Introduction
Triple-negative breast cancers (TNBCs), which are
characterized by the lack of estrogen receptor (ER), progesterone
receptor (PR), and human epidermal growth factor receptor (Her2),
account for 15–20% of all breast cancers (1). These cancers exhibit a more aggressive
phenotype and a poorer clinical outcome compared to other breast
cancer subtypes, and that is because of the high propensity for
metastatic progression and the absence of specific targeted
treatment options (2,3). Besides, the molecular heterogeneity of
TNBC increases the difficulty in improving survival rates and
developing specific targeted therapy.
Due to the lack of ER, PR, and Her2 receptors,
patients with TNBC could not benefit from hormonal and
trastuzumab-based targeted therapies and thus conventional
chemotherapy such as taxanes and anthracyclines remains the
mainstay for the treatment of TNBC (4). Although a significant number of TNBC
patients respond well to the conventional chemotherapy, the
prognosis of TNBC patients remains poor and alternative therapeutic
approaches are therefore highly needed.
Heat shock proteins 90 (Hsp90) is an adenosine
triphosphate (ATP) dependent molecular chaperone protein, which is
widely expressed in breast cancers (5–7). Hsp90
plays a critical role in the correct folding, stability, and
function of its substrate proteins, referred to as ‘client
proteins’ (8–10). These client proteins include
epidermal growth factor receptor (EGFR/ErbB1), human epidermal
growth factor receptor 2 (Her2/ErbB2), mesenchymal-epithelial
transition factor (Met), anaplastic lymphoma kinase (Alk), protein
kinase B (Akt/PKB), cellular rapidly accelerated fibrosarcoma
(c-Raf), cyclin-dependent kinase 4 (Cdk4), hypoxia-inducible factor
1 (Hif-1α), matrix metalloproteinase 2 (MMP2) (11–14).
Hsp90 has received significant attention and emerged as an
attractive target for cancer therapy, in that the inhibition of
single Hsp90 protein induces client protein degradation via the
ubiquitin-proteasome pathway, and subsequently results in
simultaneous blockage of multiple signaling pathways in the
heterogeneous cancers.
Chalcones represent an important group of naturally
occurring molecules, which are especially abundant in edible plants
such as green tea, licorice, and bean sprouts. Chemically,
chalcones are aromatic ketone with two phenyl linked by
three-carbon enone moiety (Fig. 1).
Chalcones exhibit a wide spectrum of biological activities
including anti-oxidative (15),
anti-inflammatory (16), and
anticancer activities (17–21). More importantly, chalcones have been
shown to interfere with each step of carcinogenesis including
initiation, promotion and progression, suggesting that chalcones
and their derivatives could serve as promising candidates for
anticancer drugs.
In our ongoing effort to develop a new Hsp90
inhibitor (22–26), we have recently found that a
chalcone-based small molecule
(E)-3-(2-bromo-3,4,5-trimethoxyphenyl)-1-(2,4-dihydroxyphenyl)prop-2-en-1-one
(BDP) impairs the growth of cancer cells and this observation
prompted us to direct our efforts toward investigating its
biological activities and the underlying mechanisms of action.
Materials and methods
Cell culture and material
Triple-negative breast cancer cells MDA-MB-231 were
grown in DMEM high glucose, supplemented with streptomycin (500
mg/ml), penicillin (100 U/ml), and 10% fetal bovine serum (FBS).
Cells were grown to confluence at 37°C in humidified atmosphere
with 5% CO2. BDP was prepared following the previously
reported procedure (26). For in
vitro studies, BDP and geldanamycin (Alexis Biochemical,
Farmingdale, NY, USA) were dissolved in DMSO. Antibodies for EGFR,
Her2, Met, Akt, c-Raf, Cdk4, Hsp90, Hsp70, PARP, caspase 3, cleaved
caspase 8, Bax and β-actin were purchased from Cell Signaling
Technology (Beverly, MA, USA). Anti-Bcl-2, anti-mouse, and
anti-rabbit antibodies were purchased from Santa Cruz Biothechnolgy
(Dallas, TX, USA).
Cell proliferation assay
MDA-MB-231 cells (2×103 cells/well) were
seeded in 96-well plate, the medium volume was brought to 100 µl,
and the cells were allowed to attach for 24 h. The cells were then
incubated with BDP (10, 30, 50, 70 and 100 µM) or GA (1 µM) at 37°C
with 5% CO2 for 1, 2 and 3 days. CellTiter 96 Aqueous
One Solution reagent (Promega, Madison, WI, USA) was added into
each well following the manufacturer's instructions. The absorbance
of each sample was determined by Tecan Infinite F200 Pro plate
reader at 490 and 690 nm as the reference wavelength.
Assessment of cell morphology
MDA-MB-231 cells (1×105 cells/well) were
seeded in 12-well plate, and the cells were allowed to attach for
24 h. Culture medium was then changed to fresh complete medium
containing BDP (10, 30 and 50 µM). After being incubated for 24 h,
the cell morphology was observed with inverted phase contrast
microscope (Olympus, Japan) at 20x objective.
Western blotting
After being treated with BDP (10, 30, 50 and 70 µM)
or GA (1 µM) for 24 h, MDA-MB-231 cells were harvested and lysed in
ice-cold lysis buffer (25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP40,
1% sodium deoxycholate, 0.1% SDS, 1 mM phenylmethylsulfonyl
fluoride). The 30 µg of lysate per lane was separated by SDS-PAGE
and followed by transferring to a PVDF membrane (Bio-Rad, Hercules,
CA, USA). After being blocked with 5% skim milk in TBS with 0.1%
Tween-20 (TBS-T), the membrane was incubated with the corresponding
antibodies in TBS-T at 4°C overnight. Proteins were visualized by
using enhanced chemiluminescence (ECL) reagent according to the
manufacturer's instructions (GE Healthcare, Pittsburgh, PA,
USA).
Reverse transcriptase-polymerase chain
reaction (RT-PCR)
RT-PCR was performed using the RT-PCR kit (Bio-Rad)
following the manufacturer's protocol. Briefly, total RNA was
extracted from cultured cells using TRIzol reagent (Fisher
Scientific, Hampton, NH, USA), reverse transcribed, and then
subjected to PCR. The following primers were used for the
amplification of human Met, Akt and β-actin: Met,
5′-AAGAGGGCATTTTGGTTGTG-3′ (forward) and 3′-GATGATTCCCTCGGTCAGAA-5′
(reverse); Akt, 5′-TTTTATTTCTCGGGTGCAT-3′ (forward) and
3′-CATTTCCCTACGTGAATCGG-5′ (reverse); β-actin,
5′-AGAAAATCTGGCACCACACC-3′ (forward) and 3′-CCATCTCTTGCTCGAAGTCC-5′
(reverse).
Apoptosis assay
After being treated with BDP (10, 30, 50 and 70 µM)
or GA (1 µM) for 24 h, MDA-MB-231 cells were resuspended in 1X
Annexin V binding buffer, and stained with Annexin V for 15 min.
The cells were treated with FACS buffer and propidium iodide prior
to FACS analysis. Apoptotic cells were analyzed by
fluorescent-activated cell sorting (FACS) flow cytometer (BD
Bioscience, San Jose, CA, USA) and BD CellQuest™ Pro software.
Cell cycle arrest
For cell cycle assay, MDA-MB-231 cells were treated
with BDP (10, 30, 50 and 70 µM) or GA (1 µM) for 24 h. The cells
were resuspended in 300 µl of PBS, treated with 700 µl of 95%
ethanol, and gently vortexed. The cells were then incubated at 4°C
for 2 h, washed with PBS, resuspended in 500 µl of PBS containing
50 µg/ml of propidium iodide and 1 µg/ml of RNAse A. After being
incubated for an additional 30 min at room temperature in the dark,
the cells were analyzed by FACS flow cytometer and BD CellQuest Pro
software.
Wound healing assay
MDA-MB-231 cells (3×105 cells/well) were
seeded in 6-well plate, and the cells were allowed to attach for 24
h. The 80% confluent cells were wounded with a linear scratch by
using disposable 200 µl micropipette tip. The cells were washed
with medium to remove cell debris and covered with serum-free
medium containing BDP (10 µM). After being incubated for 24 h,
migrated cells were determined under inverted phase contrast
microscope (Olympus) at 10x objective.
Gelatin zymography assay
MDA-MB-231 cells (5×105 cells/dish) were
seeded in 60 mm dish and the cells were allowed to attach for 24 h.
The cells were then washed with PBS and incubated with serum-free
medium containing BDP (10 and 30 µM) or GA (1 µM) for 24 h.
Conditioned media from cell cultures treated with BDP or GA were
collected, centrifuged, and mixed with sample buffer (60 mM
Tris-HCl pH 6.8, 25% glycerol, 2% SDS, 0.1% bromophenol) without
reducing agents. The corresponding samples were loaded on 10%
polyacrylamide gels with gelatin (1 mg/ml) and separated by gel
electrophoresis. Gels were then washed with 2.5% Triton X-100 for
40 min and incubated in incubation buffer (50 mM Tris, 0.15 M NaCl,
10 mM CaCl2, 0.05% sodium azide) at 37°C for 72 h. Gels
were stained with 1% coomassie staining solution containing 10%
acetic acid and 20% methanol, and incubated at room temperature for
1 h. Proteolytic activity was detected as clear band against the
background stain of undigested substrate.
Statistical analysis
Quantitative data are presented as mean value ± SD.
The statistical significance of compared values was determined by
using Student's t-test, and p<0.001, p<0.01, and p<0.05
were considered to indicate statistically significant results.
Results
BDP has anti-proliferative effect on
MDA-MB-231 cells
We first evaluated the dose- and time-dependent
effect of BDP on the growth of MDA-MB-231 cells. MDA-MB-231 cells
were treated with various concentrations (0, 10, 30, 70 and 100 µM)
of BDP for 1, 2 and 3 days and cell viability was determined using
MTS colorimetric assay (Fig. 2A).
The assay indicated that BDP afforded a potent growth-inhibitory
effect on MDA-MB-231 cells in a dose- and time-dependent manner.
The treatment of cells with BDP (30 µM) effectively impaired nearly
50% of MDA-MB-231 cell growth.
We also observed the morphology changes of
MDA-MB-231 cells (Fig. 2B).
MDA-MB-231 cells became round and floating upon the treatment of
cells with 30 or 50 µM of BDP for 24 h, which indicated typical
characteristic of apoptosis.
BDP inhibits the chaperone function of
Hsp90 and downregulates the Hsp90 client proteins through the
proteasomal pathway
To determine whether the observed anti-proliferative
effect of BDP was associated with Hsp90 inhibition, we next
evaluated the dose-dependent effect of BDP on the cellular
biomarkers of Hsp90 inhibition. Since Hsp90 is responsible for
maintaining the stability of EGFR, Her2, Met, Akt, c-Raf, and Cdk4,
the inhibition of Hsp90 will induce the degradation of Hsp90 client
proteins through the ubiquitin-proteasome pathway. As shown in
Fig. 3A, the treatment of
MDA-MB-231 cells with the indicated concentration of BDP for 24 h
caused a dose-dependent decrease of EGFR, Her2, Met, Akt, c-Raf,
and Cdk4. Interestingly, Her2 and Cdk4 more sensitively responded
to the exposure of cells with BDP than other client proteins. The
expression levels of Her2 and Cdk4 were almost completely depleted
with 30 µM of BDP. On the contrary, BDP upregulated the cellular
protein level of Hsp70, the upregulation of which is considered a
cellular hallmark of Hsp90 inhibition.
 | Figure 3.BDP induces the degradation of Hsp90
client proteins and the upregulation of Hsp70. (A) MDA-MB-231 cells
were treated with the indicated concentration of BDP for 24 h and
the expression of EGFR, Her2, Met, Akt, c-Raf, Cdk4, Hsp70, Hsp90,
and β-actin was analyzed using western blotting. Geldanamycin (GA,
1 µM) and DMSO (D, 0.5%) were employed as a positive and a negative
control, respectively. (B) MDA-MB-231 cells were pretreated with 1
µM of MG-132 for 3 h and then incubated with 30 µM of BDP for
additional 24 h. The expression of Met, Akt, and β-actin were
analyzed using western blotting. (C) Effect of BDP on the
transcriptional levels of Met and Akt were measured by
semi-quantitative RT-PCR. MDA-MB-231 cells were treated with
indicated concentration of BDP or GA (1 µM) for 24 h. Total RNA was
isolated from the cells, reverse transcribed to cDNA, and then
assessed by semi-quantitative RT-PCR. |
To further determine whether the observed
downregulation of Hsp90 client proteins was a consequence of
Hsp90-mediated proteasomal degradation, we performed a recovery
experiment using a proteasomal inhibitor, MG-132 (Fig. 3B). BDP (30 µM) significantly
decreased the protein level of Met and Akt, whereas the
pretreatment of MDA-MB-231 cells with 1 µM of MG-132 recovered the
protein abundance of these proteins.
We also investigated the dose-dependent effect of
BDP on the transcriptional level of Met and Akt (Fig. 3C). As expected, BDP did not affect
the transcriptional levels of Met and Akt, suggesting the
downregulation of the Hsp90 client proteins, Met and Akt was not
associated with the transcriptional regulation, but the proteasomal
degradation.
Collectively, this result clearly suggested that BDP
inhibited the chaperone function of Hsp90 and downregulated the
Hsp90 client proteins via Hsp90-mediated proteasomal degradation
pathway.
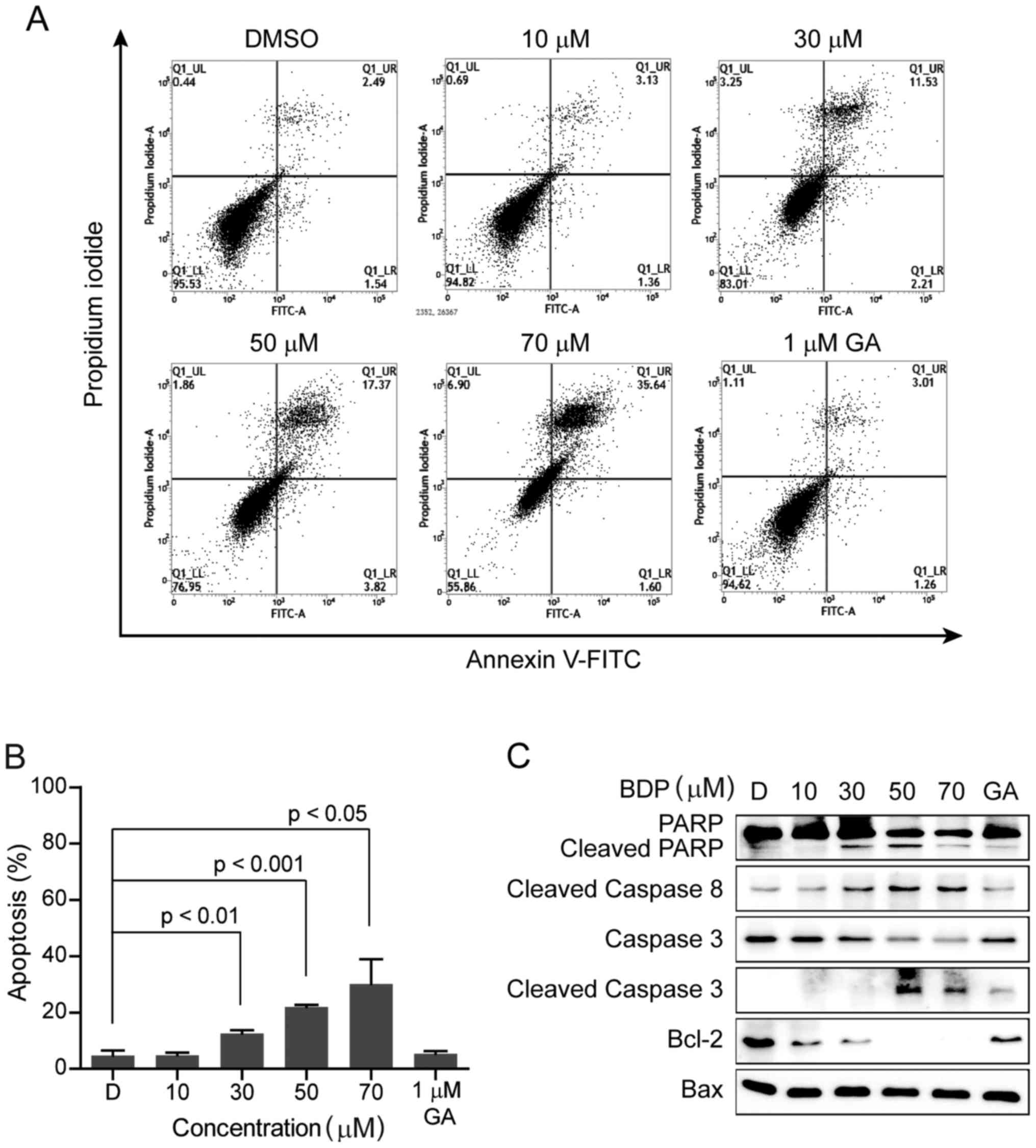
BDP induces apoptotic cell death in
MDA-MB-231 breast cancer cells
To address the question of whether the
anti-proliferative effect of BDP was associated with the induction
of apoptotic cell death, MDA-MB-231 cells were treated with the
indicated concentration of BDP for 24 h, stained with Annexin V and
propidium iodide, and then analyzed by fluorescence-activated cell
sorting (FACS). As shown in Fig. 4A and
B, the exposure of MDA-MB-231 cells with BDP induced the early
and late apoptosis in a dose-dependent manner.
The activation of caspases is an important indicator
of apoptosis, which is stimulated by various apoptotic stimuli. To
elucidate the mechanism of BDP-induced apoptotic cell death in
MDA-MB-231 cells, we next examined the cellular levels of apoptotic
biomarker proteins including poly(ADP-ribose) polymerase (PARP),
cleaved PARP, caspase 3, cleaved caspase 3, cleaved caspase 8,
B-cell lymphoma 2 (Bcl-2), and Bcl-2 associated X protein (Bax),
shown in Fig. 3C. Upon the exposure
to BDP, the cleavage of PARP, caspase 3, and caspase 8 were
remarkably induced and the anti-apoptotic protein, Bcl-2 was
significantly decreased in a dose-dependent manner, while the
pro-apoptotic protein, Bax was unchanged. The result clearly
suggested that BDP efficiently inhibited the growth of MDA-MB-231
cells through inducing apoptosis.
BDP induces cell cycle arrest at the
G2/M phases in MDA-MB-231 cells
To determine whether BDP affected the cell cycle, we
analyzed the cell cycle distribution using flow cytometry. After
exposure to the indicated concentration of BDP for 24 h, the cell
cycle distribution of MDA-MB-231 cells was analyzed. As shown in
Fig. 5, the treatment of cells with
BDP remarkably induced the cell cycle arrest at the G2/M
phases compared to the DMSO control. Twenty-four-hour exposure to
10 µM BDP increased the G2/M fraction from 21.3 to 30.1%
and this increase was more marked when exposed to 30 µM BDP
(G2/M fraction, 47.9%). Similar results were also
observed when exposed to 50 µM BDP (G2/M fraction,
43.1%) or 70 µM BDP (G2/M fraction, 46.2%).
Consequently, the treatment of cells with BDP results in a
reduction of the cell population at the G0/G1
phases, while the cell population at the S phase was not
significantly altered. Taken together, these findings revealed that
BDP significantly induced cell cycle arrest at the G2/M
phases in MDA-MB-231 cells.
BDP impairs the migration of
MDA-MB-231 cells
An increase of mobility has been associated with the
metastatic potential of cancer cells. We thus evaluated the impact
of BDP on the mobility of MDA-MB-231 cells. Wounds were formed by
scratching the cell monolayer with a pipette tip and wound closure
of MDA-MB-231 monolayer in the presence or absence of 10 µM BDP was
measured by counting the number of cells that had infiltrated the
wounded area at 24 h. As shown in Fig.
6A and B, the treatment of cells with 10 µM BDP for 24 h
suppressed the migration of MDA-MB-231 cells, in that the number of
migrated cells was significantly decreased (p<0.01) in the
BDP-treated group, compared to the non-treated group without
impacting cell viability (Fig. 6C).
The assay indicated that BDP inhibited the migration of MDA-MB-231
cells.
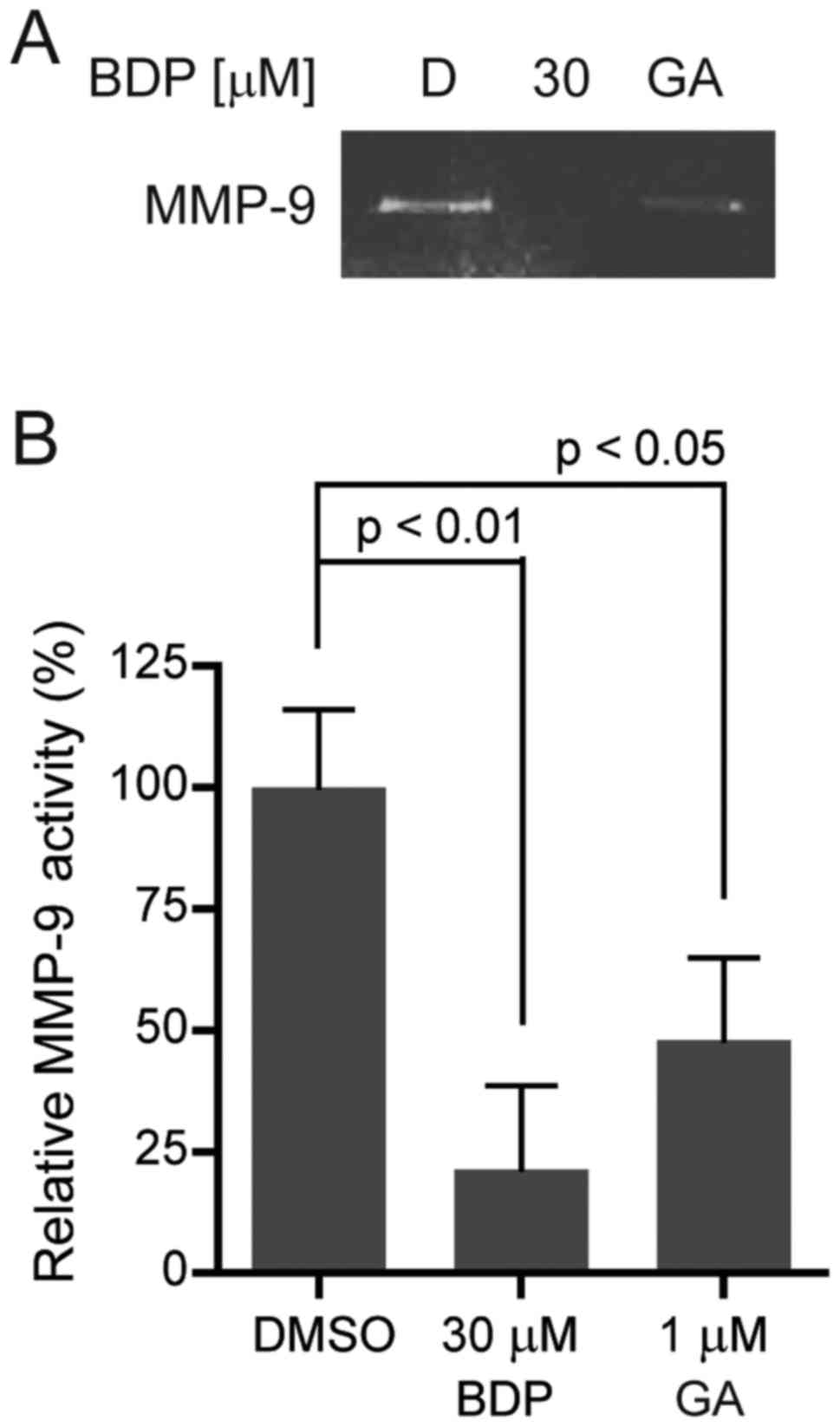
BDP inhibits the activity of MMP-9 in
MDA-MB-231 cells
Gelatinases, such as MMP-2 and MMP-9, play a
critical role in regulating extracellular matrix degradation. One
of the gelatinases, MMP-9 is secreted by various types of malignant
cells and contributes to tumor metastasis by breaking down various
extracellular matrix molecules, which allows metastatic cells to be
more invasive. In order to investigate the effect of BDP on MMP-9
activity, we performed gelatin zymography. As shown in Fig. 7A and B, BDP efficiently inhibited
MMP-9 activity in MDA-MB-231 cells, compared to the DMSO control.
BDP (30 µM) significantly decreased the activity of MMP-9 to 21.3%
(p<0.01). The result clearly indicated that BDP inhibited the
activity of MMP-9 in MDA-MB-231 cells.
Discussion
TNBC exhibits an aggressive subtype and a poor
prognosis because these cancers lack ER, PR and Her2 receptors
(27–29). Therefore, TNBC does not respond to
hormone therapies or Her2 targeted therapies, which makes it
difficult to treat TNBC (30). The
molecular chaperone protein Hsp90 is widely expressed in breast
cancers and plays a key role in regulating the stability and
functions of many oncogenic proteins (31). Therefore, Hsp90 inhibition
represents a promising anticancer strategy to treat TNBCs.
In this study, we investigated the biological
activity of a chalcone-based small molecule, BDP and found that BDP
efficiently impaired the growth of MDA-MB-231 breast cancer cells.
Our data indicated that BDP treatment of MDA-MB-231 cells
significantly led to the degradation of Hsp90 client proteins such
as EGFR, Her2, Met, Akt, c-Raf, and Cdk4, while BDP upregulated
Hsp70, which is a cellular hallmark of Hsp90 inhibition (32,33).
The downregulation of Hsp90 client proteins, Met and Akt could be
reversed by adding a proteasome inhibitor, MG-132 indicating that
BDP caused the degradation of Hsp90 client proteins by
ubiquitin-proteasome pathway. The investigation of the
transcription level demonstrated that BDP did not alter mRNA level
of Met and Akt, further suggesting that the downregulation of the
Hsp90 client proteins, Met and Akt was not associated with the
transcriptional regulation, but the proteasomal degradation.
Apoptosis is a tightly regulated cell suicide
program that plays an essential role in maintaining the
physiological balance between life and death of cells (34,35).
Accordingly, the proliferation of cancer can be suppressed by
triggering apoptotic signaling pathways. As expected, BDP treatment
of MDA-MB-231 cells induced the cleavage of PARP, caspase 3, and
caspase 8, while the anti-apoptotic protein, Bcl-2 was
significantly downregulated in a dose-dependent manner. The result
clearly suggested that BDP efficiently induced the apoptosis of
MDA-MB-231 cells. BDP also caused cell cycle arrest in
G2/M phase in MDA-MB-231 cells. As the percentage of
cells in G2/M phase increased, the percentage of cells
in G0/G1 phase decreased.
An increase of mobility has been associated with the
metastatic potential of cancer cells and patients with TNBC have a
tendency to metastasize to bone, lung, liver, and brain, which
contributes to the poor prognosis with short overall survival
(36,37). In particular, MMPs are associated
with cancer invasion and metastasis. Scratch wound healing assay
and gelatin zymography assay demonstrated that BDP significantly
abrogated the migratory and invasive capacity of MDA-MB-231
cells.
In conclusion, BDP suppressed the proliferation of
triple-negative MDA-MB-231 breast cancer cells by inducing
apoptosis, coupled with augmented G2/M phase arrest.
Moreover, BDP displayed significant degradation of Hsp90 client
proteins, including EGFR, Her2, Met, Akt, c-Raf, and Cdk4, and the
upregulation of Hsp70. Our data also indicated the treatment with
BDP attenuated the migratory and invasive capacity of MDA-MB-231
cells. Overall, these findings strongly supported that BDP could
serve as a potential drug candidate to treat TNBC.
Acknowledgements
This study was supported by Basic Science Research
Program through the National Research Foundation of Korea (NRF)
funded by the Ministry of Education (NRF-2016R1A6A1A03011325 and
2016R1D1A1B01009559), and Korea Institute of Planning and
Evaluation for Technology in Food, Agriculture, Forestry and
Fisheries (IPET) through Animal Disease Management Technology
Development Program, funded by Ministry of Agriculture, Food and
Rural Affairs (MAFRA) (116102–03).
References
|
1
|
Anders C and Carey LA: Understanding and
treating triple-negative breast cancer. Oncology (Williston Park).
22:1233–1239; discussion 1239–1240, 1243. 2008.PubMed/NCBI
|
|
2
|
Osborne CK and Schiff R: Mechanisms of
endocrine resistance in breast cancer. Annu Rev Med. 62:233–247.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baselga J: Targeting the
phosphoinositide-3 (PI3) kinase pathway in breast cancer.
Oncologist. 16 Suppl 1:12–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: NCCN Guidelines Insights Breast Cancer, Version
1.2016. J Natl Compr Canc Netw. 13:1475–1485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haffty BG, Yang Q, Reiss M, Kearney T,
Higgins SA, Weidhaas J, Harris L, Hait W and Toppmeyer D:
Locoregional relapse and distant metastasis in conservatively
managed triple negative early-stage breast cancer. J Clin Oncol.
24:5652–5657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng Q, Chang JT, Geradts J, Neckers LM,
Haystead T, Spector NL and Lyerly HK: Amplification and high-level
expression of heat shock protein 90 marks aggressive phenotypes of
human epidermal growth factor receptor 2 negative breast cancer.
Breast Cancer Res. 14:R622012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sankhala KK, Mita MM, Mita AC and Takimoto
CH: Heat shock proteins: A potential anticancer target. Curr Drug
Targets. 12:2001–2008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bishop SC, Burlison JA and Blagg BS:
Hsp90: A novel target for the disruption of multiple signaling
cascades. Curr Cancer Drug Targets. 7:369–388. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zuehlke A and Johnson JL: Hsp90 and
co-chaperones twist the functions of diverse client proteins.
Biopolymers. 93:211–217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mahalingam D, Swords R, Carew JS, Nawrocki
ST, Bhalla K and Giles FJ: Targeting HSP90 for cancer therapy. Br J
Cancer. 100:1523–1529. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Normant E, Paez G, West KA, Lim AR, Slocum
KL, Tunkey C, McDougall J, Wylie AA, Robison K, Caliri K, et al:
The Hsp90 inhibitor IPI-504 rapidly lowers EML4-ALK levels and
induces tumor regression in ALK-driven NSCLC models. Oncogene.
30:2581–2586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mishra L, Sinha R, Itokawa H, Bastow KF,
Tachibana Y, Nakanishi Y, Kilgore N and Lee KH: Anti-HIV and
cytotoxic activities of Ru(II)/Ru(III) polypyridyl complexes
containing 2,6-(2′-benzimidazolyl)-pyridine/chalcone as co-ligand.
Bioorg Med Chem. 9:1667–1671. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen M, Christensen Brøgger S, Zhai L,
Rasmussen MH, Theander TG, Frøkjaer S, Steffansen B, Davidsen J and
Kharazmi A: The novel oxygenated chalcone,
2,4-dimethoxy-4′-butoxychalcone, exhibits potent activity against
human malaria parasite Plasmodium falciparum in vitro and rodent
parasites Plasmodium berghei and Plasmodium yoelii in vivo. J
Infect Dis. 176:1327–1333. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ducki S: The development of chalcones as
promising anticancer agents. IDrugs. 10:42–46. 2007.PubMed/NCBI
|
|
18
|
Chua AW, Hay HS, Rajendran P, Shanmugam
MK, Li F, Bist P, Koay ES, Lim LH, Kumar AP and Sethi G: Butein
downregulates chemokine receptor CXCR4 expression and function
through suppression of NF-κB activation in breast and pancreatic
tumor cells. Biochem Pharmacol. 80:1553–1562. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katsori AM and Hadjipavlou-Litina D:
Chalcones in cancer: Understanding their role in terms of QSAR.
Curr Med Chem. 16:1062–1081. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X and Go ML: Antiproliferative
properties of piperidinylchalcones. Bioorg Med Chem. 14:153–163.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Modzelewska A, Pettit C, Achanta G,
Davidson NE, Huang P and Khan SR: Anticancer activities of novel
chalcone and bis-chalcone derivatives. Bioorg Med Chem.
14:3491–3495. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeong JH, Oh YJ, Kwon TK and Seo YH:
Chalcone-templated Hsp90 inhibitors and their effects on gefitinib
resistance in non-small cell lung cancer (NSCLC). Arch Pharm Res.
40:96–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeong JH, Oh YJ, Lho Y, Park SY, Liu KH,
Ha E and Seo YH: Targeting the entry region of Hsp90's ATP binding
pocket with a novel 6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl amide.
Eur J Med Chem. 124:1069–1080. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeong CH, Park HB, Jang WJ, Jung SH and
Seo YH: Discovery of hybrid Hsp90 inhibitors and their
anti-neoplastic effects against gefitinib-resistant non-small cell
lung cancer (NSCLC). Bioorg Med Chem Lett. 24:224–227. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee T and Seo YH: Targeting the
hydrophobic region of Hsp90's ATP binding pocket with novel
1,3,5-triazines. Bioorg Med Chem Lett. 23:6427–6431. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seo YH and Jeong JH: Synthesis of butein
analogues and their anti-proliferative activity against
gefitinib-resistant non-small cell lung cancer (NSCLC) through
Hsp90 inhibition. Bull Korean Chem Soc. 35:1294–1298. 2014.
View Article : Google Scholar
|
|
27
|
Chacón RD and Costanzo MV: Triple-negative
breast cancer. Breast Cancer Res. 12 Suppl 2:S32010. View Article : Google Scholar
|
|
28
|
Elias AD: Triple-negative breast cancer: A
short review. Am J Clin Oncol. 33:637–645. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hudis CA and Gianni L: Triple-negative
breast cancer: An unmet medical need. Oncologist. 16 Suppl 1:1–11.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Andreopoulou E, Schweber SJ, Sparano JA
and McDaid HM: Therapies for triple negative breast cancer. Expert
Opin Pharmacother. 16:983–998. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sidera K and Patsavoudi E: Extracellular
HSP90: Conquering the cell surface. Cell Cycle. 7:1564–1568. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sekihara K, Harashima N, Tongu M, Tamaki
Y, Uchida N, Inomata T and Harada M: Pifithrin-μ, an inhibitor of
heat-shock protein 70, can increase the antitumor effects of
hyperthermia against human prostate cancer cells. PLoS One.
8:e787722013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Banerji U, O'Donnell A, Scurr M, Pacey S,
Stapleton S, Asad Y, Simmons L, Maloney A, Raynaud F, Campbell M,
et al: Phase I pharmacokinetic and pharmacodynamic study of
17-allylamino, 17-demethoxygeldanamycin in patients with advanced
malignancies. J Clin Oncol. 23:4152–4161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Viktorsson K, Lewensohn R and Zhivotovsky
B: Apoptotic pathways and therapy resistance in human malignancies.
Adv Cancer Res. 94:143–196. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang X and Wang X: Cytochrome
C-mediated apoptosis. Annu Rev Biochem. 73:87–106. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tseng LM, Hsu NC, Chen SC, Lu YS, Lin CH,
Chang DY, Li H, Lin YC, Chang HK, Chao TC, et al: Distant
metastasis in triple-negative breast cancer. Neoplasma. 60:290–294.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Trinca F, Inacio M, Timoteo T and Dinis R:
Triple-negative breast cancer with brain metastasis in a pregnant
woman. BMJ Case Rep 20172017. doi:10.1136/bcr-2016-218657.
|