Introduction
Tumor necrosis factor-α-related apoptosis-inducing
ligand (TRAIL/Apo2L), a member of the tumor necrosis factor (TNF)
family of apoptosis triggering proteins, is believed to selectively
induce apoptosis in cancer cells but not the normal cells (1,2).
Recombinant soluble TRAIL (rsTRAIL) initiates extrinsic apoptosis
through binding to death receptors (DRs) 4 and/or 5 expressed on
the cell surface in a variety of tumor cells (3). Preclinical and clinical studies
indicate that TRAIL is safe for potential therapeutic use (4,5).
However, TRAIL resistance has been widely reported, and this
resistance appears to be mediated through the loss of TRAIL
receptors, inhibitors such as cFLIP, X-linked inhibitor of
apoptosis protein (XIAP), cIAP, and survivin alternations in
expression of the Bcl-2 family proteins (6–9).
Combinational therapy enhancing TRAIL-induced apoptosis through
differential regulation of pro- and anti-apoptotic proteins have
been reported, including combination with
chemo-radiotherapy/biological agents. These kinds of strategies
have been shown to have favorable development prospects (2).
Plumbagin (5-hydroxy-2-methyl-1,4-napthoquinone),
which is isolated from the roots of the medicinal plant Plumbago
zeylanica L, has been safely used for centuries in Indian
Ayurvedic and Oriental medicine for the treatment of various
ailments (10,11). Plumbagin and its analogs exhibit a
variety of potent pharmacological and biological activities
including anti-microbial (12,13),
anti-malarial (14),
anti-inflammatory (15),
anti-atherosclerotic (16),
anti-diabetic (17) and
neuroprotective (18) effects. The
promising antineoplastic effect of plumbagin has also been
demonstrated in various cancer models both in vivo and in
vitro. It effectively induces apoptosis and exerts
anti-proliferation activity in diverse cancer cell lines, such as
leukemia (19,20), lung cancer (21,22),
prostate cancer (23), breast
cancer (24–26), ovarian cancer (27), cervical cancer (28) and melanoma (29). The mechanisms of its antitumor
effect are still not fully unveiled.
The balanced translocation between chromosome eight
and twenty-one [t(8;21)] is one of the most frequent chromosomal
abnormalities observed in acute myeloid leukemia (AML) and only
~50% of patients with this relatively favorable subtype can survive
for 5 years (30). The Kasumi-1
cell line with the expression of t(8;21) is widely used as a model
for the study of this AML subtype (31). Although TRAIL and/or plumbagin were
investigated in some leukemic cell lines, there is no previous
report on plumbagin, TRAIL and their combination on Kasumi-1 cell
line.
In this study, we determined the cell proliferation
inhibition activity of both agents alone and/or combination by
using in vitro and in vivo experimental models on
Kasumi-1 cells and also explored their possible mechanisms.
Collectively, our results suggest that combination treatment with
plumbagin and TRAIL might be an effective therapeutic strategy for
TRAIL resistant AML with t(8;21).
Materials and methods
Reagents
Plumbagin and NAC were purchased from Sigma-Aldrich
(USA). Plumbagin was dissolved in dimethyl sulfoxide (DMSO). The
recombinant human zinc ion positioned trimeric form of TRAIL/Apo-2L
was a gift from Shanghai Qiaer Co. Revert Aid First strand cDNA
synthesis kit was from Fermentas (EU). SYBR Green PCR master mix
was from AB Applied Biosystems (UK). For western blot analysis, the
antibodies used were: anti-DR5 (Abcam), anti-caspase-3,
anti-caspase-8, anti-caspase-9 (R&D), anti-Bid (Abcam),
anti-bcl-2 (R&D), anti-bax (R&D), anti-GAPDH (Cell
Signaling Technology), anti-cFLIP (Stressgen) and
peroxidase-conjugated goat anti-rabbit and anti-mouse antibodies
(Chemicon Co., USA and Canada). For flow cytometry antibodies used
were: anti-DR4 (LifeSpan BioSciences, Inc., USA), anti-DR5
(LifeSpan BioSciences, Inc.), anti-TRAIL (Biolegend, USA),
anti-DcR1 (LifeSpan BioSciences, Inc.), anti-DcR1 (LifeSpan
BioSciences, Inc.). Neutralizing anti-TRAIL-R2 (DR5) was purchased
from Diaclone (French).
Cell cultures, cell morphology and
TUNEL assay
Human leukemic Kasumi-1 cell lines were kindly
provided by Professor J.X. Wang (Institute of Hematology and Blood
Diseases Hospital, CAMS and PUMC) or J. Zhu (Shanghai Institute of
Hematology) and confirmed by morphology, cytogenetics,
immunophenotype and/or molecular analysis. Kasumi-1 cells were
cultured in RPMI-1640 medium supplemented with 20% fetal bovine
serum (FBS). Cultures were maintained in a humidified atmosphere of
95% air/5% CO2 at 37°C. Cell morphology was evaluated by
Wright's staining of cells prepared by cytospin centrifugation.
Cells were assessed by imaging the TUNEL-positive cells under a
light microscope after incubating for 12 h using TUNEL kit
(Chemicon Co.).
Cell proliferation assay
Cell proliferation was analyzed using a Cell
Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan). In brief, cells
were seeded on a 96-well plate (Corning Inc., Corning, NY, USA) at
a concentration of 5×105 cells/well in a volume of 200
µl. The cells were incubated with plumbagin, rsTRAIL or the
combination of plumbagin and rsTRAIL at different concentrations
for 12, 24 or 48 h and the absorbance was measured at 490 nm by
spectrophotometry.
Flow cytometry analysis of Annexin
V/PI, cell cycle, mitochondrial membrane potential, and death
receptors
A total of 2×104 cells were evaluated by
Annexin V/PI kit (BD Pharmingen, USA) according to FACSCanto™ flow
cytometer. To assess the cell cycle, cells were collected, washed
in PBS and fixed overnight in 75% ethanol at −20°C, incubated with
PI/RNase staining buffer (BD Pharmingen, USA) for 15 min at room
temperature. For the detection of mitochondrial membrane potential,
1×106 cells were washed twice with PBS, incubated with
JC-1 kit (BD Pharmingen) for 15 min at 37°C in the dark. Cell
surface expression of DR4 and DR5 was detected by incubation of
Kasumi-1 cells with primary antibodies (anti-DR4, anti-DR5) with
FITC (fluorescein isothiocyanate) for 15 min at room temperature.
Fluorescence intensity was monitored using a Beckman flow
cytometry. All experiments were performed in triplicate and data
were analyzed by software.
Quantitative real-time RT-PCR
Total RNA was isolated using TRIzol (Invitrogen Co.,
Carlsbad, CA, USA). Total RNA template (2 µg) was used per 1 µl of
reverse transcriptase reaction by AMV reverse transcriptase
(Fermentas, EU) using olido(dT) primers at 42°C for 1 h according
to the manufacturer's instructions. For semi-quantitative real-time
RT-PCR, duplicate 1 µl samples of each cDNA using SYBR Green mix
were amplified as follows: 95°C for 10 min, 40 cycles at 95°C for
15 sec, and 60°C for 60 sec. The relative amount of gene expression
was calculated using the expression of β-actin as an internal
standard. DR4: sense, 5′-GGCTGAGGACAATGCTCACA-3′; antisense,
5′-TTGCTGCTCAGAGACGAAAGTG-3′.DR5: sense,
5′-CCAGCCCTCCCTCAGATGTAC-3′; antisense,
5′-TGTAAGGTTGTCCAGTTCAAAAGACT-3′. β-actin: sense,
5′-CCAGCTCACCATGGATGATG-3′; antisense, 5′-ATGCCGGAGCCGTTGTC-3′.
Data were normalized to the level of β-actin expression in each
individual sample according to ∆∆Ct
(CtDR4/DR5-Ctβ-actin) value.
Western blot analysis
Cells treated with plumbagin alone, rsTRAIL alone,
or their combinations were harvested, washed with PBS, and treated
with RIPA lysis buffer (Merck Millipore, Darmstadt, Germany). The
lysates were centrifuged at 10,000 × g for 15 min at 4°C and the
concentration of protein in each lysate was determined using BCA
protein assay reagent (Biyuntian) following the manufacturer's
protocol. After being mixed with loading buffer, 30 µg of protein
sample in each group was loaded for SDS-polyacrylamide gel
electrophoresis and subsequently transferred to a PVDF membrane.
Then, PVDF membrane was blocked with appropriate blocking solution
for 2 h at room temperature (RT), incubated with primary antibody
overnight at 4°C, and followed with HRP conjugated secondary
antibody for 2 h at RT. After being incubated with
electrochemiluminescence substrate (Immobilon Western
Chemiluminescent HRP substrate), PVDF membrane was visualized and
scanned via FluorChem E system (Bio-Techne, San Jose, CA, USA)
Glutathione determination
Kasumi-1 cells were treated with diverse
concentration of plumbagin. The cells were centrifuged with 800 × g
for 10 min. The concentrations of total glutathione (T-GSH),
reduced glutathione (GSH) and oxidized disulfide (GSSG) in
different group cells were measured by an enzymatic method
according to the commercial assay kit procedure (Beyotime Institute
of Biotechnology, Jiangsu, China).
In vivo tumor xenograft study
NOD/SCID male mice (4–6-week-old) (Beijing HFK
Bioscience Co. Ltd., Beijing, China) were housed under
pathogen-free conditions with a 12-h light/12-h dark schedule and
fed with an autoclaved diet and water ad libitum. All animal
procedures adhered to the National Institutes of Health Guidelines
for the Care and Use of Laboratory Animals with all efforts to
minimize animal number and suffering. Kasumi-1 cells were injected
s.c. into the flanks of the mice (2.5×106 in 2 ml) and
tumors were allowed to develop for 18–30 days until they reached
50–100 mm3, then treatment was initiated. Mice (n=25)
were randomly assigned to one of the following treatment groups: i)
mice (n=5) was treated daily with vehicle (0.4 ml physiological
saline) as a normal saline group; ii) Ara-C group (n=5) was treated
daily with Ara-C at doses of 50 mg/kg body weight in 0.4 ml PBS;
iii) mice (n=5) had an injection of rsTRAIL alone (10 mg/kg body
weight in 0.1 ml PBS); while iv) plumbagin alone group (n=5) were
treated with plumbagin (6 mg/kg of body weight) in a volume of PBS
0.4 ml every day; v) mice (n=5) were treated daily with rsTRAIL (10
mg/kg) and plumbagin (6 mg/kg) in a final volume of 0.6 ml for two
weeks. Tumor volumes and weights were measured twice a week. Tumor
size was based on the formula: tumor volume (TV, mm3) =
1/2 (long diameter) × (short diameter)2, measured by
calipers. After 3 weeks of treatment, tumor-bearing mice were
sacrificed. Xenograft tumors, as well as other vital organs of the
treated and control mice were fixed in 4% paraformaldehyde,
embedded in paraffin and cut into 3–5-µm sections. The pathological
slices of tumor and organs of mouse were stained with H&E
method and observed under a microscope. The apoptotic rate and the
expression of TRAIL and TRAIL receptors on the surface of the cells
were detected by flow cytometry.
Statistical analysis
Data are expressed as mean values ± standard
deviation (SD). Analyses were carried out using Student's t-tests.
Values of P<0.05 were considered statistically significant.
Results
Combined treatment with plumbagin and
rsTRAIL decreased the cell viability and induced cell apoptosis in
human leukemic Kasumi-1 cells
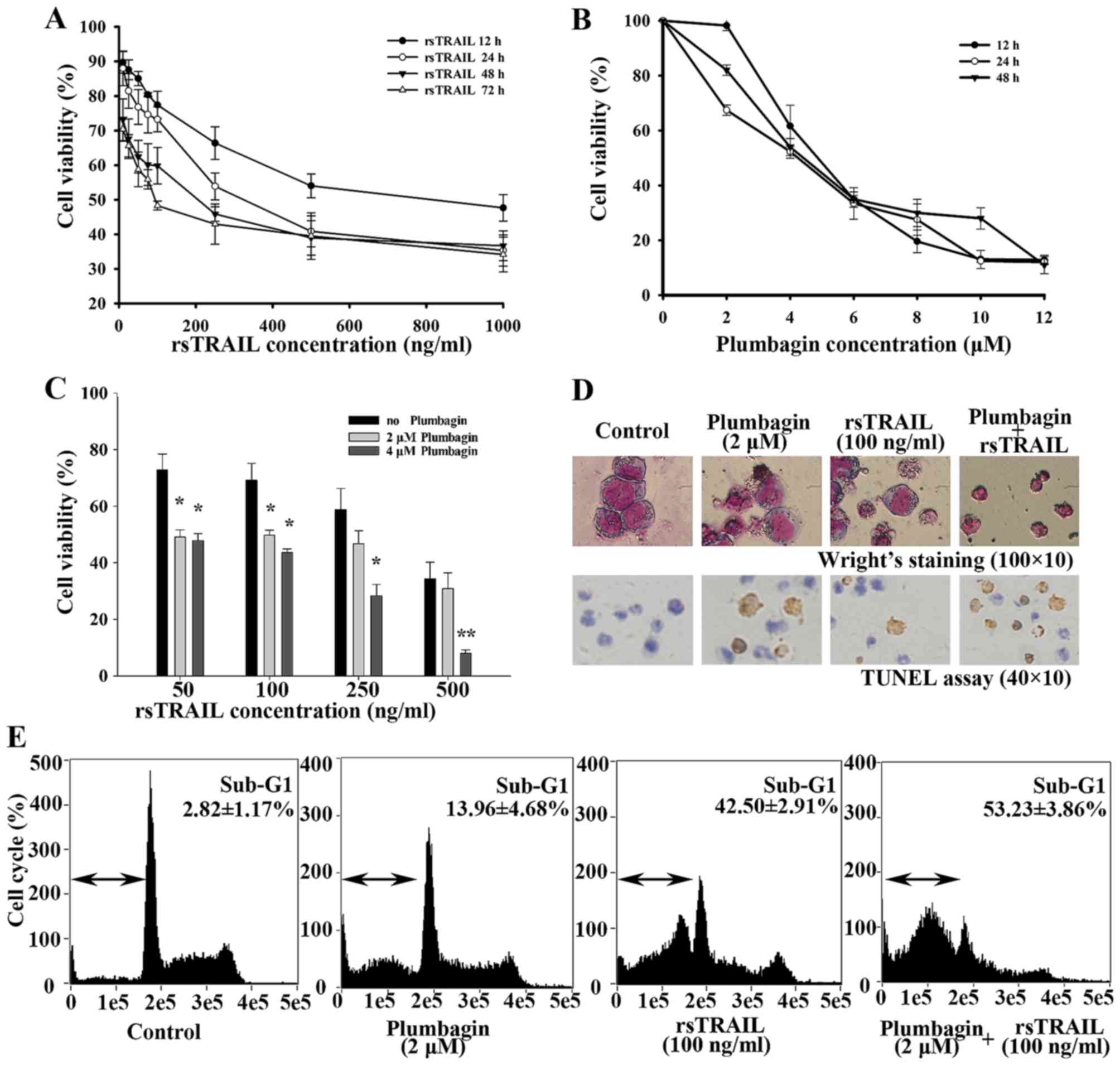
Using CCK-8 assay, we first treated Kasumi-1 cells
with different concentrations of rsTRAIL or plumbagin alone for
different incubation times to determine cell viability. rsTRAIL
alone from 10 to 1,000 ng/ml could inhibit cell proliferation in a
in a dose- and time-dependent manner after 12–72 h (Fig. 1A). Plumbagin inhibited cell
proliferation in Kasumi-1 cells with a dosage of 2–12 µM (Fig. 1B). Then, we tested their
combinational effects. As shown in Fig.
1C, rsTRAIL-inhibited cell viability of Kasumi-1 cells were
dosage and time-dependently enhanced by 2 and 4 µM plumbagin, which
is slightly inhibited in Kasumi-1 cells.
To confirm whether the cell viability decrease was
caused by apoptosis, cell morphology and TUNEL staining assay were
performed. We exposed Kasumi-1 cells to various concentrations of
rsTRAIL alone or in combination with 2 µM pumbagin for 12 h. Many
more cells showed characteristic changes with plumbagin plus
rsTRAIL, such as cell shrinkage, nuclear condensation, and
formation of apoptotic bodies (Fig.
1D). As shown in Fig. 1D, more
cells with brown precipitate were toward the combination group than
rsTRAIL or plumbagin alone. Cell cycle analysis also revealed a
markedly increased sub-G1 population in the combined treatment with
plumbaign and rsTRAIL, compared with the results of plumbagin or
rsTRAIL alone (Fig. 1E).
Plumbagin upregulated expression of
DR5 and contributed to the enhancement of TRAIL-induced cell
death
Death receptors (DRs) play key roles in
TRAIL-induced apoptosis (32,33).
It has been reported that sensitivity to TRAIL-induced apoptosis
may result from the differential expression of death receptors, and
a positive correlation of the sensitivity to TRAIL with the
expression of DR4 and DR5 is documented (1,34). We,
therefore, investigated whether these receptors are similarly
regulated by plumbagin in Kasumi-1 cells. We examined the effect of
plumbagin on the expression of death receptors at both mRNA and
protein levels by using real-time PCR, flow cytometry and western
blot analyses. We found that treatment of Kasumi-1 cells with 1 and
2 µM plumbagin for 4, 8 and 12 h resulted in an increased mRNA
level expression of DR5 but not DR4 in a dose- and time-dependent
manner (Fig. 2A). The surface
levels of DR4 and DR5 were also detected by flow cytometry
analysis, and upregulation of DR5 was further confirmed as shown in
Fig. 2B. Then we extracted protein
of Kasumi-1 cells treated by 1 and 2 µM plumbagin for 4 and 8 h.
Western blot analysis was performed and clearly showed the
upregulated expression of DR5 (Fig.
2C). To observe the upregulation of DR5 in both agents
combination-induced apoptosis, Kasumi-1 cells were further treated
by plumbagin alone, rsTRAIL alone, and their combination for 4, 8
and 12 h. As shown in Fig. 2D,
upregulation of DR5 expression was observed in plumbagin alone and
combination with rsTRAIL group but not in rsTRAIL group.
To investigate the functional upregulation of DR5
induced by plumbagin in Kasumi-1 cells, we used an anti-DR5
neutralizing antibody to inhibit DR5 activity by flow cytometry
analysis. We demonstrated that the apoptosis induced by rsTRAIL
alone and the combination of plumbagin and rsTRAIL were
significantly abolished in the presence of anti-DR5 neutralizing
antibody but not pumbagin alone (Fig.
2E). The results demonstrated that upregulation of DR5 by
plumbagin contribute to apoptosis of Kasumi-1 cells induced by
TRAIL.
Caspase activation, mitochondrial
damage and decreased cFLIP expression were involved in the
enhancement effect of plumbagin on rsTRAIL-induced Kasumi-1 cell
apoptosis
Both the death receptor (extrinsic) pathway and the
mitochrochria (intrinsic) pathway are involved in TRAIL-induced
apoptosis, and TRAIL apoptosis signaling pathway finally results in
the activation of caspases (35).
Cellular FLICE-inhibitory protein (c-FLIP) is major inhibitor of
TRAIL-mediated apoptosis and is involved in the resistance to
TRAIL-induced apoptosis (2). We
then analyzed the caspases, Bcl-2, Bid, Bax and cFLIP by western
blot analysis. As shown in Fig. 3A,
combined treatment led to more significantly increasing caspase-8,
−3 and −9 activities than treatment with rsTRAIL alone, indicating
that combined treatment induces apoptosis of Kasumi-1 cells through
a caspases-dependent pathway. Upregulation of Bax and activation of
Bid were also observed in the combined group, suggesting intrinsic
pathway is involved (Fig. 3B). In
parallel to the western blot analysis, combined treatment with
plumbagin and rsTRAIL also significantly decreased the MMP
(Fig. 3C). As shown in Fig. 3B, the expressions of c-FLIP was
decreased in plumbagin group and the combined group, but not in
rsTRAIL alone group, suggesting that downregulation of c-FLIP might
be involved in plumbagin-induced apoptosis of Kasumi-1 cells and
might be used as the sensitizer of TRAIL resistance cells.
 | Figure 3.Plumbagin and rsTRAIL induces caspase
activation, bax upregulation, cFLIP downregulation and MMP loss.
(A) Western blot analysis of caspase-8, −3, and −9 in Kasumi-1
cells treated with plumbagin (2 µM), rsTRAIL (100 ng/ml), or both
reagents for 8 h. (B) Western blot analysis for Bid, Bax, Bcl-2,
and cFLIP. Kasumi-1 cells were treated with plumbagin (2 µM),
rsTRAIL (100 ng/ml), or both reagents for 8 h. (C) The cells
stained with JC-1 were exposed to the indicated concentrations of
plumbagin and rsTRAIL for 12 h and then were analyzed by flow
cytometry. The data are representative of three separate
experiments. |
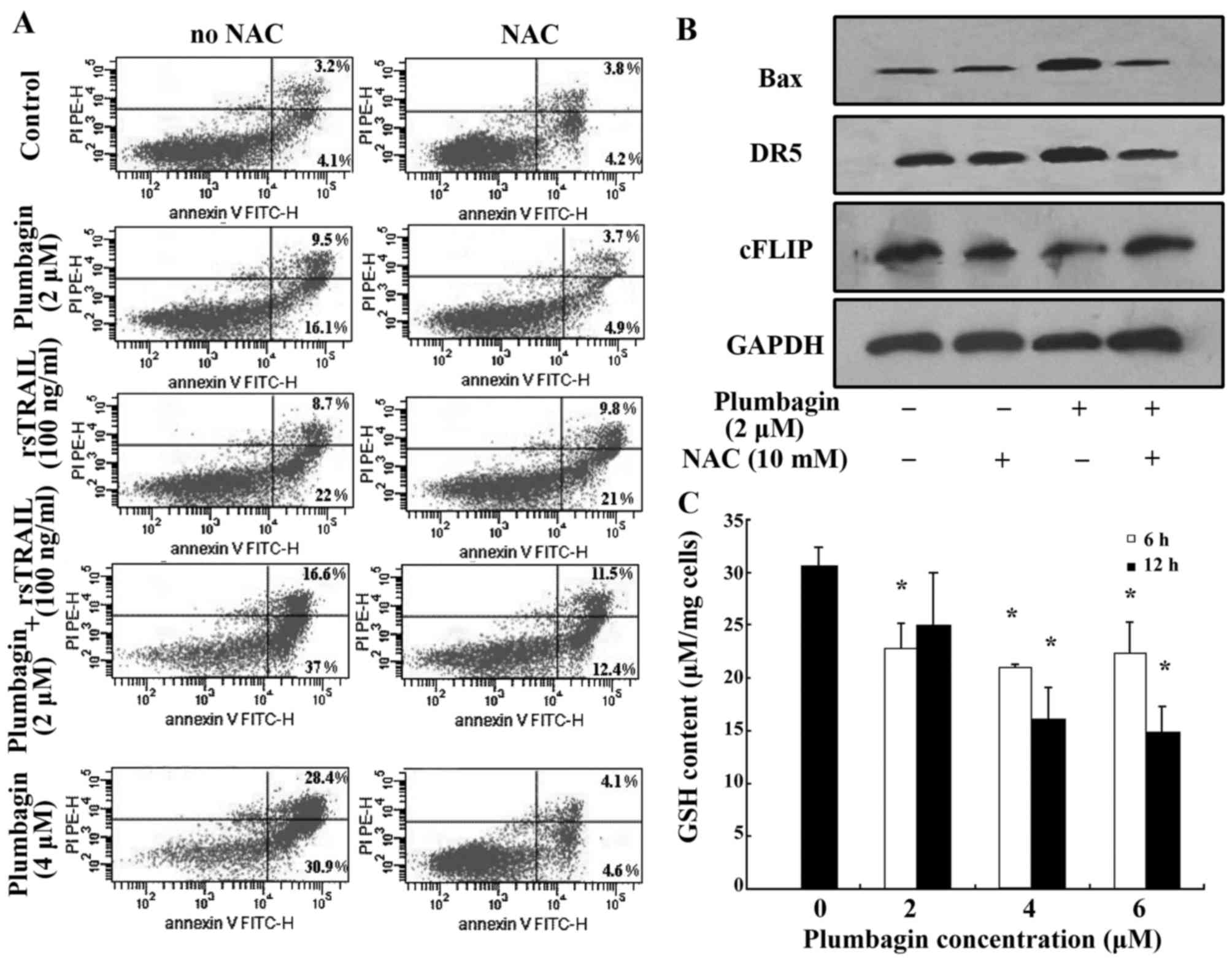
The effects of plumbagin on the
expression of DR5, Bax and cFLIP is partially abolished by ROS
scavenger NAC in Kasumi-1 cells
Plumbagin contains a quinone nucleus which could
transfer one electron in aerobic metabolism under oxidative stress,
and the semiquinone radical participates in redox cycling to
generate reactive oxygen species (ROS) like superoxide anion
(O2) and hydrogen peroxide (H2O2)
(36). Published data reported that
plumbagin effectively induced hematological malignancies, in which
ROS levels play an important role in mediating apoptosis (19,20,37).
We postulated that the mechanism might also underlie
plumbagin-induced apoptosis in Kasumi-1 cells. Then, ROS scavenger
NAC was combined with plumbagin to induce apoptosis of Kasumi-1
cells. Apoptosis of Kasumi-1 cells induced by plumbagin (4 µM)
alone or plumbagin (2 µM) plus rsTRAIL (100 ng/ml) were inhibited
by NAC from 59.3 to 8.7 and 53.6 to 23.9%, respectively (Fig. 4A). However, NAC did not show a
significant effect on rsTRAIL (100 ng/ml) alone induced apoptosis
of Kasumi-1 cells (Fig. 4A). Cell
lysates containing equal amounts of total protein from cells
treated by plumbagin with or without NAC were assayed for western
blot analysis, and the expression of DR5, Bax and cFLIP were
detected. Plumbagin-induced DR5 upregulation was attenuated by the
ROS scavenger NAC, the expression of Bax and cFLIP was also
influenced by the ROS scavenger NAC (Fig. 4B).
GSH depletion by plumbagin increases
the production of ROS
It has been shown that the decrease of GSH content
is a common feature in apoptotic cell death (38). Previous studies reported that
plumbagin regulates the cellular redox state by modulation of GSH
(36,39). In this study, we observed the
content of GSH in Kasumi-1 cells treated by plumbagin for 6 and 12
h. The content of GSH was significantly decreased in groups of
plumbagin treatment. The decrease was negatively correlated with
plumbagin concentrations (Fig.
4C).
Combined treatment with plumbagin and
rsTRAIL inhibits tumor growth in vivo
To determine whether combined treatment with
plumbagin and rsTRAIL can inhibit tumor growth in vivo, we
injected human leukemic Kasumi-1 cells s.c. into NOD/SCID male
mice. Combined treatment with plumbagin and rsTRAIL had a
synergistic effect and was found to markedly inhibit tumor growth
compared with the control group, plumbagin alone, or TRAIL alone
(P<0.05) (Fig. 5A). The tumor
volume was 1783.12±891.28 mm3 in the control group and
293.51±86.06 mm3 in combined group (P<0.01) (Fig. 5B). Moreover, the average volume of
the tumors in combined group was reduced by 19.44% when compared
with the control group (P<0.01) (Fig. 5B). As shown in Fig. 5C, the decrease of tumor weight is
obvious in Ara-C group, plumbagin alone group and combined group.
Apart from the infiltration of tumor cells in spleen and bone
marrow, there was no obvious toxic pathologic change in the heart,
liver or kidney tissues in any of the groups (Fig. 5D).
To gain insight into the mechanism of combined
treatment with plumbagin and rsTRAIL inhibition of tumor growth
in vivo, we harvested the Kasumi-1 tumor xenografts from the
treated mice. Comparing with the control mice, a significantly
increased number of apoptotic cells was observed in the combined
treated mice (P<0.01) (Fig. 5E).
As shown in Fig. 5F, combined
treatment with plumbagin and rsTRAIL could increase the expression
of both DR4 and DR5 receptors and decrease the DcR2 receptors
expression on the surface of the cells. In addition to this,
plumbagin not only increased the expression of DR5 in cells of
xenograft tumors, but also increased the expression of DR4, which
was different with the expression of DRs in vitro. There
were no obvious difference on the expression of both TRAIL and DcR1
receptors between combined groups and control groups. These data
indicated that the administration of plumbagin plus rsTRAIL induces
tumor regression associated with apoptosis in vivo.
Discussion
AML with t(8;21) accounts for ~15% of AML. It is
generally considered as a subtype of better outcomes, but
resistance to drugs is still a tough problem in clinic. So the
approach of finding new agents and/or methods is important to
conquer this problem. In this study, we used Kasumi-1 cells, which
is a classic cell line derived from t(8;21) positive acute leukemia
patient, as model to study the effect of two agents, the rsTRAIL
and plumbagin. Although rsTRAIL could significantly induce
apoptosis of Kasumi-1 cells, resistance to TRAIL apoptotic pathway
is a common phenomenon in refractory/relapse AML patients (35). Here we report for the first time,
that plumbagin could enhance TRAIL-induced apoptosis on acute
myeloid leukemia cells with t(8;21). This was observed not only in
well-established cell lines in vitro, but also in a murine
xenograft tumor model. Combined treatment with plumbagin and
rsTRAIL decreased the cell viability mainly due to the induction of
apoptosis, which has been demonstrated by morphological assay and
increase of apoptotic cells both in vitro and in
vivo. The mechanisms by which plumbagin mediates its effects on
TRAIL-induced apoptosis appears to involve the induction of TRAIL
receptor upregulation, activation of caspase-8 and inhibition of
cFLIP.
Firstly, we found upregulation of DR5 expression was
closely involved in the enhancement effect of plumbagin on
TRAIL-induce apoptosis of Kasumi-1 cells both in vitro and
in vivo. This further supports the notion that TRAIL
receptor expression is related to TRAIL sensitivity in tumor cells
(35). As shown on Fig. 1B, the concentration of plumbagin
<2 µM could not induce significant apoptosis of Kasumi-1 cells
within 12 h. Then, the concentrations of plumbagin at 1 and 2 µM
were applied to test the effect on TRAIL-induced apoptosis of
Kasumi-1 cells and their influence of TRAIL receptors as well as
death signaling pathway. The results of real-time PCR showed that
upregulation of DR5 mRNA level, but not DR4, by plumbagin was
significant in plumbagin-induced apoptosis of Kasumi-1 cells. Flow
cytometry and western blot analysis further confirmed the findings
at protein levels. Similar results were seen by Li et al
previously finding that plumbagin upregulated death receptor mRNA
and protein expression of human melanoma A375 cells (40).
Although expressed on the cell surface, DR4 and DR5
may not be functional, and both functional and non-functional TRAIL
receptors are detected by FCM and western blot analysis (2). Then, we used DR5 neutralizing antibody
to observe its effect on Kasumi-1 cells. The enhancement effect of
plumbagin on TRAIL-induced apoptosis of Kasumi-1 cells was
significantly decreased after the neutralization of DR5. It means
upregulation of DR5 by plumbagin is functionally taken effect among
TRAIL-induced apoptosis of Kasumi-1 cells. Then we asked whether
these findings could be translated into in vivo situations.
Interestingly, our study found that plumbagin could increase the
expression of both DR4 and DR5 in murine xenograft tumors, which
was different from the results that only the DR5 expression on the
cell surface in vitro. Recently, von Karstedt et al
(41) found that high TRAIL-R2
expression correlates with invasion of pancreatic ductal
adenocarcinoma into lymph vessels. Upregulation of DR4 not only DR5
might imply more clinical importance and need to be further
investigated.
Secondly, the augmented effect of plumbagin on
TRAIL-induced apoptosis of Kasumi-1 cell is ROS-dependent, and ROS
levels are mainly negatively regulated by content of GSH in the
cell has been reported. Plumbagin can induce ROS-mediated apoptosis
in various leukemic cells in vitro (20,37).
In this study, plumbagin was also confirmed to induce significant
apoptosis of leukemic Kasumi-1 cells in vitro, which was
completely abolished by NAC treatment.
There are two main mechanisms with the therapeutic
activity of quinones including the production of the semiquinone
radical under aerobic conditions which participates in redox
cycling to generate ROS, like superoxide anion
(O2·) and hydrogen peroxide
(H2O2) and as a potent electrophile reacting
with the thiol groups of proteins and GSH (36). Plumbagin is a bicycling
naphthoquinone, its cytotoxic properties is related to its quinone
core and contribute to its therapeutic activity.
Castro et al (36) reported the toxicity of plumbagin
acts mainly as a potent electrophile in Saccharomyces
cerevisiae, and then decrease the concentration of GSH. The GSH
level is an important factor in the protection against several
stress conditions, and low GSH levels can be associated with
decrease in cell proliferation, protection against apoptosis and
ROS elimination. The contents of GSH in Kasumi-1 cells after
treated with plumbagin were negatively correlated with the
concentration of plumbagin from 2 to 6 µM. Therefore, we postulate
that plumbagin-induced ROS-mediated apoptosis of leukemic Kasumi-1
cells are mainly through reacting with thiol group of GSH.
Glutathione-S-transferases (GST), which is related to the contents
of GSH in cells and is important in quinone detoxification, may
also be inactivated by plumbagin. This modulation is paralleled by
ROS formation (42).
Thirdly, we found the activation of both extrinsic
and intrinsic caspase pathway is involved in plumbagin-induced
apoptosis of Kasumi-1 cells, which in turn may potentially promote
TRAIL-induced apoptosis in Kasumi-1 cells. As shown in Fig. 3A, both intrinsic and extrinsic
caspases could be activated in plumbagin alone and the combination
with rsTRAIL-induced apoptosis of Kasumi-1 cells. It was
interesting that caspase-8 (the substrate of extrinsic pathway)
could be activated by plumbagin alone at the concentration <2 µM
in our study, and the result was consistent with the finding of Xu
and Lu that caspase-8 could be activated after caspase-9 activation
in NB4 cells (19). McKallip et
al (43) also suggested that
the observed effects on caspase-8 activity following plumbagin
exposure were mediated through the production of ROS. Wieder et
al (44) reported that
drug-induced caspase-8 activation in B-lymphoma cells is
independent of death receptor signaling and is mediated by
postmitochondrial caspase-3 activation. Plumbagin is generally
considered as an intrinsic apoptosis inducer, our results supported
that there is cross-talk between the extrinsic and intrinsic
apoptotic pathways in plumbagin-induced apoptosis of tumor cells.
Therefore, we think plumbagin-induced caspase-8 activation might
also involve in its enhancement effect on TRAIL-induced apoptosis
of Kasumi-1 cells.
In conclusion, we found that plumbagin could enhance
TRAIL-induced apoptosis in Kasumi-1 cells, and the mechanisms
include ROS-mediated upregulation of DR5 expression, caspase-8
activation and inhibition of cFLIP expression. Although overcoming
TRAIL resistance still remains a challenge, our in vitro and
in vivo data suggest that rational combination of plumbagin
and TRAIL is a regimen that would optimize the anti-leukemic
activity in refractory/relapse AML with t(8;21) in future.
Acknowledgements
This study was supported by a grant from National
Natural Science Foundation of China (NSFC, no. 30672415), Science
and Technology Commission of Shanghai Municipality (STCSM, no.
054119528) and Japanese Society for the Promotion of Science
Ronpaku program (2004-CSC-2).
References
|
1
|
LeBlanc HN and Ashkenazi A: Apo2L/TRAIL
and its death and decoy receptors. Cell Death Differ. 10:66–75.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mahalingam D, Szegezdi E, Keane M, de Jong
S and Samali A: TRAIL receptor signalling and modulation: Are we on
the right TRAIL? Cancer Treat Rev. 35:280–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sessler T, Healy S, Samali A and Szegezdi
E: Structural determinants of DISC function: New insights into
death receptor-mediated apoptosis signalling. Pharmacol Ther.
140:186–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ashkenazi A, Holland P and Eckhardt SG:
Ligand-based targeting of apoptosis in cancer: The potential of
recombinant human apoptosis ligand 2/Tumor necrosis factor-related
apoptosis-inducing ligand (rhApo2L/TRAIL). J Clin Oncol.
26:3621–3630. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ashkenazi A, Pai RC, Fong S, Leung S,
Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert
A, et al: Safety and antitumor activity of recombinant soluble Apo2
ligand. J Clin Invest. 104:155–162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L and Fang B: Mechanisms of
resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther.
12:228–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Geelen CMM, de Vries EGE and de Jong
S: Lessons from TRAIL-resistance mechanisms in colorectal cancer
cells: Paving the road to patient-tailored therapy. Drug Resist
Updat. 7:345–358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Kane HF, Watson CJ, Johnston SR, Petak
I, Watson RW and KE; O Kane HF Williamson: Targeting death
receptors in bladder, prostate and renal cancer. J Urol.
175:432–438. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng J, Hylander BL, Baer MR, Chen X and
Repasky EA: Multiple mechanisms underlie resistance of leukemia
cells to Apo2 Ligand/TRAIL. Mol Cancer Ther. 5:1844–1853. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Padhye S, Dandawate P, Yusufi M, Ahmad A
and Sarkar FH: Perspectives on medicinal properties of plumbagin
and its analogs. Med Res Rev. 32:1131–1158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krishnaswamy M and Purushothaman KK:
Plumbagin: A study of its anticancer, antibacterial &
antifungal properties. Indian J Exp Biol. 18:876–877.
1980.PubMed/NCBI
|
|
12
|
Mishra BB, Singh DD, Kishore N, Tiwari VK
and Tripathi V: Antifungal constituents isolated from the seeds of
Aegle marmelos. Phytochemistry. 71:230–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abdul KM and Ramchender RP: Modulatory
effect of plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) on
macrophage functions in BALB/c mice. I. Potentiation of macrophage
bactericidal activity. Immunopharmacology. 30:231–236. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pradeepa V, Sathish-Narayanan S,
Kirubakaran SA and Senthil-Nathan S: Antimalarial efficacy of
dynamic compound of plumbagin chemical constituent from Plumbago
zeylanica Linn (Plumbaginaceae) against the malarial vector
Anopheles stephensi Liston (Diptera: Culicidae). Parasitol Res.
113:3105–3109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Checker R, Patwardhan RS, Sharma D, Menon
J, Thoh M, Sandur SK, Sainis KB and Poduval TB: Plumbagin, a
vitamin K3 analogue, abrogates lipopolysaccharide-induced oxidative
stress, inflammation and endotoxic shock via NF-κB suppression.
Inflammation. 37:542–554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharma I, Gusain D and Dixit VP:
Hypolipidaemic and antiatherosclerotic effects of plumbagin in
rabbits. Indian J Physiol Pharmacol. 35:10–14. 1991.PubMed/NCBI
|
|
17
|
Sunil C, Duraipandiyan V, Agastian P and
Ignacimuthu S: Antidiabetic effect of plumbagin isolated from
Plumbago zeylanica L. root and its effect on GLUT4 translocation in
streptozotocin-induced diabetic rats. Food Chem Toxicol.
50:4356–4363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Son TG, Camandola S, Arumugam TV, Cutler
RG, Telljohann RS, Mughal MR, Moore TA, Luo W, Yu QS, Johnson DA,
et al: Plumbagin, a novel Nrf2/ARE activator, protects against
cerebral ischemia. J Neurochem. 112:1316–1326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu KH and Lu DP: Plumbagin induces
ROS-mediated apoptosis in human promyelocytic leukemia cells in
vivo. Leuk Res. 34:658–665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gaascht F, Teiten MH, Cerella C, Dicato M,
Bagrel D and Diederich M: Plumbagin modulates leukemia cell redox
status. Molecules. 19:10011–10032. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsu YL, Cho CY, Kuo PL, Huang YT and Lin
CC: Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) induces
apoptosis and cell cycle arrest in A549 cells through p53
accumulation via c-Jun NH2-terminal kinase-mediated phosphorylation
at serine 15 in vitro and in vivo. J Pharmacol Exp Ther.
318:484–494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shieh JM, Chiang TA, Chang WT, Chao CH,
Lee YC, Huang GY, Shih YX and Shih YW: Plumbagin inhibits
TPA-induced MMP-2 and u-PA expressions by reducing binding
activities of NF-kappaB and AP-1 via ERK signaling pathway in A549
human lung cancer cells. Mol Cell Biochem. 335:181–193. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aziz MH, Dreckschmidt NE and Verma AK:
Plumbagin, a medicinal plant-derived naphthoquinone, is a novel
inhibitor of the growth and invasion of hormone-refractory prostate
cancer. Cancer Res. 68:9024–9032. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kawiak A, Zawacka-Pankau J and Lojkowska
E: Plumbagin induces apoptosis in Her2-overexpressing breast cancer
cells through the mitochondrial-mediated pathway. J Nat Prod.
75:747–751. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuo PL, Hsu YL and Cho CY: Plumbagin
induces G2-M arrest and autophagy by inhibiting the AKT/mammalian
target of rapamycin pathway in breast cancer cells. Mol Cancer
Ther. 5:3209–3221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee JH, Yeon JH, Kim H, Roh W, Chae J,
Park HO and Kim DM: The natural anticancer agent plumbagin induces
potent cytotoxicity in MCF-7 human breast cancer cells by
inhibiting a PI-5 kinase for ROS generation. PLoS One.
7:e450232012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sinha S, Pal K, Elkhanany A, Dutta S, Cao
Y, Mondal G, Iyer S, Somasundaram V, Couch FJ, Shridhar V, et al:
Plumbagin inhibits tumorigenesis and angiogenesis of ovarian cancer
cells in vivo. Int J Cancer. 132:1201–1212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nair S, Nair RRK, Srinivas P, Srinivas G
and Pillai MR: Radiosensitizing effects of plumbagin in cervical
cancer cells is through modulation of apoptotic pathway. Mol
Carcinog. 47:22–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang CCC, Chiang YM, Sung SC, Hsu YL,
Chang JK and Kuo PL: Plumbagin induces cell cycle arrest and
apoptosis through reactive oxygen species/c-Jun N-terminal kinase
pathways in human melanoma A375.S2 cells. Cancer Lett. 259:82–98.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marcucci G, Mrózek K, Ruppert AS, Maharry
K, Kolitz JE, Moore JO, Mayer RJ, Pettenati MJ, Powell BL, Edwards
CG, et al: Prognostic factors and outcome of core binding factor
acute myeloid leukemia patients with t(8;21) differ from those of
patients with inv(16): A Cancer and Leukemia Group B study. J Clin
Oncol. 23:5705–5717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Asou H, Tashiro S, Hamamoto K, Otsuji A,
Kita K and Kamada N: Establishment of a human acute myeloid
leukemia cell line (Kasumi-1) with 8;21 chromosome translocation.
Blood. 77:2031–2036. 1991.PubMed/NCBI
|
|
32
|
Pan G, O'Rourke K, Chinnaiyan AM, Gentz R,
Ebner R, Ni J and Dixit VM: The receptor for the cytotoxic ligand
TRAIL. Science. 276:111–113. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Walczak H, Degli-Esposti MA, Johnson RS,
Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA,
Smith CA, et al: TRAIL-R2: A novel apoptosis-mediating receptor for
TRAIL. EMBO J. 16:5386–5397. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ashkenazi A: Targeting death and decoy
receptors of the tumour-necrosis factor superfamily. Nat Rev
Cancer. 2:420–430. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fulda S: Cell death in hematological
tumors. Apoptosis. 14:409–423. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Castro FA, Mariani D, Panek AD, Eleutherio
EC and Pereira MD: Cytotoxicity mechanism of two naphthoquinones
(menadione and plumbagin) in Saccharomyces cerevisiae. PLoS One.
3:e39992008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun J and McKallip RJ: Plumbagin treatment
leads to apoptosis in human K562 leukemia cells through increased
ROS and elevated TRAIL receptor expression. Leuk Res. 35:1402–1408.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Franco R and Cidlowski JA: Glutathione
efflux and cell death. Antioxid Redox Signal. 17:1694–1713. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Inbaraj JJ and Chignell CF: Cytotoxic
action of juglone and plumbagin: A mechanistic study using HaCaT
keratinocytes. Chem Res Toxicol. 17:55–62. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li J, Shen Q, Peng R, Chen R, Jiang P, Li
Y, Zhang L and Lu J: Plumbagin enhances TRAIL-mediated apoptosis
through up-regulation of death receptor in human melanoma A375
cells. J Huazhong Univ Sci Technol. 30:458–463. 2010. View Article : Google Scholar
|
|
41
|
von Karstedt S, Conti A, Nobis M,
Montinaro A, Hartwig T, Lemke J, Legler K, Annewanter F, Campbell
AD, Taraborrelli L, et al: Cancer cell-autonomous TRAIL-R signaling
promotes KRAS-driven cancer progression, invasion, and metastasis.
Cancer Cell. 27:561–573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Turella P, Cerella C, Filomeni G, Bullo A,
De Maria F, Ghibelli L, Ciriolo MR, Cianfriglia M, Mattei M,
Federici G, et al: Proapoptotic activity of new glutathione
S-transferase inhibitors. Cancer Res. 65:3751–3761. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McKallip RJ, Lombard C, Sun J and
Ramakrishnan R: Plumbagin-induced apoptosis in lymphocytes is
mediated through increased reactive oxygen species production,
upregulation of Fas, and activation of the caspase cascade. Toxicol
Appl Pharmacol. 247:41–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wieder T, Essmann F, Prokop A, Schmelz K,
Schulze-Osthoff K, Beyaert R, Dörken B and Daniel PT: Activation of
caspase-8 in drug-induced apoptosis of B-lymphoid cells is
independent of CD95/Fas receptor-ligand interaction and occurs
downstream of caspase-3. Blood. 97:1378–1387. 2001. View Article : Google Scholar : PubMed/NCBI
|