Introduction
Cervical cancer is the most common malignant tumor
of the female reproductive system. It is the second most commonly
diagnosed cancer and the third leading cause of cancer-related
death among females in developing countries (1). Radiotherapy is the major treatment for
cervical cancer. For patients with early-stage disease,
radiotherapy and surgery alone have equal effects. In recent years,
chemoradiotherapy has gradually become a new treatment pattern for
patients with locally advanced disease. It has been demonstrated
that radiotherapy plays a crucial role in the treatment of cervical
cancer. However, patients who suffer pelvic recurrence account for
~70% of the cases of radiotherapy failure (2). Thus, the identification of new factors
that influence radiosensitivity has important significance in
cervical cancer treatment.
Homeobox containing 1 (HMBOX1), a new member of the
homeobox genes, was identified and isolated from the human
pancreatic cDNA library. HMBOX1 was described as a transcription
repressor (3). In the ALT
(alternative lengthening of telomeres) cell line WI38-VA13 and
telomerase-positive HeLa cells, HMBOX1 was identified as a
double-stranded telomeric DNA binding protein (4) and acted as a positive regulator of
telomere length (5). In U2OS (ALT
cells), HMBOX1 was found to modulate telomere maintenance without
influencing telomere length (6).
However, the further function of HMBOX1 is not fully clear.
Telomeres, nucleoprotein complexes at the end of
eukaryotic chromosomes, are composed of 5′-TTAGGG-3′ repeats and
are maintained by telomerase. Telomeres contain protein complexes
and shelterin, and play a crucial role in protecting chromosome
ends from DNA damage. Moreover, telomere length may serve as a
target in predicting the individual radiosensitivity of patients
with cancers (7–11).
However, to date, the relationship between HMBOX1
and radiosensitivity remains unclear. Therefore, the present study
aimed to investigate whether HMBOX1 modulates radiosensitivity in
cervical cancer cells and to explore the potential mechanism.
Materials and methods
Cell lines and cell culture
The human cervical cancer cell lines (HeLa and C33A)
were maintained by the Key Laboratory of Tumor Biological Behavior
of Hubei Province. All cells were cultured in minimum essential
medium (MEM) (HyClone, Logan, UT, USA) supplemented with 10% fetal
bovine serum (Gibco, Grand Island, NY, USA), 100 U/ml penicillin
and 100 µg/ml streptomycin (BioSharp, Hefei, China) in 5%
CO2 at 37°C.
Transfections, lentiviral shRNA and
plasmids
HeLa and C33A cells were transfected with lentiviral
shRNA (GenePharma, Shanghai, China). The sequences of shRNA were:
shGFP, 5′-GTGGTACCAGCATCAGCCTT-3′, and shHMBOX1,
5′-GGACCTAGATGTAGATGAT-3′. Forty-eight hours after transfection,
the cells were treated with 1 µg/ml puromycin for 2 weeks. The
stable transfected cell lines were named as HeLa-NC, C33A-NC,
HeLa-HMBOX1 and C33A-HMBOX1, respectively. HeLa-HMBOX1 cells were
transfected with pHBLV-CMVIE-ZsGreen-TERT using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA).
Western blot analysis and real-time
PCR
Radioimmunoprecipitation assay (RIPA) lysis buffer
and phenylmethylsulfonyl fluoride (PMSF) were used to extract total
protein from the cultured cells. Western blotting was performed as
previously described (12). The
following antibodies were used: GAPDH (10494-1-AP), tubulin
(11224-1-AP), HMBOX1 (16123-1-AP) (all from Proteintech, Wuhan,
China), TERT (H231; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
BRCA1 (20649-1-AP) (Proteintech), ATM (2873), ATR (2790),
phospho-ATM (13050), phospho-ATR (2853), caspase-3 (9662) (all from
Cell Signaling Technology, Inc., Danvers, MA, USA) and γH2AX
(ab11174; Abcam, Cambridge, MA, USA).
Real-time PCR was used to detect the
relative mRNA level and telomere length
Total RNA was extracted from cultured cells using
TRIzol reagent (BioSharp). cDNA was synthesized using the
PrimeScript™ II First Strand cDNA Synthesis kit (Takara, Dalian,
China). Total DNA was extracted using the E.Z.N.A. Tissue DNA kit
(Omega Bio-Tek, Inc., Norcross, RA, USA). Then, cDNA and DNA were
amplified using the CFX96 Real-Time PCR Detection System (Bio-Rad,
Hercules, CA, USA) by SYBR Premix Ex Taq™ (Takara). The protocols
of GAPDH, HMBOX1 and TERT were performed as follows:
pre-degeneration at 95°C for 5 sec, 40 cycles of 95°C for 15 sec,
60°C for 30 sec.
36B4 and telomere primers were used to detect
relative telomere length as follows (13): pre-degeneration at 95°C for 10 min,
40 cycles of 95°C for 15 sec, 54°C for 2 min. All primer sequences
were synthesized by Tsingke Biological Technology (Wuhan, China):
GAPDH (forward, 5′-TGGAAGGACTCATGACCACA-3′ and reverse,
5′-TTCAGCTCAGGGATGACCTT-3′); HMBOX1 (forward,
5′-CTTCAGCGACTTCGGCGTA-3′ and reverse,
5′-ATCATAACTGTTGCTAGGTGACG-3′); TERT (forward,
5′-CATTTCATCAGCAAGTTTGGAAG-3′ and reverse,
5′-TTTCAGGATGGAGTAGCAGAGG-3′); tel (tel1,
5′-GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT-3′ and tel2,
5′-TCCCGACTATCCCTATCCCTATCCCTATCCCTATCCCTA-3′); 36B4 (36B4u,
5′-CAGCAAGTGGGAAGGTGTAATCC-3′ and 36B4d,
5′-CCCATTCTATCATCAACGGGTACAA-3′).
Colony formation assay
Cells were harvested from culture flasks and plated
into 6-well plates at appropriate dilutions, and with different
viable cells at 100, 100, 200, 400, 800, 1,000 and 2,000/well of
HeLa-NC and HeLa-HMBOX1 cells, respectively. Each plate was
irradiated with 0, 1, 2, 4, 6, 8, 10 Gy of X-rays after 12 h,
respectively. After culturing for 14 days, the cells were fixed and
stained using paraformaldehyde and Giemsa; clones were considered
to be viable cells when containing at least 50 cells. Different
cell lines have different plating efficiencies after IR exposure.
C33A cells have a higher intrinsic radiosensitivity compared with
HeLa, thus C33A-NC and C33A-HMBOX1 cells were plated with viable
cells at 100, 150, 300, 1,000, 6000, 20,000 and 100,000/well,
respectively. Other conditions were the same as for the HeLa cells
(12,14,15).
Data of the survival fraction were fitted into multi-target and
single-hit models, and survival curves were drawn using GraphPad
Prism 5.0 software.
Apoptosis assay
Apoptosis was assessed in the cells with or without
6 Gy X-ray exposure, and then cultured for 48 h. Apoptosis assay
was performed using an Annexin V-PE and 7-AAD apoptosis analysis
kit (Sungene Biotech Co., Ltd., Tianjin, China) according to the
protocol, and then analyzed by a flow cytometer (Beckman Coulter,
Brea, CA, USA).
Immunofluorescence
γH2AX was identified as a molecular marker of DSBs,
detected by immunofluorescence. Cells were divided into 4 groups:
HeLa-NC, HeLa-HMBOX1, HeLa-NC + 4Gy and HeLa-HMBOX1 + 4Gy. Thirty
minutes later, the cells with and without IR exposure were fixed
with paraformaldehyde for 15 min, permeabilized with 0.2% Triton
X-100 for 20 min, blocked with goat serum for 2 h and incubated
with the primary antibody overnight at 4°C, and then washed with
phosphate-buffered saline (PBS) and incubated with the secondary
antibody for 2 h away from light at room temperature. Nuclei were
stained with DAPI (Sigma, St. Louis, MO, USA). Images were obtained
using a confocal microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
Statistical analysis
All data were obtained from 3 independent
experiments. SPSS 17.0 and GraphPad Prism 5.0 were used to analyze
data. Data are expressed as mean ± SD. Independent samples t-test
was used for the comparison of data, and P<0.05 was considered
to indicate a statistically significant difference.
Results
Efficiency of HMBOX1 knockdown in
cervical cancer cells
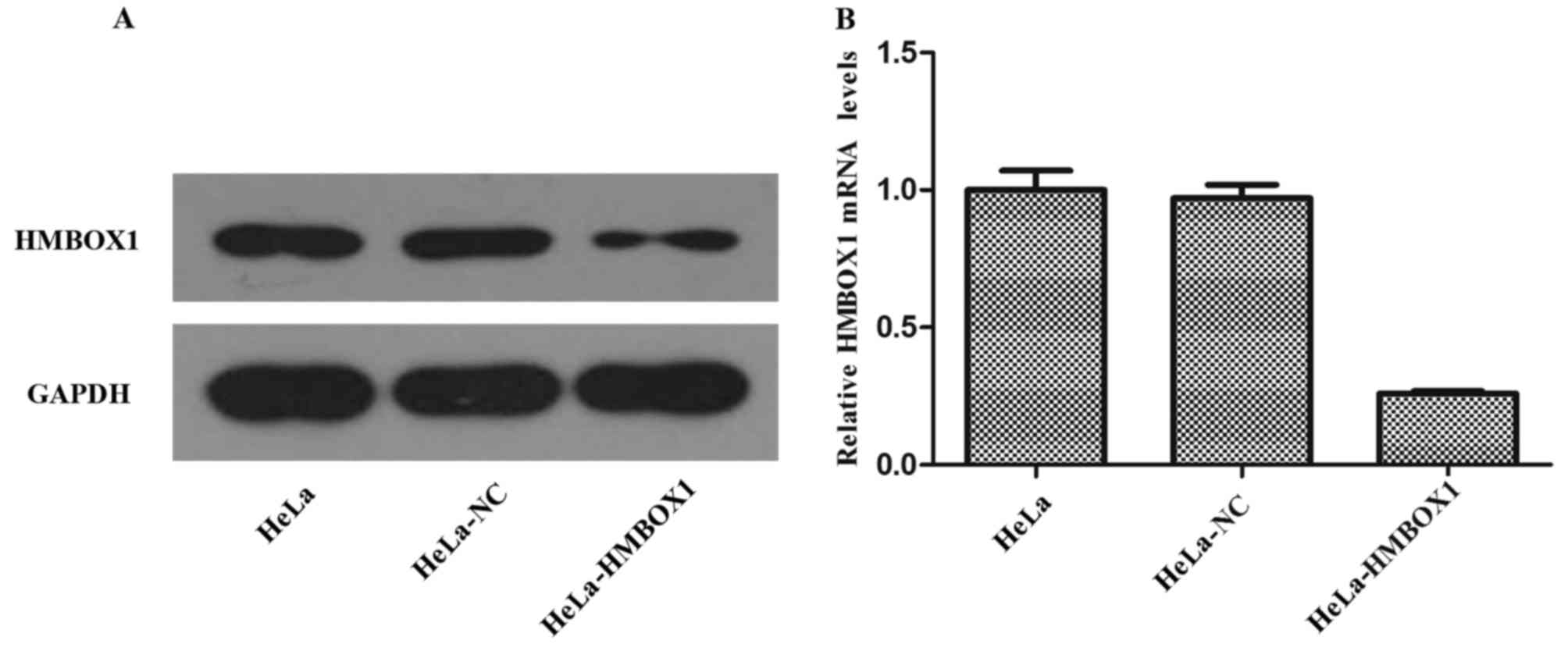
The expression of protein and mRNA was assessed
using western blotting and real-time PCR, respectively. As shown in
Fig. 1A and B, the expression of
HMBOX1 was obviously decreased both at the protein and mRNA level
in the HeLa cells. The results for the C33A cells were similar as
those for the HeLa cells. These results indicated that we
successfully established stable transfected cell lines of HeLa and
C33A.
Knockdown of HMBOX1 increases the
radiosensitivity of HeLa and C33A cells
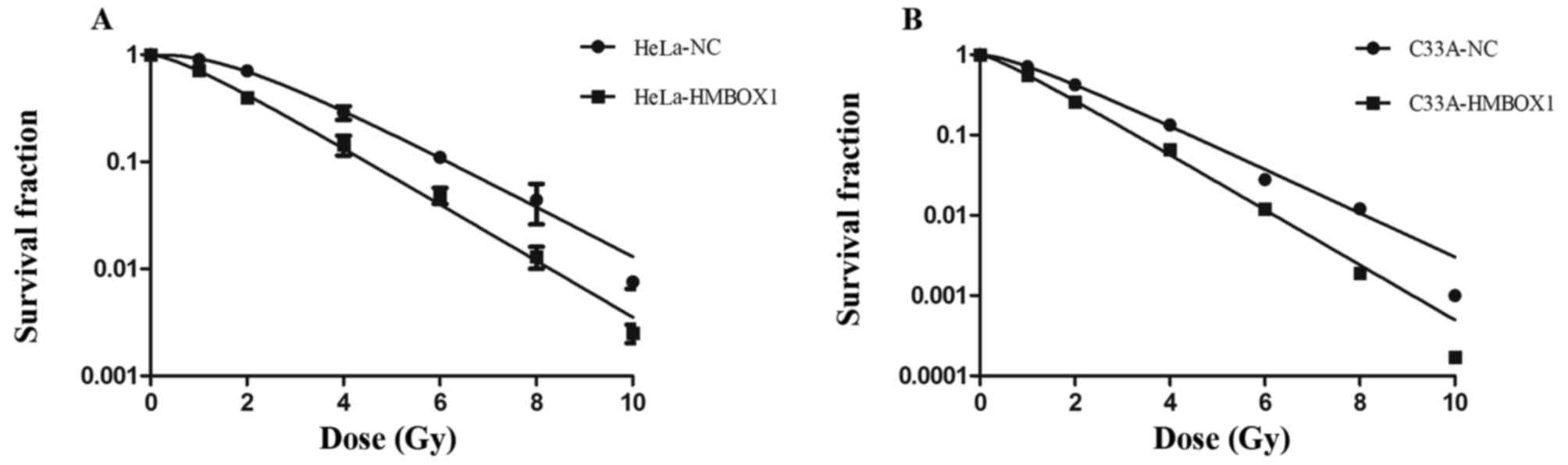
Colony formation assay is a cell survival assay that
reflects the ability to grow into a colony of cells. The survival
fraction after irradiation with 2 Gy X-rays (SF2) is a main target
in assessing the radiosensitivity of cancer cells. Compared with
the negative control cells, the knockdown cells had a significantly
lower SF2 (Fig. 2A and B). The
knockdown cell lines showed higher radiosensitivity compared with
that noted in the negative control cell lines.
Knockdown of HMBOX1 increases
spontaneous and radiation-induced apoptosis
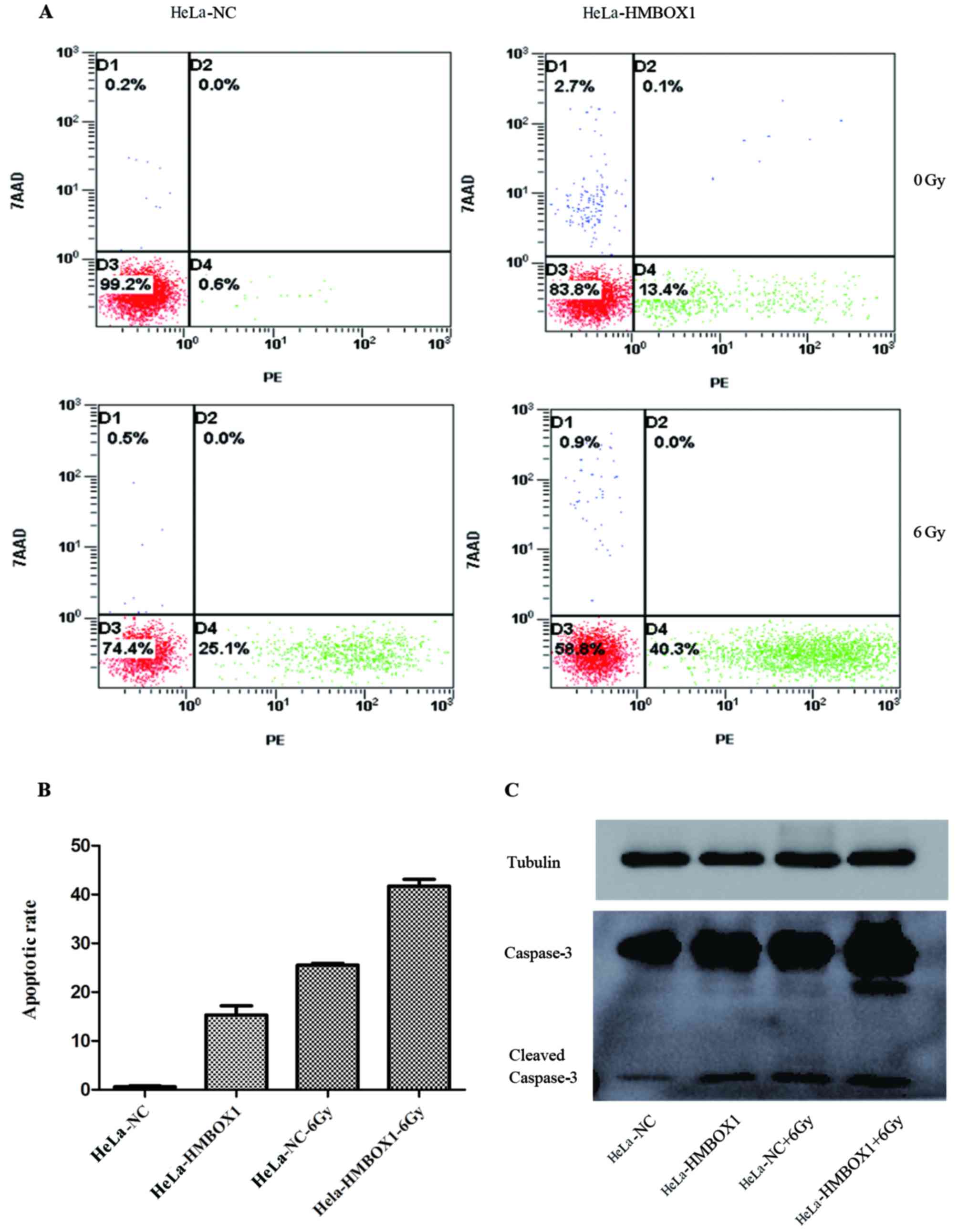
Flow cytometry was used to evaluate the effect of
HMBOX1 knockdown on the apoptosis rate in HeLa cells. The results
of flow cytometry showed that when compared with the negative
control groups, there was an increase in the apoptosis rate both in
the HeLa-HMBOX1 cell line with or without IR exposure (Fig. 3A and B). The expression levels of
apoptosis-related protein caspase-3 were consistent with the
results of the flow cytometry (Fig.
3C).
Knockdown of HMBOX1 reduces the
ability to repair DNA damage induced by irradiation
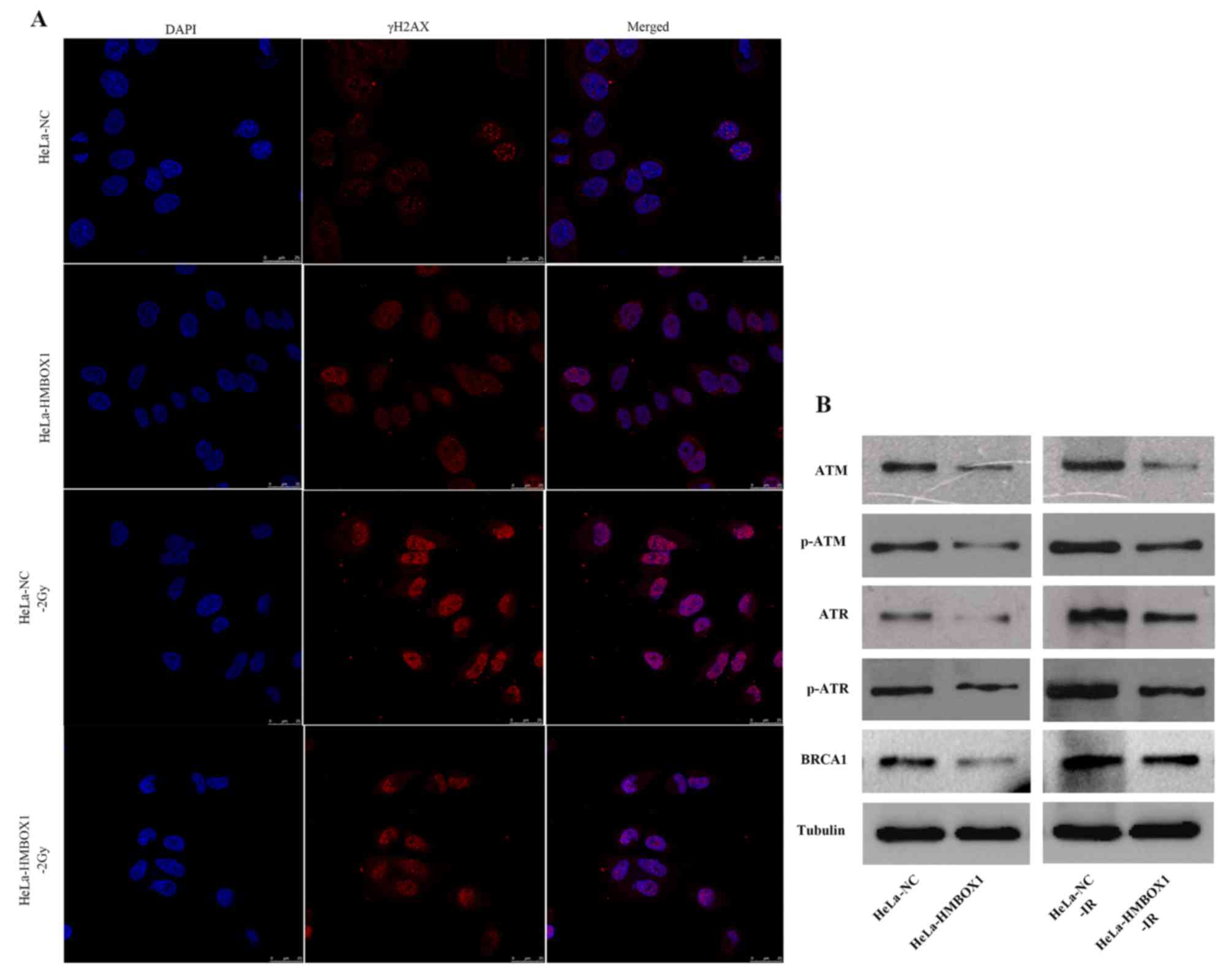
To investigate the effect of HMBOX1 knockdown on the
DNA damage response, we used immunofluorescence assay to detect DNA
damage foci, and found that HMBOX1 knockdown significantly
decreased radiation-induced DNA damage foci (γH2AX) compared with
the negative control group after irradiation. Furthermore, there
was no difference between the two cell lines without IR exposure
(Fig. 4A). Regarding DNA damage
repair proteins, HMBOX1 knockdown significantly reduced the
expression of ATM, ATR, p-ATM, p-ATR and BRCA1 (Fig. 4B).
Knockdown of HMBOX1 shortens telomere
length through TERT
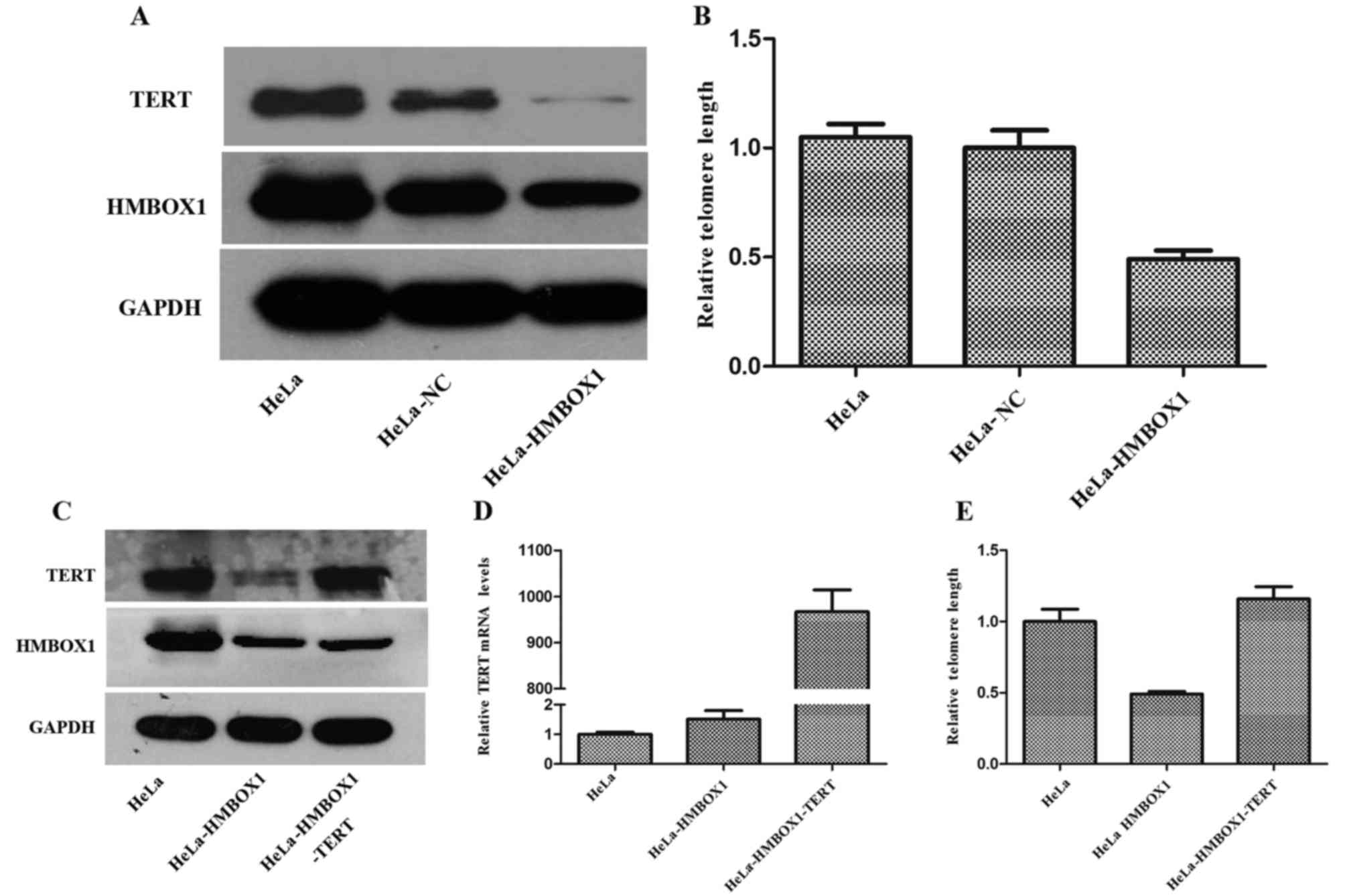
As shown in Fig. 5B,
we verified that knockdown of HMBOX1 shortened telomere length in
HeLa cells in accordance with a previous study (5). Moreover, the expression of TERT was
significantly decreased in the HeLa-HMBOX1 cells (Fig. 5A). To investigate the role of TERT
in the process of telomere shortening by HMBOX1 knockdown,
HeLa-HMBOX1 cells were transfected with pHBLV-CMVIE-ZsGreen-TERT
(Fig. 5C and D). TERT
overexpression rescued telomere shortening in the HeLa-HMBOX1 cells
(Fig. 5E).
Discussion
In the present study, we demonstrated that knockdown
of HMBOX1 increased the radiosensitivity of cervical cancer HeLa
and C33A cells through telomere shortening.
A previous study confirmed that HMBOX1 could
directly bind to double-stranded telomeric DNA (4) and participate in telomere length
regulation (5). In the present
study, we repeated a previous procedure and revealed that knockdown
of HMBOX1 could negatively regulate telomere length in HeLa cells.
Telomeres play a crucial role in radiotherapy. Telomere length can
be used to predict the radiosensitivity of patients with cancers
(7). Therefore, we hypothesized
that the radiosensitivity of HeLa and C33A cells could be increased
by HMBOX1 knockdown through telomere shortening.
To test this hypothesis, we established stable
transfected cell lines of HeLa and C33A. We found that HMBOX1
knockdown shortened telomere length and decreased ATM, ATR, BRCA1
protein expression levels which play a crucial role in DNA damage
repair response. Colony formation assay revealed that knockdown of
HMBOX1 increased radiosensitivity of the HeLa and C33A cells. These
data confirmed that HMBOX1 knockdown was closely related to the
radiosensitivity of cervical cancer cells.
There is scarce research referring to the
relationship between HMBOX1 and the DNA damage repair response.
Immunofluorescence showed that HMBOX1 knockdown decreased γH2AX
induced by irradiation which was identified as a molecular marker
of DSBs (16). After IR exposure,
H2AX becomes phosphorylated within a few minutes and forms foci at
DNA break sites. γH2AX plays a critical role in the recruitment of
repair factors to nuclear foci after DNA damage such as Rad50,
Rad51 and BRCA1. Moreover, the process facilitates further
recruitment of other DNA damage response factors (PI-3 protein
family, p53 and NBS1) to DNA break sites (7,17–19).
In mammalian cells, nonhomologous end joining
(NHEJ), and homologous recombination (HR) are primary repair
pathways in DNA DSBs. ATM and ATR protein kinase are the main
upstream checkpoint kinases in the HR pathway, while BRCA1 is an
important downstream protein. Radiation-induced DNA damage contains
double- and single-stranded breaks. The ATM pathway is thought to
respond primarily to double-stranded breaks, whereas ATR is
primarily required in single-stranded breaks (16). We found that knockdown of HMBOX1
decreased the expression of ATM, ATR and BRCA1 with or without
ionizing radiation exposure, as well as phospho-ATM and
phospho-ATR. Previous studies have verified that ATM, and ATR
expression levels are closely related to radiosensitivity in cancer
cells (20–24), and inhibition of radiation-induced
DNA damage repair is believed to lead to radiosensitization
(25). Our data indicate that
knockdown of HMBOX1 resulted in lower expression of ATM, ATR and
BRCA1 in the HR pathway.
Previous studies and our studies have demonstrated
that knockdown of HMBOX1 leads to telomere shortening. Telomere
attrition serves as a suppression mechanism of tumors, and
suppresses the proliferative capacity of cells resulting in the
suppression of cell clone growth (7). Short telomeres contain less shelterin
complex that contributes to telomere maintenance. Telomere
attrition may lead to telomere dysfunction due to the failing of
shelterins to adhere to short telomeres (7,26).
Telomere shortening directly contributes to the DNA damage, which
leads to chromosomal breaks, while chromatin structure influences
DNA repair process in turn (16,19).
In U2OS cells, knockdown of HMBOX1 increased both telomere
dysfunction-induced foci (TIFs)/cell and the percentage of
TIF-positive cells (6). Although we
did not repeat the experiment, we believe that knockdown of HMBOX1
has the same effect in HeLa cells based on this evidence. Thus, we
proposed that knockdown of HMBOX1 may result in the DNA damage
response through telomere shortening, which may be a main mechanism
of radiosensitization.
Telomerase reverse transcriptase (TERT) and
telomerase RNA component (TERC) are the two core elements of
telomerase which works as a ribonucleoprotein complex. TERT serves
as an enzyme with reverse transcription activity that is essential
for adding telomeric repeats to the chromosome end (5). Previous studies have verified that
there is a negative correlation between TERT and the
radiosensitivity of cancer cells (11,14).
We found that expression of TERT also decreased while HMBOX1 was
downregulated. Thus, TERT may act as a mediator between HMBOX1 and
telomere length. Notably, overexpression of TERT in HeLa-HMBOX1
cells rescued telomere shortening, which provided forceful evidence
of the link between HMBOX1 and telomere length. However, knockdown
of HMBOX1 in U2OS cells (ALT cell) did not influence telomere
length, which indirectly confirmed that TERT plays a crucial role
in the process of the regulation and telomere length by HMBOX1.
Ma et al showed that knockdown of HMBOX1
induced apoptosis in vascular endothelial cells in mice (27). We found that HMBOX1 had the same
effect in HeLa cells. Moreover, HeLa-HMBOX1 cells showed a
significantly higher apoptosis rate compared with HeLa-NC cells
after ionizing radiation exposure. Furthermore, telomere shortening
induced p53-dependent and -independent apoptosis (28,29).
Previous studies have verified that endothelial cell apoptosis and
antiangiogenic therapy enhance the tumor response to radiotherapy
(30–32). Combined effects of antiangiogenic
and HMBOX1 knockdown in radiotherapy need further investigation.
These results indicated that increasing spontaneous and
radiation-induced apoptosis via telomere shortening may contribute
to radiosensitization.
The main aim of the present study was to assess the
relationship between HMBOX1 and radiosensitivity. The present study
verified that knockdown of HMBOX1 increased radiosensitivity
through telomere shortening in cervical cancer cells. We
demonstrated the correlation among HMBOX1, telomere length and
TERT. Furthermore, the present study enhanced our understanding of
the link between DNA damage repair response and HMBOX1. Finally,
the combination of HMBOX1 knockdown and ionizing radiation may have
a synergistic effect on apoptosis. Together, our data suggest a
model in which HMBOX1 knockdown shortens telomere length that
limits repair of DNA damage and induces cell apoptosis, thereby
providing a reasonable explanation for the increased
radiosensitivity of cervical cancer cells.
Acknowledgements
The authors thank Sulin Mi for the editorial
assistance.
Glossary
Abbreviations
Abbreviations:
|
DSBs
|
DNA double-stranded breaks
|
|
γH2AX
|
phosphorylated histone H2AX
|
|
IR
|
ionizing radiation
|
|
ATM
|
ataxia telangiectasia-mutated
|
|
ATR
|
ataxia telangiectasia rad3-related
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zuliani AC, Esteves SC, Teixeira LC,
Teixeira JC, de Souza GA and Sarian LO: Concomitant cisplatin plus
radiotherapy and high-dose-rate brachytherapy versus radiotherapy
alone for stage IIIB epidermoid cervical cancer: A randomized
controlled trial. J Clin Oncol. 32:542–547. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen S, Saiyin H, Zeng X, Xi J, Liu X, Li
X and Yu L: Isolation and functional analysis of human HMBOX1, a
homeobox containing protein with transcriptional repressor
activity. Cytogenet Genome Res. 114:131–136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Déjardin J and Kingston RE: Purification
of proteins associated with specific genomic Loci. Cell.
136:175–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kappei D, Butter F, Benda C, Scheibe M,
Draškovič I, Stevense M, Novo CL, Basquin C, Araki M, Araki K, et
al: HOT1 is a mammalian direct telomere repeat-binding protein
contributing to telomerase recruitment. EMBO J. 32:1681–1701. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng X, Luo Z, Jiang S, Li F, Han X, Hu Y,
Wang D, Zhao Y, Ma W, Liu D, et al: The telomere-associated
homeobox-containing protein TAH1/HMBOX1 participates in telomere
maintenance in ALT cells. J Cell Sci. 126:3982–3989. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palm W and de Lange T: How shelterin
protects mammalian telomeres. Annu Rev Genet. 42:301–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Lange T: Protection of mammalian
telomeres. Oncogene. 21:532–540. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blackburn EH: Switching and signaling at
the telomere. Cell. 106:661–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McIlrath J, Bouffler SD, Samper E,
Cuthbert A, Wojcik A, Szumiel I, Bryant PE, Riches AC, Thompson A,
Blasco MA, et al: Telomere length abnormalities in mammalian
radiosensitive cells. Cancer Res. 61:912–915. 2001.PubMed/NCBI
|
|
11
|
Mirjolet C, Boidot R, Saliques S,
Ghiringhelli F, Maingon P and Créhange G: The role of telomeres in
predicting individual radiosensitivity of patients with cancer in
the era of personalized radiotherapy. Cancer Treat Rev. 41:354–360.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang H, Wu L, Ke S, Wang W, Yang L, Gao X,
Fang H, Yu H, Zhong Y, Xie C, et al: Downregulation of
ubiquitin-conjugating enzyme UBE2D3 promotes telomere maintenance
and radioresistance of Eca-109 human esophageal carcinoma cells. J
Cancer. 7:1152–1162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cawthon RM: Telomere measurement by
quantitative PCR. Nucleic Acids Res. 30:e472002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao X, Wang W, Yang H, Wu L, He Z, Zhou S,
Zhao H, Fu Z, Zhou F and Zhou Y: UBE2D3 gene overexpression
increases radiosensitivity of EC109 esophageal cancer cells in
vitro and in vivo. Oncotarget. 7:32543–32553. 2016.PubMed/NCBI
|
|
15
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
d'Adda di Fagagna F, Reaper PM,
Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G,
Carter NP and Jackson SP: A DNA damage checkpoint response in
telomere-initiated senescence. Nature. 426:194–198. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paull TT, Rogakou EP, Yamazaki V,
Kirchgessner CU, Gellert M and Bonner WM: A critical role for
histone H2AX in recruitment of repair factors to nuclear foci after
DNA damage. Curr Biol. 10:886–895. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shiloh Y: ATM and related protein kinases:
Safeguarding genome integrity. Nat Rev Cancer. 3:155–168. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Drissi R, Wu J, Hu Y, Bockhold C and Dome
JS: Telomere shortening alters the kinetics of the DNA damage
response after ionizing radiation in human cells. Cancer Prev Res.
4:1973–1981. 2011. View Article : Google Scholar
|
|
20
|
Yang L, Wang W, Hu L, Yang X, Zhong J, Li
Z, Yang H, Lei H, Yu H, Liao Z, et al: Telomere-binding protein
TPP1 modulates telomere homeostasis and confers radioresistance to
human colorectal cancer cells. PLoS One. 8:e810342013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tribius S, Pidel A and Casper D: ATM
protein expression correlates with radioresistance in primary
glioblastoma cells in culture. Int J Radiat Oncol Biol Phys.
50:511–523. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rainey MD, Charlton ME, Stanton RV and
Kastan MB: Transient inhibition of ATM kinase is sufficient to
enhance cellular sensitivity to ionizing radiation. Cancer Res.
68:7466–7474. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang L, Graham PH, Hao J, Ni J, Bucci J,
Cozzi PJ, Kearsley JH and Li Y: PI3K/Akt/mTOR pathway inhibitors
enhance radiosensitivity in radioresistant prostate cancer cells
through inducing apoptosis, reducing autophagy, suppressing NHEJ
and HR repair pathways. Cell Death Dis. 5:e14372014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alao JP and Sunnerhagen P: The ATM and ATR
inhibitors CGK733 and caffeine suppress cyclin D1 levels and
inhibit cell proliferation. Radiat Oncol. 4:512009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu WG, Seno JD, Beck BD and Dynlacht JR:
Translocation of MRE11 from the nucleus to the cytoplasm as a
mechanism of radiosensitization by heat. Radiat Res. 156:95–102.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loayza D and De Lange T: POT1 as a
terminal transducer of TRF1 telomere length control. Nature.
423:1013–1018. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma H, Su L, Yue H, Yin X, Zhao J, Zhang S,
Kung H, Xu Z and Miao J: HMBOX1 interacts with MT2A to regulate
autophagy and apoptosis in vascular endothelial cells. Sci Rep.
5:151212015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ribero S, Sanna M, Visconti A, Navarini A,
Aviv A, Glass D, Spector TD, Smith C, Simpson M, Barker J, et al:
Acne and telomere length: A new spectrum between senescence and
apoptosis pathways. J Invest Dermatol. 137:513–515. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Wang X, Flores ER, Yu J and Chang
S: Dysfunctional telomeres induce p53-dependent and independent
apoptosis to compromise cellular proliferation and inhibit tumor
formation. Aging Cell. 15:646–660. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mauceri HJ, Hanna NN, Beckett MA, Gorski
DH, Staba MJ, Stellato KA, Bigelow K, Heimann R, Gately S, Dhanabal
M, et al: Combined effects of angiostatin and ionizing radiation in
antitumour therapy. Nature. 394:287–291. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Garcia-Barros M, Paris F, Cordon-Cardo C,
Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z and Kolesnick R:
Tumor response to radiotherapy regulated by endothelial cell
apoptosis. Science. 300:1155–1159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meng MB, Jiang XD, Deng L, Na FF, He JZ,
Xue JX, Guo WH, Wen QL, Lan J, Mo XM, et al: Enhanced radioresponse
with a novel recombinant human endostatin protein via tumor
vasculature remodeling: Experimental and clinical evidence.
Radiother Oncol. 106:130–137. 2013. View Article : Google Scholar : PubMed/NCBI
|