Introduction
Breast cancer is one of the most common malignancies
among females worldwide. As a result of early detection and
improved treatment, the mortality of breast cancer patients has
been declining. However, metastasis is still the leading cause of
death among breast cancer patients, accounting for more than 90% of
cancer-related mortality (1,2).
Although many studies have focused on the mechanisms of
carcinogenesis and metastasis in breast cancer, the exact mechanism
remains unclear.
Chemokines and their receptors have been widely
known to play important roles in multiple pathways of tumor
progression, including cancer cell proliferation, survival,
cellular senescence, angiogenesis and metastasis (3). Among the four subfamilies (CXC, CC,
CX3C and XC) of chemokines, the subfamily of CC chemokines has
attracted more and more attention in recent years. For example,
CCL18 was reported to promote breast cancer metastasis (4) and induce epithelial-to-mesenchymal
transition in lung cancer (5). CCL2
(MCP-1) was shown to facilitate breast cancer metastasis (6), promote cancer stem cell (CSC)-mediated
disease progression (7) and enhance
breast cancer cell survival (8).
CCL28, also known as mucosa-associated epithelial
chemokine (MEC), which is a ligand for CCR3/CCR10, was initially
recognized as a chemokine predominantly produced by epithelial
cells in diverse mucosal tissues (9), including the salivary gland, mammary
gland, trachea, colon, and to a lesser extent in the small
intestine (10). Moreover, its
expression can be increased after stimulation with pro-inflammatory
cytokines and bacterial products (11,12).
CCL28 appears to have dual functions in different types of human
cancer. For example, Dimberg et al (13) found that CCL28 protein expression in
colon tumors was significantly lower than that in normal tissues,
suggesting that suppression of CCL28 expression was related to
colorectal carcinogenesis. Moreover, CCL28 mRNA and protein
expression were markedly reduced in pleomorphic adenomas and
adenolymphomas compared with the levels in normal adjacent tissue
(14). On the contrary, several
studies have indicated that overexpression of CCL28 promoted tumor
tolerance and angiogenesis (15).
Although CCL28 was found to be produced in the mammary gland, the
role of CCL28 in breast carcinogenesis and metastasis has not been
explored. In a previous study, CCL28 mRNA expression was highly
reduced or eliminated in human breast tumors compared to that noted
in normal adjacent tissues, but the relevance needs to be further
studied (16). In this study, we
used a stably expressing CCL28 breast cancer cell line
MDA-MB-231HM/CCL28 derived from parental MDA-MB-231HM cells to
investigate the effects of CCL28 on breast cancer cell
proliferation, migration and invasion in vitro and in
vivo. We found for the first time that CCL28 promotes breast
cancer growth and metastasis at least in part through
mitogen-activated protein kinase (MAPK) signal-mediated
anti-apoptosis and promoted invasiveness.
Materials and methods
Cell lines and culture
Human breast cancer cell line MDA-MB-231 was
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). The MDA-MB-231HM cell line was established by a subclone
selection procedure derived from MDA-MB-231 cells in our institute.
The MDA-MB-231HM cell line has a high potential to metastasize to
the lung and its establishment has been described previously
(17). Cells were routinely
maintained in the recommended Dulbeccos modified Eagles medium
(DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin and 100 µg/ml streptomycin. The cultures were incubated
at 37°C in a humidified 5% CO2 atmosphere. Cell culture
medium and FBS were purchased from Life Technologies, Inc.
(Rockville, MD, USA). RT-PCR reagents were obtained from Takara
Biotechnology Co., Ltd.
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was
used to extract total RNA according to the manufacturer's
recommendations. CCL28 cDNA was produced by reverse
transcription-polymerase chain reaction. First, reverse
transcription was performed with random hexamer primers by using
the PrimeScript™ RT kit (Takara Biotechnology Co., Ltd.). The
primers used to amplify CCL28 cDNA were 5-TAT
GGATCCATGCAGCAGAGAGGACTCGCCAT-3′ (sense, boldface type
indicates the BamHI site) and 5-ACGCGTCGAC
CTAATAAGGAGTTTTATGGCCGTA-3′ (antisense, boldface type indicates the
SalI site). Conditions for the polymerase chain reaction
were 94°C for 2 min, 94°C for 30 sec, 56°C for 1 min, and 72°C for
1 min for a total of 35 cycles, followed by 72°C for a 10-min
extension. The CCL28 reverse transcription-polymerase chain
reaction product was purified on a 1% agarose gel, digested with
BamHI and SalI, and ligated to a pBabe/puromycin
retroviral vector that had been digested with the same enzymes.
After transformation of the plasmid in Escherichia coli
(DH5α), positive clones were selected and DNA sequencing analysis
was performed at the DNA sequencing corporation. The control vector
used in this study was an empty pBabe/puromycin retroviral vector.
All of these plasmids were transfected into amphotropic Phoenix
packaging cells to generate retroviruses, which were used to infect
the corresponding cell lines by using previously described
protocols (18). Retroviruses
carrying CCL28 cDNA were used to infect the MDA-MB-231HM cells.
RNA extraction and real-time
quantitative PCR
TRIzol reagent (Invitrogen) was used to extract
total RNA according to the manufacturer's recommendations. In
brief, 1.0 µg of total RNA was applied for reverse transcription in
20 µl. The quantification of mRNA levels was carried out using DNA
Engine Opticon 2 real-time PCR detection system (MJ Research) with
SYBR-Green. Two microliters of diluted cDNA was used as a template;
10 µl 2X SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.) was
mixed with the template and primers. The total reaction volume was
20 µl. Cycling conditions were denaturation at 95°C for 30 sec,
annealing at 60°C for 30 sec, and elongation at 72°C for 45 sec.
Plate reading was at 72 and 82°C. The specific primers used in the
experiment were as follows: CCL28 forward,
5′-TGCACGGAGGTTTCACATCAT-3′ and reverse,
5′-TTGGCAGCTTGCACTTTCATC-3′; and GAPDH forward,
5′-ACCCACTCCTCCACCTTTGA-3′ and reverse,
5′-CATACCAGGAAATGAGCTTGACAA-3′. All experiments were repeated in
triplicate. To ensure that the correct product was amplified in the
reaction, all PCR products were also separated using 1.2% agarose
gel electrophoresis.
Western blot analysis
Total protein extract for each cell line was
obtained by using a lysis buffer as described elsewhere (19), and equal amounts (30 µg/load) were
analyzed by immuno-blotting. The antibody against β-actin was
obtained from Sigma-Aldrich (A5441, 1:20,000). Antibodies against
Bak (sc-832, 1:1,000), Bcl-2 (sc-7382, 1:500), matrix
metalloproteinase (MMP)-9 (sc-6840, 1:1,000), MMP-2 (sc-13594,
1:1,000), vascular endothelial growth factor (VEGF, sc-507,
1:1,000) and JNK (sc-571, 1:1,000) were from Santa Cruz
Biotechnology. Antibodies against ERK1/2 (9102, 1:1,000),
phospho-ERK1/2 (4376, 1:1,000), MEK1/2 (9126, 1:1,000),
phospho-MEK1/2 (2338, 1:1,000) and phospho-JNK (4668, 1:1,000) were
obtained from Cell Signaling Technology. The antibody against
Bcl-XL (#AM05, 1:1,000) was from Calbiochem. The antibody against
CCL28 (18214-1-AP, 1:500) was from Proteintech. The antibody
against E-cadherin (BD 610182, 1:1,000) was obtained from BD
Labware (Franklin Lakes, NJ, USA). The secondary antibodies were
F(ab)2 fragment of donkey anti-mouse immunoglobulin
(product NA931) or of donkey anti-rabbit immunoglobulin (product
NA9340) linked to horseradish peroxidase from Amersham Biosciences
(Little Chalfont, Buckghamshire, UK).
Proliferation assay
Cell proliferation was detected using Cell Counting
Kit-8 (CCK-8; Dojindo, Kumamoto, Japan). The cells were plated in
96-well plates at a density of 2,500/well (100 µl) and cultured in
growth medium. The number of cells was counted according to the
protocol of the kit from the company.
Wound healing assay
To evaluate cell motility, a wound healing assay was
performed. The cells were cultured in a 6-well culture plate.
Twenty four hours later, when the cells reached 90% confluence, a
single wound was created in the center of the cell monolayer by
gently scratching the attached cells with a sterile 10-µl
micropipette tip. The debris was removed by washing at least twice
with phosphate-buffered saline (PBS). After 12 or 20 h of
incubation, the cells which migrated into the wounded area or
protruded from the border of the wound were visualized and
photographed under an inverted microscope. Each experiment was
performed at least three times independently.
In vitro invasion
Invasion experiments were carried out with a
Matrigel invasion chamber (BD Labware). Each well insert was
layered with 120 ml of a 1:3 mixture of Matrigel/DMEM (1,400 mg
Matrigel/cm2). An amount of 105 cells were
incubated in an incubator at 37°C for 24 h. Invasion was assessed
by counting the cells that had traveled across the filter and that
had attached to the bottom side of the filter. Then, the filter was
fixed in 4% formalin and stained with 1% Crystal violet. Cells that
had invaded through the Matrigel and reached the lower surface of
the filter were counted under a light microscope at a magnification
of ×200. Five fields were counted for each sample.
Anchorage-independent colony
formation
The soft agar assay was carried out as described
previously (19). Briefly,
2×104 cells were suspended in 2 ml of medium with 0.7%
agarose LMP (A8350; Solarbio, Amresco), and the suspension was
placed on top of 3 ml of solidified 1.4% agarose. Triplicate
cultures of each well type were maintained for 14 days at 37°C in
an atmosphere of 5% CO2 and 95% air, with fresh medium
being added at day 7. The number of colonies that were larger than
50 µm (~100 cells) in diameter in each dish was counted at 14–20
days. The assay was repeated three times with duplicate
samples.
Xenograft tumors in nude mice
All mouse experiments were carried out in accordance
with the NIH ‘Guide for the Care and Use of Laboratory Animals’.
The study protocol was approved by the Shanghai Medical
Experimental Animal Care Committee. All applicable international,
national, and institutional guidelines for the care and use of
animals were followed. Breast tumor cells were implanted into the
mammary fat pad as described previously (20). Briefly, we harvested
3×106 cells by incubation in trypsin-EDTA, washed the
cells twice with PBS and resuspended the cells in 0.15 ml of PBS.
The tumor growth of the modified and control cell lines was
monitored until the day that the mice were sacrificed. The date on
which the first grossly visible tumor appeared for subcutaneous
injection was recorded, and the tumor size was measured every 3
days. Two-dimensional measurements were taken with an electronic
caliper after injection, and tumor volume was calculated with the
use of the following formula: tumor volume (in mm3) = a
× b2 × 0.52, where a is the longest diameter, b is the
shortest diameter, and 0.52 is a constant to calculate the volume
of an ellipsoid. Mice were sacrificed when observed for lethargy,
poor appetite and feebleness. Metastasis formation was assessed by
macroscopic observation of all major organs for secondary tumors
and confirmed by histological examination of the organs. All tumor
nodules were counted and dissected; each primary tumor nodule was
also weighed and its volume was determined as described above for
subcutaneous tumors.
Immunohistochemical staining for
animal xenografts
Sections from the xenografts of the
MDA-MB-231HM/CCL28 and MDA-MB-231HM/vector groups were used for
CCL28 and related protein detection. Antibodies used for
immunohistochemical staining included anti-CCL28,
anti-phospho-ERK1/2 and anti-β-catenin. Antibody binding was
detected using avidinbiotin-peroxidase methods. Briefly, tissue
slides were deparaffinized in xylene and rehydrated in a graded
series of ethanol, and sections were subjected to antigen retrieval
by boiling in 0.01 mol/l sodium citrate buffer (pH 6.0) in a
microwave oven for 10 min. After blocking endogenous peroxidase
activity with 0.3% hydrogen peroxide and blocking nonspecific
protein binding with 1.5% normal goat serum, the sections were
incubated overnight with an antibody at 4°C in a humid chamber.
Then, antibodies were localized by incubating sections with
biotinylated goat anti-mouse or goat anti-rabbit IgG for 30 min and
detected with 3,3-diaminobenzidine (DAB). The lung and lymph
tissues were serially cut into 5-µm slices, and every 10th section
was stained with hematoxylin and eosin (H&E) to evaluate the
presence or the absence of lung metastasis. Two independent
pathologists calculated the number of metastasis in whole
lungs.
Statistical analysis
Statistical analysis was performed using Statistical
Package for the Social Sciences (SPSS) software version 16.0 for
Windows (SPSS Inc., Chicago, IL, USA). ANOVA and Student's t-test
were used to determine the statistical significance of differences
between experimental groups in vitro. Values of p<0.05
were considered as statistically significant. Graphs were created
with GraphPad Prism 5.
Results
Expression of CCL28 in human breast
cancer cell lines
CCL28 mRNA expression was detected by reverse
transcription-PCR in 8 breast cancer cell lines (MCF-7, MDA-MB-468,
ZR-75-30, MDA-MB-231, MDA-MB-231HM, SKBR3, Bcap37, T47D) and a
normal mammary epithelial cell line (MCF-10A) (Fig. 1A). To further confirm the difference
in CCL28 mRNA expression in these cells, we detected CCL28 mRNA
expression by quantitative RT-PCR (Fig.
1B). We found that CCL28 mRNA expression was high in the
MDA-MB-231HM and T47D cell lines, moderate in the MDA-MB-231,
MDA-MB-468, ZR-75-30 and MCF-10A cell lines, and low in the other
cell lines. It was clear that CCL28 mRNA expression was higher in
high-metastatic breast cancer cell line MDA-MB-231HM than that in
low-metastatic cell line MDA-MB-231 and in MCF-7 a non-metastatic
cell line, which indicated a potential correlation between the
expression of CCL28 and the metastatic ability of breast cancer
cells.
Stable transfection of CCL28 cDNA into
MDA-MB-231HM cells
In order to investigate the role of CCL28 in breast
cancer cell tumorigenesis and metastasis, we transfected CCL28
expression vector pBabe/CCL28 into MDA-MB-231HM cells and generated
stable transfectants. As shown by reverse transcription-PCR
(Fig. 2A), western blot analysis
(Fig. 2B) and immunohistochemistry
(Fig. 2C) in the tissues generated
from animals injected with MDA-MB-231HM/CCL28 or
MDA-MB-231HM/vector, CCL28 was markedly over-expressed in the cells
respectively treated with CCL28 cDNA (MDA-MB-231HM/CCL28) compared
with the control cells treated with the empty vector
(MDA-MB-231HM/vector). These results suggest that a stable
expressing CCL28 breast cancer cell line was successfully
generated, and it can be used in further experiments.
CCL28 promotes breast cancer cell
proliferation, tumorigenesis and tumor growth
To investigate whether expression of CCL28 is
associated with tumor growth of breast cancer cells in
vitro, we performed proliferation and anchorage-independent
growth assays in MDA-MB-231HM/CCL28 cells and compared the results
with the parental cells. As shown in Fig. 3A, overexpression of CCL28
significantly promoted breast cancer cell proliferation.
Furthermore, the number of colonies formed in soft agar was
increased in the MDA-MB-231HM/CCL28 cells compared to that noted in
the control cells (Fig. 3B).
Furthermore, western blot analysis showed that the expression of
anti-apoptotic protein Bcl-2 was higher in the MDA-MB-231HM/CCL28
cells when compared with that in the control cells, whereas no
changes were observed in the expression of Bcl-XL. The
pro-apoptotic protein BAK was decreased in the MDA-MB-231HM/CCL28
cells (Fig. 3C).
Next, the effect of CCL28 on tumor growth was
further investigated using an orthotropic xenograft tumor model in
nude mice. The results showed that MDA-MB-231HM/CCL28 cell-derived
tumors grew much faster than the MDA-MB-231HM/vector cell-derived
tumors in the nude mice (Fig. 3D).
When the mice were xenografted with MDA-MB-231HM/CCL28 cells, as
shown in Fig. 3E, the primary tumor
volume was much larger than that noted for the control cells.
Furthermore, the average tumor weight was also increased in the
mice bearing MDA-MB-231HM/CCL28 xenografts as compared with the
control xenografts (Fig. 3F).
Collectively, these data suggest that CCL28 enhances breast cancer
cell proliferation, tumorigenesis and tumor growth.
CCL28 enhances breast cancer cell
migration, invasion and tumor metastasis
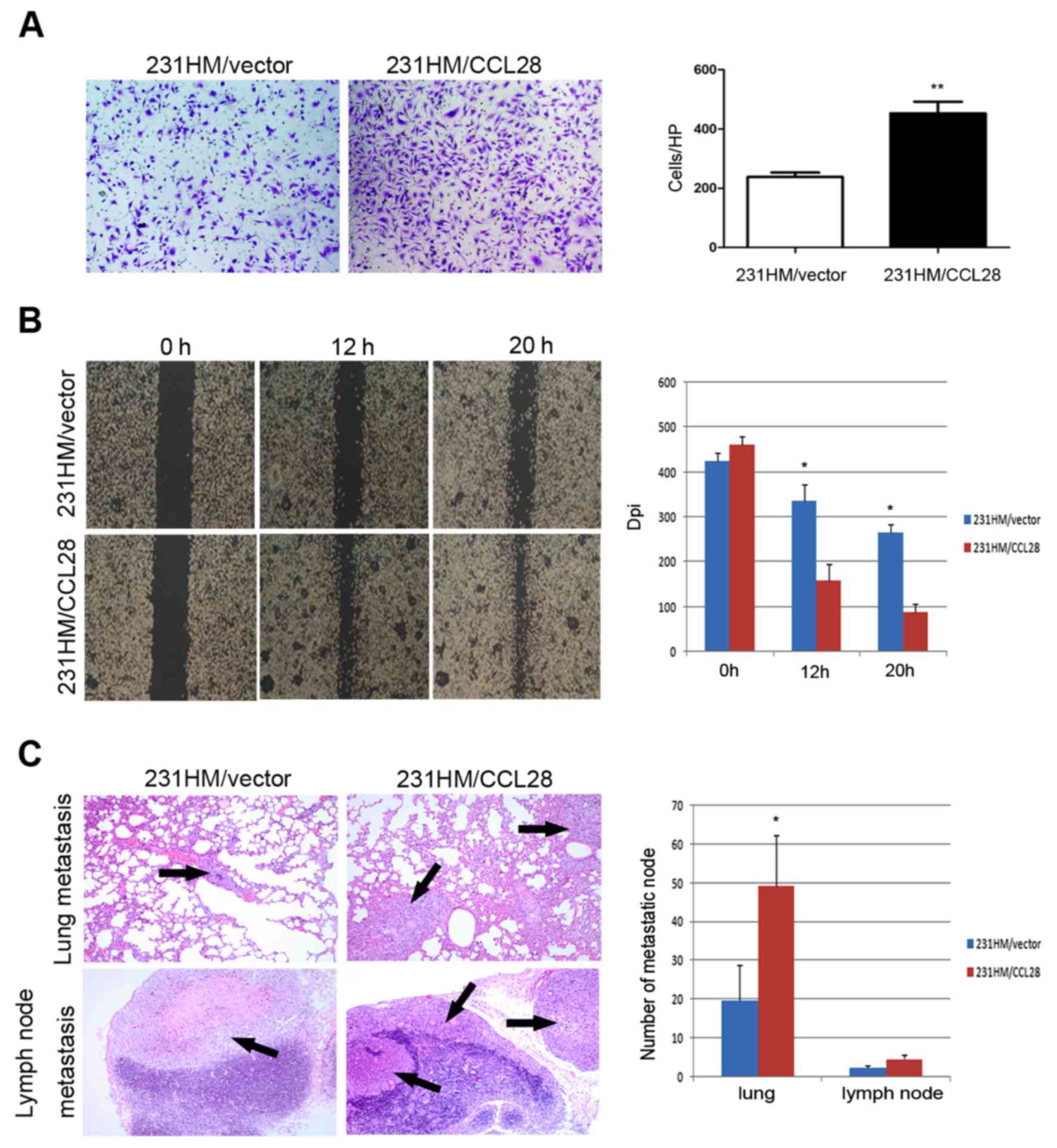
We then assessed the invasiveness of
MDA-MB-231HM/CCL28 and parental cells using Transwell chambers. The
results showed that more MDA-MB-231HM/CCL28 cells intruded into the
bottom chamber than the number of MDA-MB-231HM/vector cells
(Fig. 4A). A wound-healing assay
was used to evaluate the effect of CCL28 expression on breast
cancer cell migration. As shown in Fig.
4B, MDA-MB-231HM/CCL28 cells migrated more rapidly close to the
scratched wound when compared to the MDA-MB-231HM/vector cells
within 20 h. Thus, overexpression of CCL28 increased cell migration
and invasion in vitro.
We also investigated the effect of CCL28 on the
metastasis of tumors in vivo. As shown in Fig. 4C, H&E staining showed that CCL28
led to more massive metastasis in the lungs and lymph node of the
mice injected with MDA-MB-231HM/CCL28 cells than the metastasis of
mice injected with the control cells. Injection of
MDA-MB-231HM/CCL28 cells also increased the number of metastatic
nodules in the lungs and lymph nodes (Fig. 4C).
MAPK pathway is involved in
CCL28-mediated tumor metastasis
To investigate the potential mechanism of CCL28 in
breast cancer metastasis, we first examined the expression of
metastasis-associated proteins in cells overexpressing CCL28, and
found that no significant changes could be detected in the levels
of MMP2, MMP9 and VEGF, while the expression of β-catenin was
decreased (Fig. 5A). β-catenin is a
dual function protein, involved in the regulation and coordination
of cell-cell adhesion and gene transcription. The induced
expression is related to breast cancer (21). In this study, further
immunohistochemistry analysis showed that β-catenin expression was
decreased in the xenograft mouse tumor tissues overexpressing
CCL28, which was consistent with the western blot results (Fig. 5B). These data suggest that CCL28
promotes breast cancer cell metastasis through these molecules.
Since the MAPK pathway is associated with the
promotion of tumor metastasis, we examined the expression of signal
molecules involved in the MAPK pathway in the CCL28 cDNA-treated
cells as well as in their corresponding control cells. As shown in
Fig. 5C, there were no changes in
the expression of ERK1/2, MEK1/2 and JNK1/2, however, levels of
phosphorylated ERK1/2 (Thr202/Tyr204), MEK1/2 (Ser212/221) and
JNK1/2 (Thr183/Tyr185) were markedly increased after overexpression
of CCL28 compared with the control cell line. To confirm this
observation, we then examined pERK expression in the tissues
generated from animals injected with the MDA-MB-231HM/CCL28 and
MDA-MB-231HM/vector by immunohistochemistry. As expected, the
expression of pERK was significantly increased (Fig. 5D). These results suggest that CCL28
promotes breast cancer metastasis by suppressing β-catenin, which
may be mediated through upstream signaling of MAPK.
Discussion
Chemokines are a family of small chemotactic
cytokines, which have been divided into four main subfamilies: CXC,
CC, CX3C and XC. Chemokines and their receptors have been widely
investigated in human cancers, including breast cancer. In
addition, they play a dual role - positive and negative - in cancer
progression. For example, atypical chemokine receptors DARC
(22), D6 (23) and CCX-CKR (24) have been found to inhibit breast
cancer growth and metastasis and are positively correlated with
enhanced survival, whereas CXCR2 has been demonstrated to promote
ovarian cancer growth through multiple pathways (18). CCL28 is one of the newly discovered
CC subfamily chemokine. Previous studies have shown that CCL28 is
constitutively and inductively expressed in diverse mucosal sites,
and is suggested to take part in mucosal immunity (25). Moreover, it also has a broad
spectrum of antimicrobial activity (26,27).
The balance of proliferation and apoptosis plays a
significant role in the control of tumor growth. In general,
progression of tumor growth is characterized by a net increase in
the number of tumor cells. This could be due to increased
proliferation and/or decreased apoptosis, or both (28). Bcl-2 and BCL-xL facilitate G0
quiescence by decreasing RNA content and cell size and upregulating
p27 protein (29). In mammary tumor
and hepatocellular carcinogenesis models, Bcl-2 plays a
tumor-suppressing role by inhibiting proliferation (30). The MAPK pathway is highly conserved
among eukaryotes and transmits signals from cell surface receptors
for nuclear transcription. The MAPK family consists of ERK1/2, JNK
and p38. Generally, upregulation and activation of ERK1/2 and JNK
tends to promote tumor development (31,32).
In this study, we demonstrated that CCL28 plays an important role
in breast cancer progression by promoting breast cancer cell
proliferation, tumorigenesis, migration and invasion in
vitro, and enhancing tumor growth and metastasis in vivo
via the MAPK signaling pathway. We showed that overexpression of
CCL28 increased the expression of anti-apoptotic Bcl-2, and
suppressed the expression of pro-apoptotic Bak, leading to
decreased cellular apoptosis. Furthermore, CCL28 promoted cell
invasiveness and tumor metastasis by suppressing the expression of
cell adhesion protein β-catenin.
As a CC subfamily chemokine, CCL28 and its receptors
CCR3/CCR10 have been extensively investigated in terms of
eosinophil recruitment in inflammation-induced diseases (25,33).
Hanamoto et al found that H-RS cells in 15 of 19 cases were
positive for CCL28. Among them, 7 cases were also positive for
CCR10, suggesting a potential autocrine effect (34). CCL28 is a key regulator of IgA ASC
accumulation in the mammary gland and thus controls the passive
transfer of IgA antibodies from mother to infant (35), but there is no report about whether
it has an effect on cancer cells in an autocrine or a paracrine
manner. There is much evidence to show that CCR3 and CCR10, two
receptors for CCL28, play important roles in numerous types of
malignancies (36–38), whereas the function of their ligand
in epithelial cancer has not been well defined. A study in asthma
demonstrated that phosphorylation of NF-κB is associated with the
expression of inducible CCL28 (39). Kagami et al (40) demonstrated that CCL28 production was
downregulated in keratinocytes by inhibition of ERK/MAPK and NF-κB
pathway. We showed here that CCL28 may promote breast tumorigenesis
by regulating the MAPK signaling pathway, which ultimately controls
cell apoptosis and metastasis.
To our knowledge, no investigation has focused on
the effect of CCL28 on breast cancer cell proliferation and
invasive abilities in vitro and in vivo. In our
study, we showed for the first time that overexpression of CCL28
led to increased cell proliferation and tumor growth, and the
effect was at least in part attributed to increased anti-apoptotic
factor Bcl-2. This is consistent with a previous study in human
hematopoietic stem and progenitor cells (HSPCs), which showed that
CCL28 is a growth factor that directly stimulates the proliferation
of primitive hematopoietic cells (41). However, Sun et al (42) obtained the opposite results and
found that CCL28 induced the apoptosis of decidual stromal cells in
human spontaneous abortion. The reason for the different result may
be due to the different source of CCL28, different
microenvironment, and different types of diseases, which need to be
further studied in the future. Furthermore, our data showed that
overexpression of CCL28 not only promoted the growth of tumors, but
also elevated the metastatic ability by increasing the number of
lung metastatic nodules and the weight of wet lung.
Immunohistochemistry indicated that CCL28 positively regulated
cancer metastasis by suppressing the expression of β-catenin. The
result was confirmed by western blot analysis as following the
overexpression of CCL28, the expression of β-catenin was decreased.
In addition, our data showed that VEGF protein expression did not
change in terms of CCL28 expression, which was not consistent with
a study by Facciabene et al. They demonstrated that CCL28
production was upregulated by hypoxia, and then recruited Treg
cells expressing CCR10, and ultimately promoted tumor immune
tolerance and angiogenesis (15).
This discrepancy may suggest that CCL28 regulates tumor progression
through different mechanisms.
In summary, we demonstrated for the first time that
overexpression of CCL28 promotes breast cancer proliferation and
tumor growth by activating Bcl-2 expression, and increases breast
cancer invasiveness and metastatic ability by suppressing β-catenin
expression, which are positively related to activation of the MAPK
signaling pathway. Thus, targeting CCL28 may effectively inhibit
breast cancer cell growth and metastasis and may be a valuable
therapeutic strategy against breast cancer.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant no. 81172506), and the Shanghai
Committee of Science and Technology, China (grant no.
12DZ2260100).
References
|
1
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mantovani A, Savino B, Locati M, Zammataro
L, Allavena P and Bonecchi R: The chemokine system in cancer
biology and therapy. Cytokine Growth Factor Rev. 21:27–39. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen J, Yao Y, Gong C, Yu F, Su S, Chen J,
Liu B, Deng H, Wang F, Lin L, et al: CCL18 from tumor-associated
macrophages promotes breast cancer metastasis via PITPNM3. Cancer
Cell. 19:541–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ploenes T, Scholtes B, Krohn A, Burger M,
Passlick B, Müller-Quernheim J and Zissel G: CC-chemokine ligand 18
induces epithelial to mesenchymal transition in lung cancer A549
cells and elevates the invasive potential. PLoS One. 8:e530682013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qian BZ, Li J, Zhang H, Kitamura T, Zhang
J, Campion LR, Kaiser EA, Snyder LA and Pollard JW: CCL2 recruits
inflammatory monocytes to facilitate breast-tumour metastasis.
Nature. 475:222–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsuyada A, Chow A, Wu J, Somlo G, Chu P,
Loera S, Luu T, Li AX, Wu X, Ye W, et al: CCL2 mediates cross-talk
between cancer cells and stromal fibroblasts that regulates breast
cancer stem cells. Cancer Res. 72:2768–2779. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang WB, Jokar I, Zou A, Lambert D,
Dendukuri P and Cheng N: CCL2/CCR2 chemokine signaling coordinates
survival and motility of breast cancer cells through Smad3 protein-
and p42/44 mitogen-activated protein kinase (MAPK)-dependent
mechanisms. J Biol Chem. 287:36593–36608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang W, Soto H, Oldham ER, Buchanan ME,
Homey B, Catron D, Jenkins N, Copeland NG, Gilbert DJ, Nguyen N, et
al: Identification of a novel chemokine (CCL28), which binds CCR10
(GPR2). J Biol Chem. 275:22313–22323. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan J, Kunkel EJ, Gosslar U, Lazarus N,
Langdon P, Broadwell K, Vierra MA, Genovese MC, Butcher EC and
Soler D: A novel chemokine ligand for CCR10 and CCR3 expressed by
epithelial cells in mucosal tissues. J Immunol. 165:2943–2949.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shibata S, Maeda S, Maeda S, Chimura N,
Kondo N and Fukata T: Augmentation of CCL17 and CCL28 gene
expression by TNF-alpha, IL-1beta, or IFN-gamma in cultured canine
keratinocytes. Res Vet Sci. 88:422–426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ogawa H, Iimura M, Eckmann L and Kagnoff
MF: Regulated production of the chemokine CCL28 in human colon
epithelium. Am J Physiol Gastrointest Liver Physiol.
287:G1062–G1069. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dimberg J, Hugander A and Wågsäter D:
Protein expression of the chemokine, CCL28, in human colorectal
cancer. Int J Oncol. 28:315–319. 2006.PubMed/NCBI
|
|
14
|
Liu GX, Lan J, Sun Y, Hu YJ and Jiang GS:
Expression of the chemokine CCL28 in pleomorphic adenoma and
adenolymphoma of the human salivary glands. Exp Ther Med. 4:65–69.
2012.PubMed/NCBI
|
|
15
|
Facciabene A, Peng X, Hagemann IS, Balint
K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, et
al: Tumour hypoxia promotes tolerance and angiogenesis via CCL28
and T(reg) cells. Nature. 475:226–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mickanin CS, Bhatia U and Labow M:
Identification of a novel β-chemokine, MEC, down-regulated in
primary breast tumors. Int J Oncol. 18:939–944. 2001.PubMed/NCBI
|
|
17
|
Li JY, Ou ZL, Yu SJ, Gu XL, Yang C, Chen
AX, Di GH, Shen ZZ and Shao ZM: The chemokine receptor CCR4
promotes tumor growth and lung metastasis in breast cancer. Breast
Cancer Res Treat. 131:837–848. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang G, Rosen DG, Liu G, Yang F, Guo X,
Xiao X, Xue F, Mercado-Uribe I, Huang J, Lin SH, et al: CXCR2
promotes ovarian cancer growth through dysregulated cell cycle,
diminished apoptosis, and enhanced angiogenesis. Clin Cancer Res.
16:3875–3886. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang G, Rosen DG, Mercado-Uribe I,
Colacino JA, Mills GB, Bast RC Jr, Zhou C and Liu J: Knockdown of
p53 combined with expression of the catalytic subunit of telomerase
is sufficient to immortalize primary human ovarian surface
epithelial cells. Carcinogenesis. 28:174–182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jones LW, Viglianti BL, Tashjian JA,
Kothadia SM, Keir ST, Freedland SJ, Potter MQ, Moon EJ, Schroeder
T, Herndon JE II, et al: Effect of aerobic exercise on tumor
physiology in an animal model of human breast cancer. J Appl
Physiol (1985). 108:343–348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aaltomaa S, Kärjä V, Lipponen P, Isotalo
T, Kankkunen JP, Talja M and Mokka R: Reduced alpha- and
beta-catenin expression predicts shortened survival in local
prostate cancer. Anticancer Res. 25:4707–4712. 2005.PubMed/NCBI
|
|
22
|
Wang J, Ou ZL, Hou YF, Luo JM, Shen ZZ,
Ding J and Shao ZM: Enhanced expression of Duffy antigen receptor
for chemokines by breast cancer cells attenuates growth and
metastasis potential. Oncogene. 25:7201–7211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu FY, Ou ZL, Feng LY, Luo JM, Wang LP,
Shen ZZ and Shao ZM: Chemokine decoy receptor d6 plays a negative
role in human breast cancer. Mol Cancer Res. 6:1276–1288. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng LY, Ou ZL, Wu FY, Shen ZZ and Shao
ZM: Involvement of a novel chemokine decoy receptor CCX-CKR in
breast cancer growth, metastasis and patient survival. Clin Cancer
Res. 15:2962–2970. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eksteen B, Miles A, Curbishley SM,
Tselepis C, Grant AJ, Walker LS and Adams DH: Epithelial
inflammation is associated with CCL28 production and the
recruitment of regulatory T cells expressing CCR10. J Immunol.
177:593–603. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hieshima K, Ohtani H, Shibano M, Izawa D,
Nakayama T, Kawasaki Y, Shiba F, Shiota M, Katou F, Saito T, et al:
CCL28 has dual roles in mucosal immunity as a chemokine with
broad-spectrum antimicrobial activity. J Immunol. 170:1452–1461.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang P, Lan J, Hu Y, Li D and Jiang G:
Enhancing CCL28 expression through the gene transfer to salivary
glands for controlling cariogenic microbe. Cytokine. 59:94–99.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mattern J and Volm M: Imbalance of cell
proliferation and apoptosis during progression of lung carcinomas.
Anticancer Res. 24:4243–4246. 2004.PubMed/NCBI
|
|
29
|
Kordezangeneh M, Irani S, Mirfakhraie R,
Esfandyari-Manesh M, Atyabi F and Dinarvand R: Regulation of
BAX/BCL2 gene expression in breast cancer cells by docetaxel-loaded
human serum albumin nanoparticles. Med Oncol. 32:2082015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eom YH, Kim HS, Lee A, Song BJ and Chae
BJ: BCL2 as a subtype-specific prognostic marker for breast cancer.
J Breast Cancer. 19:252–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen J, Hou R, Zhang X, Ye Y, Wang Y and
Tian J: Calycosin suppresses breast cancer cell growth via
ERβ-dependent regulation of IGF-1R, p38 MAPK and PI3K/Akt pathways.
PLoS One. 9:e912452014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang XL, Lin FJ, Guo YJ, Shao ZM and Ou
ZL: Gemcitabine resistance in breast cancer cells regulated by
PI3K/AKT-mediated cellular proliferation exerts negative feedback
via the MEK/MAPK and mTOR pathways. Onco Targets Ther. 7:1033–1042.
2014.PubMed/NCBI
|
|
33
|
Takeda A, Baffi JZ, Kleinman ME, Cho WG,
Nozaki M, Yamada K, Kaneko H, Albuquerque RJ, Dridi S, Saito K, et
al: CCR3 is a target for age-related macular degeneration diagnosis
and therapy. Nature. 460:225–230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hanamoto H, Nakayama T, Miyazato H,
Takegawa S, Hieshima K, Tatsumi Y, Kanamaru A and Yoshie O:
Expression of CCL28 by Reed-Sternberg cells defines a major subtype
of classical Hodgkin's disease with frequent infiltration of
eosinophils and/or plasma cells. Am J Pathol. 164:997–1006. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wilson E and Butcher EC: CCL28 controls
immunoglobulin (Ig)A plasma cell accumulation in the lactating
mammary gland and IgA antibody transfer to the neonate. J Exp Med.
200:805–809. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miyagaki T, Sugaya M, Murakami T, Asano Y,
Tada Y, Kadono T, Okochi H, Tamaki K and Sato S: CCL11-CCR3
interactions promote survival of anaplastic large cell lymphoma
cells via ERK1/2 activation. Cancer Res. 71:2056–2065. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kakinuma T and Hwang ST: Chemokines,
chemokine receptors, and cancer metastasis. J Leukoc Biol.
79:639–651. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Murakami T, Cardones AR, Finkelstein SE,
Restifo NP, Klaunberg BA, Nestle FO, Castillo SS, Dennis PA and
Hwang ST: Immune evasion by murine melanoma mediated through CC
chemokine receptor-10. J Exp Med. 198:1337–1347. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
O'Gorman MT, Jatoi NA, Lane SJ and Mahon
BP: IL-1beta and TNF-alpha induce increased expression of CCL28 by
airway epithelial cells via an NFkappaB-dependent pathway. Cell
Immunol. 238:87–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kagami S, Saeki H, Komine M, Kakinuma T,
Nakamura K, Tsunemi Y, Sasaki K, Asahina A and Tamaki K: CCL28
production in HaCaT cells was mediated by different signal pathways
from CCL27. Exp Dermatol. 15:95–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karlsson C, Baudet A, Miharada N, Soneji
S, Gupta R, Magnusson M, Enver T, Karlsson G and Larsson J:
Identification of the chemokine CCL28 as a growth and survival
factor for human hematopoietic stem and progenitor cells. Blood.
121:3838–3842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun C, Zhang YY, Tang CL, Wang SC, Piao
HL, Tao Y, Zhu R, Du MR and Li DJ: Chemokine CCL28 induces
apoptosis of decidual stromal cells via binding CCR3/CCR10 in human
spontaneous abortion. Mol Hum Reprod. 19:676–686. 2013. View Article : Google Scholar : PubMed/NCBI
|