Introduction
Leukemia is also known as blood cancer and has a
high mortality rate. It is pathologically characterized by the
massive proliferation of abnormal hematopoietic stem cells
(1,2). Although the precise pathogenesis of
leukemia remains to be elucidated, various genetic mutations
occurring in response to factors including genetic factors, immune
deficiency, toxic agents and the leukosis virus in the process of
leukocyte differentiation have been widely accepted as the main
causes of leukemia (3). Genetic
mutations may lead to the unlimited proliferation of leukemia cells
in bone marrow and other hematopoietic tissue (4). As a result, the development of mature
blood cells is disrupted. Due to the high mortality rate, leukemia
is considered as a malignant cancer which seriously threatens human
health. It has also been reported that the incidence rate of
leukemia is relatively high in the elderly population and children
in China (5). At present, the main
treatment methods for leukemia are combined chemotherapy and marrow
transplantation. However, the high expense, donor deficiency and
immunological rejection are important issues in marrow
transplantation (6–8). Thus, combined chemotherapy is the most
important treatment for leukemia. However, among conventional
chemotherapeutic agents, cisplatin is also widely used in combined
chemotherapy for leukemia (9).
Unfortunately, evidence has shown that with treatment more and more
cancer cells become resistant to cisplatin (10). Therefore, it is urgent to further
investigate the precise mechanism of cisplatin resistance.
Platinum-based drugs are widely used in the
treatment of cancer such as leukemia, lymphomas, melanoma,
head-neck cancer, bladder cancer and gynecological tumors (11). Cisplatin is one of the first
platinum-based drugs discovered in the 1960s (12). Cisplatin interacts with DNA double
strands by forming interstrand and intrastrand adducts, thereby
inducing apoptosis in cancer cells through the interference with
DNA replication and gene transcription (13). Similar to other chemotherapeutic
agents, the effect of cisplatin is commonly limited by the
resistance of cancer cells. Cisplatin resistance can be intrinsic
or acquired. Intrinsic resistance means that cancer cells retain
certain featured gene expression profiles contributing to
resistance prior to cisplatin treatment. In contrast, acquired
resistance occurs in cancer cells after cisplatin-induced
epigenetic modulation and gene mutation (13). In clinical treatment, cisplatin
often results in the development of chemoresistance, despite a
consistent rate of initial responses. Acquired cisplatin resistance
is also the most common cause of therapeutic failure, and leads to
leukemia recurrence.
A primary study revealed that cisplatin-induced cell
death was mostly caused by nuclear DNA damage (14). However, it was recently discovered
that mitochondial DNA, or other mitochondrial targets may be more
important than nuclear DNA in cisplatin-induced cell death
(15). Mitochondria are well known
for their essential function in the production of ATP. In fact,
mitochondria are involved in a variety of cellular processes,
including survival, proliferation and apoptosis (16–18).
Mitochondria are also highly dynamic organelles and move through
the cell with frequent fission and fusion events (19). Various highly conserved
dynamin-related GTPases are identified as the mediators of
mitochondrial dynamics. Dynamin-related protein 1 (Drp1) is
involved in the process of mitochondrial fission, while mitofusin
1/2 (Mfn1/2) and OPA1 are required for mitochondrial outer or inner
membrane fusion in mammalian cells, respectively (20). In addition, recent studies have
suggested the involvement of mitochondrial dynamics in the acquired
cisplatin resistance or sensitivity in several cancer cell lines
(21,22). It has been reported that
OPA-1-mediated mitochondrial fusion is potentially responsible for
cisplatin-induced resistance in neuroblastoma B50 rat cells
(21). By contrast, Drp1-dependent
mitochondrial fission was found to regulate piceatannol-induced
cisplatin sensitivity in ovarian cancer (22). Therefore, it is of interest to
investigate the role of mitochondrial dynamics in the
antineoplastic activity of cisplatin in leukemia cells.
In the present study, we established the L1210/DDP
cell line, and found that the IC50 value of cisplatin in
the L1210/DDP cells was increased 10-fold. In addition,
mitochondrial outer membrane fusion proteins, Mfn1 and Mfn2 were
upregulated in L1210/DDP cells. In addition, mitofusins were also
upregulated in the parental L1210 cells subjected to cisplatin
stress. The Drp1 inhibitor, Mdivi-1, efficiently attenuated
cisplatin-induced cell death, caspase activation and intracellular
ROS increase in L1210 cells. Our data indicate that mitofusins and
Drp1-mediated mitochondrial dynamics may be involved in the
antineoplastic activity of cisplatin in L1210 cells, and suggest
that mitochondria may be potential targets used to improve the
clinical outcomes of leukemia in the future.
Materials and methods
Cell culture
Leukemia cell line L1210 was obtained from the China
Center for Type Culture Collection (CCTCC; Wuhan, China). The
L1210/DDP cell line was generated according to the dose-escalation
strategy as previously described (23,24).
In brief, the parental L1210 cells were treated with cisplatin at
an IC90 concentration, and the surviving cells were
cloned in soft agar. A clone was selected and cultured in medium
supplemented with a first dose of 0.4 mg/l of cisplatin
(Sigma-Aldrich, St. Louis, MO, USA). The dose of cisplatin in the
medium for the L1210 cells was increased 50% at every time-point of
subculture. The surviving cells followed by six subcultures were
cloned again in soft agar. A clone was selected as the
cisplatin-resistant cell line for culture in the medium. The cells
were grown in suspension in medium (Gibco, Grand Island, NY, USA)
supplemented with 10% fetal bovine serum (FBS; TransGen Biotech,
Beijing, China) and 1% penicillin/streptomycin (P/S) (Solarbio,
Beijing, China), and maintained in a humidified incubator (Thermo
Fisher Scientific, Waltham, MA, USA) at 37°C with an atmosphere
containing 5% CO2.
Cell viability assay
Cell viability was determined by trypan blue
exclusion assay as previously described (25). In brief, L1210 and L1210/DDP cells
were firstly seeded at 5×105 in 24-well plates and
maintained in suspension culture. At 72 h after incubation with DDP
or Mdivi-1 (Sigma-Aldrich) + DDP, samples were centrifuged at 1,000
× g for 5 min at 25°C. The cells were stained with 0.04% trypan
blue (Sigma-Aldrich) after being washed with phosphate-buffered
saline (PBS). The number of dead cells (blue) and viable cells
(uncolored) were counted using a hemacytometer. The ratio of the
number of dead cells/all counted cells represented the percentage
of cell death. The IC50 value of cisplatin to L1210 or
L1210/DDP was determined in the same way.
Western blot analysis
L1210 and L1210/DDP cells were harvested and lyzed
using radioimmunoprecipitation assay (RIPA) lysis buffer (Solarbio)
according to the manufacturer's instructions. Whole cell lysates
were mixed with an equal volume of 2X loading buffer (25% glycerol,
2% sodium dodecyl sulfate, 5% β-mercaptoethanol, 0.01% bromophenol
blue and 1 M Tris-HCl), sonicated, boiled for 5 min and stored at
−20°C prior to use. The cell lysates were subjected to sodium
dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE).
After electrophoresis, the proteins were transferred onto
polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica,
MA, USA). The membranes were blocked with 5% skim milk in
Tris-buffered saline with Tween-20 (TBST) buffer for 1 h at room
temperature, and then, immunoblotted for 2 h at room temperature
with the following primary antibodies: rabbit anti-Drp1, Mfn2 and
caspase-3 (1:1,000; Cell Signaling, Boston, MA, USA), rabbit
anti-Mfn1 and OPA-1 (1:1,000; Abcam, Cambridge, UK), and rabbit
anti-GAPDH (1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). After three washes with TBST, the membranes were further
incubated with an HRP-conjugated goat anti-rabbit secondary
antibody (1:2,000; TransGen Biotech) for 2 h at room temperature. A
chemiluminescence assay was carried out with Amersham ECL Prime
Western Blotting Detection reagents (CWBIO, Beijing, China), and
the immunoblotting signal was detected using Molecular
Imager® ChemiDoc™ XRS+ system (Bio-Rad, Hercules, CA,
USA).
Annexin V-FITC/PI apoptosis assay
Subsequent to the indicated treatments, the cells
were harvested from each group for the apoptosis assay using
Annexin V-fluorescein isothiocyanate (Annexin V-FITC) and propidium
iodide (PI) (4A Biotech Co., Ltd., Beijing, China) double staining.
The cells were resuspended in 100 µl of binding buffer with 5 µl of
Annexin V-FITC and 200 ng of PI, and incubated for 15 min at room
temperature in the dark. Then, the samples were subjected to the
apoptosis assay using flow cytometry, and the data were processed
using the Guawa Nexin software (Guava, Millipore Corp.).
Detection of the intracellular ROS
level
To examine the role of Drp1-dependent mitochondrial
fission in intracellular ROS production, cells were pretreated with
5 µM Mdivi-1 for 2 h prior to CDDP treatment. At 72 h after
cisplatin treatment, the cells were incubated with 10 µM of the
fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate
(DCFH-DA; Sigma-Aldrich) for 30 min at 37°C in the dark. After
incubation, the cells were washed twice with PBS and harvested. The
fluorescence intensity was assessed using flow cytometry with the
excitation source at 488 nm and the emission wavelength at 525 nm,
thus detecting the intracellular ROS. Data analysis was carried out
using inCyte software (both from Guava, Millipore Corp.).
Statistical analysis
The quantitative data are displayed as the mean ±
SD. Data were analyzed using either Student's t-test to compare two
conditions or ANOVA followed by planned comparisons of multiple
conditions, and p<0.05 was considered to indicate a
statistically significant result.
Results
Establishment of the L1210/DDP cell
line and confirmation of its cisplatin resistance
In order to investigate the mechanism of cisplatin
resistance, we successfully generated the L1210/DDP cell line
according to a dose-escalation strategy (23,24).
The parental cells were grown in suspension in RPMI-1640 medium
supplemented with 10% FBS and 1% P/S, whereas the L1210/DDP cells
were maintained in medium containing 4 mg/l of cisplatin to retain
their resistance. The cisplatin resistance of the L1210/DDP cells
was determined by trypan blue exclusion assay. As shown in Fig. 1A and Table I, the parental L1210 cells were
sensitive to 0.8–6.4 mg/l of cisplatin. In contrast, the L1210/DDP
cells were sensitive to 4–64 mg/l of cisplatin (Fig. 1B and Table II). In addition, the dose-response
curves of the two cell lines to cisplatin were generated (Fig. 1A and B), and the IC50
values for cisplatin were also calculated as 0.795 and 8.131 mg/l
in the L1210 and L1210/DDP cell lines, respectively. Compared to
the parental cell line, the IC50 value for cisplatin in
the L1210/DDP cells was increased 10-fold.
 | Table I.Cisplatin cytotoxicity in the L1210
cells. |
Table I.
Cisplatin cytotoxicity in the L1210
cells.
|
|
Cisplatin
concentration in the L1210 cells (mg/l) |
|---|
|
|
|
|---|
|
| 0 | 0.025 | 0.05 | 0.1 | 0.2 | 0.4 | 0.8 | 1.2 | 1.6 | 2 | 3.2 | 6.4 |
|---|
| Percentage of cell
death (%) | 13.43 | 23.77 | 27.46 | 28.18 | 30.70 | 39.53 | 49.28 | 53.23 | 65.30 | 73.25 | 86.78 | 90.57 |
 | Table II.Cisplatin cytotoxicity in the
L1210/DDP cells. |
Table II.
Cisplatin cytotoxicity in the
L1210/DDP cells.
|
|
Cisplatin
concentration in the L1210/DDP cells (mg/l) |
|---|
|
|
|
|---|
|
| 0 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 |
|---|
| Percentage of cell
death (%) | 9.84 | 13.06 | 15.84 | 16.73 | 26.41 | 44.44 | 51.89 | 74.48 | 60.74 | 93.85 |
Expression of mitochondrial
dynamic-related proteins in L1210 and L1210/DDP cells
It has been reported that mitochondrial dynamics are
involved in acquired cisplatin resistance (7). To investigate the possible role of
mitochondrial dynamics in the cisplatin resistance of leukemia
cells, the expression level of mitochondrial dynamic-related
proteins was examined in the two cell lines using western blot
assay. Notably, we found that both Mfn1 and Mfn2 were upregulated
in L1210/DDP cells. However, there was no significant difference in
the expression level of Drp1 and OPA1 in the two cell lines
(Fig. 2). Since both Mfn1 and Mfn2
are important components required for mitochondrial outer membrane
fusion, the results revealed the possibility that
mitofusin-mediated mitochondrial fusion may contribute to the
mechanism of cisplatin resistance in leukemia cells.
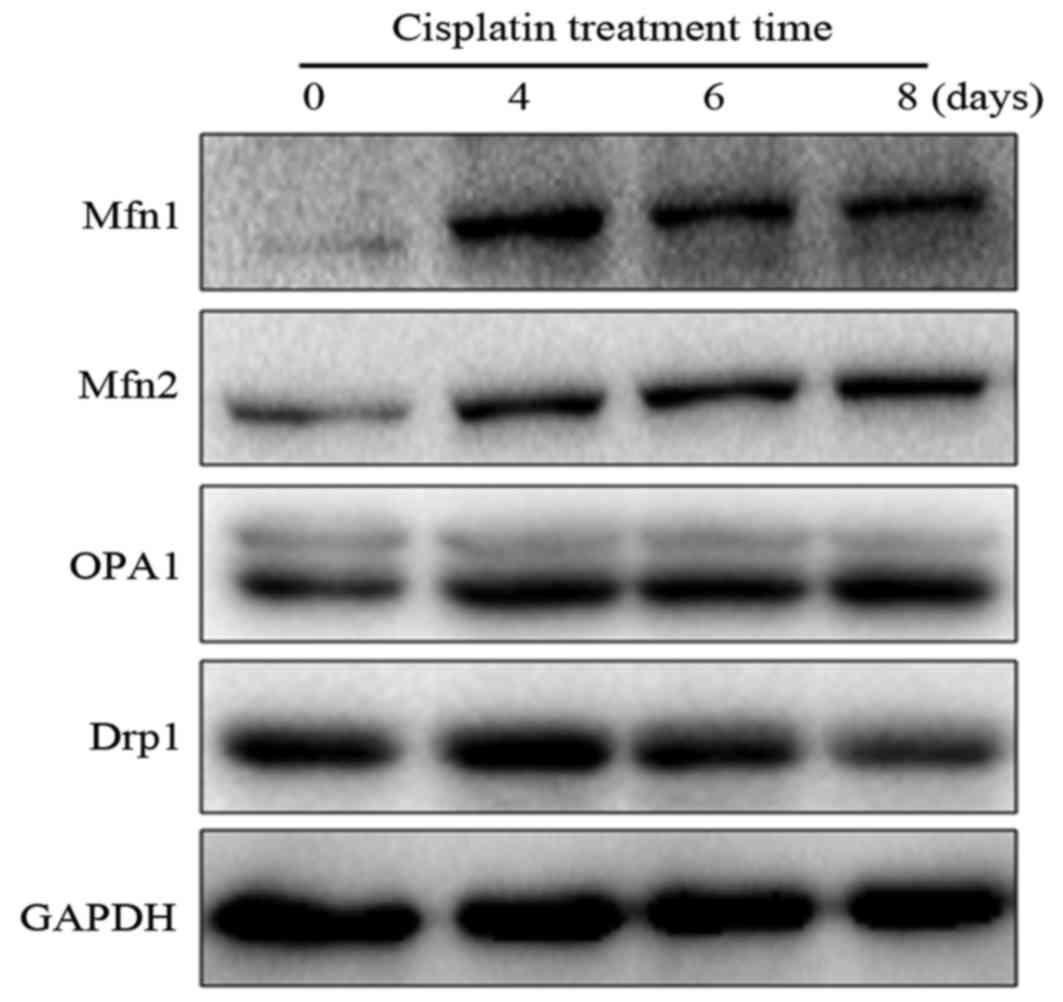
Effect of cisplatin stress on
mitochondrial dynamic-related protein expression in the parental
L1210 cells
Although mitofusins were upregulated in the
L1210/DDP cells, direct evidence that mitofusin-mediated
mitochondrial fusion leads to the development of cisplatin
resistance in leukemia cells remains unclear. Therefore, to further
confirm the involvement of mitofusins in the development of
cisplatin resistance in leukemia cells, cisplatin stress at 0.4
mg/l (the concentration below the IC50 value) was
introduced to the parental L1210 cells. The expression of
mitochondrial dynamic-related proteins in cells was examined at 0,
4, 6 and 8 days after treatment with 0.4 mg/l of cisplatin. As
shown in Fig. 3, it was found that
both Mfn1 and Mfn2 were obviously upregulated during the period of
cisplatin stress, whereas the expression of OPA1 was not
significantly altered. In contrast, Drp1 was downregulated at 8
days after cisplatin stress. Although there were a few differences
in the expression patterns of mitochondrial dynamic-related
proteins, the results revealed that the shift of mitochondrial
dynamics to fusion may contribute to the development of cisplatin
resistance in L1210 cells.
Drp1 inhibitor Mdivi-1 efficiently
attenuates cisplatin-induced cell death in L1210 cells
The aforementioned data revealed that mitochondrial
fusion may contribute to the antineoplastic activity of cisplatin
in leukemia cells. To further investigate the role of mitochondrial
dynamics in the sensitivity of leukemia cells to cisplatin, we used
different concentrations of Mdivi-1, a Drp1 inhibitor, to decrease
Drp1-dependent mitochondrial fission, and examined the effect of
Mdivi-1 on cisplatin-induced cell death in L1210 cells. In the
trypan blue exclusion assay, the percentage of cell death induced
by 0.4 mg/l of cisplatin was ~40%. Mdivi-1 (5 µM) significantly
attenuated 0.4 mg/l of cisplatin-induced cell death in L1210 cells,
with the exception of 2.5 and 10 µM of Mdivi-1 (Fig. 4). In the Annexin V-FITC/PI apoptosis
assay, it was also revealed that 5 µM Mdivi-1 significantly
inhibited cisplatin-induced cell death in L1210 cells (Fig. 5). The results demonstrated that
inhibition of mitochondrial fission contributes to the tolerance of
leukemia cells to cisplatin.
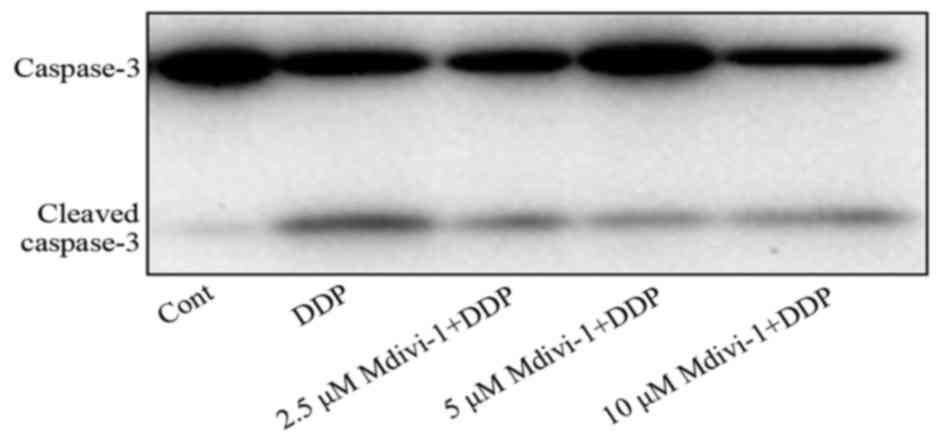
Effects of Mdivi-1 on
cisplatin-induced ROS production and caspase activation in L1210
cells
It has been reported that cisplatin induces cancer
cell death through the promotion of intracellular ROS production
and caspase activation (26,27).
The aforementioned data revealed that mitofusins were upregulated
in L1210/DDP cells, and that the Drp1 inhibitor, Mdivi-1 attenuated
cisplatin-induced L1210 cell death. It was necessary to further
investigate the mechanism of mitochondrial dynamics in
cisplatin-induced cell death in L1210 cells. Thus, the effect of
Mdivi-1 on cisplatin-induced ROS production and caspase activation
was examined in L1210 cells. Consistent with previous studies
(26,27), 4 mg/l of cisplatin significantly
increased the intracellular ROS level in L1210 cells. Pretreatment
with 5 µM of Mdivi-1 significantly attenuated cisplatin-induced
intracellular ROS production in L1210 cells, although it did not
completely block the increment of ROS (Fig. 6). Moreover, the results of western
blotting revealed that 4 mg/l of cisplatin markedly stimulated the
cleavage of caspase-3. Pretreatment with 5 µM of Mdivi-1 attenuated
the cleavage of caspase-3 in L1210 cells (Fig. 7). These data demonstrated that
mitochondrial dynamics may be involved in cisplatin-induced L1210
cell death through the regulation of intracellular ROS production
and the caspase pathway.
Discussion
Leukemia is the most common hematopoietic
malignancy. The development and progression of leukemia is a
multifactorial and multi-step process that involves genetic and
epigenetic changes (28). Leukemia
is also one of the most common malignant tumors, particularly in
children. At present, combined chemotherapy is the major treatment
method for leukemia, and cisplatin is one of the most common agents
used for combined chemotherapy in clinical treatment. Cisplatin was
also the first platinum-based drug to obtain approval of the US
Food and Drug Administration (29).
The antineoplastic effects of cisplatin have been linked to its
ability to crosslink with DNA, then interfere with DNA repair
mechanisms, cause DNA damage and eventually lead to apoptosis in
cancer cells (14,24). In contrast, mitochondria are also
important targets of cisplatin. Cisplatin can induce the decline of
ATP enzyme activity and activate the endogenous apoptosis pathway
(26). Cisplatin is currently
applied for the clinical management of patients suffering from
leukemia, testicular, ovarian, head and neck, colorectal and lung
cancers (11,30–32).
However, the resistance of cancer cells to cisplatin has become a
serious problem, and greatly limits its therapeutic effect in
clinical treatment.
In the present study, the role of mitochondrial
dynamics in cisplatin resistance of leukemia L1210 cells was
investigated. Firstly, we established the L1210/DDP cell line using
the dose-escalation strategy. The tolerance of L1210/DDP cells to
cisplatin was increased 10-fold, compared to the parental cells
(Fig. 1). Notably, mitofusins Mfn1
and Mfn2 were obviously upregulated in L1210/DDP cells (Fig. 2). Mfn1 and Mfn2 are vital components
required for mitochondrial outer membrane fusion (33). In addition, it has been reported
that mitofusins are involved in apoptosis by interacting with Bak
and Bax (34–36). To examine the role of mitofusins in
the development of cisplatin in L1210 cells, the expression of
mitochondrial dynamic-related proteins was detected after cisplatin
stress. Similarly, Mfn1 and Mfn2 were upregulated in L1210 cells
subjected to cisplatin stress (Fig.
3). The results revealed the upregulation of mitofusins not as
an effect, but as a cause in the development of cisplatin
resistance. To clarify the role of mitofusin-mediated mitochondrial
fusion in cisplatin resistance, it is necessary to knockdown
mitofusins in the L1210/DDP cells or overexpress mitofusins in
parental L1210 cells, and examine the antineoplastic effects of
cisplatin in these cells. Unfortunately, we failed to transfect any
siRNAs or plasmids into L1210 cells, and failed to infect L1210
cells with a lentivirus or adenovirus (data not shown). Thus, it is
impossible to knockdown or overexpress mitofusins in L1210 cells
using gene manipulation technology.
The balance between mitochondrial fission and fusion
is important to maintain mitochondrial morphology. Thus, we
disrupted the dynamic balance using chemical agents, to further
investigate the mechanism of mitochondrial dynamics in the
development of cisplatin resistance. It has been well documented
that Mdivi-1 is a potent Drp1 inhibitor, and is most widely used
(37,38). Obvious mitochondrial fusion in cells
can be observed when Drp1-dependent mitochondrial fission was
inhibited by Mdivi-1 (39,40). In the present study, 5 µM of Mdivi-1
efficiently attenuated cisplatin-induced cell death, intracellular
ROS production and caspase activation in L1210 cells. These results
demonstrated that mitochondrial dynamics play an important role in
the antineoplastic activity of cisplatin in leukemia cells.
However, there are some deficiencies that warrant improvement in
the present study. First, it is necessary to observe and compare
mitochondrial morphology in L1210 and L1210/DDP cells, since
mitofusins are upregulated in L1210/DDP cells. The two common
methods used to label transfected mitochondria are Mito-DsRed and
MitoTracker staining (41,42). As L1210 cells are difficult to be
transfected, we stained cells with MitoTracker, and found that
mitochondria were clustered around the perinuclear region in the
two cell lines. The two cell lines cultured in suspension appear as
small spheres. As a result, it was difficult to distinguish the
morphology of clustered mitochondria in the two cell lines (data
not shown). Therefore, it may be interesting to further investigate
the role of mitochondrial dynamics in the development of cisplatin
resistance in other adherent cancer cells with high transfection
efficiency.
In conclusion, our data revealed that mitofusins
Mfn1 and Mfn2 were upregulated in leukemia L1210 cells resistant to
cisplatin. In addition, cisplatin stress increased the expression
of mitofusins in parental L1210 cells. Inhibition of Drp1-dependent
mitochondrial fission by Mdivi-1 efficiently attenuated
cisplatin-induced cell death, intracellular ROS production and the
activation of the caspase pathway in L1210 cells. These results
demonstrate that mitochondrial dynamics play an important role in
the antineoplastic effects of cisplatin in leukemia cells. Thus,
targeting mitochondrial dynamics may provide a novel strategy in
order to improve the chemotherapeutic effect of cisplatin in the
future.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (81660607), the Natural Science
Foundation of Jiangxi (20161ACB20019), the Foundation of the Health
and Family Planning Commission of Jiangxi Province (20161052), the
Foundation of the Education Department of Jiangxi Province
(150271), the Foundation of The Second Affiliated Hospital of
Nanchang University (2014YNQN12012), and the Science Project of the
Education Department of Hunan Province (16C0177).
References
|
1
|
Benton CB, Nazha A, Pemmaraju N and
Garcia-Manero G: Chronic myelomonocytic leukemia: Forefront of the
field in 2015. Crit Rev Oncol Hematol. 95:222–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chavez-Gonzalez A, Bakhshinejad B,
Pakravan K, Guzman ML and Babashah S: Novel strategies for
targeting leukemia stem cells: sounding the death knell for blood
cancer. Cell Oncol. 40:1–20. 2017. View Article : Google Scholar
|
|
3
|
Calado RT and Young NS: Telomere
maintenance and human bone marrow failure. Blood. 111:4446–4455.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Safe S, Jin UH, Hedrick E, Reeder A and
Lee SO: Minireview: Role of orphan nuclear receptors in cancer and
potential as drug targets. Mol Endocrinol. 28:157–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen BA, Huang ZH, Zhang XP, Ou-Yang J, Li
JY, Zhai YP, Sun XM, Xu YL, Lu Q, Wang JM, et al: An
epidemiological investigation of leukemia incidence between 2003
and 2007 in Nanjing, China. J Hematol Oncol. 3:212010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Welch HG and Larson EB: Cost effectiveness
of bone marrow transplantation in acute nonlymphocytic leukemia. N
Engl J Med. 321:807–812. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lenarsky C and Parkman R: Bone marrow
transplantation for the treatment of immune deficiency states. Bone
Marrow Transplant. 6:361–369. 1990.PubMed/NCBI
|
|
8
|
Godder KT, Abhyankar SH, Lamb LS, Best RG,
Geier SS, Pati AR, Gee AP and Henslee-Downey PJ: Donor leukocyte
infusion for treatment of graft rejection post partially mismatched
related donor bone marrow transplant. Bone Marrow Transplant.
22:111–113. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kobayashi T, Yoshida J, Ishiyama J, Noda
S, Kito A and Kida Y: Combination chemotherapy with cisplatin and
etoposide for malignant intracranial germ-cell tumors. An
experimental and clinical study. J Neurosurg. 70:676–681. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borst P, Rottenberg S and Jonkers J: How
do real tumors become resistant to cisplatin? Cell Cycle.
7:1353–1359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wong E and Giandomenico CM: Current status
of platinum-based antitumor drugs. Chem Rev. 99:2451–2466. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Milacic V, Fregona D and Dou QP: Gold
complexes as prospective metal-based anticancer drugs. Histol
Histopathol. 23:101–108. 2008.PubMed/NCBI
|
|
13
|
Chen H, Hardy TM and Tollefsbol TO:
Epigenomics of ovarian cancer and its chemoprevention. Front Genet.
2:672011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y, Bhattacharyya D, King CL,
Baskerville-Abraham I, Huh SH, Boysen G, Swenberg JA, Temple B,
Campbell SL and Chaney SG: Solution structures of a DNA dodecamer
duplex with and without a cisplatin 1,2-d(GG) intrastrand
cross-link: Comparison with the same DNA duplex containing an
oxaliplatin 1,2-d(GG) intrastrand cross-link. Biochemistry.
46:6477–6487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Podratz JL, Knight AM, Ta LE, Staff NP,
Gass JM, Genelin K, Schlattau A, Lathroum L and Windebank AJ:
Cisplatin induced mitochondrial DNA damage in dorsal root ganglion
neurons. Neurobiol Dis. 41:661–668. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oakes SA and Korsmeyer SJ: Untangling the
web: Mitochondrial fission and apoptosis. Dev Cell. 7:460–462.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Szabadkai G, Simoni AM, Chami M,
Wieckowski MR, Youle RJ and Rizzuto R: Drp-1-dependent division of
the mitochondrial network blocks intraorganellar Ca2+
waves and protects against Ca2+-mediated apoptosis. Mol
Cell. 16:59–68. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shaw JM and Nunnari J: Mitochondrial
dynamics and division in budding yeast. Trends Cell Biol.
12:178–184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wan YY, Zhang JF, Yang ZJ, Jiang LP, Wei
YF, Lai QN, Wang JB, Xin HB and Han XJ: Involvement of Drp1 in
hypoxia-induced migration of human glioblastoma U251 cells. Oncol
Rep. 32:619–626. 2014.PubMed/NCBI
|
|
20
|
Chan DC: Mitochondrial fusion and fission
in mammals. Annu Rev Cell Dev Biol. 22:79–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Santin G, Piccolini VM, Barni S, Veneroni
P, Giansanti V, Dal Bo V, Bernocchi G and Bottone MG: Mitochondrial
fusion: A mechanism of cisplatin-induced resistance in
neuroblastoma cells? Neurotoxicology. 34:51–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Farrand L, Byun S, Kim JY, Im-Aram A, Lee
J, Lim S, Lee KW, Suh JY, Lee HJ and Tsang BK: Piceatannol enhances
cisplatin sensitivity in ovarian cancer via modulation of p53,
X-linked inhibitor of apoptosis protein (XIAP), and mitochondrial
fission. J Biol Chem. 288:23740–23750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kato S, Ideguchi H, Muta K, Nishimura J
and Nawata H: Mechanisms involved in the development of adriamycin
resistance in human leukemic cells. Leuk Res. 14:567–573. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zwelling LA, Michaels S, Schwartz H,
Dobson PP and Kohn KW: DNA cross-linking as an indicator of
sensitivity and resistance of mouse L1210 leukemia to
cis-diamminedichloroplatinum(II) and
L-phenylalanine mustard. Cancer Res. 41:640–649.
1981.PubMed/NCBI
|
|
25
|
Horvathova K, Novotny L and Vachalkova A:
The free radical scavenging activity of four flavonoids determined
by the comet assay. Neoplasma. 50:291–295. 2003.PubMed/NCBI
|
|
26
|
Choi YM, Kim HK, Shim W, Anwar MA, Kwon
JW, Kwon HK, Kim HJ, Jeong H, Kim HM, Hwang D, et al: Mechanism of
cisplatin-induced cytotoxicity is correlated to impaired metabolism
due to mitochondrial ROS generation. PLoS One. 10:e01350832015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaushal GP, Kaushal V, Hong X and Shah SV:
Role and regulation of activation of caspases in cisplatin-induced
injury to renal tubular epithelial cells. Kidney Int. 60:1726–1736.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bai J, He A, Huang C, Yang J, Zhang W,
Wang J, Yang Y, Zhang P, Zhang Y and Zhou F: Serum peptidome based
biomarkers searching for monitoring minimal residual disease in
adult acute lymphocytic leukemia. Proteome Sci. 12:492014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muggia F: Platinum compounds 30 years
after the introduction of cisplatin: Implications for the treatment
of ovarian cancer. Gynecol Oncol. 112:275–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prestayko AW, D'Aoust JC, Issell BF and
Crooke ST: Cisplatin (cis-diamminedichloroplatinum II).
Cancer Treat Rev. 6:17–39. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lebwohl D and Canetta R: Clinical
development of platinum complexes in cancer therapy: An historical
perspective and an update. Eur J Cancer. 34:1522–1534. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Galanski M: Recent developments in the
field of anticancer platinum complexes. Recent Patents Anticancer
Drug Discov. 1:285–295. 2006. View Article : Google Scholar
|
|
33
|
Chen H and Chan DC: Physiological
functions of mitochondrial fusion. Ann NY Acad Sci. 1201:21–25.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Renault TT, Floros KV, Elkholi R, Corrigan
KA, Kushnareva Y, Wieder SY, Lindtner C, Serasinghe MN, Asciolla
JJ, Buettner C, et al: Mitochondrial shape governs BAX-induced
membrane permeabilization and apoptosis. Mol Cell. 57:69–82. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brooks C, Wei Q, Feng L, Dong G, Tao Y,
Mei L, Xie ZJ and Dong Z: Bak regulates mitochondrial morphology
and pathology during apoptosis by interacting with mitofusins. Proc
Natl Acad Sci USA. 104:11649–11654. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Whelan RS, Konstantinidis K, Wei AC, Chen
Y, Reyna DE, Jha S, Yang Y, Calvert JW, Lindsten T, Thompson CB, et
al: Bax regulates primary necrosis through mitochondrial dynamics.
Proc Natl Acad Sci USA. 109:6566–6571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wen S, Zhu D and Huang P: Targeting cancer
cell mitochondria as a therapeutic approach. Future Med Chem.
5:53–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tanaka A and Youle RJ: A chemical
inhibitor of DRP1 uncouples mitochondrial fission and apoptosis.
Mol Cell. 29:409–410. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han XJ, Yang ZJ, Jiang LP, Wei YF, Liao
MF, Qian Y, Li Y, Huang X, Wang JB, Xin HB, et al: Mitochondrial
dynamics regulates hypoxia-induced migration and antineoplastic
activity of cisplatin in breast cancer cells. Int J Oncol.
46:691–700. 2015.PubMed/NCBI
|
|
40
|
Kim H, Lee JY, Park KJ, Kim WH and Roh GS:
A mitochondrial division inhibitor, Mdivi-1, inhibits mitochondrial
fragmentation and attenuates kainic acid-induced hippocampal cell
death. BMC Neurosci. 17:332016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Akita M, Suzuki-Karasaki M, Fujiwara K,
Nakagawa C, Soma M, Yoshida Y, Ochiai T, Tokuhashi Y and
Suzuki-Karasaki Y: Mitochondrial division inhibitor-1 induces
mitochondrial hyperfusion and sensitizes human cancer cells to
TRAIL-induced apoptosis. Int J Oncol. 45:1901–1912. 2014.PubMed/NCBI
|
|
42
|
Han XJ, Lu YF, Li SA, Kaitsuka T, Sato Y,
Tomizawa K, Nairn AC, Takei K, Matsui H and Matsushita M: CaM
kinase I alpha-induced phosphorylation of Drp1 regulates
mitochondrial morphology. J Cell Biol. 182:573–585. 2008.
View Article : Google Scholar : PubMed/NCBI
|