Introduction
As one of the common complications, bone invasion is
frequently diagnosed with the progression of bone invasion by OSCC
(1,2). Although local bone invasion and
distant bone metastasis are closely related pathological processes,
invasion of bone by OSCC has its own special characteristics
(3). Initially, proteases help to
degrade the extracellular matrix (ECM) of soft tissue and
facilitate the entry of malignant keratinocytes. Secondly,
osteoclasts are recruited by cytokines from tumour cells, and take
the main role to resorb the bone. Lastly, osteoclasts mediate the
bone resorption and liberate growth factors, promote the growth of
neoplastic cells, thus driving a vicious cycle to accelerate the
invasion (4).
Of the growth factors released, TGF-β is embedded in
the reservoirs of mineralized bone matrix and easily secreted
during the degradation. TGF-β is the TGF super family member, which
normally controls tissue homeostasis by limiting cell proliferation
(5). Three isoforms of TGF-β have
been identified in human: TGF-β1, TGF-β2, TGF-β3. Since cancer
cells usually have specific gene mutations or loss of TGF-β
signaling components; they can antagonize the inhibitory effects
and selectively shut down its suppression arm (4). Additionally, TGF-β has been proved to
stimulate artificial EMT of epithelial cells, as well as malignant
cells in vitro (6). Based on
the well known report that induction of EMT by TGF generates cancer
cells with higher stem cell properties, TGF has been utilized as a
research tool to generate artificial EMT in a variety of studies
(7). Previous work of our group has
found that long-term treatment of recombinant human TGF-β1
(rhTGF-β1) triggers the EMT of OSCC cells in vitro (8). Typical changes include cell phenotype
changing from slabstone to spindle shape, also with EMT marker
expression changes of Snail, Slug, E-cad and N-cad. These EMT
changes were further found to be associated with bone invasion of
OSCC, and we suppose that TGF-β1 may not only induce EMT to
increase the invasive ability of OSCC cells, but also promote
expression of osteoclastic factors and prolong osteoclast survival
(9).
Recently, a report of osteoclast fusion machinery by
Fiorino and Harrison found the protein of E-cad was expressed
during early stages of osteoclastogenesis in both monocytes and
primary macrophages (10). Blocking
E-cad with neutralizing antibodies significantly diminished
multinucleared osteoclast differentiation. E-cad-GFP overexpressing
macrophages displayed rapid differentiation of mature osteoclasts.
Since TGF-β1 could induce artificial EMT of cancer cells with
‘cadherin switch’, and our previous research demonstrated the loss
of E-cad protein in the progression of bone invasion by OSCC
(9), we hypothesized that E-cad may
be utilized by monocytes to fuse and differentiate into
osteoclasts. Therefore, in the present study, we used two research
models to explore our hypothesis. On one hand, we use OSCC cells of
SCC25 to establish an animal model of bone invasion by OSCC, and
investigated whether E-cad ‘disappear’ in vivo; on the other
hand, we used the indirect cell co-culture model of SCC25 and RAW
264.7, with the treatment of TGF-β1, to evaluate whether E-cad
protein is ‘hijacked’ in vitro.
Materials and methods
Reagents
Dubecco's modified Eagle's medium (DMEM), α-MEM,
foetal bovine serum (FBS), trypsin-EDTA, antibiotics and phosphate
buffered saline (PBS) were purchased from Thermo Fisher Scientific
(Waltham, MA, USA). Primary antibody of mouse anti-human monoclonal
E-cad (cat. no. 4A2) and N-cad (cat. no. 13A9) were obtained from
Cell Signaling Technology, Inc. (Danvers, MA, USA), rabbit
anti-human polyclonal Cytokeratin (CK, cat. no. BA2266-1) and
Vimentin (Vim, cat. no. PB0378) from Boster (Wuhan, China), mouse
anti-human monoclonal Snail1 (cat. no. sc-271977) was from Santa
Cruz Biotechnology (Santa Cruz, CA, USA), and mouse anti-human
monoclonal α-tublin (cat. no. ab15246) was from Abcam (Cambridge,
MA, USA). The secondary antibody with horseradish peroxidase
(HRP)-conjugated (cat. nos. STAR137P and STAR121P) was supplied by
Bio-Rad Laboratory (Hercules, CA, USA). Recombinant cytokines of
TGF-β1 and RANKL were from R&D Systems (Minneapolis, MN, USA)
and Pepro Tech (Rocky Hill, NJ, USA) respectively. TRAP staining
kit was obtained from Sigma-Aldrich (St. Louis, MO, USA). The OSCC
cell line of SCC25 was obtained from American Type Tissue
Collection (ATCC, Rockvile, MD, USA), maintained in DMEM with 10%
FBS and antibiotics (100 U/ml of penicillin G and 100 mg/ml of
streptomycin) at 37°C in an incubator (5% CO2/20%
O2). The murine macrophage cell line of RAW 264.7 was
kindly given by Dr Hongwei Jiang from Sun yat-sen University. RAW
264.7 cells were cultured in α-MEM with 10% FBS at 37°C in a
humidified 5% CO2 atmosphere.
Immunohistochemistry
OSCC tissue from 10 patients with bone invasion was
examined to validate the protein expression of E-cad, Vim and
Snail1. Informed consent was obtained from each patient. Serial
paraffin tissue sections (5 µm) were dewaxed, rehydrated and
treated with 0.3% hydrogen peroxiode in PBS. Antigen retrieval was
performed by heating sections in a microwave oven (2×4 min) with
0.2% citrate buffer (pH=6). After non-specific binding was blocked
with 5% BSA in PBS for 30 min, sections were incubated with primary
antibody of E-cad (1:100), Vim (1:100), Snail1 (1:200) overnight at
4°C. Sections were then treated with anti-mouse/rabbit secondary
antibodies (Envision+Systems) (Dako, Carpinteria, CA, USA) for 30
min, followed by diaminobenzidine (DAB) detection solution for a
few minutes at room temperature. Primary antibodies were replaced
by non-immune serum as negative control. Sections were
counterstained with Mayer's haematoxylin, dehydrated and mounted
with mounting medium. The final results were visualized by light
microscopy and photographed using a digital camera. Immunostaining
intensity was scored according to the percentage of tumour cells
positively-stained: and designated as + where 20% of cells were
stained; ++ where 40% were stained; +++ where 60% were stained.
In vivo animal model of bone invasion
by OSCC
Balb-c nude mice were purchased from the animal
resources center, housed in animal facility, and cared for by
animal house staff. All protocols were reviewed and approved by
university ethics committee (2016-334QX). At 6–7 weeks old, these
mice were utilized to develop an animal model of bone invasion by
OSCC (3). Under sterile condition,
OSCC cells of SCC25 (5×106/100 µl) were injected
subcutaneously overlaying the calvaria. Mice were randomly divided
into 2 groups (n=6/group): the negative control group received PBS
(Group 1: PBS); the positive control group received cells of SCC25
(Group 2: SCC25). Body weight and tumour volume were recorded every
week. All animals were sacrificed at week 6.
Micro-computed tomography (μCT)
imaging
Calvariae were surgically removed from PBS treated
control, SCC25 tumours bearing nude mice, fixed in 70% ethanol and
scanned by using a µCT instrument (Scanco Medical AG, Bassersdorf,
Switzerland). µCT-analyzer software was used to analyze the
structure of calvaria with the global segmentation method.
Two-dimensional images were used to generate three-dimensional
reconstruction. The area of each calvaria was outlined for analysis
and quantification.
Histological and immunohistochemical
analysis
To perform histological analysis for the
tumour-bearing calvaria, all calvariae were firstly decalcified
with 10% EDTA (pH 7.4) for 2 weeks and then processed for paraffin
embedding. Serial 5-µm sections of paraffin-embedded calvariae were
stained by both hematoxylin and eosin (H&E) and
tartrate-resistant acid phosphatase (TRAP). Analysis of
TRAP-positive osteoclast numbers at the tumour-bone interface was
performed (3). For each section, an
area of 2 mm2 with the tumour-bone interface was defined
for counting osteoclast numbers. Four fields of this area were
randomly selected and counted to determine the numbers of
TRAP-postitive osteoclasts.
Immunohistochemial staining of sections was
performed by incubation of serial sections with the primary
antibody of E-cad (1:100), Vim (1:100), Snail (1:200) overnight,
followed by HRP labeled secondary antibody and DAB staining.
Specimens treated with non-immune serum served as negative
controls. Immunostaining intensity was scored as above.
Cell proliferation assay
SCC25 cells were seeded at a density of
5×103 cells/well in 96-well plates, allowed to attach
overnight and then treated with TGF-β1 (5 ng/ml) for 2, 4 and 6
days. Absorbance was read at 590 nm on a Biomek plate reader, after
addition of 20 µl methylthiazol tetrazolium (MTT, 5 mg/ml, Life
Technologies, Carlsbad, CA, USA) to the wells for 4 h of
incubation, followed by removal of the solution and addition of 150
µl/well of dimethyl suphoxide (DMSO, Sigma-Aldrich) to solubilize
the cells.
Cell apoptosis assay
The apoptosis analysis of SCC25 cells with or
without TGF-β1 treatment was performed by flow cytometry analysis
(FACS, Beckman Coulter, Brea, CA, USA), with the staining of
Annexin V/PI (Life Technologies) based on the standard protocol.
Cells were washed in ice-cold PBS at least three times,
re-suspended in 100 µl of binding buffer and incubated with Annexin
V-fluorescein isothiocyanate for 15 min at 4°C in the dark. Cells
were then incubated for 5 min with PI and analyzed by FACS.
Immunocytochemistry
After the treatment with TGF-β1 for 2, 4 and 6 days,
SCC25 cells were fixed with 70% ethanol for 10 min and
permeabilized by 0.1% Triton X-100 for 5 min. Non-specific binding
of the antibodies was avoided by blocking with 5% BSA in PBS for 30
min, followed by incubation with primary antibodies of CK (1:200)
and Vim (1:100) overnight at 4°C, and then with secondary
antibodies for 1 h at 37°C. Non-immune serum instead of the primary
antibody was used as negative control. Sites of binding were
visualized using liquid diaminobenzidine (DAB) substrate chromogen
system (Dako), counterstained with Mayer's haematoxylin, and
photographed by a digital camera. Immunostaining intensity was
scored as above.
Real-time PCR
Total RNA was isolated from SCC25 cells before and
after TGF-β1 treatment using PureLink RNA mini kit (Invitrogen,
Carlsbad, CA, USA), and reverse transcribed to cDNA using iScript
cDNA Synthesis kit (Bio-Rad) based on the manufacturer's
instructions. Quantitative gene analysis was performed for E-cad,
N-cad, Snail1 and Vim by Express SYBR GreenER qPCR Supermix
Universal kit (Invitrogen) and icycler iQ5 Real-time PCR system
(Bio-Rad). The data were normalized to the internal control, GAPDH
to obtain ∆Cq. Finally fold-change of genes of interest relative to
untreated samples was reported by 2−∆∆Cq method
(11). Primers used in this study
are as reported elsewhere (7,9,10).
Western blotting
Total protein was extracted from SCC25 cells before
and after TGF-β1 treatment using lysis buffer (Thermo Fisher
Scientific). The protein concentration was determined by a BCA
Protein assay kit (Pierce, Rockford, IL, USA), and 40 µg of protein
was subjected to SDS-PAGE with 10% poly-acrylamide gels. Proteins
were transferred to PVDF membranes, and blocked with 5% non-fat dry
milk in Tris-buffered saline (TBS) for 1 h at room temperature. The
membranes were then incubated with primary antibodies of E-cad
(1:200), N-cad (1:200), Snail1 (1:200), Vim (1:100) and α-tublin
(1:3000) overnight at 4°C, washed twice and incubated with
horseradish peroxidase-conjugated (HRP) secondary antibodies for 1
h at room temperature. The protein bands were detected by
SuperSignal WestPico Chemiluminescent Substrate (Thermo Fisher
Scientific) and visualized using VersaDoc-MP Imaging Systems
(Bio-Rad).
Indirect cell co-culture
Transwell inserts (0.4-µm pore, Corning, Inc.,
Corning, NY, USA) were used in the indirect cell co-culture. SCC25
cells (5×103 cells/well) were seeded in the upper
chamber with the treatment of TGF-β1 (5 ng/ml), and RAW 264.7 cells
(5×104 cells/well) were placed in the 24-well plates.
The chambers were incubated for 2, 4 and 6 days. To examine the
effects of TGF-β1, total RNA were extracted from SCC25 and RAW
264.7 on each time point and reverse transcribed to cDNA.
Quantitative gene analysis of E-cad, Snail1, RANKL for SCC25, and
E-cad, TRAP, nuclear factor of activated T-cells cytoplasmic 1
(NFATc1) for RAW 264.7 was performed by SYBR Green ER qPCR Supermix
(Invitrogen) and icycler iQ5 Real-time PCR system (Bio-Rad).
Changes of gene expression were analyzed as above.
Immunofluorescence
To further confirm the switch of E-cad protein,
immunofluorescence (IF) was utilized. Cells of SCC25 and RAW 264.7
were placed into transwell inserts and treated as above. On each
time point, both cells of SCC25 and RAW 264.7 were fixed with 75%
ethanol for 10 min, and blocked in 5% bovine serum albumin for 30
min. Primary antibody of E-cad (1:50) were incubated at 4°C
overnight, followed by detection with fluorescence-conjugated
secondary antibody (FITC, 1:200, Boster) at room temperature for 2
h. DAPI staining (Boster) was used to visualize the nuclei. Images
were acquired and photographed by a Nikon inverted microscope.
Normal serum replaced primary antibodies as negative controls.
Osteoclast differentiation from RAW
264.7 cells
Cells of SCC25 and RAW 264.7 were placed into the
transwell inserts, treated as above. To obtain osteoclasts, RAW
264.7 were supplemented with 50 ng/ml of recombinant mouse RANKL
(Pepro Tech). Medium change and new cytokine were added every 2
days, while osteoclasts appeared after 4 days of treatment. These
osteoclasts were subsequently fixed in 10% formalin. TRAP staining
and IF of F-actin staining were used to characterize osteoclasts.
TRAP positive cells of three or more nuclei were considered to be
multinucleated osteoclasts. Rhodamine-conjugated phalloidin (Life
Technologies) were used to stain F-actin. DAPI staining (Boster)
was used to visualize the nuclei. Four fields were randomly
selected and counted for osteoclast and F-actin numbers.
Statistical analysis
Results were presented as mean ± standard error (M ±
SE) of at least 3 independent experiments. Data analysis was
performed using SPSS software (Version 20.0, IBM, USA). Student's
t-test was used to compare two means. One way analysis of variance
was applied to compare two or more means, followed by
Student-Newan-Keulls test. A p-value of <0.05 was regarded as
statistically significant.
Results
Weak staining of E-cad protein is
observed in tissue samples from OSCC patients with bone
invasion
OSCC tissue samples with bone invasion were obtained
from 10 patients and used for IHC. Results showed weak staining of
E-cad protein was found in cytoplasm of tumour cells, moderate
staining of Vim and strong staining of Snail1 was observed in cell
cytoplasm (Fig. 1A). A summary of
staining density was shown in Fig.
1B.
Animal model of OSCC with bone
invasion established by using SCC25 cells
The animal model of OSCC with bone invasion was
established by using SCC25 cells injected through the central area
of calvariae (Fig. 2A). No
significant differences of body weight were found between PBS
control group and SCC25 group (Fig.
2B). µCT imaging found typical bone invasion of SCC25 group,
while the control group had not formed a tumour (Fig. 2A and B). Histological analysis
showed plenty of osteoclasts accumulating in the tumour-bone
interface, while less osteoclasts were observed in the bone marrow
of the control group (Fig. 3A and
B).
Validation of the E-cad protein in
animal tissue samples with bone invasion
IHC of animal tissue samples further confirmed the
loss of E-cad protein. Results detected less staining of E-cad
protein was found in cytoplasm of tumour cells, with moderate
staining of Vim and strong staining of Snail1 (Fig. 4A). A summary of staining results was
supplied in Fig. 4B.
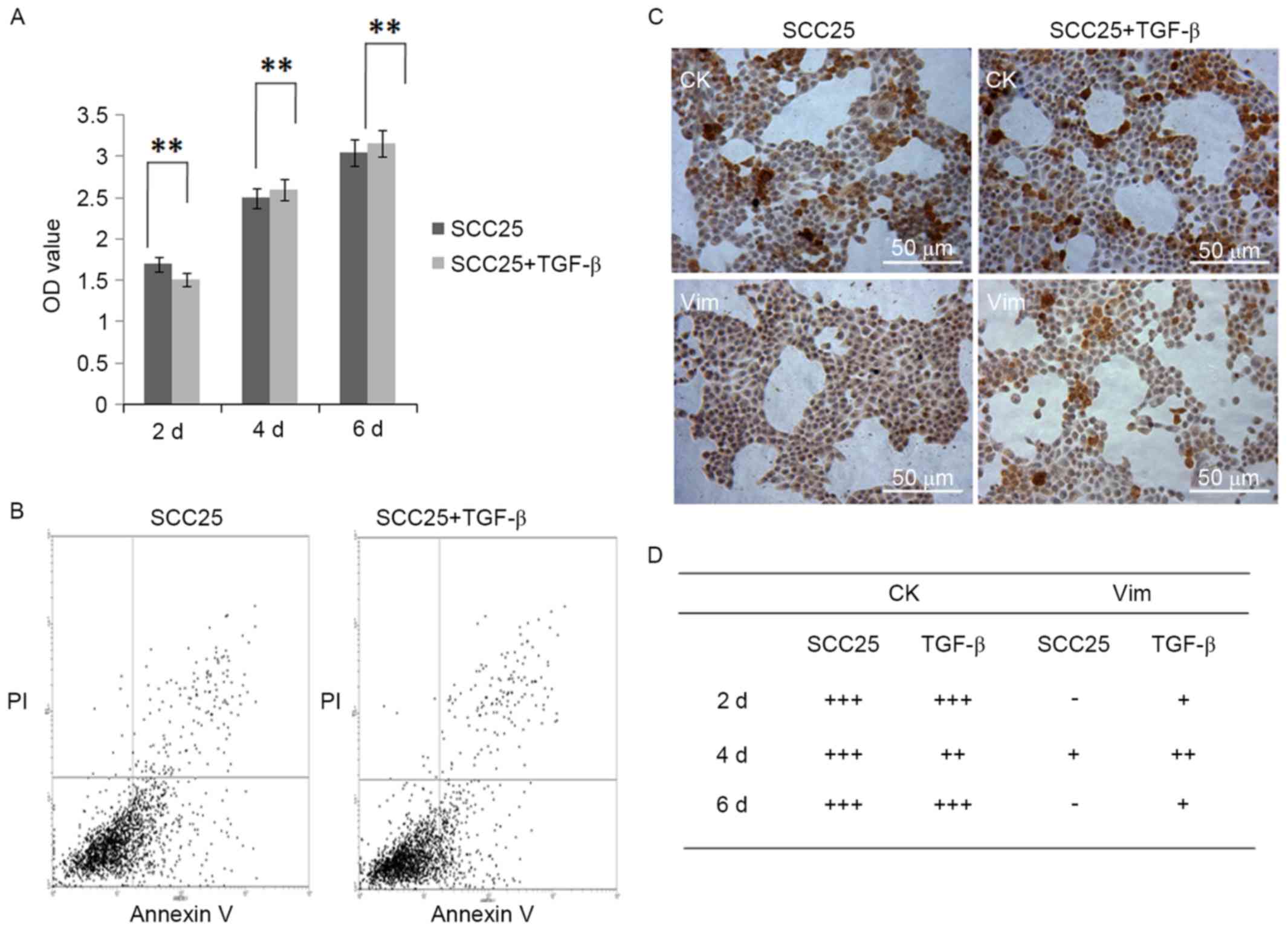
TGF-β1 has no inhibitory effect on
proliferation and apoptosis of SCC25 cells
MTT assay demonstrated that 5 ng/ml of TGF-β1 had no
inhibitory effect on the proliferation of SCC25 cells during 6 days
of treatment (Fig. 5A). FACS
analysis showed the apoptotic subpopulation of SCC25 cells with
TGF-β1 treatment was similar with that of normal SCC25 cells
(Fig. 5B).
Artificial EMT is induced in SCC25
cells with TGF-β1 treatment
Since TGF-β1 could induce the artificial EMT, 5
ng/ml of TGF-β1 was used to treat SCC25 cells for 2, 4 and 6 days.
The cell morphology was not obviously changed, still slab stone
cell shape (Fig. 5C). IHC found CK
staining remained the same before and after TGF-β1 treatment, and
slight staining of Vim was observed in cytoplasm of SCC25 cells
(Fig. 5C and D). Real-time PCR was
utilized to examine mRNA expression of selected EMT markers: E-cad
decreased while N-cad increased, Snail1 decreased then increased
while Vim increased (Fig. 6A).
Western blotting further confirmed these EMT marker changes at the
protein level (Fig. 6B).
The indirect co-culture model is
utilized in cells of SCC25 and RAW 264.7
The indirect cell co-culture of SCC25 and RAW 264.7
was performed with 2, 4 and 6 days, and on each time point both
cells were obtained to extract RNA and real-time PCR was performed.
Results showed that E-cad mRNA decreased in SCC25 cells on day 4
and day 6, while Snail1 and RANKL increased (Fig. 7A). Conversely, E-cad mRNA increased
in RAW 264.7 cells on day 4 and day 6, while TRAP and NFATc1
increased from day 4 to day 6 (Fig.
7A).
Switch of E-cad staining is observed
in SCC25 and RAW 264.7 cells
To further confirm the changes of E-cad protein in
the indirect co-culture model, SCC25 and RAW 264.7 cells were
stained with E-cad and observed by IF. Results indicated a switch
change of E-cad staining in both cell types: the staining of E-cad
was observed to decrease in SCC25 cells, while increase in RAW
264.7 cells from day 4 to day 6 during the cell co-cultures
(Fig. 7B).
More osteoclasts are generated from
RAW 264.7 cells with RANKL treatment
With the increase of E-cad in RAW 264.7 cells, next
we asked whether more osteoclasts would differentiate with the
supplement of RANKL. We still used the indirect cell co-culture and
added the cytokine of RANKL within RAW 264.7 cells. Results of TRAP
staining showed some giant cells on day 2, while more osteoclasts
differentiated on day 4, and a maximum number of osteoclasts was
found on day 6 (Fig. 8A and B). The
F-actin staining suggested the same trend with TRAP staining, and
more staining of F-actin was observed in osteoclasts on day 6 by IF
(Fig. 8A and B).
Discussion
Cadherins, involved in cell-cell contact, are
regulated trans-membrane calcium-dependent proteins. One of the
main cadherins is E-cad, which is responsible for intercellular
adhesion between epithelial cells (12). For several decades, E-cad has been
reported as a major constituent of adherens junction, mediating
adhesion between epithelial cells, therefore safeguarding
epithelial barrier integrity (13).
If carcinogens induce the ligation of epithelial layer, E-cad would
no longer work and maintain the normal function. The neoplastic
cells at the invasive tumour front lose their epithelial cell
phenotype and acquire mesenchymal-like phenotype referred to as
EMT. The loss of E-cad and the acquisition of N-cad, so-called
‘cadherin switch’, is regarded as a hallmark of EMT (8).
Except for epithelial cells, recent progress has
uncovered a critical role of E-cad in mononuclear phagocyte
function. It becomes increasingly clear that E-cad and its
associated catenins are expressed in dendritic cells, langerhans
cells, macrophages and osteoclasts (13). For example, interleukin-4 (IL-4)
stimulated macrophages, which fuse to become multinucleated foreign
body giant cells, increase expression of E-cad protein (14). Additionally, multiple studies have
implicated M- and N-cad during myoblast fusion and differentiation
(15). As for osteoclasts,
Mbalaviele et al suggested that E-cad participated in the
resorptive function (16). The
study by Fiorino and Harrison demonstrated a role for E-cad-based
signaling of osteoclast differentiation (10).
With E-cad and its related reports on osteoclasts,
we re-considered our research focus, since bone invasion by OSCC is
crosstalk between osteoblasts, osteoclasts, and tumour cells.
Especially, the loss of E-cad protein is observed in OSCC tissue
samples from patients with bone invasion. This was confirmed in our
previous reports (9) and our
current research. In the present study, we also constructed an
animal model of OSCC with bone invasion by using SCC25 cells. After
6 weeks this model was successfully established, and histological
analysis also found similar changes of E-cad protein, which further
confirm our hypothesis that loss of E-cad may be utilized by
osteoclast precursors. We perform double staining of E-cad and TRAP
to locate osteoclasts, but we did not get any staining of E-cad on
osteoclasts. We considered that we could not get time course window
on human tissue samples to capture the switch of E-cad protein.
What we observed are the terminal stage of mature osteoclasts,
which could not mimic the procedure how E-cad moves from tumour
cells to osteoclast precursors. Therefore, we used the indirect
cell co-culture model to further explore our hypothesis.
To induce the loss of E-cad in OSCC cells, we
utilized TGF-β1 as the inducer since TGF has long been reported to
be a key initiator of EMT. EMT process can be categorized into
three well-defined sub-types and TGF-β are involved in all three
types (5). Type 1 EMT is known as
developmental EMT and disruption of TGF-β isoforms and their
receptors has been associated with defects in type 1 EMT. Type 2
EMT is induced in response to inflammation, particularly wound
healing and tissue regeneration. TGF-β plays an instrumental role
in mediating this process. Type 3 EMT is most prevalent for
oncogenically transformed cells which are capable of metastasizing.
Studies of in vitro suggest a major role of TGF-β signaling
in the induction of EMT in cancer cells.
By using TGF-β1 in the current study, we confirm the
artificial EMT of SCC25 cells, with EMT marker changes, and slight
cell phenotype changes. This is the basis of simulation, and it is
possible to check whether E-cad would be utilized by RAW 264.7
cells only if E-cad deceases in SCC25 cells. Since TGF-β1 has no
inhibitory effects on RAW 264.7 cells, we used the indirect cell
co-culture model to check whether there are changes of E-cad
between these two cell types. Real-time PCR showed E-cad mRNA
increased in monocytes and IF found the obvious switch of E-cad
staining from SCC25 to RAW 264.7. This may be explained by two
potential mechanisms. On the one hand, E-cad may be shaded by
proteases such as matrix metalloproteinases (MMPs) and secreted
into extracellular medium, which are further ‘hijacked’ by RAW
264.7 cells. On the other hand, whether TGF-β1 has stimulative
effects on E-cad expression of RAW 264.7 cells needs to be further
investigated. Therefore, whether the switch of E-cad is the result
of simple protein movement, or the innate response between two
types of cells is the next research question to be addressed in the
near future.
With the increased amount of E-cad in RAW 264.7
cells, we want to confirm if more osteoclasts would differentiate
with the supplement of RANKL, which is the key transcriptional
factor for osteoclast development (17). Actually, within the cell co-culture,
real-time PCR detected increased expression of RANKL mRNA in SCC25
cells. Additionally, results of TRAP and F-actin staining confirmed
the increased number of osteoclasts after adding the cytokine of
RANKL. Being consistent with the report by Fiorino and Harrison
(10), these results confirmed the
important roles of E-cad in the early differentiation of
osteoclasts and proved the key roles of RANKL in osteoclast
maturation.
E-cad is not only the key component within cell
adherens junction but also the starter of cellular signaling for
bone cell fate. As for osteoclastogenesis, a possible explanation
of how E-cad works with other factors especially chemokines is
that, the chemokines may recruit and mobilize monocytes first, and
then E-cad connects them more tightly to fuse, which would easily
differentiate into osteoclasts in a short time. These may supply
therapeutic implications for blocking agent development in
vivo, and specific peptides, antibodies or mutant proteins
would be developed in an effort for potential clinical use
(18). Nonetheless, the underling
mechanisms have to be discovered through a variety of experiments
in vitro.
Taken together, our study found the switch of E-cad
protein was observed in the progression of bone invasion by OSCC.
The loss of E-cad in tumour cells may be utilized by monocytes to
differentiate into osteoclasts, further explaining the mechanisms
of bone invasion by OSCC, and indicating that cross-talk between
tumour cells, osteoblasts, and osteoclasts really exist, which may
supply clues for future molecular biotherapies.
Acknowledgements
This study was supported by Foundation of Sun
Yat-sen University (13ykpy41), Medical Scientific Research
Foundation of Guangdong Province (B2014164) and National Natural
Science Foundation of China (81500839).
References
|
1
|
Hwang YS, Ahn SY, Moon S, Zheng Z, Cha IH,
Kim J and Zhang X: Insulin-like growth factor-II mRNA binding
protein-3 and podoplanin expression are associated with bone
invasion and prognosis in oral squamous cell carcinoma. Arch Oral
Biol. 69:25–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quan J, Zhou C, Johnson NW, Francis G,
Dahlstrom JE and Gao J: Molecular pathways involved in crosstalk
between cancer cells, osteoblasts and osteoclasts in the invasion
of bone by oral squamous cell carcinoma. Pathology. 44:221–227.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quan J, Morrison NA, Johnson NW and Gao J:
MCP-1 as a potential target to inhibit the bone invasion by oral
squamous cell carcinoma. J Cell Biochem. 115:1787–1798. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quan J, Johnson NW, Zhou G, Parsons PG,
Boyle GM and Gao J: Potential molecular targets for inhibiting bone
invasion by oral squamous cell carcinoma: A review of mechanisms.
Cancer Metastasis Rev. 31:209–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Syed V: TGF-β signaling in cancer. J Cell
Biochem. 117:1279–1287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiao B, Johnson NW and Gao J:
Epithelial-mesenchymal transition in oral squamous cell carcinoma
triggered by transforming growth factor-beta1 is Snail
family-dependent and correlates with matrix metalloproteinase-2 and
−9 expressions. Int J Oncol. 37:663–668. 2010.PubMed/NCBI
|
|
9
|
Quan J, Elhousiny M, Johnson NW and Gao J:
Transforming growth factor-β1 treatment of oral cancer induces
epithelial-mesenchymal transition and promotes bone invasion via
enhanced activity of osteoclasts. Clin Exp Metastasis. 30:659–670.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fiorino C and Harrison RE: E-cadherin is
important for cell differentiation during osteoclastogenesis. Bone.
86:106–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−∆∆C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pereira CH, Morais MO, Martins AF, Soares
MQ, Alencar RC, Batista AC, Leles CR and Mendonça EF: Expression of
adhesion proteins (E-cadherin and β-catenin) and cell proliferation
(Ki-67) at the invasive tumor front in conventional oral squamous
cell and basaloid squamous cell carcinomas. Arch Oral Biol.
61:8–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van den Bossche J, Malissen B, Mantovani
A, De Baetselier P and Van Ginderachter JA: Regulation and function
of the E-cadherin/catenin complex in cells of the
monocyte-macrophage lineage and DCs. Blood. 119:1623–1633. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moreno JL, Mikhailenko I, Tondravi MM and
Keegan AD: IL-4 promotes the formation of multinucleated giant
cells from macrophage precursors by a STAT6-dependent, homotypic
mechanism: Contribution of E-cadherin. J Leukoc Biol. 82:1542–1553.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gavard J, Marthiens V, Monnet C, Lambert M
and Mège RM: N-cadherin activation substitutes for the cell contact
control in cell cycle arrest and myogenic differentiation:
Involvement of p120 and beta-catenin. J Biol Chem. 279:36795–36802.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mbalaviele G, Chen H, Boyce BF, Mundy GR
and Yoneda T: The role of cadherin in the generation of
multinucleated osteoclasts from mononuclear precursors in murine
marrow. J Clin Invest. 95:2757–2765. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikeda K and Takeshita S: The role of
osteoclast differentiation and function in skeletal homeostasis. J
Biochem. 159:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marie PJ, Haÿ E and Saidak Z: Integrin and
cadherin signaling in bone: Role and potential therapeutic targets.
Trends Endocrinol Metab. 25:567–575. 2014. View Article : Google Scholar : PubMed/NCBI
|