Introduction
Human malignant glioma, as the most common primary
central nervous system tumor, has an adverse prognosis for patients
(1,2). Even with a variety of treatments,
patients affected by glioblastoma multiforme (GBM), which is
regarded as the highest malignant degree of astrocytic tumors (WHO
grade IV), survive only 12 months on average after diagnosis
(3). Unfortunately, despite
enormous advances in neurosurgery and chemoradiotherapy in recent
years, the prognosis of malignant gliomas is still poor. The rapid
proliferation of glioma cells and their strong invasive ability are
the critical reasons for the resistance of conventional treatments
(4,5). Therefore, it is urgent to explore the
molecular pathological mechanism concerning the occurrence and
development of gliomas, and to apply specific molecules that may be
invoked as new diagnostic markers and therapeutic targets.
Piwi-like RNA-mediated gene silencing 2 (PIWIL2)
protein is a member of the Argonaute family (6). Argonaute proteins, as a highly
conserved protein family, are subdivided into two subfamilies, Piwi
and Ago (7). The Piwi subfamilies
characterized by highly conserved Piwi/Argonaute/Zwille (PAZ) and
P-element induced wimpytestis (PIWI) domains, were found to play
critical roles in stem cell self-renewal, RNA interference,
spermatogenesis, chromatin remodeling and translational regulation
(6,8,9). In
humans, the Ago/Piwi family is comprised of PIWIL1 (HIWI), PIWIL2
(HILI, CT80, Miwi like), PIWIL3 (HIWI3), and PIWIL4 (HIWI2)
(10). Among the PIWI subfamily
members, PIWIL2 has been demonstrated to play an important role in
the pathological process of various malignant tumors (11). It has been reported that PIWIL2 is
stably expressed in precancerous stem cells (pCSCs) in breast
cancer, and may participate in the regulation of pCSC development
and differentiation (12).
Moreover, PIWIL2 was found to be mainly expressed in breast cancer
stem cells, and inhibited their apoptosis through the Stat3/Bcl-XL
pathway, and thus could be used as a biomarker for breast cancer
(13,14). In addition, recent research also
indicated that PIWIL2 is highly expressed in lung, gastric,
bladder, prostate, colorectal and papillary thyroid cancer, and is
also closely associated with the initiation, development and
metastasis of tumors (15–18). Notwithstanding the research of
PIWIL2 in diverse human cancers which has yielded fruitful results,
we also detected the expression of PIWIL2 in glioma tissues and
cells. Yet, its function and mechanism in the occurrence and
development of glioma still remain to be clarified by further
research.
In the present study, we ascertained the high
expression of PIWIL2 in human glioma tissues and cell lines, and
indicated that overexpression of PIWIL2 predicts a poor prognosis
in patients with glioma. Furthermore, we explored the possible
impact of PIWIL2 on cellular proliferation and migration in U87
glioma cells. Our research demonstrated that PIWIL2 plays an
important role in the pathological process of human gliomas, and we
expect to provide a novel molecular target for glioma diagnosis and
therapies in the future.
Materials and methods
Patients and tissue specimens
A total of 97 glioma specimens (including WHO grade
II–IV) was collected from patients diagnosed with glioma for the
first time and who had received no prior therapy. All the tissues
were obtained from the Affiliated Hospital of Nantong University
between 2011–2015 and their clinicopathological data were provided
by the Department of Pathology. Normal brain tissue was donated by
patients who had succumbed to traffic accidents and were
ascertained to be without any pathological changes. All the tissues
were collected and applied in accordance with the ethical standards
of the World Medical Association and all of the studies were
approved by the Ethics Committee of the Affiliated Hospital of
Nantong University. Specimens used for immunoblot analysis were
frozen in liquid nitrogen immediately after surgical removal, and
then stored at −80°C. Some specimens were fixed in 10% formalin
before they were sliced into paraffin sections for
immunohistochemical analysis.
Cell culture
The human glioma cell lines H4, A172, U251, U87 and
U118 were purchased from the Cell Library of the Chinese Academy of
Sciences. All the cells were cultured in Dulbecco's modified Eagles
medium (DMEM; Gibco-BRL, Grand Island, NY, USA) with 10% fetal
bovine serum (FBS), 2 mM L-glutamine and 100 U/ml of a
penicillin/streptomycin mixture (Gibco-BRL) at 37°C, and 5%
CO2.
Antibodies
The antibodies used for the western blot analysis
were as follows: anti-PIWIL2 (diluted 1:500), anti-proliferating
cell nuclear antigen (PCNA, diluted 1:500), anti-p53 (diluted
1:500), anti-p21 (diluted 1:500), anti-cyclin-dependent kinase 2
(CDK2, diluted 1:500), anti-cyclin D1 (diluted 1:1,000), and
anti-cyclin E polyclonal antibody (diluted 1:500) (all from Santa
Cruz Biotechnology, CA, USA), anti-cleaved caspase 3 (diluted
1:200; Cell Signaling Technology, Danvers, MA, USA), anti-B-cell
CLL/lymphoma 2 (Bcl-2, diluted 1:1,000),
anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, diluted
1:1,000), anti-N-cadherin (diluted 1:1,000), anti-E-cadherin
(diluted 1:1,000), anti-vimentin (diluted 1:1,000), and anti-matrix
metallopeptidase 9 (MMP9 diluted 1:1,000) (all from Santa Cruz
Biotechnology). The antibodies used for immunohistochemistry were:
anti-PIWIL2 (diluted 1:200), and anti-Ki-67 (1:300) (both from
Santa Cruz Biotechnology).
Immunohistochemical staining
Glioma tissues were fixed in 10% formalin, and then
embedded in paraffin to produce paraffin sections. The glioma
paraffin sections with xylene dewaxing were rehydrated in graded
ethanol. Then, we used 0.3% hydrogen peroxide to block endogenous
peroxidase activity. The sections were then processed in 0.1 M
citrate buffer (pH 6.0) and heated to 120°C in an autoclave for 5
min for antigen retrieval. As the sections cooled, they were rinsed
in the phosphate-buffered saline (PBS) (pH 7.2), then incubated
with 5% bovine serum 1.5 h at room temperature to block nonspecific
reactions. The tissue sections were incubated with anti-PIWIL2
antibody and anti-Ki-67 antibody overnight at 4°C. Meanwhile, the
same concentration of non-specific immunoglobulin IgG (Sigma
Chemical Co., St. Louis, MO, USA) was used as the first antibody
for the negative control group. According to the manufacturer's
instructions, the horseradish peroxidase-conjugated anti-rabbit or
anti-mouse Ig polymer was used as a second antibody (EnVision kit;
Dako) to incubate the tissue sections for 30 min at room
temperature. After being rinsed in PBS, the slides were
counterstained with hematoxylin, dehydrated, and mounted with
resin.
Immunohistochemical analysis
Without any knowledge of the clinicopathological
characteristics of the patients, all of the immunohistochemical
staining sections were evaluated by three pathologists in a blinded
manner, and discrepancies were resolved by consensus. At least five
high-power fields (Leica microscope; Leica, Wetzlar, Hessen,
Germany) were chosen randomly in each specimen, and more than 300
cells in each field were counted to determine the mean percentage
of signal-positive cells. In order to evaluated the expression of
PIWIL2 and Ki-67 in glioma, the staining intensity score and the
proportion of positive tumor cells were both graded with different
scores, and the product of the two scores was used to evaluate the
staining results. An intensity score representing the average
intensity of positive cells: 0 (negative staining); 1 (weak
staining: light yellow); 2 (moderate staining: yellow brown) and 3
(strong staining: brown), and the percentage of tumor cells stained
positive were scored as follows: 0 (0–5% tumor cells stained), 1
(6–25% tumor cells stained), 2 (26–50% tumor cells stained), 3
(51–75% tumor cells stained) and 4 (76–100% tumor cells stained).
Then intensity and staining scores were multiplied to create an
immunoreactivity score (IS), and the IS was further divided as
follows: 0–1 (−); 2–4 (+); 5–7 (++); >8 (+++). For statistical
analysis, ‘−’ and ‘+’ were considered low levels of
immunoreactivity, while ‘++’ and ‘+++’ were considered high levels
of immunoreactivity (19).
Western blot analysis
Glioma tissue samples and cell protein were
homogenized in a homogenization buffer (1 M TrisHCl pH 7.5, 1%
Triton X-100, 1% Nonidet p-40, 10% sodium dodecyl sulfate, 0.5%
sodium deoxycholate, 0.5 M EDTA, 10 µg/ml leupeptin, 10 µg/ml
aprotinin, and 1 mM PMSF) on the ice, and centrifuged at 13,000 × g
for 10 min at 4°C to collect the supernatant liquid. Then the
samples were denatured at 100°C for 15 min and used immediately or
stored at −20°C. Protein concentrations were assessed with a
Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). The protein
extracts were separated by sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene
difluoride filter (PVDF) membranes (Millipore, Bedford, MA, USA).
Then the membranes were blocked in 5% fat-free milk with TBST (150
mM NaCl, 20 mM Tris, 0.05% Tween-20) for 2 h at room temperature,
and incubated with the corresponding primary antibodies for 6–8 h
at 4°C. Subsequently, the membranes were rinsed with TBST three
times, 5 min each time, and then incubated with horseradish
peroxidase-linked IgG as the secondary antibody for 2 h at room
temperature. Finally, the membranes were detected by a computer
imaging system (Imaging Technology, Ontario, Canada). There were at
least three independent experiments performed to ensure the
rigorousness of the results.
Transient transfection
The PIWIL2-shRNAs were designed and synthesized by
GenechemCo. Ltd. (Shanghai, China). Full-length PIWIL2 was
amplified using PCR and subcloned into pcDNA3.1 construct
(Invitrogen, Carlsbad, CA, USA). PIWIL2-shRNA#1 target sequence
was: 5-ACCGGC UGGGUUGAACUAAA-3, PIWIL2-shRNA#2 target sequence was:
5-ACAGCCUCAAACACUGUGCAC-3, and control-shRNA target sequence was:
5-GUACCGCAC GUCAUUCGUAUC-3. The glioma cells were transfected with
PIWIL2-shRNAs or negative-control shRNA using Lipofectamine 2000
(Invitrogen) according to the manufacturer's instructions, and the
corresponding glioma cells without any treatment were used as mock
groups.
Flow cytometric analysis of cell
apoptosis and the cell cycle
For the cell apoptosis analysis, U87 cells
transfected with PIWIL2-shRNAs and negative control-shRNA were
cultured for 48 h. PE Annexin V apoptosis detection kit I (BD
Pharmingen, San Jose, CA, USA) and flow cytometer BD FACScan
(Becton Dickinson, San Jose, CA, USA) were used in conjunction
according to the manufacturer's instructions to detect apoptosis.
For the cell cycle analysis, after transfection for 48 h, U87 cells
were collected and fixed with 70% ethanol for 24 h at −4°C. Then
the cells were rinsed with cold PBS and incubated with 10 mg/ml PI
and 0.5 mg/ml RNase A for 20 min at 37°C. The DNA contents of the
labeled cells were analyzed by a Becton Dickinson flow cytometer BD
FACScan (Becton Dickinson).
Colony formation assay
U87 cells transfected with PIWIL2-shRNA and
negative-control shRNA were seeded in 6-cm culture plates (2,000
cells/plate). After being cultured for 14 days at 37°C, the
colonies were washed with PBS, fixed with methanol for 30 min, and
stained with 0.5% crystal violet (Solarbio, Beijing, China) for 30
min. Then, the number of colonies was counted under a microscope,
and colonies containing >50 cells were considered as positive
for growth.
Wound healing assay
U87 cells transfected with PIWIL2-shRNA#1 or
control-shRNA, respectively, were seeded in 6-well plates and grown
to confluence. Subsequently, the cells were serum starved for 12 h,
and scratched on the monolayer by using a sterile 10-µl
micropipette tip. Then the U87 cells were washed twice with PBS,
and cultured in serum-free DMEM medium at 37°C and 5%
CO2, for an additional 24 h. Images were captured using
an inverted Leica phase contrast microscope (Leica DFC 300 FX) at
0- and 24-h time-points.
Transwell migration assay
U87 cells transfected with PIWIL2-shRNA#1 and
negative-control shRNA were suspended in DMEM containing 0.1%
bovine serum albumin, respectively. Cells (1×105) were
added to the top chambers of 24-well Transwell plates (8-µm pore
size; Corning), and DMEM containing 10% FBS was added to the bottom
chambers. Following incubation at 37°C for 24 h, the cells which
migrated to the bottom chambers were fixed with paraformaldehyde
and stained with crystal violet. The number of migrating cells in 5
fields were counted under an ×200 magnification for all the
chambers. Experimental data were obtained from triplicate chambers
and the experiments were repeated three times.
Statistical analysis
The statistical correlations between PIWIL2 and
Ki-67 expression and the clinicopathological features were analyzed
by the Chi-square (χ2) test or Fisher's exact test.
Kaplan-Meier survival plots were constructed to analyze the
survival rate of patients with glioma, and the log-rank test was
performed to compare the curves. Statistical analysis was performed
using SPSS 22.0 statistical software (SPSS, Inc., Chicago, IL,
USA). All values are expressed as the mean ± SEM, and p<0.05 was
considered to be statistically significant.
Results
PIWIL2 overexpression in glioma
tissues
In order to explore the possible role of PIWIL2 in
the progression of glioma, we first evaluated the expression of
PIWIL2 in two normal brain specimens and three pairs of glioma
specimens from different pathological grades (WHO grade II–IV) by
western blot analysis. It was found that PIWIL2 was overexpressed
in the glioma tissues, and its expression level exhibited an
increasing trend from low-grade glioma tissues to high-grade glioma
tissues (Fig. 1A and B).
Furthermore, the expression of PIWIL2 and proliferation marker
Ki-67 (20) were examined in 97
glioma tissue samples by immunohistochemistry. Similar to the
expression of Ki-67, PIWIL2 was highly expressed in the glioma
samples with a high degree of malignancy and its expression was
significantly higher than that in the low-grade malignancy samples
(Fig. 1C).
 | Figure 1.Relationship between Piwi-like
RNA-mediated gene silencing 2 (PIWIL2) and glioma pathological
grade, Ki-67 and glioma prognosis. (A) The protein expression
levels of PIWIL2 were detected in glioma tissue of different grades
(grades II–IV) and normal brain tissues by western blot analysis.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a
cross-reference. (B) The bar graph displays the ratio of PIWIL2
protein to GADPH for the aforementioned tissues by densitometry.
The data are expressed as the mean ± SEM of three independent
experiments (n=3, *,ˆp<0.01). (C) Immunohistochemical
staining. Paraffin-embedded glioma tissue sections (grades II, III,
and IV) were stained with anti-PIWIL2 antibodies and anti-Ki-67
antibodies, and then counterstained with hematoxylin
(magnification, ×400; scale bar, 50 µm). (D) The correlation
between PIWIL2 and Ki-67. (E and F) Kaplan-Meier survival curves of
97 glioma patients with high PIWIL2 expression vs. low PIWIL2
expression. Based on the mean PIWIL2 percentages, patients were
divided into a high PIWIL2 expression group and a low PIWIL2
expression group. According to the WHO grades, the patients were
divided into three groups (WHO grade II, III and IV). Patients in
the high-expression PIWIL2 group had a significantly shorter
overall survival (P<0.05). |
Correlation between the expression of
PIWIL2 and the survival of glioma patients
According to the results obtained with
immunohistochemical analysis, the data revealed that PIWIL2 and
Ki-67 were expressed with a positive correlation in glioma patients
(Fig. 1D). Subsequently, we further
analyzed the immunohistochemical results and the
clinicopathological data of 97 patients with glioma to explore the
clinical significance of PIWIL2 expression (Table I). We observed that the expression
of PIWIL2 was clearly associated with the clinicpathological grade
of the glioma patients (P=0.001) and Ki-67 expression (P=0.001),
but there was no obvious correlation between the expression of
PIWIL2 and other clinicopathological patient factors such as age
(P=0.577), sex (P=0.443), tumor location (P=0.848), tumor size
(P=0.800) and extent of resection (P=0.626). Subsequently, Cox's
proportional hazards model was constructed and it revealed that a
high expression level of PIWIL2 and WHO grade were prognostic
indicators of the survival time of the glioma patients (P<0.05,
Table II). By using Kaplan-Meier
survival curves, we observed that the expression of PIWIL2 was
negatively correlated with the overall survival of the glioma
patients (Fig. 1E and F). In
summary, we conclude that a high expression of PIWIL2 was an
important determinant of poor prognosis in patients with
glioma.
 | Table I.PIWIL2 expression and
clinicopathological variables in 97 glioma specimens. |
Table I.
PIWIL2 expression and
clinicopathological variables in 97 glioma specimens.
|
|
| PIWIL2
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Variables | Total | Low | High | χ2
value | P-value |
|---|
| Age (years) |
|
<50 | 59 | 20 | 39 | 0.312 | 0.577 |
|
≥50 | 38 | 15 | 23 |
|
|
| Sex |
|
Male | 56 | 22 | 34 | 0.589 | 0.443 |
|
Female | 41 | 13 | 28 |
|
|
| Tumor location |
|
Frontal | 23 | 10 | 13 | 1.379 | 0.848 |
|
Parietal | 9 | 2 | 7 |
|
|
|
Occipital | 21 | 7 | 14 |
|
|
|
Temporal | 17 | 6 | 11 |
|
|
|
Unknown | 27 | 10 | 17 |
|
|
| Tumor size
(cm) |
|
<4 | 51 | 19 | 32 | 0.064 | 0.800 |
| ≥4 | 46 | 16 | 30 |
|
|
| Extent of
resection |
| Total
resection | 33 | 13 | 20 | 0.238 | 0.626 |
|
Subtotal resection | 64 | 22 | 42 |
|
|
| WHO grade |
| II | 22 | 15 | 7 | 13.675 | 0.001a |
|
III | 30 | 6 | 24 |
|
|
| IV | 45 | 14 | 31 |
|
|
| Ki-67 |
| Low
expression | 28 | 17 | 11 | 10.355 | 0.001a |
| High
expression | 69 | 18 | 51 |
|
|
 | Table II.Contribution of various potential
prognostic factors to survival by Cox regression analysis in 97
glioma specimens. |
Table II.
Contribution of various potential
prognostic factors to survival by Cox regression analysis in 97
glioma specimens.
| Characteristic | Hazard ratio | 95% CI | P-value |
|---|
| Age | 1.157 | 0.643–2.081 |
0.627 |
| Sex | 0.765 | 0.429–1.366 |
0.365 |
| Tumor location | 1.018 | 0.844–1.226 |
0.855 |
| Tumor size | 0.902 | 0.505–1.611 |
0.727 |
| WHO grade | 2.792 | 1.708–4.562 |
<0.001a |
| PIWIL2
expression | 4.780 | 2.320–9.852 |
<0.001a |
| Ki-67
expression | 2.865 | 1.450–5.662 | 0.002a |
Expression of PIWIL2 in human glioma
cell lines and the promotion of G1/S phase transition
In our preliminary investigation, we determined that
the expression of PIWIL2 was positively correlated with the
expression of Ki-67 in glioma specimens. Thus, we hypothesized that
PIWIL2 may be involved in the cell cycle progression of glioma
cells. Due to the fact that PIWIL2 is expressed at the highest
level in U87 cells, we selected it as our main experimental cell
line in the following experiments (Fig.
2A and B). In order to ascertain our conjecture, we built a
serum starvation and re-feeding model. U87 cells were controlled in
the serum-free environment for 72 h, and the results of the flow
cytometric analysis demonstrated that the highest proportion of U87
cells was arrested in the G0/G1 phase. Subsequently, we added 10%
FBS and harvested the cells at 0, 4, 8, 12 and 24 h. Flow
cytometric analysis revealed that most U87 cells shifted from the
G0/G1 phase to the S phase gradually with the readdition of serum
(Fig. 2C). Meanwhile, we detected
changes in the expression levels of PIWIL2 at different time-points
during cell progression. Furthermore, the expression of two cell
cycle-related proliferation markers, PCNA and cyclin D1 (21), were assessed by western blot
analysis at the corresponding time-points. As expected, we found
that the expression of PIWIL2 increased gradually after serum
readdition, and a similar trend was obtained with these cell
cycle-related proliferation markers (Fig. 2D and E). Taking into consideration
the aforementioned experimental results, we put forward a
preliminary conclusion that PIWIL2 may be related to the
proliferation of glioma in a cell cycle-dependent pathway.
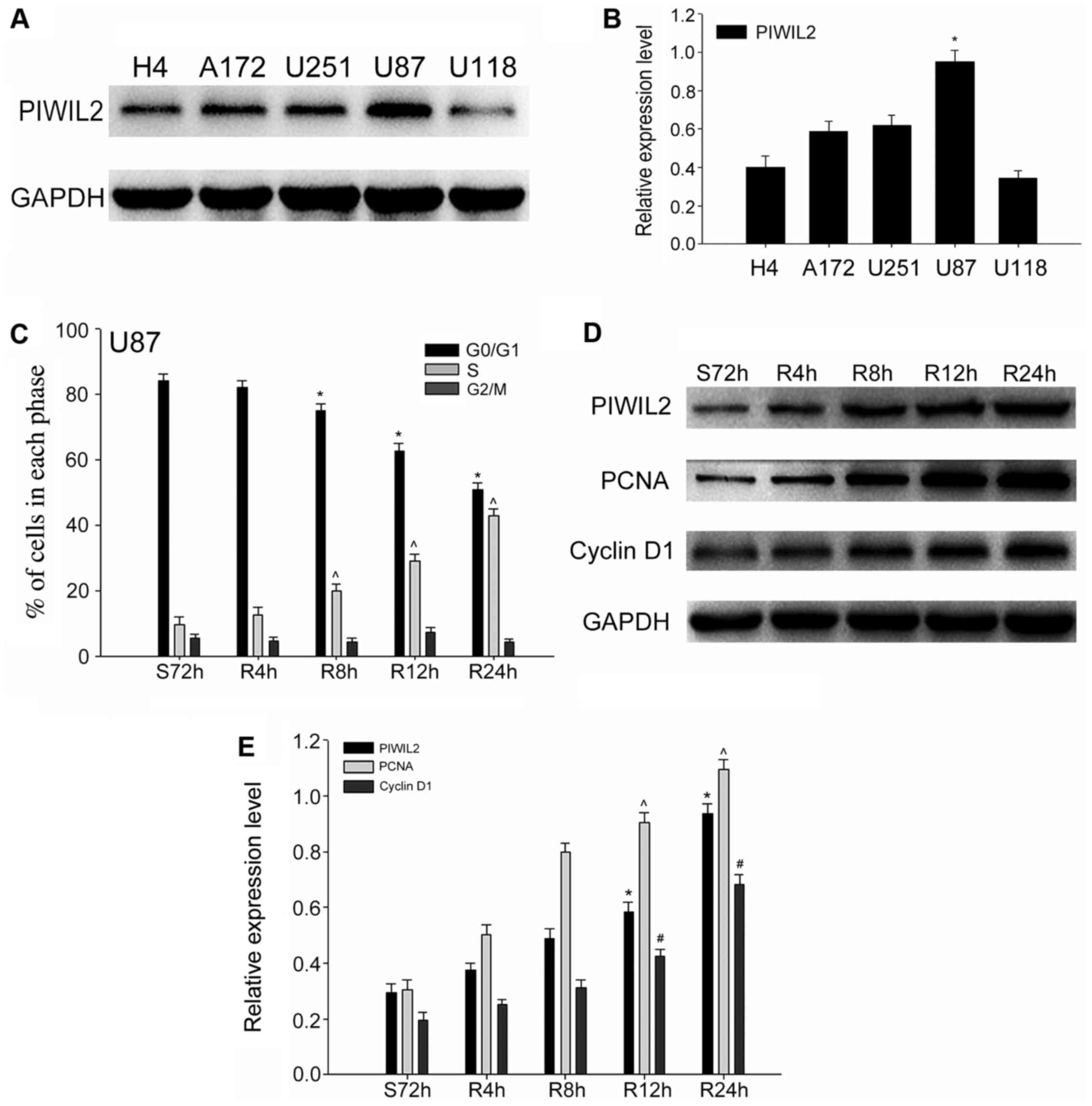 | Figure 2.The expression of Piwi-like
RNA-mediated gene silencing 2 (PIWIL2) and cell cycle-related
molecules in glioma cells. (A and B) The expression level of PIWIL2
in five glioma cell lines (H4, A172, U251, U87 and U118);
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an
internal standard. Data are expressed as the mean ± SEM (n=3,
*p<0.05). (C) Flow cytometry revealed the cell cycle progression
of U87 cells in the serum starvation (S) and re-feeding (R) model.
Data are expressed as the mean ± SEM (n=3,
*,^p<0.05). (D and E) The expression of PIWIL2 and
two cell cycle and proliferation markers (PCNA and cyclin D1) were
detected concomitantly with western blot analysis at different
time-points. GAPDH was used as a protein loading control. The ratio
of PCNA and cyclin D1 compared to GAPDH was determined by
densitometry, which is displayed in the form of a bar graph. The
data are expressed as the mean ± SEM [n=3,
*,ˆ,#p<0.01, compared with the control cell
serum-starved control for 72 h (S72 h)]. |
Downregulation of PIWIL2 inhibits cell
growth and induces the delay of G1/S transition
To further clarify the function of PIWIL2, we
investigated the effect of the interference of PIWIL2 expression on
the proliferation of glioma cells. We transfected U87 cells with
PIWIL2-shRNAs or control-shRNA, respectively, and then detected the
expression of PIWIL2 protein by western blot analysis to determine
the effectiveness of all the PIWIL2-shRNAs (Fig. 3A and B). The results revealed that
PIWIL2-shRNA#1 exhibited the most effective decrease, thus we
selected it as our main shRNA in the following experiments. In
comparison to the control group, flow cytometry indicated that a
larger proportion of U87 cells transfected with PIWIL2-shRNA#1 were
arrested in the G0/G1 phase, which meant that PIWIL2 may contribute
to the G1/S phase transition of U87 cells (Fig. 3C and D). Previous studies
demonstrated that PIWIL2 could facilitate tumor cell (HeLa and
HepG2 cells) transformation from the G0/G1 phase to the S phase
(22). Moreover, it could also
inhibit the expression of p53, which is considered to be an
important regulator of the cell cycle (11). In our study, western blot analysis
indicated that silencing of PIWIL2 promoted the expression of p53
and p21, and inhibited the expression of cyclin D1 and CDK2
concomitantly (Fig. 3E and F).
Furthermore, colony formation assay revealed that the proliferation
of U87 glioma cells was significantly suppressed after PIWIL2
knockdown (Fig. 3G). These results
demonstrated that PIWIL2 may regulate the cell cycle via
dysregulation of the p53-p21-cyclin E/Cdk2 pathway, and interfering
with its expression could block glioma cell cycle progression, and
then inhibit its proliferation.
 | Figure 3.Knockdown of Piwi-like RNA-mediated
gene silencing 2 (PIWIL2) inhibits the proliferation of U87 cells.
(A and B) Detection of the expression of PIWIL2 in
PIWIL2-shRNA-transfected-U87 cells by western blot analysis. The
results revealed that PIWIL2-shRNA1 exhibited a stronger
downregulation effect. Data are expressed as the mean ± SEM (n=3,
*p<0.05 compared with the control groups). (C and D)
PIWIL2-shRNA1-transfected U87 cells, control shRNA transfected U87
cells and untreated U87 cells were pretreated by serum starvation
to maintain cell cycle synchronization. The cell cycle distribution
was assessed by flow cytometry 48 h later. Data are expressed as
the mean ± SEM (n=3, *,^p<0.05 compared with the
control groups). (E and F) Western blot analysis revealed the
influence of PIWIL2 knockdown on p53, p21, cyclin E and
cyclin-dependent kinase 2 (CDK2) protein expression. Data are
expressed as the mean ± SEM (n=3, *,ˆ,#,$,&p<0.05
compared with the control groups). (G) Silencing of PIWIL2
inhibited cell growth as determined by colony formation assay. |
Knockdown of PIWIL2 expression induces
apoptosis in glioma cells
A decrease in of apoptosis is an important mechanism
for the occurrence and development of cancer. Consequently, flow
cytometric assay was utilized to explore whether PIWIL2 could
affect the apoptosis of glioma cells. The results demonstrated that
the proportion of apoptotic cells in the PIWIL2-shRNA1-transfected
U87 cells was significantly higher than that in the control group
(Fig. 4A and B). Then, further
investigation by western blotting assay was performed to compare
the expression of PIWIL2 with some previously reported
apoptosis-related molecules, such as cleaved caspase-3 and Bcl-2
(which is considered to be an antiapoptotic protein) (23,24).
The results revealed that knockdown of PIWIL2 increased the
expression of active caspase-3, and inhibited the expression of
Bcl-2 concomitantly (Fig. 4C and
D). In conclusion, these results indicated that PIWIL2 may
promote the development of glioma through its antiapoptotic
effect.
PIWIL2 enhances glioma cell migration
in vitro
Strong invasion and migration abilities are
considered as significant characteristics of human gliomas.
Moreover, previous studies have demonstrated that PIWIL2 promotes
tumor cell migration (17,25). Based on this, we speculated that
PIWIL2 may be associated to the migration of glioma cells. Through
the wound-healing experiment, we observed that the migration of U87
cells transfected with PIWIL2-shRNA1 was significantly slower than
that of the control group (Fig.
5A). In addition, the result of the Transwell experiment
demonstrated that knockdown of the expression of PIWIL2 inhibited
the ability of U87 cell migration to the bottom chambers (Fig. 5B). To further clarify the function
of PIWIL2 in the migration of glioma cells, we compared the
expression of certain migration-related molecules in the
PIWIL2-shRNA1-transfected U87 and control cells. Notably, we
determined that when PIWIL2 was silenced by shRNA1, the expression
levels of two mesenchymal markers (N-cadherin and vimentin) were
downregulated, while epithelial marker E-cadherin expression was
upregulated (26). The results
demonstrated that PIWIL2 may be involved in the
epithelial-mesenchymal transition (EMT) of glioma cells. In
addition, PIWIL2 was reported to promote the invasion and migration
of colon and prostate cancer cells through the regulation of the
expression of matrix metalloproteinase-9 (MMP9) (17,25).
In our study, we also observed that silencing of PIWIL2 decreased
the expression of MMP9 (Fig. 5C and
D). MMP9 is considered to be an important factor in the
regulation of the migration of gliomas (27). Collectively the aforementioned
experiments demonstrated that PIWIL2 was associated to the
migration of gliomas.
 | Figure 5.Silencing of Piwi-like RNA-mediated
gene silencing 2 (PIWIL2) inhibits the migration of glioma cells.
(A) Monolayer U87 cells transfected with control or PIWIL2 shRNA,
as well as the mock group were analyzed by wound-healing assays.
The migration of cells into the wound was assessed at 0 and 24 h.
(B) U87 cells were stained with crystal violet, and the migration
ability of glioma cells was analyzed by Transwell experiment after
24 h. (C and D) Western blot analysis of PIWIL2, E-cadherin,
N-cadherin, vimentin and MMP9 levels in the mock, control and
shRNA1 groups. Data are expressed as the mean ± SEM (n=3,
*,ˆ,#,$,&p<0.05 compared with the control
groups). |
Discussion
PIWIL2, as a member of the Piwi gene family, is
considered to be closely related with RNA interference, stem cell
self-renewal and translational regulation in germ cells (6). Moreover, it was also reported to be
involved in the pathological process of the development and
differentiation of cancer stem cells (CSCs) (13,28).
CSCs are regarded as the major determinant of tumor initiation,
propagation, metastasis and recurrence (29). Previous studies have demonstrated
that PIWIL2 may be associated with the proliferation, invasion and
migration of a variety of malignancies and predicts the poor
prognosis of patients (17,25). In this study, we investigated the
potential role of PIWIL2 in the promotion of proliferation and
migration of human gliomas, as well as demonstrated the clinical
significance of its expression.
Through western blot and immunohistochemical
analyses, we found that PIWIL2 was significantly overexpressed in
glioma tissues. Furthermore, the expression level of PIWIL2
exhibited an increasing trend, and was tightly associated with the
increase in the pathological grade of the gliomas (WHO grade
II–IV), which indicates that PIWIL2 may be involved in the
pathological process of gliomas. Subsequently, the
clinicopathological data of the corresponding patients with glioma
were analyzed systematically. Through Kaplan-Meier analysis as well
as univariate analysis by Cox proportional hazards model, it was
revealed that the high expression of PIWIL2 indicated a poor
prognosis with a significantly shorter overall survival.
From a macro point of view, the expression of PIWIL2
may contribute to the progression of gliomas. We further explored
the effect of PIWIL2 on the proliferation and migration of human
glioma cells in vitro. Cell cycle acceleration and apoptosis
inhibition are considered to be important factors for the rapid
proliferation of tumor cells (30,31).
Previous studies demonstrated that PIWIL2 could accelerate G1/S
phase transformation of tumor cells (HeLa and HepG2 cells) by
inducing c-Myc expression (22). In
the current study, serum starvation and re-feeding experiments
determined that the expression of PIWIL2 was gradually increased
after serum readdition, consistent with two cell cycle-related
proteins, PCNA and cyclin D1. Thus, we believe that PIWIL2 is
involved in the progression of the cell cycle of glioma cells. In
addition, flow cytometric analysis displayed that a higher
proportion of U87 cells was arrested in the G0/G1 phase when the
expression of PIWIL2 was silenced by RNAi technique. PIWIL2 was
reported to function in the inhibition of p53 expression (11). p53, known as an important
cancer-inhibiting protein, has an intimate connection with tumor
cell cycle regulation and apoptosis (32). The p53 protein upregulates the
expression of p21, which then blocks the cell cycle in the S phase
by inhibiting the activation of the cyclin E/Cdk2 complex (33). Based on this theory, we found that
silencing of PIWIL2 led to the upregulation of p53 and p21
expression, and the downregulation of the expression of cyclin E
and Cdk2. These results demonstrated that PIWIL2 may induce glioma
G1/S phase transition via dysregulation of the p53-p21-cyclin
E/Cdk2 pathway. Moreover, flow cytometric analysis revealed that
there was a significantly higher proportion of apoptotic cells in
the PIWIL2-depleted U87 cells. PIWIL2 has already been demonstrated
to inhibit cell apoptosis through the activation of the
Stat3/Bcl-XL pathway (34). Similar
to Bcl-XL, Bcl-2 and caspase 3 are also regulated by STAT3, which
leads to cell apoptosis (35–37).
In our study, we determined that downregulated expression of PIWIL2
led to the increased expression of cleaved caspase 3 while it
inhibited Bcl-2 expression, which meant that PIWIL2 may inhibit
glioma cell apoptosis by suppressing nuclear to mitochondrial
translocation as well as the subsequent caspase activation.
Migration and invasion is another prominent feature
of gliomas (38). In the study,
wound healing and Transwell assays demonstrated that PIWIL2
promoted the migration of glioma cells. Previous studies have
reavealed that EMT is an important process in which tumor cells can
enhance their migration ability (39). Therefore, research concerning the
effect of PIWIL2 on the EMT would be helpful to elucidate its
mechanism in the migration of gliomas. Loss of cell-cell adhesion,
increased tumor cell mobility, downregulated expression of
epithelial markers and overexpression of mesenchymal markers are
usually recognized as the main biological characteristics of EMT
(40,41). Our study revealed that inhibition of
PIWIL2 expression in glioma cells increased epithelial marker
E-cadherin expression, while it decreased the expression of
mesenchymal markers N-cadherin and vimentin. Therefore,
PIWIL2-induced EMT may be an important mechanism for the migration
of glioma cells. Moreover, it was revealed that PIWIL2 promoted
MMP9 expression by regulating its transcriptional activity, thereby
enhancing the invasion and migration of colon cancer cells
(25). MMP9 is also considered to
be an important regulatory factor for the migration and invasion of
gliomas (27). Western blot
analysis demonstrated that silencing of PIWIL2 decreased the
expression of MMP9 in U87 cells. Thus, we believe that the
promotion of MMP9 expression was also one of the mechanisms through
which PIWIL2 enhanced the migration of glioma cells. Nevertheless,
specific mechanisms of PIWIL2 regulation on MMP9 expression, as
well as the influence on its activity have yet to be elucidated
through further experimental research. In conclusion, our research
demonstrated that PIWIL2 is highly expressed in glioma tissues and
provides important predictive information for patients with
gliomas. Additionally, PIWIL2 was also positively correlated with
the proliferation and migration of glioma cells. Nonetheless, the
precise molecular mechanisms of PIWIL2 in gliomas require further
investigation, with the hope to provide a new molecular target for
the diagnosis and therapies of glioma in the future.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81372687).
References
|
1
|
Wang Y, Liu F, Mao F, Hang Q, Huang X, He
S, Wang Y, Cheng C, Wang H, Xu G, et al: Interaction with cyclin
H/cyclin-dependent kinase 7 (CCNH/CDK7) stabilizes C-terminal
binding protein 2 (CtBP2) and promotes cancer cell migration. J
Biol Chem. 288:9028–9034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu K, Pei H, Zhang Z, Dong S, Fu RJ, Wang
WM and Wang H: FoxO3a mediates glioma cell invasion by regulating
MMP9 expression. Oncol Rep. 36:3044–3050. 2016.PubMed/NCBI
|
|
3
|
Batchelor TT, Reardon DA, De Groot JF,
Wick W and Weller M: Antiangiogenic therapy for glioblastoma:
Current status and future prospects. Clin Cancer Res. 20:5612–5619.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tao T, Cheng C, Ji Y, Xu G, Zhang J, Zhang
L and Shen A: Numbl inhibits glioma cell migration and invasion by
suppressing TRAF5-mediated NF-κB activation. Mol Biol Cell.
23:2635–2644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu B, Nandhu MS, Sim H, Agudelo-Garcia PA,
Saldivar JC, Dolan CE, Mora ME, Nuovo GJ, Cole SE and Viapiano MS:
Fibulin-3 promotes glioma growth and resistance through a novel
paracrine regulation of Notch signaling. Cancer Res. 72:3873–3885.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sasaki T, Shiohama A, Minoshima S and
Shimizu N: Identification of eight members of the Argonaute family
in the human genome. Genomics. 82:323–330. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Höck J and Meister G: The Argonaute
protein family. Genome Biol. 9:2102008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuramochi-Miyagawa S, Kimura T, Ijiri TW,
Isobe T, Asada N, Fujita Y, Ikawa M, Iwai N, Okabe M, Deng W, et
al: Mili, a mammalian member of piwi family gene, is essential for
spermatogenesis. Development. 131:839–849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cox DN, Chao A, Baker J, Chang L, Qiao D
and Lin H: A novel class of evolutionarily conserved genes defined
by piwi are essential for stem cell self-renewal. Genes Dev.
12:3715–3727. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen C, Liu J and Xu G: Overexpression of
PIWI proteins in human stage III epithelial ovarian cancer with
lymph node metastasis. Cancer Biomark. 13:315–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu Y, Zhang K, Li C, Yao Y, Tao D, Liu Y,
Zhang S and Ma Y: Piwil2 suppresses p53 by inducing phosphorylation
of signal transducer and activator of transcription 3 in tumor
cells. PLoS One. 7:e309992012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen L, Shen R, Ye Y, Pu XA, Liu X, Duan
W, Wen J, Zimmerer J, Wang Y, Liu Y, et al: Precancerous stem cells
have the potential for both benign and malignant differentiation.
PLoS One. 2:e2932007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JH, Schütte D, Wulf G, Füzesi L,
Radzun HJ, Schweyer S, Engel W and Nayernia K: Stem-cell protein
Piwil2 is widely expressed in tumors and inhibits apoptosis through
activation of Stat3/Bcl-XL pathway. Hum Mol Genet. 15:201–211.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu JJ, Shen R, Chen L, Ye Y, He G, Hua K,
Jarjoura D, Nakano T, Ramesh GK, Shapiro CL, et al: Piwil2 is
expressed in various stages of breast cancers and has the potential
to be used as a novel biomarker. Int J Clin Exp Pathol. 3:328–337.
2010.PubMed/NCBI
|
|
15
|
Qu X, Liu J, Zhong X, Li X and Zhang Q:
PIWIL2 promotes progression of non-small cell lung cancer by
inducing CDK2 and cyclin A expression. J Transl Med. 13:3012015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Liu Y, Shen X, Zhang X, Chen X,
Yang C and Gao H: The PIWI protein acts as a predictive marker for
human gastric cancer. Int J Clin Exp Pathol. 5:315–325.
2012.PubMed/NCBI
|
|
17
|
Yang Y, Zhang X, Song D and Wei J: Piwil2
modulates the invasion and metastasis of prostate cancer by
regulating the expression of matrix metalloproteinase-9 and
epithelial-mesenchymal transitions. Oncol Lett. 10:1735–1740.
2015.PubMed/NCBI
|
|
18
|
Oh SJ, Kim SM, Kim YO and Chang HK:
Clinicopathologic implications of PIWIL2 expression in colorectal
cancer. Korean J Pathol. 46:318–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mu H, Lin KX, Zhao H, Xing S, Li C, Liu F,
Lu HZ, Zhang Z, Sun YL, Yan XY, et al: Identification of biomarkers
for hepatocellular carcinoma by semiquantitative
immunocytochemistry. World J Gastroenterol. 20:5826–5838. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Preusser M, Hoeftberger R, Woehrer A,
Gelpi E, Kouwenhoven M, Kros JM, Sanson M, Idbaih A, Brandes AA,
Heinzl H, et al: Prognostic value of Ki67 index in anaplastic
oligodendroglial tumours - a translational study of the European
Organization for Research and Treatment of Cancer Brain Tumor
Group. Histopathology. 60:885–894. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Darzynkiewicz Z, Zhao H, Zhang S, Lee MY,
Lee EY and Zhang Z: Initiation and termination of DNA replication
during S phase in relation to cyclins D1, E and A, p21WAF1, Cdt1
and the p12 subunit of DNA polymerase δ revealed in individual
cells by cytometry. Oncotarget. 6:11735–11750. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yao Y, Li C, Zhou X, Zhang Y, Lu Y, Chen
J, Zheng X, Tao D, Liu Y and Ma Y: PIWIL2 induces c-Myc expression
by interacting with NME2 and regulates c-Myc-mediated tumor cell
proliferation. Oncotarget. 5:8466–8477. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang S, Wang L and Kong Q: Depression of
focal adhesion kinase induces apoptosis in rat osteosarcoma OSR-6
cells in a caspase-dependent pathway. Cell Biochem Biophys.
70:765–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Al-Shenawy HA: Expression of beclin-1, an
autophagy-related marker, in chronic hepatitis and hepatocellular
carcinoma and its relation with apoptotic markers. APMIS.
124:229–237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li D, Sun X, Yan D, Huang J, Luo Q, Tang H
and Peng Z: Piwil2 modulates the proliferation and metastasis of
colon cancer via regulation of matrix metallopeptidase 9
transcriptional activity. Exp Biol Med (Maywood). 237:1231–1240.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tian L, Lu ZP, Cai BB, Zhao LT, Qian D, Xu
QC, Wu PF, Zhu Y, Zhang JJ, Du Q, et al: Activation of pancreatic
stellate cells involves an EMT-like process. Int J Oncol.
48:783–792. 2016.PubMed/NCBI
|
|
27
|
Veeravalli KK and Rao JS: MMP-9 and uPAR
regulated glioma cell migration. Cell Adhes Migr. 6:509–512. 2012.
View Article : Google Scholar
|
|
28
|
Litwin M, Dubis J, Arczyńska K, Piotrowska
A, Frydlewicz A, Karczewski M, Dzięgiel P and Witkiewicz W:
Correlation of HIWI and HILI expression with cancer stem cell
markers in colorectal cancer. Anticancer Res. 35:3317–3324.
2015.PubMed/NCBI
|
|
29
|
Akbari-Birgani S, Paranjothy T, Zuse A,
Janikowski T, Cieślar-Pobuda A, Likus W, Urasińska E, Schweizer F,
Ghavami S, Klonisch T, et al: Cancer stem cells, cancer-initiating
cells and methods for their detection. Drug Discov Today.
21:836–842. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bunt J, De Haas TG, Hasselt NE,
Zwijnenburg DA, Koster J, Versteeg R and Kool M: Regulation of cell
cycle genes and induction of senescence by overexpression of OTX2
in medulloblastoma cell lines. Mol Cancer Res. 8:1344–1357. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang C, Song T, Li J, Ao F, Gong X, Lu Y,
Zhang C, Chen L, Liu Y, He H and Huang O: RAS promotes
proliferation and resistances to apoptosis in meningioma. Mol
Neurobiol. 779–787. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu H, Chen F, Zhang L, Zhou Q, Gui S and
Wang Y: A novel all-trans retinoic acid derivative 4-amino 2
trifluoromethyl-phenyl retinate inhibits the proliferation of human
hepatocellular carcinoma HepG2 cells by inducing G0/G1 cell cycle
arrest and apoptosis via upregulation of p53 and ASPP1 and
downregulation of iASPP. Oncol Rep. 36:333–341. 2016.PubMed/NCBI
|
|
33
|
Zolota V, Sirinian C, Melachrinou M,
Symeonidis A and Bonikos DS: Expression of the regulatory cell
cycle proteins p21, p27, p14, p16, p53, mdm2, and cyclin E in bone
marrow biopsies with acute myeloid leukemia. Correlation with
patients' survival. Pathol Res Pract. 203:199–207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee JH, Jung C, Javadian-Elyaderani P,
Schweyer S, Schütte D, Shoukier M, Karimi-Busheri F, Weinfeld M,
Rasouli-Nia A, Hengstler JG, et al: Pathways of proliferation and
antiapoptosis driven in breast cancer stem cells by stem cell
protein piwil2. Cancer Res. 70:4569–4579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dlamini Z, Rupnarain C, Naicker S, Hull R
and Mbita Z: Expression analysis and association of RBBP6 with
apoptosis in colon cancers. J Mol Histol. 47:169–182. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jin H, Chen H, Yu K, Zhang J, Li B, Cai N
and Pan J: Resveratrol inhibits phosphorylation within the signal
transduction and activator of transcription 3 signaling pathway by
activating sirtuin 1 in SW1353 chondrosarcoma cells. Mol Med Rep.
14:2685–2690. 2016.PubMed/NCBI
|
|
37
|
Baral R, Bose A, Ray C, Paul S, Pal S,
Haque E, Mishra B, Pal D, Nagpal JK, Panda CK, et al: Association
of early phase of colorectal carcinogenesis with STAT3 activation
and its relevance in apoptosis regulation. Exp Mol Pathol.
87:36–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liang Q, Ma C, Zhao Y, Gao G and Ma J:
Inhibition of STAT3 reduces astrocytoma cell invasion and
constitutive activation of STAT3 predicts poor prognosis in human
astrocytoma. PLoS One. 8:e847232013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Song X, Han P, Liu J, Wang Y, Li D, He J,
Gong J, Li M, Tu W, Yan W, et al: Up-regulation of SPOCK1 induces
epithelial-mesenchymal transition and promotes migration and
invasion in esophageal squamous cell carcinoma. J Mol Histol.
46:347–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen HN, Yuan K, Xie N, Wang K, Huang Z,
Chen Y, Dou Q, Wu M, Nice EC, Zhou ZG, et al: PDLIM1 stabilizes the
E-cadherin/β-catenin complex to prevent epithelial-mesenchymal
transition and metastatic potential of colorectal cancer cells.
Cancer Res. 76:1122–1134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|



















