Introduction
Gastric cancer, the third leading cause of cancer
death worldwide, is very common in Eastern Asia, and most are
gastric adenocarcinoma (1–3). The 5-year survival rate for gastric
adenocarcinoma has increased, possibly due to surgical resection
and normalized combined chemotherapy (4–6).
However, a large population remains resistant to chemotherapy, this
suggests that there is still significant room for improvement of
diagnosis and therapy in gastric adenocarcinoma patients (7,8). To
our knowledge, it is clear that some patients who are positive of
human epidermal growth factor receptor-2 (Her-2), P53 and Survivin
will indicate poor prognosis due to chemotherapy resistance
(9–14). Therefore, the relationships between
these chemotherapy-related biomarkers and the SUVmax of
18F-FDG PET/CT are needed, to predict the occurrence of
chemotherapy resistance and create personalized therapeutic
regimens of gastric adenocarcinoma patients before chemotherapy
treatment.
18F-fluorodeoxyglucose
(18F-FDG) positron emission tomography/computed
tomography (PET/CT) has been used as a non-invasive efficient tool
for diagnosing, staging, monitoring the response to chemotherapy
and identifying the recurrence following treatment in various types
of tumors (14,15). Recent studies have demonstrated that
there are some significant correlation between specific tumor
markers and SUVmax in pancreatic cancer and non-small cell lung
cancer (NSCLC), in which EGFR and P53 mutations are associated with
tumor progression or chemotherapy resistance (16,17).
Besides, several studies have reported the relationship between the
SUVmax and some serum tumor biomarker levels in many kinds of
cancers, such as CA19-9, CA72-4, and CEA (18–20).
In a previous study, we investigated the predictive
significance of the SUVmax measured by 18F-FDG PET/CT in
NSCLC patients, and found that SUVmax was significantly correlated
with P53 expression, and PET/CT could be considered as a simple and
effective non-invasive method for predicting P53-related
chemotherapy resistance at the cut-off value of 5.15 (17). However, the relationship between
SUVmax and chemotherapy resistance tumor markers in gastric
adenocarcinoma patients, including Her-2, P53 and Survivin, has not
been studied.
Hence, we examined Her-2, P53 and Survivin status
using immunohistochemical staining, and found the correlation
between these markers and SUVmax. Subsequently, we designed the
linear correlation analysis to detect the relationships between
SUVmax and serum tumor markers (CA19-9, CA72-4, and CEA), and to
establish an equation of SUVmax with CA19-9 and CA125 as
independent variables. Thus, based on the above data, we could
establish an equation, which could provide the quantitative
relation between serum tumor markers and chemotherapy-related tumor
markers. According to these quantitative relations, SUVmax could be
equation-generated before PET/CT scanning, and this method might
screen the high-risk patients for examination, besides, combining
the relationship between SUVmax and chemotherapy-related tumor
markers, 18F-FDG PET/CT would give us more clinical
information on gastric adenocarcinoma patients.
Materials and methods
Study population
Sixty-four gastric adenocarcinoma patients who
underwent preoperative 18F-FDG PET/CT and were naive to
chemotherapy from January 1, 2014 to December 31, 2015 were
enrolled in this study at Cancer Center of the First Affiliated
Hospital of Xi'an Jiaotong University. This study was approved by
the Institutional Ethics Committee of Xi'an Jiaotong University,
including the patients' written informed consent.
18F-FDG PET/CT imaging
All patients were instructed to fast at least 6 h
before the 18F-FDG PET/CT scans (Gemini 64TF, Philips,
Cleveland, OH, USA). Furthermore, they were also requested to drink
at least 500 ml water in order to distend the stomach before
scanning. Emission scans were initiated 1 h following nearly
simultaneous intravenous administration of FDG (3.7 MBq/kg).
All of the PET/CT images were evaluated by two
experienced physicians, and the measuring of SUVmax referred to
previous described standard methods. The image results of the
patients were visually evaluated and were classified as positive or
negative according to 18F-FDG uptake of the cancer
lesions. A positive 18F-FDG uptake was considered as
increased 18F-FDG uptake of lesions exceeded the uptake
of the surrounding normal stomach wall or corresponded with cancer
lesions which were diagnosed by contrast-enhanced CT or
gastroduodenoscopy. Conversely, a negative 18F-FDG
uptake was considered as no visible increased 18F-FDG
uptake compared with the surrounding normal stomach wall.
Additionally, focally increased 18F-FDG, which did not
correspond with cancer lesions diagnosing by contrast-enhanced CT
or gastroduodenoscopy and histopathological findings, were excluded
(21,22). Region of interest (ROI) were drawn
exactly with outline of the primary tumor on the transaxial slices,
and the calculation of SUV was performen by the following equation:
SUV = Tumor activity concentration/(Injected dose/Body weight)
(23).
Immunohistochemistry staining
Her-2 was detected by using a mouse monoclonal
antibody (ab8054, Abcam, Cambridge, MA, USA), P53 was detected by
using a mouse monoclonal antibody (ZM-0408, Thermo Fisher
Scientific, Waltham, MA, USA), and Survivin was detected with a
mouse monoclonal antibody (ab93274, Abcam). The sections were
independently evaluated by two pathologists.
For P53 and Survivin, the positive expression was
considered with nucleus and/or cytoplasm staining, and Her-2
expression was considered positive when cell membrane staining was
observed. Decision criteria for P53 and Survivin were the
following: intensity of staining was scored as 0 (no staining), +
(weak staining), ++ (intermediate staining), +++ (strong staining).
The percentage of positive cells was scored as 0 (0%), 1 (1–9%), 2
(10–49%), and 3 (50–100%). The staining intensity and percentage of
positive cells defined as the immunohistochemistry (IHC) score from
0 to 9. When the IHC score was ≥1, the marker expression was judged
as positive expression (24,25).
For Her-2, the IHC score was defined as: no staining or <10%
tumor cell positive staining as 0; faintly or barely perceptible
staining on ≥10% tumor cell membrane as +; weak to moderate
positive staining on ≥10% tumor cell membrane as ++; and cohesive
moderate to strong staining on ≥10% tumor cell membrane as +++. In
this study, we classified IHC +++ as Her 2-positive (26,27).
Serum assays for tumor markers
Serum samples for CA19-9, CA72-4, and CEA
(accessories of E170 analyzer, Roche Diagnostics, Rotkreuz,
Switzerland) levels were measured when the patients never had any
kind of therapy including operation and chemotherapy. Serum levels
of CA19-9, CA72-4, and CEA were assayed with
electrochemiluminescence (ECL) method (E170 analyzer, Roche
Diagnostics). The cut-off values were 6.7 U/ml, 37.0 U/ml and 5.0
ng/ml for CA19-9, CA72-4, and CEA, which were all based on the the
manufacturer's instructions (28).
When the marker serum levels were higher than the cut-off value,
they were judged as positive expression.
Statistical analysis
All data were presented as mean ± standard error
mean (SEM). Student's t-test was used to determine the Her-2, P53
and Survivin positivity dependent differences. Receiver operating
characteristics (ROC) curve analysis was performed to assess a
cut-off value for SUVmax with the most appropriate sensitivity and
specificity. Spearman correlation analysis was used to evaluate the
relationships between different serum markers and
chemotherapy-resistance related markers, and the value of R was
considered as follows: 0.8–1.0, highly strong correlation; 0.6–0.8,
strong correlation; 0.4–0.6, moderate correlation; 0.2–0.4 weak
correlation; 0.0–0.2, no correlation. The equations for SUVmax with
serum CA72-4, CA19-9 parameters were established by the linear
regression models. All calculations and statistical analyses were
performed using SPSS software (version 20.0, IBM Corp., Armonk, NY,
USA). p<0.05 was considered as a statistically significant
difference.
Results
Clinical characteristics of
patients
The demographic and clinical characteristics of the
patients are summarized in Table I.
The ages of the patients ranged from 42 to 82 years (average age,
63.0 years), There were 33 male (average age, 62.4 years), and 31
female (average age, 63.7 years). All the patients who were naive
to chemotherapy were diagnosed as gastric adenocarcinoma by biopsy
or operation at the cancer center of our hospital from January 1,
2014 to December 31, 2015. 18F-FDG PET/CT examination
and serum test were performed within one week before biopsy or
operation. The representative 18F-FDG PET/CT images of
the patient are shown in Fig. 1,
and the positive expression of Her-2, P53 and Survivin were 43.8,
46.9 and 57.8% in gastric adenocarcinoma patients, respectively
(Fig. 2).
 | Table I.Patients and tumor
characteristics. |
Table I.
Patients and tumor
characteristics.
|
| No. of patients
(%) |
|---|
| Sex |
|
|
Male | 33 |
|
Female | 31 |
| Age |
|
|
<60 | 22 |
|
≥60 | 42 |
| Clinical stage |
|
| I | 10 |
| II | 13 |
|
III | 20 |
| IV | 21 |
| Her-2 status |
|
|
Positive | 28 |
|
Negative | 36 |
| P53 status |
|
|
Positive | 30 |
|
Negative | 34 |
| Survivin
status |
|
|
Positive | 37 |
|
Negative | 27 |
The relationships between SUVmax and
chemotherapy-related markers
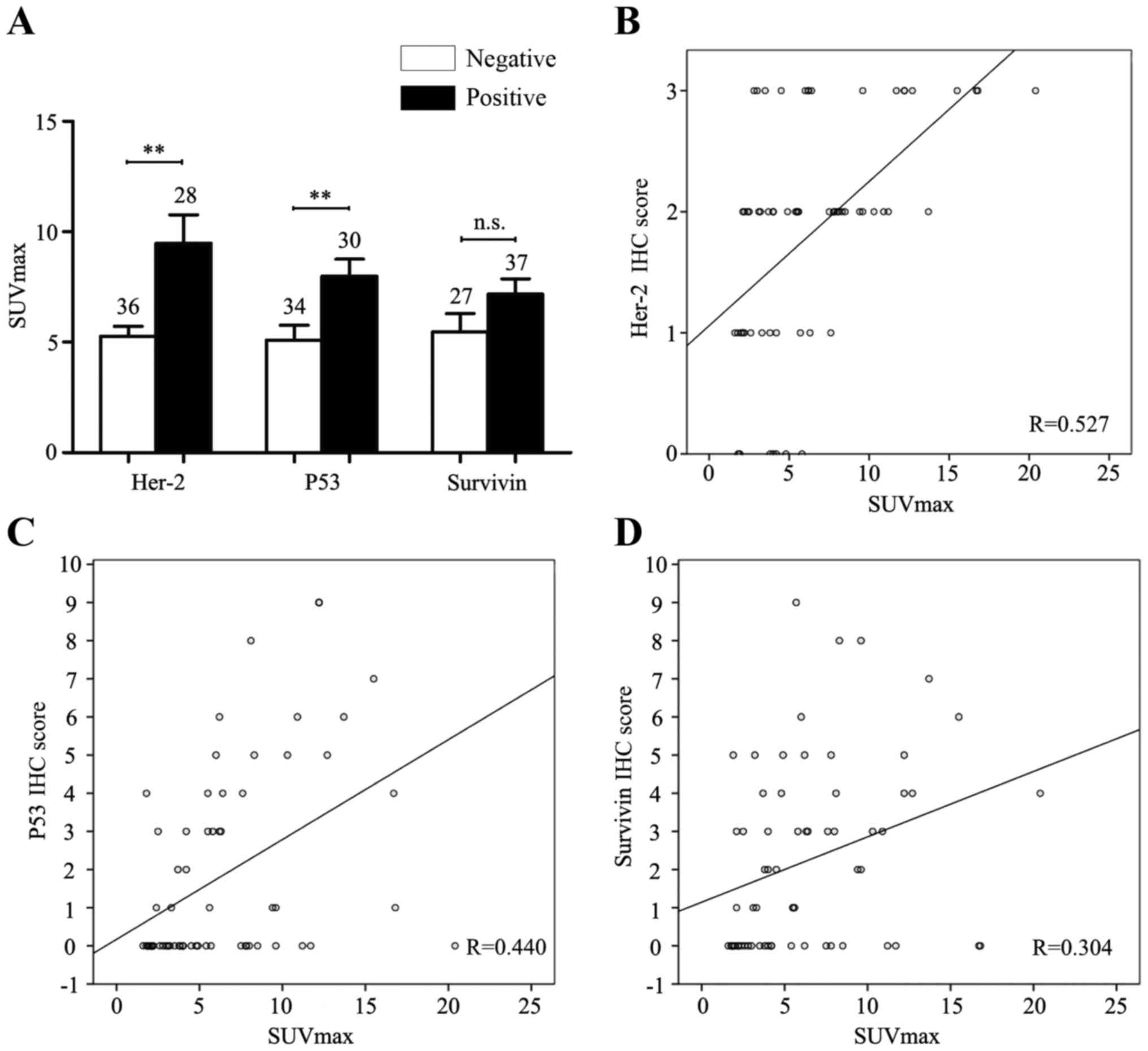
Furthermore, Student's t-test was used to clarify
the relationships between SUVmax and expression levels of
chemotherapy-related tumor markers. We found significantly higher
SUVmax in Her-2-positive (SUVmax: 9.225±1.260) or P53-positive
(SUVmax: 7.600±0.859) group of gastric adenocarcinoma patients,
compared to Her-2-negative (SUVmax: 5.075±0.412) or P53-negative
(SUVmax: 5.020±0.877) group (p<0.05), but there was no SUVmax
difference between Survivin-positive group (SUVmax: 6.855±0.633)
and Survivin-negative (SUVmax: 5.223±0.909) group (p>0.05)
(Fig. 3A). Moreover, the Spearman
correlation analysis showed that high SUVmax was associated with
higher expression level of Her-2 or P53, based on the moderate
relevant Pearson correlation coefficient (R=5.27 or R=0.440,
respectively), but for Survivin expression, the relevant Pearson
correlation coefficient was small (R=0.304) (Fig. 3B-D). Next, ROC curve analysis
revealed that the area under the curve for predicting
Her-2-positivity was 0.737 with the 95% confidence interval (CI)
ranging from 0.617 to 0.857, and for predicting P53-positive, the
area under the curve was 0.778 with 95% CI between 0.667 and 0.890.
When the optimized cut-off value of SUVmax was set at 3.25, the
sensitivity and specificity of SUVmax showed Her 2-positive were
96.4 and 44.4%, respectively. For predicting P53-positivity, the
sensitivity and specificity of SUVmax were 73.3 and 67.6%, when we
set the optimized cut-off value of SUVmax at 5.45 (Fig. 4). We suggested that the higher
SUVmax was related to the positive expression level of Her-2 or
P53. Hence, we cautiously showed a hypothesis that the abnormal
expression of Her-2 and P53 caused aberrant metabolic activity in
tumor cells, and this process ultimately resulted in aberrant
glycometabolism, which could be detect by 18F-FDG PET/CT
scanning in gastric adenocarcinoma patients.
The relationships between SUVmax and
serum tumor markers
Serum CA72-4, CA19-9 and CEA were measured
preoperatively, and the mean values were 60.3±6.8, 28.2±2.5 and
4.6±0.9 ng/ml in enrolled gastric adenocarcinoma patients,
respectively. Interestingly, based on the cut-off values mentioned
in methods above for these serum tumor makers, the mean values of
serum CA72-4 significantly increased, however, the increase of
serum CA19-9 and CEA were not found in our study. Next, the
Spearman correlation analysis was used to evaluate the linear
relationships of SUVmax and serum tumor markers of gastric
adenocarcinoma patients. The results showed that SUVmax was
significantly linearly correlated with serum CA72-4, CA19-9 and CEA
(Table II). Furthermore, we found
that SUVmax was correlated with CEA with small relevant Pearson
correlation coefficient (R=0.346), but SUVmax was strongly
correlated with CA72-4 and CA19-9 (R=0.676 and R=0.691,
respectively, Fig. 5). So we
applied linear regression models to establish an equation for
SUVmax using CA72-4 and CA19-9 as independent variables. SUVmax =
1.701+0.092 × CA72-4+0.033 × CA19-9.
 | Table II.The correlation between SUVmax and
serum CA19-9, CA72-4, and CEA. |
Table II.
The correlation between SUVmax and
serum CA19-9, CA72-4, and CEA.
|
| Pearson correlation
coefficient | CA19-9 | CA72-4 | CEA |
|---|
| SUVmax | R value | 0.691 | 0.676 | 0.346 |
|
| p-value | 0.000 | 0.000 | 0.005 |
According to this equation, we suggest that CA72-4
and CA19-9 could be used as parameters to estimate the value of
SUVmax, if 18F-FDG PET/CT was not available or for
screening the high-risk patients.
Discussion
18F-FDG PET/CT, one of the non-invasive
methods, is used to detect glucose metabolism in malignant tumors
and indicated by increased 18F-FDG PET/CT uptake which
is represented by an increased SUVmax (29). Currently, 18F-FDG PET/CT
scans are widely used in cancer diagnosis, assessment of treatment
response, and as a prognostic marker (30–32).
However, the underlying relationships between some chemotherapy
resistant markers and the clinical observation of
18F-FDG accumulation have not yet been elucidated. To
the best of our knowledge, it has been proved that the positive
expression of Her-2, P53 and Survivin is closely related with
chemotherapy resistance in gastric cancer patients, but the
relationships are still unknown between these positive tumor
markers and the abnormal SUVmax detected by 18F-FDG
PET/CT scanning.
Her-2 related signal transduction pathway takes part
in many kinds of chemotherapy-resistance mechanisms (33). In SGC7901 and BGC823 cell lines of
gastric cancer, transfection of Her-2 resulted in
chemotherapy-resistance for various drugs including paclitaxel,
adriamycin, fluorouracil, platinum-based chemotherapy and
camptothecin (34). However, the
patients Her-2-positive also have some benefits. Trastuzumab, one
of anti-Her-2 antibodies from human protooncogene, has the ability
to inhibit the Her-2 related signal transduction pathway to elevate
chemotherapy sensitivity for many patients, in order to enhance
response effect and prolong survival period (35,36).
In previous studies, SUVmax of Her-2-positive and Her-2-negative
phenotype subgroups in breast cancer were significantly different,
and this indicated that there was a relationship between the Her-2
expression and SUVmax (37,38).
P53 plays an important role in the chemotherapy
response of various gastric cancer cell lines and clinical
treatment, including cisplatin, carboplatin, paclitaxel and
gemcitabine (10,39). In our previous study, we found that
P53 expression was significantly related to SUVmax, and SUVmax in
P53-positive cases was statistically higher than that of
P53-negative cases in non-small cell lung cancer (NSCLC) patients
(17). Furthermore, in lung
adenocarcinoma patients, the diffusivity and intensity of P53
staining had a significant relationship with the SUVmax (17). However, in one triple-negative
breast cancer study, there was no association between P53
expression and SUVmax. The difference might result from the
different types of tumor or pathological patterns (40). In conclusion, it was meaningful and
interesting to clarify the underlying relationship between P53
expression and SUVmax, for making chemotherapy regimens in gastric
cancer.
Survivin, an inhibitor of apoptosis
repeat-containing 5 (BIRC5), is found in almost all human tumors
(40,41). Overexpression of it mainly indicates
inhibition of apoptosis and cell cycle control (42). One study found that Survivin was a
positive biomarker for predicting the sensitivity of paclitaxel
treatment in gastric cancer patients by western blotting method,
however, overexpression of Survivin was closely related with
cisplatin and 5-FU treatment sensitivity in gastric SGC7901 cancer
cells, and in nude mice, and knockdown of Survivin expression by
shRNA, the cisplatin and 5-FU treatment sensitivity of gastric
SGC7901 cancer cells and the nude mice enhanced significantly
(13,43,44).
The present study of 64 gastric adenocarcinoma
patients was the first report that the SUVmax was significantly
higher in the positive expression of Her-2 and P53, compared to
that of negative expression in gastric cancer patients. The results
suggested that SUVmax might reflect the expression level of
chemotherapy resistant-related markers, such as Her-2 and P53 when
the cut-off values were set at 3.25 and 5.45. Interestingly, higher
SUVmax was not found in the positive Survivin expression group, and
we considered SUVmax might not be suitable for determining
Survivin-related chemotherapy resistance for gastric cancer
patients.
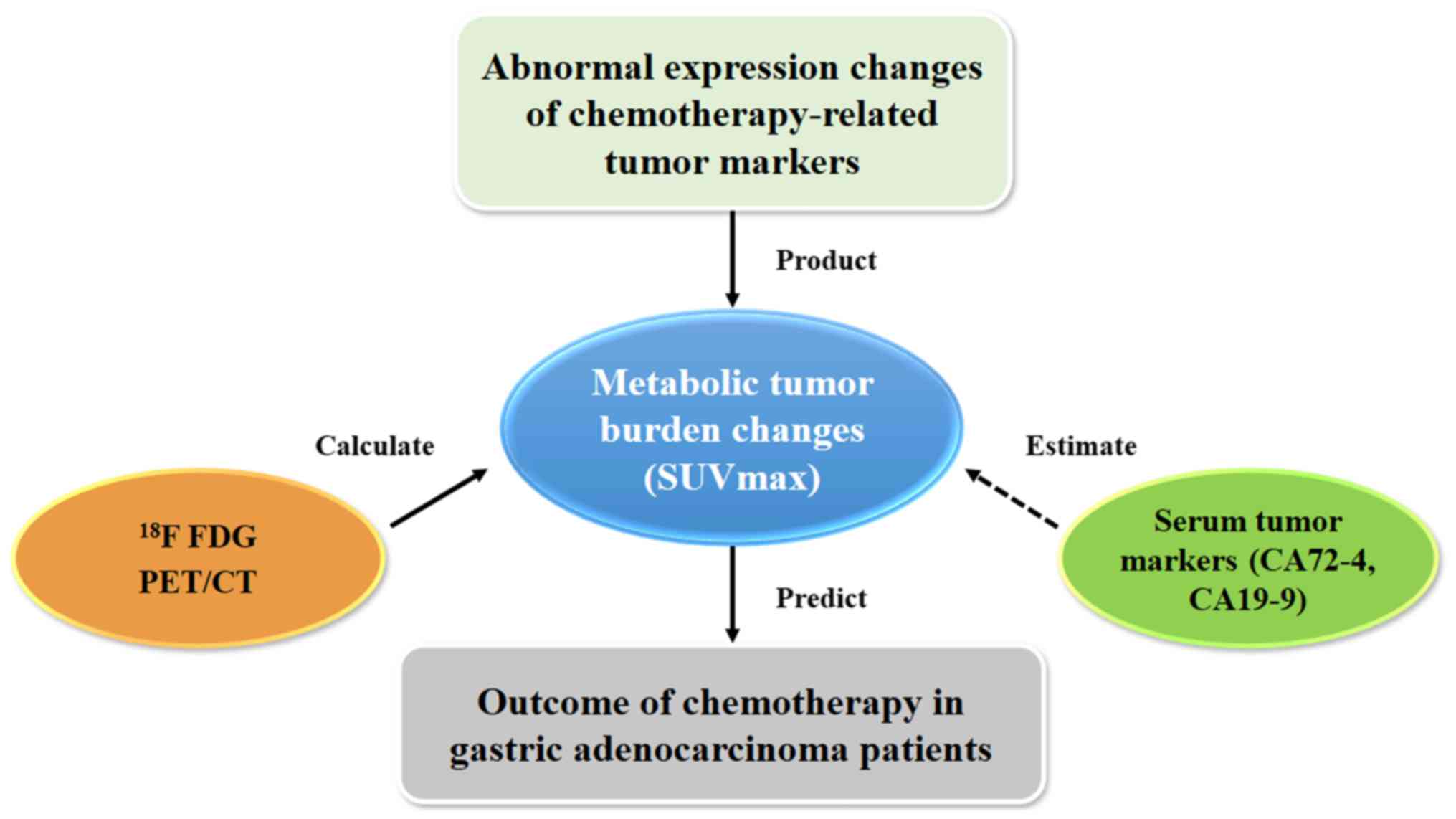
In clinic, high expression of CA72-4, CA19-9 and CEA
could be used to evaluate patients' prognosis and efficacy of
chemotherapy, and serum examination is more convenient and
affordable, compared with 18F-FDG PET/CT. If certain
specific quantitative relationship between SUVmax and the values of
different serum tumor markers were detected, we could use the
results of serum tumor markers to predict the range of SUVmax
approximately. In this study, we found that CA72-4 and CA19-9
significantly linearly related to SUVmax, but not CEA. Linear
regression models were used to establish equations for SUVmax using
CA72-4 and CA19-9 as independent variables. Thus, CA72-4 and CA19-9
could be used as parameters to estimate the value of SUVmax. Hence,
it is suitable for monitoring of treatment response in gastric
adenocarcinoma patients evaluated by 18F-FDG PET/CT or
estimated by CA19-9 and CA72-4 (45) (Fig.
6).
However, we recognized that there were some
shortcomings in our study: i) clinical cases were limited, further
studies were needed to expand the number of cases. ii) The
mechanism that the positive expression of Her-2 and P53 induced
high metabolic tumor burden was still unclear in gastric
adenocarcinoma patients. iii) We considered that the relationship
between SUVmax and serum tumor markers was very complicated. In
this study, only 3 types of serum tumor markers were performed.
Further studies are needed to expand the types of serum tumor
markers, more sufficient data provided, including more accurate
equations. So the results should be accepted cautiously.
In conclusion, SUVmax is associated with the
expression level of Her-2 and P53, which were closely related to
chemotherapy resistance in gastric adenocarcinoma patients. SUVmax,
either calculated by 18F-FDG PET/CT or estimated by
serum tumor markers of CA72-4 and CA19-9, could be used to predict
and evaluate Her-2 or P53 related chemotherapy resistance of
gastric patients.
Acknowledgements
This study was supported by National Natural Science
Foundation of China (no. 81471710).
Glossary
Abbreviations
Abbreviations:
|
18F-FDG
|
18F-fludrodeoxyglucose
|
|
PET/CT
|
positron emission tomography/computed
tomography
|
|
SUVmax
|
maximal measuring standardized uptake
value
|
|
NSCLC
|
non-small cell lung cancer
|
|
Her-2
|
human epidermal growth factor
receptor-2
|
References
|
1
|
Joensuu H, Hohenberger P and Corless CL:
Gastrointestinal stromal tumour. Lancet. 382:973–983. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khanderia E, Markar SR, Acharya A, Kim Y,
Kim YW and Hanna GB: The influence of gastric cancer screening on
the stage at diagnosis and survival: A meta-analysis of comparative
studies in the far East. J Clin Gastroenterol. 50:190–197. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sugano K: Screening of gastric cancer in
Asia. Best Pract Res Clin Gastroenterol. 29:895–905. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Markar SR, Mikhail S, Malietzis G,
Athanasiou T, Mariette C, Sasako M and Hanna GB: Influence of
surgical resection of hepatic metastases from gastric
adenocarcinoma on long-term survival: Systematic review and pooled
analysis. Ann Surg. 263:1092–1101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen XZ, Wen L, Rui YY, Liu CX, Zhao QC,
Zhou ZG, Hu JK and Liu Y: Long-term survival outcomes of
laparoscopic versus open gastrectomy for gastric cancer: A
systematic review and meta-analysis. Medicine (Baltimore).
94:e4542015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coccolini F, Cotte E, Glehen O, Lotti M,
Poiasina E, Catena F, Yonemura Y and Ansaloni L: Intraperitoneal
chemotherapy in advanced gastric cancer. Meta-analysis of
randomized trials. Eur J Surg Oncol. 40:12–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan IB, Ivanova T, Lim KH, Ong CW, Deng N,
Lee J, Tan SH, Wu J, Lee MH, Ooi CH, et al: Intrinsic subtypes of
gastric cancer, based on gene expression pattern, predict survival
and respond differently to chemotherapy. Gastroenterology.
141:476–485, e1-485.e11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun Z, Song X, Li X, Su T, Qi S, Qiao R,
Wang F, Huan Y, Yang W, Wang J, et al: In vivo multimodality
imaging of miRNA-16 iron nanoparticle reversing drug resistance to
chemotherapy in a mouse gastric cancer model. Nanoscale.
6:14343–14353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Endo F, Nishizuka SS, Kume K, Ishida K,
Katagiri H, Ishida K, Sato K, Iwaya T, Koeda K and Wakabayashi G: A
compensatory role of NF-κB to p53 in response to 5-FU-based
chemotherapy for gastric cancer cell lines. PLoS One. 9:e901552014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang X, Hu G, Xu C, Ouyang K, Fang W,
Huang W, Zhang J, Li F, Wang K, Qin X, et al: HZ08 reverse the
aneuploidy-induced cisplatin-resistance in Gastric cancer by
modulating the p53 pathway. Eur J Pharmacol. 720:84–97. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JY, Hong M, Kim ST, Park SH, Kang WK,
Kim KM and Lee J: The impact of concomitant genomic alterations on
treatment outcome for trastuzumab therapy in HER2-positive gastric
cancer. Sci Rep. 5:92892015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Won E, Janjigian YJ and Ilson DH: HER2
directed therapy for gastric/esophageal cancers. Curr Treat Options
Oncol. 15:395–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong H, Liu G, Jiang B, Guo J, Tao G, Yiu
W, Zhou J and Li G: Overexpression of the Survivin gene in SGC7901
cell resistance to cisplatin. Oncol Lett. 8:1953–1956.
2014.PubMed/NCBI
|
|
14
|
Sun XP, Dong X, Lin L, Jiang X, Wei Z,
Zhai B, Sun B, Zhang Q, Wang X, Jiang H, et al: Up-regulation of
survivin by AKT and hypoxia-inducible factor 1α contributes to
cisplatin resistance in gastric cancer. FEBS J. 281:115–128. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Spick C, Herrmann K and Czernin J: 18F-FDG
PET/CT and PET/MRI perform equally well in cancer: Evidence from
studies on more than 2,300 patients. J Nucl Med. 57:420–430. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kitasato Y, Yasunaga M, Okuda K, Kinoshita
H, Tanaka H, Okabe Y, Kawahara A, Kage M, Kaida H and Ishibashi M:
Maximum standardized uptake value on
18F-fluoro-2-deoxy-glucose positron emission
tomography/computed tomography and glucose transporter-1 expression
correlates with survival in invasive ductal carcinoma of the
pancreas. Pancreas. 43:1060–1065. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duan XY, Wang W, Wang JS, Shang J, Gao JG
and Guo YM: Fluorodeoxyglucose positron emission tomography and
chemotherapy-related tumor marker expression in non-small cell lung
cancer. BMC Cancer. 13:5462013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao JG, Hu Y, Liao Q, Niu ZY and Zhao YP:
Prognostic significance of SUVmax and serum carbohydrate antigen
19-9 in pancreatic cancer. World J Gastroenterol. 20:5875–5880.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Caglar M, Yener C and Karabulut E: Value
of CT, FDG PET-CT and serum tumor markers in staging recurrent
colorectal cancer. Int J CARS. 10:993–1002. 2015. View Article : Google Scholar
|
|
20
|
Tomita M, Shimizu T, Ayabe T and Onitsuka
T: Maximum SUV on positron emission tomography and serum CEA level
as prognostic factors after curative resection for non-small cell
lung cancer. Asia Pac J Clin Oncol. 8:244–247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuruva M, Mittal BR, Abrar ML, Kashyap R
and Bhattacharya A: Multivariate analysis of various factors
affecting background liver and mediastinal standardized uptake
values. Indian J Nucl Med. 27:20–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wahl RL, Jacene H, Kasamon Y and Lodge MA:
From RECIST to PERCIST: Evolving considerations for PET response
criteria in solid tumors. J Nucl Med. 50 Suppl 1:S122–S150. 2009.
View Article : Google Scholar
|
|
23
|
Hwang JP, Lim I, Kong CB, Jeon DG, Byun
BH, Kim BI, Choi CW and Lim SM: Prognostic value of SUVmax measured
by pretreatment fluorine-18 fluorodeoxyglucose positron emission
tomography/computed tomography in patients with Ewing sarcoma. PLoS
One. 11:e01532812016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
25
|
Cheng AN, Jiang SS, Fan CC, Lo YK, Kuo CY,
Chen CH, Liu YL, Lee CC, Chen WS, Huang TS, et al: Increased Cdc7
expression is a marker of oral squamous cell carcinoma and
overexpression of Cdc7 contributes to the resistance to
DNA-damaging agents. Cancer Lett. 337:218–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wesoła M and Jeleń M: A comparison of IHC
and FISH cytogenetic methods in the evaluation of HER2 status in
breast cancer. Adv Clin Exp Med. 24:899–903. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hofmann M, Stoss O, Shi D, Büttner R, van
de Vijver M, Kim W, Ochiai A, Rüschoff J and Henkel T: Assessment
of a HER2 scoring system for gastric cancer: Results from a
validation study. Histopathology. 52:797–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim JH, Jun KH, Jung H, Park IS and Chin
HM: Prognostic value of preoperative serum levels of five tumor
markers (carcinoembryonic antigen, CA19-9, alpha-fetoprotein,
CA72-4, and CA125) in gastric cancer. Hepatogastroenterology.
61:863–869. 2014.PubMed/NCBI
|
|
29
|
Schmidt-Hansen M, Baldwin DR, Hasler E,
Zamora J, Abraira V and Roqué i Figuls M: PET-CT for assessing
mediastinal lymph node involvement in patients with suspected
resectable non-small cell lung cancer. Cochrane Database Syst Rev.
11:CD0095192014.
|
|
30
|
Coleman RE: Value of FDG-PET scanning in
management of lung cancer. Lancet. 359:1361–1362. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Avril S, Muzic RF Jr, Plecha D, Traughber
BJ, Vinayak S and Avril N: 18 F-FDG PET/CT for monitoring of
treatment response in breast cancer. J Nucl Med. 57(Suppl 1):
S34–S39. 2016. View Article : Google Scholar
|
|
32
|
Kim JW, Oh JS, Roh JL, Kim JS, Choi SH,
Nam SY and Kim SY: Prognostic significance of standardized uptake
value and metabolic tumour volume on 18F-FDG PET/CT in
oropharyngeal squamous cell carcinoma. Eur J Nucl Med Mol Imaging.
42:1353–1361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Agelaki S, Kalykaki A, Markomanolaki H,
Papadaki MA, Kallergi G, Hatzidaki D, Kalbakis K, Mavroudis D and
Georgoulias V: Efficacy of lapatinib in therapy-resistant
HER2-positive circulating tumor cells in metastatic breast cancer.
PLoS One. 10:e01236832015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cui H, Cheng Y, Piao SZ, Xu YJ, Sun HH,
Cui X, Li XZ, Zhang SN, Piao LZ, Jin YM, et al: Correlation between
HER-2/neu (erbB-2) expression level and therapeutic effect of
combination treatment with HERCEPTIN and chemotherapeutic agents in
gastric cancer cell lines. Cancer Cell Int. 14:102014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meza-Junco J, Au HJ and Sawyer MB:
Trastuzumab for gastric cancer. Expert Opin Biol Ther. 9:1543–1551.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gong J, Liu T, Fan Q, Bai L, Bi F, Qin S,
Wang J, Xu N, Cheng Y, Bai Y, et al: Optimal regimen of trastuzumab
in combination with oxaliplatin/capecitabine in first-line
treatment of HER2-positive advanced gastric cancer (CGOG1001): A
multicenter, phase II trial. BMC Cancer. 16:682016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim JY, Lee SH, Kim S, Kang T and Bae YT:
Tumour 18F-FDG uptake on preoperative PET/CT may predict
axillary lymph node metastasis in ER-positive/HER2-negative and
HER2-positive breast cancer subtypes. Eur Radiol. 25:1172–1181.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoon HJ, Kang KW, Chun IK, Cho N, Im SA,
Jeong S, Lee S, Jung KC, Lee YS, Jeong JM, et al: Correlation of
breast cancer subtypes, based on estrogen receptor, progesterone
receptor, and HER2, with functional imaging parameters from
68Ga-RGD PET/CT and 18F-FDG PET/CT. Eur J
Nucl Med Mol Imaging. 41:1534–1543. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim CW, Lu JN, Go SI, Jung JH, Yi SM,
Jeong JH, Hah YS, Han MS, Park JW, Lee WS, et al: p53 restoration
can overcome cisplatin resistance through inhibition of Akt as well
as induction of Bax. Int J Oncol. 43:1495–1502. 2013.PubMed/NCBI
|
|
40
|
Koo HR, Park JS, Kang KW, Han W, Park IA
and Moon WK: Correlation between (18)F-FDG uptake on PET/CT and
prognostic factors in triple-negative breast cancer. Eur Radiol.
25:3314–3321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen X, Duan N, Zhang C and Zhang W:
Survivin and tumorigenesis: Molecular mechanisms and therapeutic
strategies. J Cancer. 7:314–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shen X, Zheng JY, Shi H, Zhang Z and Wang
WZ: Survivin knockdown enhances gastric cancer cell sensitivity to
radiation and chemotherapy in vitro and in nude mice. Am J Med Sci.
344:52–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vallböhmer D, Drebber U, Schneider PM,
Baldus S, Bollschweiler E, Brabender J, Warnecke-Eberz U, Mönig S,
Hölscher AH and Metzger R: Survivin expression in gastric cancer:
Association with histomorphological response to neoadjuvant therapy
and prognosis. J Surg Oncol. 99:409–413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Malibari N, Hickeson M and Lisbona R:
PET/computed tomography in the diagnosis and staging of gastric
cancers. PET Clin. 10:311–326. 2015. View Article : Google Scholar : PubMed/NCBI
|