Introduction
Oral cancer is one of the most common malignancies
worldwide. For patients with locally advanced or metastatic stages,
chemotherapy is recommended as the standard therapy. Cisplatin
alone or cisplatin-based chemotherapy or concurrent
radiochemotherapy remains the first choice for the treatment of
advanced stage cancers, in particular unresectable tumors (1). Unfortunately, acquired or intrinsic
tumor resistance to cisplatin therapy greatly hampers its
therapeutic efficacy (2). Several
genes and pathways involved in apoptosis, drug efflux, growth,
proliferation and angiogenesis appear to influence sensitivity to
cisplatin (3,4), but overcoming drug resistance in
patients remains a challenge. Understanding the molecular
mechanisms underlying cisplatin resistance is essential for solving
this problem.
Recently, mounting evidence has demonstrated that
microRNAs (miRNAs) play a role in chemoresistance (5–7). For
example, overexpression of miR-21 suppresses PDCD4, TPM1 and PTEN
expression and induces cisplatin resistance, representing a poor
prognosis in tongue cancer (8,9).
Upregulation of miR-200b and miR-15b reverses
epithelial-to-mesenchymal transition in chemoresistant tongue
cancer cells by inhibiting BMI1 (10). However, the role of miRNAs in oral
cancer cell resistance to chemotherapy is not fully understood and
necessitates further investigation.
MicroRNA-218 (miR-218) is an intronic miRNA that is
coexpressed with its host genes: tumor suppressor genes SLIT2/3
(11). Evidence suggests that
miR-218 is frequently downregulated in various solid tumors and
functions as a tumor suppressor (12–16).
Concordantly, decreased miR-218 expression has been found to
correlate with a poor prognosis in non-small cell lung cancer
(NSCLC) (17), cervical (18) and oral cancer (19). Moreover, reduced miR-218 levels have
been detected in chemoresistant cancer cells, and restoration of
miR-218 expression can sensitize cells to chemotherapeutic agents
including cisplatin (20,21) and carboplatin (22). Conversely, increased expression of
miR-218 has been reported in acute lymphocytic leukemia (ALL)
(23), glioma (24) and metastatic breast cancer (25). A recent study also showed higher
expression levels of plasma miR-218 in advanced NSCLC patients with
epidermal growth factor receptor (EGFR) mutation, suggesting a
potential role of this miRNA in primary resistance to epidermal
growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) in
advanced NSCLC patients with EGFR mutation (26). These contradictory findings indicate
the complex function of miR-218 in mediating networks in
tumorigenesis and progression.
Dysregulation of the Wnt signaling pathway plays a
key role in cancer chemoresistance. Hassan et al found that
miR-218 activates the Wnt pathway by downregulating its inhibitors
sclerostin (SOST), Dickkopf2 (DKK2) and secreted frizzled-related
protein 2 (SFRP2), thus, promoting osteomimicry of metastatic
breast cancer cells (25). Protein
phosphatase 2A (PP2A) and its B56 subunit α (encoded by the
PPP2R5A gene) has been reported to control cell growth and
division in several critical pathways, including the Wnt pathway
(27). PP2A was originally thought
to be a tumor suppressor in Wnt signaling by negatively regulating
β-catenin and GSK3β (28,29). However, the precise biological
function of PP2A, as well as PPP2R5A in cancer progression and
chemoresistance, remains elusive. In the present study, we found
that miR-218 expression was upregulated in cisplatin-resistant oral
cancer cell lines and tissues. Furthermore, downregulation of
miR-218 inhibited Wnt signaling in oral cancer cells and sensitized
tumor cells to cisplatin-induced apoptosis by targeting
PPP2R5A.
Materials and methods
Patients and specimens
After obtaining written informed consent, human oral
cancer tissues (n=61) were collected from patients undergoing
standard surgical procedures at The Affiliated Stomatological
Hospital of Sun Yat-sen University (Guangzhou, China) between
January 2008 and December 2012. The diagnosis was based on
pathological evidence, and the specimens were immediately
snap-frozen in liquid nitrogen. All patients received
cisplatin-based neoadjuvant chemotherapy before surgical excision.
The study protocol was approved by the Ethics Committee of The
Affiliated Stomatological Hospital of Sun Yat-sen University.
Cell lines and culture
Human oral cancer UM1, UM2, Cal27 and MD1386Ln cell
lines were provided by Dr Zhou, University of Illinois at Chicago
(Chicago, IL, USA). SCC9, SCC15 and SCC25 cell lines were obtained
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). The Tca8113 cell line was obtained from the Chinese Oral
Tissue Culture and Collection Center (Shanghai, China). The cells
were maintained in Dulbeccos modified Eagles medium (DMEM)/F12
(Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS) and 1% penicillin/streptomycin (Gibco, Grand
Island, NY, USA) in a humid atmosphere containing 5% CO2
at 37°C.
Reagents and antibodies
cis-Diammineplatinum (II) dichloride was
purchased from Sigma-Aldrich (St. Louis, MO, USA; P/N: P4394).
Antibodies against β-catenin, GSK3β and β-tubulin were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). The
antibody against PPP2AB56α was purchased from ProteinTech (Chicago,
IL, USA) (P/N: 12675-2-AP). Antibodies against MRP1, ABCG2,
P-glycoprotein and topoisomerase IIβ were obtained from Abcam
(Cambridge, MA, USA). The antibody against EZH2 was purchased from
OriGene (Rockville, MD, USA).
Cell transfection
miR-218 mimic (P/N: miR10000275) and inhibitor
(anti-miR-218; P/N: miR20000275), PPP2R5A siRNA (P/N: stQ0004381-1)
and β-catenin siRNA (P/N: stQ0002532-1) were obtained from RiboBio
(Guangzhou, China). Cells were plated and cultured for 24 h and
transfected with miR-218 mimic (20 nM), anti-miR-218 (50 nM) or
siRNAs (50 nM). The transfection was performed using Lipofectamine
RNAi Max (Invitrogen, Carlsbad, CA, USA). Lentiviral PPP2R5A
(LV-PPP2R5A) and empty lentiviral vector (LV-NC) were designed and
constructed by GenePharma Co., Ltd. (Shanghai, China), and they
were transfected into tumor cells according to the manufacturer's
instructions.
Quantitative real-time reverse
transcription-PCR
Total RNA was isolated from oral cancer tissues or
cells using TRIzol reagent (Invitrogen). miR-218 was quantified
using the mirVana™ qRT-PCR miRNA detection kit (Ambion) as
previously described (Liu et al, 2009) according to the
manufacturers instructions. Human U6 rRNA served as an internal
control. To determine the relative mRNA levels of PPP2R5A and GAPDH
(used as an internal control), 1 µg of total RNA was subjected to
reverse transcription using a PrimeScript RT reagent kit (Takara
Bio, Shiga, Japan). Real-time PCR was performed using a
quantitative 2-step RT-PCR assay (Roche Diagnostics, Basel,
Switzerland) according to the standardized protocol. The sequences
of the specific primers for mRNA amplification were as follows:
PPP2R5A forward primer, 5′-CTAGTCCCTCCTGACCCA-3′ and reverse
primer, 5′-TAAATGCTGCTCAATCCC-3′; GAPDH forward primer,
5′-AACGGATTTGGTCGTATTG-3′ and reverse primer,
5′-GGAAGATGGTGATGGGATT-3′. The thermal cycling parameters were as
follows: pre-incubation at 95°C for 5 min, followed by 40 cycles of
amplification (95°C for 10 sec, 60°C for 20 sec, and 72°C for 20
sec), melting curve (95°C for 5 sec, 65°C for 1 min, and 97°C), and
cooling (40°C for 10 sec). The relative expression level was
computed using the 2−ΔΔCt analysis method. All qRT-PCR
reactions were evaluated in triplicate. All primers were ordered
from Invitrogen.
Cell viability assay
Cell viability was measured using a tetrazolium
salt-based Cell Counting Kit-8 assay (CCK-8; Dojindo Laboratories,
Kumamoto, Japan) according to the manufacturer's protocol. Briefly,
the cells (5×103/well) were seeded in 96-well plates and
allowed to attach overnight. They were then cultured in fresh
medium containing different concentrations of cisplatin (0, 0.25,
0.5, 1, 2, 4, 8, 16 and 32 µg/ml). After incubation for another 72
h, 10 µl of CCK-8 solution was added to each well and incubated at
37°C for 2 h. The absorbance of each well was assessed on a
microplate reader (Tecan GENios, Zurich, Switzerland) at 450 nm.
Cell viability was calculated as the ratio of each treatment group
to the untreated control group. The IC50 values were
determined as the concentration resulting in a 50% reduction in
cell viability compared with the control. All experiments were
performed 3 times in quadruplicate.
Cell apoptosis assay
Cells were grown in 6-well plates, transfected with
the desired reagents for 48 h and treated with cisplatin after
transfection. After treatment for 48 h, the cells were collected,
washed with ice-cold phosphate-buffered saline (PBS) twice and
gently resuspended in 1X binding buffer. After addition separately
of 5 µl of Annexin V-FITC and 10 µl of propidium iodide, the cells
were gently vortexed and incubated for 5 min in the dark. Flow
cytometry (FACScan; Beckman Coulter, Brea, CA, USA) was used to
assess apoptotic cells. Apoptotic cells were quantified using
CellQuest software.
Western blot analysis
Total cell lysates were prepared using RIPA lysis
buffer (50 mM Tris-HCl pH 7.4, 10 mM NaCl, 1% NP-40, 0.5% sodium
deoxycholate and 0.1% SDS). The protein concentration was measured
using the BCA method (Beyotime, Beijing, China). Proteins were
resolved by SDS-PAGE (10% gel) and subjected to immunoblot analysis
using antibodies against MRP1 (1:1,000), ABCG2 (1:1,000),
P-glycoprotein (1:500), topoisomerase IIβ (1:1,000), EZH2
(1:1,000), β-catenin (1:1,000), GSK3β (1:1,000), β-tubulin
(1:2,000) or PPP2AB56α (1:1,000). The membrane was further probed
with horseradish peroxidase-conjugated goat anti-rabbit IgG
(1:1,000 dilution; Beyotime) or goat anti-mouse IgG (1:1,000; Cell
Signaling Technology, Inc.), and the bands were visualized using a
Novex ECL HRP chemiluminescent substrate reagent kit (Invitrogen).
The protein bands were quantified using Alpha Innotech imaging
software (San Leandro, CA, USA).
Dual luciferase reporter assay
A 55-bp fragment from the 3′ untranslated region
(3′UTR) of the PPP2R5A gene (position 2586 to 2640;
NM_006243.3) containing the miRNA-218 binding site was cloned into
the XbaI site of the pGL3 firefly luciferase reporter vector
(Promega, Madison, WI, USA). A corresponding mutant construct was
created by mutating the seed region of the miR-218 binding site.
The constructs were then verified by sequencing. Cells were
transfected with the reporter constructs containing the targeting
sequence from the PPP2R5A 3′UTR (named pGL-PPP2R5A WT) or its
mutant (named pGL-PPP2R5A Mut) using Lipofectamine 2000
(Invitrogen). The pRL-TK vector (Promega) was co-transfected as an
internal control for normalization of the transfection efficiency.
The luciferase activities were then determined as previously
described (30) using a Lumat LB
9507 luminometer (Berthold Technologies GmbH & Co. KG, Bad
Wildbad, Germany).
Statistical analysis
All numerical data are presented as the mean ±
standard deviation (SD), and all error bars represent the SD of the
mean. The Student's t-test and one-way analysis of variance (ANOVA)
were used to determine differences. The Chi-square or Fisher's
exact tests were used to analyze the association between miR-218
and PPP2AB56α expression. All statistical analyses were performed
using SPSS 16.0 software. A P-value <0.05 was considered
significant.
Results
miR-218 expression is upregulated in
cisplatin-resistant oral cancer cells
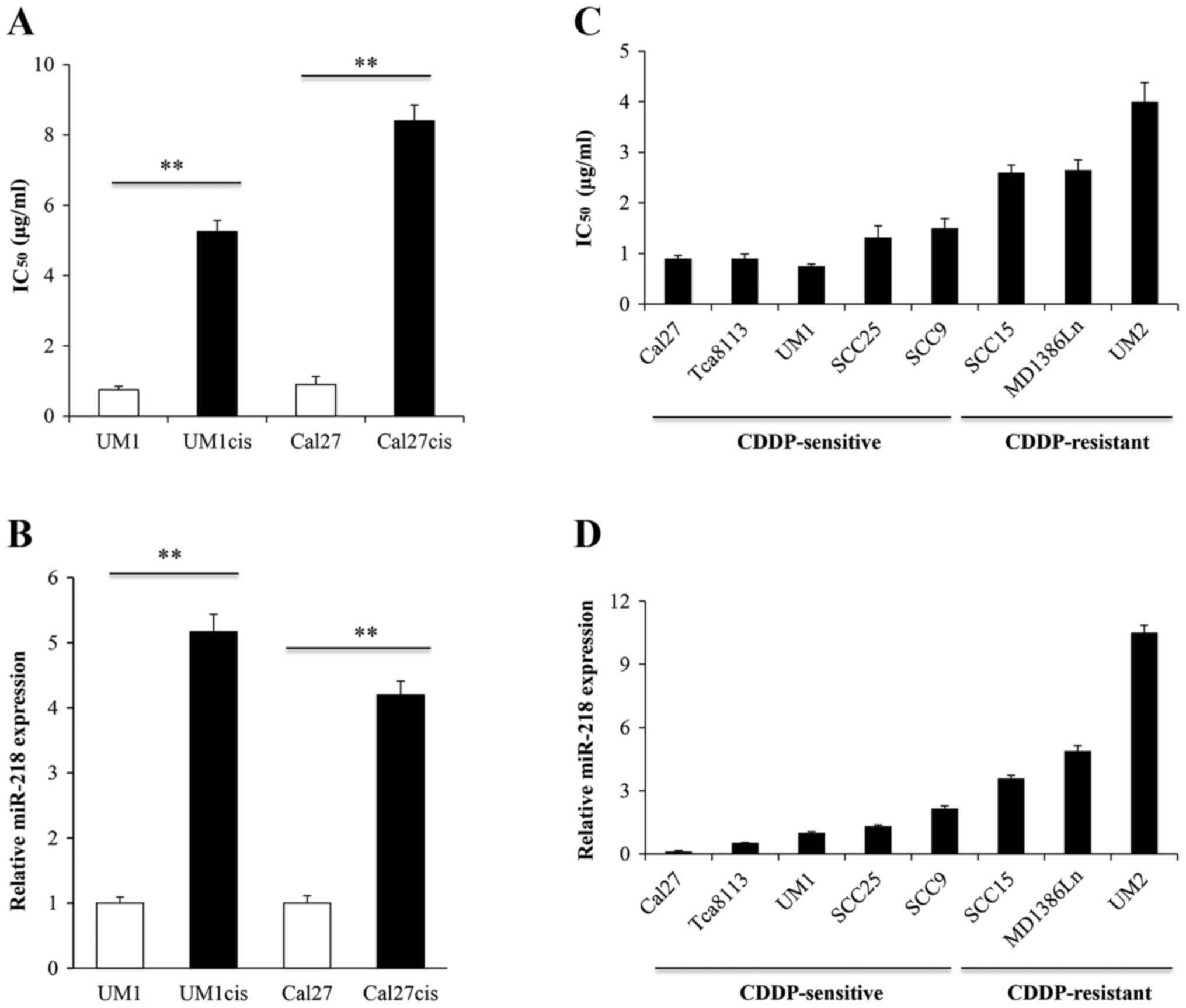
First, we determined whether upregulation of miR-218
expression was a common feature of cisplatin-resistant oral cancer
cells. UM1 and Cal27 cells, both of which are sensitive to
cisplatin (cisplatin IC50 values of 0.75 and 0.9 µg/ml,
respectively), were continuously exposed to a gradient
concentration of cisplatin for ~6 months until the cells acquired
cisplatin resistance. Cell viability was determined using the CCK-8
assay in cell lines exposed to increasing concentrations of CDDP
for 48 h. As illustrated in Fig.
1A, UM1cis and Cal27cis cells were more resistant to cisplatin
than the parental cell lines, with IC50 values of 5.23
and 8.42 µg/ml, respectively, which were ~7- and 9-fold higher than
the IC50 values for UM1 and Cal27. miR-218 expression
was determined by quantitative real-time RT-PCR in cisplatin
sensitive and resistant UM1 and Cal27 cells. As shown in Fig. 1B, cisplatin-sensitive cells
expressed lower levels whereas cisplatin-resistant cell lines
exhibited markedly increased levels of miR-218. Next, we
investigated the endogenous miR-218 expression in an oral cancer
cell line panel. As expected, the levels of miR-218 were
significantly lower in CDDP-sensitive cell lines compared with
CDDP-resistant cell lines (Fig. 1C and
D). To confirm these results, we used oral cancer tissues from
chemosensitive and non-sensitive patients and compared the
expression profiles of miR-218. Chemosensitive or non-sensitive was
determined based on the clinical assessment of patients treated
with cisplatin-based neoadjuvant chemotherapy; those with a good
therapeutic outcome were classified as sensitive, others were
non-sensitive. It was found that miR-218 was increased in the
non-sensitive oral cancers (4.16±0.51 vs. 1.38±0.25; P<0.05,
data not shown).
Downregulation of miR-218 increases
the sensitivity of oral cancer cells to cisplatin
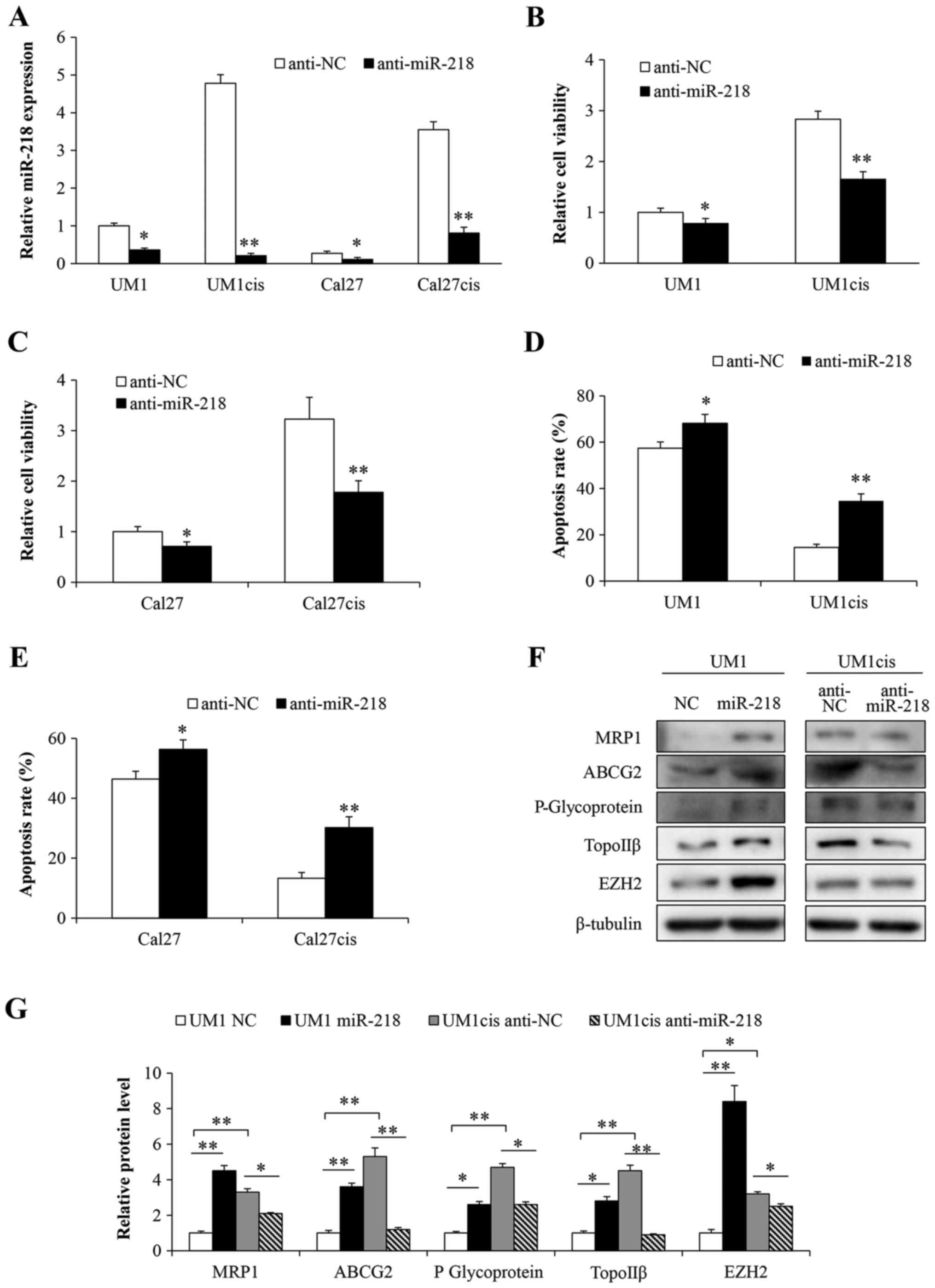
To determine whether the modulation of miR-218
levels influences the response to cisplatin, we suppressed miR-218
expression in cisplatin-resistant cell lines by transfecting cells
with anti-miR-218 (Fig. 2A). Both
UM1 and Cal27 cells became more sensitive to cisplatin compared
with the negative control group (Fig.
2B and C). In addition, a marked increase in apoptosis was
observed in the anti-miR-218-transfected cells (Fig. 2D and E). No significant difference
between the mock and the negative control group were found (data
not shown). Furthermore, we also detected the expression levels of
chemoresistance-related proteins. As expected, increased protein
levels of MRP1, ABCG2, P-glycoprotein, topoisomerase IIβ (TopoIIβ)
and EZH2 were found in the UM1cis cell line compared to the
parental cell line UM1 (Fig. 2F and
G). However, levels of these proteins were reduced when UM1cis
cells were transfected with anti-miR-218 compared with levels in
the anti-NC-transfected cells, whereas levels were increased in the
UM1 cells transfected with the miR-218 mimic (Fig. 2F and G). These results indicate that
miR-218 expression induces oral cancer resistance to cisplatin
treatment.
PPP2R5A is a direct target of miR-218
in oral cancer cells
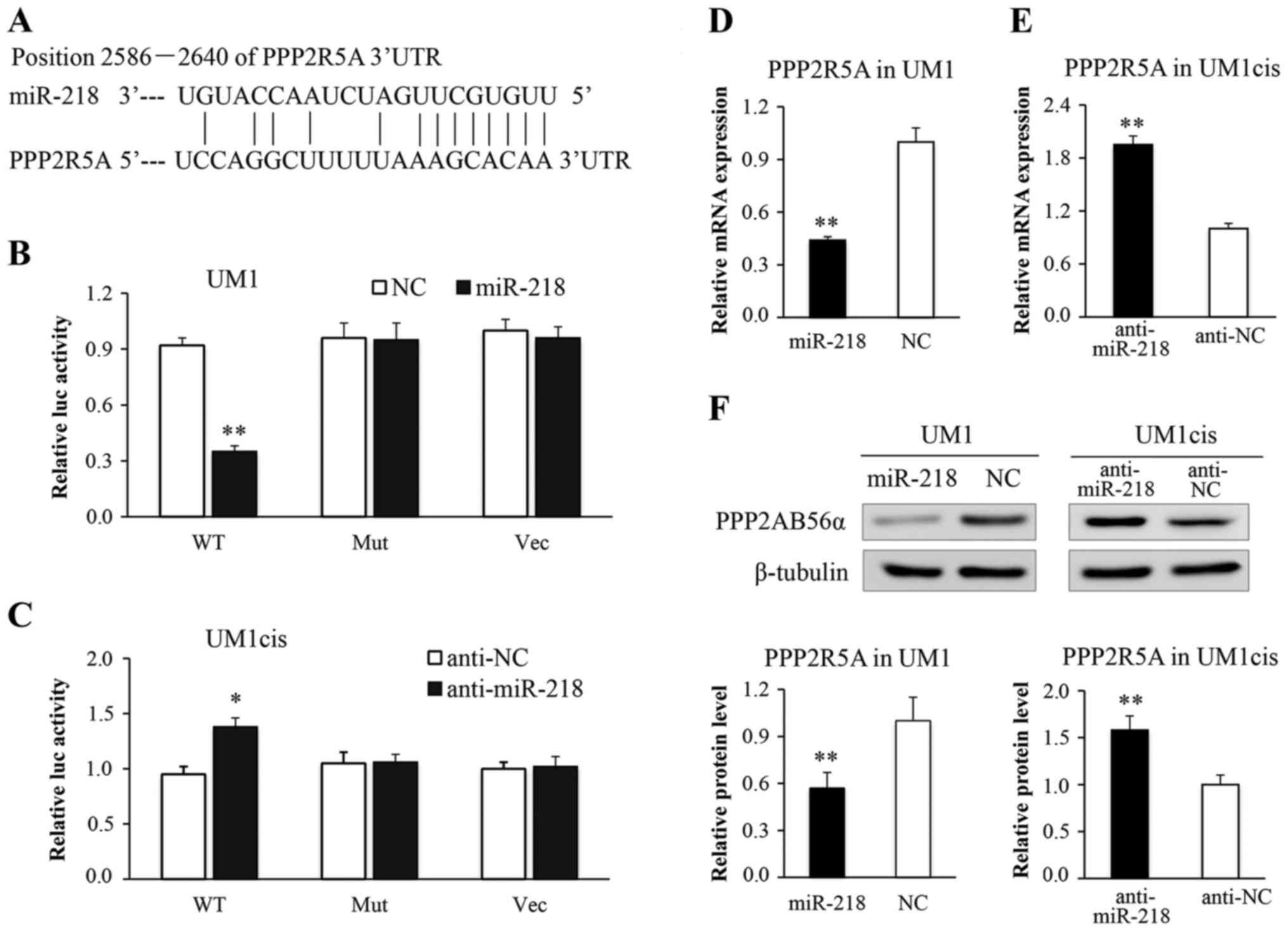
To explore the potential target through which
miR-218 induces cisplatin resistance in oral cancer cells, we
predicted putative miR-218 targets using miRWalk, PicTar,
TargetScan and miRanda. Our analysis identified PPP2R5A, a key
subunit of PP2A, as a candidate miR-218 target (Fig. 3A). The putative targeted sequence
found in the PPP2R5A 3′UTR [wild-type, (WT)] or the mutant sequence
[mutant type (MUT)] was cloned into the luciferase reporter
plasmid, respectively. Our results showed that miR-218 reduced the
luciferase activity in the reporter construct containing wild-type
PPP2R5A, but not the mutant 3′UTR in UM1 cells (Fig. 3B). Furthermore, the luciferase
activity of the construct containing the wild-type 3′UTR, but not
the mutant 3′UTR was increased in the UM1cis cells transfected with
anti-miR-218 (Fig. 3C). The cells
were further transfected with miR-218 or anti-miR-218 and were
examined for PPP2R5A expression by qRT-PCR and western blot
analysis. As shown in Fig. 3D and
F, miR-218 transfection led to an obvious decrease in PPP2R5A
mRNA and protein expression. Conversely, anti-miR-218 transfection
significantly increased the expression level of PPP2R5A (Fig. 3E and F). These data confirmed that
PPP2R5A is a direct target of miR-218 and may contribute to the
effect of miR-218 on oral cancer chemoresistance.
PPP2R5A renders oral cancer cells more
sensitive to cisplatin
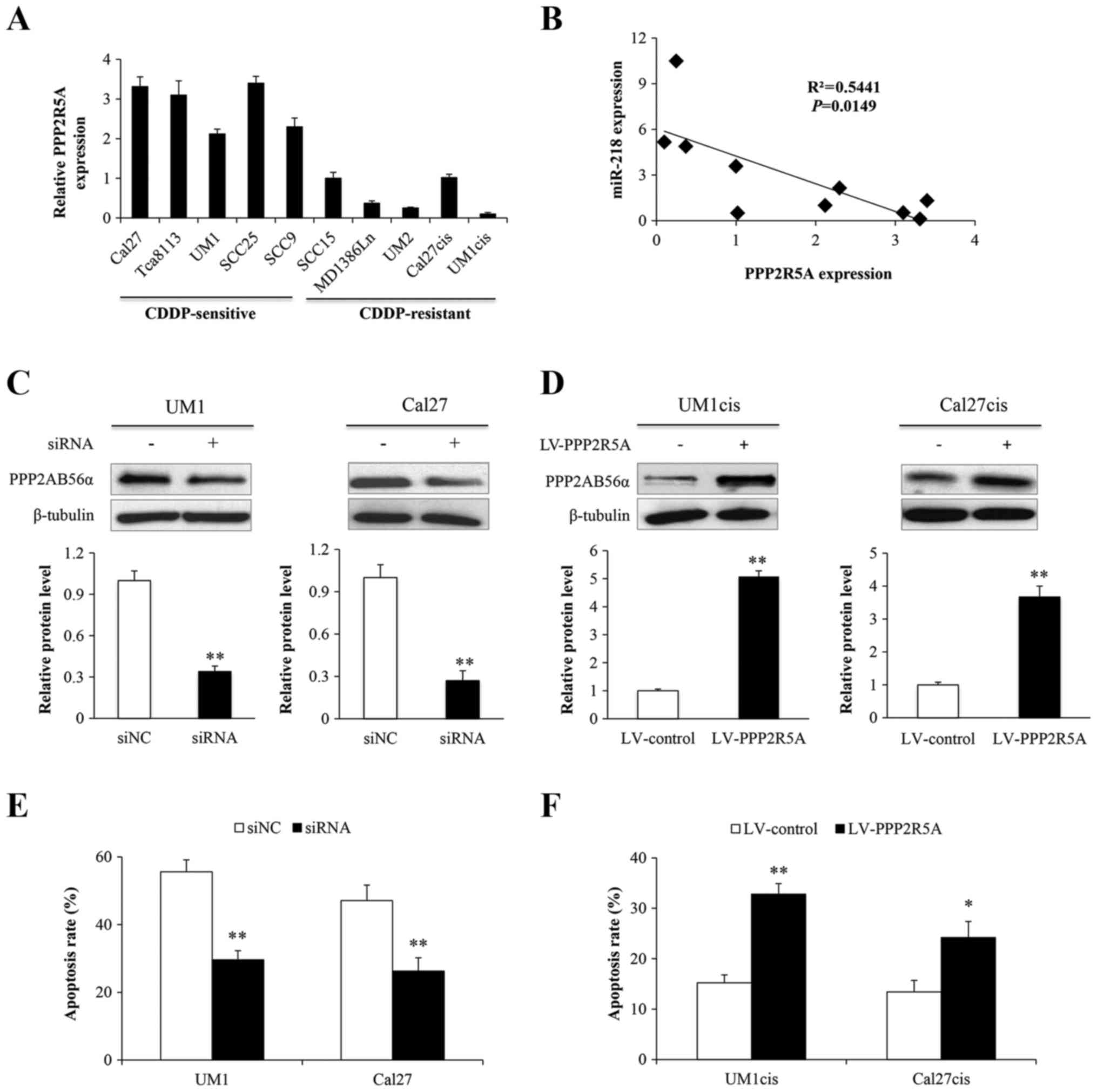
To determine whether the expression levels of
PPP2R5A influence the cisplatin response of oral cancer cells, we
investigated the endogenous PPP2R5A expression in an oral cancer
cell line panel. As shown in Fig.
4A, the levels of PPP2R5A were significantly higher in the
CDDP-sensitive cell lines compared with levels in the
CDDP-resistant cell lines. Moreover, a negative correlation between
expression of PPP2R5A and miR-218 was identified (P=0.0149;
Fig. 4B). Then, the UM1 and Cal27
cells were transfected with an siRNA against PPP2R5A or its control
(siNC) and PPP2R5A protein (PPP2AB56α) was knocked down (Fig. 4C). Alternatively, UM1cis and
Cal27cis cells were infected with lentiviruses expressing PPP2R5A
(LV-PPP2R5A) or its control (LV-control) and PPP2AB56α was indeed
overexpressed (Fig. 4D). The
treated UM1, UM1cis, Cal27 and Cal27cis cells were further exposed
to cisplatin to assess cell apoptosis. The results showed that
suppression of PPP2R5A inhibited apoptosis in the sensitive cells
(Fig. 4E), and overexpression of
PPP2R5A induced apoptosis in the resistant cells (Fig. 4F).
miR-218 induces cisplatin resistance
in oral cancer cells by targeting PPP2R5A
To further explore whether miR-218 plays a role as
an oncomiR through PPP2R5A, we evaluated the expression of PPP2R5A
protein and its correlation with miR-218 expression in human oral
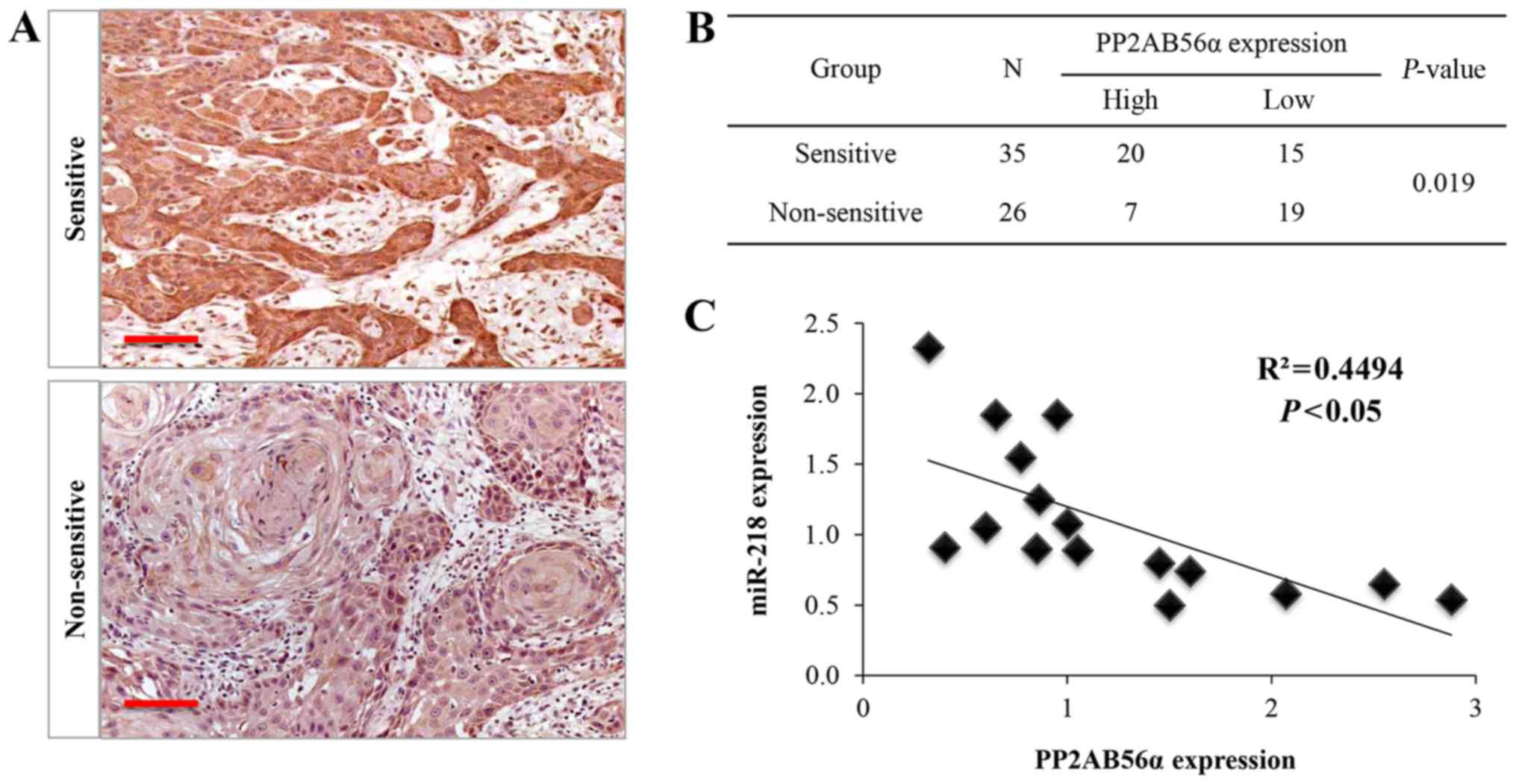
cancer tissues. As expected, PPP2AB56α was upregulated in
chemosensitive oral cancer tissues (Fig. 5A and B). Furthermore, a reverse
correlation between PPP2AB56α and miR-218 expression was noted
(Fig. 5C). Next, we performed
rescue experiments to validate that PPP2R5A targeting is involved
in miR-218-mediated drug resistance. To achieve this aim, UM1 and
Cal27 cells were infected with miR-218 mimics or combined with
LV-PPP2R5A, and cell viability was analyzed using the CCK-8 assay.
It was found that miR-218 enhanced cell viability, and LV-PPP2R5A
coexpression partially abrogated miR-218-induced cell growth
(Fig. 6A). In contrast, cell
viability results showed that anti-miR-218 could restore cisplatin
sensitivity in a concentration-dependent manner in vitro,
which could be partially reversed by the knockdown of PPP2R5A
(Fig. 6B). Western blot analysis
confirmed that PPP2AB56α in these cells was modulated by miR-218
(Fig. 6C). Taken together, our
results revealed that miR-218 acts as an oncomiR and induces
cisplatin resistance in oral cancers by targeting PPP2R5A.
miR-218 targets PPP2R5A, leading to
activation of the Wnt signaling pathway
Given that PPP2R5A is a repressor of Wnt signaling,
we postulated that miR-218 overexpression in oral cancer cells may
play a role in Wnt signaling. In UM1 and Cal27 cells, in which
miR-218 is expressed at low levels and Wnt signaling remains
inactive, we found that miR-218 overexpression resulted in
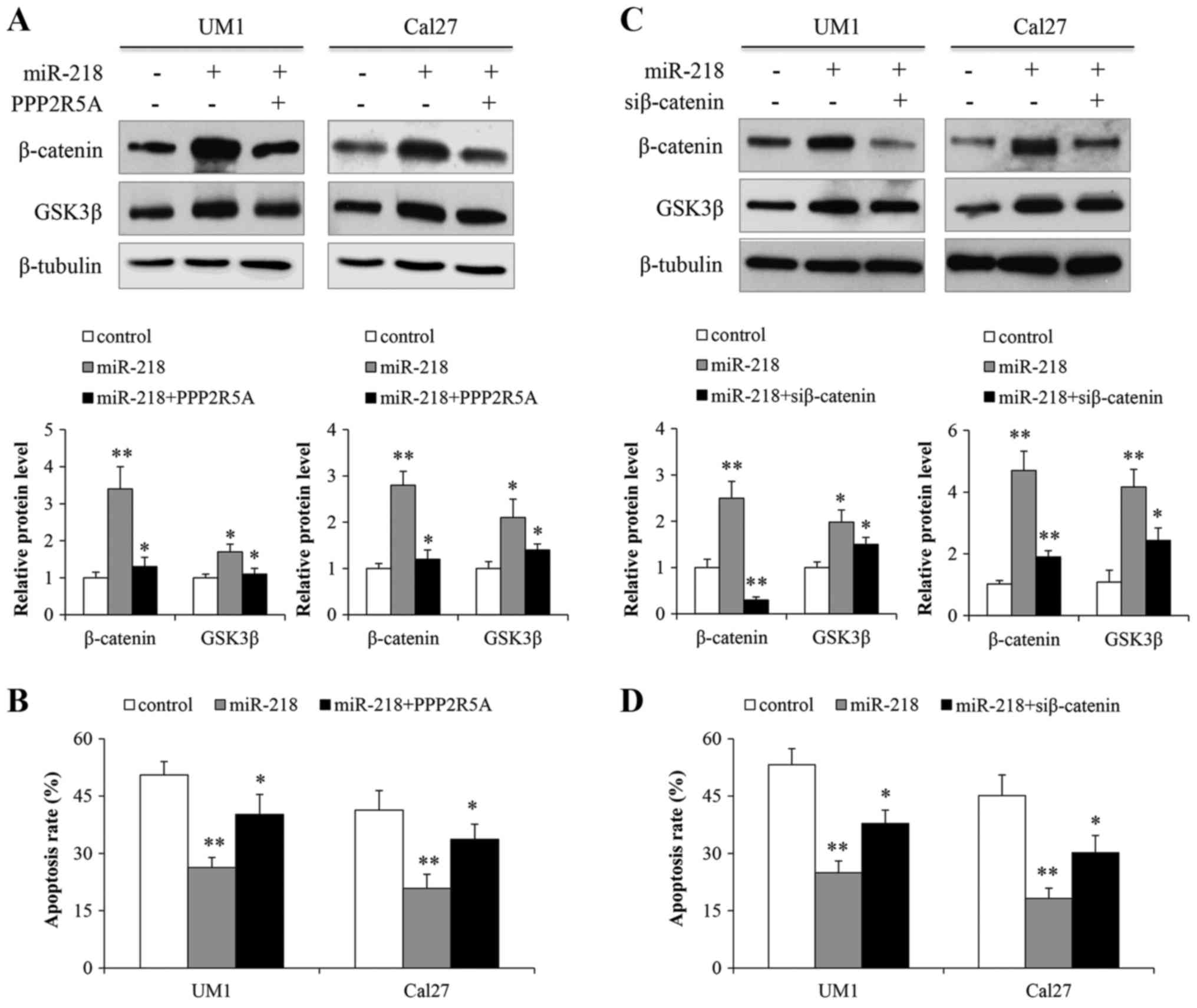
increased levels of β-catenin and GSK3β proteins. When the cells
were simultaneously transfected with miR-218 and LV-PPP2R5A,
β-catenin protein levels were not affected (Fig. 7A). To determine whether miR-218
impairs cisplatin sensitivity specifically through the Wnt
signaling pathway, we transfected UM1 and Cal27 cells with miR-218,
miR-218 + LV-PPP2R5A, or a control prior to cisplatin treatment.
Results from the flow cytometric analysis showed increased
apoptosis of cells transfected with miR-218 + LV-PPP2R5A compared
with those transfected with miR-218 (Fig. 7B). Finally, we transfected siRNA
against β-catenin to validate the miR-218-induced Wnt activation.
As shown in Fig. 7C and D, miR-218
activated Wnt signaling, whereas the siRNA against β-catenin
abrogated the miR-218-promoting activation of Wnt signaling and
cell survival. Collectively, these data indicate that miR-218 may
increase cisplatin resistance via the Wnt signaling pathway in oral
cancer cells by suppressing PPP2R5A expression.
Discussion
Resistance to platinum-based chemotherapy is still
the major barrier to the treatment of oral cancer. In the present
study, we provide evidence of the role of miR-218 in the resistance
of oral cancer cells to cisplatin through the Wnt signaling pathway
by targeting PPP2R5A. We showed that miR-218 was significantly
upregulated in cisplatin-resistant oral cancer cell lines, and in
oral cancer patients with failed neoadjuvant chemotherapy. We also
demonstrated that miR-218 decreased PPP2R5A levels and increases
β-catenin and GSK3β expression. Importantly, inhibition of miR-218
restored the sensitivity of oral cancer cells to cisplatin therapy.
Moreover, sensitivity to cisplatin was partially restored when
cells transfected with miR-218 were cotransfected with LV-PPP2R5A
or siRNA against β-catenin compared with the control. Thus, our
results suggest that miR-218 regulates the cisplatin response of
oral cancers through the PPP2R5A/Wnt signaling pathway.
In the present study, UM1 and Cal27 cells, both
sensitive to cisplatin, were continuously exposed to a gradient
concentration of cisplatin for ~6 months. The IC50 of
UM1cis and Cal27cis cells increased by 7- and 9-fold, respectively,
as compared with their parental cell lines. Our results were
consistent with the findings of Sun et al (10) and Gosepath et al (31). We also determined the expression
levels of chemoresistance-related proteins, including EZH2, ABCG2,
MRP1, P-glycoprotein and topoisomerase IIβ. These results further
identified the resistance to cisplatin of the two resistant cells.
To an extent, UM1cis and Cal27cis cells may serve as a tool for
further investigation on the molecular mechanism of drug resistance
of oral cancer cells.
Various microRNAs (miRNAs) have been demonstrated to
regulate the resistance of oral cancer cells to cisplatin, acting
as either oncogenes or tumor suppressors (8–10,32–34).
Reduced miR-218 has been shown to potentiate the malignant
progression of many types of cancers by targeting Robo1 (12), survivin (13,18),
BMI1 (15) and laminin 332
(35). Restoration of the
expression of miR-218 was found to reduce the proliferation,
migration and invasion and increase the sensitivity of tumor cells
to chemotherapeutic agents (20,22).
Similarly, decreased expression of miR-218 has also been observed
in oral cancers. Patients with lower levels of miR-218 have a
higher risk of a poor outcome (19,36,37).
However, the activity of miR-218 appears to be functionally
different in breast cancer and glioma (24,25).
These conflicting data suggest that miR-218 may act as either an
oncogene or a tumor suppressor, depending on the tumor type, tumor
microenvironment and its targets.
To date, the role of miR-218 in the chemoresistance
of oral cancer is not clear. In the present study, we observed a
significantly higher expression of miR-218 in chemoresistant oral
cancer cells both in vitro and in vivo. Our results
are consistent with those obtained in a recent study by Wang et
al (26). Given that miRNAs are
important molecular regulators in gene expression through the
repression of transcription and translation, we further explored
the possible biological link between miR-218 and its potential
targets. Notably, a total of 7 Wnt-associated genes were predicted
to be miR-218 targets by bioinformatic analysis. Of which, a clear
association between PPP2R5A and miR-218 expression was identified
in oral cancer cells both in vitro and in vivo.
Importantly, PPP2R5A was experimentally validated as a direct
target of miR-218. Ectopic miR-218 expression or PPP2R5A
downregulation reduced cisplatin-induced apoptosis of oral cancer
cells. In contrast, inhibition of miR-218 sensitized oral cancer
cells to cisplatin, and PPP2R5A sensitized the cells to cisplatin.
These results indicated that miR-218 is involved in the regulation
of cisplatin resistance in oral cancer.
Aberrant Wnt signaling can promote malignant
progression in many epithelial cancers, including oral cancer.
Recently, several studies have demonstrated a role for miRNAs in
oral cancer via modulation of Wnt pathway components. For example,
Shiah et al reported that decreased expression of miR-329
and miR-410, which target a canonical Wnt ligand, Wnt7b, increased
β-catenin activity (38). Moreover,
miR-21 promoted Wnt/β-catenin signaling activation by directly
targeting Dkk2. Decreased miR-21 suppressed the invasive potential
of tongue cancer cells with upregulated Dkk2 protein levels
(39). In the present study, we
found that sensitivity to cisplatin was decreased by upregulation
of miR-218. The effects of miR-218 on the chemosensitivity of oral
cancer cells may be explained by its indirect activation of Wnt
signaling via inhibition of PPP2R5A, which counteracts the
inhibitory effects of cisplatin on the same pathway. This notion is
supported by the observation that cotransfection of LV-PPP2R5A or
administration of the specific siRNA against β-catenin blocked the
effects of miR-218.
MicroRNAs such as miR-218, may exert different
biological functions depending on the microenviroment, but the
mechanisms are largely unknown. Previous studies have shown
decreased expression of miR-218 in human malignancies including
oral cancers (19,36,37).
Hence, miR-218 may act as a tumor suppressor in these types of
cancers. Currently, the role of miR-218 in the chemoresistance of
oral cancer is not clear. In the present study, we report that
miR-218 promotes cisplatin resistance in oral cancer. We propose
several reasons for our inconsistent results. Firstly, miR-218 is
an intronic miRNA that is co-expressed with its host genes SLIT2/3
(11). The expression of miR-218
and SLIT2/3 can be regulated via epigenetic mechanisms, such as DNA
methylation (40,41). Numerous studies have demonstrated
substantial epigenetic alterations in drug-resistant cancer cells
(42). Secondly, the oncogene E6 of
the high-risk HPV likely contributes to miR-218 expression in
cervical and non-small cell lung cancer (17,43).
Oral cancer, particularly oropharyngeal cancer, is closely related
to HPV infection, which may have an effect on the role of miR-218
in chemoresistance. Finally, the function of miR-218 depends on its
targets. An miRNA can affect the expression levels of thousands of
genes. Several studies have demonstrated that miR-218 inhibits
metastasis via targeting multiple components of the Wnt/β-catenin
pathway (44). Conversely, miR-218
activates the Wnt pathway by targeting its inhibitors SOST and
DKK2, thus, promoting metastasis (25,45).
These controversial findings reflect the complexity of miR-218
regulation in cancers. Based on our results, we envision that
miR-218-induced Wnt signaling activation may also contribute to its
induction of resistance to cisplatin. Nevertheless, we must note
that oral tumorigenesis and chemoresistance are complicated
processes and involve a variety of regulators. Further studies are
needed to determine the precise mechanisms underlying cisplatin
resistance in oral cancer.
In summary, we identified miR-218 as an important
mediator of the cisplatin response in oral cancer. miR-218
decreased cisplatin sensitivity at least in part through the Wnt
signaling pathway in oral cancer by directly targeting PPP2R5A.
Based on the potential of miRNA therapy, inhibition of miR-218 may
be a therapeutic approach to improve cisplatin sensitivity in oral
cancer.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (81172567, 81272949,
81202136, 81372885, 81572661 and 81572939), the Major Special
Research Collaborative Innovation of Guangzhou (201604020160), and
the Pear River S&T Nova Program of Guangzhou
(2014J2200045).
Glossary
Abbreviations
Abbreviations:
|
miR-218
|
microRNA-218
|
|
3′UTR
|
3′ untranslated region
|
|
PPP2R5A
|
protein phosphatase 2 regulatory
subunit B-α
|
|
GSK3β
|
glycogen synthase kinase 3β
|
|
TopoIIβ
|
τopoisomerase IIβ
|
References
|
1
|
Wang C, Liu XQ, Hou JS, Wang JN and Huang
HZ: Molecular mechanisms of chemoresistance in oral cancer. Chin J
Dent Res. 19:25–33. 2016.PubMed/NCBI
|
|
2
|
Zhang P, Zhang Z, Zhou X, Qiu W, Chen F
and Chen W: Identification of genes associated with cisplatin
resistance in human oral squamous cell carcinoma cell line. BMC
Cancer. 6:2242006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang B, Liu M, Tang HK, Ma HB, Wang C,
Chen X and Huang HZ: The expression and significance of MRP1, LRP,
TOPOIIβ, and BCL2 in tongue squamous cell carcinoma. J Oral Pathol
Med. 41:141–148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olszewski U and Hamilton G: A better
platinum-based anticancer drug yet to come? Anticancer Agents Med
Chem. 10:293–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Donzelli S, Mori F, Biagioni F, Bellissimo
T, Pulito C, Muti P, Strano S and Blandino G: MicroRNAs: Short
non-coding players in cancer chemoresistance. Mol Cell Ther.
2:162014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garofalo M and Croce CM: MicroRNAs as
therapeutic targets in chemoresistance. Drug Resist Updat.
16:47–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu ZW, Zhong LP, Ji T, Zhang P, Chen WT
and Zhang CP: MicroRNAs contribute to the chemoresistance of
cisplatin in tongue squamous cell carcinoma lines. Oral Oncol.
46:317–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ren W, Wang X, Gao L, Li S, Yan X, Zhang
J, Huang C, Zhang Y and Zhi K: MiR-21 modulates chemosensitivity of
tongue squamous cell carcinoma cells to cisplatin by targeting
PDCD4. Mol Cell Biochem. 390:253–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Huang H, Sun L, Yang M, Pan C, Chen
W, Wu D, Lin Z, Zeng C, Yao Y, et al: MiR-21 indicates poor
prognosis in tongue squamous cell carcinomas as an apoptosis
inhibitor. Clin Cancer Res. 15:3998–4008. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun L, Yao Y, Liu B, Lin Z, Lin L, Yang M,
Zhang W, Chen W, Pan C, Liu Q, et al: MiR-200b and miR-15b regulate
chemotherapy-induced epithelial-mesenchymal transition in human
tongue cancer cells by targeting BMI1. Oncogene. 31:432–445. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tatarano S, Chiyomaru T, Kawakami K,
Enokida H, Yoshino H, Hidaka H, Yamasaki T, Kawahara K, Nishiyama
K, Seki N, et al: miR-218 on the genomic loss region of
chromosome 4p15.31 functions as a tumor suppressor in bladder
cancer. Int J Oncol. 39:13–21. 2011.PubMed/NCBI
|
|
12
|
Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S,
Guo X, Wang B, Gang Y, Zhang Y, et al: MiR-218 inhibits invasion
and metastasis of gastric cancer by targeting the Robo1 receptor.
PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alajez NM, Lenarduzzi M, Ito E, Hui AB,
Shi W, Bruce J, Yue S, Huang SH, Xu W, Waldron J, et al: MiR-218
suppresses nasopharyngeal cancer progression through downregulation
of survivin and the SLIT2-ROBO1 pathway. Cancer Res. 71:2381–2391.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Venkataraman S, Birks DK, Balakrishnan I,
Alimova I, Harris PS, Patel PR, Handler MH, Dubuc A, Taylor MD,
Foreman NK, et al: MicroRNA 218 acts as a tumor suppressor by
targeting multiple cancer phenotype-associated genes in
medulloblastoma. J Biol Chem. 288:1918–1928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tu Y, Gao X, Li G, Fu H, Cui D, Liu H, Jin
W and Zhang Y: MicroRNA-218 inhibits glioma invasion, migration,
proliferation, and cancer stem-like cell self-renewal by targeting
the polycomb group gene Bmi1. Cancer Res. 73:6046–6055. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mathew LK, Skuli N, Mucaj V, Lee SS, Zinn
PO, Sathyan P, Imtiyaz HZ, Zhang Z, Davuluri RV, Rao S, et al:
miR-218 opposes a critical RTK-HIF pathway in mesenchymal
glioblastoma. Proc Natl Acad Sci USA. 111:291–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu DW, Cheng YW, Wang J, Chen CY and Lee
H: Paxillin predicts survival and relapse in non-small cell lung
cancer by microRNA-218 targeting. Cancer Res. 70:10392–10401. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kogo R, How C, Chaudary N, Bruce J, Shi W,
Hill RP, Zahedi P, Yip KW and Liu FF: The microRNA-218~Survivin
axis regulates migration, invasion, and lymph node metastasis in
cervical cancer. Oncotarget. 6:1090–1100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu DW, Chuang CY, Lin WL, Sung WW, Cheng
YW and Lee H: Paxillin promotes tumor progression and predicts
survival and relapse in oral cavity squamous cell carcinoma by
microRNA-218 targeting. Carcinogenesis. 35:1823–1829. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie J, Yu F, Li D, Zhu X, Zhang X and Lv
Z: MicroRNA-218 regulates cisplatin (DPP) chemosensitivity in
non-small cell lung cancer by targeting RUNX2. Tumour Biol.
37:1197–1204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian H, Hou L, Xiong YM, Huang JX, She YJ,
Bi XB and Song XR: miR-218 suppresses tumor growth and enhances the
chemosensitivity of esophageal squamous cell carcinoma to
cisplatin. Oncol Rep. 33:981–989. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zarogoulidis P, Petanidis S, Kioseoglou E,
Domvri K, Anestakis D and Zarogoulidis K: MiR-205 and miR-218
expression is associated with carboplatin chemoresistance and
regulation of apoptosis via Mcl-1 and Survivin in lung cancer
cells. Cell Signal. 27:1576–1588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zanette DL, Rivadavia F, Molfetta GA,
Barbuzano FG, Proto-Siqueira R, Silva-Jr WA, Falcão RP and Zago MA:
miRNA expression profiles in chronic lymphocytic and acute
lymphocytic leukemia. Braz J Med Biol Res. 40:1435–1440. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng Z, Zhang L, Zhou J, Zhou S, Li L, Guo
X, Feng G, Ma Z, Huang W and Huang F: mir-218-2 promotes
glioblastomas growth, invasion and drug resistance by targeting
CDC27. Oncotarget. 8:6304–6318. 2017.PubMed/NCBI
|
|
25
|
Hassan MQ, Maeda Y, Taipaleenmaki H, Zhang
W, Jafferji M, Gordon JA, Li Z, Croce CM, van Wijnen AJ, Stein JL,
et al: miR-218 directs a Wnt signaling circuit to promote
differentiation of osteoblasts and osteomimicry of metastatic
cancer cells. J Biol Chem. 287:42084–42092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang S, Su X, Bai H, Zhao J, Duan J, An T,
Zhuo M, Wang Z, Wu M, Li Z, et al: Identification of plasma
microRNA profiles for primary resistance to EGFR-TKIs in advanced
non-small cell lung cancer (NSCLC) patients with EGFR activating
mutation. J Hematol Oncol. 8:1272015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wlodarchak N and Xing Y: PP2A as a master
regulator of the cell cycle. Crit Rev Biochem Mol Biol. 51:162–184.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar A, Pandurangan AK, Lu F, Fyrst H,
Zhang M, Byun HS, Bittman R and Saba JD: Chemopreventive
sphingadienes downregulate Wnt signaling via a PP2A/Akt/GSK3β
pathway in colon cancer. Carcinogenesis. 33:1726–1735. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mitra A, Menezes ME, Pannell LK, Mulekar
MS, Honkanen RE, Shevde LA and Samant RS: DNAJB6 chaperones PP2A
mediated dephosphorylation of GSK3β to downregulate β-catenin
transcription target, osteopontin. Oncogene. 31:4472–4483. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Wang C, Chen Z, Jin Y, Wang Y,
Kolokythas A, Dai Y and Zhou X: MicroRNA-138 suppresses
epithelial-mesenchymal transition in squamous cell carcinoma cell
lines. Biochem J. 440:23–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gosepath EM, Eckstein N, Hamacher A,
Servan K, von Jonquieres G, Lage H, Györffy B, Royer HD and Kassack
MU: Acquired cisplatin resistance in the head-neck cancer cell line
Cal27 is associated with decreased DKK1 expression and can
partially be reversed by overexpression of DKK1. Int J Cancer.
123:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu M, Wang J, Huang H, Hou J, Zhang B and
Wang A: miR-181a-Twist1 pathway in the chemoresistance of tongue
squamous cell carcinoma. Biochem Biophys Res Commun. 441:364–370.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou X, Ren Y, Liu A, Jin R, Jiang Q,
Huang Y, Kong L, Wang X and Zhang L: WP1066 sensitizes oral
squamous cell carcinoma cells to cisplatin by targeting
STAT3/miR-21 axis. Sci Rep. 4:74612014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peng F, Zhang H, Du Y and Tan P: miR-23a
promotes cisplatin chemoresistance and protects against
cisplatin-induced apoptosis in tongue squamous cell carcinoma cells
through Twist. Oncol Rep. 33:942–950. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kinoshita T, Hanazawa T, Nohata N, Kikkawa
N, Enokida H, Yoshino H, Yamasaki T, Hidaka H, Nakagawa M, Okamoto
Y, et al: Tumor suppressive microRNA-218 inhibits cancer cell
migration and invasion through targeting laminin-332 in head and
neck squamous cell carcinoma. Oncotarget. 3:1386–1400. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peng SC, Liao CT, Peng CH, Cheng AJ, Chen
SJ, Huang CG, Hsieh WP and Yen TC: MicroRNAs MiR-218, MiR-125b, and
Let-7g predict prognosis in patients with oral cavity squamous cell
carcinoma. PLoS One. 9:e1024032014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jamali Z, Aminabadi Asl N, Attaran R,
Pournagiazar F, Oskouei Ghertasi S and Ahmadpour F: MicroRNAs as
prognostic molecular signatures in human head and neck squamous
cell carcinoma: A systematic review and meta-analysis. Oral Oncol.
51:321–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shiah SG, Hsiao JR, Chang WM, Chen YW, Jin
YT, Wong TY, Huang JS, Tsai ST, Hsu YM, Chou ST, et al:
Downregulated miR329 and miR410 promote the proliferation and
invasion of oral squamous cell carcinoma by targeting Wnt-7b.
Cancer Res. 74:7560–7572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kawakita A, Yanamoto S, Yamada S, Naruse
T, Takahashi H, Kawasaki G and Umeda M: MicroRNA-21 promotes oral
cancer invasion via the Wnt/β-catenin pathway by targeting DKK2.
Pathol Oncol Res. 20:253–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dickinson RE, Dallol A, Bieche I, Krex D,
Morton D, Maher ER and Latif F: Epigenetic inactivation of
SLIT3 and SLIT1 genes in human cancers. Br J Cancer.
91:2071–2078. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Narayan G, Goparaju C, Arias-Pulido H,
Kaufmann AM, Schneider A, Dürst M, Mansukhani M, Pothuri B and
Murty VV: Promoter hypermethylation-mediated inactivation of
multiple Slit-Robo pathway genes in cervical cancer progression.
Mol Cancer. 5:162006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Roberti A, La Sala D and Cinti C: Multiple
genetic and epigenetic interacting mechanisms contribute to
clonally selection of drug-resistant tumors: Current views and new
therapeutic prospective. J Cell Physiol. 207:571–581. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Martinez I, Gardiner AS, Board KF, Monzon
FA, Edwards RP and Khan SA: Human papillomavirus type 16 reduces
the expression of microRNA-218 in cervical carcinoma cells.
Oncogene. 27:2575–2582. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Iwai S, Yonekawa A, Harada C, Hamada M,
Katagiri W, Nakazawa M and Yura Y: Involvement of the Wnt-β-catenin
pathway in invasion and migration of oral squamous carcinoma cells.
Int J Oncol. 37:1095–1103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang WB, Zhong WJ and Wang L: A
signal-amplification circuit between miR-218 and Wnt/β-catenin
signal promotes human adipose tissue-derived stem cells osteogenic
differentiation. Bone. 58:59–66. 2014. View Article : Google Scholar : PubMed/NCBI
|