Introduction
Non-small cell lung cancer (NSCLC) is a leading
cause of cancer-related death in most of the industrial countries.
Chemotherapy, radiation therapy and surgery are the main
therapeutic options. Despite these therapies, the prognosis for
NSCLC has not improved as expected, and the mortality rate remains
high. Therefore, additional therapeutic options are needed.
Recently, cancer immunotherapy has been developed
and shown promising results against some malignancies in clinical
trials (1–4). One of immuno-check-point proteins, the
programmed cell death 1/programmed cell death 1/ligand 1
(PD-1/PD-L1) pathway has attract attention as playing a main role
in cancer immunology (5). PD-1
(also known as CD279) is a receptor expressed on immune cells, and
when combined with its ligand, PD-L1 (also known as CD274)
expressed on cancer cells, induces the immunosuppression of cancer
cells and blocks the attack by host immunity (5). Anti-PD-1/PD-L1 immunotherapies have
recently been developed and has shown promising results against
several malignancies (1–4). The expression of PD-L1 in cancer cells
reportedly is a useful predictive factor for the therapeutic effect
of anti-PD-1 or anti-PD-L1 antibody immunotherapies (6).
However, the mechanism of PD-L1 expression in cancer
cells remains unclear. In addition, immunotherapy has other
problems to be solved (e.g. the selection of suitable patients and
the timing of administration of the immunotherapy). Elucidation of
the mechanism of PD-L1 expression may provide useful information to
solve those problems.
Epithelial-mesenchymal transition (EMT) is a key
process in cancer progression and is induced by several factors,
including transforming growth factor (TGF-β). We previously showed
that chemo-treatment increased TGF-β expression in human
adenocarcinoma cell lines, and this autocrine TGF-β lead to higher
malignant characteristics of cancer cells via EMT process (7,8). We
also revealed the clinical significance of the EMT in NSCLC after
induction chemotherapy (7,8). In the present study, we explored the
mechanism by which TGF-β controls PD-L1 expression and elucidated
the clinical relationships between the PD-L1 expression and EMT
status in NSCLC after induction chemotherapy.
Materials and methods
Cell culture, reagents and
antibodies
The human lung adenocarcinoma cell lines A549 and
NCI-H358 were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA) and maintained in RPMI-1640 medium with
10% fetal bovine serum (FBS) and streptomycin and penicillin.
Carboplatin was purchased from Sigma-Aldrich (St. Louis, MO, USA;
cat, no. C2538) and TGF-β1 was from R&D Systems (Minneapolis,
MN, USA; cat. no. 240-B). SB 431542, a specific and selective
inhibitor of TGF-β1 receptor kinase inhibitor, was purchased from
Tocris Bioscience (St. Louis, MO, USA; cat. no. 1614).
Reversion assay
[mesenchymal-epithelial transition assay (MET)]
To determine whether there is a causal relationship
between PD-L1 expression EMT, reversion assay was performed. We
analyzed the gene expression of PD-L1 and N-cadherin in A549 cells
under MET (EMT reverse process). First, we induced EMT in the cells
by using culture medium including TGF-β1 (1 ng/ml) for 3 days, and
then we promoted MET by changing to culture medium without
TGF-β1.
Immunohistochemical staining analysis
of EMT status and PD-L1 expression in clinical samples
IHC staining was performed as follows.
Formalin-fixed paraffin-embedded tissue sections of non-small cell
lung cancer from patients who underwent surgical resection after
induction chemotherapy were deparaffinized and rehydrated. For
antigen retrieval, the sections were brought to boil in 1 mM EDTA
pH 8.0 and then maintained for 15 min at a sub-boiling temperature.
EMT status was evaluated according to N-cadherin, E cadherin and
TGF-β1 staining intensities. The evaluation of EMT status and PD-L1
were based on a previous study (9).
In brief, the stained specimens were scored in a semi-quantitative
manner (H score): the staining percentages (0–100%) and the
intensity 0 (no staining), +1 (weak staining), +2 (distinct
staining), +3 (very strong staining) H score was calculated by
multiplying the percentage by the intensity. In addition, H score
was classified as 0 (score <10), +1 (≥10 or <30), or +2 (≥30
or <70), +3 (>70). We defined a positive change in EMT status
as either or both H score classifications decrease in staining for
E-cadherin and increase in the staining for N-cadherin,
respectively, between the biopsy samples before induction
chemotherapy and the surgical samples after induction chemotherapy
(CT). The PD-L1 immunohistochemistry was evaluated based on the
method described by Koh et al (9). Briefly, the intensity and proportion
of membranous and/or cytoplasmic staining in tumor cells were
scored as follows: 0, negative; 1, weak or moderate in <10% of
tumor cells; 2, moderate in ≥10% of tumor cells; 3, strong (more
intense than alveolar macrophages for PD-L1) in ≥10% of tumor
cells. Cases with scores of 2 or 3 were deemed positive for PD-L1
expression. The antibody used for immunohistochemistry (IHC) were
as follows: monoclonal mouse anti-human N-cadherin
(6G11/M3613,1/50; Dako, Glostrup, Denmark), monoclonal mouse
anti-human E-cadherin (NCH-38/M3612; Dako), polyclonal rabbit
anti-human TGF-β1 (ab66043; Abcam, Cambridge, UK) and polyclonal
rabbit anti-human PD-L1 (EIL3N/13684, 1/200; Cell Signaling
Technology, Inc., Danvers, MA, USA).
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was isolated from cell lines by using an
RNeasy Mini kit (Qiagen, Tokyo, Japan). Real-time RT-PCR was
conducted with a TaqMan assay; the relative expression levels were
calculated by the comparative Ct method. The TaqMan gene
assays (Applied Biosystems, Carlsbad, CA, USA) for GAPDH
(Hs02758991_g1), E-cadherin (Hs01023894_m1), N-cadherin
(Hs00983056_m1), PD-L1 (Hs01125301_m1) and TGF-β (Hs00998133_m1)
were used. All experiments were performed in triplicate and the
results are presented as means ± SD. The significance of
differences between the untreated cells and the treated cells was
tested with the Mann-Whitney U test.
Western blot analysis
Monolayers of cultured cells were treated with TGF-β
or carboplatin, and the proteins were extracted with RIPA buffer
(Cell Signaling Technology; cat. no. 9806). Cell extracts were
separated with sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and immunoblotted as previously
described (10,11). Western blotting after extraction of
membrane proteins was performed by using a Cell Surface Protein
Isolation kit (P74008; Takara Bio, Tokyo, Japan). The following
antibodies were used for detection: mouse anti-human E-cadherin
(monoclonal, M106, 2 µg/ml; Takara), mouse anti-human N-cadherin
(monoclonal, sc-59987; Santa Cruz Biotechnology, Santa Cruz, CA,
USA), rabbit anti-human GAPDH (monoclonal, G9545; Sigma-Aldrich)
and rabbit anti-human TGF-β (polyclonal ab66043; Abcam).
Study population
Twenty-eight patients underwent induction
chemotherapy and pulmonary resection between 1996 and 2010 at the
Osaka University Hospital. Osaka University Hospital Review Board
approved this retrospective study, and written informed consent for
this retrospective study and surgery was obtained from each
patient. Each patient received two cycles of cisplatin- or
carboplatin-based chemotherapy every 4 weeks in one of three
regimens: cisplatin at 80 mg/m2 on day 1 and vindesine
at 3 mg/m2 on days 1 and 8, with or without mitomycin at
8 mg/m2 on day 1 (PV(M) regimen), cisplatin at 80
mg/m2 on day 1 and vinorelbine at 20 mg/m2 on
days 1 and 8 (nPV regimen), or cisplatin at 80 mg/m2 on
day 1 and docetaxel at 60 mg/m2 on day 1 (DP regimen)
(Table I). A surgical resection was
performed 6–8 weeks after induction chemotherapy.
 | Table I.Patients characteristics. |
Table I.
Patients characteristics.
| Variables | N |
|---|
| Age (mean years) | 63.1 |
| Sex |
|
| Male | 22 |
|
Female | 6 |
| Clinical stage |
|
| II | 3 |
| III | 25 |
| Pathological
stage |
|
| I | 9 |
| II | 6 |
| III | 13 |
| Histopathology |
|
|
Adenocarcinoma | 14 |
|
Squamous cell carcinoma | 12 |
|
Others | 2 |
| Chemotherapy |
|
|
1st |
|
|
CDDP | 13 |
|
CBDCA | 15 |
| 2nd |
|
|
ETP | 1 |
|
PTX | 10 |
|
TXT | 9 |
|
VDS | 6 |
|
VNR | 2 |
| Surgical
procedure |
|
|
Lobectomy | 23 |
|
Pneumonectomy | 2 |
|
Bi-lobectomy | 3 |
Statistical design and data
analysis
A χ2 test, Mann-Whitney U test, or
repeated-measures analysis of variance were used to compare the
RT-PCR results. The correlations between PD-L1 IHC status and EMT
markers were analyzed by the Pearson's Chi-squared test.
Disease-free survival (DFS) and overall survival (OS) were analyzed
by using the Kaplan-Meier method, and the log-rank test was used to
compare the survival distributions of subgroups. All statistical
analyses were performed by using the JMP version 11 for Windows
(SAS Institute, Inc., Cary, NC, USA). P<0.05 is considered to be
statistically significant.
Results
PD-L1 is enhanced in TGF-β induced EMT
and decreased in MET induced by removal of TGF-β stimulation
To examine the relationship between PD-L1 and EMT
status, we induced EMT in A549 and NCI-H358 cells by using TGF-β1
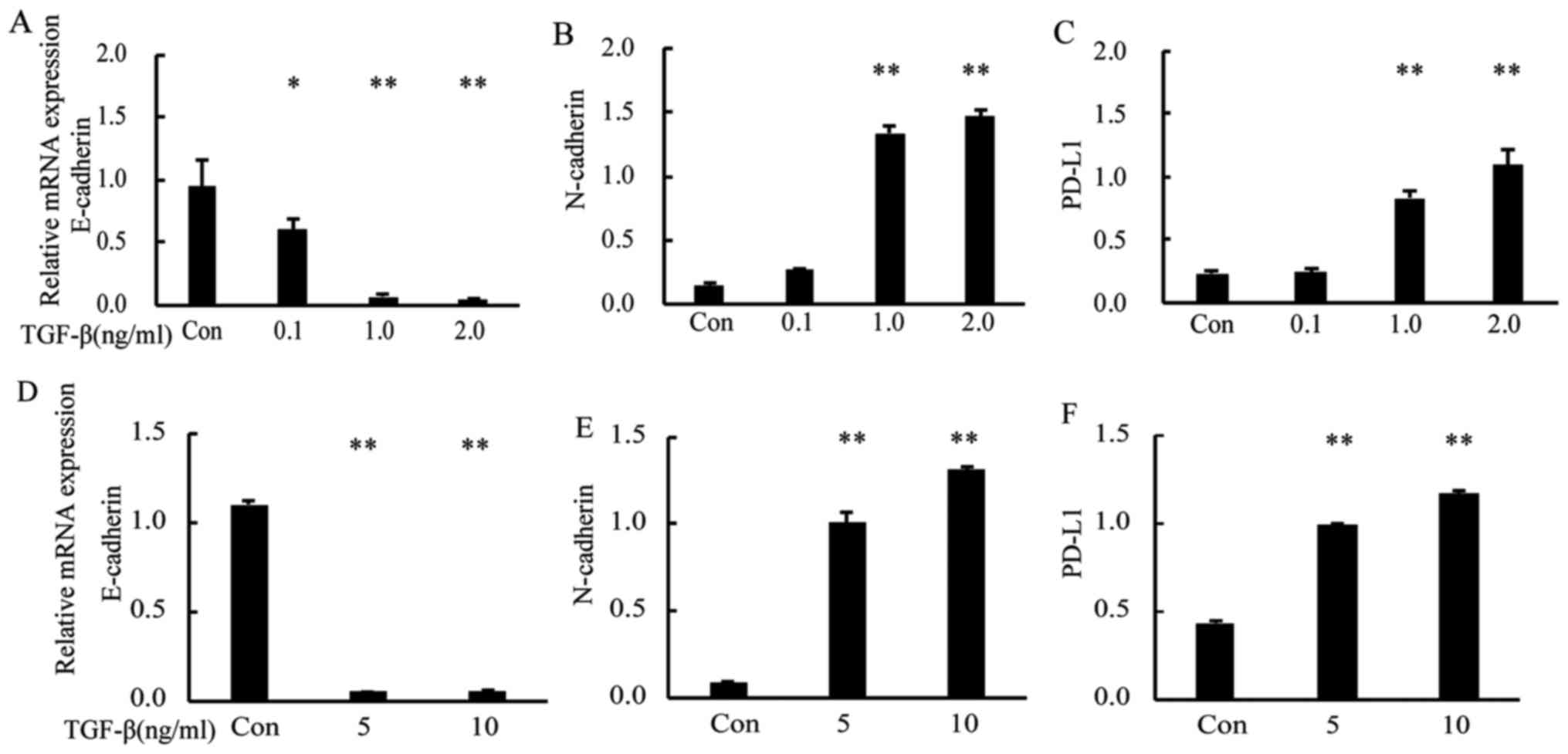
at 0.1–2 ng/m. RT-PCR analysis showed that, after TGF-β1 treatment,
the gene expression of E-cadherin (as the epithelial marker) was
downregulated compared with that in control cells, whereas that of
N-cadherin (as the mesenchymal marker) and PD-L1 were upregulated
in a dose-dependent manner (Fig.
1A-C; P<0.05 and P<0.01 vs. Con). We also analyzed the
PD-L1 gene expression in the human Caucasian bronchioalveolar
carcinoma cell line H358 and obtained similar results (Fig. 1D-F; P<0.01 vs. Con). Western blot
analysis of A549 with TGF-β1 revealed that equivalent differences
were observed at the protein level (Fig. 2). Immunofluorescence analysis showed
that the expression levels of PD-L1 and N-cadherin were enhanced by
TGF-β treatment and those on the cell surface became co-localized
(Fig. 3).
To determine whether there is a causal relationship
between the PD-L1 expression and TGF-β induced EMT, reversion assay
was performed. We used RT-PCR assays to analyze the gene expression
of PD-L1, E-cadherin and N-cadherin in the cells under EMT and MET
(Fig. 4A-C). The gene expression
levels of PD-L1, E-cadherin and N-cadherin changed with significant
difference as compared with the pre-treatment levels (Con; control)
(P<0.01 vs. Con) and reverted to pre-treatment
(TGF-β−) levels after the change to culture medium
without TGF-β (##P<0.01 vs. TGF-β+). These
results suggest that the PD-L1 expression is regulated by the TGF-β
signaling and changes in parallel with the EMT status.
TGF-β inhibitors block the expression
of PD-L1
Next, to examine whether the PD-L1 expression was
regulated through the TGF-β signal pathway, we treated A549 cells
for 2 days with medium alone (Fig.
4D-F, Con; TGF-β−/SB−), the TGF-β1
receptor kinase inhibitor; SB 431542 (Fig. 4D-F,
TGF-β−/SB+), or TGF-β1 plus SB
431542(Fig. 4D-F,
TGF-β+/SB+). Next, we examined PD-L1,
E-cadherin and N-cadherin expression by RT-PCR and western blot
analysis. The RT-PCR results showed that the upregulation of PD-L1
and N-cadherin mRNA expression after TGF-β1 treatment was
suppressed by SB 431542 (Fig. 4D-F;
P<0.01 vs. TGF-β−/SB−, P<0.01 vs.
TGF-β+/SB−) and the western blot analysis
demonstrated that the PD-L1 protein level was suppressed by SB
431542 (Fig. 5, lane 2 vs. 3).
Taken together, these results suggest that the PD-L1 expression is
regulated through the TGF-β signal pathway.
PD-L1 expression was enhanced by
chemo-induced TGF-β pathway
We previously reported that chemo-treatment
increased the expression of TGF-β and the autocrine TGF-β induced
EMT in A549 cells (12,13). In this study, we hypothesized that
chemo-treatment enhanced PD-L1 expression through the TGF-β
pathway. We evaluated the TGF-β, EMT markers and PD-L1 mRNA and
protein expression in the A549 cells treated with carboplatin (25
µM) for 4 days. The RT-PCR results indicated that, for E-cadherin,
N-cadherin and PD-L1, the expression patterns of the control versus
treatment subline (Fig. 6A-C) were
similar to those for control vs. TGF-β treatment (P<0.05; vs.
Con). Next, to examine whether PD-L1 upregulation under
chemo-treatment was via TGF-β pathway, we examined PD-L1 expression
of the cells after treatment with carboplatin alone (CB) and
carboplatin plus SB 43152 (CB+SB), by RT-PCR and western blot
analysis. Fig. 6E showed the
results of RT-PCR that PD-L1 gene expression after chemo-treatment
(CB) increased significantly as compared with no-treated cell lines
(Con). In contrast, the upregulation of the mRNA levels of PD-L1
and N-cadherin was attenuated by SB 431542 (CB+SB) (Fig. 6D-E, P<0.05 vs. Con, P<0.01 vs.
CB). While, TGF-β mRNA expression was upregulated by carboplatin as
previously described (12)
(Fig. 6F; P<0.05 vs. Con);
however, TGF-β upregulation with carboplatin treatment was not
attenuated by SB 431542. Fig. 6F
shows that there was no significant difference in TGF-β mRNA levels
between carboplatin alone (CB) and carboplatin plus SB 431542
treatment (CB+SB). Moreover, in western blot analysis, the
expression of PD-L1 was upregulated by chemo-treatment in a
dose-dependent manner (Fig. 7A),
and this upregulation was abolished by SB 431542 (Fig. 7B; lane 4 vs. 5). These results
suggest that the PD-L1 gene expression is upregulated through the
chemo-induced TGF-β pathway.
 | Figure 6.Chemo-treatment enhances PD-L1
expression via TGF-β signaling. RT-PCR analysis of untreated A549
cells (Con) and those cells treated with carboplatin (25 µM) for 4
days (CB). Total RNA was extracted from the untreated A549 cells
(Con) and the cells treated with carboplatin (CB), and E-cadherin,
N-cadherin, PD-L1 and GAPDH transcripts were quantified by RT-PCR
analysis (A-C). (*P<0.05 vs. Con). (D-F) Control A549 cells
(Con), the cells treated with carboplatin alone (CB), and the cells
treated with carboplatin and SB 43152 (SB; 10 µM) (CB+SB) underwent
RT-PCR analysis for quantification of TGF-β, N-cadherin, PD-L1 and
GAPDH mRNA levels. (N-cad; *P<0.05 vs. CB, PD-L1; *P<0.05 vs.
Con, **P<0.01 vs. CB, TGF-β; *P<0.05 vs. Con). |
High expression of PD-L1 in non-small
cell lung cancer cases after induction chemotherapy
To elucidate the relationship between PD-L1 and EMT
status in clinical samples of NSCLC, we performed IHC staining of
clinical specimens obtained from samples resected from 28 patients
with NSCLC after induction chemotherapy.
The patient characteristics are listed in Table I. IHC staining was used to analyze
the expression of EMT markers, TGF-β and PD-L1. Fig. 8A shows the representative images of
low expression of PD-L1 and Fig. 8B
shows those of high expression of PD-L1. Fig. 8C-E shows the representative images
of E-cadherin, N-cadherin and TGF-β positive IHC. A total of 28
patients with NSCLC underwent induction chemotherapy followed by
complete surgical resection. All cases underwent carboplatin or
cisplatin-based doublet chemotherapy. The patients comprised 22 men
and 6 women with a mean age of 63.1.
Eight cases (28.6%) showed a positive EMT change. Of
them, 7 (87.5%) showed positive results for PD-L1 staining. The
proportion of EMT-change positive cases among PD-L1-high expression
cases was significantly higher than that among PD-L1-low expression
cases (Fig. 9A, Pearson's
chi-square test; P=0.01). In TGF-β IHC analysis, there were also
significant relationships between PD-L1 expression and TGF-β
expression (Fig. 9B, Pearson's
chi-square test; P=0.04). The DFS (measured as no recurrence of
cancer) in PD-L1-high expression cases was significantly worse than
that of PD-L1-low expression cases (Fig. 9C, P=0.02; log-rank test); however,
the OS rate was not significantly related to PD-L1 status (Fig. 9D, P=0.2; log-rank test).
Univariate and multivariate analyses showed that
high PD-L1 expression was an independent prognostic factor for
NSCLC surgically resected after induction chemotherapy (Table II).
 | Table II.Univariate and multivariate analyses
of disease-free survival. |
Table II.
Univariate and multivariate analyses
of disease-free survival.
| A, Univariate
analysis of disease-free survival |
|---|
|
|---|
| Factors | Hazard ratio | 95% CI | P-value |
|---|
| Sex |
|
Male | 1 |
|
|
|
Female | 0.59 | 0.03–2.93 | 0.58 |
| Histology |
|
Adeno | 1 |
|
|
| Sq | 0.79 | 0.28–2.32 | 0.66 |
|
other | 0.42 | 0.02–2.31 | 0.36 |
| p-Stage |
|
I+II | 1 |
|
|
|
III+IV | 2.29 | 0.83–6.27 | 0.1 |
| Down-stage |
|
Positive | 1 |
|
Negative | 2.27 | 0.73–10.03 | 0.16 |
| PD-L1 |
|
Low | 1 |
|
|
|
High | 2.97 | 1.13–8.54 | 0.02 |
|
| B, Multivariate
analysis of disease-free survival |
|
| Factors | Hazard ratio | 95% CI | P-value |
|
| PD-L1 |
|
|
|
|
Low | 1 |
|
|
|
High | 2.8 | 1.00–8.41 | 0.04 |
| Histology |
|
|
|
|
Adeno | 1 |
|
|
| Sq | 0.8 | 0.26–2.42 | 0.7 |
|
other | 0.7 | 0.1–3.17 | 0.69 |
| p-Stage |
|
I+II | 1 |
|
|
|
III+IV | 2.95 | 0.83–11.7 | 0.09 |
| Down-stage |
|
Positve | 1 |
|
|
|
Negative | 1.07 | 0.32–3.47 | 0.9 |
Discussion
In the present study, we showed that PD-L1 was
regulated by TGF-β, and that chemo-treatment enhanced the PD-L1
expression through the chemo-induced TGF-β signal pathway in NSCLC
cell lines. We also performed IHC staining of surgically resected
NSCLC after induction chemotherapy and thus revealed a significant
relationship between PD-L1 expression and EMT status.
To overcome advanced NSCLC, surgery and chemotherapy
are the most important therapeutic options; however, their results
are far from satisfactory. Immunotherapy has been recently
developed as another option; in particular, anti-PD-1/PD-L1
blockade agents have exhibited dramatic antitumor efficacy in
clinical trials for patients with a variety of cancer types
(14). However, immunotherapy
likewise is associated with several problems to be solved, as
outlined below.
The first problem is the selection of suitable
patients. The current selection method depends on PD-L1 IHC results
as a predictor of therapeutic effect. PD-L1 expression by IHC has
been also reported to be a useful prognostic indicator (15); however, staining intensity and
sensitivity vary according to the type of PD-L1 IHC antibody
(15,16). The next problem is when to start
this immunotherapy. Understanding the mechanism of PD-L1 expression
will yield important information regarding potential solutions to
these problems. To elucidate this mechanism, we focused on EMT.
EMT plays a crucial role in cancer progression, and
this phenomenon can be found in this cancer microenvironment. EMT
is promoted by TGF-β secreted from not only the cancer cell itself
but also from other cell types, and it gives cancer cells the
invasive and metastatic abilities necessary for successful
metastasis (7,8,12,17).
In the present study, we demonstrated that PD-L1 expression in
cancer cells was regulated by TGF-β signal pathway. Other groups
showed a significant relationship between PD-L1 expression and EMT
status by IHC staining analysis on several malignancies including
NSCLC (18,19). Together, these data support our
notion that the TGF-β pathway is an important regulator of PD-L1
expression, and EMT markers may be useful for selecting patients
that would likely benefit from immunotherapy.
We previously reported that chemo-treatments
increased TGF-β production and autocrine TGF-β induced EMT in human
lung adenocarcinoma cell lines (7,12).
Based on our previous results, we showed that chemo-treatment
enhanced PD-L1 expression through the chemo-induced TGF-β signal
pathway. Moreover, from the IHC analysis, we revealed the
significant relationship between PD-L1 expression and EMT status of
resected samples after induction chemotherapy. Zhang et al
(20) demonstrated that
chemo-preventive agents induced PD-L1 in human breast cancer cells
and promoted PD-L1-mediated INF-γ by T-cell apoptosis. Hecht et
al (21) also showed that PD-L1
expressing cells in rectal adenocarcinoma were upregulated after
chemoradiotherapy (CRT) as compared with before CRT. Other groups
have shown that several stimuli, such as hypoxia and radiation
induction, enhance PD-L1 expression (22–24).
Those results suggested that PD-L1 is enhanced by chemo-treatment
and the PD-L1 blockade after chemotherapy or in combination with
chemotherapy could be a more effective anticancer activity than the
monotherapy.
This study has some limitations. The limitation is
the small sample size analyzed. In our hospital, adjuvant
chemo-radiation therapy was usually performed in most of advanced
cases. As mentioned above, the radiation, like chemotherapy,
enhances the expression of PD-L1 (22–24).
Since the effect of radiation cannot be ignored, we analyzed only
cases after induction chemotherapy alone. Moreover, the predictive
role of PD-L1 expression is still controversial. Our results showed
the PD-L1 expression was a prognostic indicator. However, our
sample size was small as mentioned. Therefore, further
investigation will be needed.
In conclusion, the present study provides new and
important information regarding the mechanism of PD-L1 expression.
The results suggest that EMT markers could be a surrogate marker
for the selection of patients for immunotherapy and that a
combination of immunotherapy and chemotherapy could be a new
therapeutic option to overcome NSCLC.
Acknowledgements
The present study was supported by KAKENHI
(Grants-in-Aid for Scientific Research) 16K10680.
References
|
1
|
Bordon Y: Immunotherapy: Checkpoint
parley. Nat Rev Cancer. 15:32015. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: KEYNOTE-024 Investigators: Pembrolizumab versus
chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl
J Med. 375:1823–1833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weber JS, DAngelo SP, Minor D, Hodi FS,
Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD,
et al: Nivolumab versus chemotherapy in patients with advanced
melanoma who progressed after anti-CTLA-4 treatment (CheckMate
037): A randomised, controlled, open-label, phase 3 trial. Lancet
Oncol. 16:375–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iwai Y, Ishida M, Tanaka Y, Okazaki T,
Honjo T and Minato N: Involvement of PD-L1 on tumor cells in the
escape from host immune system and tumor immunotherapy by PD-L1
blockade. Proc Natl Acad Sci USA. 99:12293–12297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang Y, Pang Z, Ding N, Dong W, Ma W, Li
Y, Du J and Liu Q: The efficacy and potential predictive factors of
PD-1/PD-L1 blockades in epithelial carcinoma patients: A systematic
review and meta analysis. Oncotarget. 7:74350–74361.
2016.PubMed/NCBI
|
|
7
|
Shintani Y, Okimura A, Sato K, Nakagiri T,
Kadota Y, Inoue M, Sawabata N, Minami M, Ikeda N, Kawahara K, et
al: Epithelial to mesenchymal transition is a determinant of
sensitivity to chemoradiotherapy in non-small cell lung cancer. Ann
Thorac Surg. 92:1794–1804; discussion 1804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shintani Y, Abulaiti A, Kimura T, Funaki
S, Nakagiri T, Inoue M, Sawabata N, Minami M, Morii E and Okumura
M: Pulmonary fibroblasts induce epithelial mesenchymal transition
and some characteristics of stem cells in non-small cell lung
cancer. Ann Thorac Surg. 96:425–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koh J, Go H, Keam B, Kim MY, Nam SJ, Kim
TM, Lee SH, Min HS, Kim YT, Kim DW, et al: Clinicopathologic
analysis of programmed cell death-1 and programmed cell
death-ligand 1 and 2 expressions in pulmonary adenocarcinoma:
Comparison with histology and driver oncogenic alteration status.
Mod Pathol. 28:1154–1166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shintani Y, Maeda M, Chaika N, Johnson KR
and Wheelock MJ: Collagen I promotes epithelial-to-mesenchymal
transition in lung cancer cells via transforming growth factor-beta
signaling. Am J Respir Cell Mol Biol. 38:95–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McCarty KS Jr, Szabo E, Flowers JL, Cox
EB, Leight GS, Miller L, Konrath J, Soper JT, Budwit DA, Creasman
WT, et al: Use of a monoclonal anti-estrogen receptor antibody in
the immunohistochemical evaluation of human tumors. Cancer Res. 46
(Suppl):4244s–4248s. 1986.PubMed/NCBI
|
|
12
|
Shintani Y, Fujiwara A, Kimura T, Kawamura
T, Funaki S, Minami M and Okumura M: IL-6 decreted from
cancer-associated fibroblasts mediates chemoresistance in NSCLC by
increasing epithelial-mesenchymal transition signaling. J Thorac
Oncol. 11:1482–1492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abulaiti A, Shintani Y, Funaki S, Nakagiri
T, Inoue M, Sawabata N, Minami M and Okumura M: Interaction between
non-small-cell lung cancer cells and fibroblasts via enhancement of
TGF-β signaling by IL-6. Lung Cancer. 82:204–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schats KA, Van Vré EA, De Schepper S,
Boeckx C, Schrijvers DM, Waelput W, Fransen E, Vanden Bempt I,
Neyns B, De Meester I, et al: Validated programmed cell death
ligand 1 immunohistochemistry assays (E1L3N and SP142) reveal
similar immune cell staining patterns in melanoma when using the
same sensitive detection system. Histopathology. 70:253–263. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith J, Robida MD, Acosta K, Vennapusa B,
Mistry A, Martin G, Yates A and Hnatyszyn HJ: Quantitative and
qualitative characterization of Two PD-L1 clones: SP263 and E1L3N.
Diagn Pathol. 18:442016. View Article : Google Scholar
|
|
17
|
Thomson S, Petti F, Sujka-Kwok I, Mercado
P, Bean J, Monaghan M, Seymour SL, Argast GM, Epstein DM and Haley
JD: A systems view of epithelial-mesenchymal transition signaling
states. Clin Exp Metastasis. 28:137–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimoji M, Shimizu S, Sato K, Suda K,
Kobayashi Y, Tomizawa K, Takemoto T and Mitsudomi T: Clinical and
pathologic features of lung cancer expressing programmed cell death
ligand 1 (PD-L1). Lung Cancer. 98:69–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ock CY, Kim S, Keam B, Kim M, Kim TM, Kim
JH, Jeon YK, Lee JS, Kwon SK, Hah JH, et al: PD-L1 expression is
associated with epithelial-mesenchymal transition in head and neck
squamous cell carcinoma. Oncotarget. 7:15901–15914. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang P, Su DM, Liang M and Fu J:
Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1)
surface expression in breast cancer cells and promote
PD-L1-mediated T cell apoptosis. Mol Immunol. 45:1470–1476. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hecht M, Büttner-Herold M,
Erlenbach-Wünsch K, Haderlein M, Croner R, Grützmann R, Hartmann A,
Fietkau R and Distel LV: PD-L1 is upregulated by radiochemotherapy
in rectal adenocarcinoma patients and associated with a favourable
prognosis. Eur J Cancer. 65:52–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Noman MZ, Desantis G, Janji B, Hasmim M,
Karray S, Dessen P, Bronte V and Chouaib S: PD-L1 is a novel direct
target of HIF-1α, and its blockade under hypoxia enhanced
MDSC-mediated T cell activation. J Exp Med. 211:781–790. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dovedi SJ, Adlard AL, Lipowska-Bhalla G,
McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M,
Stewart R, et al: Acquired resistance to fractionated radiotherapy
can be overcome by concurrent PD-L1 blockade. Cancer Res.
74:5458–5468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deng L, Liang H, Burnette B, Beckett M,
Darga T, Weichselbaum RR and Fu YX: Irradiation and anti-PD-L1
treatment synergistically promote antitumor immunity in mice. J
Clin Invest. 124:687–695. 2014. View
Article : Google Scholar : PubMed/NCBI
|