Introduction
Multidrug resistance (MDR) is one of the primary
problems to efficient cancer therapy (1). Cancer cells may employ several
mechanisms of drug resistance such as apoptosis, autophagy, DNA
repair, efflux transporters, and epigenetic regulation to acquire
multidrug resistance (2,3).
In 1980s, Hedgehog (Hh) signal transduction pathway
research made remarkable progress in fruit fly (4). The Hedgehog signaling pathway
regulates cell proliferation, cell fate and patterning, stem and
progenitor cell maintenance, as well as self-renewal and tissue
repair (5,6). It is now known that Hh signaling has
two distinct facets of action mechanisms: the canonical and
non-canonical pathways (7,8). The canonical pathway components
include ligands (sonic Hh, Indian Hh, and desert Hh), patched
receptors (PTCH1, PTCH2), signal transducer smoothened (SMO), and
transcription factors (Gli1, Gli2, Gli3) (9). In brief, it is about binding of
Hedgehog ligands secreted from one cell and the PTCH receptor on
another cell. The non-canonical pathway initiates Hedgehog
signaling, via Shh-mediated ERK activation, Wnt signaling results
in expression and activation of Gli proteins, and the atypical
mutual interaction of core Hh pathway components (10–12).
Traditional Chinese Medicines (TCMs) have been used
as medicines, or health supplements in China and in East Asia for
millennia. Traditional Chinese prescriptions and formulae, based on
TCM principles, have been considered to target multiple pathways,
and have been used to treat cancer, such as breast carcinoma
(13), gastric cancer (14) and colorectal cancer (15). Similar efficiency as the
chemotherapeutic drugs, traditional Chinese prescriptions and
formulae are capable of effectively controling cancer progression,
improving quality of life and prolonging survival times.
ZZR, a TCM empirical prescription developed in
Shanghai General Hospital, which is a recipe derived from a classic
TCM principle (nvigorating spleen and detoxification and opening
collateral). Some studies illustrated that a Chinese formula, named
JPJD, played an important role in liver cancer cure through
upregulating expression levels of ABCC2 and downregulating levels
of OATP1B2 (16). Additionally, our
in vivo study demonstrated that intragastric administration
of ZZR remarkedly inhibited P-gp expression levels and increased
the sensitivity to chemotherapy drugs in CRC MDR cells. Also, the
underlying mechanisms of the anti-metastatic effects associated
with this formula remain unknown.
Hh signaling pathway can be considered an actionable
target for therapeutic intervention in CRC. The present study
explored how Hh signaling pathway regulated ABC
transporter-mediated MDR and potential mechanisms of ZZR extract by
evaluating antitumor effects of this formula both in vitro
and in vivo.
Materials and methods
Preparation of the extracts for
ZZR
ZZR extract was prepared according to our previously
published method (17), including
Huang-Qi (Radix Astragali), Nv-Zhen-Zi (Fructus Ligustri
Lucidi), Yi-yi-ren (Semen Coicis), Shi-Jian-Chuan
(Salvia Chinensis), Ye-Pu-Tao-Teng (Vitis quinquangularis
Rehd), Teng-Li-Gen (Actinidia arguta), and Zhi-Xiang-Fu
(Cyperus rotundus L.). In brief, the above mentioned 6 herbs
were mixed at a ratio of 6:3:6:6:6:6:2 for a total dry weight of
175 g. The herb concoction was immersed in 55% ethanol (1:10 w/v)
for 4 h and refluxed for 1.5 h. After filtration, the residue was
again refluxed with 55% ethanol (1:8, w/v) for 1 h and filtered.
The two decoctions were dried by lyophilization to obtain the ZZR
extract with a yield of dried powder of 24.4%. Simultaneous
quantification of five major active constituents in the extract was
conducted by high-performance liquid chromatography (HPLC). The
extract was stored at 4°C, and its preparations were standardized,
regulated and quality controlled according to the guidelines
defined by Chinese State Food and Drug Administration (SFDA).
Cell culture and reagents
The human colorectal cancer HCT-116 and HCT-8
parental cell lines were purchased from the Shanghai Cell
Collection (Shanghai, China). HCT-116/L-OHP cell line and
HCT-8/5-FU cell line were generated and maintained in our
laboratory. Cells were maintained in RPMI-1640 containing 10% FBS,
penicillin (100 U/ml), and streptomycin (100 mg/ml) (Invitrogen,
Carlsbad, CA, USA) at 37°C in a 5% CO2 humidified
atmosphere. HCT-116/L-OHP cells were routinely maintained in a
medium containing 5,000 ng/ml oxaliplatin (L-OHP), and HCT-8/5-FU
cells were in 1,000 ng/ml (5-FU). L-OHP was purchased from Shenzhen
Main Luck Pharmaceuticals Co., Ltd. (Shenzhen, China), 5-FU was
purchased from Shanghai Xudong Haipu Pharmaceutical Co., Ltd.
(Shanghai, China). Monoclonal antibodies against Ptch1, Gli1, Gli2,
and GAPDH were products of Cell Signaling Technology (Beverly, MA,
USA). GANT61 and SAG were purchased from Selleck (Houston, TX,
USA).
Cell viability asays
Cell proliferation assay was conducted using the
cell count kit CCK-8 (Sigma), as previously described (17). Briefly, cells were seeded in 96-well
plates at 1×104 cells/well. When the cells reached 60%
confluence, the medium was removed and replaced with fresh medium
containing varying concentrations of ZZR with or without
chemotherapy drugs (L-OHP and 5-FU) and incubated for 48 h. The
absorbance was read at 450 nm using a microplate enzyme-linked
immunosorbent assay reader (Labsystems Dragon, Wellscan). All
experiments were performed with 5 wells per experiment and repeated
at least three times. IC10, IC20,
IC50 (the half maximal inhibitory concentration) was
estimated according to the following formula respectively:
IC50=lg-1[Xm-i(ΣP-0.5)]; Xm, the maximum concentration;
i, logarithm of the concentration; ΣP, sum of growth inhibition
rate. IC10 and IC20 was calculated according
to SPSS statistical software.
Apoptosis assay
Cells were plated in 6-well plates (1×106
cells/well) and after treatment were trypsinized and analyzed using
an Annexin V-fluorescein isothiocyanate detection kit (eBioscience
Inc., San Diego, CA, USA). Three concentrations of ZZR (obtained
from the result of ZZR Cell Viability assays) and L-OHP (10 µM)
were added for 24 h. The samples were immediately processed using
flow cytometry to detect the relative amount of Annexin
V-FITC-positive-PI-negative cells, as previously described
(17).
Western blot analysis
Equal protein per lysate was resolved on
Tris-glycine gel, transferred onto nitrocellulose membrane, and
blocked for 1 h with 5% non-fat dry milk. Membranes were incubated
with desired primary antibody: Ptch1, Gli1, and Gli2 with 1:1,000
(Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at
4°C and then with appropriate secondary antibody as previously
reported (18). Equal loading was
confirmed with GAPDH (0.1 µg/ml). Densitometric analysis was
performed using the Scion Imaging software (Scion Corp.). GAPDH was
used as a control for each sample.
Animal experiments
Male athymic nude mice (NCr-nu), 8–12 weeks old,
were purchased from Sino-British SIPPR/BK Lab Animal Co., Ltd.
(Shanghai, China, license no. SCXK 2010–0012), and maintained under
specific-pathogen-free conditions. All animal protocols were
approved by the Institutional Animal Use and Care Committee. All
the experiments and animal care were approved by Shanghai Medical
Experimental Animal Care Commission and in accordance with the
Provision and General Recommendation of Chinese Experimental
Animals Administration Legislation.
Mice were subcutaneously injected with
1.0×106 HCT-116/L-OHP cells per animal. When the tumors
reach an average size of 100 mm3, the mice were
randomized into 5 groups (n=6 per group) and received intragastric
administration of vehicle control, L-OHP and amixture of ZZR and
L-OHP. Briefly, L-OHP was given as an intraperitoneal injection
every 2 days and the injection dosage (5 mg/kg) was according to
half of the maximum tolerated dose (MTD) of oxaliplatin as
previously described (19). ZZR was
given every day at the doses of 13.27, 26.54 and 53.08 g/kg. In the
clinical practice of Chinese herbal medicine, ZZR is usually
prescribed at a daily dose of 175 g of herbal materials. When this
human dose was converted into an animal dose (a person of 60 kg,
and a conversion factor of 9.1 between human and mouse), it was
equivalent to the middle dose (26.54 g/kg) used in this study.
The body weight of the animals and the two
perpendicular diameters (A and B) of tumor were recorded every 3
days and tumor volume (V) was estimated according to the following
formula (17):
V=π/6x[(A+B)/2]3. The curve of tumor growth was drawn
according to tumor volume and time of implantation. Six mice were
sacrificed by decapitation in each group on 28th day after
treatment.
Immunohistochemical analysis
Hydrated paraffin sections were incubated in a
blocking solution (10% donkey serum + 5% non-fat dry milk + 4% BSA
+ 0.1% Triton X-100) for 10 min, and then incubated at 4°C
overnight with anti-Gli1 (1:500). The analysis was conducted as
described previously (19).
Statistical analysis
All quatitative data were expressed as mean ±
standard of at least three independent experiments. Statistical
analyses were conducted using the Student's t-test. P<0.05 or
P<0.01 were considered statistically significant.
Results
Analysis of active compounds in
ZZR
To ensure the quality and stability of ZZR formula,
we characterized the active components and their concentration
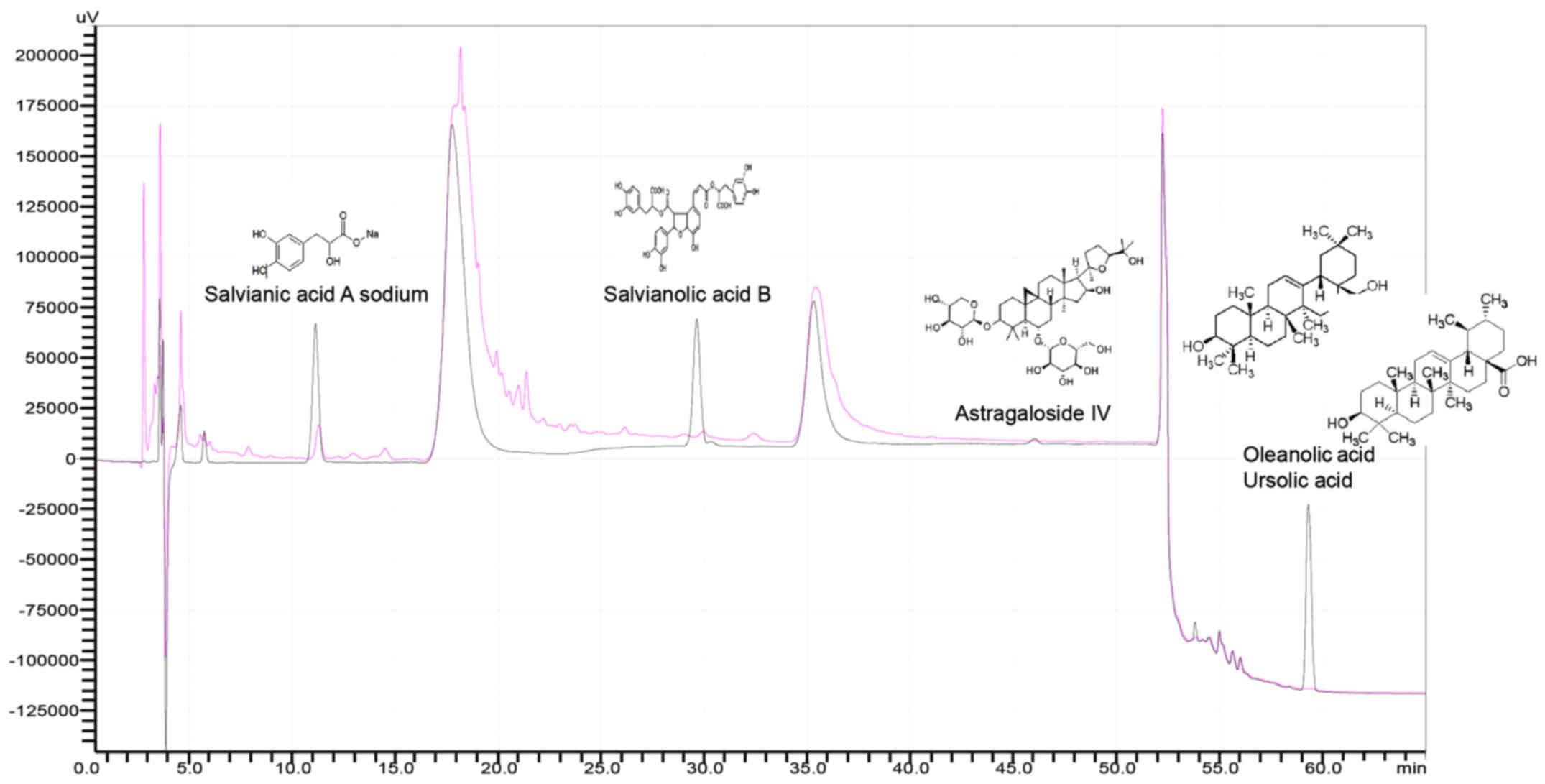
using HPLC. As shown in Fig. 1,
compared to standards, the amount of salvianic acid A sodium,
salvianolic acid B and astragaloside IV are lower in ZZR ethanol
extraction, but higher than that of ZZR water extract (data not
shown). Thus, in this study, 55% ethanol was adopted for ZZR
extraction. As the ursolic acid and oleanolic acid isomers are
inseparable (Fig. 1), salvianic
acid A sodium, salvianolic acid B and astragaloside IV were
therefore predicted to be the major antitumor components of ZZR
solution.
Non-cytotoxic dose of ZZR
To rule out the possibility of cytotoxicity-induced
inhibition of cell proliferation, we determined the cytotoxic
effect of ZZR on colorectal cancer cells HCT-116 and its MDR
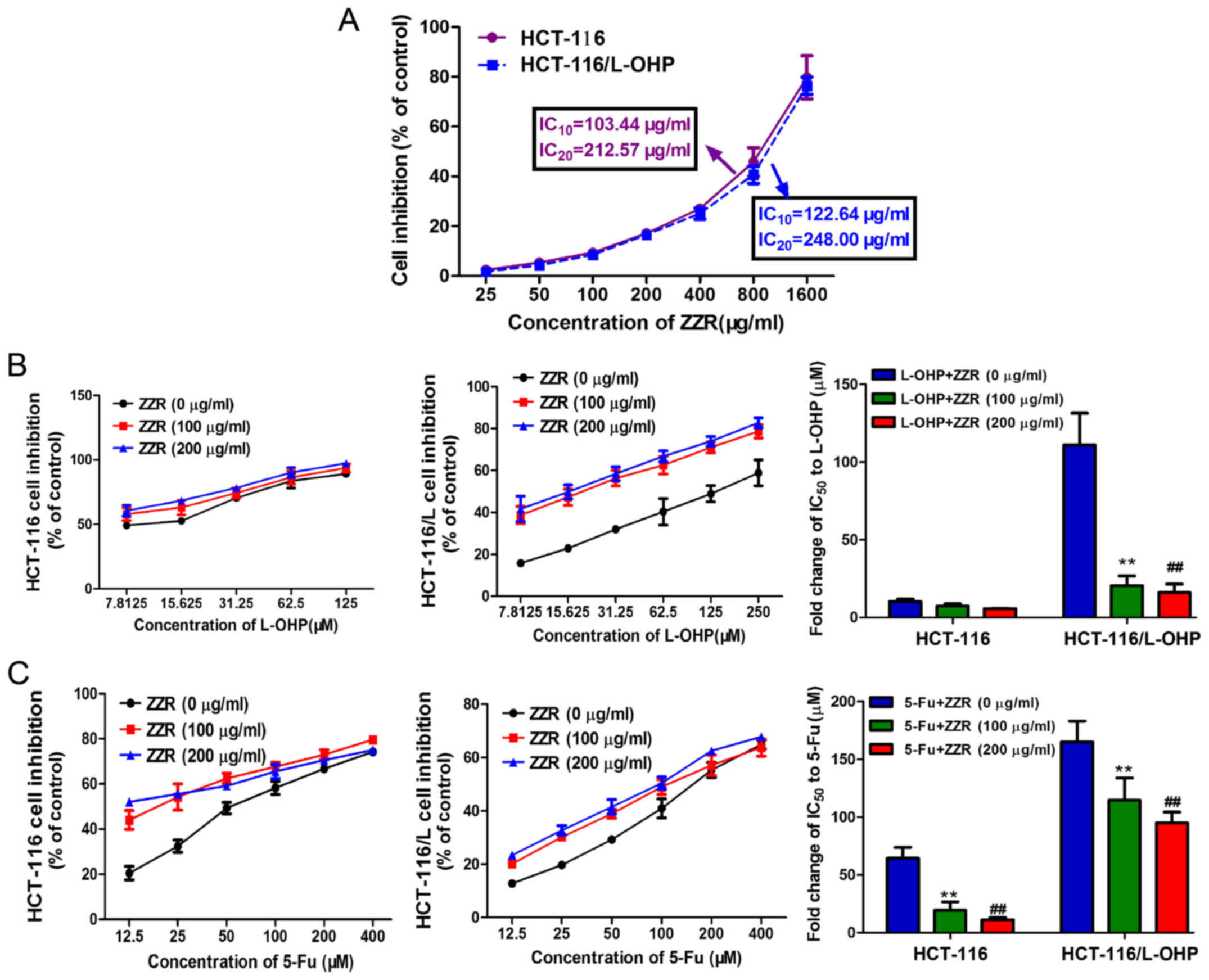
derived cells HCT-116/L-OHP. Our data revealed: the doses of
IC10 and IC20 in HCT-116 cells were 103.44
and 212.57 µg/ml, 122.64 and 248.00 µg/ml in HCT-116/L-OHP cells,
respectively (Fig. 2A). In any
lower doses, little effect was observed on cell survival, thus for
the following experiments, cells were treated with ZZR below the
dose of 200 µg/ml which was roughly IC20.
ZZR enhances chemo-sensitivity of
colorectal cancer cells
To determine if ZZR synergized with chemotherapeutic
drugs in treating colorectal cancer cells, HCT-116 and
HCT-116/L-OHP cells were treated with ZZR and L-OHP and 5-FU at
IC50. As shown in Fig.
2B, ZZR induced significant cytotoxicity in HCT-116/L-OHP
cells, decreasing L-OHP IC50 from 111.03±20.60 to
20.48±6.19 µM and 16.23±5.31 µM with 100 or 200 µg/ml of ZZR
respectively, close to the IC50 in the parental cells
which was 10.30±1.53 µM. Similarly, the IC50 of 5-FU
decreased from 165.03±17.97 to 114.65±19.08 µM and 95.03±9.22 µM
with 100 or 200 µg/ml of ZZR in a dose-dependent manner (Fig. 2C). These results suggested that ZZR
had synergistic effect in combination with chemotherapy in treating
human colorectal cancer.
ZZR induces apoptosis without
disturbing cell cycle colorectal cancer cells
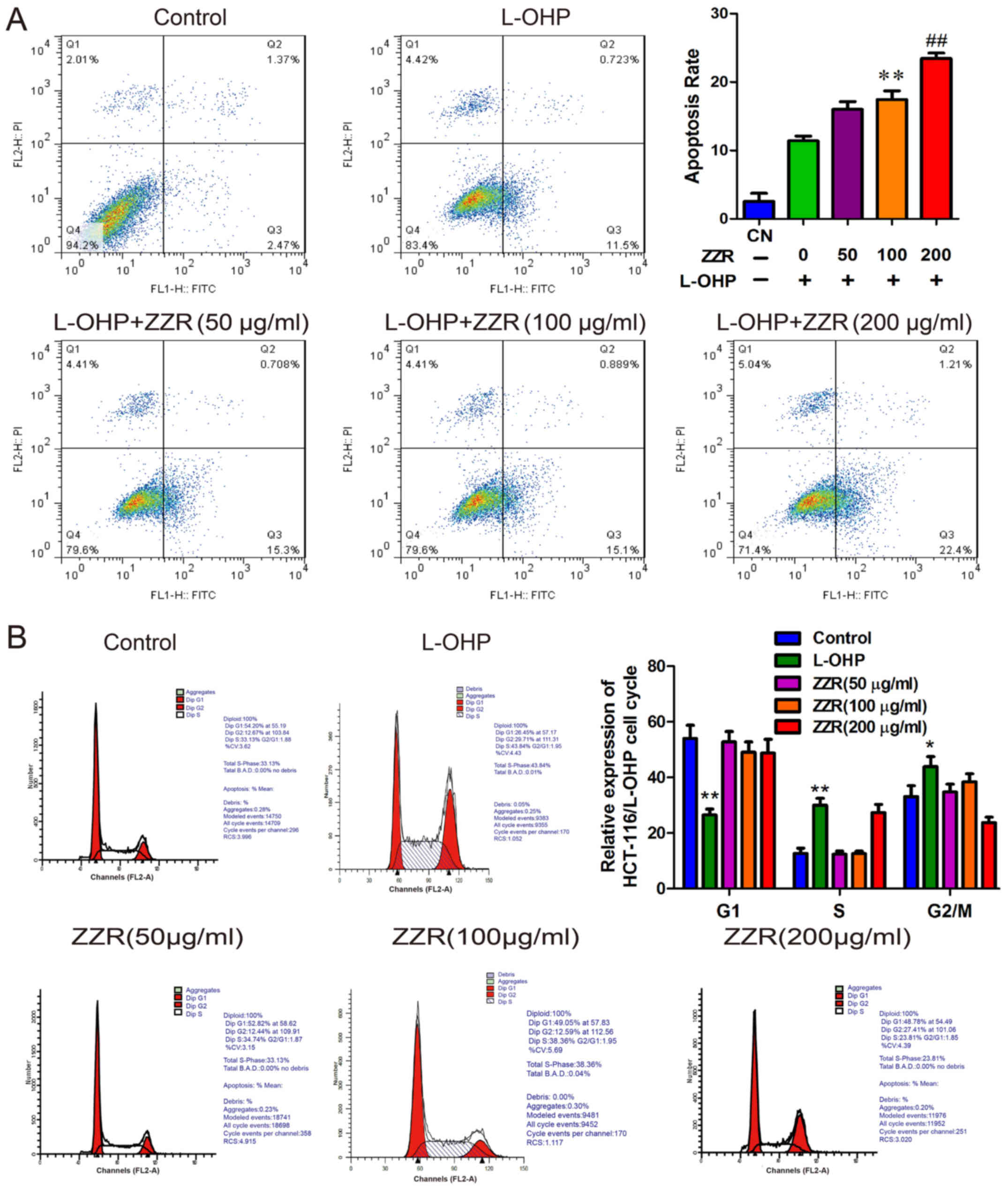
To explore the mechanisms of antiproliferative
effect of ZZR, flow cytometry analysis was performed to determine
if apoptosis was induced by L-OHP/ZZR combination. As shown in
Fig. 3A, the percentages of
apoptotic cells (including the early and late apoptotic cells)
induced by 0, 50, 100 and 200 µg/ml of ZZR were 11.41±0.57,
16.01±0.92, 17.45±1.03 and 23.43±0.66%, respectively. This
indicates that ZZR augmented L-OHP induced cell apoptosis in a
dose-dependent manner. However, the cell cycle analyses obtained
from the flow cytometric analysis showed little impact of ZZR
(Fig. 3B). These data suggested
that the antiproliferative effect of ZZR was mostly via the direct
induction of apoptosis.
Effects of ZZR on the Hedgehog pathway
in the chemotherapeutic reagent-sensitive, and -insensitive
colorectal cancer cells
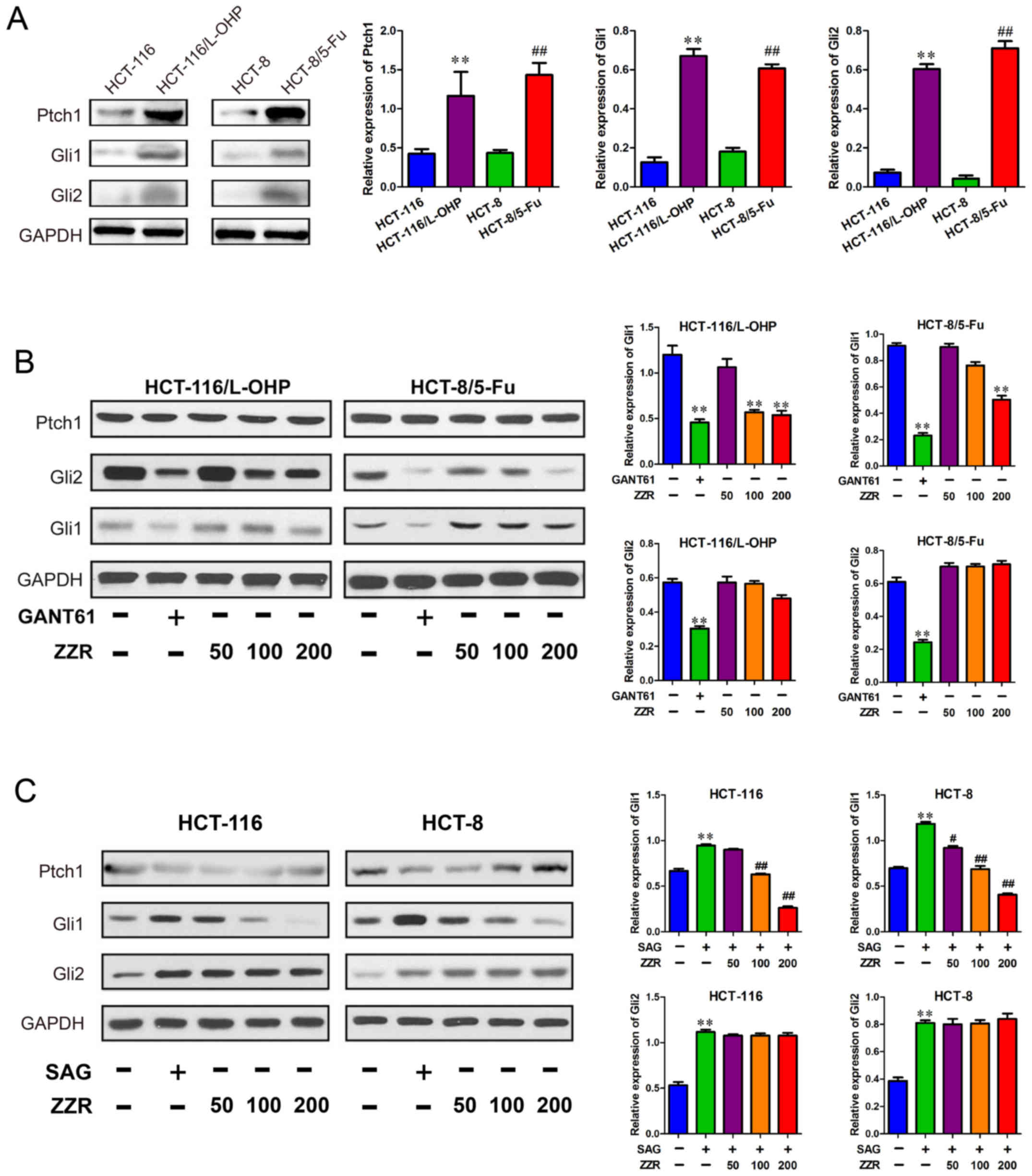
To further elucidate the action mechanisms of ZZR in
MDR colorectal cancer cells, we investigated if ZZR affected
Hedgehog signaling pathway, protein levels of Ptch1, Gli1, and Gli2
were interrogated. As shown in Fig.
4A, the Ptch1, Gli1, and Gli2 protein levels were higher in
colorectal cancer MDR cells than that of the sensitive cells. Next,
we found ZZR attenuated levels of Gli1 protein in HCT-116/L-OHP
cells, without affecting Ptch1 and Gli2 (Fig. 4B). The data suggested that ZZR did
not alter the upstream signaling of Hedgehog pathway, such as
Ptch1, in HCT-116/L-OHP cells. To determine whether the ZZR
modulated Hedgehog pathway activation, we used a specific agonist
SAG in the colorectal cancer sensitive cells. As expected, Hedgehog
pathway was activated by SAG, but the Gli1 protein level was
decreased by ZZR in a dose-dependent manner, suggesting the
activation of Hedgehog pathway was dampened by ZZR (Fig. 4C).
ZZR synergizes with chemotherapeutic
agents through downregulating Gli1 in vivo
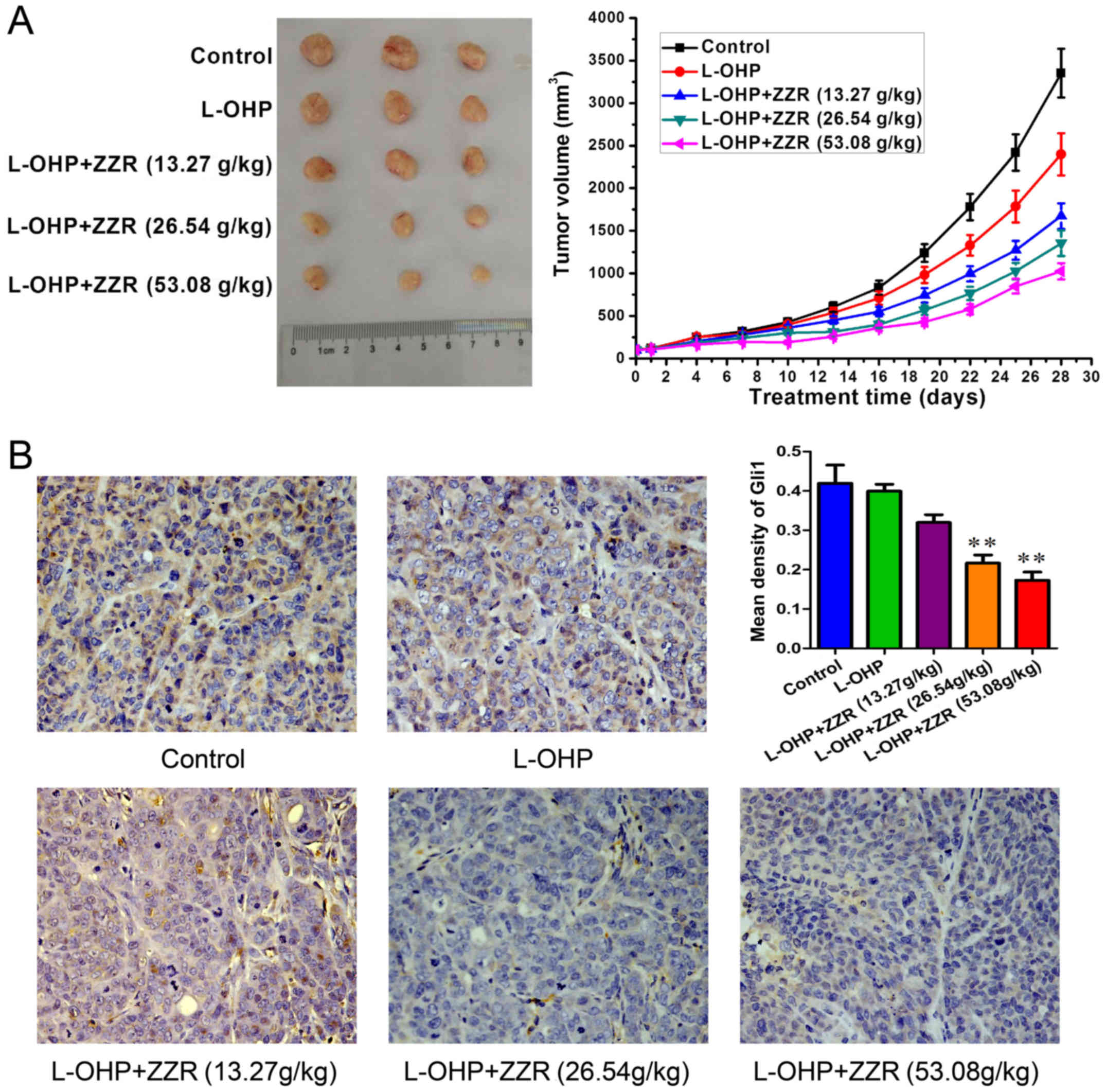
The in vivo MDR-antagonizing effect of ZZR
was evaluated by using colorectal MDR cancer xenograft mice. As
shown in Fig. 5A, ZZR and L-OHP
combination treatment resulted in a 69.17% reduction in average
tumor volume compared with the controls (1025±95 vs. 3352±285
mm3). These data indicated that ZZR enhanced the
inhibitory effect of L-OHP in vivo. Immunohistochemistry
analyses confirmed the lowered levels of Gli1 in tumors treated by
ZZR (Fig. 5B). These findings
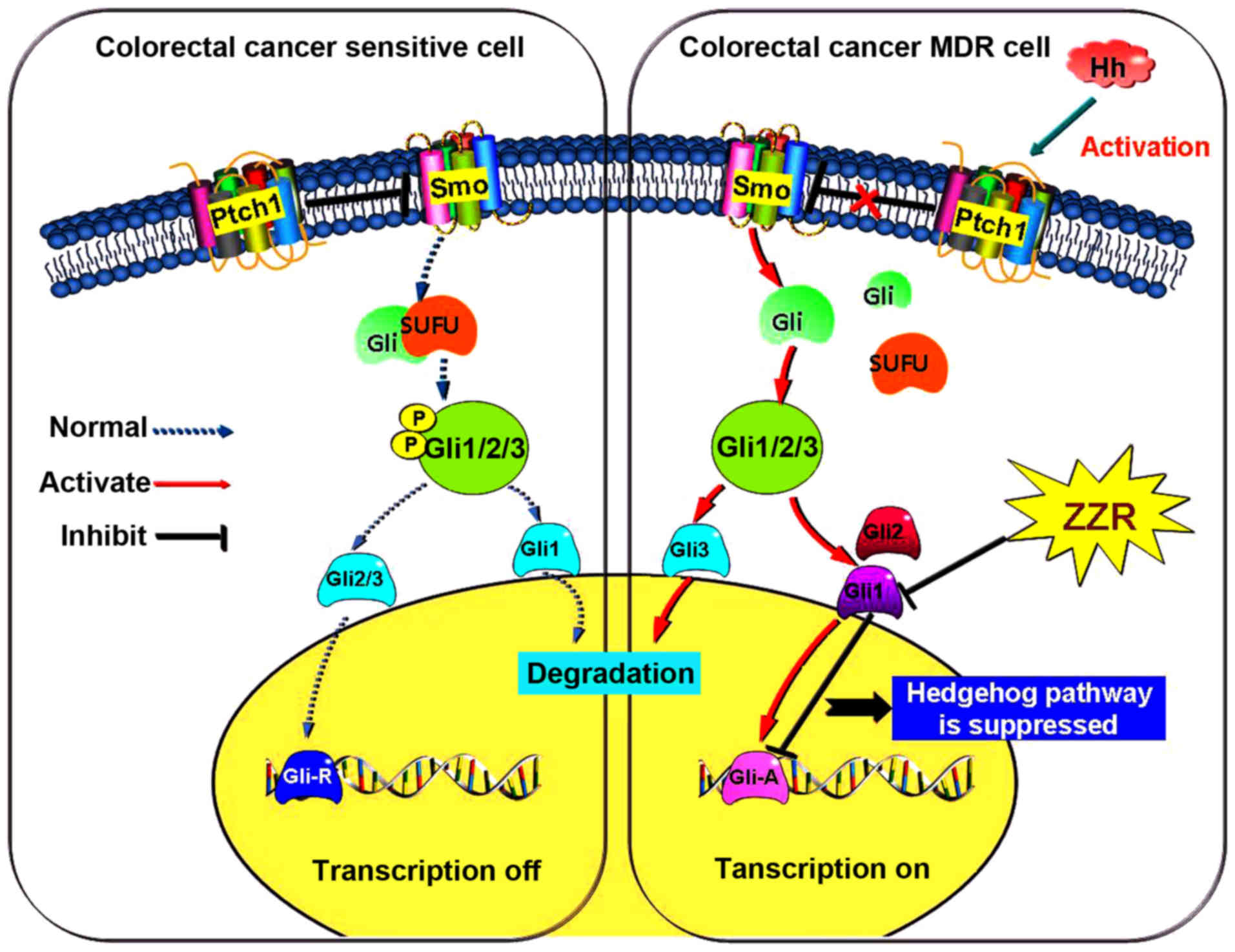
suggested that ZZR was a MDR inhibitor repressing the activation of
Hedgehog pathway incolorectal cancer (Fig. 6).
Discussion
MDR is one of the main factors for colorectal cancer
chemotherapy failure (20–22). To overcome MDR, efforts have been
made to thwart drug membrane transporters, but broadly applicable
inhibitors are to no avail due to severe side effects of the drugs
(23,24). In China, Chinese herbal medicines,
often prescribed as concoctions, are commonly used as complementary
approaches for the prevention and treatment of colorectal cancer
(25). Published reports have
indicated that adjunctive TCM therapies display remarkable
advantages, including maintaining organ functions, improving life
quality, ameliorating symptoms, reducing side effects of
chemotherapy or radiotherapy, and extending survival time in
patients with advanced colorectal cancer (26–28).
In our previous study, we found that ZZR, a traditional Chinese
herb, has significant anticancer effects in enhancing efficacy of
chemotherapeutic drugs. Based on this clinical finding, we aimed to
explore the anti-MDR effect of ZZR ethanol extract using in
vitro and in vivo models.
In our study, the herbal components of ZZR extract,
including Huang-Qi (Radix Astragali), Nv-Zhen-Zi (Fructus
Ligustri Lucidi), Yi-yi-ren (Semen Coicis),
Shi-Jian-Chuan (Salvia Chinensis), Ye-Pu-Tao-Teng (Vitis
quinquangularis Rehd), Teng-Li-Gen (Actinidia arguta),
and Zhi-Xiang-Fu (Cyperus rotundus L.), were analyzed by
HPLC/ESI-MS. To investigate the anti-MDR effects of ZZR on human
colorectal cancer, HCT-116 and its MDR counterpart HCT-116/L-OHP
cells were treated with ZZR, and the non-toxic dose of ZZR was
determined. The result suggested that concentrations of ZZR <200
µg/ml are safe in experiments in vitro. Furthermore, cell
viability assays were performed in HCT-116 and HCT-116/L-OHP cells
to determine their relative sensitivity in increasing
concentrations of chemotherapy drugs, L-OHP and 5-FU. Data showed
that HCT-116/L-OHP cells are much more sensitive to death induced
by chemotherapeutics after ZZR treament, when compared with its
parental cells.
In our study, we found ZZR synergized with
chemotherapeutic drugs to enhance cell apoptosis in a
time-dependent manner, consistent with what several previous
reports have shown with traditional Chinese prescriptions and
formulae (29,30). However, in our study with ZZR, we
did not observe any disturbance on cell cycle. One possible
explanation is because of the interaction between different herb
constituents of this formula which could lead to complicated
effects. These findings are in accordance with several reports
showing opposite effects for some antitumor drugs, causing
apoptosis but not cell cycle alterations (17,31).
Earlier study from our laboratory revealed an
association between Hedgehog pathway and P-gp in MDR colorectal
cancer cells (32). Here we
extended that study by using another two MDR colorectal cancer cell
lines, and found ZZR perturbed the expression levels of Ptch1, Gli1
and Gli2. We uncovered that expression levels of Ptch1, Gli1 and
Gli2 were higher in resistant HCT-116/L-OHP and HCT-8/5-FU cells
than that of their parental sensitive cells. It was reported that
Hedgehog signaling pathway was activated in MDR cancer cells
(33). In this study, in
HCT-116/L-OHP and HCT-8/5-FU cells, we observed elevated protein
levels of Ptch1, Gli1, and Gli2, among which ZZR treatment
specifically repressed the levels of Gli1.
We next investigated the in vivo therapeutic
potential of ZZR. Indeed, animal experiment results showed a smilar
synergistic anticancer effect of ZZR with chemotherapy drugs on
resistant colorectal cancer cell xenografts. Our data further
confirmed that ZZR markedly inhibited Gli1 protein level in the
xenograft tumors of MDR colorectal cancer cells.
In conclusion, the present study for the first time
demonstrated that ZZR suppresssed Gli1 protein levels in colorectal
cancer through inhibiting Hedgehog signaling pathway. The
traditional Chinese prescription and formula ZZR, used alone or
incombination with chemotherapeutic agents, may provide promising
approach for the treatment of drug-resistant colorectal cancer.
Acknowledgements
This research was supported by the project from
Shanghai Municipal Commission of Health and Family Planning (no.
ZY3CCCX11009), the National Natural Science Foundation of China
(nos. 81373862, 81403360 and 81573805), and Program from Xinjiang
Autonomous Region (no. 2013911125).
References
|
1
|
Sui H, Cai GX, Pan SF, Deng WL, Wang YW,
Chen ZS, Cai SJ, Zhu HR and Li Q: miR200c attenuates P-gp-mediated
MDR and metastasis by targeting JNK2/c-Jun signaling pathway in
colorectal cancer. Mol Cancer Ther. 13:3137–3151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sui H, Zhu L, Deng W and Li Q:
Epithelial-mesenchymal transition and drug resistance: Role,
molecular mechanisms, and therapeutic strategies. Oncol Res Treat.
37:584–589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang YK, Zhang GN, Wang YJ, Patel BA,
Talele TT, Yang DH and Chen ZS: Bafetinib (INNO-406) reverses
multidrug resistance by inhibiting the efflux function of ABCB1 and
ABCG2 transporters. Sci Rep. 6:256942016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nüsslein-Volhard C and Wieschaus E:
Mutations affecting segment number and polarity in
Drosophila. Nature. 287:795–801. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee RT, Zhao Z and Ingham PW: Hedgehog
signalling. Development. 143:367–372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flemban A and Qualtrough D: The potential
role of Hedgehog signaling in the luminal/basal phenotype of breast
epithelia and in breast cancer invasion and metastasis. Cancers
(Basel). 7:1863–1884. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laukkanen MO and Castellone MD: Hijacking
the Hedgehog pathway in cancer therapy. Anticancer Agents Med Chem.
16:309–317. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kling JC and Blumenthal A: Roles of WNT,
NOTCH, and Hedgehog signaling in the differentiation and function
of innate and innate-like lymphocytes. J Leukoc Biol jlb.
1MR0616-272R. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie J, Bartels CM, Barton SW and Gu D:
Targeting Hedgehog signaling in cancer: Research and clinical
developments. Onco Targets Ther. 6:1425–1435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee MY, Sun L and Veltmaat JM: Hedgehog
and Gli signaling in embryonic mammary gland development. J Mammary
Gland Biol Neoplasia. 18:133–138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shevde LA and Samant RS: Nonclassical
Hedgehog-GLI signaling and its clinical implications. Int J Cancer.
135:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malhotra GK, Zhao X, Band H and Band V:
Shared signaling pathways in normal and breast cancer stem cells. J
Carcinog. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li WY, Chan SW, Guo DJ, Chung MK, Leung TY
and Yu PH: Water extract of Rheum officinale Baill. induces
apoptosis in human lung adenocarcinoma A549 and human breast cancer
MCF-7 cell lines. J Ethnopharmacol. 124:251–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rasul A, Yu B, Yang LF, Ali M, Khan M, Ma
T and Yang H: Induction of mitochondria-mediated apoptosis in human
gastric adenocarcinoma SGC-7901 cells by kuraridin and
Nor-kurarinone isolated from Sophora flavescens. Asian Pac J Cancer
Prev. 12:2499–2504. 2011.PubMed/NCBI
|
|
15
|
Sui H, Zhu HR, Wu J, Nikitin AY, Cai JF,
Fan ZZ and Li Q: Effects of Jianpi Jiedu Recipe on reversion of
P-glycoprotein-mediated multidrug resistance through COX-2 pathway
in colorectal cancer. Chin J Integr Med. 20:610–617. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun B, Chen Y, Xiang T, Zhang L, Chen Z,
Zhang S, Zhou H and Chen S: The Chinese herb Jianpijiedu
contributes to the regulation of OATP1B2 and ABCC2 in a rat model
of orthotopic transplantation liver cancer pretreated with food
restriction and diarrhea. BioMed Res Int. 2015:7528502015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sui H, Liu X, Jin BH, Pan SF, Zhou LH, Yu
NA, Wu J, Cai JF, Fan ZZ, Zhu HR, et al: Zuo Jin Wan, a traditional
Chinese herbal formula, reverses P-gp-mediated MDR in vitro and in
vivo. Evid Based Complement Alternat Med. 2013:9570782013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sui H, Xu H, Ji Q, Liu X, Zhou L, Song H,
Zhou X, Xu Y, Chen Z, Cai J, et al: 5-hydroxytryptamine receptor
(5-HT1DR) promotes colorectal cancer metastasis by regulating
Axin1/β-catenin/MMP-7 signaling pathway. Oncotarget. 6:25975–25987.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kolinsky K, Shen BQ, Zhang YE, Kohles J,
Dugan U, Zioncheck TF, Heimbrook D, Packman K and Higgins B: In
vivo activity of novel capecitabine regimens alone and with
bevacizumab and oxaliplatin in colorectal cancer xenograft models.
Mol Cancer Ther. 8:75–82. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martins A, Sipos P, Dér K, Csábi J, Miklos
W, Berger W, Zalatnai A, Amaral L, Molnár J, Szabó-Révész P, et al:
Ecdysteroids sensitize MDR and non-MDR cancer cell lines to
doxorubicin, paclitaxel, and vincristine but tend to protect them
from cisplatin. BioMed Res Int. 2015:8953602015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang S, Wang L, Shi Z, Zhong Z, Chen M and
Wang Y: Evodiamine synergizes with doxorubicin in the treatment of
chemoresistant human breast cancer without inhibiting
P-glycoprotein. PLoS One. 9:e975122014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z, Sun X, Feng Y, Liu X, Zhou L, Sui
H, Ji QeQ, Chen J, Wu L, et al: Dihydromyricetin reverses
MRP2-mediated MDR and enhances anticancer activity induced by
oxaliplatin in colorectal cancer cells. Anticancer Drugs.
28:281–288. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharom FJ: ABC multidrug transporters:
Structure, function and role in chemoresistance. Pharmacogenomics.
9:105–127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilkens S: Structure and mechanism of ABC
transporters. F1000Prime Rep. 7:142015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng W, Sui H, Wang Q, He N, Duan C, Han
L, Li Q, Lu M and Lv S: A Chinese herbal formula, Yi-Qi-Fu-Sheng,
inhibits migration/invasion of colorectal cancer by down-regulating
MMP-2/9 via inhibiting the activation of ERK/MAPK signaling
pathways. BMC Complement Altern Med. 13:652013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao P, Selvan SR, Akkol Küpeli E, Wang N,
Yang H and Cheng X: Complementary and alternative medicine in
cancer prevention and therapy. Evid Based Complement Alternat Med.
2015:6393722015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao CJ, Chow JM, Yang CM, Kuo HC, Chang
CL, Lee HL, Lai IC, Chuang SE and Lai GM: Chinese herbal mixture,
Tien-Hsien liquid, induces G2/M cycle arrest and radiosensitivity
in MCF-7 human breast cancer cells through mechanisms involving
DNMT1 and Rad51 downregulation. Evid Based Complement Alternat Med.
2016:32510462016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen T, Wang Q, Li Y, Huang H and Hu W:
Chinese herbal formula QHF inhibits liver cancer cell invasion and
migration. Exp Ther Med. 11:2413–2419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li J, Sun GZ, Lin HS, Pei YX, Qi X, An C,
Yu J and Hua BJ: The herb medicine formula ‘Yang Wei Kang Liu’
improves the survival of late stage gastric cancer patients and
induces the apoptosis of human gastric cancer cell line through
Fas/Fas ligand and Bax/Bcl-2 pathways. Int Immunopharmacol.
8:1196–1206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu G, Chu J, Huang Z, Ye J, Chen P, Zheng
C, Li X, Liu X and Wu M: Xiao Jin Wan, a traditional Chinese herbal
formula, inhibits proliferation via arresting cell cycle
progression at the G2/M phase and promoting apoptosis via
activating the mitochondrial-dependent pathway in U-2OS human
osteosarcoma cells. Int J Oncol. 42:1070–1080. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sui H, Pan SF, Feng Y, Jin BH, Liu X, Zhou
LH, Hou FG, Wang WH, Fu XL, Han ZF, et al: Zuo Jin Wan reverses
P-gp-mediated drug-resistance by inhibiting activation of the
PI3K/Akt/NF-κB pathway. BMC Complement Altern Med. 14:2792014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang W, Wang H, Tang Y, Cai SB, Zhang X
and Wang SP: The effect and mechanism of Zhizhen Recipe on
multidrug resistance in human colorectal cancer cell lines through
Hedgehog signaling pathway. Chin J Integr Trad W Med Dig.
23:151–155. 2015.(In Chinese).
|
|
33
|
Zhang XL, Shi HJ, Wang JP, Tang HS and Cui
SZ: MiR-218 inhibits multidrug resistance (MDR) of gastric cancer
cells by targeting Hedgehog/smoothened. Int J Clin Exp Pathol.
8:6397–6406. 2015.PubMed/NCBI
|