Introduction
Inflammation is a beneficial host response to
foreign challenge or tissue injury that leads ultimately to the
restoration of tissue structure and function (1). The response consists of a sequential
release of mediators and recruitment of circulating leukocytes
which become activated at the inflammatory site and then release
further mediators (2). TNF-α is now
known more generally as a mediator of inflammatory responses. The
pro-inflammatory effects of TNF-α are primarily due to its ability
to activate NF-κB (3). NF-κB is
responsible for the transcription of the genes encoding many
pro-inflammatory cytokines and chemokines (2). In resting cells, NF-κB complexes are
inactive, residing predominantly in the cytoplasm in a complex with
inhibitory IκBs (1). Upon
appropriate stimulation for example by TNF-α, IκB is phosphorylated
by IκB kinases (IKKs), polyubiquitinated by a ubiquitin ligase
complex, and degraded by the 26S proteasome (4,5). It
has been shown that TNF-α can activate the PI3K/Akt pathway, which
in turn has been shown to be able to activate the NF-κB signaling
pathway through IKKα/β in HeLa cells (6,7).
Reactive oxygen species (ROS) are constantly
generated and eliminated in the biological system and are required
to drive regulatory pathways (8).
Under normal physiologic conditions, cells control ROS levels by
balancing the generation of ROS with their elimination by
scavenging system. But under oxidative stress conditions, excessive
ROS can damage cellular proteins, lipids and DNA, leading to fatal
lesions in cells that contribute to carcinogenesis. It has been
shown that ROS can mediate the TNF-α-induced inflammation through
the Akt-mediated activation of NF-κB (9).
Imperatorin [9-(3-methylbut-2-enyloxy)-7H-furo
[3,2-g]chromen-7-one] is a naturally occurring furanocoumarin,
which can be found in selected herbal medicines, namely in the
roots and fruits of Angelica dahurica and Angelica
archangelica (Umbelliferae, Apiaceae) (10). It has multiple therapeutic
activities and is used to treat antibacterial activity, cancer,
inflammation, and antiviral activity. The anti-inflammatory effects
of imperatorin have also been reported (11). However, the effect of imperatorin on
PI3K/Akt/NF-κB pathway has not been studied. Herein, we were able
to show that imperatorin not only inhibited the activation of
NF-κB, which in turn induced the expression of NF-κB target genes,
such as angiogenesis (VEGF), invasion (MMP-9), proliferation
(cyclin D1 and COX-2) and major inflammatory cytokines (IL-6), but
also potentiated TNF-α-induced apoptosis (cIAP-1, Bcl-xL
and Bcl-2) the production of reactive oxygen species (ROS) and
phosphorylation of Akt, IKKα/β, and IκBα, trans-activity of NF-κB.
These findings showed that the suppression of NF-κB activation by
imperatorin is a possible strategy to inhibit inflammation.
Materials and methods
Cell culture and reagents
HeLa cells and 293 cells were purchased from
American Type Culture Collection (ATCC, Manassas, VA, USA). HeLa
cells and 293 cells were cultured in DMEM containing 10% FBS
(Gibco, Grand Island, NY, USA) and 1% penicillin/streptomycin
(Invitrogen, Carlsbad, CA, USA) at 37°C with 5% CO2
atmosphere in a humidified incubator. TNF-α was obtained from
R&D Systems (Minneapolis, MN, USA). N-acetyl-L-cysteine (NAC)
was from Sigma (St. Louis, MO, USA). The primary antibodies for
IκBα, phosphor (Ser32)-specific IκBα, (Ser536)-specific p65, PARP,
cyclin D1, caspase-8, c-IAP2, Akt1, phospho-Akt (Ser473),
phosphor-IKKα/β were purchased from Cell Signaling Technology
(Beverly, MA, USA). Antibodies for COX-2, MMP-9, VEGF, IKKα and
Topo-I were obtained from Santa Cruz Biotechnology (Santa Cruz, CA,
USA). Antibody for α-tubulin was from Sigma. Imperatorin was bought
from the National Institutes for Food and Drug Control (NIFDC,
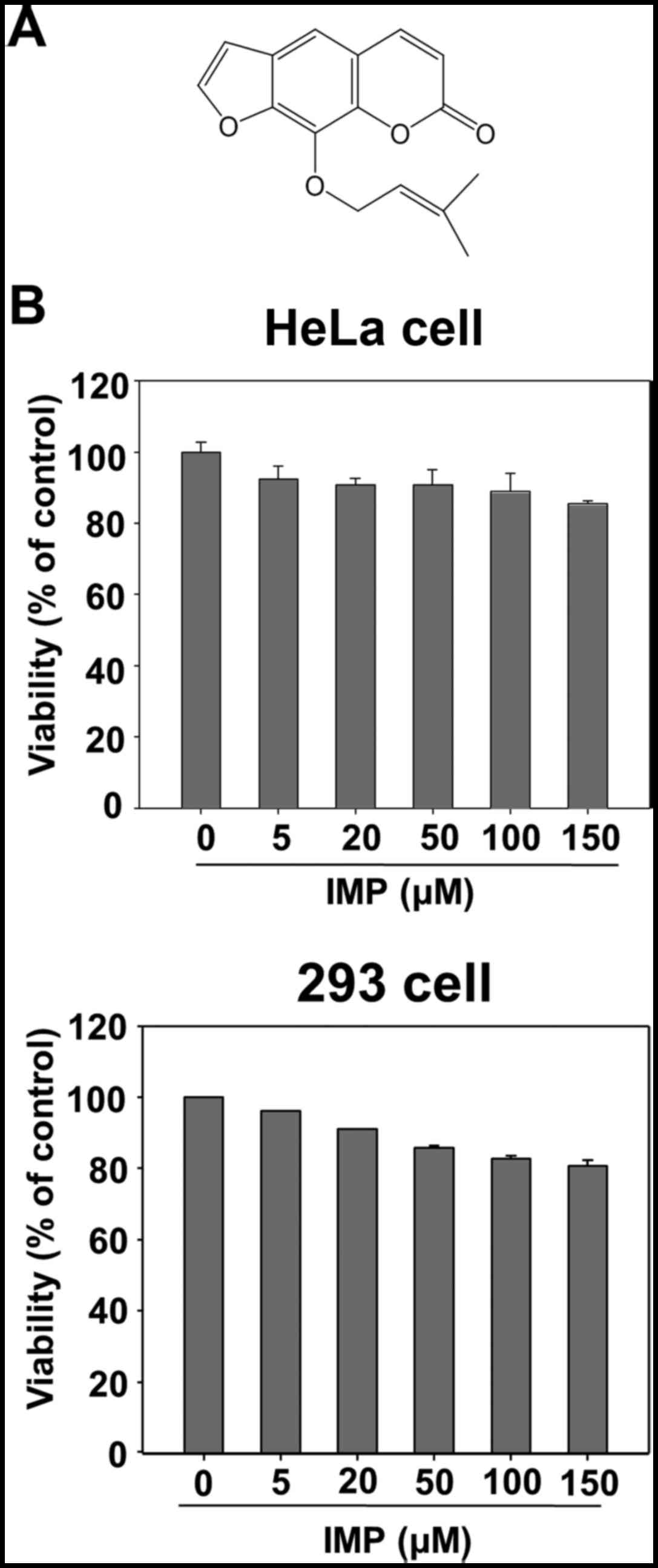
Beijing, China) and its structure is shown in Fig. 1A. The purity of imperatorin was over
99% in HPLC analysis.
Measurement of cell viability by MTT
assay
Cell viability was measured by a MTT assay
(Sigma-Aldrich). MTT assays were performed as previously described
(12). Briefly, the cells were
treated with imperatorin (5–150 µM) in triplicate wells for 24 h
followed by the addition of MTT to the cells. The MTT formazan
crystals were dissolved in DMSO. The absorbance at 570 nm was
measured by microplate reader.
Plasmids, transfections, and
luciferase reporter assay
A pNF-κB-Luc plasmid for NF-κB luciferase reporter
assay was obtained from Strategene (La Jolla, CA, USA).
Transfections were performed as previously described (13). In brief, at 50–80% confluence, HeLa
cells, were cotransfected with the vectors for
pGL3-NF-κB-Luciferase plasmid using Lipofectamine 2000 reagent
(Invitrogen). Cells were lysed and luciferase activity was
determined using the Dual Luciferase Reporter Assay system.
Enzyme-linked immunosorbent assay
(ELISA)
HeLa cells were plated in 96-well plate at a density
of 1×105 cells per well and treated with various
concentrations of imperatorin for 12 h and then incubated with
TNF-α for 12 h. The IL-6 and MMP-9 levels in the culture
supernatant were determined by ELISA kit (Cusabio Biotech Co.,
Ltd., Newark, NJ, USA) according to the manufacturer's
instructions.
Apoptosis assays
Apoptosis assays were performed as previously
described (14). The HeLa cells
were stained with Annexin V-FITC using a FITC Annexin V apoptosis
detection kit (BD Biosciences, San Jose, CA, USA). The cells were
washed twice with PBS (pH 7.4) and re-suspended in binding buffer.
The pooled cells were stained with Annexin V-FITC in the dark for
15 min at room temperature. Last, 2 µg/ml Propidium Iodide (PI) was
added, and the suspension was incubated in the dark for 5 min at
37°C. After 400 µl of binding buffer was added, the samples were
analyzed by flow cytometry. The data were analyzed by Cell Quest
software (Becton-Dickinson).
Measurement of ROS production
Intracellular ROS production was measured using 2′,
7′-dichlorodihydrofluorescein-diacetate (H2DCFDA; Life
Technologies). HeLa cells were treated with imperatorin for 24 h
and then 10 ng/ml TNF-α for 30 min. Then HeLa cells were incubated
with H2DCFDA (10 µM) for 30 min at 37°C. After removal of the
medium and washing of the cells, images were obtained using a
fluorescence microscopy.
Western blot analysis
Cell lysates were separated by SDS-polyacrylamide
gels and transferred to a PVDF (Millipore). The blots were blocked
and then incubated with specific antibodies against indicated
primary antibodies. Proteins were visualized by enhanced
chemiluminescence according to the instructions of the manufacturer
(Amersham Pharmacia Biotech, Buckinghamshire, UK).
Real-time PCR
The total RNA was extracted using RNeasy Mini kits
according to the manufacturer's instructions (Qiagen, CA, USA).
RT-PCR was performed with a Qiagen one-step RT-PCR system kit
(Qiagen OneStep RT-PCR kit handbook). In brief, 1 µg of total RNA
from each sample was added to 50 µl of a reaction mixture
containing 0.4 mM dNTP, 0.6 µM sense and antisense specific
primers, 5 units of RNase inhibitor, 2 µl of Qiagen One-step RT-PCR
Enzyme Mix including Omniscript™ and Sensiscript™ reverse
transcriptase. The following primer pairs were used for reverse
transcription-PCR amplification: human interleukin-6 (IL-6),
5′-ACAAAGCCAGAGTCCTTCAGAGA-3′ and 5′-CTGTTAGGAGAGCATTGGAAATTG-3′;
human TNF-α, 5′-CTGCCCCAATCCCTTTATT-3′ and
5′-CCCAATCTCTTTTTGAGCC-3′; Bcl-xL, 5′-
GTAAACTGGGGTCGCATTGT-3′ and 5′-TGCTGCATTGTTCCCATAGA-3′; GAPDH,
5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′. The
reaction mixture was incubated for 30 min at 50°C for the reverse
transcription reaction, for 15 min at 95°C for the inactivation of
reverse transcriptase and the activation of HotStarTaq DNA
polymerase, and then amplified using a three-temperature PCR system
consisting of denaturation at 94°C for 30 sec, primer annealing at
55°C for 30 sec, and extension at 72°C for 1 min. The number of
cycles was 25–40. The cycle threshold values (Ct values) were used
to calculate the fold differences using the ∆∆CT method,
and GAPDH expression was used as the internal control.
Immunofluorescence assay
HeLa cells were grown directly on cover slips in
24-well plates (1×104 cells/well) for 24 h, then cells
were pretreated with imperatorin (150 µM) for 24 h, whereafter,
treated with 10 ng/ml TNF-α. Cell treated with DMSO was used as
negative control and treated with 10 ng/ml TNF-α alone was used as
positive control. After treatment, the cells were washed in PBS,
fixed at room temperature with 4% paraformaldehyde, and
permeabilized with 0.2% Triton X-100. Immunofluorescence staining
was performed according to standard procedures. Briefly, the
treated cells were first stained with the anti-p65 antibody
followed by incubation with FITC conjugated anti-rabbit IgG
secondary antibody and co-staining with DAPI.
Statistical analysis
All results are expressed as mean ± SD at least
three independent experiments. A comparison of the results was
analyzed with one-way ANOVA followed by Tukey's multiple comparison
tests (Graphpad Software, Inc, San Diego, CA, USA). P-value
<0.05 was considered to be statistically significant.
Results
Effect of imperatorin on the viability
of HeLa and 293 cells
In order to assess imperatorin toxicity in cell
cultures, preliminary experiments were carried out using MTT test.
As shown in Fig. 1B, imperatorin
did not adversely affect cell viability on HeLa and 293 cells up to
150 µM.
Imperatorin downregulates
TNF-α-induced NF-κB-regulated gene products
To address effects of imperatorin on the expression
of NF-κB targets genes, we evaluated TNF-α-induced expression of
antiapoptotic protein c-IAP2, proliferative proteins COX-2 and
cyclin D1, adhesion proteins ICAM-1 and VCAM-1, and metastatic
protein MMP-9 and angiogenic protein VEGF by western blotting.
Imperatorin inhibited TNF-α-induced expression of all these
proteins in a concentration-dependent manner (Fig. 2A). Because NF-κB regulates major
inflammatory cytokines, including IL-6, Bcl-xL and
TNF-α, many of which are potent activators for NF-κB (15–17),
we determined whether imperatorin affected the expression of
TNF-α-induced IL-6, Bcl-xL and TNF-α mRNA levels, the
results suggest that imperatorin blocked TNF-α-induced expression
of anti-inflammatory gene products in a dose-dependent manner
(Fig. 2B). Moreover, we measured
the release of some cytokines in cell supernatant, such as IL-6 and
MMP-9, imperatorin could suppress the levels of these cytokines in
a concentration-dependent manner (Fig.
2C).
 | Figure 2.Effect of imperatorin (IMP) on the
expression of TNF-α-induced NF-κB-regulated target genes. (A) HeLa
cells were incubated with indicated concentrations of imperatorin
(IMP) for 12 h and then incubated with TNF-α (10 ng/ml) for 12 h.
Whole cell extracts were analyzed by western blotting using
indicated antibodies for c-IAP2, COX-2, cyclin D1, Bcl-2, VCAM-1,
ICAM-1, MMP-9, VEGF, and tubulin. (B) HeLa cells were incubated
with indicated concentrations of imperatorin (IMP) for 12 h and
then incubated with TNF-α (10 ng/ml) for 12 h. The mRNA expression
of Bcl-XL, IL-6, and TNF-α was measured by real-time PCR
as described in Materials and methods. (C) IL-6, and MMP-9 proteins
expression were evaluated by ELISA in culture supernatant of HeLa
cells after exposure to TNF-α (10 ng/ml) for 24 h in the presence
or absence of the indicated concentrations of imperatorin (IMP).
Data presented as mean ± standard deviation of three independent
experiments. *p<0.05, **p<0.01, ***p<0.001, significant
with respect to control. |
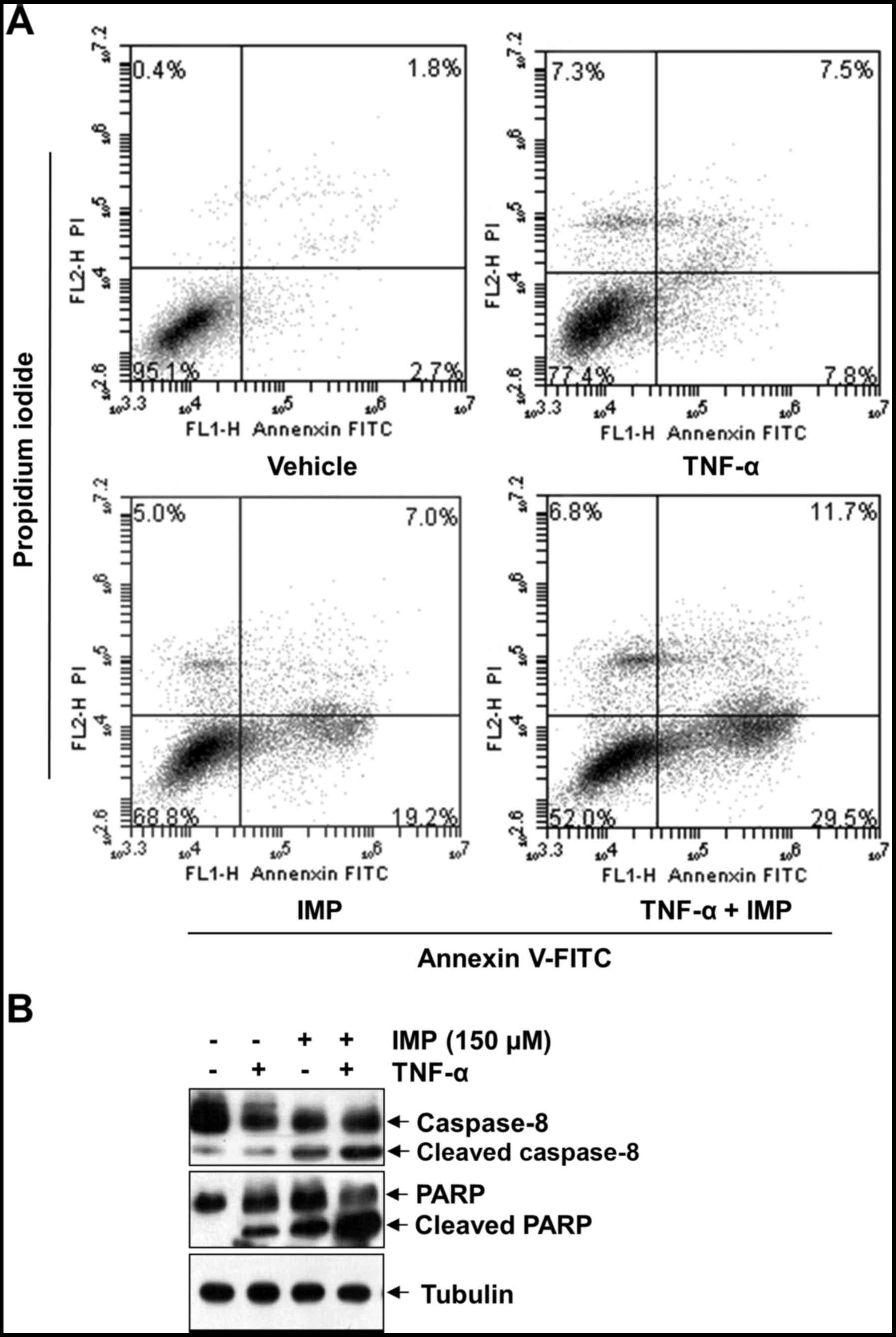
Imperatorin promotes TNF-α-induced
apoptosis
We used Annexin V-FITC/PI double staining to assess
the ability of imperatorin (150 µM) to TNF-α-induced apoptosis. The
Annexin V-positive cell population (41.2%) significantly increased
when treated with TNF-α and imperatorin, whereas no treatment
(4.5%), treatment with TNF-α alone (15.3%) or imperatorin alone
(26.2%) had only slight influence on cell apoptosis, as shown in
Fig. 3A. In addition, cleavage of
caspase-8 and PARP are considered hallmarks of apoptosis. Thus, we
examined TNF-α-induced caspase-8 and PARP levels after imperatorin
treatment in HeLa cells by western blotting. As shown in Fig. 3B, imperatorin alone had little
effect on caspase-8 and PARP cleavage. However, combined treatment
of TNF-α with imperatorin resulted in potentiated activation. These
results together indicate that imperatorin enhances the apoptotic
effect of HeLa cells by TNF-α.
Imperatorin inhibits TNF-α-induced
NF-κB activation
To test whether imperatorin inhibits the effect of
imperatorin on TNF-α-induced NF-κB activation, we performed NF-κB
reporter assay. After cells were transiently transfected with the
NF-κB-regulated luciferase reporter vector, the cells were further
incubated with TNF-α in the presence of various concentrations of
imperatorin. We discovered that imperatorin can substantially block
TNF-α-stimulated NF-κB reporter activity in a
concentration-dependent manner (Fig.
4A). Then we inspected whether imperatorin regulates
TNF-α-induced translocation of p65 to the nucleus. HeLa cells were
treated with TNF-α (10 ng/ml) and various concentrations of
imperatorin. After a 24 h incubation, NF-κB p65 was assessed by
western blot assay. The results revealed that imperatorin also
suppressed TNF-α-induced translocation of p65 to the nucleus in a
concentration-dependent manner (Fig.
4B). In HeLa cells, TNF-α-induced p65 nucleus translocation was
completed in 15 min (Fig. 4C). To
validate the suppression of NF-κB nuclear translocation by
imperatorin, we implemented immunofluorescence to survey p65
nuclear tranlocation in HeLa cells. NF-κB p65 was located at the
cytoplasm without incubation (Fig.
4D, top panel, control). HeLa cells were treated with TNF-α,
p65 was activated and translocated into the nucleus (Fig. 4D, middle panel, TNF-α).
TNF-α-stimulated p65 nuclear tranlocation was inhibited by
imperatorin (150 µM) (Fig. 4D
bottom panel). These results suggested that imperatorin can
suppress the activation of NF-κB, which induced by TNF-α, through
inhibiting nuclear translocation of p65.
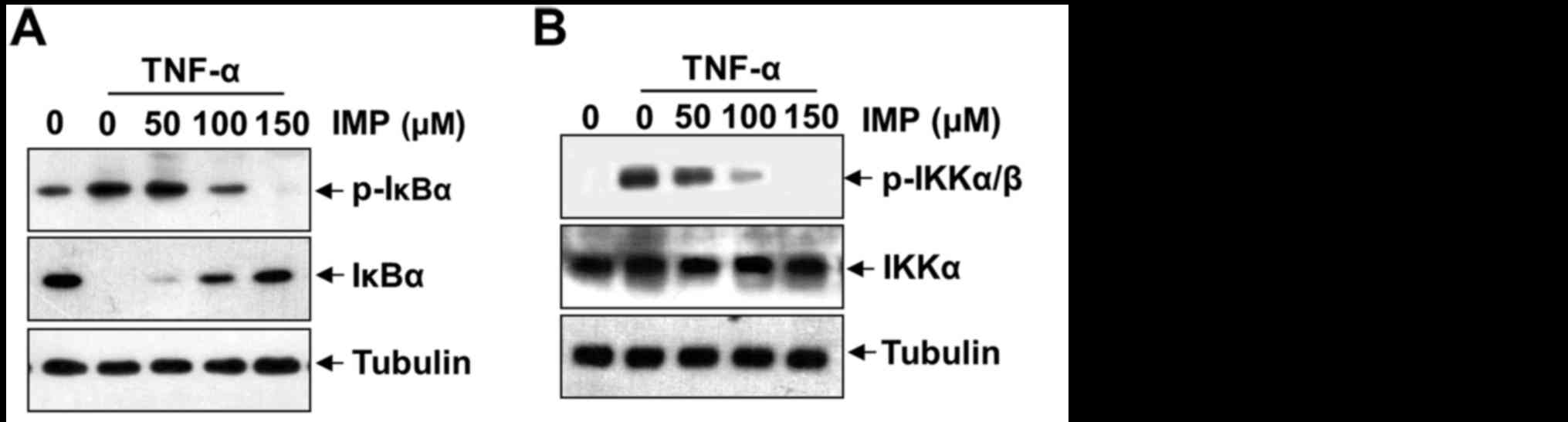
Imperatorin inhibits TNF-α-induced
NF-κB activation by modulating phosphorylation and degradation of
IκB, phosphorylation of IKKα/β and activation of PI3K/Akt
The translocation of NF-κB to the nucleus is
preceded by the phosphorylation, ubiquitination, and proteolytic
degradation of IκBα (18). To
determine whether inhibition of TNF-α-induced NF-κB activation by
imperatorin is caused by inhibition of IκBα degradation, we exposed
the HeLa cells to imperatorin and then treated with TNF-α. As shown
in Fig. 5A, imperatorin
convictively repressed the phosphorylation and degradation of IκBα
by incubation with TNF-α in a concentration-dependent manner.
IKKα/β can regulate IκB phosphorylation and appears to be vital in
NF-κB transcriptional responses. To further investigate the
mechanism of imperatorin suppression of NF-κB activation, we
examined the effect of imperatorin on the TNF-α-induced
phosphorylation of IKKα/β. As shown in Fig. 5B, imperatorin can inhibit TNF-α
induced phosphorylation of IKKα/β. These results sufficiently
certify that imperatorin can repress activation of NF-κB pathway
through the canonical signaling cascade. To ascertain activation of
PI3K-Akt in response to TNF-α, we examined the phosphorylation of
Akt after stimulation of imperatorin with TNF-α. As shown in
Fig. 5C, a dose-dependent
phosphorylation of Akt was observed, while non-phosphorylated Akt
remained the same.
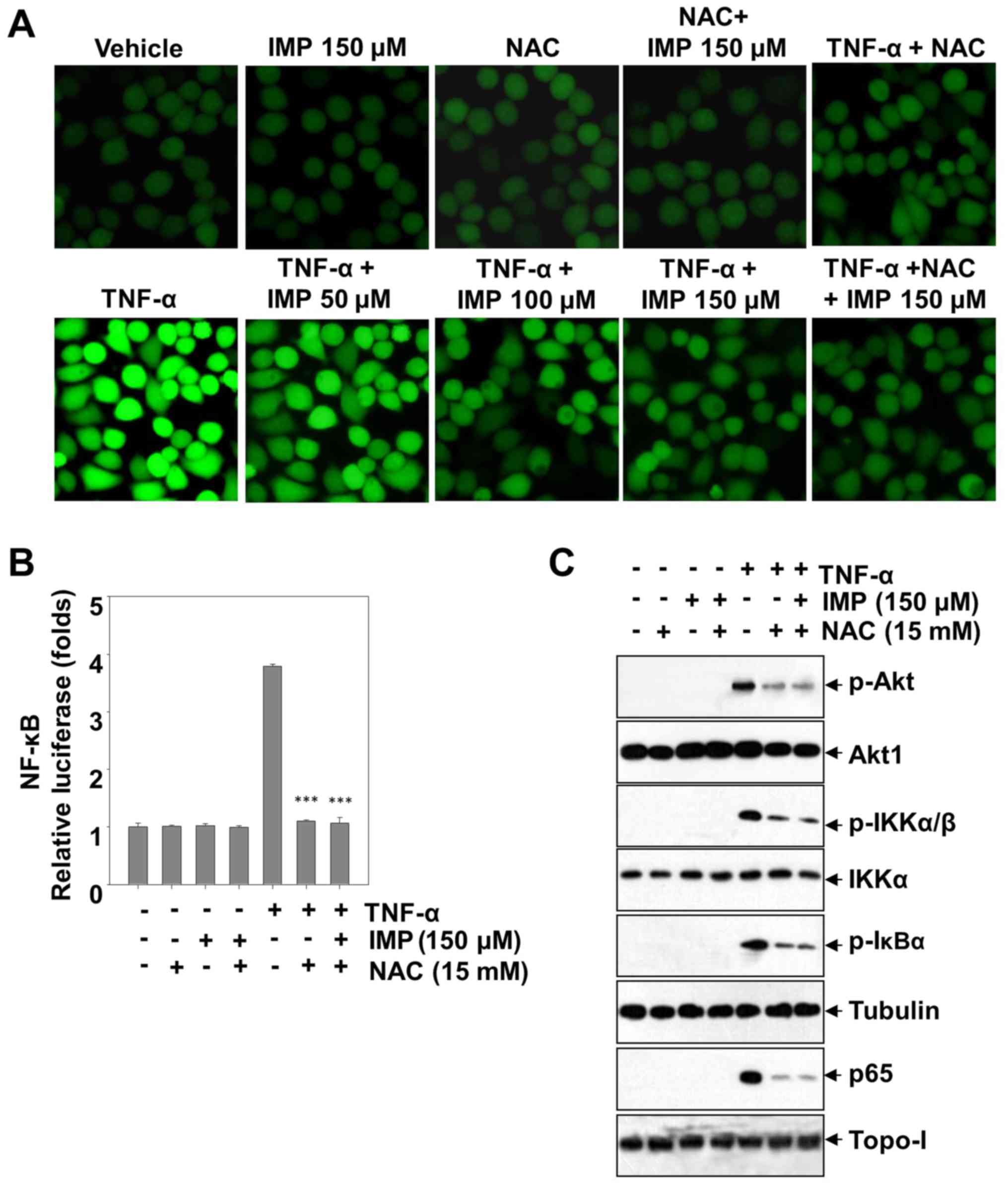
Anti-oxidative effects of imperatorin
on ROS production
Reactive oxygen species (ROS) activate various
transcription factors such as NF-κB, AP-1, hypoxia-inducible
factor-1α and STAT3, leading to expression of proteins that control
inflammation. In order to investigate whether this is also true in
HeLa cells and can be regulated by imperatorin, we used
CM-H2DCF-DA, a fluorescent probe, to detect the level of ROS
production. Treatment with TNF-α improves the endocellular level of
ROS, however, imperatorin could blunt the promotion in a
dose-dependent manner. After adding NAC (an inhibitor of ROS), the
TNF-α-stimulated ROS production was absolutely blunted. The level
of ROS production has not been ulteriorly decreased by co-treatment
with NAC and imperatorin, which showing that imperatorin inhibited
the level of ROS similarly to NAC. Furthermore, treatment with
imperatorin abrogated the TNF-α induced phosphorylation of Akt,
IKKα/β and IκBα, and NF-κB activation, and markedly decreased the
NF-κB-dependent luciferase expression induced by TNF-α (Fig. 6B and C). Taken together, these
results suggest that imperatorin can inhibit the TNF-α-stimulated
inflammation in HeLa cells through blunting the ROS-regulated
PI3K/Akt and NF-κB activation.
Discussion
In previous study, Kang et al identified
imperatorin is a type of coumarin compound with antibacterial and
antiviral activities from Angelica dahurica, which has been
used to treat headache of common cold, nasal stuffiness,
supraorbital neuralgia, painful swelling on the body, leukorrhea
and arthralgia due to wind-dampness in Chinese traditional medicine
(19). However, the molecular
mechanism of anti-inflammatory effect of imperatorin among these
pharmacological activities has not been adequately explained. We
not only identified imperatorin as an inhibitor of NF-κB and
PI3K-Akt activation, but also investigated how this compound
works.
It has been reported that imperatorin can attenuate
inflammation via weakening LPS-induced macrophages and
oxLDL-induced U937 foam cells (11,19,20).
In our results, we show that imperatorin suppressed inflammation by
block TNF-α-induced PI3K/Akt/NF-κB signaling pathway. First of all,
imperatorin can promote TNF-α-induced apoptosis, efficient
phagocytosis of apoptotic cells is of great importance in
vivo, because the clearance of apoptotic cells prior to lysis
is critical to prevent inflammation (21–23).
In this study, imperatorin inhibited TNF-α-induced expression of
anti-apoptotic proteins such as c-IAP2, Bcl-2 and
Bcl-xL, which are known to be regulated by NF-κB, then
activated caspase protein family, prompted PARP cleavage, and
finally induced apoptosis by mitochondrial pathway. On the other
hand, imperatorin promoted TNF-α-induced apoptosis analyzed with
Annexin V/PI staining by flow cytometry.
NF-κB also controls the gene expression which is
important for the cell cycle, adhesion and proliferation (24). Cyclin D1 takes part in many
biological processes and play critical roles in NF-κB mediated
tumorigenesis, such as regulating the mitotic cell cycle. ICAM-1
and VCAM-1 participate in the recruitment of the immune cells
(25,26). COX-2-induced in HeLa cells by
pro-inflammatory cytokines, may be responsible for the edema and
vasodilation associated with cellular proliferation and survival.
Our results also indicate that imperatorin blocks the expression of
VCAM-1, ICAM-1, VEGF, MMP-9, COX-2 and cyclin D1, which have been
shown to be expressed in response to NF-κB activation. Besides,
based on the current study, we detected that imperatorin can reduce
the TNF-α induced expression of IL-6 and TNF-α, which are typical
cytokines and have a wide variety of biological functions in the
regulation of immune response, homeostasis, and inflammation
(27). As shown in Fig. 2, imperatorin dose-dependently
inhibited expression of these genes. The activation of NF-κB
requires phosphorylation of IκB, which then targets IκB for
ubiquitination and degradation. Inhibition of Akt, which was
demonstrated as diminished Akt phosphorylation in the present
experiment, caused decreased phosphorylation of IκB and attenuated
the degradation of IκB in HeLa cells. This might inhibit
translocation of NF-κB to the nucleus, where it normally activates
gene transcription.
In addition to adaptor molecules, kinases or
ubiquitinases and de-ubiquitinases, other classes of molecules were
reported to have an influence on NF-κB activity: These include
reactive oxygen species (ROS), which are compounds containing free
electrons usually linked to oxygen atoms that are not part of an
atomic bond (28). ROS are capable
of eliciting a variety of pathological changes, including apoptotic
and peroxidation of lipids, proteins, and DNA. In general, moderate
oxidative stress induces apoptosis, whereas anti-apoptotic system
is triggered when cells have a higher exposure to ROS (29). The high level of ROS can activate
PI3K/Akt signaling pathways, so NF-κB-regulated Bcl-2 proteins will
be produced, while apoptosis will be inhibited (30–32).
Therefore, modulators of ROS production and their signaling
pathways could represent potential targets for anti-inflammatory
intervention (33). Then, it was
proved that imperatorin suppresses the production of TNF-α-induced
ROS, indicating its anti-inflammatory function in the
ROS/PI3K/Akt/NF-κB pathway.
Taken together, our findings indicate that
imperatorin can affect activation of each step of the NF-κB
signaling pathway and NF-κB-regulated gene products, which are
connected with anti-angiogenic, anti-proliferative, anti-invasive,
anti-oxidation pro-apoptotic and anti-inflammatory effects. Based
on our results, we have provided preclinical evidence of
imperatorin as a potential agent against inflammatory diseases.
Acknowledgements
This work was partially supported by National
Natural Science Foundation of China, no. 81360496 and 81460193.
This study also received assistance from Jilin Province Science and
Technology Development Plan item (20150101229JC) and Project of
Education Department of Jilin Province (2016.281).
Glossary
Abbreviations
Abbreviations:
|
ROS
|
reactive oxygen species
|
|
NF-κB
|
nuclear factor-κB
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IκBα
|
inhibitor of NF-κBα
|
|
IL-6
|
interleukin-6
|
|
c-IAP2
|
cellular inhibitor of apoptosis-2
|
|
ICAM-1
|
intercellular adhesion molecule-1
|
|
VCAM-1
|
vascular cell adhesion molecule-1
|
|
Bcl-2
|
B-cell lymphoma-2
|
|
Bcl-xL
|
cell lymphoma/leukemia-xl
|
|
COX-2
|
cyclooxygenase-2
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Lawrence T, Willoughby DA and Gilroy DW:
Anti-inflammatory lipid mediators and insights into the resolution
of inflammation. Nat Rev Immunol. 2:787–795. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayden MS and Ghosh S: NF-κB in
immunobiology. Cell Res. 21:223–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sethi G, Sung B and Aggarwal BB: TNF: A
master switch for inflammation to cancer. Front Biosci.
13:5094–5107. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–663. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen J and Chen ZJ: Regulation of NF-κB by
ubiquitination. Curr Opin Immunol. 25:4–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seo SH and Jeong GS: Fisetin inhibits
TNF-α-induced inflammatory action and hydrogen peroxide-induced
oxidative damage in human keratinocyte HaCaT cells through
PI3K/AKT/Nrf-2-mediated heme oxygenase-1 expression. Int
Immunopharmacol. 29:246–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bai C, Yang X, Zou K, He H, Wang J, Qin H,
Yu X, Liu C, Zheng J, Cheng F, et al: Anti-proliferative effect of
RCE-4 from Reineckia carnea on human cervical cancer HeLa cells by
inhibiting the PI3K/Akt/mTOR signaling pathway and NF-κB
activation. Naunyn Schmiedebergs Arch Pharmacol. 389:573–584. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dickinson BC and Chang CJ: Chemistry and
biology of reactive oxygen species in signaling or stress
responses. Nat Chem Biol. 7:504–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murillo MM, Carmona-Cuenca I, Del Castillo
G, Ortiz C, Roncero C, Sánchez A, Fernández M and Fabregat I:
Activation of NADPH oxidase by transforming growth factor-beta in
hepatocytes mediates up-regulation of epidermal growth factor
receptor ligands through a nuclear factor-kappaB-dependent
mechanism. Biochem J. 405:251–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baek NI, Ahn EM, Kim HY and Park YD:
Furanocoumarins from the root of Angelica dahurica. Arch Pharm Res.
23:467–470. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo W, Sun J, Jiang L, Duan L, Huo M, Chen
N, Zhong W, Wassy L, Yang Z and Feng H: Imperatorin attenuates
LPS-induced inflammation by suppressing NF-κB and MAPKs activation
in RAW 264.7 macrophages. Inflammation. 35:1764–1772. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mi C, Shi H, Ma J, Han LZ, Lee JJ and Jin
X: Celastrol induces the apoptosis of breast cancer cells and
inhibits their invasion via downregulation of MMP-9. Oncol Rep.
32:2527–2532. 2014.PubMed/NCBI
|
|
13
|
Hwangbo C, Kim J, Lee JJ and Lee JH:
Activation of the integrin effector kinase focal adhesion kinase in
cancer cells is regulated by crosstalk between protein kinase
Calpha and the PDZ adapter protein mda-9/Syntenin. Cancer Res.
70:1645–1655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin HR, Jin SZ, Cai XF, Li D, Wu X, Nan
JX, Lee JJ and Jin X: Cryptopleurine targets NF-κB pathway, leading
to inhibition of gene products associated with cell survival,
proliferation, invasion, and angiogenesis. PLoS One. 7:e403552012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aggarwal BB: Nuclear factor-kappaB: The
enemy within. Cancer Cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baeuerle PA and Henkel T: Function and
activation of NF-kappaB in the immune system. Annu Rev Immunol.
12:141–179. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang G and Ghosh S: Toll-like
receptor-mediated NF-kappaB activation: A phylogenetically
conserved paradigm in innate immunity. J Clin Invest. 107:13–19.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109 Suppl:S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang OH, Lee GH, Choi HJ, Park PS, Chae
HS, Jeong SI, Kim YC, Sohn DH, Park H, Lee JH, et al: Ethyl acetate
extract from Angelica dahuricae Radix inhibits
lipopolysaccharide-induced production of nitric oxide,
prostaglandin E2 and tumor necrosis factor-alphavia
mitogen-activated protein kinases and nuclear factor-kappaB in
macrophages. Pharmacol Res. 55:263–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang PY, Rui YC, Li K, Huang XH, Jiang JM
and Yu L: Expression of intercellular adhesion molecule-1 in U937
foam cells and inhibitory effect of imperatorin. Acta Pharmacol
Sin. 23:327–330. 2002.PubMed/NCBI
|
|
21
|
Savill J: Apoptosis. Phagocytic docking
without shocking. Nature. 392:442–443. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Savill J, Dransfield I, Gregory C and
Haslett C: A blast from the past: Clearance of apoptotic cells
regulates immune responses. Nat Rev Immunol. 2:965–975. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rovere P, Sabbadini MG, Vallinoto C,
Fascio U, Recigno M, Crosti M, Ricciardi-Castagnoli P, Balestrieri
G, Tincani A and Manfredi AA: Dendritic cell presentation of
antigens from apoptotic cells in a proinflammatory context: Role of
opsonizing anti-beta2-glycoprotein I antibodies. Arthritis Rheum.
42:1412–1420. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-kappaB functions as a tumour promoter in
inflammation-associated cancer. Nature. 431:461–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pober JS and Sessa WC: Evolving functions
of endothelial cells in inflammation. Nat Rev Immunol. 7:803–815.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hermida N and Balligand JL: Low-density
lipoprotein-cholesterol-induced endothelial dysfunction and
oxidative stress: The role of statins. Antioxid Redox Signal.
20:1216–1237. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yeom M, Kim JH, Min JH, Hwang MK, Jung HS
and Sohn Y: Xanthii fructus inhibits inflammatory responses in
LPS-stimulated RAW 264.7 macrophages through suppressing NF-κB and
JNK/p38 MAPK. J Ethnopharmacol. 176:394–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saito Y, Nishio K, Ogawa Y, Kimata J,
Kinumi T, Yoshida Y, Noguchi N and Niki E: Turning point in
apoptosis/necrosis induced by hydrogen peroxide. Free Radic Res.
40:619–630. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Porta C and Figlin RA:
Phosphatidylinositol-3-kinase/Akt signaling pathway and kidney
cancer, and the therapeutic potential of
phosphatidylinositol-3-kinase/Akt inhibitors. J Urol.
182:2569–2577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen J, Yang H, Wen J, Luo K, Liu Q, Huang
Y, Zheng Y, Tan Z, Huang Q and Fu J: NHE9 induces chemoradiotherapy
resistance in esophageal squamous cell carcinoma by upregulating
the Src/Akt/β-catenin pathway and Bcl-2 expression. Oncotarget.
6:12405–12420. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim JH, Na HJ, Kim CK, Kim JY, Ha KS, Lee
H, Chung HT, Kwon HJ, Kwon YG and Kim YM: The non-provitamin A
carotenoid, lutein, inhibits NF-kappaB-dependent gene expression
through redox-based regulation of the phosphatidylinositol
3-kinase/PTEN/Akt and NF-kappaB-inducing kinase pathways: Role of
H(2)O(2) in NF-kappaB activation. Free Radic Biol Med. 45:885–896.
2008. View Article : Google Scholar : PubMed/NCBI
|