Introduction
The morbidity of esophageal carcinoma (EC) ranks
eighth among malignant tumors globally, and is the sixth leading
cause of cancer-related death. According to statistical studies,
there were ~455,800 new cases worldwide, with 400,200 deaths in
2012. Eastern Asia, particularly the North Central region of China,
is a high-risk area (1). Most ECs
in China are esophageal squamous cell carcinoma (ESCC), which
account for 90% of all cases of EC worldwide. ESCC is different
from adenocarcinoma, which is prevalent in Western countries and
presents different pathogenesis, epidemiology, tumor biology and
prognosis (2). ESCC has a high
degree of malignancy. Despite advances in surgical therapy for
ESCC, most patients have locally advanced or disseminated disease
at diagnosis (3). To treat this
unresectable disease, chemotherapy alone or in combination with
other antineoplastic agents may be an optimal choice.
5-Fluorouracil (5-FU) is the inhibitor of
thymidylate synthetase (TS), which catalyzes the rate-limiting step
in DNA synthesis. Therefore, 5-FU decreases the biosynthesis of
pyrimidine nucleotides (4).
However, 5-FU can be misincorporated into RNA and then inhibit RNA
synthesis (5). 5-FU is used to
treat many types of malignant tumors, including ESSC. The
combination of 5-FU and cisplatin (CDDP) is a successful
therapeutic strategy (6). However,
the success of chemotherapy depends on the sensitivity of the tumor
to chemotherapeutic drugs, such as 5-FU. During treatment, ESCC
cells often acquire resistance to drugs and turn into
chemoresistant cancer cells (CCCs), which leads to the failure of
therapy. There are currently 4 known mechanisms of 5-FU resistance.
The first involves intracellular changes in 5-FU metabolism,
including anabolic and catabolic processes. To exert its
cytotoxicity, the anabolic processes of 5-FU take part in nucleic
acid synthesis by combining with intracellular-related enzymes,
such as orotate phosphoribosyltransferase (OPRT) and uridine
phosphorylase (UP). The downregulation of OPRT and UP contributes
to the 5-FU resistance of cancer cells (7). However, 5-FU is degraded by
dihydropyrimidine dehydrogenase (DPD) to its nontoxic metabolites,
which include α-fluoro-β-ureido-propionic acid (FUPA), in the
catabolism of 5-FU. The upregulation of DPD also contributes to
5-FU resistance (8). Thus, tumor
cells became resistant to 5-FU by inhibiting anabolic processes and
activating catabolic processes. In addition, since TS is the main
cellular target of 5-FU (9),
increased TS activity is detected in 5-FU-resistant cancer cells
(10). Second, drug-resistant cells
decrease the cellular concentration of the chemotherapeutic drug
and reduce its effectiveness via the activation and overexpression
of drug efflux pathways. ATP-binding cassette (ABC) transporters
are plasma membrane-associated and energy-dependent efflux pumps,
including multidrug resistance 1 (MDR1) (11), multidrug resistance-associated
protein 1 (MRP1) (12), and
ATP-binding cassette superfamily G member 2 (ABCG2) (13). Their upregulation helps cancer cells
to enhance cell survival against drugs and resist the cytotoxic
effects of anticancer agents. Third, epithelial-to-mesenchymal
transition (EMT) is closely related to chemoresistance (14). EMT is initially observed in
embryonic development, in which cells lose epithelial
characteristics and gain mesenchymal properties to increase
motility and invasiveness (15).
The molecular characteristics of EMT include the downregulation of
cell adhesion molecules (E-cadherin) and the upregulation of
vimentin. EMT is important in tumor progression, metastasis and
chemoresistance (15) and is
induced by various growth factors, such as transforming growth
factor-β, hepatocyte and epidermal growth factor (16). Fourth, chemoresistant cancer cells
(CCCs) may be derived from cancer stem cells (CSCs). CSCs are a
non-differentiated subset of cancer cells isolated from several
different tumors and characterized by an intrinsic resistance to
apoptosis as well as by unusual phenotypic plasticity (17). When environmental condition became
unfavorable (such as adding antitumor drugs) for cells,
drug-resistant stem-like cells may originate from other cancer
cells (18). The characteristics of
stem cells facilitate the resistance to injury from anticancer
agents and result in drug resistance. Several molecular markers are
related to cancer stem-cell subpopulations, such as prominin-1
(CD133), hyaluronate receptor (CD44) and aldehyde dehydrogenase 1
A1 (19). The upregulation of CD133
and CD44 was recently detected in 5-FU-resistant gastric cancer
cells (20), but has not been
reported in 5-FU-resistant ESCC cells. Notably, EMT is considered a
key process for CSC generation (21). The presence of CCCs is often
associated with poor prognosis (22).
Overcoming drug resistance is a large issue in
cancer treatment. Drug-resistant cell lines established in
vitro could be useful tools to simulate the process of cell
resistance, illuminate resistance mechanisms and provide a research
model to select sensitizers to chemotherapy. Many 5-FU-resistant
cancer cells have been established, including mucoepidermoid
carcinoma (12), gastric (20) and colorectal cancer (23), hepatocellular (24) and oral squamous cell carcinoma
(25), and human endometrial
adenocarcinoma cell lines (26). As
for ESCC 5-FU-resistant cells, TE-5 (8), TE-11 (27), KYSE410 (28) and KYSE150 (29) have been reported, and their drug
resistance mechanisms have also been studied. Compared with tumor
parental cells, 5-FU-resistant ESCC cells were found to exhibit
upregulation of DPD (8) and
PI3K/AKT (29), specific miRNA
signatures (30), and the
activation of Id1-E2F1-IGF2/TS (28). To overcome resistance to 5-FU,
researchers have demonstrated that photodynamic therapy (PDT) could
induce potent cytotoxicity in 5-FU-resistant ESCC cells (TE-5R and
TE-11R) independent of their differentiation grade or 5-FU
resistance (27). In addition,
upregulation of miR-148a attenuated resistance in
chemotherapy-resistant variants, including 5-FU-resistant cells
(31).
Despite these developments, there are still some
unclear questions concerning 5-FU-resistant ESCC cells. First, the
establishment of 5-FU-resistant sublines and determination of their
mechanism of resistance have only been reported in 2 ESCC cell
lines (KYSE and TE). Since Eca-109 was found to be more sensitive
to antitumor drugs (both CDDP and paclitaxel) than KYSE cells
(32), it may be easier to choose a
chemotherapy sensitizer in the use of 5-FU-resistant Eca-109/5-FU
cells. However, are the ABC transporters highly expressed in
5-FU-resistant ESCC cells? Sasada et al (33) explored the metabolomics of
5-FU-resistant gastric cancer cells, in which the metabolite
profile is different from that of parental cells.
To further explore the ESSC resistance mechanism for
5-FU, we established a 5-FU-resistant ESCC cell line Eca-109/5-FU,
which was prepared by stepwise exposure to increasing 5-FU
concentrations. As for Eca-109/5-FU, the decreased susceptibility
to 5-FU and lower proliferation were determined in vitro and
in vivo. Drug resistance-related, EMT-related and
CSC-related proteins were significantly highly expressed. These
results provide an experimental model for further steps in
selecting chemotherapy sensitizers.
Materials and methods
Reagents
The chemotherapeutic drugs CDDP and 5-FU were
purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). The
anti-MRP1 (BA0567), E-cadherin (PB0583), vimentin (BM0135), CD133
(BA3992) and CD144 (PB0287) antibodies were purchased from Boster
Biological Technology Ltd. (Wuhan, China). The anti-ABCG2 (RLT0053)
antibody was purchased from Ruiying Bio (Suzhou, China). The
anti-β-actin (BE0021) antibody was purchased from BioEasy
Technology Co. Ltd. (Beijing, China). The antiproliferating cell
nuclear antigen (PCNA) (ZM-0213) and secondary antibodies were
purchased from Zhongshan Golden Bridge Biotechnology (Beijing,
China).
Cell culture
The ESCC cell line Eca-109 was kindly provided by Dr
XiaoFei Zheng of the Beijing Institute of Radiation Medicine.
Eca-109 was checked by short tandem repeat (STR) analysis at the
Beijing Microread Gene Technology (Beijing, China) and maintained
in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS), 100 µg/ml penicillin/streptomycin, 2
mmol/l L-glutamine and HEPES buffer.
Establishment of the 5-FU-resistant
cell line Eca-109/5-FU
The resistant cell line was established in
vitro by intermittent exposure of human Eca-109 to different
concentrations of 5-FU in stepwise increments of time. Starting
with a concentration of 0.1 µg/ml, 5-FU was added to the cells when
they grew to 70–80% confluence. When the majority of cells began to
die, the remaining cells were then washed 3 times with
phosphate-buffered saline (PBS) and cultured in 5-FU-free growth
medium. The dead cells were washed out with PBS and fresh medium
was added daily. After incubating for a few days at 37°C in a
humidified air atmosphere containing 5% CO2, the
surviving cells were restored to exponential growth without
pressure from 5-FU, and the next concentration of 5-FU was then
added. The drug concentration was increased to 0.2, 0.4 and 1
µg/ml, and the maximal concentration was used 6 times until cells
could live in a 5-FU maximal concentration for >4 days. The
5-FU-resistant cell line Eca-109/5-FU was established 12 months
after the treatment was initiated, and the resistant phenotype was
developed. The resistant cells were frozen in liquid nitrogen.
Freeze-stored cells were recovered after 3 passages and were
subjected to the identification of biological characteristics via
the following experiments.
Cell viability assay
Cell viability was assessed by MTT assay. Cells were
seeded in 96-well plates (5,000 cells/well) for 24 h at 37̊C and
treated with 5-FU at different final concentrations (0, 0.31,
0.625, 1.25, 2.5, 5, 10, 20, 40 and 80 µg/ml). Four days after
treatment, the medium was removed and 5 mg/ml MTT was added to each
well. The cells were maintained at 37̊C for 4 h; then, 150 µl of
dimethyl sulfoxide (DMSO) was added to each well and mixed
thoroughly. The absorbance was read on a Bio-Rad microplate reader
Model 550 at 490 nm. The MTT absorbance of the untreated control
cells was set to 1 to calculate the relative number of viable
cells. The experiments were repeated at least 3 times to ensure
reproducibility and statistical significance.
Apoptosis assay
Cell apoptosis was detected via the Annexin V
binding assay and flow cytometry (FCM). Cells (Eca-109 and
Eca-109/5-FU) were seeded at 1×105 cells/well in 6-well
plates and treated with 5-FU at different final concentrations (0,
2.5, 5, 10, 20 and 40 µg/ml) the following day. On day 4 after
treatment, the cells were stained with FITC-labeled Annexin V and
propidium iodide (PI) according to the manufacturer's instructions
(KGA105-50, Annexin V-FITC apoptosis detection kit; Nanjing KeyGen
Biotech, Nanjing, China) and FCM was performed immediately after
staining. For the xenograft tumor tissue, the apoptosis of a
section was analyzed by terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) staining using
the DeadEnd™ Colorimetric TUNEL system (G7130, Promega Corporation,
Beijing, China) following the manufacturer's protocol. Hematoxylin
was applied as a counterstain. In each sample, the number of
apoptotic tumor cells from 50 different fields was evaluated at
high magnification (x200).
Growth curves
Cell viability was assessed by MTT assay. Cells were
seeded in 96-well plates (2,000 cells/well) with 0.2 ml of medium
at 37°C in an incubator with a humidified air atmosphere containing
5% CO2. We detected the MTT absorbance every 24 h for 9
days as described above. At the same time, cells were seeded in a
96-well plate with different cell numbers (doubling the dilution
from 1×105 to 200 cells/well). When they adhered to the
plates after 4 h, the MTT assay was performed, the standard curve
was drawn (x-axis indicates the OD value; y-axis indicates the cell
number) and empirical formulas were determined.
Eca-109: y = 0.1289x2 + 0.0317x + 0.0032,
R2 = 0.9999
Eca-109/5-FU: y = 0.1414x2 + 0.0317x +
0.0027, R2 = 1
The experiments were repeated at least 3 times to
ensure reproducibility and statistical significance.
Cell cycle distribution
Cells were harvested during the exponential growth
phase following trypsinization without ethylenediaminetetraacetic
acid (EDTA). The cells were washed with cold PBS and fixed by
suspending the cells in 1 ml stationary liquid (30% PBS and 70%
ethanol) at −20°C. After 3 days, the fixed cells were washed and
resuspended in 300 µl PI (50 µg/ml) and 10 µl RNase A (10 mg/ml).
Then, the samples were incubated at 37°C for 30 min in the darkness
and the DNA content was analyzed by FCM.
Metabolic analysis
Metabolomic profiles were obtained to assess the
relative distribution of various cellular metabolites of Eca-109
and Eca-109/5-FU cells. Cells were collected at 0.5 g wet weight
and quickly frozen in liquid N2. Further study was
performed by Anachro Technologies Inc. (Wuhan, China) using their
described methods (34,35), including sample preparation,
metabolic profiling, peak identification, curation and date
analysis.
Western blotting
Cell lysates were treated with RIPA lysis buffer and
the protein concentrations were determined using Pierce BCA protein
assay kit (Thermo Scientific, Waltham, MA, USA). Total protein
(20–50 µg) was separated by 15% SDS-PAGE and transferred to a
nitrocellulose membrane. After incubation in 5% non-fat milk
solution, the membranes were incubated in each of the following
antibodies: MRP1 (1:100), ABCG2 (1:1,000), CD133 (1:200), CD44
(1:500), vimentin (1:200), E-cadherin (1:500) and β-actin (1:1,000)
at 4°C overnight. The proteins were visualized on Kodak X-ray film
(Kodak, Rochester, NY, USA) by the application of the enhanced
chemiluminescence western blotting detection system (Thermo
Scientific).
Evaluation of tumor growth in
vivo
The effect of 5-FU on the proliferation of Eca-109
and Eca-109/5-FU cells in vivo was investigated by xenograft
tumors in nude mice as previously described (36). All applicable international,
national and/or institutional guidelines for the care and use of
animals were followed. All procedures performed in studies
involving animals were in accordance with ethical standards of the
Center of Biomedical Analysis of Tsinghua University, where the
studies were conducted. First, the therapeutic dose of 5-FU was
tested by i.p. injection of 8 and 25 mg/kg body weight. The
Eca-109-derived tumors in athymic BALB/c female nude mice were
induced by injecting s.c. 5×106 cells resuspended in 200
µl sterile PBS into the lower right flank of each mouse. Each group
consisted of 3 animals, drugs were administered every 3 days for a
total of 28 days, and tumor growth was monitored for 30 days. Tumor
length and width were measured using a Vernier caliper every other
day without knowledge of the treatment groups, and the tumor volume
was calculated by ‘length×width2/2’. Then, the
therapeutic effects of 5-FU on Eca-109-induced and
Eca-109/5-FU-induced tumors were evaluated. Sixteen mice received
Eca-109 cells (5×106 cells/200 µl), and the same number
of mice received Eca-109/5-FU cells with the same cell
concentrations. When the size of tumors reached 50–100
mm3 (nearly 5 days), 16 animals (bearing Eca-109 or
Eca-109/5-FU) were randomly divided into 2 groups (control and
experimental group), each containing 8 mice. The animals in the
control group were injected i.p. with 0.1 ml PBS, and the animals
in the experimental group were injected i.p. with 0.1 ml 5-FU (25
mg/kg). The tumor volume was monitored as described above. After 30
days, the animals were euthanized, and the tumors were analyzed by
immunohistochemical staining with hematoxylin and eosin (H&E),
PCNA and TUNEL staining.
Immunohistochemical analysis
Immunohistochemical labeling was performed on 10%
formalin-fixed, paraffin-embedded tumor tissue cut into 4-µm thick
sections. After being deparaffinized in xylene and rehydrated in
graded concentrations of ethanol, the sections were recovered in
citrate buffer and blocked with 3% H2O2 and
10% serum. The primary antibody PCNA (ZM-0213, 1:500) was added and
incubated overnight at 4̊C. Then, the slides were incubated with
the secondary antibody and the horseradish peroxidase-streptavidin
complex reagent for 30 min, respectively (Beijing Golden Bridge
Biotechnology, Beijing, China). Immunolabeling was developed with
chromogen 3,3′-diaminobenzidine tetrahydrochloride (DAB).
Hematoxylin was applied as a counterstain. Tissue sections stained
without the primary antibody served as negative controls. The
staining of tissue sections was analyzed and quantified, and the
results were interpreted in a blinded manner.
Statistical analysis
All experiments were performed at least 3 times.
Statistical comparisons between groups were performed using
unpaired Student's t-test with Statistical Package for the Social
Sciences (SPSS) software version 15 (SPSS, Inc., Chicago, IL, USA).
Values of p<0.05 or p<0.01 were considered to indicate
statistically significant results.
Results
Decreased sensitivity of the
drug-resistant cell line Eca-109/5-FU to 5-FU in vitro and in
vivo
Eca-109/5-FU cells were prepared in vitro by
intermittently exposing Eca-109 cells to increasing concentrations
of 5-FU (0.1, 0.2, 0.4 and 1 µg/ml) for 12 months. After the
generation of Eca-109/5-FU, the cells were cultured in 5-FU-free
growth medium for 3 passages (~1 week), and then frozen in liquid
nitrogen. Cell viability was determined by the MTT method, and the
data indicated that Eca-109/5-FU was more resistant to 5-FU than
Eca-109. The IC50 5-FU values for the Eca-109 and
Eca-109/5-FU cells were 10.5 and 45 µg/ml, respectively. The
resistance index (RI, IC50 of drug-resistant
cells/IC50 of parental cells) was 4.3, which means that
Eca-109/5-FU was 4.3-fold more resistant to 5-FU than Eca-109
(Fig. 1A). We also determined the
cell viability with CDDP, and there was almost no inhibition of
Eca-109/5-FU cell viability when the concentration of CDDP was
below 5 µg/ml, which was significantly different in comparison with
the parental cells (Fig. 1B). All
of these results implied that Eca-109/5-FU may be a
multidrug-resistant cell line. To detect the 5-FU-induced apoptotic
death in Eca-109/5-FU and Eca-109 cells, Annexin V-FITC and PI
staining were performed, followed by FCM. The results indicated
that the apoptotic proportion of Eca-109/5-FU cells was much lower
than that of the Eca-109 cells when treated with 5-FU, and the
difference was significant at p<0.01. After being treated with
low doses of 5-FU (2.5 and 5 µg/ml), the apoptotic proportion of
Eca-109 was ~40%, while that of Eca-109/5-FU was below 10%. When
the dose of 5-FU increased to 10 µg/ml, the apoptotic proportion of
Eca-109 reached 74.44%, while that of Eca-109/5-FU was 16.36%. When
the cells were supplemented with the highest dose of 5-FU, the
apoptotic proportion of Eca-109 was 94.76%, but that of
Eca-109/5-FU was 57.26% (Fig. 1C).
Furthermore, we used nude mouse xenograft models (Eca-109-bearing
and Eca-109/5-FU-bearing mice) to determine whether Eca-109/5-FU
was also resistant to 5-FU in vivo. When the xenograft
tumors reached 50–100 mm3, mice from the experimental
groups were treated with 5-FU. When analyzed 28 days later, the
volume and weight of the Eca-109-induced tumors decreased, but
there was no change in the volume and weight of the
Eca-109/5-FU-induced tumors (Fig. 1D
and E). There was no significant difference in the tumor cell
mitotic index or tissue infiltration and tumor cell proliferative
index between the control groups and the experimental groups in the
Eca-109-induced tumors or Eca-109/5-FU-induced tumors as determined
by H&E and PCNA staining, respectively (Fig. 1F and G). Compared with tumor tissues
of the control group, a higher number of TUNEL-positive stained
cells was observed in the Eca-109-induced tumors treated with 5-FU,
but no significant difference was found in the Eca-109/5-FU-induced
tumors treated with 5-FU or PBS (Fig.
1H and I; p<0.01). These experimental results suggested that
Eca-109/5-FU cells were resistant to 5-FU in vitro and in
vivo.
Proliferation of Eca-109/5-FU cells
was slower than Eca-109 cells in vitro and in vivo
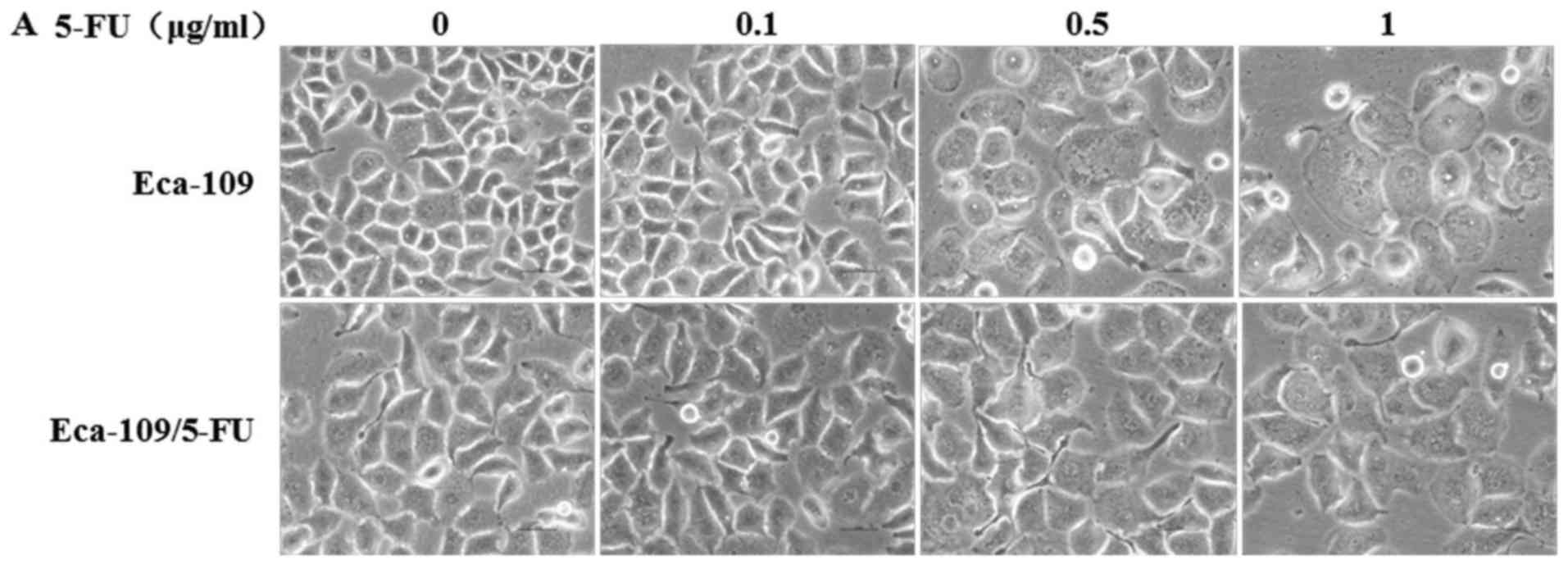
Under a bright field of vision without 5-FU
treatment, Eca-109 showed clear borders, while the borders of
Eca-109/5-FU were relatively fuzzy. The cell size of Eca-109/5-FU
was a bit larger than the parental Eca-109 cells. Under increasing
drug concentrations, the size of Eca-109 cells gradually increased
and the cell edges became indistinct, similarly to Eca-109/5-FU
cells (Fig. 2A). The cell growth
curve indicated that the proliferation of Eca-109 cells began to
exceed that of Eca-109/5-FU at 2 days (Fig. 2B). In vivo, we established
nude mouse xenograft models (Eca-109-bearing and
Eca-109/5-FU-bearing mice). When the xenograft tumors reached
50–100 mm3, the volume of xenograft tumors was recorded
every 3 days for a total of 28 days. The results showed that after
16 days, the proliferation of the Eca-109 cells became faster than
Eca-109/5-FU, and they were significantly different at 28 days
(Fig. 2C). Therefore, Eca-109/5-FU
cells grew more slowly than Eca-109 cells in vitro and in
vivo. To determine the cause of this result, the cell cycle
distribution of Eca-109 and Eca-109/5-FU was determined by FCM. As
shown in Fig. 2D, the percentages
of Eca-109 and Eca-109/5-FU cells in the G0/G1, S and G2/M phase
were 33.72 and 51.45%, 26.68 and 33.7%, and 39.6 and 14.85%,
respectively. Compared with the parental cells (Eca-109),
Eca-109/5-FU cells exhibited G0/G1 phase and S phase arrest with a
concomitantly decreased cell percentage in the G2/M phase; the
difference was significant at p<0.01. The decreased cell
percentage of the G2/M phase could be considered as an adverse
factor in the proliferation of Eca-109/5-FU cells.
Significant drug resistance-related
characteristics of Eca-109/5-FU
To determine the drug resistance mechanism of the
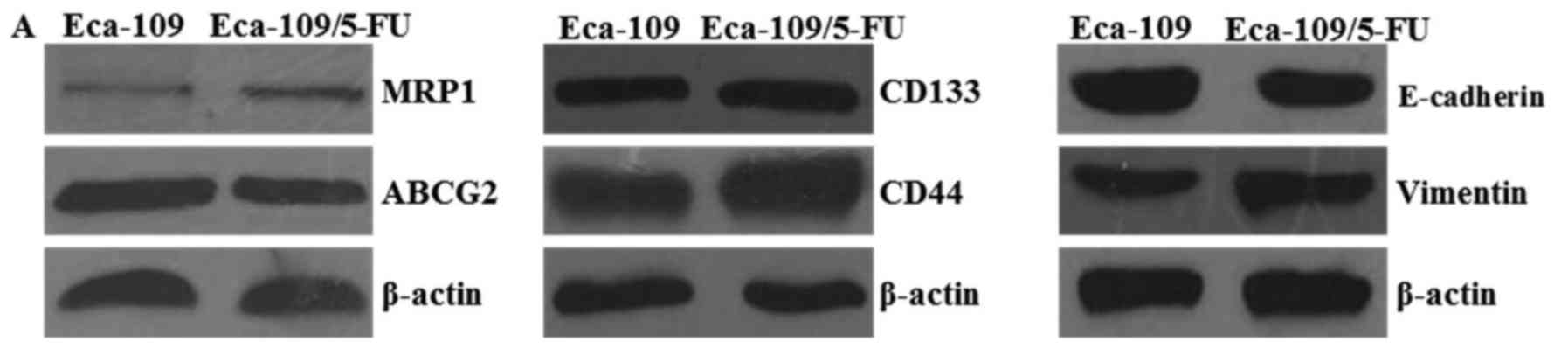
Eca-109/5-FU cell line, western blotting was used to determine the
expression level of various protein markers. Since the upregulation
of ABC transporters in cancer cells is considered to be a primary
determinant of the drug-resistant phenotype (37), we detected the expression levels of
two main ABC transporters, MRP1 and ABCG2. The expression level of
MRP1 was markedly increased in the Eca-109/5-FU cells relative to
the parental cells (Eca-109), while ABCG2 showed no obvious changes
(Fig. 3A). However, the EMT-related
marker vimentin was upregulated and another related marker
E-cadherin was downregulated. The stem-like markers CD133 and CD44
were upregulated in the Eca-109/5-FU cells. These findings suggest
that Eca-109/5-FU exhibited characteristics of EMT and CSCs, which
contribute to drug resistance (Fig.
3A). To look for statistically significant differences, the
MRP1/β-actin, ABCG2/β-actin, CD133/β-actin, CD44/β-actin,
vimentin/β-actin and E-cadherin/β-actin ratios were calculated and
a t-test was performed. The results confirmed that MRP1, CD133,
CD44 and vimentin protein expression levels were markedly higher in
the Eca-109/5-FU than levels in the Eca-109 cells, while E-cadherin
and ABCG2 expression levels were lower than these in the parental
cells (p<0.05, p<0.01) (Fig.
3B). Nuclear magnetic resonance (NMR) was used to determine
differences in the contrast of the metabolic products between
Eca-109/5-FU and Eca-109, and the analysis showed that the
metabolite profiles between Eca-109/5-FU and Eca-109 were
significantly different. This implied that the metabolism of
5-FU-resistant cells had changed, and this may be a characteristic
of drug tolerance (Fig. 3C).
Fifteen metabolites contributed to the difference in metabolite
profiles: lactate, glutamate, taurine, glutamine, proline,
aspartate, methanol, cystine, glycine, uracil, glutathione,
cysteine, alanine, valine and isoleucine (Fig. 3D and E). Glutamine is a major source
of carbon and energy for cancer cells; thus, glutaminolysis in
cells is crucial for cancer cell metabolism (38). The metabolic tests showed that
glutamate was reduced, while glutamine was significantly increased
in the Eca-109/5-FU cells compared with the parental cells, and
these changes suggested that Eca-109/5-FU cells have a stronger
survival ability than parental cells.
Discussion
Chemotherapy is widely used in cancer treatment. As
a classical chemotherapeutic drug, 5-fluorouracil (5-FU) can be
utilized to treat ESCC (6).
However, during treatment, ESCC cells often develop resistance to
5-FU, which leads to the failure of the therapy. To improve the
disease prognosis in cancer patients, it is essential to overcome
the resistance to cancer chemotherapy. The drug-resistant phenotype
of cancer cells may be intrinsic or acquired after chemotherapy
treatment using cytotoxic drugs (39). Establishing drug-resistant cancer
cells through constant or intermittent drug stimulation has been an
effective method for the investigation of drug resistance. Various
5-FU-resistant ESCC cell lines have been established and the
mechanism of 5-FU resistance has been explored (8,28).
However, since Eca-109 is a relatively more chemotherapeutic
sensitive ESCC cell line (32), it
has not produced 5-FU-resistant cell lines, only CDDP-resistant
cell lines (13) and
paclitaxel-resistant cell lines (40). Therefore, we prepared 5-FU-resistant
Eca-109/5-FU cells through stepwise exposure to increasing 5-FU
concentrations. During the process of preparation, most Eca-109
cells were gradually enlarged with more vacuole formation when
treated with low doses of 5-FU (0.1 µg/ml). Then, medium was
removed and fresh medium without 5-FU was added for cell recovery.
After one week, the surviving cells resumed exponential growth and
the above treatment was repeated. Gradually, cells acquired
tolerance to 5-FU and the RI reached 4.3. Notably, Eca-109/5-FU's
endurance capacity to CDDP also increased nearly 3 times compared
with the parental cells. To date, the most common method for
establishing resistant ESCC cell lines was an intermittent
incremental induction method with various and inconsistent final
dosages, such as 1.3 (8), 10.4
(28) and 5.2 µg/ml (29). However, Dinicola et al
(41) reported that the final
concentration of 5-FU could refer to the clinical plasma
concentration of 2.0 µg/ml. The other commonly used method is the
pulse treatment method, which was described by Hummel et al
(30) with a relatively low-dose of
5-FU (0.65 µg/ml). The resistant cells selected by the intermittent
incremental method were more resistant than the cells selected by
the pulse method (42).
Our experimental results indicated that the
proliferation of Eca-109/5-FU cells was slower than Eca-109 cells
in vitro and in vivo. The reason may be that
Eca-109/5-FU cells exhibited G0/G1 phase and S phase arrest with a
concomitantly decreased proportion of cells in the G2/M phase when
compared with Eca-109 cells, which means that the number of
proliferating cells decreased. There is a critical balance between
cell cycle arrest (promoting DNA repair and survival) and cell
death after chemotherapy. Various genes may be activated or
inactivated in response to DNA damage to induce cell cycle arrest
in the G1 phase, in which damaged DNA could be repaired (43). The slow proliferation and arrest of
G0/G1 and S phase have also been found in other tumor cells
resistant to 5-FU, such as 5-FU-resistant colorectal cancer cells
HCT116-5FR, SW480-5FR (23) and
HT29R (44); the 5-FU-resistant
gastric cancer cells SGC-7901-FR (20) and SNU620/5-FU (45); the 5-FU-resistant liver cancer cells
HLF-R2 and HLF-R10 (46). However,
there was no significant difference in cellular proliferation
between 5-FU-resistant oral squamous cancer cells HSC2/FU and
parental cells (25). The
CDDP-resistant or paclitaxel-resistant Eca-109 cells also exhibited
slow proliferation and arrest at the G0/G1 phase and S phase
compared with Eca-109. The difference in proliferation implied that
the underlying drug resistance-related mechanisms may be different,
but all the changes contribute to cushioning the damage of
cytotoxic drugs.
The ABC transporter family has always been a hotspot
in the study of drug resistance mechanisms. Its powerful function
increases drug efflux and decreases intracellular drug
concentration, which eventually contributes to drug resistance. As
a member of the ABC transporter family, MRP1 reduces the drug
concentration in the nucleus by actively transporting drugs into
subcellular organelles or indirectly affecting the distribution of
drugs, which thereby avoids DNA injury. It can also form chloride
ion channels or change channel activity to decrease the pH in the
cytoplasm or organelles, which produces an acidic environment in
which protonated drugs are largely discharged. However, MRP1 can
move drugs out of cells through vesicle transportation or
exocytosis (43). MRP1 is also
upregulated in a variety of human malignancies, including different
esophageal cancer cell lines or cancer tissues (47). It has been reported that the
percentage of MRP1-positive samples in esophageal cancer was
significantly higher than that in gastric cancers and colorectal
cancers, which implies that MRP1 may play a large role in the drug
resistance in esophageal cancer (48). A number of clinical studies have
observed correlations between high ABCG2 activity and the failure
of a variety of cytotoxic and targeted therapies (49). High ABCG2 activity is also linked to
a decreased clinical survival rate (50). Researchers have gradually assigned
importance to the relationship between drug resistance and ABCG2.
Our western blotting results indicated that the expression level of
MRP1 was markedly increased in the Eca-109/5-FU cells relative to
that noted in the parental Eca-109 cells, which indicates that
Eca-109/5-FU has a stronger ability to pump out intracellular
drugs, while ABCG2 showed significant downregulated in Eca-109/5-FU
cells. Various studies have shown that the expression level of ABC
transporters (MRP1 and ABCG2) in drug-resistant cancer cells
exhibited no differences with parental cancer cells (42) and even lower levels (13). EMT is a prerequisite event for
invasion in cancers, which plays a large role in 5-FU resistance
(25). However, there are no
studies of 5-FU-resistant ESCC. Our western blotting results
indicated that the expression level of vimentin increased and that
of E-cadherin decreased in the Eca-109/5-FU cells compared to these
level in the Eca-109 cells. This means that EMT also occurs in
5-FU-resistant ESCC cells. EMT has also been associated with
stem-like traits, such as self-renewal capacity, the expression of
stemness markers and the formation of anchorage-independent spheres
in several cancer models (44).
Therefore, EMT is considered a key process for cancer stem cell
generation (21). In addition,
mechanisms of CSC resistance may also include the preferential
activation of DNA damage checkpoints (51) and increasing drug exclusion by
efflux pumps (52).
CD133+ colon cancer cells have been confirmed to possess
stem cell properties and have inherently higher resistance to 5-FU
and oxaliplatin (53). As another
stem cell marker, CD44 is used to identify CSCs in ESCC and
CD44+ cells show more resistance to chemotherapy than
the CD44-ESCC cells KYSE-30 (54).
The upregulation of CD33 and CD44 in Eca-109/5-FU indicates that
the acquired drug resistance may be connected to CSCs. The
formation of EMT occurs together with metabolic alterations in
various pathways, such as glycolysis and oxidative phosphorylation
(55), and the reprogramming of
metabolic pathways is one of the important mechanisms of
chemoresistance (56). Sasada et
al (33) showed that the
5-FU-resistant gastric cancer cell line MKN45/F2R exhibited
significant changes in small molecule metabolism compared with
parental cells. As the action time of 5-FU was prolonged, some of
the concentrations of small molecules showed regular changes, such
as gradual reductions in alanine, asparagine, valine, proline and
citrulline; a gradual increase was also detected in parental cells
when supplemented with 5-FU (33).
Various studies confirmed that enhanced glutamine metabolism
occurred in both patients and cell lines resistant to erlotinib
(57). To further analyze the
metabolic changes of 5-FU-resistant ESCC cells, we determined the
metabolic products of Eca-109/5-FU and Eca-109 cells by NMR, and
the metabolite profiles between the two were significantly
different. Compared with the parental cell line Eca-109, the
content of glutamine was significantly increased in the
Eca-109/5-FU cells, while the content of glutamic acid was
significantly reduced. Glutaminolysis and glutaminase (GLS) have
been identified to be indispensable for the development and
progression of cancer, including drug-resistant cancer cells
(58). The high concentration of
glutamine indicates a stronger reserved capacity for glutamine in
Eca-109/5-FU cells relative to Eca-109 cells, and this phenomenon
implies that Eca-109/5-FU may more easily adapt to the severe
environment. The provision of energy is dependent on glycolysis in
cancer cells, with greater production of lactate regardless of
oxygen availability; this effect is called the Warburg effect
(59). In our experience, there was
a greater generation of lactate in the Eca-109/5-FU cells compared
with that in the Eca-109 cells. This shows that the glycolytic
pathway in Eca-109/5-FU cells is more active with more ATP
acquired, and it is well known that the intracellular repair of
cell structure and DNA and drug efflux through ABC transporters are
all ATP-dependent.
The 5-FU-resistance mechanism in tumor cells is
still unknown. There have been many attempts to increase cell
sensitivity to chemotherapeutic agents. For example, curcumin
mediates chemosensitization to 5-FU through the miRNA-induced
suppression of EMT in chemoresistant SW480-5-FUR colorectal cancer
cells (23), and tetrandrine (TET)
can decrease the expression levels of MRP1 and MDR1 in
5-FU-resistant ESCC YES-2/DDP cells (37). Low-dose all-trans retinoic
acid enhances the cytotoxicity of CDDP and 5-fluorouracil in
CD44+ cancer stem cells (54); as a GLS inhibitor compound, 968 can
reverse acquired erlotinib resistance in non-small cell lung cancer
(58). RNAi-mediated EZH2 depletion
(11), Annonaceous
acetogenins (60) and
astragaloside IV (61) could
reverse the drug resistance of 5-FU-resistant liver cancer cells
BEL-7402/5-FU by decreasing MRP1 and MDR1 expression. Hedyotis
diffusa Willd overcomes 5-FU resistance in human colorectal
cancer HCT-8/5-FU cells by downregulating the expression of MDR1
and ABCG2 (62). These results
provided evidence of the screening of chemotherapy sensitizers for
Eca-109/5-FU.
In conclusion, we established the 5-FU-resistant
ESCC cell line Eca-109/5-FU by stepwise exposure to increasing 5-FU
concentrations in vitro and preliminarily identified its
drug resistance mechanism. Eca-109/5-FU cells were resistant to
5-FU in vitro and in vivo and showed lower
proliferation rates. These cells exhibit characteristics of EMT and
CSCs. Higher intracellular concentrations of glutamine and lactate
were detected, which implies that Eca-109/5-FU cells have a strong
potential to survive when living in a severe environment. All of
these results should contribute to further research using the
drug-resistant Eca-109/5-FU cell line for selecting chemotherapy
sensitizers.
Acknowledgements
The present study was supported by the Beijing
Natural Science Foundation (grant no. 7142117) to Q.F. Ma and the
National High Technology Research and Development Program of China
(863 Program, grant no. 2014AA021605) to Z.L. Wang.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rustgi AK and El-Serag HB: Esophageal
carcinoma. N Engl J Med. 371:2499–2509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stein HJ, Sendler A, Fink U and Siewert
JR: Multidisciplinary approach to esophageal and gastric cancer.
Surg Clin North Am. 80:659–686. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Copur S, Aiba K, Drake JC, Allegra CJ and
Chu E: Thymidylate synthase gene amplification in human colon
cancer cell lines resistant to 5-fluorouracil. Biochem Pharmacol.
49:1419–1426. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scherf U, Ross DT, Waltham M, Smith LH,
Lee JK, Tanabe L, Kohn KW, Reinhold WC, Myers TG, Andrews DT, et
al: A gene expression database for the molecular pharmacology of
cancer. Nat Genet. 24:236–244. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Denlinger CS, Ligibel JA, Are M, Baker KS,
Broderick G, Demark-Wahnefried W, Friedman DL, Goldman M, Jones LW,
King A, et al: NCCN Guidelines Insights: Survivorship, Version
1.2016. J Natl Compr Canc Netw. 14:715–724. 2016.PubMed/NCBI
|
|
7
|
Inaba M, Mitsuhashi J, Sawada H, Miike N,
Naoe Y, Daimon A, Koizumi K, Tsujimoto H and Fukushima M: Reduced
activity of anabolizing enzymes in 5-fluorouracil-resistant human
stomach cancer cells. Jpn J Cancer Res. 87:212–220. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kikuchi O, Ohashi S, Nakai Y, Nakagawa S,
Matsuoka K, Kobunai T, Takechi T, Amanuma Y, Yoshioka M, Ida T, et
al: Novel 5-fluorouracil-resistant human esophageal squamous cell
carcinoma cells with dihydropyrimidine dehydrogenase
overexpression. Am J Cancer Res. 5:2431–2440. 2015.PubMed/NCBI
|
|
9
|
Look KY, Moore DH, Sutton GP, Prajda N,
Abonyi M and Weber G: Increased thymidine kinase and thymidylate
synthase activities in human epithelial ovarian carcinoma.
Anticancer Res. 17:2353–2356. 1997.PubMed/NCBI
|
|
10
|
Nakamura A, Nakajima G, Okuyama R,
Kuramochi H, Kondoh Y, Kanemura T, Takechi T, Yamamoto M and
Hayashi K: Enhancement of 5-fluorouracil-induced cytotoxicity by
leucovorin in 5-fluorouracil-resistant gastric cancer cells with
upregulated expression of thymidylate synthase. Gastric Cancer.
17:188–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang B, Zhang Y, Liang R, Gao Z, Sun D and
Wang L: RNAi-mediated EZH2 depletion decreases MDR1 expression and
sensitizes multidrug-resistant hepatocellular carcinoma cells to
chemotherapy. Oncol Rep. 29:1037–1042. 2013.PubMed/NCBI
|
|
12
|
Cai B, Miao Y and Liu Y, Xu X, Guan S, Wu
J and Liu Y: Nuclear multidrug-resistance related protein 1
contributes to multidrug-resistance of mucoepidermoid carcinoma
mainly via regulating multidrug-resistance protein 1: A human
mucoepidermoid carcinoma cells model and Spearman's rank
correlation analysis. PLoS One. 8:e696112013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wen J, Zheng B, Hu Y, Zhang X, Yang H, Luo
KJ, Zhang X, Li YF and Fu JH: Establishment and biological analysis
of the EC109/CDDP multidrug-resistant esophageal squamous cell
carcinoma cell line. Oncol Rep. 22:65–71. 2009.PubMed/NCBI
|
|
14
|
Yang AD, Fan F, Camp ER, van Buren G, Liu
W, Somcio R, Gray MJ, Cheng H, Hoff PM and Ellis LM: Chronic
oxaliplatin resistance induces epithelial-to-mesenchymal transition
in colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elliott BE, Hung WL, Boag AH and Tuck AB:
The role of hepatocyte growth factor (scatter factor) in
epithelial-mesenchymal transition and breast cancer. Can J Physiol
Pharmacol. 80:91–102. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elshamy WM and Duhé RJ: Overview: Cellular
plasticity, cancer stem cells and metastasis. Cancer Lett. 341:2–8.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He K, Xu T and Goldkorn A: Cancer cells
cyclically lose and regain drug-resistant highly tumorigenic
features characteristic of a cancer stem-like phenotype. Mol Cancer
Ther. 10:938–948. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lloyd RV, Hardin H, Montemayor-Garcia C,
Rotondo F, Syro LV, Horvath E and Kovacs K: Stem cells and cancer
stem-like cells in endocrine tissues. Endocr Pathol. 24:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu ZY, Tang JN, Xie HX, Du YA, Huang L, Yu
PF and Cheng XD: 5-Fluorouracil chemotherapy of gastric cancer
generates residual cells with properties of cancer stem cells. Int
J Biol Sci. 11:284–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen S, Song X, Chen Z, Li X, Li M, Liu H
and Li J: CD133 expression and the prognosis of colorectal cancer:
A systematic review and meta-analysis. PLoS One. 8:e563802013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Toden S, Okugawa Y, Jascur T, Wodarz D,
Komarova NL, Buhrmann C, Shakibaei M, Boland CR and Goel A:
Curcumin mediates chemosensitization to 5-fluorouracil through
miRNA-induced suppression of epithelial-to-mesenchymal transition
in chemoresistant colorectal cancer. Carcinogenesis. 36:355–367.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng L, Luo S, Jin C, Ma H, Zhou H and
Jia L: FUT family mediates the multidrug resistance of human
hepatocellular carcinoma via the PI3K/Akt signaling pathway. Cell
Death Dis. 4:e9232013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harada K, Ferdous T and Ueyama Y:
Establishment of 5-fluorouracil-resistant oral squamous cell
carcinoma cell lines with epithelial to mesenchymal transition
changes. Int J Oncol. 44:1302–1308. 2014.PubMed/NCBI
|
|
26
|
Tanaka T, Bai T and Toujima S:
Establishment and characterization of monoclonal
5-fluorouracil-resistant cell lines derived from human endometrial
adenocarcinoma. Int J Oncol. 37:731–736. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohashi S, Kikuchi O, Tsurumaki M, Nakai Y,
Kasai H, Horimatsu T, Miyamoto S, Shimizu A, Chiba T and Muto M:
Preclinical validation of talaporfin sodium-mediated photodynamic
therapy for esophageal squamous cell carcinoma. PLoS One.
9:e1031262014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li B, Xu WW, Guan XY, Qin YR, Law S, Lee
NP, Chan KT, Tam PY, Li YY, Chan KW, et al: Competitive binding
between Id1 and E2F1 to Cdc20 regulates E2F1 degradation and
thymidylate synthase expression to promote esophageal cancer
chemoresistance. Clin Cancer Res. 22:1243–1255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li B, Tsao SW, Chan KW, Ludwig DL,
Novosyadlyy R, Li YY, He QY and Cheung AL: Id1-induced IGF-II and
its autocrine/endocrine promotion of esophageal cancer progression
and chemoresistance - implications for IGF-II and IGF-IR-targeted
therapy. Clin Cancer Res. 20:2651–2662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hummel R, Sie C, Watson DI, Wang T, Ansar
A, Michael MZ, Van der Hoek M, Haier J and Hussey DJ: MicroRNA
signatures in chemotherapy resistant esophageal cancer cell lines.
World J Gastroenterol. 20:14904–14912. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hummel R, Watson DI, Smith C, Kist J,
Michael MZ, Haier J and Hussey DJ: Mir-148a improves response to
chemotherapy in sensitive and resistant oesophageal adenocarcinoma
and squamous cell carcinoma cells. J Gastrointest Surg. 15:429–438.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shen LY, Wang H, Dong B, Yan WP, Lin Y,
Shi Q and Chen KN: Possible prediction of the response of
esophageal squamous cell carcinoma to neoadjuvant chemotherapy
based on gene expression profiling. Oncotarget. 7:4531–4541.
2016.PubMed/NCBI
|
|
33
|
Sasada S, Miyata Y, Tsutani Y, Tsuyama N,
Masujima T, Hihara J and Okada M: Metabolomic analysis of dynamic
response and drug resistance of gastric cancer cells to
5-fluorouracil. Oncol Rep. 29:925–931. 2013.PubMed/NCBI
|
|
34
|
Weljie AM, Newton J, Mercier P, Carlson E
and Slupsky CM: Targeted profiling: Quantitative analysis of 1H NMR
metabolomics data. Anal Chem. 78:4430–4442. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ouattara DA, Prot JM, Bunescu A, Dumas ME,
Elena-Herrmann B, Leclerc E and Brochot C: Metabolomics-on-a-chip
and metabolic flux analysis for label-free modeling of the internal
metabolism of HepG2/C3A cells. Mol Biosyst. 8:1908–1920. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma Q, Jin B, Zhang Y, Shi Y, Zhang C, Luo
D, Wang P, Duan C, Song H, Li X, et al: Secreted recombinant human
IL-24 protein inhibits the proliferation of esophageal squamous
cell carcinoma Eca-109 cells in vitro and in vivo.
Oncol Rep. 35:2681–2690. 2016.PubMed/NCBI
|
|
37
|
Wang TH, Wan JY, Gong X, Li HZ and Cheng
Y: Tetrandrine enhances cytotoxicity of cisplatin in human
drug-resistant esophageal squamous carcinoma cells by inhibition of
multidrug resistance-associated protein 1. Oncol Rep. 28:1681–1686.
2012.PubMed/NCBI
|
|
38
|
Moghanibashi M, Jazii FR, Soheili ZS, Zare
M, Karkhane A, Parivar K and Mohamadynejad P: Proteomics of a new
esophageal cancer cell line established from Persian patient. Gene.
500:124–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baguley BC: Multidrug resistance in
cancer. Methods Mol Biol. 596:1–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang C, Guo LB, Ma JY, Li YM and Liu HM:
Establishment and characterization of a paclitaxel-resistant human
esophageal carcinoma cell line. Int J Oncol. 43:1607–1617.
2013.PubMed/NCBI
|
|
41
|
Dinicola S, Pasqualato A, Proietti S,
Masiello MG, Palombo A, Coluccia P, Canipari R, Catizone A, Ricci
G, Harrath AH, et al: Paradoxical E-cadherin increase in
5FU-resistant colon cancer is unaffected during
mesenchymal-epithelial reversion induced by γ-secretase inhibition.
Life Sci. 145:174–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yan XD, Li M, Yuan Y, Mao N and Pan LY:
Biological comparison of ovarian cancer resistant cell lines to
cisplatin and Taxol by two different administrations. Oncol Rep.
17:1163–1169. 2007.PubMed/NCBI
|
|
43
|
Longley DB and Johnston PG: Molecular
mechanisms of drug resistance. J Pathol. 205:275–292. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Denise C, Paoli P, Calvani M, Taddei ML,
Giannoni E, Kopetz S, Kazmi SM, Pia MM, Pettazzoni P, Sacco E, et
al: 5-Fluorouracil resistant colon cancer cells are addicted to
OXPHOS to survive and enhance stem-like traits. Oncotarget.
6:41706–41721. 2015.PubMed/NCBI
|
|
45
|
Kim NH, Kim SN, Oh JS, Lee S and Kim YK:
Anti-mitotic potential of
7-diethylamino-3(2′-benzoxazolyl)-coumarin in
5-fluorouracil-resistant human gastric cancer cell line
SNU620/5-FU. Biochem Biophys Res Commun. 418:616–621. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Uchibori K, Kasamatsu A, Sunaga M, Yokota
S, Sakurada T, Kobayashi E, Yoshikawa M, Uzawa K, Ueda S, Tanzawa
H, et al: Establishment and characterization of two
5-fluorouracil-resistant hepatocellular carcinoma cell lines. Int J
Oncol. 40:1005–1010. 2012.PubMed/NCBI
|
|
47
|
Langer R, Ott K, Feith M, Lordick F,
Specht K, Becker K and Hofler H: High pretherapeutic thymidylate
synthetase and MRP-1 protein levels are associated with nonresponse
to neoadjuvant chemotherapy in oesophageal adenocarcinoma patients.
J Surg Oncol. 102:503–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Takebayashi Y, Akiyama S, Natsugoe S,
Hokita S, Niwa K, Kitazono M, Sumizawa T, Tani A, Furukawa T and
Aikou T: The expression of multidrug resistance protein in human
gastrointestinal tract carcinomas. Cancer. 82:661–666. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim YK, Lee SS, Jeong SH, Ahn JS, Yang DH,
Lee JJ, Shin MG and Kim HJ: OCT-1, ABCB1, and
ABCG2 expression in imatinib-resistant chronic myeloid
leukemia treated with dasatinib or nilotinib. Chonnam Med J.
50:102–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Benderra Z, Faussat AM, Sayada L, Perrot
JY, Tang R, Chaoui D, Morjani H, Marzac C, Marie JP and Legrand O:
MRP3, BCRP, and P-glycoprotein activities are prognostic factors in
adult acute myeloid leukemia. Clin Cancer Res. 11:7764–7772. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ke CC, Liu RS, Yang AH, Liu CS, Chi CW,
Tseng LM, Tsai YF, Ho JH, Lee CH and Lee OK: CD133-expressing
thyroid cancer cells are undifferentiated, radioresistant and
survive radioiodide therapy. Eur J Nucl Med Mol Imaging. 40:61–71.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Todaro M, Alea MP, Di Stefano AB,
Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G,
Medema JP, et al: Colon cancer stem cells dictate tumor growth and
resist cell death by production of interleukin-4. Cell Stem Cell.
1:389–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Najafzadeh N, Mazani M, Abbasi A,
Farassati F and Amani M: Low-dose all-trans retinoic acid enhances
cytotoxicity of cisplatin and 5-fluorouracil on CD44+
cancer stem cells. Biomed Pharmacother. 74:243–251. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Thomson S, Petti F, Sujka-Kwok I, Mercado
P, Bean J, Monaghan M, Seymour SL, Argast GM, Epstein DM and Haley
JD: A systems view of epithelial-mesenchymal transition signaling
states. Clin Exp Metastasis. 28:137–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cioce M, Valerio M, Casadei L, Pulito C,
Sacconi A, Mori F, Biagioni F, Manetti C, Muti P, Strano S, et al:
Metformin-induced metabolic reprogramming of chemoresistant
ALDHbright breast cancer cells. Oncotarget. 5:4129–4143.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Makinoshima H, Takita M, Matsumoto S,
Yagishita A, Owada S, Esumi H and Tsuchihara K: Epidermal growth
factor receptor (EGFR) signaling regulates global metabolic
pathways in EGFR-mutated lung adenocarcinoma. J Biol Chem.
289:20813–20823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xie C, Jin J, Bao X, Zhan WH, Han TY, Gan
M, Zhang C and Wang J: Inhibition of mitochondrial glutaminase
activity reverses acquired erlotinib resistance in non-small cell
lung cancer. Oncotarget. 7:610–621. 2016.PubMed/NCBI
|
|
59
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Qian JQ, Sun P, Pan ZY and Fang ZZ:
Annonaceous acetogenins reverses drug resistance of human
hepatocellular carcinoma BEL-7402/5-FU and HepG2/ADM cell lines.
Int J Clin Exp Pathol. 8:11934–11944. 2015.PubMed/NCBI
|
|
61
|
Wang PP, Xu DJ, Huang C, Wang WP and Xu
WK: Astragaloside IV reduces the expression level of P-glycoprotein
in multidrug-resistant human hepatic cancer cell lines. Mol Med
Rep. 9:2131–2137. 2014.PubMed/NCBI
|
|
62
|
Li Q, Wang X, Shen A, Zhang Y, Chen Y,
Sferra TJ, Lin J and Peng J: Hedyotis diffusa Willd
overcomes 5-fluorouracil resistance in human colorectal cancer
HCT-8/5-FU cells by downregulating the expression of P-glycoprotein
and ATP-binding casette subfamily G member 2. Exp Ther Med.
10:1845–1850. 2015.PubMed/NCBI
|