (TBP), β-actin and protein kinase cGMP-dependent, type I
(PRGK1). Finally, based on Ct values, we chose the first
of these because the GAPDH gene expression was similar in all
tissues analyzed. Therefore, the mRNA levels of STAT5a and ERK2 are
expressed as the ratio of STAT5a/GAPDH and ERK2/GAPDH,
respectively. The relative quantification was performed using the
∆∆Ct method (31). As
calibrator we used cDNA from healthy patients. The primer sequences
used for the real-time PCR to GAPDH, TBP, β-actin and PRGK1 are
listed in Table II.
Statistical analysis
Statistical analysis of the results was performed
using Statistica 9.0 software. The distribution of all results was
analysed with Kolmogorov-Smirnov test with Lilliefors correction
and with the Shapiro-Wilk test. Finally, the significance of
differences was analysed with the use of non-parametric
Kruskal-Wallis test for multiple comparison for results with
non-normal distribution. Differences were considered statistically
significant at a p-value <0.05.
Results
The presence, spatial distribution and relative
abundance of STAT5 and ERK proteins which are the elements of
JAK2/STAT and Raf/MEK/ERK signalling pathways were traced by the
IHC and dot-blot detection by using monoclonal (phosphoSTAT5) and
polyclonal (ERK2 and ERK1/2) antibodies. Additionally, relative
expression level of pSTAT5a and ERK2 gene were examined in order to
answer the question whether expression levels of pSTAT5 and ERK2
proteins and their mRNA level overlap. Three types of MPNs were
involved in the study with the aim to establish whether the
expression level of the studied proteins and genes are similar or
different between PV, ET, PMF and control cases. Furthermore, it
was also important to determine whether there are differences in
expression of the studied proteins and genes between tissues with
wild-type and V617F version of JAK2 kinase.
Correlation between the presence of
V617F and expression of pSTAT5a protein
Generally, IHC detection of STAT5 protein,
phosphorylated on tyrosine residue 694 (Tyr694) under the light
microscope revealed less pronounced expression level in patients
with wild-type of JAK2 kinase (Fig.
1A-C), as compared to the patients expressing JAK2 with V617F
mutation (Fig. 1E-G). However,
these differences were not statistically significant
(p=0.566–0.956; Table III).
Results were statistically significant when we compared pSTAT5
expression level between control cases and PV or ET with wild-type
of JAK2 kinase (p=0.001 and 0.003, respectively; Table III); and between control cases and
PV or ET with V617F mutation (p=0.001 for both subtypes of MPNs;
Table III).
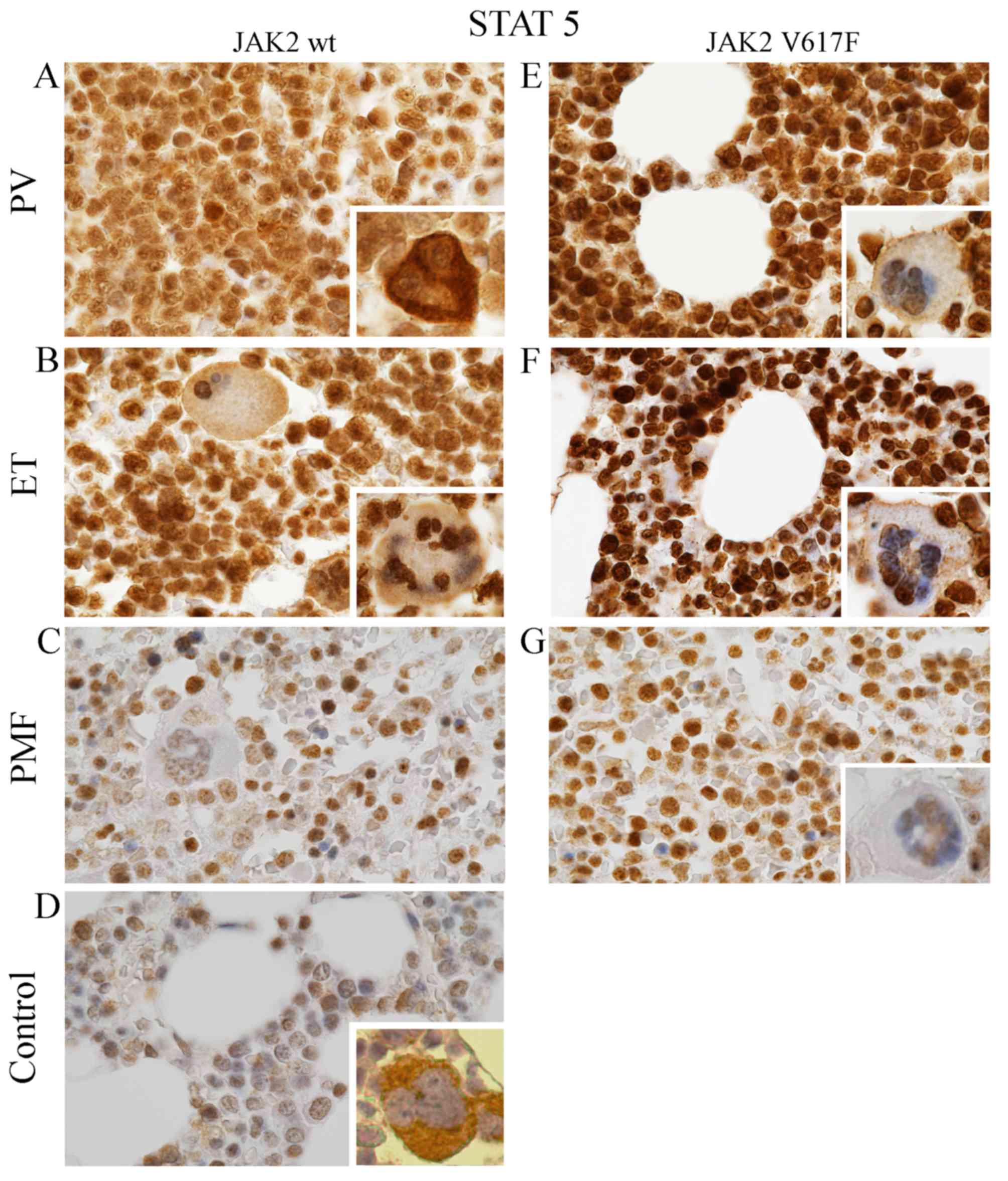 | Figure 1.Expression of pSTAT5 protein in MPNs
and in normal cases. Bone marrow trephine specimens derived from
patients both with wild-type (A-D) and mutated (E-G) form of JAK2
kinase were evaluated by using Remmele and Stegner scoring system
(30). Signal was different in
intensity and intracellular localization. Generally, pSTAT5
expression was distinctly lower in samples with wild-type JAK2
(A-D) compared to JAK2V617F (E-G). Moreover, positive expression of
this antigen was relatively high in PV (A and E) and ET samples (B
and F) compared to PMF (C and G) and to control cases (D). Except
PV with wild-type JAK2 (A inside image), pSTAT5 was mainly
localized in the nuclei of MPNs megakaryocytes, whereas the
cytoplasm is only slightly labelled (B and C, E-F, mainly inside
images) or free of pSTAT5 antigens (G inside). Inversely, in
control cases (D, inside), pSTAT5 is primarily located in the
cytoplasm of megakaryocytes. Similarly in PV with JAK2-negative,
pSTAT5 antigens is primarily localized in the cytoplasm, but the
signal from the nuclei is lower (A, inside images). All images were
taken under microscopic magnification of ×1000. |
 | Table III.Comparative scoring of pSTAT5, ERK2
and pERK1/2 in patients with PV, ET, PMF and patients from control
group. All results manifest non-normal distribution and are
presented in the form of median and upper-lower quartiles
range. |
Table III.
Comparative scoring of pSTAT5, ERK2
and pERK1/2 in patients with PV, ET, PMF and patients from control
group. All results manifest non-normal distribution and are
presented in the form of median and upper-lower quartiles
range.
|
|
| Expression level of
group 1 |
| Expression level of
group 2 |
|
|---|
|
|
|
|
|
|
|
|---|
| Antigen | Group 1 | Median | Upper-lower
quartiles | Group 2 | Median | Upper-lower
quartiles | Significance
between group 1 and 2 (P-value) |
|---|
| pSTAT5 | PV
JAK2− | 10.00 | 9.6–10.40 | PV
JAK2+ | 11.70 | 11.35–11.90 | 0.956b |
|
| ET
JAK2− | 8.40 | 8.0–9.00 | ET
JAK2+ | 11.40 | 11.0–11.70 | 0.566b |
|
| PMF
JAK2− | 5.20 | 4.6–5.70 | PMF
JAK2+ | 6.10 | 5.6–6.30 | 0.730b |
|
| PV
JAK2− | 10.00 | 9.6–10.40 | Control | 3.15 | 2.6–3.60 | 0.001a |
|
| ET
JAK2− | 8.40 | 8.0–9.00 | Control | 3.15 | 2.6–3.60 | 0.003a |
|
| PMF
JAK2− | 5.20 | 4.6–5.70 | Control | 3.15 | 2.6–3.60 | 0.430b |
|
| PV
JAK2+ | 11.70 | 11.35–11.90 | Control | 3.15 | 2.6–3.60 | 0.001a |
|
| ET
JAK2+ | 11.40 | 11.0–11.70 | Control | 3.15 | 2.6–3.60 | 0.001a |
|
| PMF
JAK2+ | 6.10 | 5.6–6.30 | Control | 3.15 | 2.6–3.60 | 0.574b |
|
| PV
JAK2− | 10.00 | 9.6–10.40 | ET
JAK2− | 8.40 | 8.0–9.00 | 1.000b |
|
| PV
JAK2− | 10.00 | 9.6–10.40 | PMF
JAK2− | 5.20 | 4.6–5.70 | 0.001a |
|
| ET
JAK2− | 8.40 | 8.0–9.00 | PMF
JAK2− | 5.20 | 4.6–5.70 | 0.004a |
|
| PV
JAK2+ | 11.70 | 11.35–11.90 | ET
JAK2+ | 11.40 | 11.0–11.70 | 1.000b |
|
| PV
JAK2+ | 11.70 | 11.35–11.90 | PMF
JAK2+ | 6.10 | 5.6–6.30 | 0.001a |
|
| ET
JAK2+ | 11.40 | 11.0–11.70 | PMF
JAK2+ | 6.10 | 5.6–6.30 | 0.001a |
| ERK2 | PV
JAK2− | 7.25 | 7.05–8.80 | PV
JAK2+ | 4.40 | 3.3–4.60 | 0.005a |
|
| PV
JAK2− | 7.25 | 7.05–8.80 | ET | 11.25 | 11.0–11.30 | 0.425b |
|
| PV
JAK2− | 7.25 | 7.05–8.80 | PMF | 6.80 | 6.2–7.50 | 0.931b |
|
| PV
JAK2− | 7.25 | 7.05–8.80 | Control | 8.55 | 7.6–9.60 | 1.000b |
|
| PV
JAK2+ | 4.40 | 3.3–4.60 | ET | 11.25 | 11.0–11.30 | 0.001a |
|
| PV
JAK2+ | 4.40 | 3.3–4.60 | PMF | 6.80 | 6.2–7.50 | 1.000b |
|
| PV
JAK2+ | 4.40 | 3.3–4.60 | Control | 8.55 | 7.6–9.60 | 0.003a |
|
| ET | 11.25 | 11.0–11.30 | PMF | 6.80 | 6.2–7.50 | 0.958b |
|
| ET | 11.25 | 11.0–11.30 | Control | 8.55 | 7.6–9.60 | 0.630b |
|
| PMF | 6.80 | 6.2–7.50 | Control | 8.55 | 7.6–9.60 | 1.000b |
| ERK1/2 | PV | 7.10 | 6.95–8.00 | ET | 9.80 | 9.3–10.20 | 0.908b |
|
| PV | 7.10 | 6.95–8.00 | PMF | 7.20 | 6.9–7.40 | 1.000b |
|
| PV | 7.10 | 6.95–8.00 | Control | 10.20 | 9.7–11.00 | 0.830b |
|
| ET | 9.80 | 9.3–10.20 | PMF | 7.20 | 6.9–7.40 | 1.000b |
|
| ET | 9.80 | 9.3–10.20 | Control | 10.20 | 9.7–11.00 | 1.000b |
|
| PMF | 7.20 | 6.9–7.40 | Control | 10.20 | 9.7–11.00 | 0.986b |
Additionally, it should be distinctly noted that the
highest expression level of pSTAT5 protein was observed
predominantly in approximately 75% of the nucleus of bone marrow
cells expressing JAK2V617F (Fig.
1E-G). It was clearly seen mainly in ET (Fig. 1F) and slightly less pronounced in
PMF (Fig. 1G). Their nuclei were
rich in pSTAT5 antigen, and the cytoplasm was completely lacking
the signal or only scarcely labelled (Fig. 1F). In contrast, PV bone marrow cells
with V617 mutation were characterized by widespread distribution of
epitopes that recognized pSTAT5 antibody (Fig. 1E). Although they were present both
in the nucleus and in the cytoplasm, their intracellular expression
was relatively stronger in the nucleus (Fig. 1E). However, among studied MPNs with
wild-type JAK2 kinase, brown signal derived from pSTAT5 was
observed (similar to PV with JAK2V617F) both in the nucleus and in
the cytoplasm (Fig. 1A-C) but their
intracellular expression was on similar level (Fig. 1A-C). Thereby, the pSTAT5 signal
derived from bone marrow cells was homogeneous (Fig. 1A-C).
Additionally, we observed that, regardless of the
JAK2V617F presence, expression of pSTAT5 protein in PV and ET
concerned almost all bone marrow cells (Fig. 1A, B, E and F), whereas in PMF it was
observed only in approximately 50% bone marrow cells. Other cells
were unmarked (Fig. 1C and G).
Regarding the statistical significant relationships between
particular MPNs with wild-type of JAK2 kinase and between
particular MPNs with JAKV617F mutation, statistical significance
was detected between expression level of pSTAT5 protein in PV and
PMF (p=0.001; Table III) and also
between ET and PMF (p=0.001–0.004; Table III). On the contrary, no
significant correlations were found between PV and ET (p=1.000;
Table III).
pSTAT5a protein expression on
megakaryocytes
Further differences observed between MPNs entities
are reflected in pSTAT5 expression pattern in megakaryocytes
(Fig. 1, only inside images). In
normal cases, pSTAT5 expression is limited to the cytoplasm of bone
marrow cells (Fig. 1D, inside).
While, in all analyzed MPNs, epitopes that bind STAT5 are mainly
localized in the nucleus of megakaryocytes (Fig. 1A-C and E-G, inside). However, the
cytoplasm of MPNs revealed different expression pattern of this
protein. In all analysed MPNs with JAK2 V617F positive, the
cytoplasm showed only trace amount of recognized epitope. In PV, ET
and PMF without JAK2 V617F mutation we could distinguish several
grades of expression: extremely rich in PV (Fig. 1A, inside), strong in ET (Fig. 1B, inside) and present in trace
amount or absence in PMF (Fig. 1C,
inside).
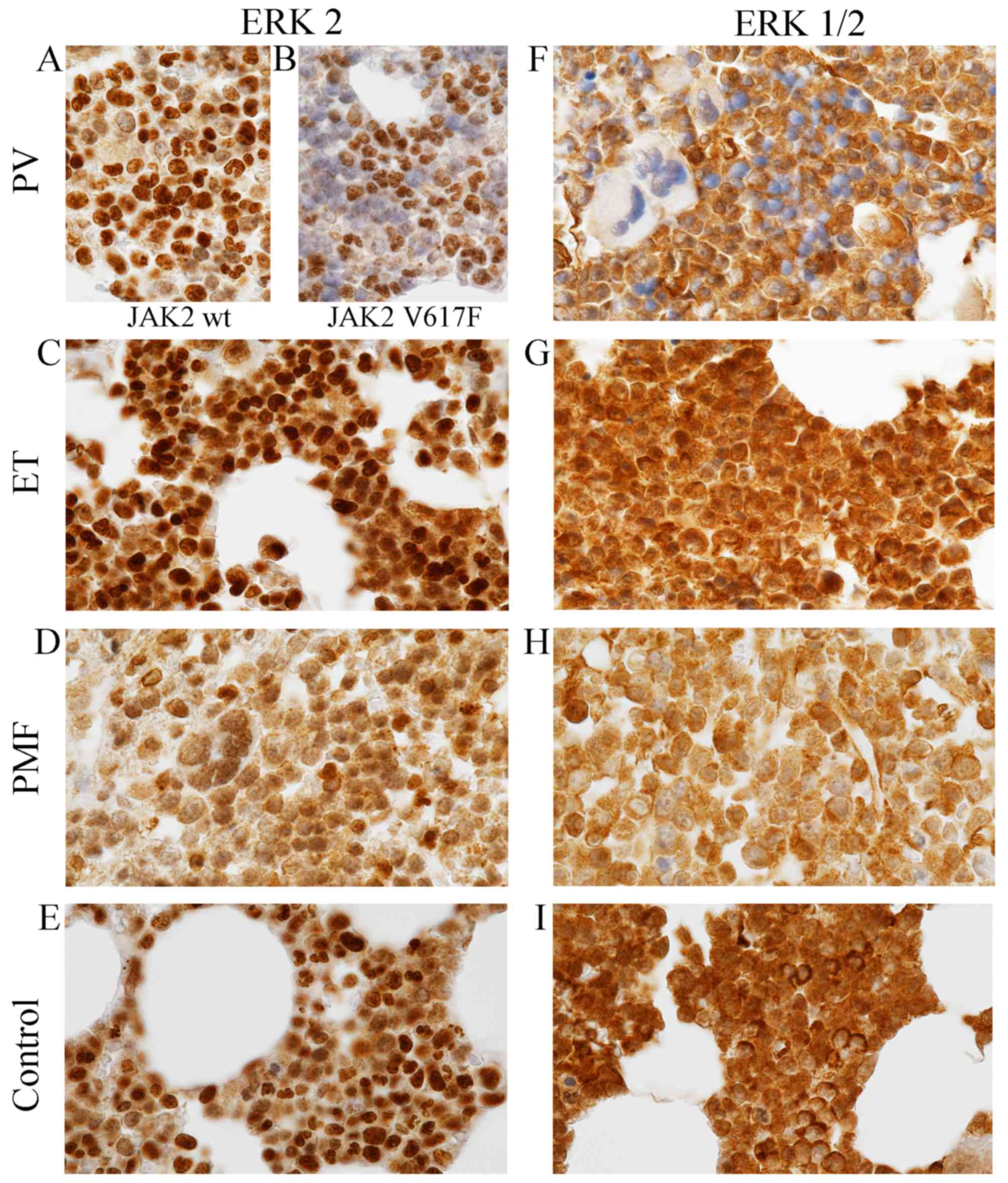
Diverse expression patterns of
unphosphorylated ERK2 and ERK1/2 antigens in MPNs
Immunohistochemical comparison of the three
disorders among MPNs revealed that both similarities and
differences exist between them in terms of presence, relative
abundance and cellular localization of two ERK antigens (Fig. 2; Tables III and IV). These antigens were recognized by two
different antibodies, ERK2 and ERK1/2 derived from Abcam and Cell
Signaling Technology, respectively. Detailed information on
specificity of antibodies used are presented in Table I.
 | Table IV.The presence and relative abundance
of ERK2 and ERK1/2 antigens in megakaryocytes observed after IHC
reaction onto slides derived from patients with PV, ET, PMF and
healthy controls. |
Table IV.
The presence and relative abundance
of ERK2 and ERK1/2 antigens in megakaryocytes observed after IHC
reaction onto slides derived from patients with PV, ET, PMF and
healthy controls.
|
| ERK2 | ERK1/2 |
|---|
|
|
|
|
|---|
| Patients | Nucleus | Cytoplasm | Nucleus | Cytoplasm |
|---|
| PV | ++/+++ | + | – | ++ |
| ET | +++ | ++ | ++ | ++ |
| PMF | + | + | + | +/++ |
| Control | ++ | + | ++ | ++/+++ |
The similarities were the relative abundance of
antigens recognized by ERK antibodies. Both antigens are richly
present within the bone marrow cells of PV, ET, PMF and healthy
individuals (Fig. 2; Tables III and IV), and small differences in their
expression levels were not statistically significant
(p=0.425–1.000; Table III),
except ERK2 expression level in PV. When we compared differences in
expression level of ERK2 between tissues with and without V617F
mutation of JAK2 kinase, results were statistically significant
(p=0.005). Additionally statistical differences concerning ERK2
expression level were indicated between PV with JAK2V617F and ET
(p=0.001) and between PV with JAK2-positive and control group
(p=0.003).
The most important differences, but not
statistically measured, are connected with intracellular
localization of ERK antigens. Although, the ERK proteins were
detected both in the nucleus and cytoplasm of bone marrow cells,
their expression level within these cell compartments were
different in each MPN entity (Fig.
2; Table IV).
The nucleus exhibits differential expression
patterns of ERK2 within the MPNs (Fig.
2; Table IV). The highest
signal derived from cell nucleus was observed in PV and ET
(Fig. 2A-C; Table IV), slightly lower in normal
trephine biopsies (Fig. 2E;
Table V), and the lowest in the PMF
(Fig. 2D, Table IV). Additionally, it is worth
noting that the highest expression levels of ERK2 antigens in PV
were observed only in approximately 30% nucleus with JAK2V617F
mutation and in approximately 80% nucleus without V617F version of
JAK2 kinase (Fig. 2A and B). The
cytoplasm of MPNs exhibit equally diverse pattern of ERK2 protein
expression level. Distinct differences were demonstrated in PV
between trephine biopsies with wild-type JAK2 (Fig. 2A; Table
IV) and JAK2V617F (Fig. 2B;
Table IV). In the first case, the
cytoplasm was characterized by relatively high expression level of
ERK2 (Fig. 2A; Table IV), whereas in the second case,
ERK2 epitopes were absent or only scarcely represented in the
cytoplasm (Fig. 2B; Table IV). In contrast to PV (Fig. 2A and B; Table IV), in the cytoplasm of ET, PMF and
control cells (Fig. 2C-E; Table IV) we did not observe differences
depending on presence JAK2V617F mutation concerning expression
pattern of ERK2 antibody. In ET they could be identified in the
cytoplasm in relatively high levels (Fig. 2C; Table
IV), slightly higher than in PMF and control cells (Fig. 2D and E; Table IV).
 | Table V.The presence and relative abundance
of phosphorylated STAT5 protein and unphosphorylated ERK2 and
ERK1/2 proteins in the protein extracts isolated from patients with
PV, ET, PMF and healthy controls. |
Table V.
The presence and relative abundance
of phosphorylated STAT5 protein and unphosphorylated ERK2 and
ERK1/2 proteins in the protein extracts isolated from patients with
PV, ET, PMF and healthy controls.
|
| phospho-STAT5 |
|
|
|---|
|
|
|
|
|
|---|
| Patients | JAK2 wt | JAK2 V617F | ERK2 | Total-ERK1/2 |
|---|
| PV | + | ++ | ++ | ++ |
| ET | + | ++ | ++ | ++ |
| PMF | + | ++ | ++ | ++ |
| Control | tr. | nd. | ++ | ++ |
A differ situation was observed in localization of
both ERK1 and ERK2 antigens recognized by ERK1/2 antibody (Fig. 2F-I; Table IV). In contrast to ERK2, the
expression patterns of ERK1/2 antibody in PV and other analysed
subtypes of MPN were independent of the presence of JAK2V617F
mutation. The analysed epitopes were not observed in most cell
nuclei of PV (Fig. 2F; Table IV), whereas in the nucleus of ET,
PMF and control group they occurred richly (Fig. 2G-I; Table IV). The cytoplasm of all examined
tissues were characterized by high expression of epitopes that
react with ERK1/2 antibody (Fig.
2F-I; Table IV). They could be
detected in high levels in the cytoplasm of ET and in normal cells
(Fig. 2G and I; Table IV); smaller amounts was present in
PV cells (Fig. 2F, Table IV) and only trace amounts could be
localized in PMF cells (Fig. 2H;
Table IV). Single cells of normal
trephine biopsies were extremely rich in these epitopes; expression
in these single cells was higher than expression in the nucleus of
these cells (Fig. 2I). The control
IHC reaction performed by omitted primary antibodies resulted in a
complete lack of the brown signal (not shown).
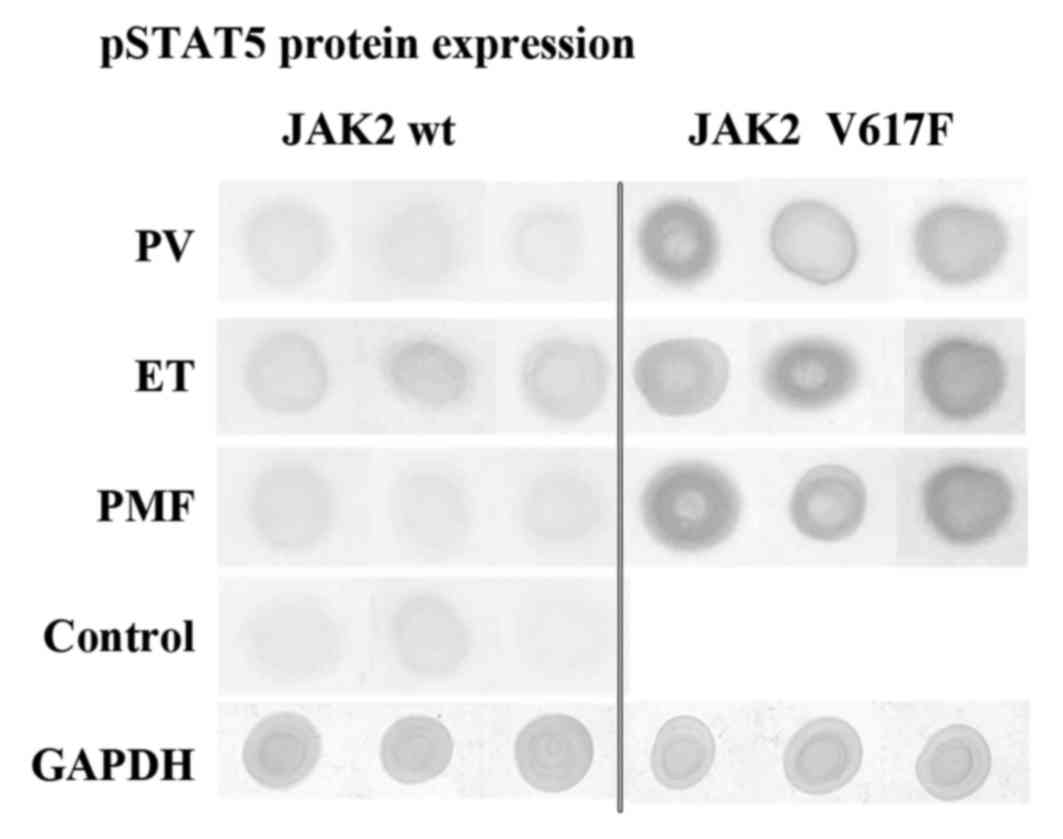
Dot blot assay results
Data obtained from our IHC studies was further
confronted by dot blot analysis. As illustrated in Fig. 3 and Table V, we observed that protein extracts
derived from JAK2V617F positive cases of PV, ET and PMF were
characterized by the abundant occurrence of pSTAT5 antigens
(Fig. 3). However, proteins from
JAK2 wild-type cases showed relatively lower pSTAT5 antigen
content. Control cases are only scarcely represented by protein of
interest (Fig. 3, Table V). These results coincide with the
results of IHC reactions (Fig. 1),
which are described in the Results section.
Additionally, we did not see differences between
expression levels of ERK antigens bound by ERK2 or ERK1/2
antibodies. These epitopes appear in abundance in all analysed
proteins, extracted from bone marrow tissues (Table V).
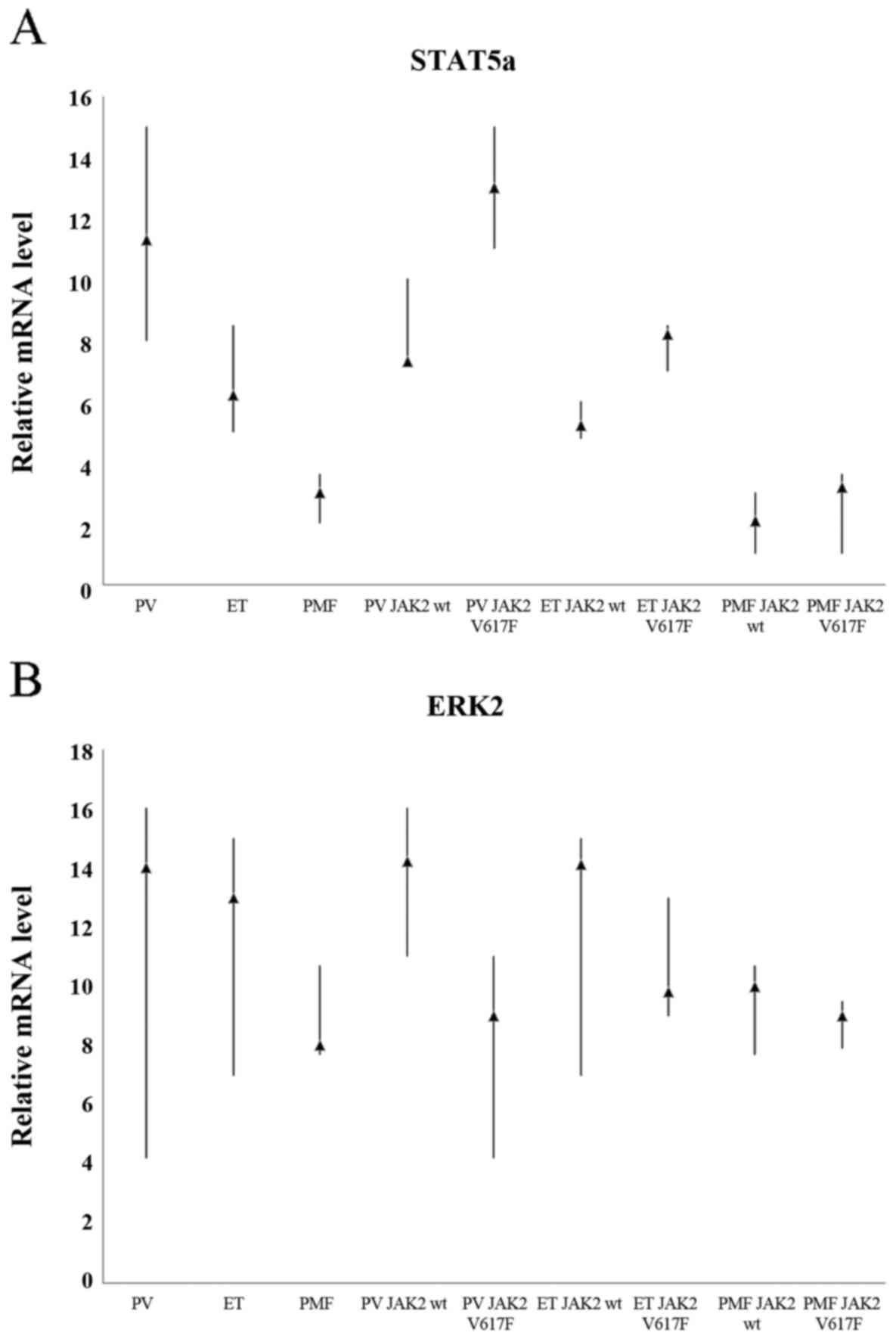
Comparison of the expression levels of
STAT5a gene between all analysed MPN subtypes
We determined whether statistically significant
differences of STAT5a and ERK2 protein expression, which were
observed between various disease among MPNs, after IHC and dot-blot
reactions are reflected in the RNA level.
Whichever of eleven calibrators was chosen in order
to determinate relative STAT5a gene expression level, the results
were always similar and statistically significant (p<0.001).
When we did not take into consideration JAK2V617F mutation status,
we could observe approximately 5-fold higher expression level of
STAT5a gene in PV in comparison to the patients with PMF and
approximately 2-fold higher than in ET. Similarly, patients with ET
displayed approximately 2-fold higher STAT5a mRNA levels compared
to patients with PMF (Fig. 4A).
Further comparison, revealed that in any case the highest STAT5a
gene expression level was positively correlated with the presence
of JAK2V617F mutation; patients who were tested positive for
JAK2V617F were characterized by high expression of STAT5a gene.
RT-qPCR results coincide with the results of the IHC and dot-blot
staining for the presence of phosphorylated form of STAT5
protein.
Relative levels of ERK2 transcripts
are similar in each of the three MPN subtypes
We also examined the expression levels of ERK2 gene
as shown in Fig. 4B. ERK2
expression in patients with ET and PV was approximately 1.5 times
higher as compared to patients with PMF. Whichever of the eleven
calibrators was selected, the results were similar and not
statistically significant (p=0.566–1.000; Table IV). These results only partially
overlap with IHC and dot-blot studies. Statistical analysis
confirmed the observations performed under a light microscope.
Discussion
The results of our study demonstrate different
levels as well as various expression patterns of antigens
recognized by phospho-STAT5, ERK2 and total ERK1/2 antibodies
within bone marrow trephine biopsies from patients with PV, ET, PMF
and in healthy subjects. Differential expressions of these proteins
were observed both in the megakaryocytes and in other haemopoietic
cells. It seems that these differences of proteins/genes
expressions, visible under the light microscope, onto membrane
after performing dot-blot reaction and after RT-qPCR could allow to
classify each sample to one of 3 types of MPNs in a simpler manner,
sometimes without checking JAK2V617F mutation status.
We believe that our findings are particularly
important, especially because, as it is well known, the most common
MPN JAK2V617F mutation alone cannot explain the phenotypic
heterogeneity of PV, ET and PMF. It should also be noted that even
in healthy persons, the V617F version of JAK2 kinase may be
recognize, but with a low frequency (10%). Phenotypic manifestation
of MPNs depends on V617F dosage, therefore in these people clinical
signs of MPNs were never developed (32). In the MPN patients who lack V617F
mutation, other but less common genetic alternations have been
described: including mutations in exon 12 of JAK2 (33) and in exon 10 of thrombopoietin
receptor gene (MPL) (34). The
first one may be found only in PV with a low frequency, estimated
at approximately 3% (33,35). The second alternation, resulting in
several common variants of mutation in codon 515 of MPL gene,
W515A/K/L, are described only in patients with ET and PMF but also
in only small percentage, approximately 1% and 10%, respectively
(34,36–38).
Furthermore, in ET and PMF patients other than the
above-mentioned mutations in MPL gene have been described: MPL
S505N, W515Ki, W515Kii, W515R and W515A. They were present both in
patients who are V617F-negative and V617F- positive, and also in
low frequency, up to 12% (39). To
date, in MPNs, aberrations were additionally described in several
subsequent genes, e.g. in TET2, ASXL1, DNMT3A, LNK IDH1, IDH2, CBL,
CALR, SH2B3 and EZH2 gene (40,41),
with different, but always relatively low frequency in PV, ET and
PMF entities, in JAK− and/or JAK+,
MPL− and/or MPL+ patients. The evidence
described above encourages to pursue other morphological and
molecular features to be used for better characterization of the
three phenotypes of MPN. This, in turn, can help to provide: a much
better understanding of MPNs pathophysiology, and the establishment
of appropriate diagnostic algorithm to monitor diseases.
We selected STAT5 molecule to our study because out
of seven members of STAT family (STAT1, STAT2, STAT3, STAT4,
STAT5a, STAT5b, STAT6) only three, STAT3, STAT5a and STAT5b were
predominantly phosphorylated by JAK2V617F kinase in hematopoietic
disorders (42). As a result of
phosphorylation, closely related STAT5a and STAT5b proteins are
activated. They form heterodimers (43) and then they are translocated from
the cytoplasm into the nucleus where they act as transcription
factors regulating multiple target gene expression with
anti-apoptotic function. These target genes are responsible for
cell cycle progression, cell growth and survival (43). JAK2V617F phosphorylate STAT5a only
at tyrosine 694 and STAT5b only at tyrosine 699. In addition, STAT5
proteins could also directly lead to the activation of RAF/MEK/ERK
signalling cascades, where ERK1 and ERK2 are downstream proteins
(44,45). These proteins were phosphorylated at
threonine 202 (ERK1) and tyrosine 204 (ERK2) and they are ready to
activate the next downstream elements of signaling pathway. Reduced
STAT5, STAT3 and ERK1/2 expression in human HEL cells with
JAK2V617F mutation, was observed as a result of JAK2 inhibition by
WP1066 inhibitor treatment (46).
This result shows that STAT5 and ERK proteins are associated with
JAK2 kinase pathway. In addition, Pircher and colleagues (44,45)
indicated that ERK proteins cooperate with STAT5a and they are
necessary for full activation of STAT5a protein.
Upon examining the bone marrow trephines derived
from patients with PV, ET and PMF we found some interesting data
concerning STAT5 protein and gene expressions. The first one and,
at the same time, the easiest to observe is not surprising, because
it had also been shown by others investigators. STAT5 protein
expression phosphorylated on 694 tyrosine residues was enhanced
only in MPNs with JAKV617F mutation. Similar results have been
reported by James et al (11) and Shide et al (23), who observed an increasing level of
phospho-STAT5 proteins in transgenic mice expressing JAKV617F.
Sometimes high level of STAT5 expression is used as surrogate
marker of JAKV617F mutation (47).
In our study we reported the differences in pSTAT5
protein expression level and intracellular expression pattern in
megakaryocytes and other hematopoietic cells. Previously, these
differences have not been described in detail. The differences in
expression level of STAT5 between the wild-type-JAK2 and JAK2V617F
were clear in almost every cases, but they were statistically
significant only for ET and PMF. We reported that in ET and PMF
patients, with JAK2V617F mutation, pSTAT5 antigens are concentrated
primarily in the nucleus of bone marrow cells, whereas the
cytoplasm is completely devoid of signal or only scarcely labelled
(Fig. 1F-G). However, in PV with
JAK2V617F and in PV, ET, PMF with wild-type JAK2, pSTAT5 is
observed both in the nucleus and in the cytoplasm of hematopoietic
cells (Fig. 1B), and the expression
pattern of these antigens is different in each disease among MPNs.
We have also observed different patterns of pSTAT5 protein
expression in megakaryocytes.
In most MPN cases (except PV with JAK2-negative),
pSTAT5 were present predominantly in the nucleus of megakaryocytes,
and their cytoplasm was usually free of signal (Fig. 1B and C, E-G, inside images).
However, in PV with wild-type of JAK2 kinase pSTAT5 gave positive
signal in both cell organelles, but in the cytoplasm expression was
more abundant (Fig. 1A, inside). As
reported by others (48,49), in the cytoplasm, STAT5a protein
cooperated with Gab2 protein and it was associated with cell
proliferation through activation of PIK3/AKT signalling cascade.
High nuclear expression of pSTAT5 in megakaryocytes of PV, ET and
PMF has already been reported and interpreted by others researchers
as abnormal (50) because pSTAT5
protein expression in megakaryocytes of healthy cases is restricted
only to the cytoplasm. The results of our experiment in this
respect are in agreement with previous reported by Gibson et
al (50). On the contrary,
Grimwade and co-authors (51) have
suggested that intracellular localization of STAT5 protein,
phosphorylated on 694 tyrosine residue, in megakaryocytes depends
on the type of pSTAT5 antibody used.
This work also demonstrated extremely high
transcription level of STAT5a gene by using RT-qPCR in patients
with JAK2V617 mutation compared to the individuals without this
mutation. Expression level of STAT5a gene in PV patients revealed
approximately 5 times higher RNA level compared to the patients
with PMF and approximately 2-fold higher than in ET. These results
were of course statistically significant, and simultaneously they
suggest that STAT5a RT-qPCR assay could be a good diagnostic tool
even without checking JAK2V617F status.
We suggest that when we bind these results from IHC
studies concerning phosphor-STAT5 protein expression with RT-qPCR
analysis, clear distinction of the three subtypes of MPNs is
possible and relatively simple. However, our studies should be
broaden by increasing the number of subjects in order to answer the
question whether different patterns of pSTAT5 expression are in
fact characteristic for each entities or not. To the best of our
knowledge, this kind of intracellular difference between the three
phenotypes of MPNs observed and described here by us has not been
described in detail before.
We also compared the expression of antigens
recognized by ERK2 and ERK1/2 antibodies in MPNs and in healthy
controls and we found a variety of differences. To the best of our
knowledge, this is the first report which demonstrated different
intracellular localization of ERK proteins in MPNs. At first, we
showed that ERK expression is independent of the presence of
JAK2V617F mutation, with one exception, in PV where clearly visible
differences in distribution of ERK2 antigens were visible (Fig. 2A and B). The highest expression
levels of ERK2 antigens were observed only in approximately 30% of
the nucleus with JAK2V617F mutation and in approximately 80% with
wild-type JAK2 kinase (Fig. 2A and
B). The cytoplasm in JAK2V617F cells is free of signal. This
kind of ERK2 expression pattern in PV patients allows to
distinguish tissue samples derived from patients with and without
JAK2V617F mutation. However, localization of both ERK1 and ERK2
antigens by using total ERK1/2 antibodies revealed opposite
results. Firstly, the expression was independent of the presence of
JAKV617F mutation and secondly it was localized only in the
cytoplasm. Nuclei were free of signal. Such significant differences
in intracellular localization of ERK2 and ERK1/2 antigens may
result from the fact that, as suggested by the manufacturer of
ERK1/2 antibodies, these molecules could recognize ERK1 more
readily than ERK2 antigens. It seems that in our experiment this
situation appears to be most likely. It is worth noting at this
moment that, as reported by others, ERK2 has pro-proliferative
effects on the cells, while ERK1 is anti-proliferative (52).
We concluded that differences in expression pattern
of ERK2 antigens may be helpful in distinguishing PV and ET from
PMF. ERK2 expression in PV and ET were relatively stronger than in
PMF and, additionally, concentrated rather in the nucleus (Fig. 2A-C). Cytoplasm was less marked. In
PMF, the intensity of signal both in nucleus and cytoplasm was on a
similar level (Fig. 2A-D). It
should be observed that dot-blot analysis and RT-qPCR did not
revealed differences between expression levels of ERK2 gene among
the MPN entities. In our opinion, using antibodies binding to both
ERK1 and ERK2 proteins also allows to distinguish PV and ET from
PMF. In most of PV cells, the signal is concentrated in the
cytoplasm, whereas in ET and PMF it was localized both in the
cytoplasm and nucleus. Additionally, we observed that in ET the
expression level is relatively stronger than in PMF but these
differences were not statistically significant. The mechanisms
leading to the accumulation of STAT5 and ERK2 proteins in the cell
nucleus remain poorly elucidated. We speculate that it could be
associated with the presence JAKV617F and/or MPL W515 alterations
because these molecules (i.e. JAK2 and MPL) are known as regulators
of JAK/STAT and RAF/MAP/ERK signalling pathways.
However, in future research, we should increase the
number of subjects and the number of antibodies directed to
different epitopes of STAT5 and ERK1/2 proteins and then again
describe in details the expression levels and patterns of these
antigens, similarly to this report. Here we used only small number
of cases of each disease and only one antibody to pSTAT5 protein
and two antibodies directed to ERK protein.
Acknowledgements
This study was supported by the Polish National
Science Center (NCN), project no. N N401 15 3538 and by the Grant
of Nicolaus Copernicus University, project no. 05/2010. The authors
would like to thank Anna Michalska-Orlik for her professional
linguistic editing of the manuscript.
References
|
1
|
Kavalerchik E, Goff D and Jamieson CH:
Chronic myeloid leukemia stem cells. J Clin Oncol. 26:2911–2915.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li S, Kralovics R, De Libero G,
Theocharides A, Gisslinger H and Skoda RC: Clonal heterogeneity in
polycythemia vera patients with JAK2 exon12 and JAK2-V617F
mutations. Blood. 111:3863–3866. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laibe S, Tadrist Z, Arnoulet C, Sainty D
and Mozziconacci MJ: A myeloproliferative disorder may hide another
one. Leuk Res. 33:1133–1136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tam CS, Nussenzveig RM, Popat U,
Bueso-Ramos CE, Thomas DA, Cortes JA, Champlin RE, Ciurea SE,
Manshouri T, Pierce SM, et al: The natural history and treatment
outcome of blast phase BCR-ABL- myeloproliferative neoplasms.
Blood. 112:1628–1637. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abdel-Wahab O, Mullally A, Hedvat C,
Garcia-Manero G, Patel J, Wadleigh M, Malinge S, Yao J, Kilpivaara
O, Bhat R, et al: Genetic characterization of TET1, TET2, and TET3
alterations in myeloid malignancies. Blood. 114:144–147. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Campbell PJ and Green AR: The
myeloproliferative disorders. N Engl J Med. 355:2452–2466. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baxter EJ, Scott LM, Campbell PJ, East C,
Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM and Curtin
N: Cancer Genome Project: Acquired mutation of the tyrosine kinase
JAK2 in human myeloproliferative disorders. Lancet. 365:1054–1061.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kralovics R, Passamonti F, Buser AS, Teo
SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M and Skoda RC: A
gain-of-function mutation of JAK2 in myeloproliferative disorders.
N Engl J Med. 352:1779–1790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Levine RL, Wadleigh M, Cools J, Ebert BL,
Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, et
al: Activating mutation in the tyrosine kinase JAK2 in polycythemia
vera, essential thrombocythemia, and myeloid metaplasia with
myelofibrosis. Cancer Cell. 7:387–397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz
SB and Zhao ZJ: Identification of an acquired JAK2 mutation in
polycythemia vera. J Biol Chem. 280:22788–22792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
James C, Ugo V, Le Couédic JP, Staerk J,
Delhommeau F, Lacout C, Garçon L, Raslova H, Berger R,
Bennaceur-Griscelli A, et al: A unique clonal JAK2 mutation leading
to constitutive signalling causes polycythaemia vera. Nature.
434:1144–1148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pradhan A, Lambert QT, Griner LN and
Reuther GW: Activation of JAK2-V617F by components of heterodimeric
cytokine receptors. J Biol Chem. 285:16651–16663. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dahabreh IJ, Giannouli S, Zoi C, Zoi K,
Voulgarelis M and Moutsopoulos HM: Management of hypereosinophilic
syndrome: A prospective study in the era of molecular genetics.
Medicine (Baltimore). 86:344–354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dahabreh IJ, Giannouli S, Zoi C, Zoi K,
Loukopoulos D and Voulgarelis M: Hypereosinophilic syndrome:
Another face of janus? Leuk Res. 32:1483–1485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jones AV, Kreil S, Zoi K, Waghorn K,
Curtis C, Zhang L, Score J, Seear R, Chase AJ, Grand FH, et al:
Widespread occurrence of the JAK2 V617F mutation in chronic
myeloproliferative disorders. Blood. 106:2162–2168. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Steensma DP, Dewald GW, Lasho TL, Powell
HL, McClure RF, Levine RL, Gilliland DG and Tefferi A: The JAK2
V617F activating tyrosine kinase mutation is an infrequent event in
both ‘atypical myeloproliferative disorders and myelodysplastic
syndromes. Blood. 106:1207–1209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tefferi A and Vardiman JW: Classification
and diagnosis of myeloproliferative neoplasms: The 2008 World
Health Organization criteria and point-of-care diagnostic
algorithms. Leukemia. 22:14–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baker SJ, Rane SG and Reddy EP:
Hematopoietic cytokine receptor signaling. Oncogene. 26:6724–6737.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morgan KJ and Gilliland DG: A role for
JAK2 mutations in myeloproliferative diseases. Annu Rev Med.
59:213–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wernig G, Mercher T, Okabe R, Levine RL,
Lee BH and Gilliland DG: Expression of Jak2V617F causes a
polycythemia vera-like disease with associated myelofibrosis in a
murine bone marrow transplant model. Blood. 107:4274–4281. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lacout C, Pisani DF, Tulliez M, Gachelin
FM, Vainchenker W and Villeval JL: JAK2V617F expression in murine
hematopoietic cells leads to MPD mimicking human PV with secondary
myelofibrosis. Blood. 108:1652–1660. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tiedt R, Hao-Shen H, Sobas MA, Looser R,
Dirnhofer S, Schwaller J and Skoda RC: Ratio of mutant JAK2-V617F
to wild-type Jak2 determines the MPD phenotypes in transgenic mice.
Blood. 111:3931–3940. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shide K, Shimoda HK, Kumano T, Karube K,
Kameda T, Takenaka K, Oku S, Abe H, Katayose KS, Kubuki Y, et al:
Development of ET, primary myelofibrosis and PV in mice expressing
JAK2 V617F. Leukemia. 22:87–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Steensma DP, McClure RF, Karp JE, Tefferi
A, Lasho TL, Powell HL, DeWald GW and Kaufmann SH: JAK2 V617F is a
rare finding in de novo acute myeloid leukemia, but STAT3
activation is common and remains unexplained. Leukemia. 20:971–978.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vizmanos JL, Ormazábal C, Larráyoz MJ,
Cross NC and Calasanz MJ: JAK2 V617F mutation in classic chronic
myeloproliferative diseases: A report on a series of 349 patients.
Leukemia. 20:534–535. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akada H, Yan D, Zou H, Fiering S,
Hutchison RE and Mohi MG: Conditional expression of heterozygous or
homozygous Jak2V617F from its endogenous promoter induces a
polycythemia vera-like disease. Blood. 115:3589–3597. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi S-E, Hong SW and Yoon SO: Proposal of
an appropriate decalcification method of bone marrow biopsy
specimens in the era of expanding genetic molecular study. J Pathol
Transl Med. 49:236–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dziaman T, Ludwiczak H, Ciesla JM,
Banaszkiewicz Z, Winczura A, Chmielarczyk M, Wisniewska E,
Marszalek A, Tudek B and Olinski R: PARP-1 expression is increased
in colon adenoma and carcinoma and correlates with OGG1. PLoS One,.
9:e1155582014. View Article : Google Scholar
|
|
29
|
Burduk PK, Bodnar M, Sawicki P, Szylberg
Ł, Wiśniewska E, Kaźmierczak W, Martyńska M and Marszałek A:
Expression of metalloproteinases 2 and 9 and tissue inhibitors 1
and 2 as predictors of lymph node metastases in oropharyngeal
squamous cell carcinoma. Head Neck. 37:418–422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
31
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sidon P, El Housni H, Dessars B and
Heimann P: The JAK2V617F mutation is detectable at very low level
in peripheral blood of healthy donors. Leukemia. 20:16222006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Scott LM, Tong W, Levine RL, Scott MA,
Beer PA, Stratton MR, Futreal PA, Erber WN, McMullin MF, Harrison
CN, et al: JAK2 exon 12 mutations in polycythemia vera and
idiopathic erythrocytosis. N Engl J Med. 356:459–468. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pikman Y, Lee BH, Mercher T, McDowell E,
Ebert BL, Gozo M, Cuker A, Wernig G, Moore S, Galinsky I, et al:
MPLW515L is a novel somatic activating mutation in myelofibrosis
with myeloid metaplasia. PLoS Med. 3:e2702006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pardanani A, Lasho TL, Finke C, Hanson CA
and Tefferi A: Prevalence and clinicopathologic correlates of JAK2
exon 12 mutations in JAK2V617F-negative polycythemia vera.
Leukemia. 21:1960–1963. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pardanani AD, Levine RL, Lasho T, Pikman
Y, Mesa RA, Wadleigh M, Steensma DP, Elliott MA, Wolanskyj AP,
Hogan WJ, et al: MPL515 mutations in myeloproliferative and other
myeloid disorders: A study of 1182 patients. Blood. 108:3472–3476.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chaligné R, Tonetti C, Besancenot R, Roy
L, Marty C, Mossuz P, Kiladjian JJ, Socié G, Bordessoule D, Le
Bousse-Kerdilès MC, et al: New mutations of MPL in primitive
myelofibrosis: Only the MPL W515 mutations promote a G1/S-phase
transition. Leukemia. 22:1557–1566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Beer PA, Campbell PJ, Scott LM, Bench AJ,
Erber WN, Bareford D, Wilkins BS, Reilly JT, Hasselbalch HC, Bowman
R, et al: MPL mutations in myeloproliferative disorders: Analysis
of the PT-1 cohort. Blood. 112:141–149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boyd EM, Bench AJ, Goday-Fernández A,
Anand S, Vaghela KJ, Beer P, Scott MA, Bareford D, Green AR, Huntly
B, et al: Clinical utility of routine MPL exon 10 analysis in the
diagnosis of essential thrombocythaemia and primary myelofibrosis.
Br J Haematol. 149:250–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Michiels JJ, Valster F, Wielenga J,
Schelfout K and De Raeve H: European vs 2015-World Health
Organization clinical molecular and pathological classification of
myeloproliferative neoplasms. World J Hematol. 4:16–53. 2015.doi:
10.5315/wjh.v4.i3.16. View Article : Google Scholar
|
|
41
|
Pasquier F, Cabagnols X, Secardin L, Plo I
and Vainchenker W: Myeloproliferative neoplasms: JAK2 signaling
pathway as a central target for therapy. Clin Lymphoma Myeloma
Leuk. 14:(Suppl). S23–S35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ihle JN and Gilliland DG: Jak2: Normal
function and role in hematopoietic disorders. Curr Opin Genet Dev.
17:8–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ihle JN: STATs: Signal transducers and
activators of transcription. Cell. 84:331–334. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pircher TJ, Flores-Morales A, Mui AL,
Saltiel AR, Norstedt G, Gustafsson JA and Haldosén LA:
Mitogen-activated protein kinase kinase inhibition decreases growth
hormone stimulated transcription mediated by STAT5. Mol Cell
Endocrinol. 133:169–176. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pircher TJ, Petersen H, Gustafsson JA and
Haldosén LA: Extracellular signal-regulated kinase (ERK) interacts
with signal transducer and activator of transcription (STAT) 5a.
Mol Endocrinol. 13:555–565. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Verstovsek S, Manshouri T, Quintás-Cardama
A, Harris D, Cortes J, Giles FJ, Kantarjian H, Priebe W and Estrov
Z: WP1066, a novel JAK2 inhibitor, suppresses proliferation and
induces apoptosis in erythroid human cells carrying the JAK2 V617F
mutation. Clin Cancer Res. 14:788–796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Aboudola S, Murugesan G, Szpurka H,
Ramsingh G, Zhao X, Prescott N, Tubbs RR, Maciejewski JP and Hsi
ED: Bone marrow phospho-STAT5 expression in non-CML chronic
myeloproliferative disorders correlates with JAK2 V617F mutation
and provides evidence of in vivo JAK2 activation. Am J Surg Pathol.
31:233–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nyga R, Pecquet C, Harir N, Gu H,
Dhennin-Duthille I, Régnier A, Gouilleux-Gruart V, Lassoued K and
Gouilleux F: Activated STAT5 proteins induce activation of the PI
3-kinase/Akt and Ras/MAPK pathways via the Gab2 scaffolding
adapter. Biochem J. 390:359–366. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rosa Santos SC, Dumon S, Mayeux P,
Gisselbrecht S and Gouilleux F: Cooperation between STAT5 and
phosphatidylinositol 3-kinase in the IL-3-dependent survival of a
bone marrow derived cell line. Oncogene. 19:1164–1172. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gibson SE, Schade AE, Szpurka H, Bak B,
Maciejewski JP and Hsi ED: Phospho-STAT5 expression pattern with
the MPL W515L mutation is similar to that seen in chronic
myeloproliferative disorders with JAK2 V617F. Hum Pathol.
39:1111–1114. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Grimwade LF, Happerfield L, Tristram C,
McIntosh G, Rees M, Bench AJ, Boyd EM, Hall M, Quinn A, Piggott N,
et al: Phospho-STAT5 and phospho-Akt expression in chronic
myeloproliferative neoplasms. Br J Haematol. 147:495–506. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Levy DE and Darnell JE Jr: Stats:
Transcriptional control and biological impact. Nat Rev Mol Cell
Biol. 3:651–662. 2002. View
Article : Google Scholar : PubMed/NCBI
|