Introduction
Ovarian cancer ranks among the most lethal of
gynecological malignancies (1–3).
Symptoms of early ovarian cancer are not obvious, and 70–80%
patients with ovarian cancer are diagnosed at the advanced or
terminal stage (4). In addition,
ovarian cancer has a low 5-year survival rate (1,5).
Invasion and proliferation are the significant attributes of
malignant cancer that result in high mortality in ovarian cancer.
Thus far, the underlying molecular mechanism of ovarian cancer
remains unclear. A better understanding of the underlying mechanism
of the invasion and proliferation in ovarian cancer could provide
novel insights for the treatment of ovarian cancer.
Annexin A2, which is also known as Annexin II, p36,
p39 and lipocortin II, is a member of the Annexin family (6–8). The
Annexin family is a Ca2+-dependent phospholipid and
membrane binding protein family (9). Annexin A2 is present in human
endothelial cells, monocytes, macrophages, neuron, and some tumor
cells (8,10–12).
The transformed cells express high levels of Annexin A2, and the
terminally differentiated cells have low expression of Annexin A2
(13). In the cells of tumors such
as breast, liver, prostate and gastric cancers, Annexin A2 has been
reported to be upregulated (14–16).
It is indicated that Annexin A2 is closely related to cell
migration, invasion and adhesion in cancers (8,17,18).
Studies have demonstrated that Annexin A2 promoted invasion of
tumor cells containing glioma cells, and human hepatocellular
carcinoma cells (8,19). Additionally, it is reported that
Annexin A2 accelerated proliferation and invasion of human breast
cancer SK-BR-3 cells (18). Annexin
A2 is indicated to be a predictor of serous ovarian cancer outcome
and accelerates ovarian cancer metastasis (20). However, the underlying mechanism of
Annexin A2 in cell invasion and proliferation in ovarian cancer is
yet to be elucidated.
β-catenin is a key regulatory factor of the Wnt
signaling pathway, and it is claimed that β-catenin could be
regulated by Annexin A2 in hepatoma cells (21). The abnormal function and regulation
of β-catenin could lead to aberrant activation of the Wnt signaling
pathway, resulting in abnormalities of gene expression, cell
adhesion, and cancer progression (22). Additionally, studies have reported
that β-catenin is closely related to tumorigenesis (23,24).
In normal cells, there is only a small amount of free intracellular
β-catenin and it cannot enter the cell nucleus to regulate gene
expression. Many studies have shown that β-catenin is upregulated
in many cancers and is associated with poor prognosis (25). The upregulation of β-catenin induces
cell proliferation and invasion in cancers such as ovarian cancer,
breast cancer, colorectal cancer, and gastric carcinoma (26–30).
It has been shown that β-catenin has an effect on the regulation of
EMT (31).
Epithelial-mesenchymal transition (EMT) is
demonstrated to be a process whereby epithelial cells change to
mesenchymal cells with decreased E-cadherin and increased
N-cadherin (32). E-cadherin is the
adhesion molecule of epithelial cell surface, and plays an
important role in the adhesion between cells (33). The downregulation of E-cadherin is
an important feature of EMT (34).
Additionally, EMT is a significant cause of tumor invasion and
metastasis. Studies have demonstrated that EMT induced cell
invasion and proliferation in various cancers such as prostate
cancer, breast cancer, colon cancer, and lung cancer (35,36).
Moreover, EMT has been claimed to be induced and promote the cell
invasion and proliferation in ovarian cancer (37,38).
In this study, we focused on identifying the
molecular mechanism of Annexin A2 in cell invasion and
proliferation in ovarian cancer. We investigated the expression of
Annexin A2 in ovarian cancer tissues and cell lines and revealed
the effect of Annexin A2 inhibition on cell invasion and
proliferation in ovarian cancer. Additionally, we found that the
function of Annexin A2 was realized by regulating EMT via
β-catenin. Annexin A2 may thus be a promising molecular target for
the treatment of ovarian cancer.
Materials and methods
Cell lines and tissues
Human ovarian cancer cell lines SKOV3 and UACC-1598
were purchased from American Type Culture Collection (ATCC,
Manassas, VA, USA), and were cultured in RPMI-1640 (Gibco,
Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; PAA Laboraties
GmbH, Pasching, Austria) in an atmosphere of 5% CO2 at
37°C. The early passages of normal human ovarian epithelial cell
line (HOSEpiC) was obtained from ScienCell Research Laboratories
(San Diego, CA, USA). HOSEpiC was incubated in the Ovarian
Epithelial Cell Medium (OEpiCM) obtained from ScienCell Research
Laboratories in the conditions of 5% CO2 at 37°C.
Additionally, ovarian cancer tissues (n=10) and the matched
adjacent normal tissues (n=10) from ovarian cancer patients (aged
30–45 years) with an average age of 39 years in stage II and III
were obtained following cryopreservation from the Third Affiliated
Hospital of Zhengzhou University along with written informed
consent of the patients. This research was approved by the ethics
committee at the Third Affiliated Hospital of Zhengzhou
University.
Real-time quantitative polymerase
chain reaction (RT-qPCR)
The total RNA was extracted by using TRIzol (Takara
Biotechnology, Dalian, China), and the extraction of RNA from
tissues was performed as previously described (39). Then, the cDNA was synthesized as per
the instructions of the reverse transcription kit (Invitrogen,
Carlsbad, CA, USA), and the RT-qPCR was subsequently performed. The
25-µl reactive volume contains 10-µl SsoFast TMEvaGreen Supermix
(Bio-Rad, Hercules, CA, USA), and the qRT-PCR protocol was: 94°C
for 30 sec; 35 cycles of 95°C for 30 sec, 57°C (Annexin A2 and
β-catenin) or 59°C (E-cadherin and N-cadherin) for 30 sec and 72°C
for 30 sec; and a final step at 72°C for 10 min. GAPDH was the
internal reference gene. The primers were: Annexin A2 forward,
5′-TAACTTTGATGCTGAGCGGG-3′ and reverse, 5′-TAATTTCCTGCAGCTCCTGG-3′;
β-catenin forward, 5′-AAGACATCACTGAGCCTGCCAT-3′ and reverse,
5′-CGATTTGCGGGACAAAGGGCA A-3′; and GAPDH forward,
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse, 5′-GACAAGCTTCCCGTTCTCAG-3′.
The relative levels of gene expression were estimated by the
2−ΔΔCt method.
Western blotting
Proteins were extracted from the cells by using the
lysate and then quantified by using the BCA kit (Pierce, Rockford,
IL, USA). The nuclear protein extracts were separated from the
homogenates using the NXTRACT CelLytic NuCLEAR Extraction kit
(Sigma, St. Louis, MO, USA) according to the standard instructions.
A total of 20 µg protein was added and isolated in the sodium
dodecyl sulfate polyacrylamide gel electrophoresis (12%). The
isolated proteins were transferred to polyvinylidene fluoride
membrane (PVDF; Bio-Rad) following electrophoresis. Next, the
membrane with proteins was blocked in 5% non-fat dry milk in
Tris-buffered saline for 1.5 h. The blocked membrane was then
incubated with the primary antibodies (Cell Signaling Technology,
Danvers, MA, USA) at 4°C overnight. The membrane was washed with
TBS before incubating with horseradish peroxidase conjugated
secondary antibody (Cell Signaling Technology). Finally, blots were
analyzed in the Bio-Rad ChemiDoc apparatus. The relative protein
expression was detected using Image-Pro Plus 6.0.
Construction of recombinant
plasmids
Reverse transcription PCR (RT-PCR) was used to
amplify the full-length β-catenin (GenBank accession no. Z19054),
and treated with the restriction enzymes EcoRI and
BamHI. The β-catenin fragment was then inserted into the
plasmid pcDNA.3.1 (Invitrogen) after which pcDNA.3.1 was treated
with EcoRI and BamHI enzymes. Next, the recombinant
plasmids were transfected into E. coli DH5α (Takara
Biotechnology) and amplified overnight. Then, the amplified
recombinant plasmids were extracted followed by sequencing, and the
correct plasmids were designated pcDNA.3.1-β-catenin.
Transfection of plasmids and
siRNA
SKOV3 and UACC-1598 cell lines that were in good
condition were respectively plated in the 6-well plate followed by
culturing in the atmosphere of 5% CO2 at 37°C for 12 h.
The transfection was then conducted when the cell fusion reached
70%-80%. pcDNA.3.1-β-catenin, Annexin A2 siRNA
(5′-GGTCTGAATTCAAGAGAAA-3′), non-specific siRNA or pcDNA.3.1 was
diluted in the 150-µl FBS-free RPMI-1640 medium with 5 µl TurboFect
(Thermo Fisher Scientific, Waltham, MA, USA) per well.
Subsequently, the transfected cells were cultured in 5%
CO2 at 37°C for 48 h. The transfection efficiency was
detected via RT-qPCR and western blot assays.
Cell growth
For the detection of cell growth, cells were seeded
into a 96-well plate (1×105 cells/well) and subjected to
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. In brief, the transfected cells were cultured in humid
atmosphere of 5% CO2 at 37°C for 48 h, then the culture
medium was replaced by MTT (5 g/l) diluted in phosphate-buffered
saline (PBS) per well (23 µl) and incubated at 37°C for 5 h. A
total of 200 µl dimethyl sulfoxide was added per well to dissolve
the formazan, and the absorbance (OD) values were read using an
SpectroFluor Plus multiwell plate reader (Tecan, Research Triangle
Park, NC, CA, USA) at a wavelength of 490 nm.
Bromodeoxyuridine (BrdU)
Cell proliferation was assessed by a BrdU cell
proliferation assay kit (Millipore, Billerica, MA, USA) according
to the instructions provided. Briefly, the cells were plated in the
96-well plate followed by reaction (1 h) with 10 µl of BrdU
solution per well. Thereafter, a total of 100 µl denaturing
solution was added per well and reacted for 25 min. Cells were then
stained with anti-BrdU antibody for 1.5 h at room temperature
followed by staining with secondary antibody solution. Finally, the
results were detected at 450 nm using a SpectroFluor Plus multiwell
plate reader (Tecan).
Cell invasion assay
The cell invasion ability was measured by Matrigel
invasion assay. Transwell chambers (Corning Incorporated, Toledo,
NY, USA) were coated with Matrigel (BD Bioscience, San Jose, CA,
USA). The transfected cells were cultured in an incubator (Thermo
Fisher Scientific) with 5% CO2 at 37°C for 48 h. Then,
the transfected cells were suspended using 300 µl serum-free medium
(1×105 cells) and added to the top chamber. Moreover,
500 µl of culture medium containing 10% FBS was added to the bottom
chamber and cultured under the conditions of 5% CO2 at
37°C for 48 h. Non-invasive cells in the top chamber were removed
and the invaded cells in the bottom chamber were fixed with 95%
ethanol and stained with trypan blue. Finally, six random fields
per membrane were chosen and the cells in the fields were counted
under a microscope, and then averaged.
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). Statistical analyses were processed with SPSS version 24.0
software (SPSS Inc., Chicago, IL, USA) with one-way analysis of
variance followed by LSD and Bonferroni test. A P-value of <0.05
was considered statistically significant.
Results
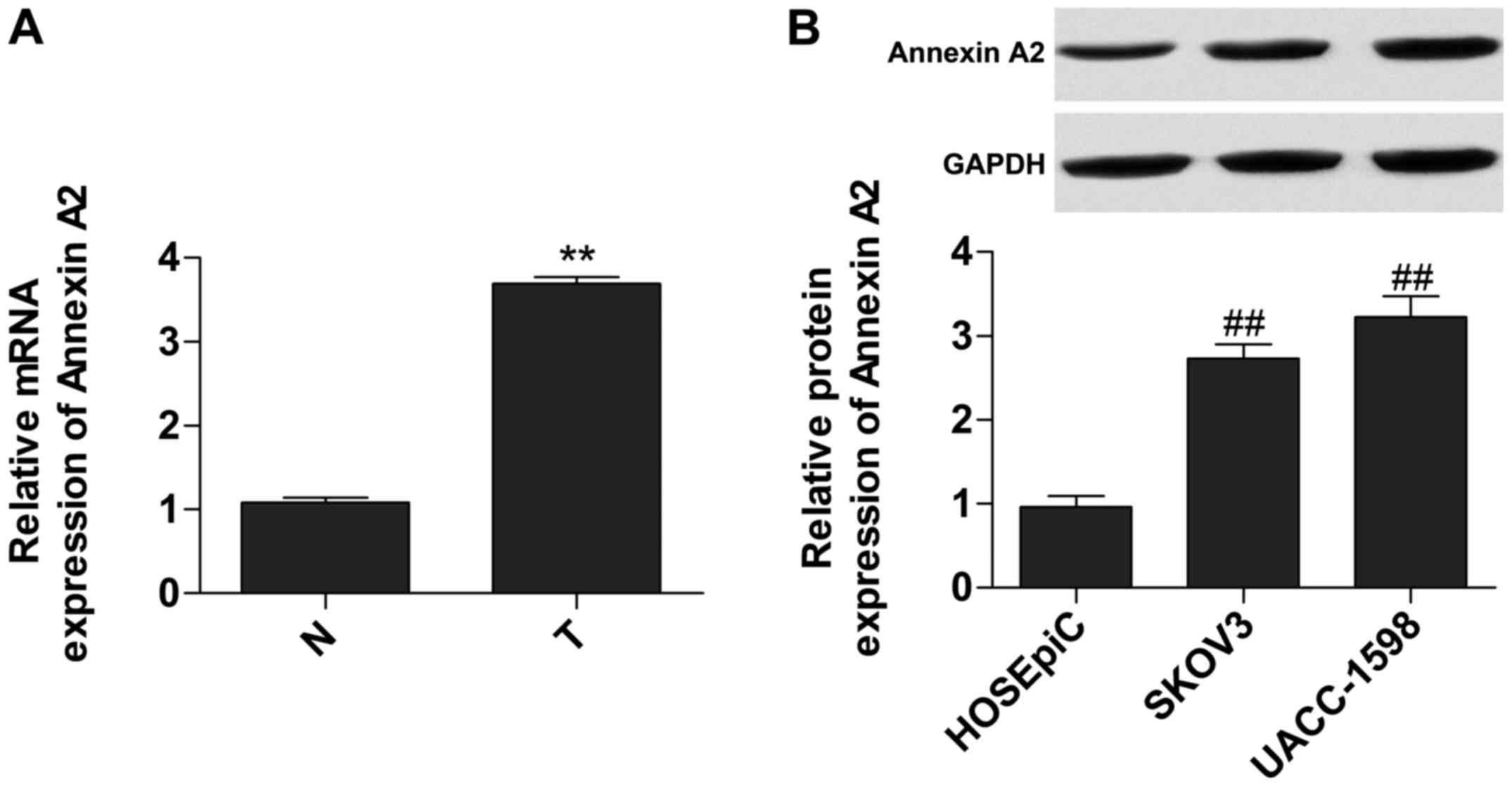
Annexin A2 is upregulated in ovarian
cancer tissues
We measured the relative expression of mRNA and
protein of Annexin A2 in tumor tissues and cell lines,
respectively, to investigate the expression of Annexin A2. Data
showed that the expression of mRNA (Fig. 1A) and protein (Fig. 1B) of Annexin A2 was significantly
increased in tumor tissues and cells.
Suppression of Annexin A2 inhibits
β-catenin
To investigate the effect of Annexin A2 on the
expression of β-catenin, we transfected Annexin A2 siRNA into SKOV3
and UACC-1598 cells to target Annexin A2. The results indicated
that expression of mRNA (Fig. 2A and
B) and protein (Fig. 2C and D)
of Annexin A2 was significantly decreased by the siRNA, and
manifested that the transfection was performed successfully.
Additionally, the data demonstrated that the relative expression of
mRNA (Fig. 2A and B) and protein
(Fig. 2C and D) of β-catenin was
markedly suppressed by Annexin A2 inhibition, and the β-catenin
protein contents in nucleus were also decreased after Annexin A2
suppression (Fig. 2E) in SKOV3 and
UACC-1598 cells.
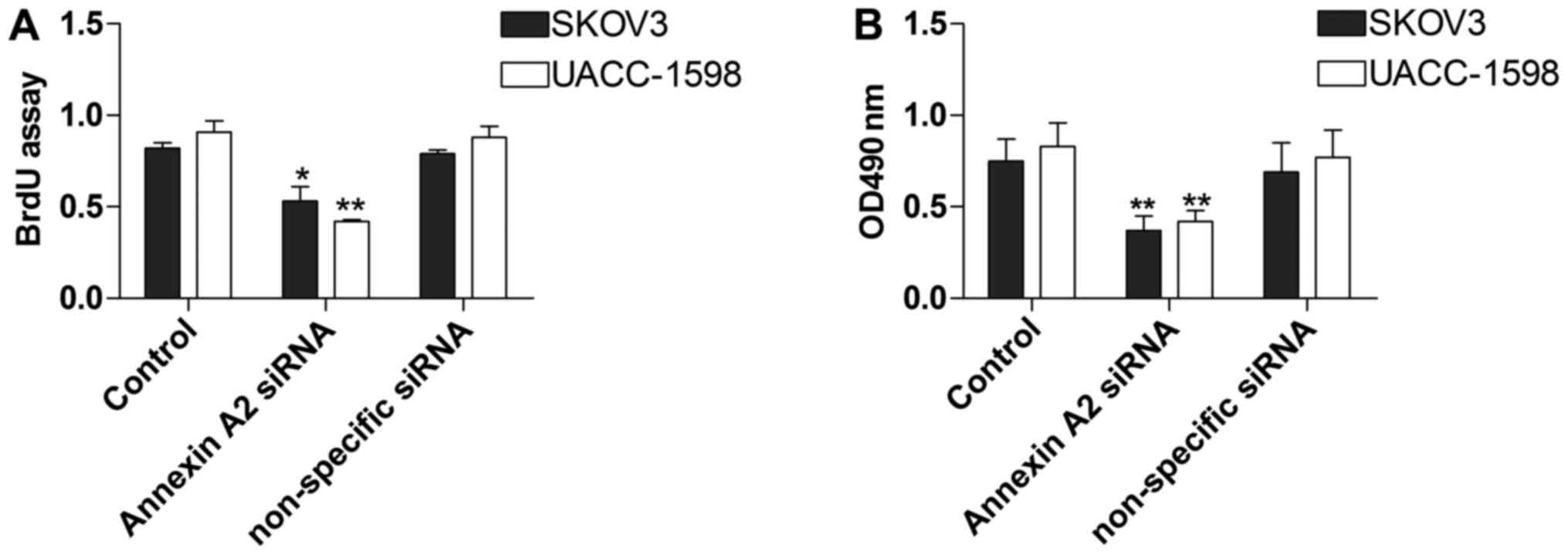
Annexin A2 inhibition restrains the
proliferation of ovarian cancer cell lines
To explore the influence of Annexin A2 on ovarian
cancer cell lines, we conducted the BrdU and MTT assays to measure
SKOV3 and UACC-1598 cell proliferation with Annexin A2 inhibition.
The data showed that the proliferation of SKOV3 and UACC-1598 cells
in the BrdU assay was obviously constrained by Annexin A2
suppression (Fig. 3A). In addition,
cell proliferation was also significantly decreased in the MTT
assay (Fig. 3B).
Annexin A2 inhibition decreases the
invasion ability of ovarian cancer cell lines
To gain insight into the potential role of Annexin
A2 in ovarian cancer cell line invasion ability, we detected the
invasion by EMT measurement and invasion assay in SKOV3 and
UACC-1598 cells with Annexin A2 inhibition. In EMT measurement by
using western blotting, we found that the protein expression of
E-cadherin was obviously increased while N-cadherin was
significantly decreased by Annexin A2 inhibition (Fig. 4A and B). In the invasion assay, cell
invasion ability was markedly constrained by the suppression of
Annexin A2 (Fig. 4C).
Annexin A2 inhibition suppresses EMT
through controlling β-catenin expression
To further explore the molecular mechanism of
Annexin A2 in the regulation of EMT in ovarian cancer cell lines,
we transfected pcDNA.3.1-β-catenin into the SKOV3 and UACC-1598
cells with Annexin A2 inhibition. The data showed that β-catenin
protein expression was markedly increased, whereas Annexin A2 was
decreased in the treated SKOV3 (Fig.
5A) and UACC-1598 (Fig. 5B),
indicating that the transfection of pcDNA.3.1-β-catenin was
successful. Additionally, we found that the suppressive effect of
Annexin A2 inhibition on EMT was apparently reversed by
pcDNA.3.1-β-catenin transfection (Fig.
6A and B). Moreover, β-catenin overexpression obviously
abolished the inhibitory influence of Annexin A2 inhibition to the
invasion (Fig. 6C) and
proliferation (Fig. 6D) of SKOV3
and UACC-1598 cells.
Discussion
Because of the dietary structure, environmental
pollution and other factors, ovarian cancer morbidity is increasing
year by year (40,41). Annexins are widely involved in
regulating cell membrane construction and material transport
(9). Additionally, Annexins have
been indicated to be involved in the development of tumors
(42). It has been demonstrated
that Annexin A2 is a potential prognostic factor, and has a
stimulatory effect on ovarian cancer. Moreover, Annexin A2 is
reported to promote cell proliferation and invasion in breast
cancer (43). Currently, the role
and underlying mechanism of Annexin A2 in cell proliferation and
invasion in ovarian cancer is unclear. In our study, Annexin A2 was
significantly increased in ovarian cancer tissues and cells in
accordance with the above reports. Besides, we found that cell
proliferation and invasion in ovarian cancer were both obviously
constrained in the loss-of-function experiment of Annexin A2.
Annexin A2 has been indicated to regulate the expression of
β-catenin.
β-catenin, which is located on human chromosome
3p21, and plays a pivotal role in the classic Wnt signaling pathway
(44). The major β-catenin in the
cytoplasm forms the adhesion complex with E-cadherin inside the
cell membrane, and the level of dissociative β-catenin content in
normal cells is low (45). It is
reported that β-catenin is increased in malignant tumors such as
breast cancer, colon cancer, and gastrointestinal cancer (46–48).
Additionally, β-catenin could regulate EMT in cancers, and is
demonstrated to promote EMT in gastric cancer (29,31,49,50).
In our study, we observed that the expression in cell and the
nucleus content of β-catenin was significantly decreased by Annexin
A2 downregulation. In addition, the results showed that β-catenin
overexpression markedly reversed the inhibitory effect of Annexin
A2 suppression on EMT. Thus, the data indicated that Annexin A2
could regulate EMT via controlling β-catenin in ovarian cancer.
Conacci-Sorrell et al indicated that the strong
β-catenin/TCF signaling promoted the expression of Slug causing the
suppression of E-cadherin expression in sparse SW480 cells,
accelerating the process of EMT (51). In our study, β-catenin inhibition
realizes the promoted function on E-cadherin, resulting in the
suppression of EMT, consisting with that found in sparse SW480
cells. Thus, we considered that β-catenin inhibition possibly
decreases Slug expression and then increases E-cadherin, resulting
in the suppression of EMT.
EMT is the morphological process where epithelial
cells transform into mesenchymal cells, and the imbalance of EMT
plays an important role in invasion and metastatic processes in
cancer (36). In the EMT process,
cancer cells access migration and invasion and obtain the
characteristics of stem cells through a loss of cell-cell adhesion
and cell polarity, transforming into the mesenchymal-like cell
morphology(51). Studies have
reported that EMT acts as the driver of invasion and metastasis in
cancers (52). Furthermore, EMT has
been revealed to modulate the invasion and proliferation of cells
in colorectal cancer (53,54). In our study, EMT was inhibited while
the loss-of-function experiment of Annexin A2 was performed, and
the invasion and proliferation were both constrained in ovarian
cancer cells. In addition, overexpression of β-catenin abolished
the suppressive function of Annexin A2 on EMT, while the cell
invasion and proliferation were promoted. Based on the above,
Annexin A2 inhibition could constrain cell invasion and
proliferation via regulating β-catenin/EMT in ovarian cancer.
In conclusion, this study found that Annexin A2
expression is accelerated in ovarian cancer tissues and cell lines.
Annexin A2 suppression has an inhibitory effect on EMT and cell
invasion and proliferation. Additionally, Annexin A2 regulation of
EMT could been realized by controlling β-catenin. Therefore, the
potential role of Annexin A2 in ovarian cancer is revealed and
provides novel insight into the treatment of ovarian cancer.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
RT-qPCR
|
real-time quantitative polymerase
chain reaction
|
|
PVDF
|
polyvinylidene fluoride membrane
|
|
PBS
|
phosphate-buffered saline
|
|
BrdU
|
bromodeoxyuridine
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
References
|
1
|
Lowe KA, Chia VM, Taylor A, O'Malley C,
Kelsh M, Mohamed M, Mowat FS and Goff B: An international
assessment of ovarian cancer incidence and mortality. Gynecol
Oncol. 130:107–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anugraham M, Jacob F, Nixdorf S,
Everest-Dass AV, Heinzelmann-Schwarz V and Packer NH: Specific
glycosylation of membrane proteins in epithelial ovarian cancer
cell lines: Glycan structures reflect gene expression and DNA
methylation status. Mol Cell Proteomics. 13:2213–2232. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liang Y, Aebi J, Nephew K and Hyder SM:
Targeting cholesterol biosynthesis pathway to inhibit growth of
drug resistant ovarian cancer cells. Cancer Res. 76 Suppl.
14:37892016. View Article : Google Scholar
|
|
4
|
Gilbert L, Basso O, Sampalis J, Karp I,
Martins C, Feng J, Piedimonte S, Quintal L, Ramanakumar AV,
Takefman J, et al: DOvE Study Group: Assessment of symptomatic
women for early diagnosis of ovarian cancer: Results from the
prospective DOvE pilot project. Lancet Oncol. 13:285–291. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar S, Meuter A, Thapa P, Langstraat C,
Giri S, Chien J, Rattan R, Cliby W and Shridhar V: Metformin intake
is associated with better survival in ovarian cancer: A
case-control study. Cancer. 119:555–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang J, Yang F, Nie J, Zou X, Tian H, Qin
Y and Liu C: Evaluation of Annexin A2 as a novel diagnostic serum
biomarker for lung cancer. Cancer Biomark. 15:205–211. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu D, Sun L, Liu S, Zhang L and Yang H:
Histological, ultrastructural and heat shock protein 70 (HSP70)
responses to heat stress in the sea cucumber Apostichopus
japonicus. Fish Shellfish Immunol. 45:321–326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Onishi M, Ichikawa T, Kurozumi K, Inoue S,
Maruo T, Otani Y, Fujii K, Ishida J, Shimazu Y, Yoshida K, et al:
Annexin A2 regulates angiogenesis and invasion phenotypes of
malignant glioma. Brain Tumor Pathol. 32:184–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu W and Hajjar KA: The Annexin A2 system
and angiogenesis. Biol Chem. 397:1005–1016. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai H, Yu Z, Fan X, Liu N, Yan M, Chen Z,
Lo EH, Hajjar KA and Wang X: Dysfunction of Annexin A2 contributes
to hyperglycaemia-induced loss of human endothelial cell surface
fibrinolytic activity. Thromb Haemost. 109:1070–1078. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bharadwaj A, Bydoun M, Holloway R and
Waisman D: Annexin A2 heterotetramer: Structure and function. Int J
Mol Sci. 14:6259–6305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamashita K, Nagai H and Toyokuni S:
Receptor role of the Annexin A2 in the mesothelial endocytosis of
crocidolite fibers. Lab Invest. 95:749–764. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kantara C, O'Connell MR, Luthra G, Gajjar
A, Sarkar S, Ullrich RL and Singh P: Methods for detecting
circulating cancer stem cells (CCSCs) as a novel approach for
diagnosis of colon cancer relapse/metastasis. Lab Invest.
95:100–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang F, Wang Z, Yuan J, Wei X, Tian R and
Niu R: RNAi-mediated silencing of Anxa2 inhibits breast cancer cell
proliferation by downregulating cyclin D1 in STAT3-dependent
pathway. Breast Cancer Res Treat. 153:263–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deng Y, Chen C, Hua M, Xi Q, Liu R, Yang
S, Liu J, Zhong J, Tang M, Lu S, et al: Annexin A2 plays a critical
role in epithelial ovarian cancer. Arch Gynecol Obstet.
292:175–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Q, Ye Z, Yang Q, He X, Wang H and
Zhao Z: Upregulated expression of annexin II is a prognostic marker
for patients with gastric cancer. World J Surg Oncol. 10:1032012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiu D, Liu L, Qiao F, Yang H, Cui L and
Liu G: Annexin A2 coordinates STAT3 to regulate the invasion and
migration of colorectal cancer cells in vitro. Gastroenterol Res
Pract. 2016:35214532016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang YQ, Zhang F, Tian R, Ji W, Zhou Y,
Sun XM, Liu Y, Wang ZY and Niu RF: Tyrosine 23 phosphorylation of
Annexin A2 promotes proliferation, invasion, and Stat3
phosphorylation in the nucleus of human breast cancer SK-BR-3
cells. Cancer Biol Med. 9:248–253. 2012.PubMed/NCBI
|
|
19
|
Zhang W, Zhao P, Xu XL, Cai L, Song ZS,
Cao DY, Tao KS, Zhou WP, Chen ZN and Dou KF: Annexin A2 promotes
the migration and invasion of human hepatocellular carcinoma cells
in vitro by regulating the shedding of CD147-harboring
microvesicles from tumor cells. PLoS One. 8:e672682013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lokman NA, Elder AS, Ween MP, Pyragius CE,
Hoffmann P, Ruszkiewicz A, Ricciardelli C and Oehler MK: Annexin
A2, a potential prognostic marker for serous ovarian cancer
promotes ovarian cancer metastasisClinical and Experimental
Medicine. Springer; Dordrecht: pp. 220. 2015
|
|
21
|
Wang C, Guo Y, Wang J and Min Z: Annexin
A2 knockdown inhibits hepatoma cell growth and sensitizes hepatoma
cells to 5-fluorouracil by regulating β-catenin and cyclin D1
expression. Mol Med Rep. 11:2147–2152. 2015.PubMed/NCBI
|
|
22
|
Amini-Nik S, Cambridge E, Yu W, Guo A,
Whetstone H, Nadesan P, Poon R, Hinz B and Alman BA:
β-Catenin-regulated myeloid cell adhesion and migration determine
wound healing. J Clin Invest. 124:2599–2610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Llado V, Nakanishi Y, Duran A,
Reina-Campos M, Shelton PM, Linares JF, Yajima T, Campos A,
Aza-Blanc P, Leitges M, et al: Repression of intestinal stem cell
function and tumorigenesis through direct phosphorylation of
β-catenin and Yap by PKCζ. Cell Rep. 10:740–754. 2015. View Article : Google Scholar
|
|
24
|
Kang DW, Choi CY, Cho YH, Tian H, Di Paolo
G, Choi KY and Min S: Targeting phospholipase D1 attenuates
intestinal tumorigenesis by controlling β-catenin signaling in
cancer-initiating cells. J Exp Med. 212:1219–1237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu G, Wang Y, Huang B, Liang J, Ding Y,
Xu A and Wu W: A Rac1/PAK1 cascade controls β-catenin activation in
colon cancer cells. Oncogene. 31:1001–1012. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen BY, Wang X, Wang ZY, Wang YZ, Chen LW
and Luo ZJ: Brain-derived neurotrophic factor stimulates
proliferation and differentiation of neural stem cells, possibly by
triggering the Wnt/β-catenin signaling pathway. J Neurosci Res.
91:30–41. 2013.PubMed/NCBI
|
|
27
|
Simic P, Zainabadi K, Bell E, Sykes DB,
Saez B, Lotinun S, Baron R, Scadden D, Schipani E and Guarente L:
SIRT1 regulates differentiation of mesenchymal stem cells by
deacetylating β-catenin. EMBO Mol Med. 5:430–440. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wend P, Runke S, Wend K, Anchondo B,
Yesayan M, Jardon M, Hardie N, Loddenkemper C, Ulasov I, Lesniak
MS, et al: WNT10B/β-catenin signalling induces HMGA2 and
proliferation in metastatic triple-negative breast cancer. EMBO Mol
Med. 5:264–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang J, Xiao D, Li G, Ma J, Chen P, Yuan
W, Hou F, Ge J, Zhong M, Tang Y, et al: EphA2 promotes
epithelial-mesenchymal transition through the Wnt/β-catenin pathway
in gastric cancer cells. Oncogene. 33:2737–2747. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagaraj AB, Joseph P, Kovalenko O, Singh
S, Armstrong A, Redline R, Resnick K, Zanotti K, Waggoner S and
DiFeo A: Critical role of Wnt/β-catenin signaling in driving
epithelial ovarian cancer platinum resistance. Oncotarget.
6:23720–23734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo Q and Qin W: DKK3 blocked
translocation of β-catenin/EMT induced by hypoxia and improved
gemcitabine therapeutic effect in pancreatic cancer Bxpc-3 cell. J
Cell Mol Med. 19:2832–2841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rogers CD, Saxena A and Bronner ME: Sip1
mediates an E-cadherin-to-N-cadherin switch during cranial neural
crest EMT. J Cell Biol. 203:835–847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McEwen AE, Maher MT, Mo R and Gottardi CJ:
E-cadherin phosphorylation occurs during its biosynthesis to
promote its cell surface stability and adhesion. Mol Biol Cell.
25:2365–2374. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang K, Yang G, Wu W, Zhang J, Xia X,
Jiang T, Cao J, Huang K, Qiu Z and Huang C: Decreased expression of
caveolin-1 and E-cadherin correlates with the clinicopathologic
features of gastric cancer and the EMT process. Recent Patents
Anticancer Drug Discov. 11:236–244. 2016. View Article : Google Scholar
|
|
35
|
Xia Y, Wu Y, Liu B, Wang P and Chen Y:
Downregulation of miR-638 promotes invasion and proliferation by
regulating SOX2 and induces EMT in NSCLC. FEBS Lett. 588:2238–2245.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rokavec M, Öner MG, Li H, Jackstadt R,
Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et
al: IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated
colorectal cancer invasion and metastasis. J Clin Invest.
124:1853–1867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mao Y, Xu J, Li Z, Zhang N, Yin H and Liu
Z: The role of nuclear β-catenin accumulation in the Twist2-induced
ovarian cancer EMT. PLoS One. 8:e782002013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lili LN, Matyunina LV, Walker LD, Wells
SL, Benigno BB and McDonald JF: Molecular profiling supports the
role of epithelial-to-mesenchymal transition (EMT) in ovarian
cancer metastasis. J Ovarian Res. 6:492013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li YL, Ye F, Hu Y, Lu WG and Xie X:
Identification of suitable reference genes for gene expression
studies of human serous ovarian cancer by real-time polymerase
chain reaction. Anal Biochem. 394:110–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie J, Poole EM, Terry KL, Fung TT, Rosner
BA, Willett WC and Tworoger SS: A prospective cohort study of
dietary indices and incidence of epithelial ovarian cancer. J
Ovarian Res. 7:1122014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
García-Pérez J, Lope V, López-Abente G,
González-Sánchez M and Fernández-Navarro P: Ovarian cancer
mortality and industrial pollution. Environ Pollut. 205:103–110.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Peng B, Guo C, Guan H, Liu S and Sun MZ:
Annexin A5 as a potential marker in tumors. Clin Chim Acta.
427:42–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu B, Zhang F, Yu M, Zhao P, Ji W, Zhang
H, Han J and Niu R: Up-regulation of Anxa2 gene promotes
proliferation and invasion of breast cancer MCF-7 cells. Cell
Prolif. 45:189–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mei XD, Su H, Song J and Dong L:
Prognostic significance of β-catenin expression in patients with
non-small cell lung cancer: A meta-analysis. Biosci Trends.
7:42–49. 2013.PubMed/NCBI
|
|
45
|
Sempou E, Biasini E, Pinzón-Olejua A,
Harris DA and Málaga-Trillo E: Activation of zebrafish Src family
kinases by the prion protein is an amyloid-β-sensitive signal that
prevents the endocytosis and degradation of E-cadherin/β-catenin
complexes in vivo. Mol Neurodegener. 11:182016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Siemens H, Neumann J, Jackstadt R,
Mansmann U, Horst D, Kirchner T and Hermeking H: Detection of
miR-34a promoter methylation in combination with elevated
expression of c-Met and β-catenin predicts distant metastasis of
colon cancer. Clin Cancer Res. 19:710–720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yan D, Avtanski D, Saxena NK and Sharma D:
Leptin-induced epithelial-mesenchymal transition in breast cancer
cells requires β-catenin activation via Akt/GSK3- and MTA1/Wnt1
protein-dependent pathways. J Biol Chem. 287:8598–8612. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zheng H, Li W, Wang Y, Liu Z, Cai Y, Xie
T, Shi M, Wang Z and Jiang B: Glycogen synthase kinase-3 beta
regulates Snail and β-catenin expression during Fas-induced
epithelial-mesenchymal transition in gastrointestinal cancer. Eur J
Cancer. 49:2734–2746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zha L, Zhang J, Tang W, Zhang N, He M, Guo
Y and Wang Z: HMGA2 elicits EMT by activating the Wnt/β-catenin
pathway in gastric cancer. Dig Dis Sci. 58:724–733. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cheung CT, Bendris N, Paul C, Hamieh A,
Anouar Y, Hahne M, Blanchard JM and Lemmers B: Cyclin A2 modulates
EMT via β-catenin and phospholipase C pathways. Carcinogenesis.
36:914–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Conacci-Sorrell M, Simcha I, Ben-Yedidia
T, Blechman J, Savagner P and Ben-Ze'ev A: Autoregulation of
E-cadherin expression by cadherin-cadherin interactions: The roles
of β-catenin signaling, Slug, and MAPK. J Cell Biol. 163:847–857.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang RY, Guilford P and Thiery JP: Early
events in cell adhesion and polarity during epithelial-mesenchymal
transition. J Cell Sci. 125:4417–4422. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Biddle A and Mackenzie IC: Cancer stem
cells and EMT in carcinoma. Cancer Metastasis Rev. 31:285–293.
2012. View Article : Google Scholar
|
|
54
|
Zou J, Luo H, Zeng Q, Dong Z, Wu D and Liu
L: Protein kinase CK2α is overexpressed in colorectal cancer and
modulates cell proliferation and invasion via regulating
EMT-related genes. J Transl Med. 9:972011. View Article : Google Scholar : PubMed/NCBI
|