Introduction
Glioblastoma is the most common and malignant type
of primary brain tumor (1,2). The median survival of GBM patients
remains ~15 months under the gold standard treatment with
temozolomide (TMZ) (1,3–5). GBM
chemoresistance has been linked to several mechanisms. The presence
of stem-like cells, overexpression of efflux proteins such as
P-glycoprotein (PGP), the methylation of MGMT promoter and
the constitutive activation of proliferative signaling pathways,
mainly phosphorylated protein kinase C (PKC), have been described
as some of the main reasons of GBM chemoresistance and contribute
to the increased proliferation, survival and motility of GBM cells
(6–13). We previously reported that the
combination of tamoxifen (TMX), a PKC inhibitor, with TMZ can
reduce the amount of phosphorylated PKC-pan and contribute to the
reduction of aggressive behaviour of the GBM cell lines U87 and
U118 (6). In fact, a large spectrum
of TMX targets other than estrogen receptors have been defined as
key mediators of signal pathways activating cell proliferation,
determining aggressive course of neoplastic disorders or tumor
chemosensitivity, namely in GBM (14). Taking into consideration the genetic
and molecular variability in GBM cell lines, we i) isolated and
characterized a human GBM cell line, termed GBM11; and ii) compared
the effect of TMX and TMZ co-treatment on this GBM cell line with
that observed in U87 and U118 cell lines in our previous study
(6). The treatment comparison
between the GBM11 cells and the U87 and U118 cells with TMX and TMZ
as chemotherapeutic compounds and their combinations could reveal
distinct cytotoxic effects among GBM cells, indicating an
individualized response to therapy.
GBM11 cell line was isolated as previously described
from surgical biopsies from a glial tumor diagnosed as GBM
(15,16). Next, we characterized the GBM11
considering their stem cell properties, i.e. expression of
stem-like cell markers, histopathological features, analysis of
GFAP and Nestin expression, properties found in the other
established cell lines. We also analysed PGP expression in GBM11,
U87 and U118 cell lines. We tested the sensitivity of GBM11 cells
to TMZ treatment alone as the gold standard for GBM treatment. We
finally evaluated the effect of TMX and TMZ co-treatment on GBM11
cells by comparing the results with U87 and U118 cell lines,
previously published by our group (6). Principally, our results showed that
our GBM11 cells presented a higher resistance to TMX and/or TMZ
treatment compared to that obtained with U87 and U118 cells,
probably due to the existence of a stem-like cell population and a
higher PGP expression. In fact, the overexpression of PGP at the
blood-brain-barrier (BBB) is discussed as a major mechanism of
pharmacoresistance in cancer, namely in GBM (17), but some studies also suggested an
intrinsic chemoresistance role of MRP1 expression in GBM
tumor cells, independent of the BBB endothelial transport system
(18).
The aim of our present study is to introduce a new
human GBM cell line, GBM11, that could serve as a patient-specific
approach to understand the mechanisms underlying chemotherapeutic
resistance expanding the resources available for preclinical
studies in GBM treatment. We believe that the introduction of this
cellular resistant model could provide a potential testing platform
to investigate new therapeutic strategies. We consider that our new
GBM cell line derived from human tumor cells, is able to introduce
the variability of a patient-specific response to therapy in a way
to reinforce the individually-designed cancer therapy approach and
circumvent the eventual impaired therapy.
Materials and methods
Materials
Dulbeccos modified Eagles medium (DMEM) and fetal
bovine serum (FBS) were supplied by Invitrogen (Paisley, UK). The
anti-mouse and anti-rabbit antibodies were obtained from GE
Healthcare (Little Chalfont, UK). Protease and phosphatase
inhibitors were supplied by Roche Diagnostics (Indianapolis, IN,
USA). Antibody for PKC-pan pan was from Cell Signaling Technology
(Beverly, MA, USA). Mouse anti-tubulin and mouse anti-actin
antibody were obtained from Boehringer (Mannheim, Germany).
Temozolomide (TMZ) and tamoxifen (TMX) were dissolved in dimethyl
sulfoxide (DMSO) at a stock concentration of 0.133 M and 3 mM,
respectively, and diluted in culture medium according to the
concentrations used. Both TMZ and TMX were from Sigma-Aldrich
Chemicals (St. Louis, MO, USA).
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) was from Sigma-Aldrich Chemicals. Glucose was from Merck
(Darmstadt, Germany). Fungizone was from Bristol-Meyers Squibb
(Princeton, NJ, USA). Penicillin/streptomycin was from Gibco
(Carlsbad, CA, USA). Rabbit anti-glial fibrillary acidic protein
(GFAP) and Nestin clone 10c2 and PVDF membranes were from Millipore
(Billerica, MA, USA). The 5-ethynyl-2-deoxyuridine (EdU) kit was
from Invitrogen (Carlsbad, CA, USA). Annexin V and propidium iodide
(PI) were from BD Biosciences (Biolegends, San Diego, CA, USA).
PI/RNase was from Immunostep (Salamanca, Spain). The phalloidin and
the anti-human PGP (fluorescein isothiocyanate (FITC) mouse
anti-human P-glycoprotein) were from BD Biosciences. NZYDNA Ladder
VI was from (NZYTech, Lisbon, Portugal). Antibodies for Nanog
(#3580), Oct-4A (#2840) and SOX2 (#D6D9), Slug (#9585) were from
Cell Signaling Technology (Beverly, MA, USA). Mouse anti-actin
antibody was from Boehringer. PVDF membranes were from Millipore;
2x Laemmli buffer and β-mercaptoethanol were obtained from Bio-Rad
Laboratórios do Brasil (São Paulo, Brazil).
Cell line culture conditions
The GBM11 cell line was established and
characterized in our laboratory as previously described for other
cell lines (15,16) using the same protocols to U87 and
U118 cell cultures. Briefly, GBM11 cells were obtained by surgical
biopsy from a 57-year-old male patient bearing a recurrent
glioblastoma previously treated with TMZ concomitantly with
radiotherapy, who had given written consent to the study. All
procedures were in agreement with the Brazilian Ministry of Health
Ethics Committee (CONEP no. 2340). The tumor cells were termed
GBM11. The tumor sample was analysed histologically by the
Pathology Service of the Federal University of Rio de Janeiro
Hospital as previously described (16). The biopsy was washed in DMEM medium,
mechanically dissociated and then plated directly plated on a
24-well plate and/or 25-cm2 tissue culture flasks with
DMEM supplemented with 3.5 mg/ml glucose, 0.1 mg/ml penicillin,
0.14 mg/ml streptomycin and 10% inactivated FBS. Cells were
maintained at 37°C in an atmosphere containing 95% air and 5%
CO2. The medium was changed every 3 days until the
culture was near confluence, approximately after 7 days. Then, cell
cultures were fixed and processed for characterization as described
in Faria et al (16). Cells
were also frozen in FBS and 10% DMSO in cryotubes and conserved in
liquid N2. For the experiments, unsynchronized cells were treated
with different concentrations of TMX or TMZ. The curve for the
calculation of each drug concentration necessary to inhibit cell
proliferation by 50% was fitted using GraphPad Prism 5 for Windows
(version 5.00; GraphPad Software, Inc., San Diego, CA, USA).
Cell viability evaluation by MTT
assay
Metabolically active cells were assessed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
reduction colorimetric assay, as described by Balça-Silva et
al (6). Briefly, GBM11 cells
were plated in 96 multi-well plates and then were incubated with
TMX and/or TMZ at different concentrations for 48 h. After 48 h of
incubation, MTT (5 mg/ml) was added to each well at a final
concentration of 0.5 mg/ml and left for 1 h. In order to dissolve
the blue formazan crystals, 200 µl of DMSO was added. GBM11 cells
were maintained in fresh medium at 37°C in an atmosphere of 95%
humidity and 5% CO2 for 48 h. The absorbance was read in
a microplate reader at 570 nm. Cytotoxicity was evaluated as the
percentage of metabolically active in relation to untreated cells.
The drug concentration required to reduce the percentage of
metabolically active cells by 50% (IC50) was estimated
with GraphPad.
Histological analysis and
immunofluorescence
Haematoxylin was used to stain and counterstain
paraffin tumor sections for histopathological analysis. Sections
were mounted with Permount™. For immunofluorescence analysis,
10×104 cells/ml were plated on 24-well plates, as
described by Khan et al (15). Briefly, cells were fixed with 4% PFA
in phosphate-buffered saline (PBS) for 15 min and then washed with
PBS and incubated with 5% BSA/PBS for 30 min. Cells were incubated
with mouse anti-Nestin (1:200), rabbit anti-GFAP (1:500). Cells
were incubated overnight at 4°C with the primary antibodies and
then washed with PBS and incubated with secondary antibodies
conjugated with Alexa Fluor 488 (goat anti-mouse; 1:250), Alexa
Fluor 488 (goat anti-rabbit; 1:250) overnight. The day after, cells
were washed with PBS, stained with DAPI, then washed with PBS again
and mounted. Negative controls were performed with non-immune
rabbit or mouse IgG. Cells were imaged using a DMi8 advanced
fluorescence microscope (Leica Microsystems, Wetzlar, Germany) and
analysed with the aid of Leica LAS AF Lite, at 63x magnification.
Imaging processing was performed sing the software ImageJ
1.49v.
Analysis of F-actin filament
organization
GBM11 cells were incubated with TMZ and/or TMX for
48 h and F-actin filament organization was studied using Alexa
Fluor 568 phalloidin staining solution (5 U/ml) as previously
described (6). Briefly, cells were
fixed with 2.5% paraformaldehyde/PBS for 20 min. Next, cells were
permeabilized with 0.1% Triton X-100/PBS for 3 min and then
incubated for 30 min with the Alexa Fluor 568 phalloidin staining
solution (5 U/ml) in PBS containing 1% BSA. Nuclei were stained
with DAPI for 2 min. Finally, the coverslips were mounted on glass
slides and inspected under a Zeiss LSM 510 Meta confocal microscope
at a magnification of ×40, using a filter set with an excitation
filter of 568 nm and a barrier filter of 585 nm. Then, cells were
viewed on a Zeiss LSM image browser (Version 4.2.0.121; Carl Zeiss,
Inc., Oberkochen, Germany).
PGP expression by flow cytometry
The expression of PGP in the different GBM cell
lines U87, U118 and GBM11 was assessed by flow cytometry using
monoclonal antibodies labelled with fluorochromes. For each assay,
106 cells were used and data on at least 10,000 events
were collected using a FACSCalibur flow cytometer, CellQuest
software (Becton-Dickinson, Franklin Lakes, NJ, USA), and analysed
using CellQuest™ software (Becton-Dickinson). Since PGP is a
membrane protein, cells were centrifuged at 300 × g and incubated
for 15 min at room temperature with the monoclonal antibodies:
anti-human PGP [fluorescein isothiocyanate (FITC) and mouse
anti-human P-glycoprotein (BD Biosciences)].
MGMT methylation pattern analysis
DNA from GBM cell lines U87, U118 and GBM11 was
extracted according to the standard procedures. One microgram of
genomic DNA was treated with sodium bisulfite using the EpiTect
Bisulfite kit (Qiagen, Hilden, Germany). Methylation-specific PCRs
of MGMT gene promoters were carried out as described by Gonçalves
et al (19) by the EpiTect
PCR control DNA kit (Qiagen) according to the manufacturers
instructions. PCR products were resolved on 4% agarose gels,
stained with ethidium bromide and observed under UV
illumination.
Western blot analysis
PKC-pan expression was analysed by western blot
analysis as described by Balça-Silva et al (6). Also, the expression of SOX2, Oct-4A
and NANOG were analysed by western blot analysis as originally
described by Towbin et al (20) and adapted by Balça-Silva et
al (6) and Kahn et al
(15) in both the GBM and GBM-SF
cell lines. Briefly, cells were centrifuged at 500 × g for 10 min
at 4°C. The supernatants were discarded. Cells were then
resuspended in RIPA buffer (50 mM Tris-HCl at pH 8.0, 150 mM NaCl,
1.0% NP-40, 0.5% sodium deoxycholate, 0.1% SDS and 2 mM EDTA,
supplemented with protease and phosphatase inhibitors and DTT) and
finally sonicated. The samples were denatured with Laemmli buffer
2x added to each sample at a 1:1 ratio. All the protein extracts
were boiled at 95°C for 5 min before use. A total of 30 µg of
protein was run on a 10% SDS-PAGE gel and transferred to a PVDF
membrane. Then, it was incubated with a solution of 5% non-fat milk
in TBST for 1 h at room temperature. The primary antibodies against
p-PKC pan (1:1,000), SOX2 (1:1,000), Oct-4A (1:1,000), and NANOG
(1:1,000) were diluted in TBST with 1% non-fat milk supplemented
with azide. After the incubation period, the immunocomplexes were
detected with anti-rabbit antibody (1:1,000) and conjugated with
horseradish peroxidase. Bands were obtained after exposing the
membranes to X-ray film and analysed through densitometry scanning.
The protein expression was quantified using ImageJ 1.49v software
(Wayne Rasband) with the expression of β-actin used as a loading
control. Each experiment was repeated three times.
Cell apoptosis analysis by flow
cytometry
Cells were collected after 48 h of incubation with
TMZ and/or TMX, washed with PBS, resuspended in binding buffer, and
incubated with Annexin V (AV) (BD Biosciences) and propidium iodide
(IP) (BioLegends) for 15 min in the dark as previously described
(6). Cells were diluted in binding
buffer and analysed using a FACSCalibur flow cytometer. The
experiments were performed in triplicate, and the results were
analysed through CellQuest™ and data analysed by modifit LTMM
software.
Cell cycle analysis by flow
cytometry
The cell cycle analysis was performed by flow
cytometry, using the detection kit PI/RNAse (Immunostep), after the
cells were incubated for 48 h with TMZ and/or TMX, as previously
described (6). Briefly, after
incubation, cells were collected and washed with PBS, and the
pellet was resuspended in cold 70% ethanol, during vortex
agitation, and finally incubated during 30 min on ice. Cells were
incubated in in PI/RNAse solution. A total of 20,000 events were
acquired, and cells were evaluated through CellQuest and data
analysed by modifit LTMM software. The results are expressed by the
percentage of cells in each phase of cell cycle with a mean ± SEM
of at least three independent experiments. A total of 20,000 events
were acquired, and cells were evaluated through CellQuest™ and were
data analysed by modifit LTMM software.
Cell proliferation using EdU
assay
Cells were plated in 6-well plates with different
TMZ and/or TMX concentrations for 48 h, and the effect on the
proliferation rate was assayed using the EdU
(5-ethynyl-2-deoxyuridine) kit, exactly as previously described
(6). In the final step, cells were
incubated with anti-EdU-antibody working solution for 30 min at
37°C in a humidified atmosphere (5% CO2). The
incorporation of EdU was analysed by flow cytometry and data were
analysed with modifit LTMM software.
Evaluation of cell migration
ability
Cell migration was studied according to the method
described by Liang et al (21). After the cells incubation with TMZ
and/or TMX at different concentrations, for 48 h, and the cell
monolayer was scraped in a straight line with a p200 pipette tip,
just as previously described (6).
The debris were removed by washing the cells with culture medium
and new culture medium was added. ImageJ software (National
Institutes of Health) was used to record the coordinates for each
scratch location using a computer-controlled stage. The mean
scratch width at 6 h was compared to the original scratch width (0
h). Each experiment was repeated three times.
Statistical analysis
Statistical analysis was performed on GraphPad Prism
5 for Windows, version 5.00. After confirmation of the assumption
of normality and homogeneity of variance across groups, the groups
were compared by nested design with analysis of variance and
post-hoc comparison, with correction of α error according to
Bonferroni probabilities to compensate for multiple comparisons.
All values were expressed as mean ± SEM, P<0.05.
Results
Establishment and characterization of
GBM11 cell line
Surgical biopsy from a glial tumor diagnosed as a
recurrent GBM was used in this study and U87 and U118 were a
generous gift from another laboratory. The diagnosis was based on
magnetic resonance imaging (MRI), which showed a typical
ring-shaped appearance with a hypodense area due to necrosis,
peripheral contrast enhancement, and edema that indicates a GBM
(Fig. 1A). Haematoxylin and eosin
(H&E) staining revealed hypercellular injury with a fibrillary
background and significant atypia, glomeruloid vessels, and
extensive areas of necrosis (Fig.
1B). Also, immunohistochemistry analysis showed GFAP-positive
cells (Fig. 1C) and negativity for
cytokeratin pool (AE1/AE3), TTF-1, chromogranin, synaptophysin,
CK7, CK20 and PSA (data not shown).
After tumor removal, cells were isolated and
cultured as previously described for other GBM cultures established
in our laboratory (16). The
presence of stem-like cell markers, namely SOX2, OCT-4A and Nanog
was also confirmed (Fig. 1D). We
also evaluated the viability of GBM11 cells in the presence of TMX
and TMZ, by MTT assay. Treatment with TMZ alone did not induce any
alteration in cell viability (Fig.
1E), whereas treatment with TMX alone showed reduction of cell
viability, with an IC50 of 25.6 µM (Fig. 1F). On the other hand, the
combination of TMX (8.8 µM) + TMZ (250 µM) induced a reduction of
49.2% in cell viability (Fig.
1G).
Resistance mechanisms evaluation of
different GBM cell lines
The U87 and U118 cell lines, previously studied, and
the new GBM11 cell line established in our laboratory revealed a
positive staining for Nestin, a neuronal marker, and for GFAP,
usually overexpressed in GBM cells, as well as a different F-actin
organization by phalloidin staining (Fig. 2A), which suggests a similar origin
of these three cell lines. Since the activity of P-glycoprotein
(PGP) may prevent the accumulation of several chemotherapeutic
drugs in glioma cells, we evaluated the expression of PGP in all
GBM cell lines (17,22,23).
The results showed that in GBM11 the PGP expression was 3-fold
higher compared with the GBM cells U87 and U118 (Fig. 2B).
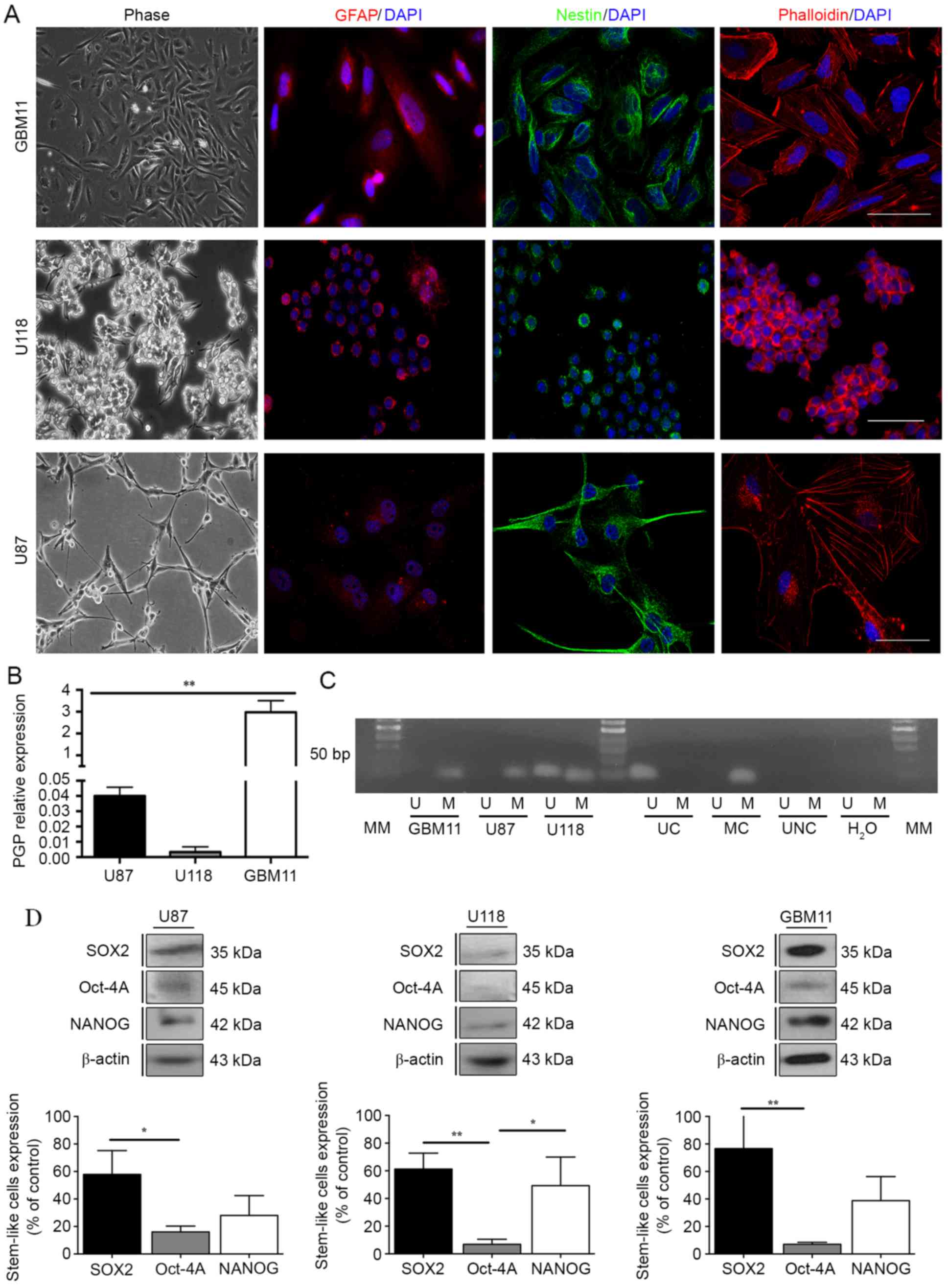 | Figure 2.Evaluation of the resistance
mechanisms in GBM cell lines. (A) Nestin, GFAP and phalloidin
positive-staining were analysed for U87 and U118 cell lines. (B)
PGP expression was quantified in the three GBM cell lines, U87,
U118 and GBM11 by flow cytometric analysis in a BD FACSCalibur
system. A total of 10,000 events were collected. Relative
expression was obtained by PGP expression compared to the
respective isotype and corresponds to the % of gated cells. (C)
MGMT methylation pattern was analysed through methylation-specific
PCRs for U87, U118 and GBM11 cells lines. UC, universal
unmethylated control (bisulfite converted); MC, universal
methylated control (bisulfite converted); UNC, universal not
converted unmethylated control (not bisulfite converted); MM,
molecular marker of 50 bp. (D) The expression of SOX2, Oct-4A and
Nanog in U87, U118 and GBM11 cell lines was quantified by western
blot analysis. Statistical analysis was performed in GraphPad Prism
5 for Windows (version 5.00; GraphPad Software, Inc., San Diego,
CA, USA). Each value represents the mean ± SEM from three
independent experiments, *P<0.05, **P<0.01. |
It is also well known that methylation of the MGMT
promoter can affect the sensitivity of cells to TMZ (24,25).
These findings led us to analyse the methylation status through a
methylation-specific PCR in the three GBM cell lines. In U87 and
GBM11 cell lines, the MGMT proved to be completely methylated and
in the U118 cell line the MGMT showed partial methylation (Fig. 2C). Regarding the stem-like cell
markers expression we noted that the U87 cell line present
61.3±11.5% of SOX2; 6.8±3.7% of Oct-4A; and 49.3±20.7% of NANOG.
The U118 cell line present 57.9±17.3% of SOX2; 16.01±4.2% of
Oct-4A; and 28.0±14.4% of NANOG. Furthermore, the GBM11 cell line
present 76.8±25.8% of SOX2; 7.0±1.5% of Oct-4A; and 38.8±17.4% of
NANOG (Fig. 2D).
Evaluation of death and cell cycle in
GBM11 cells treated with TMX and TMZ
To evaluate cell death with the combined therapy
compared with monotherapy with TMZ, we stained the cells with
Annexin V (AV), to analyse apoptotic cells, and with propidium
iodide (PI), to analyse necrotic cells, by flow cytometry (Fig. 3A). TMZ alone did not induce cell
death in GBM11 (Fig. 3B). However,
17.5 µM of TMX induced an increase of 7.5±0.7% in apoptosis and
37.5±21.5% in late apoptosis, respectively, compared to control
cells. Combined treatment with 17.5 µM TMX + 250 µM TMZ induced an
increase of 8.1±2.3% in apoptosis and 36.5±23.4% in late apoptosis,
P<0.001 (Fig. 3C). Cell cycle
analysis showed an arrest in the G1 phase when TMX was administered
alone and in combination with TMZ, P<0.001 (Fig. 3D).
 | Figure 3.Effects of TMX and TMZ combination on
cell death and cell cycle of GBM11 cells. Following the incubation
of GBM11 cells with TMX and/or TMZ for 48 h, cells were stained
with Annexin V (AV) and propidium iodide (PI) and analysed by flow
cytometry. (A) Debris was removed to obtain the viable cell region,
R1. The R1 region was used to analyse AV and PI expression by flow
cytometry. The analysis was based on the percentage of gated
positive cells. (B) TMZ alone was added to GBM11 cells in different
concentrations. (C) TMX and/or in combination with TMZ was added to
GBM11 cells in different concentrations. The chosen doses were
above the dose that inhibited growth by 50% (IC50),
respectively. The AV positive cells, PI positive cells, AV and PI
double-positive cells and the live cells (double-negative) were
immediately analysed by flow cytometry in a BD FACSCalibur system
and evaluated in the FL2 and FL1 channel, respectively. A total of
10,000 events were collected. (D) Cell cycle analysis was
determined by gating G0/G1, S and G2/M on PI-area signal by flow
cytometry after the debris were removed to obtain the R1 region.
*P<0.05, **P<0.01, ***P<0.001. |
Evaluation of EdU incorporation and
p-PKC-pan regulation in GBM11 cells treated with TMX and TMZ
EdU incorporation in 17.5 µM of TMX and in 17.5 µM
TMX + 250 µM TMZ was reduced by 90.7±8.0 and 96.2±3.4%,
respectively, compared to control cells, P<0.001 (Fig. 4A).
We verified that GBM11 cells also
express p-PKC-pan
The 17.5 µM of TMX induced a significant reduction
of 49.3±30.1% in p-PKC-pan regulation, P<0.05. When combined
with TMZ, the reduction of p-PKC-pan was 44.3±29.4% with 17.5 µM
TMX + 250 µM TMZ (P<0.01) and 65.2±20.3% with 17.5 µM TMX + 350
µM TMZ (Fig. 4B).
Study of cell migration and
organization of F-actin filaments in GBM11 cells treated with TMX
and TMZ
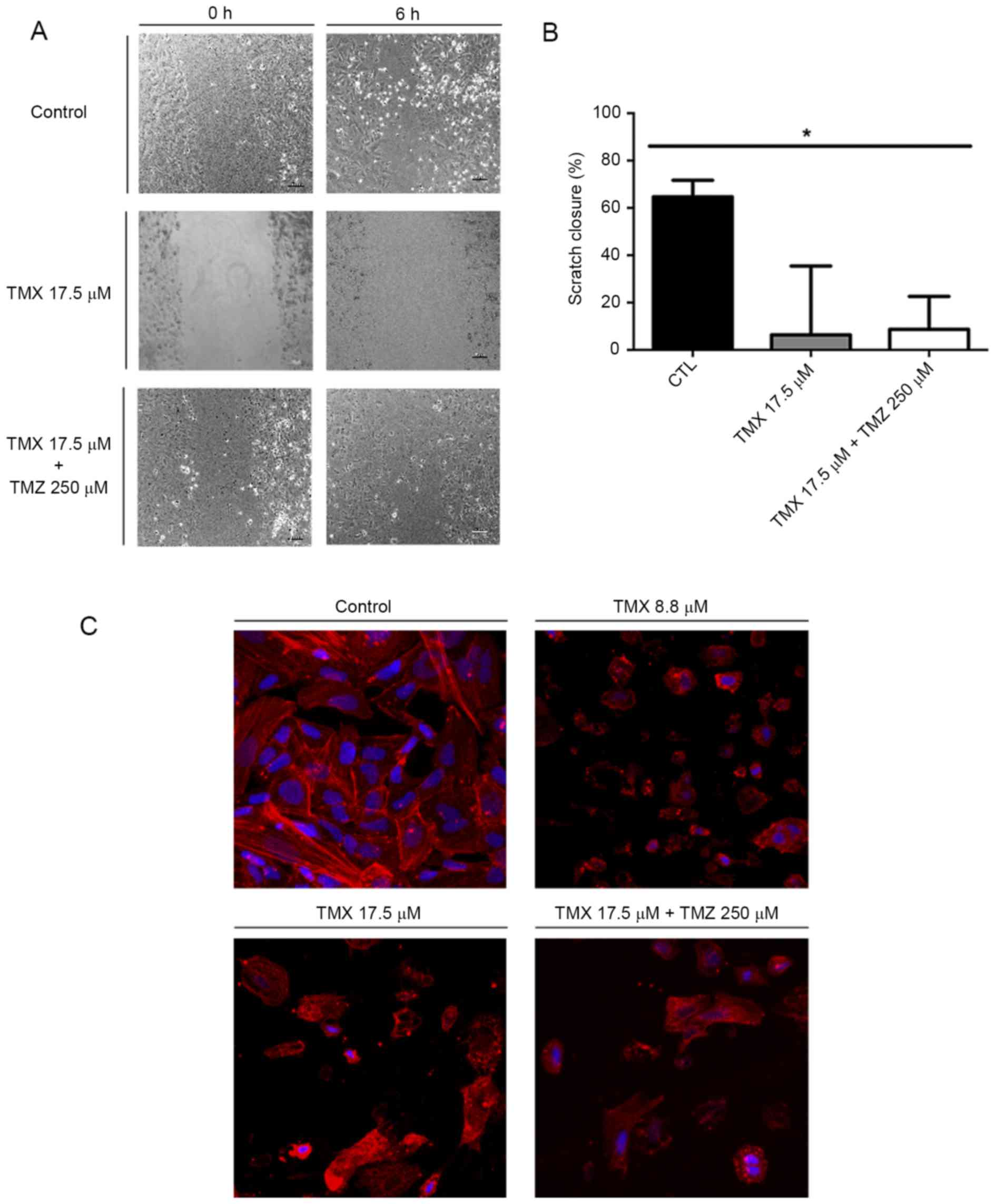
To evaluate the effect of TMX and TMZ co-treatment
on GBM11 cells, we performed the scratch assay. After 6 h, control
cells moved and filled the scratch (Fig. 5A). When cells were incubated with
17.5 µM TMX or with TMX 17.5 µM + 250 µM TMZ, cell motility was
reduced by 80.8±29.1 and 73.9±13.9% compared to control cells,
respectively, P<0.05 (Fig. 5B).
Phalloidin staining was performed to evaluate the cytoskeleton
organization in the presence of TMX and/or TMZ (Fig. 5C).
Discussion
Despite the lack of a successful response,
temozolomide (TMZ) is still considered the gold-standard for GBM
treatment. Currently, alternative treatment options for patients
with TMZ resistant GBMs are desperately needed (26). Tamoxifen (TMX) is an estrogen
receptor (ER) modulator commonly used for the treatment of
ER-positive breast cancer recently considered to have many other
antitumor actions, mainly in the phosphorylated protein kinase C
(PKC) regulation when used in higher concentrations (10,25–27).
Phosphorylated PKC is one of the most enigmatic signaling pathways
that may promote or inhibit apoptosis and cell survival, as
previously described (6). TMX is
well known to be one of p-PKC inhibitors used in in vitro
studies with glioma cells and also in clinical trials with GBM
patients. Besides the in vivo studies were disappointing
since treatment of patients with recurrent malignant glioma with
low doses of TMX did not significantly increase the survival rate
of the patients, the clinical trials using high doses of TMX alone
or in combination with other cytotoxic agents, have yielded better
results (6,28–31).
We recently described the success of TMX and TMZ combination in two
GBM cell lines, U87 and U118. In order to study the heterogeneity
between GBM cells and the variability in the chemotherapeutic
response, similarly to that observed in GBM patients, we stablished
the new GBM cell line GBM11 and compared the mechanisms underlying
chemoresistance and the response to treatment with the two cell
lines previously studied, the U87 and U118 (6).
A tumor sample from GBM was analysed histologically
by the Pathology Service of the Federal University of Rio de
Janeiro Hospital, as described by Faria et al (16). Prior to isolation of the GBM cells
and at the time of the diagnosis, the tumor was classified as GBM
due to the: i) magnetic resonance diagnosis (Fig. 1A); ii) histopathological
characteristics of GBM by staining with H&E in paraffin
sections (Fig. 1B); iii) the
positive GFAP immunostaining, which is currently used for diagnosis
of GBM (Fig. 1C) (32); and iv) existence of a previous GBM
lesion. Subsequently, the tumor cells from the biopsy sample of GBM
were isolated and established in culture. GBM is also characterized
as a heterogeneous tumor that has a subpopulation of glioma
stem-like cells (GSCs), known to be chemo- and radioresistant,
properties that are responsible for tumor recurrence (33–35).
In fact, our GBM cells also expressed stem-like cell markers,
mainly SOX2, OCT-4A and Nanog (Fig.
1D), probably because these tumor stem-like cells have been
selected through the previous TMZ treatment. GBM11 cells could also
grow in an in vivo animal model (data not shown), as
described by Garcia et al (36). This suggests that the glioma
stem-like cells are able to contribute to the tumor growing in
vivo and that the previous treatment with TMZ may have
contributed to the recurrence of a tumor endowed with a higher
number of cancer stem-like cells.
Regarding cell cytotoxicity, we noted an expected
resistance of GBM11 to TMZ treatment (6) (Fig.
1E), similarly to that observed in the U87 and U118 cell lines,
which was in accordance with the low survival rate of GBM patients
treated with TMZ (2,7,8,15).
Regarding cell cytotoxicity of TMX in GBM11 cells we observed a
higher IC50, 25.6 µM, compared, respectively, to 9.1 and
7.3 µM from U87 and U118, previously published (Fig. 1F), which suggests that this new cell
line presents a more resistant behavior when compared to U87 and
U118. The combined treatment of GBM11 with TMX and TMZ induce a
decrease in cell viability until 49.2% using doses below the
IC50 of each drug alone. Similar cytotoxicity was
observed in U87 cells, ~50.0% in cell viability decrease and a
higher one observed in U118 of 90.0% (Fig. 1G). These results are in accordance
with the higher resistance of GBM11 cells and higher sensitivity of
U118 to TMX alone.
After the analysis of the GFAP and Nestin positive
expression and F-actin filament staining, confirming a GBM
phenotype of all three GBM cells (Fig.
2A), we analysed the expression of PGP. PGP is expressed by
endothelial cells in the brain and in the newly formed blood
vessels in glioma. PGP recognizes structurally unrelated
chemotherapeutic agents such as vincristine, etoposide,
doxorubicin, taxol and temozolomide (18,37–40).
In the present study we observed that the PGP expression in GBM11
is significantly higher than compared to U87 and U118. It suggests
that PGP expression is enhanced by previous TMZ treatment and may
explain the more aggressive phenotype of recurrent gliomas. Thus,
we considered that, together with the higher IC50 of TMX
and lower cell viability of TMX plus TMZ treatment, GBM11 could be
used to evaluate the chemoresistance of different therapeutic
agents in future studies (Fig.
2B).
Regarding the mechanisms of chemoresistance of GBM
to TMZ, we evaluated the expression of O6-methylguanine-DNA
methyltransferase (MGMT) (24,41).
Accordingly, analysis of the methylation pattern revealed a total
methylation of the MGMT in GBM11 and U87 cell lines and a partial
methylation in U118 cell line, suggesting that the resistance of
TMZ is probably not related with the MGMT action. In the
U118 case, due to a partial methylation pattern of the MGMT
we could expect a higher resistance to TMZ compared with the other
cell lines (Fig. 2C). Still,
MGMT alone is not always correlated with resistance to TMZ
in GBM, which was apparently the case of these three cell lines
(37,40,41).
Accordingly, since the glioma stem-like cells are known to be
chemo- and radioresistant and so responsible for tumor recurrence
(42–45), the stem-like cell marker expression
evaluation confirmed that the GBM11 could be the more resistant
cell line since it presents higher levels of SOX2, the
overexpression of which has been correlated with poor prognosis in
gliomas, compared to U87 and U118 cell lines (Fig. 2D). In fact SOX2 levels must be
tightly controlled for proper development of the nervous system.
Specifically, deregulation of SOX2 levels in chick neural stem
cells (NSC) has been shown to disrupt their fate (46).
Similarly to the results obtained for the U87 and
U118 cell lines, we observed an induction of cell death (Fig. 3A-C) and an induction of cell cycle
arrest (Fig. 3D) in GBM11 cells
treated with TMX and TMZ, although the TMX alone treatment induce a
similar effect than the combined therapy in this cell line. It may
suggest that for recurrent tumors previously treated with TMZ
accordingly to Stupp protocol, the best choice of second-line
treatment may be only TMX to reduce putative side effects of
combined treatment with TMZ. Also, a reduction in cell
proliferation (Fig. 4A) was
observed in GBM11 cells, probably due to the decreased expression
of p-PKC-pan (Fig. 4B).
We finally observed a reduction of cell migration
(Fig. 5A and B), which could be
explained, not exclusively, but consistently, by the visible
disorganization of F-actin filaments (Fig. 5C).
Altogether, when treated with TMX, our new GBM11
cell line presented a higher IC50 of TMX alone, higher
reduction of cell proliferation, probably due to the reduction of
p-PKC expression, and cell migration capability and a higher
expression of PGP compared to U87 and U118 cell lines previously
described (Table I). The increase
of PGP expression has been correlated with a poor response to
therapy, which may justify the higher resistance of GBM11 cells
(37,39,41,47).
Also, TMX is known to inhibit drug transport since it interacts
with PGP inhibiting PGP-dependent drug transport (47). They may be more resistance also due
to the previous treatment with TMZ, but the overexpression of PGP
and the higher amount of stem-like cells are more likely to be
responsible for the GBM11 resistant profile. In this way, the
concentration of TMX required to induce an effect would be expected
to be higher compared to U87 and U118 cell lines, which is also in
accordance with our results.
 | Table I.Comparison of response of different
GBM cell lines to treatment. |
Table I.
Comparison of response of different
GBM cell lines to treatment.
|
| Cell viability (µM)
(IC50) | p-PKC reduction
(%) | EdU incorporation
reduction (%) |
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
|
| TMX | TMZ | TMX | TMX + TMZ | TMX | TMX + TMZ | Cell cycle
arrest | Cell death (%) | Cell migration
reduction (%) | F-actin filaments
disorganization | MGMT
methylation | PGP expression (%
relative to GBM11) |
|---|
| U87 | 9.1 | – |
39.1±8.9a,d |
56.9±14.8b,d | – | – | G0/G1c–e |
3.8±0.7b,d
(TMX alone) |
41.3±8.3b,d (TMX + TMZ) | – | M | 1.34 |
| U118 | 7.3 | – |
28.9±14.0a,d |
55.2±23.1c,d | – |
68.3±2.3c–e | – |
37.8±7.3c–e (TMX + TMZ) |
25.8±5.4b,d (TMX alone) | # | PM | 0.11 |
| GBM11 | 25.6 | – |
49.3±30.1a,d |
44.3±29.4b,d |
90.7±8.0c,d |
96.2±3.4c,d | G0/G1c,d |
37.5±21.5b,d (TMX alone) |
73.9±13.9a,d (TMX alone) | # | M | 100 |
However, and probably due to the higher resistance
of this new GBM cell line, the combination of TMX and TMZ did not
have a synergistic effect as observed in U87 and U118 cell lines.
In GBM11 the results between combined or monotherapy are the same,
which could represent action in another receptor site of
interaction and would be an alternative chemotherapeutic approach
for GBM. Besides TMX can actually be very important on p-PKC
regulation, and consequently, interfere with proliferation,
survival and migration of glioma cells, the contribution of
chemotherapeutic drugs depends on the GBM cell characteristics. In
the present study, we evaluated the effect of TMX on TMZ-resistant
GBM cell lines, which express different levels of PGP expression
and similar MGMT activity. The MGMT is totally
methylated in the GBM11 and U87 cell lines, which justifies the
chemoresistance of these cells to TMZ. However, in the U118 cell
line we could see only a partial methylation of MGMT, which
could explain some of the TMZ resistance behavior but a better
response of the combined therapy, since TMX could sensitize cells
to TMZ action in this cell line, which is in accordance with our
results (Table I). We found that
TMX alone significantly inhibited the viability of TMZ-resistant
GBM cells with higher expression of PGP, and induced apoptosis of
glioma cells in vitro. In GBM11 we observed no need for TMZ
addition in the chemotherapeutic approach, besides this drug
combination with TMX presented a huge advantage in U87 and U118
cells. This response suggests that TMX alone can be used as a
second-line therapy in patients bearing recurrent tumors previously
treated with TMZ, instead of a combination of TMZ and TMX (48). It also emphasizes the heterogeneity
response between GBM cells, which reflects the same variability
between different GBM, highlighting the urgent need of the
establishment of a personalized therapy (6,14,15).
Our results propose TMX alone treatment as a
successful approach in GBM cells that have previously been treated
according to the Stupp protocol (12). Since the TMX and TMZ constitutes a
beneficial combination for U87 and U118 cells, and since TMZ is
still part of the gold-standard for GBM treatment, the combination
constitutes a therapeutic approach that still reaches a wide range
of GBM cells, especially those that still have not been exposed to
TMZ. Furthermore, these results open new avenues to recognize
different cell responses according to tumor heterogeneity that
together might evidence functional differences between gliomas
entities.
Acknowledgements
The present study was financed by FEDER funds
through the Operational Programme Factors Competitiveness-COMPETE
and National Funds through FCT-Foundation for Science and
Technology under the project ‘National funds from FCT’ of the
Fundação para a Ciência e Tecnologia, under a Ph.D. fellowship to
Joana Balça Pinheiro da Costa e Silva (SFRH/BD/51993/2012); and by
the project PEst-C/SAU/LA0001/2013-2014. Additional funding was
granted by FEDER/COMPETE/FCT PTDC/EBB-EBI/120634/2010 and
PDTC/QUI-BIQ/120652/2010 and QREN: CENTRO-01-0762-FEDER-00204. This
study was also supported by the Conselho Nacional de
Desenvolvimento Científico e Tecnológico (CNPq), Fundação Carlos
Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
(FAPERJ), and Instituto Estadual do Cérebro Paulo Niemeyer (IECPN)
and Pró-Saúde Associação Beneficente de Assistência Social e
Hospitalar, Rio de Janeiro, Brazil.
Glossary
Abbreviations
Abbreviations:
|
GBM
|
glioblastoma
|
|
GSCs
|
glioma stem-like cells
|
|
TMX
|
tamoxifen
|
|
TMZ
|
temozolomide
|
|
PGP
|
P-glycoprotein
|
|
PKC
|
protein kinase C
|
References
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Hegi ME, Gilbert MR and
Chakravarti A: Chemoradiotherapy in malignant glioma: Standard of
care and future directions. J Clin Oncol. 25:4127–4136. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: European Organisation for Research and Treatment of
Cancer Brain Tumor and Radiotherapy Groups; National Cancer
Institute of Canada Clinical Trials Group: Radiotherapy plus
concomitant and adjuvant temozolomide for glioblastoma. N Engl J
Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lima FRS, Kahn SA, Soletti RC, Biasoli D,
Alves T, da Fonseca AC, Garcia C, Romão L, Brito J, Holanda-Afonso
R, et al: Glioblastoma: Therapeutic challenges, what lies ahead.
Biochim Biophys Acta. 1826:338–349. 2012.PubMed/NCBI
|
|
5
|
Huse JT, Holland E and DeAngelis LM:
Glioblastoma: Molecular analysis and clinical implications. Annu
Rev Med. 64:59–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balça-Silva J, Matias D, do Carmo A, Girão
H, Moura-Neto V, Sarmento-Ribeiro AB and Lopes MC: Tamoxifen in
combination with temozolomide induce a synergistic inhibition of
PKC-pan in GBM cell lines. Biochim Biophys Acta. 1850:722–732.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carmo A, Carvalheiro H, Crespo I, Nunes I
and Lopes MC: Effect of temozolomide on the U-118 glioma cell line.
Oncol Lett. 2:1165–1170. 2011.PubMed/NCBI
|
|
8
|
do Carmo A, Patricio I, Cruz MT,
Carvalheiro H, Oliveira CR and Lopes MC: CXCL12/CXCR4 promotes
motility and proliferation of glioma cells. Cancer Biol Ther.
9:56–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hattermann K and Mentlein R: An infernal
trio: The chemokine CXCL12 and its receptors CXCR4 and CXCR7 in
tumor biology. Ann Anat. 195:103–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou J, Atsina KB, Himes BT, Strohbehn GW
and Saltzman WM: Novel delivery strategies for glioblastoma. Cancer
J. 18:89–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Safa AR, Saadatzadeh MR, Cohen-Gadol AA,
Pollok KE and Bijangi-Vishehsaraei K: Glioblastoma stem cells
(GSCs) epigenetic plasticity and interconversion between
differentiated non-GSCs and GSCs. Genes Dis. 2:152–163. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: European Organisation for Research and Treatment
of Cancer Brain Tumour and Radiation Oncology Groups; National
Cancer Institute of Canada Clinical Trials Group: Effects of
radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thirant C, Bessette B, Varlet P, Puget S,
Cadusseau J, Tavares SR, Studler JM, Silvestre DC, Susini A, Villa
C, et al: Clinical relevance of tumor cells with stem-like
properties in pediatric brain tumors. PLoS One. 6:e163752011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bogush T, Dudko E, Bogush E, Polotsky B,
Tjulandin S and Davydov M: Tamoxifen non-estrogen receptor mediated
molecular targets. Oncol Rev. 6:e152012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kahn SA, Biasoli D, Garcia C, Geraldo LH,
Pontes B, Sobrinho M, Frauches AC, Romão L, Soletti RC, Assunção
FS, et al: Equinatoxin II potentiates temozolomide- and
etoposide-induced glioblastoma cell death. Curr Top Med Chem.
12:2082–2093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Faria J, Romão L, Martins S, Alves T,
Mendes FA, de Faria GP, Hollanda R, Takiya C, Chimelli L, Morandi
V, et al: Interactive properties of human glioblastoma cells with
brain neurons in culture and neuronal modulation of glial laminin
organization. Differentiation. 74:562–572. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Linn SC, Giaccone G, van Diest PJ,
Blokhuis WM, van der Valk P, van Kalken CK, Kuiper CM, Pinedo HM
and Baak JP: Prognostic relevance of P-glycoprotein expression in
breast cancer. Ann Oncol. 6:679–685. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calatozzolo C, Gelati M, Ciusani E,
Sciacca FL, Pollo B, Cajola L, Marras C, Silvani A,
Vitellaro-Zuccarello L, Croci D, et al: Expression of drug
resistance proteins Pgp, MRP1, MRP3, MRP5 and GST-π in human
glioma. J Neurooncol. 74:113–121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gonçalves AC, Cortesão E, Oliveiros B,
Alves V, Espadana AI, Rito L, Magalhães E, Lobão MJ, Pereira A,
Costa JM Nascimento, et al: Oxidative stress and mitochondrial
dysfunction play a role in myelodysplastic syndrome development,
diagnosis, and prognosis: A pilot study. Free Radic Res.
49:1081–1094. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Towbin H, Staehelin T and Gordon J:
Immunoblotting in the clinical laboratory. J Clin Chem Clin
Biochem. 27:495–501. 1989.PubMed/NCBI
|
|
21
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rittierodt M and Harada K: Repetitive
doxorubicin treatment of glioblastoma enhances the PGP expression -
a special role for endothelial cells. Exp Toxicol Pathol. 55:39–44.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Borowski E, Bontemps-Gracz MM and
Piwkowska A: Strategies for overcoming ABC-transporters-mediated
multidrug resistance (MDR) of tumor cells. Acta Biochim Pol.
52:609–627. 2005.PubMed/NCBI
|
|
24
|
Qiu ZK, Shen D, Chen YS, Yang QY, Guo CC,
Feng BH and Chen ZP: Enhanced MGMT expression contributes to
temozolomide resistance in glioma stem-like cells. Chin J Cancer.
33:115–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hegi ME, Diserens A-C, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He W, Liu R, Yang SH and Yuan F:
Chemotherapeutic effect of tamoxifen on temozolomide-resistant
gliomas. Anticancer Drugs. 26:293–300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Couldwell WT, Hinton DR, He S, Chen TC,
Sebat I, Weiss MH and Law RE: Protein kinase C inhibitors induce
apoptosis in human malignant glioma cell lines. FEBS Lett.
345:43–46. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang W, Couldwell WT, Song H, Takano T,
Lin JH and Nedergaard M: Tamoxifen-induced enhancement of calcium
signaling in glioma and MCF-7 breast cancer cells. Cancer Res.
60:5395–5400. 2000.PubMed/NCBI
|
|
29
|
OBrian CA, Liskamp RM, Solomon DH and
Weinstein IB: Inhibition of protein kinase C by tamoxifen. Cancer
Res. 45:2462–2465. 1985.PubMed/NCBI
|
|
30
|
Kamburoğlu G, Kiratli H, Söylemezoğlu F
and Bilgiç S: Clinicopathological parameters and expression of
P-glycoprotein and MRP-1 in retinoblastoma. Ophthalmic Res.
39:191–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hui AM, Zhang W, Chen W, Xi D, Purow B,
Friedman GC and Fine HA: Agents with selective estrogen receptor
(ER) modulator activity induce apoptosis in vitro and in vivo in
ER-negative glioma cells. Cancer Res. 64:9115–9123. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deck JH, Eng LF, Bigbee J and Woodcock SM:
The role of glial fibrillary acidic protein in the diagnosis of
central nervous system tumors. Acta Neuropathol. 42:183–190. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Altaner C and Altanerova V: Stem cell
based glioblastoma gene therapy. Neoplasma. 59:756–760. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jackson M, Hassiotou F and Nowak A:
Glioblastoma stem-like cells: At the root of tumor recurrence and a
therapeutic target. Carcinogenesis. 36:177–185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meacham CE and Morrison SJ: Tumour
heterogeneity and cancer cell plasticity. Nature. 501:328–337.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Garcia C, Dubois LG, Xavier AL, Geraldo
LH, da Fonseca AC, Correia AH, Meirelles F, Ventura G, Romão L,
Canedo NH, et al: The orthotopic xenotransplant of human
glioblastoma successfully recapitulates
glioblastoma-microenvironment interactions in a
non-immunosuppressed mouse model. BMC Cancer. 14:9232014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tóth K, Vaughan MM, Peress NS, Slocum HK
and Rustum YM: MDR1 P-glycoprotein is expressed by endothelial
cells of newly formed capillaries in human gliomas but is not
expressed in the neovasculature of other primary tumors. Am J
Pathol. 149:853–858. 1996.PubMed/NCBI
|
|
38
|
Sun H, Dai H, Shaik N and Elmquist WF:
Drug efflux transporters in the CNS. Adv Drug Deliv Rev. 55:83–105.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cordon-Cardo C, OBrien JP, Casals D,
Rittman-Grauer L, Biedler JL, Melamed MR and Bertino JR:
Multidrug-resistance gene (P-glycoprotein) is expressed by
endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci
USA. 86:695–698. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schaich M, Kestel L, Pfirrmann M, Robel K,
Illmer T, Kramer M, Dill C, Ehninger G, Schackert G and Krex D: A
MDR1 (ABCB1) gene single nucleotide polymorphism predicts
outcome of temozolomide treatment in glioblastoma patients. Ann
Oncol. 20:175–181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tomaszowski KH, Schirrmacher R and Kaina
B: Multidrug efflux pumps attenuate the effect of MGMT inhibitors.
Mol Pharm. 12:3924–3934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Diaz A and Leon K: Therapeutic approaches
to target cancer stem cells. Cancers (Basel). 3:3331–3352. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ortensi B, Setti M, Osti D and Pelicci G:
Cancer stem cell contribution to glioblastoma invasiveness. Stem
Cell Res Ther. 4:182013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Seymour T, Nowak A and Kakulas F:
Targeting aggressive cancer stem cells in glioblastoma. Front
Oncol. 5:1592015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cox JL, Wilder PJ, Desler M and Rizzino A:
Elevating SOX2 levels deleteriously affects the growth of
medulloblastoma and glioblastoma cells. PLoS One. 7:e440872012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Callaghan R and Higgins C: Interaction of
tamoxifen with the multidrug resistance P-glycoprotein. Br J
Cancer. 71:294–299. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Di Cristofori A, Carrabba G, Lanfranchi G,
Menghetti C, Rampini P and Caroli M: Continuous tamoxifen and
dose-dense temozolomide in recurrent glioblastoma. Anticancer Res.
33:3383–3389. 2013.PubMed/NCBI
|