Introduction
Non-small cell lung cancer (NSCLC) is the leading
cause of cancer-related mortality worldwide (1). New treatments for advanced and
recurrent NSCLC have been introduced, including so-called third
genetation chemotherapeutic agents such as docetaxel, paclitaxel,
vinorelbin, and CPT-11, which have contibuted to improving patient
prognosis (2). Other therapeutic
developments including molecular-targeted therapies for driver
oncogenes, such as a tyrosine kinase inhibitor targeting mutations
of the epidermal growth factor receptor gene and an inhibitor
targeting translocations of the anaplastic lymphoma kinase gene,
have improved the survival of patients with advanced and recurrent
NSCLC over the past decade (3–5).
Recently, a new therapeutic strategy, cancer immunotherapy, has
emerged to confer clinical benefits for patients.
In some types of cancer immunotherapy, immune
checkpoint inhibitors targeting the programmed cell death
1-programmed cell death-ligand 1 (PD-1-PD-L1) axis, exerted a
significant durable clinical response for advanced NSCLC (6–9).
Several clinical trials revealed that an objective response
(including complete and partial responses) was observed in
10.2–18.4% of NSCLC patients (6,9). The
immune checkpoint molecule, PD-L1, located on tumor cells, binds to
PD-1 on cytotoxic T lymphocytes (CTLs) which attack tumor cells,
and induces apoptosis and exhaust CTLs (10). Inhibiting the interaction between
PD-1 and PD-L1 prevents CTLs from unresponsiveness, and maintains
their cytotoxic activity against tumor cells.
The PD-1-PD-L1 axis is one of the immune check point
systems of tumor cells, but the mechanism of PD-L1 expression on
tumor cells remains to be fully elucidated. We previously examined
PD-L1 expression in pulmonary adenocarcinomas using
immunohistochemistry, and reported the heterogeneous distribution
of tumor cells with various levels of PD-L1 expression in a tumor
tissue section (11). These data
suggested that PD-L1 expression on NSCLC cells may be influenced by
environmental factors. PD-L1 gene expression in human peripheral
blood monocytes is upregulated by stimulation with interferon-γ
(IFN-γ) (12). IFN-γ also induces
PD-L1 expression on microvascular endothelial cells (13), while PD-L1 expression on tumor cells
is upregulated by IFN-γ secreted from CD8+ T lymphocytes
in the tumor-immune cell interaction (14,15).
This indicates that PD-L1 expression on NSCLC cells might also be
upregulated by IFN-γ released from CTLs following NSCLC cell
recognition.
For recognition by CTLs, NSCLC cells must express
the major histocompatibility complex (MHC) class I molecule, which
interact with T cell receptors (16). However, tumor cells often delete MHC
class I molecule expression to evade CTL immune surveillance in a
process known as immune editing (17). Indeed, 24.3–80.5% of tumor cells in
NSCLC tumor tissue were reported to delete or downregulate the
expression of MHC class I molecule (18–22).
Such NSCLC cells are less likely to be recognized by CTLs, or to be
exposed to IFN-γ released by CTLs; therefore, they are unlikely to
express PD-L1. In this context, we hypothesized that the de
novo expression of PD-L1 on NSCLC cells might be associated
with CTL infiltration into tumor tissue and the expression of MHC
class I molecules on NSCLC cells.
In this study, we focused on the interaction between
NSCLC cells and CD8+ TILs in a tumor microenvironment,
and aimed to clarify the mechanism of de novo PD-L1
expression on squamous cell carcinomas of the lung. The data
presented here suggest that de novo PD-L1 expression on
tumor cells is upregulated by IFN-γ secreted from CD8+
TILs following the recognition of tumor cells with MHC class I
molecules.
Materials and methods
Cells
Human pulmonary squamous cell carcinoma cell lines
LK-2 and EBC-1 (obtained from the Japanese Collection of Research
Bioresource, Tokyo, Japan) were maintained in a humidified
atmosphere of 5% CO2 at 37°C. RPMI-1640 medium was used
for LK-2 culture, and EBC-1 cells were cultured in Dulbecco's
modified Eagle's medium, each supplemented with 10% fetal bovine
serum.
Flow cytometry
To examine the dose-dependent effect of IFN-γ on
surface expression of PD-L1 on tumor cells, the cells were cultured
in medium containing 0, 31.25, 125 or 500 pg/ml recombinant human
IFN-γ (PeproTech, Rocky Hill, NJ, USA) for 48 h. To examine the
reversibility of IFN-γ-induced PD-L1 expression, the cells were
cultured in the medium containing 1.0 ng/ml IFN-γ for 48 h. The
culture medium was then replaced by fresh medium without IFN-γ, and
cell culture was continued for an additional 48 h. Those cells were
then harvested and stained with a phycoerythrin (PE)-labeled
anti-human PD-L1 antibody (clone: MIH1) (BD Biosciences, San Jose,
CA, USA) or a mouse IgG1, κ isotype control (BD Biosciences). To
examine IFN-γ receptor 1 expression on tumor cells, the cells were
stained with a PE-labeled anti-human IFN-γ receptor 1 (CD119)
antibody (clone: GIR-208) (eBioscience). Flow cytometric analysis
was performed using BD FACSCalibur, and the data were analyzed
using BD CellQuest Pro software (BD Biosciences).
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was extracted from tumor cells using
RNeasy mini kit (Qiagen, Hilden, Germany), and was subjected to the
reverse transcription reaction using cloned AMV first-strand cDNA
synthesis kit (Life Technologies, Carlsbad, CA, USA). Each cDNA was
amplified with specific paired primers as follows: PD-L1 forward,
5′-GAGCCTCCAAGCAAATCATC-3′; reverse, 5′-GCAACCAACGGTTTGATCTT-3′.
GAPDH forward, 5′-TGGAAGGACTCATGACCACA-3; reverse,
5′-CCCTGTTGCTGTAGCCAAAT-3′.
Tumor samples
Tumor samples were obtained from surgically resected
specimens of patients with squamous cell carcinoma of the lung at
Shiga University of Medical Science Hospital, between January 2008
and December 2012. Patient clinicopathological data were obtained
from medical records. The study design was approved by the Ethics
Committee of Shiga University of Medical Science (no. 25-225), and
a written informed consent was obtained from all the patients.
Immunohistochemistry
Serial sections (4-µm-thick) of formalin-fixed
paraffin-embedded tissue specimens were stained by standard
indirect immunoperoxidase procedures for PD-L1, CD8, and MHC class
I molecules, according to the manufacturer's protocols. Briefly,
each tissue section was deparaffinized in xylene, and rehydrated in
ethanol and distilled water. Antigen retrieval was performed by
microwave treatment in 10 mM sodium citrate buffer (pH 6.0) for
PD-L1 or 10 mM Tris/1 mM ethyleneaminetetraacetic acid (pH 9.0) for
CD8 and MHC class I molecules for 10 min. Endogenous peroxidase
activity was blocked by treatment with 3%
H2O2 for 10 min. After blocking with 5%
normal goat serum in Tris-buffered saline with Tween-20 for 1 h at
room temperature, the sections were incubated overnight with an
anti-human PD-L1 monoclonal antibody (clone: E1L3N, diluted at
1:200) (Cell Signaling Technology, Danvers, MA, USA), an anti-human
CD8 antibody (clone: CD8/144B, diluted at 1:200) (Dako,
Carpinteria, CA, USA), or an anti-HLA class I molecule antibody
(clone: EMR8-5, diluted at 1:500) (Hokudo, Sapporo, Japan) at 4°C
overnight. The sections were then incubated with SignalStain boost
IHC detection reagent (Cell Signaling Technology) for PD-L1 or
Envision Dako ChemMate for CD8 and MHC class I molecules. They were
visualized using the SignalStain DAB substrate kit (Cell Signaling
Technology) for PD-L1 or Envision Dako ChemMate/HRP (DAB) for CD8
and MHC class I molecules for 1 min, followed by counterstaining
with hematoxylin. As an isotype-matched control antibody, rabbit
IgG monoclonal antibody (Cell Signaling Technology) was used for
PD-L1 immunohistochemistry, and mouse IgG monoclonal antibody
(Dako) were used for MHC class I and CD8 immunohistochemistry. In
PD-L1 immunohistochemistry, paraffin-embedded cell-blocks of
PD-L1-positive H-1975 tumor cells and PD-L1-negative A549 tumor
cells were utilized for positive and negative controls,
respectively, as described in a previous report (11).
Evaluation of PD-L1, CD8, and MHC
class I expression
Serial sections of stained tumor tissue were
independently examined by two researchers, including a pathologist.
To compare the staining intensities of PD-L1, CD8, and MHC class I
molecules on each tumor cell, the cells which commonly existed in
the serial section were evaluated. Under ×200 magnification, three
representative fields of view were selected, and 30 tumor cells per
field were observed for the expression of PD-L1 and MHC class I
molecules. If the frequency of PD-L1-positive tumor cells was
>50%, we described it as a PD-L1+ tumor cell-dominant
case. If the frequency of MHC class I molecule-positive tumor cells
was >80%, we described it as an MHC class I+ tumor
cell-dominant case. For an evaluation of TILs, the number of
CD8-positive lymphocytes was counted in the fields.
Statistical analysis
The statistical analysis between groups was
determined by Chi-square test, P-values <0.05 were considered
statistically significant. All analyses were performed using SPSS
Statistics 22.0 software (IBM, Armonk, NY, USA).
Results
Patient characteristics
In total, 77 patients with squamous cell carcinomas
of the lung were included in this study (Table I). The median patient age at the
time of surgery was 71 years (range, 47–87 years). The patients
consisted of 72 males (93.5%) and five females (6.5%); 74 patients
(96.1%) had a smoking habit. Postoperative pathological stages of
squamous cell carcinoma of the lung were IA in 23 cases (29.8%), IB
in 13 (16.9%), IIA in 11 (14.3%), IIB in 13 (16.9%), IIIA in 14
(18.2%), IIIB in one (1.3%), and IV in two (2.6%). Thirty-three
patients (42.9%) received adjuvant chemotherapy for approximately
one month after surgery.
 | Table I.The patient characteristics
(n=77). |
Table I.
The patient characteristics
(n=77).
| Median age
(range) | 71 (47–87) |
| Sex, n (%) |
|
|
Male | 72 (93.5) |
|
Female | 5
(6.5) |
| Smoking status, n
(%) |
|
|
Current/former | 74 (96.1) |
|
Never | 3
(0.9) |
| Pathological stage,
n (%) |
|
| IA | 23 (29.8) |
| IB | 13 (16.9) |
|
IIA | 11 (14.3) |
|
IIB | 13 (16.9) |
|
IIIA | 14 (18.2) |
|
IIIB | 1
(1.3) |
| IV | 2
(2.6) |
Effect of IFN-γ on PD-L1 expression in
pulmonary squamous carcinoma cells
Several studies have previously reported that IFN-γ
induces PD-L1 expression in human cancer cell lines in
vitro, such as pulmonary adenocarcinomas (A549 cells) (23), hepatocytes (HEPG2 cells) (24), and ovarian cancer (SK-OV-3, ovary
1847, and OVCAR8 cells) (25). We
examined the effect of IFN-γ on PD-L1 expression in the pulmonary
squamous cell carcinoma cell lines LK2, and EBC-1. Flow cytometry
demonstrated that PD-L1 expression on those cell lines were
upregulated by IFN-γ exposure in a dose-dependent manner (Fig. 1A). Furthermore, the PD-L1 expression
induced by IFN-γ was downregulated by depletion of IFN-γ from the
culture medium (Fig. 1B),
demonstrating that PD-L1 expression on the tumor cells was
reversible. Next, we examined whether levels of PD-L1 mRNA
were affected by IFN-γ. The levels of PD-L1 mRNA in those cell
lines were also positively and reversibly regulated by IFN-γ
(Fig. 1C). In addition, we
evaluated IFN-γ receptor expression on those tumor cells. Flow
cytometry revealed that expression intensity of IFN-γ receptor 1
was higher in LK2 than EBC-1 (Fig.
1D). The mean fluorescence intensity of PD-L1-expressed tumor
cells increased 1.7 and 2.0 times by IFN-γ in LK2 and EBC1,
respectively (Fig. 1B). In the
RT-PCR, signal intensity of PD-L1 mRNA increased 1.4 and 1.8
times by IFN-γ in LK2 and EBC1, respectively. We detected the
expression of IFN-γ receptor 1 on these cells, however these levels
were less likely to affect the level of PD-L1 expression induced by
IFN-γ. These data demonstrate that PD-L1 expression in pulmonary
squamous carcinoma cells is reversibly regulated by IFN-γ.
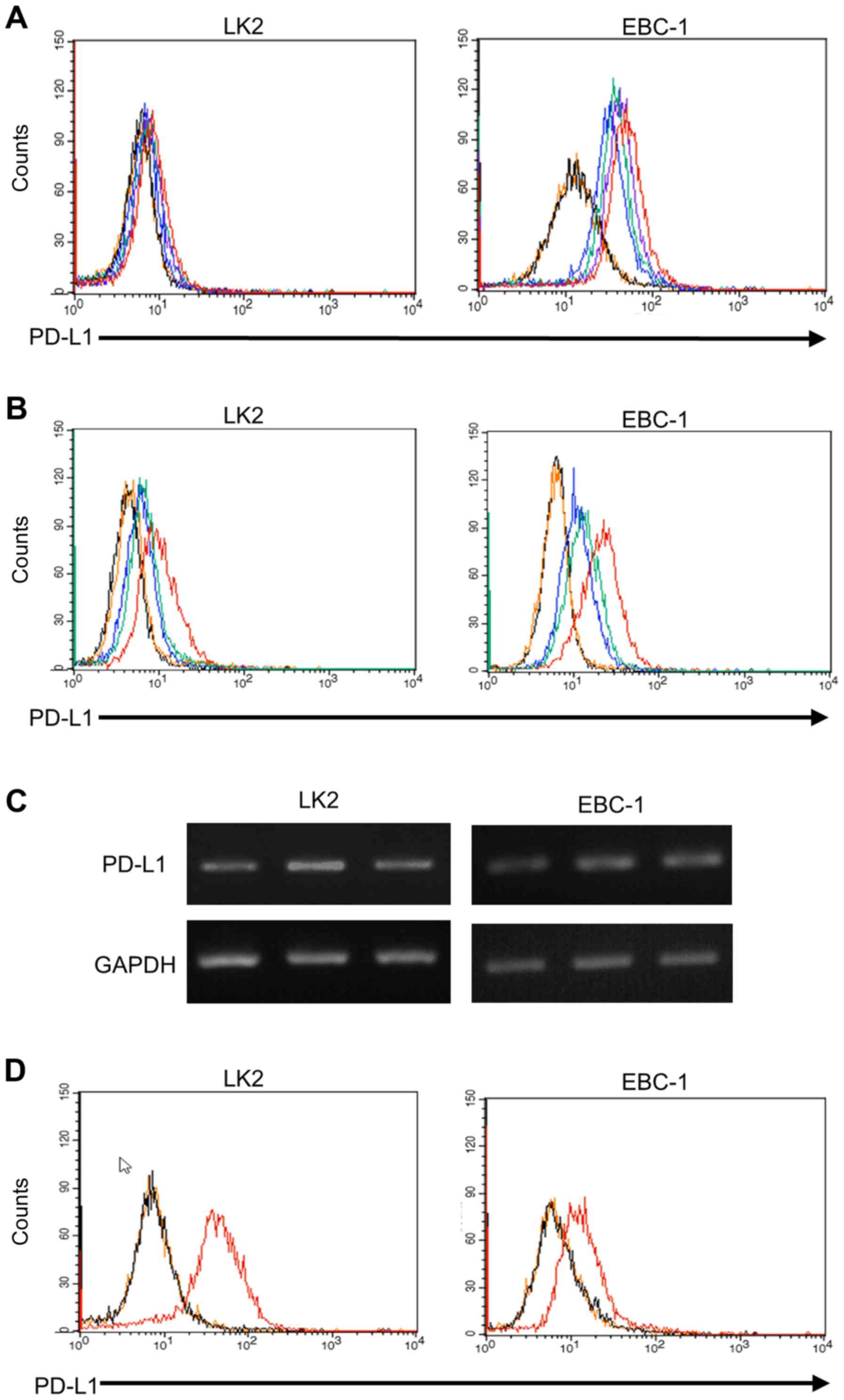 | Figure 1.Flow cytometric analysis of PD-L1
expression induced in pulmonary squamous cell carcinomas. (A) PD-L1
expression was upregulated in LK2 and EBC-1 cells following IFN-γ
exposure in a dose-depending manner (blue line, 0 pg/ml; green
line, 31.25 pg/ml; purple line, 125 pg/ml; red line, 500 pg/ml
IFN-γ; black line, non-staining; orange line, isotype control). (B)
PD-L1 expression was upregulated in LK2 and EBC-1 cells following
IFN-γ exposure for 48 h (red line), and was downregulated by IFN-γ
depletion (green line). Blue line, spontaneous level of PD-L1
expression; black line, non-staining; orange line, isotype control.
(C) The mRNA level of PD-L1 in LK2 and EBC-1 cells examined by
RT-PCR. Left lane, no treatments; middle lane, IFN-γ exposure for
48 h; right lane, IFN-γ depletion for 48 h after IFN-γ exposure.
(D) Red line, IFN-γ receptor 1 expression on LK2 and EBC-1 cells;
black line, non-staining; orange line, isotype control. |
Expression of PD-L1 and MHC class I
molecules in squamous cell carcinoma of the lung
TILs appear to be the main source of IFN-γ in the
tumor microenvironment, and their secretion of IFN-γ occurs after
recognition of MHC class I molecule expression by tumor cells
(26). On the basis of this
finding, we examined the correlation between the expression of
PD-L1 and MHC class I molecules by tumor cells (Fig. 2). The median frequency of
PD-L1+ tumor cells was 55.1% (range, 0.0–98.1%), and 45
PD-L1+ tumor cell-dominant cases (58.4%) were included
in the study. The median frequency of MHC class I molecule-positive
tumor cells was 88.7% (range, 8.8–100%), and 72 MHC class
I+ tumor cell-dominant cases (93.5%) were included in
the study. In MHC class I+ tumor cell-dominant cases
(n=72), the number of PD-L1+ and PD-L1− tumor
cell-dominant cases was 45 (62.5%) and 27 (37.5%), respectively.
The frequency of PD-L1+ tumor cell-dominant cases was
significantly higher in MHC class I+ tumor cell-dominant
cases compared with MHC class I− tumor cell-dominant
cases (45/72, 62.5% vs. 0/5, 0%, P=0.006) (Fig. 3). In contrast, in MHC class
I− tumor cell-dominant cases (n=5), no PD-L1+
tumor cell-dominant case was observed, and all cases were
PD-L1− tumor cell-dominant (Fig. 2). These data suggest that the
expression of MHC class I molecule is needed for the de novo
expression of PD-L1 on tumor cells.
Correlation between PD-L1 expression
and CD8+ TILs in squamous cell carcinomas of the
lung
Even in tumor cells expressing MHC class I molecule,
a few CTLs are unlikely to contribute to the expression of PD-L1.
In this context, we examined the correlation between PD-L1
expression on tumor cells and CD8+ TILs in MHC class
I+-dominant cases (Fig. 4A
and B). We observed median numbers of CD8+ TILs of
84.5 for PD-L1+ tumor cell-dominant cases (range,
3.3–569.7), and 73.7 for PD-L1− tumor cell-dominant
cases (range, 40.7–135.0). Significantly higher numbers of
CD8+ TILs were shown to migrate to the tumor tissues of
PD-L1+ tumor cell-dominant cases compared with
PD-L1− tumor cell-dominant cases (P=0.005) (Fig. 4C). These data suggest that the
interaction between MHC class I-expressing tumor cells and abundant
CD8+ TILs contributes to the de novo expression
of PD-L1 on tumor cells.
Discussion
In this study, we present the potential mechanism of
de novo PD-L1 expression in squamous cell carcinomas of the
lung. PD-L1 expression is reversibly regulated by IFN-γ in
vitro, and in a tumor microenvironment, it is associated with
the expression of MHC class I molecule on tumor cells and the
number of CD8+ TILs. These data suggest that
CD8+ TILs secrete IFN-γ into the tumor microenvironment
following an interaction with MHC class I-positive tumor cells,
leading to the upregulation of PD-L1 expression on tumor cells.
Tumor cells have been reported to acquire mechanisms
to evade the immune surveillance system of antitumor immune cell
types, leading to tumor progression (27). Among these immune escape systems,
the deletion of MHC class I molecules on tumor cells prevents CTLs
from recognizing them through the interaction with T cell receptors
(17). In the present study, tumor
cells with deleted MHC class I molecule ranged in frequency from
8.8 to 100% (median, 88.7%), and the frequency of cases in which
MHC class I molecule was expressed on almost all tumor cells was
found to be 66.2% (52/77). In five cases (6.5%), tumor cells with
deleted MHC class I molecule dominated the tumor tissue. In these
cases, CTLs activated following immunotherapy would exert no
cytotoxic effect on tumor cells because of few chances of
recognition. Therefore, a case in which the frequency of tumor
cells with deleted MHC class I molecule is low would be a good
candidate for immune checkpoint inhibitors.
IFN-γ is the most potent factor for PD-L1 expression
in a tumor microenvironment (28,29).
After IFN-γ binding to its receptor on tumor cells, it induces
signal transducer and activator of transcription 1 activation via
the mitogen activated protein kinase/extracellular signal-regulated
kinase pathway (30). In the
present study, flow cytometry revealed that PD-L1 expression on
tumor cells was upregulated following IFN-γ exposure for 48 h in a
dose-dependent manner. Interestingly, PD-L1 expression was
downregulated 48 h after the removal of IFN-γ from culture medium,
demonstrating that the PD-L1 response to IFN-γ was reversibly
regulated. These data suggest that the PD-L1 status of tumor cells
is affected by the level of IFN-γ in the tumor microenvironment,
and is constantly changing. Previously, we and others reported
heterogeneity in levels of PD-L1 expression between NSCLC cells
within a section of tumor tissue (11,31,32).
We propose that this reflects the distinct levels of IFN-γ in the
tumor microenvironment which affect PD-L1 expression.
When considering the cell type of the main source of
IFN-γ secretion into the tumor microenvironment, IFN-γ is usually
released from CTLs following tumor cell recognition (26); thus, IFN-γ secreted by CTLs might
affect PD-L1 expression on NSCLC cells. Given that MHC class I
molecule on tumor cells are required for their recognition by CTLs,
we examined the association between expression of PD-L1 and that of
MHC class I molecule on NSCLC cells. In all cases where NSCLC cells
with deleted MHC class I molecules were dominant (n=5), PD-L1
expression was significantly downregulated. This is to be expected
because such NSCLC cells are not recognized by CTLs, so do not
receive stimulation by IFN-γ released from CTLs, ultimately leading
to PD-L1 downregulation.
We also examined the association between expression
of PD-L1 and that of MHC class I molecule in cases where NSCLC
cells expressing MHC class I molecule were dominant. We showed that
higher PD-L1 expression on NSCLC cells was significantly associated
with an increased number of CD8+ TILs. These data
suggest that PD-L1 expression cannot be upregulated without
sufficient stimulation from IFN-γ released by CTLs, even in NSCLC
cells expressing MHC class I molecule. However, several factors or
conditions, such as enhanced PD-L1 expression by some
altered signals derived from epidermal growth factor receptor
(EGFR) mutation (33),
KRAS mutation (34), and
echinoderm microtubule-associated protein-like 4 (EML4) -
anaplastic lymphoma kinase (ALK) rearrangement (35), may be additively regulating the
PD-L1 status of tumor cells. The data presented in Fig. 4C also suggest that other mechanisms
than IFN-γ-induced de novo PD-L1 expression may be involved
in PD-L1 status of tumor cells. Although the level of IFN-γ in the
tumor microenvironment should be investigated further, de
novo PD-L1 expression in NSCLC cells expressing MHC class I
molecule seems to be upregulated by IFN-γ released from
CD8+ TILs following NSCLC cell recognition.
In the treatment of NSCLCs by immune checkpoint
inhibitors such as anti-PD-L1/PD-1 antibodies, predictive
biomarkers of a clinical response have yet to be established.
Several studies have suggested that immune checkpoint inhibitors
could be possible biomarkers. In PD-L1 inhibition, a clinical
response was associated with PD-L1 expression on tumor-infiltrating
immune cells such as macrophages, dendritic cells, and T cells
(36,37). Moreover, in anti-PD-1 therapy, an
association between the therapeutic response and PD-L1 expression
on tumor cells was reported (38).
On the basis of the present data, we propose that the status of MHC
class I molecule expression on NSCLC cells could be used as a
predictive biomarker for immune checkpoint inhibitors as well as
other types of cancer immunotherapies.
Even if antitumor immune responses are improved by
immunotherapy, NSCLC cells with deleted MHC class I molecule would
not be attacked by an improved CTL response. Therefore, the status
of MHC class I molecule expression on NSCLC cells should be
determined prior to immunotherapy. Because we suggest that both the
expression of MHC class I molecule on NSCLC cells and a sufficient
number of CD8+ TILs are required for the de novo
expression of PD-L1 on tumor cells, many CD8+
lymphocytes will infiltrate the tumor tissue when it is dominated
by PD-L1-positive tumor cells. In such cases, a PD-L1/PD-1 blockade
would be an efficient means of improving the antitumor immune
response.
In conclusion, PD-L1 expression in pulmonary
squamous cell carcinomas appears to be reversibly regulated by
IFN-γ in vitro. In cases of squamous cell carcinomas of the
lung in which MHC class I molecule-positive tumor cells are
dominant, the high frequency of PD-L1-positive tumor cells is
associated with high numbers of CD8+ TILs. This suggests
that the de novo expression of PD-L1 on MHC class I
molecule-positive tumor cells is upregulated by IFN-γ secreted by
CD8+ TILs after tumor cell recognition via MHC class I
molecules.
Acknowledgements
This study was supported in part by Grant-in-Aid for
Scientific Research (C) (16716415, 17904205), Grant-in-Aid for
Scientific Research (B) and Grant-in-Aid for Scientific Research on
Innovative Areas from The Japan Society for the Promotion of
Science (JSPS KAKENHI grant nos. JP: 15H04761 and 16H06277). Y.D.
and K.T. are members of Shiga Cancer Treatment Project supported by
Shiga Prefecture (Japan). The authors thank the staff at the
Central Research Laboratory in Shiga University of Medical Science
for their technical support.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baggstrom MQ, Stinchcombe TE, Fried DB,
Poole C, Hensing TA and Socinski MA: Third-generation chemotherapy
agents in the treatment of advanced non-small cell lung cancer: A
meta-analysis. J Thorac Oncol. 2:845–853. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seto T, Kiura K, Nishio M, Nakagawa K,
Maemondo M, Inoue A, Hida T, Yamamoto N, Yoshioka H, Harada M, et
al: CH5424802 (RO5424802) for patients with ALK-rearranged advanced
non-small-cell lung cancer (AF-001JP study): A single-arm,
open-label, phase 1–2 study. Lancet Oncol. 14:590–598. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
PROFILE 1014 Investigators: First-line crizotinib versus
chemotherapy in ALK-positive lung cancer. N Engl J Med.
371:2167–2177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus Docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Igarashi T, Teramoto K, Ishida M, Hanaoka
J and Daigo Y: Scoring of PD-L1 expression intensity on pulmonary
adenocarcinomas and the correlations with clinicopathological
factors. ESMO Open. 1:e0000832016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eppihimer MJ, Gunn J, Freeman GJ,
Greenfield EA, Chernova T, Erickson J and Leonard JP: Expression
and regulation of the PD-L1 immunoinhibitory molecule on
microvascular endothelial cells. Microcirculation. 9:133–145. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spranger S, Spaapen RM, Zha Y, Williams J,
Meng Y, Ha TT and Gajewski TF: Up-regulation of PD-L1, IDO, and
T(regs) in the melanoma tumor microenvironment is driven by CD8(+)
T cells. Sci Transl Med. 5:200ra1162013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abiko K, Mandai M, Hamanishi J, Yoshioka
Y, Matsumura N, Baba T, Yamaguchi K, Murakami R, Yamamoto A, Kharma
B, et al: PD-L1 on tumor cells is induced in ascites and promotes
peritoneal dissemination of ovarian cancer through CTL dysfunction.
Clin Cancer Res. 19:1363–1374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brenner MB, McLean J, Scheft H, Riberdy J,
Ang SL, Seidman JG, Devlin P and Krangel MS: Two forms of the
T-cell receptor gamma protein found on peripheral blood cytotoxic T
lymphocytes. Nature. 325:689–694. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dunn GP, Old LJ and Schreiber RD: The
three Es of cancer immunoediting. Annu Rev Immunol. 22:329–360.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirata S, Kubo Y, Kokubo T, Kitada M and
Nosaka T: Expression of major histocompatibility complex of
advanced non-small cell lung cancer (NSCLC). Nihon Kyobu Geka
Gakkai Zasshi. 44:138–143. 1996.(In Japanese). PubMed/NCBI
|
|
19
|
Korkolopoulou P, Kaklamanis L, Pezzella F,
Harris AL and Gatter KC: Loss of antigen-presenting molecules (MHC
class I and TAP-1) in lung cancer. Br J Cancer. 73:148–153. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramnath N, Tan D, Li Q, Hylander BL,
Bogner P, Ryes L and Ferrone S: Is downregulation of MHC class I
antigen expression in human non-small cell lung cancer associated
with prolonged survival? Cancer Immunol Immunother. 55:891–899.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki H, Higuchi M, Hasegawa T, Yonechi
A, Ohsugi J, Yamada F, Hoshino M, Shio Y, Fujiu K and Gotoh M:
Tissue array analysis of the aberrant expression of HLA class I
molecules in human non small cell lung cancer. Gan To Kagaku Ryoho.
33:1713–1716. 2006.(In Japanese). PubMed/NCBI
|
|
22
|
Hanagiri T, Shigematsu Y, Kuroda K, Baba
T, Shiota H, Ichiki Y, Nagata Y, Yasuda M, Uramoto H, So T, et al:
Prognostic implications of human leukocyte antigen class I
expression in patients who underwent surgical resection for
non-small-cell lung cancer. J Surg Res. 181:e57–e63. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI,
Park YM, Oh S, Shin JG, Yao S, Chen L, et al: Interferon regulatory
factor-1 is prerequisite to the constitutive expression and
IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett.
580:755–762. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mühlbauer M, Fleck M, Schütz C, Weiss T,
Froh M, Blank C, Schölmerich J and Hellerbrand C: PD-L1 is induced
in hepatocytes by viral infection and by interferon-alpha and
-gamma and mediates T cell apoptosis. J Hepatol. 45:520–528. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abiko K, Matsumura N, Hamanishi J,
Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I
and Mandai M: IFN-γ from lymphocytes induces PD-L1 expression and
promotes progression of ovarian cancer. Br J Cancer. 112:1501–1509.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dunn GP, Koebel CM and Schreiber RD:
Interferons, immunity and cancer immunoediting. Nat Rev Immunol.
6:836–848. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Burnet FM: The concept of immunological
surveillance. Prog Exp Tumor Res. 13:1–27. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei S, Shreiner AB, Takeshita N, Chen L,
Zou W and Chang AE: Tumor-induced immune suppression of in vivo
effector T-cell priming is mediated by the B7-H1/PD-1 axis and
transforming growth factor beta. Cancer Res. 68:5432–5438. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zou W and Chen L: Inhibitory B7-family
molecules in the tumour microenvironment. Nat Rev Immunol.
8:467–477. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J, Hamrouni A, Wolowiec D, Coiteux V,
Kuliczkowski K, Hetuin D, Saudemont A and Quesnel B: Plasma cells
from multiple myeloma patients express B7-H1 (PD-L1) and increase
expression after stimulation with IFN-{gamma} and TLR ligands via a
MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 110:296–304.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mansfield AS, Murphy SJ, Peikert T, Yi ES,
Vasmatzis G, Wigle DA and Aubry MC: Heterogeneity of programmed
cell death ligand 1 expression in multifocal lung cancer. Clin
Cancer Res. 22:2177–2182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McLaughlin J, Han G, Schalper KA,
Carvajal-Hausdorf D, Pelekanou V, Rehman J, Velcheti V, Herbst R,
LoRusso P and Rimm DL: Quantitative assessment of the heterogeneity
of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol.
2:46–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akbay EA, Koyama S, Carretero J, Altabef
A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp
EM, Pugh TJ, et al: Activation of the PD-1 pathway contributes to
immune escape in EGFR-driven lung tumors. Cancer Discov.
3:1355–1363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sumimoto H, Takano A, Teramoto K and Daigo
Y: RAS-mitogen-activated protein kinase signal is required for
enhanced PD-L1 expression in human lung cancers. PLoS One.
11:e01666262016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ota K, Azuma K, Kawahara A, Hattori S,
Iwama E, Tanizaki J, Harada T, Matsumoto K, Takayama K, Takamori S,
et al: Induction of PD-L1 expression by the EML4-ALK oncoprotein
and downstream signaling pathways in non-small cell lung cancer.
Clin Cancer Res. 21:4014–4021. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Powles T, Eder JP, Fine GD, Braiteh FS,
Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et
al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in
metastatic bladder cancer. Nature. 515:558–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Garber K: Predictive biomarkers for
checkpoints, first tests approved. Nat Biotechnol. 33:1217–1218.
2015. View Article : Google Scholar : PubMed/NCBI
|


















