Introduction
High mortality and poor prognosis due to late
diagnosis are the main characteristics of ovarian cancer (1). Cancer metastasis is also considered a
major cause of death in ovarian cancer patients (2). TLR stimulation in cancer cells is
known to be closely related with proliferation and progression in
various carcinomas (3). In
addition, TLR activation promotes migration or invasion of several
tumors (4–6). The expression of TLR2, TLR3, TLR4, and
TLR5 are observed on the surface of normal ovary epithelium as well
as benign and malignant tumor conditions (7). TLR1, TLR7, and TLR9 are also detected
in malignant cells, but expressed at low level (7). Thus, studying TLR activation and
related mechanism of cancer progression is critical for the
regulation of ovarian cancer metastasis.
High mesothelin (MSLN) expression is one of
characteristics of several human cancers, including ovarian cancer
(8). Mesothelin plays an important
role in cancer cell survival, migration, invasion and tumor
progression (9,10). Secreted mesothelin is capable of
binding to CA125/MUC16 (11) and
overexpression of CA125 on the surface of ovarian cancer cells is
strongly associated with poor survival of ovarian cancer patients
(11,12). Mesothelin/CA125 binding promotes
migration and invasion of pancreatic cancer cells via activation of
matrix metalloproteinase 7 (MMP7) (13). Mesothelin triggers cancer cell
survival, proliferation and drug resistance through
phosphatidylinositol 3-kinase (PI3K)/Wnt/NF-κB-signaling pathways
(14). The PI3K/Akt pathway
generally promotes the inhibition of apoptosis and progression of
the cell cycle (15). TLRs
stimulation is functionally associated with tumor growth and
progression (16). We also reported
that TLR4-mediated PI3K activation controls the invasion and
metastasis of ovarian cancer through the production of galectin-1
(17). However, the underlying
mechanism and association of PI3K for inducing mesothelin in
TLR5/7-activated ovarian cancer cells remains unclear.
The WASP (Wiskott-Aldrich syndrome protein) and WAVE
(WASP verprolin-homologous) families of proteins play important
roles in the morphological changes and cytokinesis through the
regulation of actin-cytoskeleton interactions (18). Although WAVE1 and WAVE2 are
essential for the formation of the peripheral leading edge in
invading fibroblasts (19), WAVE3
is also required for the process of epithelial mesenchymal
transition (EMT) of various cancers, including breast, liver, and
prostate (20–22). In addition, WAVE3 interacts with
p85, the regulatory subunit of the PI3K, to activate the downstream
signaling cascade (23). Although
WAVE3 is one of the attractive diagnostic and therapeutic targets
for anti-cancer therapy, the underlying mechanism of its induction
and association with mesothelin in TLR-stimulated ovarian cancer
remains poorly understood.
Ovarian cancer cells express a variety of TLRs
(7). Furthermore, signaling and
response induced by TLR5 activation regulates tumor growth and
progression of ovarian cancer (24). Based on these studies, we
hypothesized that the stimulation of TLR5 and TLR7 on the surface
of ovarian cancer cells would induce PI3K/Akt-mediated
epithelial-mesenchymal transition (EMT) through the regulation of
several signaling pathways. We investigated the relationship
between WAVE3 and mesothelin induction by the TLR5/7-activated
PI3K/Akt signaling pathway. We also examined the association of
TAp63 in TLR5/7-mediated ovarian cancer migration because induction
of TAp63 expression is related to promotion of migratory and
invasive activities in TLR4-mediated cancer cells (25).
Materials and methods
Cell lines and chemicals
The human ovarian cancer cell lines CaOV3 and SKOV3
were purchased from ATCC (Manassas, VA, USA). The CaOV3 cells were
maintained in DMEM medium (Corning Inc., Corning, NY, USA)
supplemented with 10% FBS (RMBIO, Missoula, MT, USA), penicillin,
streptomycin, and glutamine at 37°C in 5% CO2. The SKOV3
cells were maintained in RPMI-1640 medium (Corning Inc.)
supplemented with 10% FBS, penicillin, streptomycin, and glutamine
at 37°C in 5% CO2. Flagellin (TLR5 ligand) was purchased
from Sigma-Aldrich (St. Louis, MO, USA). Imiquimod (TLR7 ligand)
was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
A66 (p110α inhibitor), TGX-221 (p110β inhibitor), CAL-101 (p110δ
inhibitor), LY294002 (pan-p110 inhibitor), Bay 80–6946 (p110α and
p110β dual inhibitor), and pictilisib (p110α and p110δ dual
inhibitor) were obtained from Selleck Chemicals (Houston, TX,
USA).
Proliferation assay with Cell Counting
Kit-8
Cell proliferation was measured using Cell Counting
Kit-8 (CCK-8) (Enzo Life Sciences, Farmingdale, NY, USA) according
to the manufacturer's instructions. The cells were seeded into
96-well plates (2×104 cells/well) and treated with
flagellin (0.1 µg/ml) or imiquimod (4 µg/ml). For comparison,
non-treated control cells were cultured with media in the presence
of DMSO. After 24 h, the cells were stained with 10 µl of CCK-8 dye
in 90 µl of culture medium for 2 h at 37°C. The absorbance was
measured at 450 nm.
Western blotting
The cells were washed in PBS and lysed in NP-40
buffer (Elpis Biotech, Daejeon, Korea) supplemented with a protease
inhibitor cocktail (Sigma-Aldrich). Protein phosphorylation states
were preserved by the addition of phosphatase inhibitors (Cocktail
II, Sigma-Aldrich) to the NP-40 buffer. Protein concentrations were
determined by using a BCA assay kit (Pierce, Rockford, IL, USA).
The proteins (10 µg/sample) were resolved by SDS-PAGE and then
transferred onto a nitrocellulose membrane (Millipore Corp.,
Billerica, MA, USA). The membranes were blocked with 5% non-fat
milk prior to western blot analysis. Chemiluminescence was detected
using an ECL kit (Advanta Corp., Menlo Park, CA, USA) and the
Amersham Imager 600 (GE Healthcare Life Sciences, Little Chalfont,
UK). The following primary Abs were used: E-cadherin, N-cadherin,
Slug, Snail, Vimentin, TCF8/Zeb1, β-actin, MMP2, MMP9, MyD88,
p110α, p110β, p110δ, WAVE3, mesothelin, SOX2, and OCT4 (Cell
Signaling Technology, Beverly, MA, USA); α-SMA (Bioss, Woburn, MA,
USA); CA125 (Abcam, Cambridge, UK); TAp63 (BioLegend, San Diego,
CA, USA); TLR5 and TLR7 (Santa Cruz Biotechnology).
Small interfering RNA (siRNA)
transfection
Experimentally verified human WAVE3-small
interfering RNA (siRNA) duplex, human OCT4-siRNA duplex, human
SOX2-siRNA duplex, human mesothelin-siRNA duplex, human TLR5-siRNA
duplex, human TLR7-siRNA duplex, and negative control-siRNA were
obtained from Bioneer Corp. (Daejeon, Korea). Cells were seeded at
a concentration of 1×105 per well in a 6-well plate and
grown overnight. The cells were then transfected with 200 nM siRNA
using Lipofectamine RNAiMAX Reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's instructions. The cells were used
for further experiments at 36 h after transfection and then treated
with TLR agonists for 24 h.
Quantification of human cytokines by
ELISA
The concentrations of active TGF-β1, TNF-α, VEGF,
and IL-8 in the cell culture supernatants were quantified by single
cytokine ELISA assay kits (R&D Systems, Minneapolis, MN, USA).
Mesothelin was quantified with a kit from BioLegend. WAVE3, SOX2,
and OCT4 were quantified with kits from MyBioSource (San Diego, CA,
USA). The data are expressed as the average value from a number of
biological replicates ± standard deviation (SD).
Invasion assay
The invasion assay was performed using the
CultreCoat 96-well Medium BME Cell Invasion Assay kit (R&D
Systems) according to the manufacturer's protocol. Cells
(2.5×104) in serum-free RPMI-1640 or DMEM containing
0.1% FBS were seeded into the upper chamber and the lower
compartment was filled with RPMI-1640 or DMEM containing 10% FBS as
the chemoattractant. After incubation for 24 h, the non-invading
cells on the upper membrane surface were removed by wiping with a
cotton swab. The invaded cells were stained with calcein-AM and
quantified using a microplate reader.
Statistical analyses
Data are expressed as the mean ± standard deviation
(SD). Statistical analysis was conducted using one-way analysis of
the variance. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Binding to TLR5/7 induces changes in
SKOV3 cell motility and secretion of EMT-related cytokines
We first examined the expression of TLR5 and TLR7 to
determine the effect of TLR stimulation on EMT processes in ovarian
cancer cells. First, we selected the four cell lines (CaOV3,
OVCAR3, OV90 and SKOV3) as model of ovarian cancer (26) and checked the expression of TLR5 and
TLR7 as well as the proliferation after stimulation with TLR5
ligand (flagellin) or TLR7 ligand (imiquimod). Although TLR7 was
detected in all ovarian cancer cell lines, the appearance of TLR5
on CaOV3 cells was very weak compared to the other cell lines
(Fig. 1A). TLR5 or TLR7 activation
by specific agonist had no effect on the proliferation of CaOV3 and
SKOV3 cells (Fig. 1B). Based on
those results, we selected CaOV3 cells as the representative of
primary ovarian cancer and SKOV3 cells as the model of metastatic
ovarian cancer (27). Treatment
with flagellin and imiquimod of SKOV3 cells not only induced the
mesenchymal markers (N-cadherin, Vimentin, Slug, Snail, α-SMA, and
TCF8) but also reduced the epithelial marker, E-cadherin; however,
the stimulation of CaOV3 cells with TLRs had no influence on
upregulation of the mesenchymal phenotype even after stimulation
with flagellin and imiquimod (Fig.
1C). The invasive activity of the SKOV3 cells was also enhanced
by stimulation with flagellin or imiquimod (Fig. 1D). The SKOV3 cells significantly
increased the EMT-related cytokines (TGF-β1, IL-8, VEGF, and TNF-α)
compared with non-treated control cells after treatment with
flagellin (Fig. 1E) or imiquimod
(Fig. 1F). These results suggest
that activation of TLR5/7 might be one of the critical targets to
modulate ovarian cancer metastasis and invasion.
 | Figure 1.TLR5/7 agonist increases the invasion
and secretion of EMT-related cytokines of ovarian cancer cells. (A)
The protein levels of TLR5/7 on ovarian cancer cells were measured
using western blotting. β-actin was used as a loading control. (B)
Effect of TLR agonists on the proliferation of CaOV3 and SKOV3
cells. The cells were seeded into 96-well plates (2×104
cells/well) and treated with flagellin (0.1 µg/ml) or imiquimod (4
µg/ml). After incubation for 24 h, cell proliferation was
determined using the Cell Counting Kit-8 (CCK-8) according to the
manufacturer's instructions. (C-F) Cells (1.5×105/well)
were seeded into 6-well plates and grown overnight. The cells were
cultured with or without TLR agonists (0.1 µg/ml flagellin or 4
µg/ml imiquimod) for 24 h. (C) EMT markers (E-cadherin, N-cadherin,
Vimentin, Slug, Snail, α-SMA, and TCF8) were determined using
western blotting. (D) The invasiveness of the SKOV3 cells was
enhanced by TLR agonists (flagellin or imiquimod) as determined
with the BME cell invasion assay described in Materials and
methods. Each value is the mean ± standard deviation of 3
determinations. *P<0.01. (E and F) Culture supernatants were
collected 24 h after stimulation with TLR agonists (flagellin or
imiquimod), and the amounts of active TGF-β1, IL-8, VEGF, and TNF-α
were determined by ELISA. Data are presented as the mean of three
independent experiments, and the error bars represent SD of the
means. **P<0.005. The results are representative of three
independent experiments. |
TLR5/7 stimulation induces the
expression of EMT-associated signaling molecules in SKOV3
cells
Next, we examined the signaling pathway that is
influenced by the activation of TLR5/7 responsible for the
migration and invasion in ovarian cancer cells. The
TLR5/7-activated ovarian cancer cells showed activation of MyD88
expression; the levels of PI3K p110α, β, and δ isoforms were
significantly increased in the TLR5/7-stimulated SKOV3 cells
compared to the non-activated cells (Fig. 2A). Although the expression of TAp63
was inhibited, the levels of WAVE3, mesothelin, and CA125 were
upregulated in the TLR5/7-treated SKOV3 cells (Fig. 2A). However, gene silencing of TLR5
or TLR7 with siRNA profoundly blocked the expression of mesenchymal
marker (N-cadherin) and prevented the activation of downstream
signaling pathway in TLR5- or TLR7-stimulated SKOV3 cells (Fig. 2B). The secretion of mesothelin and
WAVE3 by SKOV3 cells was detected after stimulation with TLR5 and
TLR7 (Fig. 2C and D). The
TLR5/7-activated SKOV3 cells showed an induction in the expression
of SRY-related HMG-box gene 2 (SOX2) and Octamer-binding
transcription factor 4 (OCT4) proteins (Fig. 2E), which contribute to cancer
invasion (28,29). In addition, secreted SOX2 and OCT4
were detected in the culture supernatant (Fig. 2F and G). These results suggest that
TLR5/7 responses to their ligands play a major role in triggering
the EMT-related signal transduction of metastatic ovarian cancer
cells.
 | Figure 2.TLR5/7 stimulation induces the
expression of EMT-associated signaling molecules in ovarian cancer
cells. (A-G) Cells (1.5×105/well) were seeded into
6-well plates and grown overnight. The cells were cultured with or
without TLR agonists (0.1 µg/ml flagellin or 4 µg/ml imiquimod) for
24 h. (B) To determine the effect of TLR5 or TLT7 on EMT-related
signal transduction, the cells were seeded into 6-well plates
(1×105/well) and transfected with siRNA oligonucleotides
of TLR5-siRNA (200 nM) or TLR7-siRNA (200 nM) or control-siRNA for
36 h and then treated with TLR agonists for 24 h. (A, B and E)
Total cell lysates were immunoblotted with the indicated
antibodies. β-actin served as the loading control. (C, D, F and G)
The culture supernatants were collected 24 h after stimulation with
flagellin (C and F) or imiquimod (D and G), and then the amounts of
mesothelin, WAVE3, SOX2, and OCT4 were determined by ELISA. Data
are presented as the mean of three independent experiments, and the
error bars represent SD of the means. *P<0.001. **P<0.05.
#P<0.005. ##P<0.01. The results are
representative of three independent experiments. MSLN,
mesothelin. |
TLR5/7-dependent PI3K activation
regulates WAVE3-mediated cancer cell motility
Previously, we found that TLR stimulation
contributed to the activation of PI3K catalytic subunits. Thus, the
biological significance of PI3K in cancer cell metastasis and its
effect on the EMT-related signaling molecules were examined using
specific pharmacological inhibitors. Specific inhibitor against
PI3K p110α, β, and δ isoforms considerably suppressed the induction
of WAVE3 and CA125 in the SKOV3 cells after treatment with
flagellin or imiquimod, whereas the levels of TAp63 were
upregulated (Fig. 3A and B).
Additionally, the specific inhibition of each PI3K p110 isoform
efficiently reversed the expression of TAp63 and reduced the
mesenchymal properties of the TLR5/7-activated SKOV3 cells
(Fig. 3A and B). Pharmacological
inhibition of PI3K p110 isoform significantly prevented cell
motility (Fig. 3C), secretion of
mesothelin (Fig. 3D) and production
of OCT4 (Fig. 3E) and SOX2
(Fig. 3F) in the TLR5/7-treated
SKOV3 cells. These results suggest that the PI3K-mediated the
expression of WAVE3, mesothelin, SOX2, and OCT4 activates migration
and invasion capacity of the ovarian cancer cells after stimulation
with flagellin or imiquimod.
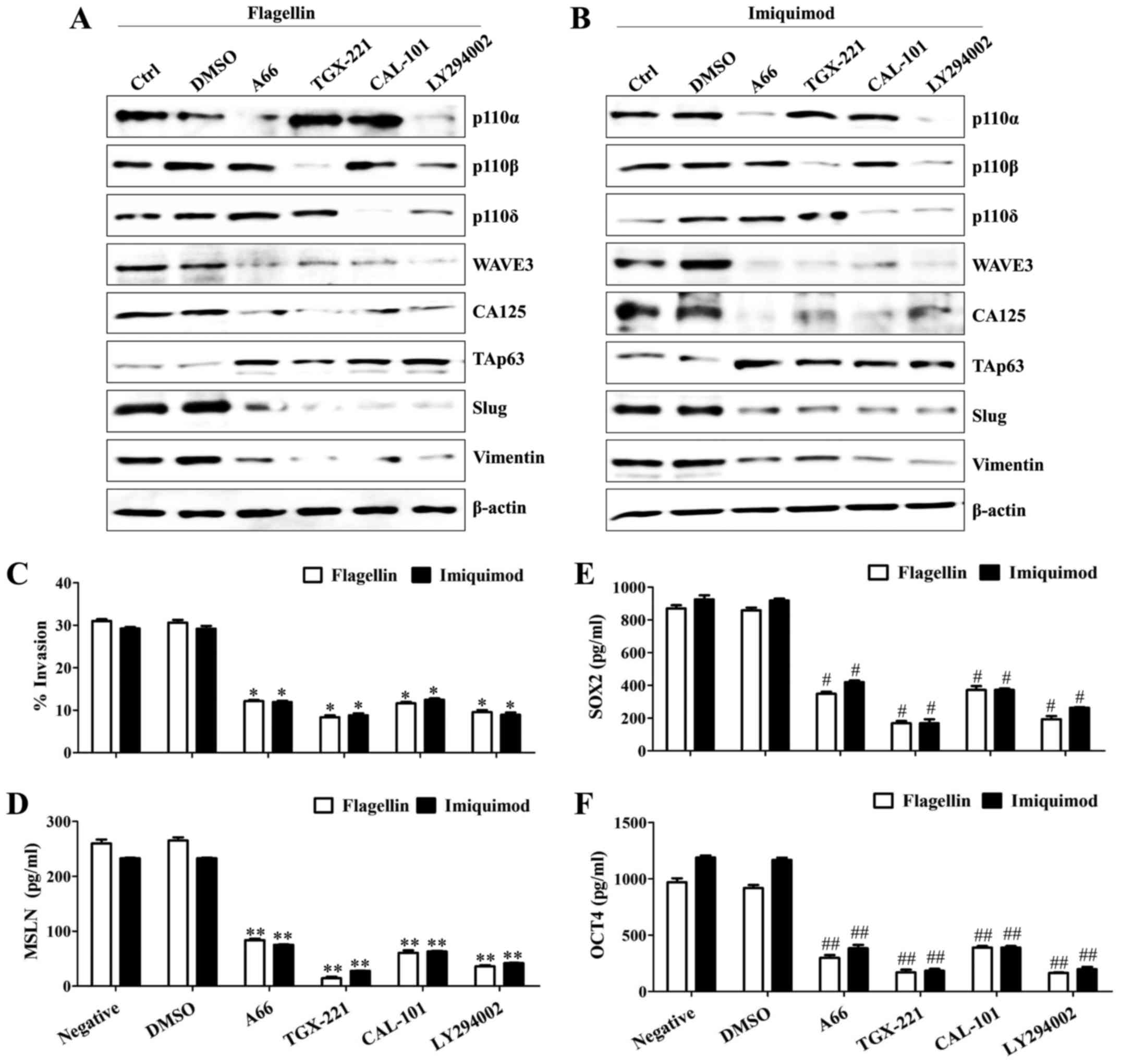 | Figure 3.TLR5/7-dependent PI3K activation
regulates WAVE3-mediated cancer cell motility. Cells
(1.5×105/well) were seeded into 6-well plates and
incubated overnight. The cells were pre-incubated with 50 µM A66,
50 µM TGX-221, 50 µM CAL-101, 50 µM LY294002, 200 nM BAY 80–6946,
or 200 nM pictilisib for 2 h and then treated with TLR agonists
(0.1 µg/ml flagellin or 4 µg/ml imiquimod) for 24 h. (A and B)
Total cell lysates were immunoblotted with the indicated
antibodies. (C) The invasiveness of the TLR5/7-stimulated SKOV3
cells was blocked by PI3K inhibitors as determined by the BME cell
invasion assay described in Materials and methods. Each value is
the mean ± SD of 3 determinations. *P<0.005. (D-F) The culture
supernatants were collected 36 h after transfection, and the
amounts of mesothelin (D), SOX2 (E), and OCT4 (F) were determined
by ELISA. Data are presented as the mean of three independent
experiments, and the error bars represent SD of the means.
**P<0.005. #P<0.01. ##P<0.01. The
results are representative of three independent experiments. MSLN,
mesothelin. |
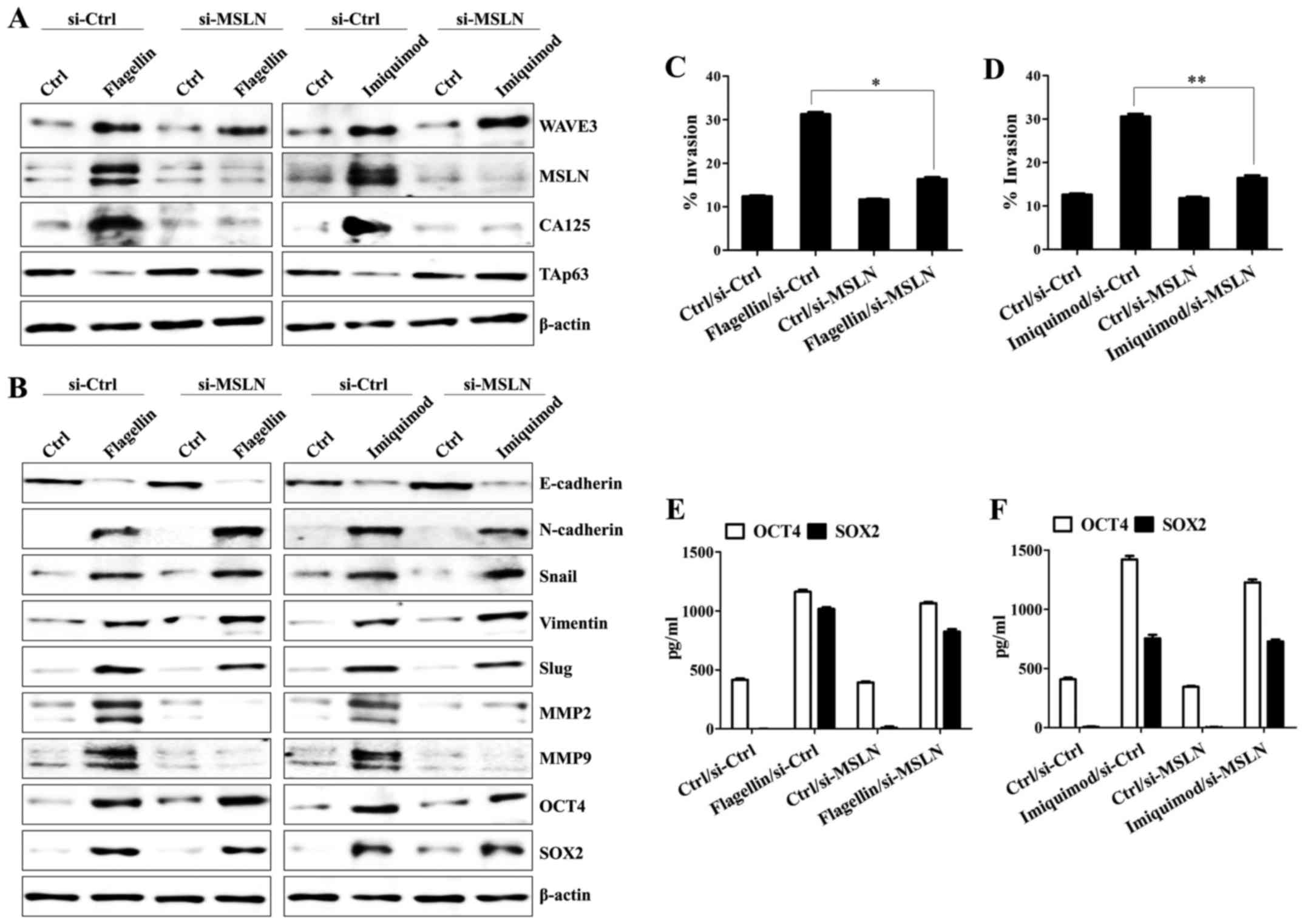
WAVE3 is required for the expression
of mesothelin/CA125 and production of OCT4/SOX2 in the
TLR5/7-mediated invasion activity of SKOV3 cells
Next, we investigated the relationship of
EMT-related molecules to better understand how the TLR5/7-mediated
signaling pathway regulates cancer cell motility. In flagellin- or
imiquimod-treated SKOV3 cells, the knockdown of WAVE3 using siRNA
resulted in the loss of expression of mesothelin and CA125;
however, the level of TAp63 was not downregulated by stimulation
with flagellin or imiquimod in the WAVE3-silenced SKOV3 cells
(Fig. 4A). Silencing of the WAVE3
in the SKOV3 cells blocked the expression of mesenchymal markers,
matrix metalloproteinase 2 (MMP2) and MMP9 (Fig. 4B) and inhibited the invasive
activity of the cells after TLR5/7 stimulation (Fig. 4C and D). In addition, downregulation
of WAVE3 markedly suppressed the expression (Fig. 4E) and secretion of OCT4 and SOX2 in
the TLR5/7-activated SKOV3 cells (Fig.
4F and G). Although WAVE3-silencing reduced the expression of
mesothelin and CA125 along with the activation of MMP2/MMP9 in the
TLR5/7-stimulated SKOV3 cells, silencing of the mesothelin gene
using siRNA decreased only the level of CA125 in flagellin- or
imiquimod-treated SKOV3 cells (Fig.
5A) but not the levels of WAVE3 or TAp63 (Fig. 5A). The expression of mesenchymal
markers, OCT4, and SOX2 were not affected by silencing of
mesothelin gene in the flagellin- or imiquimod-treated SKOV3 cells
(Fig. 5B), whereas the targeted
inhibition of mesothelin reduced the expression of MMP2 and MMP5
after the stimulation with TLR5 and TLR7 ligands (Fig. 5B). Stimulation with flagellin or
imiquimod of the mesothelin-silenced SKOV3 cells resulted in the
attenuation of their migratory activity (Fig. 5C and D); however, the SKOV3 cells
lacking mesothelin expression failed to decrease the production of
SOX2 and OCT4 after treatment with flagellin or imiquimod (Fig. 5E and F). These results suggest that
WAVE3 not only plays a critical role in the
mesothelin/CA125-mediated TAp63 downregulation and MMP upregulation
but also controls the expression of OCT4 and SOX2 in a
mesothelin-independent manner.
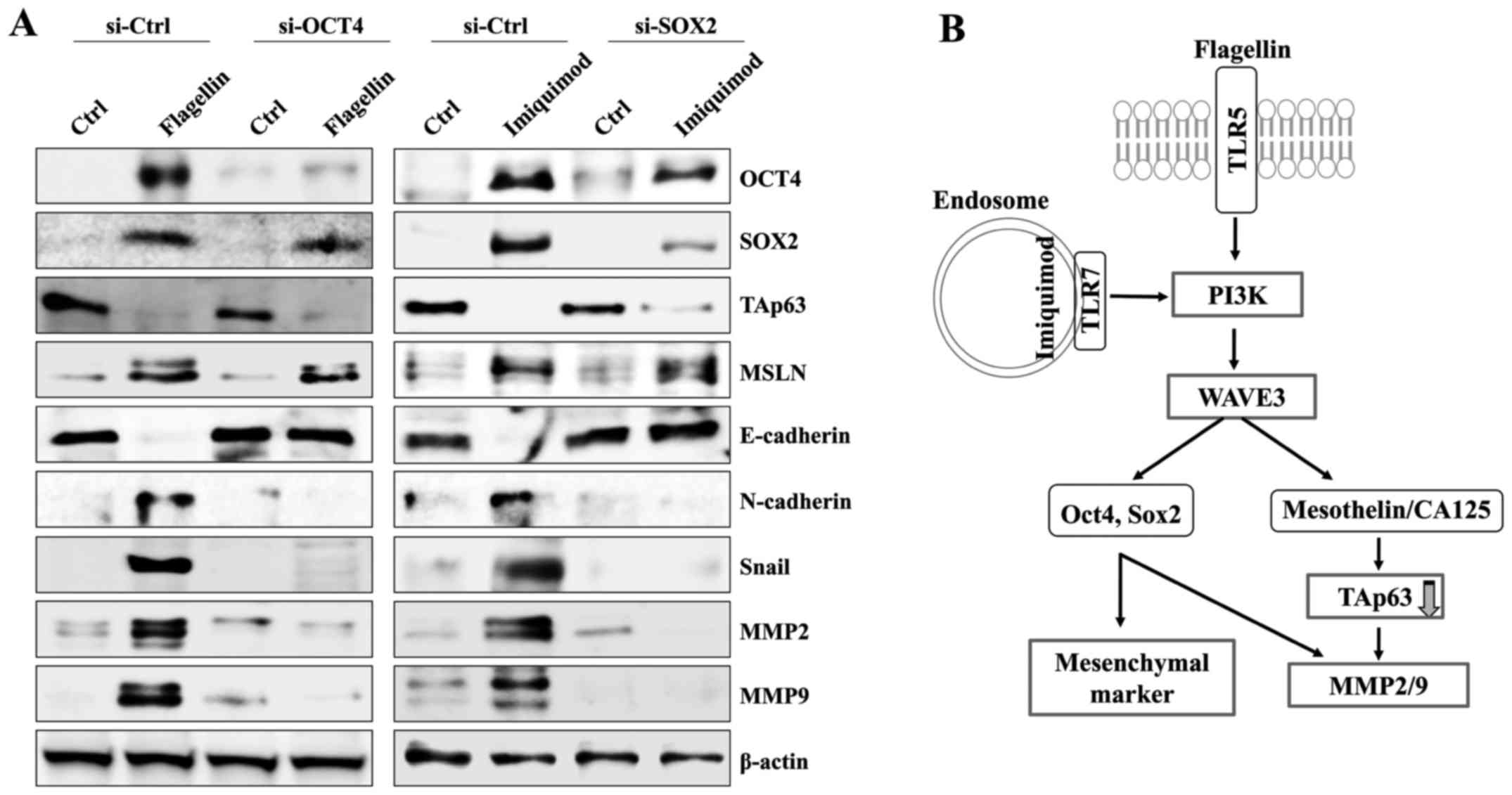
OCT4 and SOX2 modulate the expression
of mesenchymal markers in the TLR5/7-activated SKOV3 cells
Next, we investigated whether the level of OCT4 or
SOX2 has an effect on the expression of mesenchymal markers in the
TLR5/7-stimulated SKOV3 cells. The targeted inhibition of OCT4 or
SOX2 using small interference RNA had no effect on the mesothelin
upregulation and TAp63 downregulation of the flagellin- or
imiquimod-treated SKOV3 cells (Fig.
6A). Moreover, silencing of OCT4 or SOX2 genes reduced the
expression of N-cadherin and Snail in SKOV3 after treatment with
TLR5 and TLR7 ligands (Fig. 6A). In
addition, the downregulation of OCT4 or SOX2 significantly
suppressed the expression of activated MMP2 and MMP9 in the
flagellin- or imiquimod-treated SKOV3 cells. These results suggest
that the TLR5/7-mediated WAVE3 signaling pathway controls the EMT
processes through the regulation of OCT4 and SOX2 expression.
Discussion
TLR activation in cancer cells promotes metastasis
of cancer cells at various stages through the regulation of cell
adhesion, invasion, angiogenesis, and increase in vascular
permeability (3–6). The activation of TLR2:TLR6 complexes
has been shown to enhance the metastatic growth of lung cancer by
inducing TNF-α secretion in a rat model (4). The exposure of TLR4 ligands to
lipopolysaccharides (LPS), induces cancer growth and lung
metastasis of breast cancer by increasing angiogenesis (5). CpG oligonucleotide treatment decreased
the expression of tissue inhibitor of metalloproteinase-3 and
increased levels of active MMP13 in TLR9-expressing breast cancer
cells (6). TLR4 stimulation is also
involved in ovarian cancer growth and sensitivity to apoptosis
mediated by drugs or immune cells (30). Although TLR5 and TLR7 are detected
on the ovarian epithelium under benign conditions and epithelial
tumors (7), the role and signaling
mechanism of TLR5 and TLR7 in ovarian cancer metastasis has yet to
be determined.
In this study, SKOV3 cells, as representatives of
metastatic ovarian cancer, strongly expressed TLR5 and TLR7,
whereas the CaOV3 cells expressed TLR7, but their expression of
TLR5 was weak. TLR5/7-dependent signaling contributed to the
enhancement of cell motility through PI3K-mediated WAVE3
activation. Moreover, WAVE3 modulated the expression of
mesothelin/CA125, downregulated the expression of TAp63, and
promoted the expression of OCT4/SOX2-mediated mesenchymal markers
in SKOV3 (Fig. 6B). Although TLR7
was also expressed in the CaOV3 cells, stimulation of these cells
with imiquimod failed to activate downstream signaling pathways.
Furthermore, downregulation of TLR5 or TLR7 by transfection with
siRNA in SKOV3 cells prevented the initiation of TLR5- or
TLR7-mediated signaling pathway. Our findings suggest that both
TLR5 and TLR7 expression of ovarian cancer play critical roles in
triggering WAVE3-dependent EMT processes.
Mesothelin, a plasma membrane differentiation
antigen, is expressed at significantly high levels in several human
cancers, including nearly all mesotheliomas (8) and approximately 70% of ovarian cancers
(11). High mesothelin expression
is positively correlated with drug resistance and short
disease-free survival in ovarian cancer patients (31). Although mesothelin fails to provide
diagnostic information about the cancer due to its low sensitivity
and specificity as a tumor marker (32), the co-expression of CA125 and
mesothelin is strongly correlated with induction of cancer cell
metastasis and unfavorable patient outcomes in pancreatic cancer
(13,33). The molecular mechanisms that
contribute to the induction of mesothelin and CA125 expression
remain largely unknown. Furthermore, additional studies on
mesothelin induction are still needed to define its role as a
diagnostic marker for determining the clinical outcome and response
to anti-cancer therapy. The levels of mesothelin and CA125 were
upregulated after stimulation of the SKOV3 cells with flagellin or
imiquimod. In addition, the loss of WAVE3 expression led to the
complete downregulation of mesothelin and CA125 in the
TLR5/7-activated SKOV3 cells. Our data suggest that the acquisition
of WAVE3 activation is one of important steps in controlling the
expression of mesothelin/CA125.
The WASP/WAVE proteins are closely related to cell
shape changes, cytokinesis, and cell motility, through the
re-organization of cytoskeletal proteins (34). The WAVE subfamily of proteins
contains three members, WAVE1, WAVE2, and WAVE3 (35). WAVE3 has been shown to regulate
motility and invasiveness of breast cancer cells through the
activation of p38 and MMP (36).
Knockdown of WAVE3 in cells results in the inhibition of NF-κB
signaling and activation of MMP9 (37). Treatment of cells with LY294002, an
inhibitor of PI3K, also prevents WAVE3-mediated breast cancer cell
migration (23). Although PI3K/Akt
activation is involved in multiple signal transduction pathways
(38), an important question that
remains to be answered is whether the TLR5/7-mediated cancer cell
migration is also regulated by the PI3K/WAVE3 pathway.
TLR5/7-dependent PI3K activation led to the WAVE3-mediated
expression of mesothelin and CA125 and the production of OCT4 and
SOX2. Pharmacological inhibition of PI3K p110 isoform suppressed
the production of mesothelin, OCT4, and SOX2 but recovered the
expression of TAp63 in flagellin- or imiquimod-treated SKOV3 cells.
Our results suggest that the PI3K/WAVE3 signaling pathway initiates
the invasion and metastasis of ovarian cancer after stimulation
with TLR5/7.
The expression of p63 plays an important role in the
generation of epithelial tissues during the developmental stages of
both humans and murine animals (39). Furthermore, the disruption of p63
results in more aggressive and metastatic tumors through the
upregulation of genes associated with increased invasiveness and
metastasis (40). TLR4 stimulation
had been shown to promote the invasive activity of colon cancer
cells by TAp63-mediated GSK-3β activation (25). Moreover, the loss of p63 expression
accelerates the metastatic spread and aggressive invasion of the
cancer cells (41). In this study,
the TLR5/7-stimulated ovarian cancer cells also showed reduced
migratory activities along with the suppression of TAp63
expression. These results suggest that the role of TAp63 in cancer
metastasis is still controversial and cell-type dependent.
The expression of embryonic stem cell markers,
including SOX2 and OCT4 is significantly associated with the
progression of various human malignancies (42). SOX2 promotes the metastasis of
breast and prostate cancer cells by promoting
epithelial-to-mesenchymal transition (EMT) (43). Silencing of the p63 gene using siRNA
suppresses the expression of SOX2 and OCT4 in mouse embryonic
fibroblasts (44). Although the
stimulation with flagellin or imiquimod of SKOV3 cells induced the
expression of SOX2 and OCT4 in this study, the activation of TAp63
was significantly reduced. Conversely, the knockdown of WAVE3
attenuated mesothelin, OCT4, and SOX2 production, whereas the
expression of TAp63 was recovered. In addition, the targeted
inhibition of mesothelin recovered the expression of TAp63 but had
no effect on the production of OCT4 and SOX2 in the flagellin- or
imiquimod-treated SKOV3 cells.
TLR stimulation is closely associated with
carcinogenesis and progression as well as the production of various
cytokines and chemokines (3). In
this study, we also identified the critical role of TLR5/7-mediated
WAVE3 signaling in the invasion and metastasis of ovarian cancer
cells in the independent regulation of expression of
mesothelin/CA125 and OCT4 and SOX2. Therefore, these results
suggest that WAVE3 might be a promising candidate for the
development of a new targeted therapy against ovarian cancer
metastasis.
Acknowledgements
This study was supported by the Basic Science
Research Program of Ministry of Education (NRF-2015R1D1A1A01056672)
and Ministry of Science, ICT and Future Planning
(NRF-2015R1C1A2A01053732) through the National Research Foundation
(NRF) of Republic of Korea.
References
|
1
|
Brun JL, Feyler A, Chêne G, Saurel J, Brun
G and Hocké C: Long-term results and prognostic factors in patients
with epithelial ovarian cancer. Gynecol Oncol. 78:21–27. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rakoff-Nahoum S and Medzhitov R: Toll-like
receptors and cancer. Nat Rev Cancer. 9:57–63. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim S, Takahashi H, Lin WW, Descargues P,
Grivennikov S, Kim Y, Luo JL and Karin M: Carcinoma-produced
factors activate myeloid cells through TLR2 to stimulate
metastasis. Nature. 457:102–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harmey JH, Bucana CD, Lu W, Byrne AM,
McDonnell S, Lynch C, Bouchier-Hayes D and Dong Z:
Lipopolysaccharide-induced metastatic growth is associated with
increased angiogenesis, vascular permeability and tumor cell
invasion. Int J Cancer. 101:415–422. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Merrell MA, Ilvesaro JM, Lehtonen N, Sorsa
T, Gehrs B, Rosenthal E, Chen D, Shackley B, Harris KW and Selander
KS: Toll-like receptor 9 agonists promote cellular invasion by
increasing matrix metalloproteinase activity. Mol Cancer Res.
4:437–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou M, McFarland-Mancini MM, Funk HM,
Husseinzadeh N, Mounajjed T and Drew AF: Toll-like receptor
expression in normal ovary and ovarian tumors. Cancer Immunol
Immunother. 58:1375–1385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang K and Pastan I: Molecular cloning of
mesothelin, a differentiation antigen present on mesothelium,
mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 93:pp.
136–140. 1996; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang MC, Chen CA, Chen PJ, Chiang YC,
Chen YL, Mao TL, Lin HW, Lin Chiang WH and Cheng WF: Mesothelin
enhances invasion of ovarian cancer by inducing MMP-7 through
MAPK/ERK and JNK pathways. Biochem J. 442:293–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Servais EL, Colovos C, Rodriguez L, Bograd
AJ, Nitadori J, Sima C, Rusch VW, Sadelain M and Adusumilli PS:
Mesothelin overexpression promotes mesothelioma cell invasion and
MMP-9 secretion in an orthotopic mouse model and in epithelioid
pleural mesothelioma patients. Clin Cancer Res. 18:2478–2489. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rump A, Morikawa Y, Tanaka M, Minami S,
Umesaki N, Takeuchi M and Miyajima A: Binding of ovarian cancer
antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol
Chem. 279:9190–9198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Markman M, Federico M, Liu PY, Hannigan E
and Alberts D: Significance of early changes in the serum CA-125
antigen level on overall survival in advanced ovarian cancer.
Gynecol Oncol. 103:195–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen SH, Hung WC, Wang P, Paul C and
Konstantopoulos K: Mesothelin binding to CA125/MUC16 promotes
pancreatic cancer cell motility and invasion via MMP-7 activation.
Sci Rep. 3:18702013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bharadwaj U, Marin-Muller C, Li M, Chen C
and Yao Q: Mesothelin confers pancreatic cancer cell resistance to
TNF-α-induced apoptosis through Akt/PI3K/NF-κB activation and
IL-6/Mcl-1 overexpression. Mol Cancer. 10:1062011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu L and Chen S: Toll-like receptors
expressed in tumor cells: Targets for therapy. Cancer Immunol
Immunother. 57:1271–1278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park GB, Chung YH and Kim D: Induction of
galectin-1 by TLR-dependent PI3K activation enhances
epithelial-mesenchymal transition of metastatic ovarian cancer
cells. Oncol Rep. 37:3137–3145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suetsugu S and Takenawa T: Regulation of
cortical actin networks in cell migration. Int Rev Cytol.
229:245–286. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suetsugu S, Yamazaki D, Kurisu S and
Takenawa T: Differential roles of WAVE1 and WAVE2 in dorsal and
peripheral ruffle formation for fibroblast cell migration. Dev
Cell. 5:595–609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kulkarni S, Augoff K, Rivera L, McCue B,
Khoury T, Groman A, Zhang L, Tian L and Sossey-Alaoui K: Increased
expression levels of WAVE3 are associated with the progression and
metastasis of triple negative breast cancer. PLoS One.
7:e428952012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ji Y, Li B, Zhu Z, Guo X, He W, Fan Z and
Zhang W: Over-expression of WAVE3 promotes tumor invasiveness and
confers an unfavorable prognosis in human hepatocellular carcinoma.
Biomed Pharmacother. 69:409–415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moazzam M, Ye L, Sun PH, Kynaston H and
Jiang WG: Knockdown of WAVE3 impairs HGF induced migration and
invasion of prostate cancer cells. Cancer Cell Int. 15:512015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sossey-Alaoui K, Li X, Ranalli TA and
Cowell JK: WAVE3-mediated cell migration and lamellipodia formation
are regulated downstream of phosphatidylinositol 3-kinase. J Biol
Chem. 280:21748–21755. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rutkowski MR, Stephen TL, Svoronos N,
Allegrezza MJ, Tesone AJ, Perales-Puchalt A, Brencicova E,
Escovar-Fadul X, Nguyen JM, Cadungog MG, et al: Microbially driven
TLR5-dependent signaling governs distal malignant progression
through tumor-promoting inflammation. Cancer Cell. 27:27–40. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park GB, Chung YH, Gong JH, Jin DH and Kim
D: GSK-3β-mediated fatty acid synthesis enhances epithelial to
mesenchymal transition of TLR4-activated colorectal cancer cells
through regulation of TAp63. Int J Oncol. 49:2163–2172. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vergara D, Merlot B, Lucot JP, Collinet P,
Vinatier D, Fournier I and Salzet M: Epithelial-mesenchymal
transition in ovarian cancer. Cancer Lett. 291:59–66. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beaufort CM, Helmijr JC, Piskorz AM,
Hoogstraat M, Ruigrok-Ritstier K, Besselink N, Murtaza M, van IJcken
WF, Heine AA, Smid M, et al: Ovarian cancer cell line panel (OCCP):
Clinical importance of in vitro morphological subtypes. PLoS One.
9:e1039882014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leis O, Eguiara A, Lopez-Arribillaga E,
Alberdi MJ, Hernandez-Garcia S, Elorriaga K, Pandiella A, Rezola R
and Martin AG: Sox2 expression in breast tumours and activation in
breast cancer stem cells. Oncogene. 31:1354–1365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumar SM, Liu S, Lu H, Zhang H, Zhang PJ,
Gimotty PA, Guerra M, Guo W and Xu X: Acquired cancer stem cell
phenotypes through Oct4-mediated dedifferentiation. Oncogene.
31:4898–4911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kelly MG, Alvero AB, Chen R, Silasi DA,
Abrahams VM, Chan S, Visintin I, Rutherford T and Mor G: TLR-4
signaling promotes tumor growth and paclitaxel chemoresistance in
ovarian cancer. Cancer Res. 66:3859–3868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng WF, Huang CY, Chang MC, Hu YH,
Chiang YC, Chen YL, Hsieh CY and Chen CA: High mesothelin
correlates with chemoresistance and poor survival in epithelial
ovarian carcinoma. Br J Cancer. 100:1144–1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bast RC Jr: Status of tumor markers in
ovarian cancer screening. J Clin Oncol. 21 Suppl 10:200–205. 2003.
View Article : Google Scholar
|
|
33
|
Shimizu A, Hirono S, Tani M, Kawai M,
Okada K, Miyazawa M, Kitahata Y, Nakamura Y, Noda T, Yokoyama S, et
al: Coexpression of MUC16 and mesothelin is related to the invasion
process in pancreatic ductal adenocarcinoma. Cancer Sci.
103:739–746. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Millard TH, Sharp SJ and Machesky LM:
Signalling to actin assembly via the WASP (Wiskott-Aldrich syndrome
protein)-family proteins and the Arp2/3 complex. Biochem J.
380:1–17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miki H, Suetsugu S and Takenawa T: WAVE, a
novel WASP-family protein involved in actin reorganization induced
by Rac. EMBO J. 17:6932–6941. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sossey-Alaoui K, Ranalli TA, Li X, Bakin
AV and Cowell JK: WAVE3 promotes cell motility and invasion through
the regulation of MMP-1, MMP-3, and MMP-9 expression. Exp Cell Res.
308:135–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Davuluri G, Augoff K, Schiemann WP, Plow
EF and Sossey-Alaoui K: WAVE3-NFκB interplay is essential for the
survival and invasion of cancer cells. PLoS One. 9:e1106272014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang A, Kaghad M, Wang Y, Gillett E,
Fleming MD, Dötsch V, Andrews NC, Caput D and McKeon F: p63, a p53
homolog at 3q27-29, encodes multiple products with transactivating,
death-inducing, and dominant-negative activities. Mol Cell.
2:305–316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Koga F, Kawakami S, Fujii Y, Saito K,
Ohtsuka Y, Iwai A, Ando N, Takizawa T, Kageyama Y and Kihara K:
Impaired p63 expression associates with poor prognosis and
uroplakin III expression in invasive urothelial carcinoma of the
bladder. Clin Cancer Res. 9:5501–5507. 2003.PubMed/NCBI
|
|
41
|
Barbieri CE, Tang LJ, Brown KA and
Pietenpol JA: Loss of p63 leads to increased cell migration and
up-regulation of genes involved in invasion and metastasis. Cancer
Res. 66:7589–7597. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luo W, Li S, Peng B, Ye Y, Deng X and Yao
K: Embryonic stem cells markers SOX2, OCT4 and Nanog expression and
their correlations with epithelial-mesenchymal transition in
nasopharyngeal carcinoma. PLoS One. 8:e563242013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li X, Xu Y, Chen Y, Chen S, Jia X, Sun T,
Liu Y, Li X, Xiang R and Li N: SOX2 promotes tumor metastasis by
stimulating epithelial-to-mesenchymal transition via regulation of
WNT/β-catenin signal network. Cancer Lett. 336:379–389. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Alexandrova EM, Petrenko O, Nemajerova A,
Romano RA, Sinha S and Moll UM: ∆Np63 regulates select routes of
reprogramming via multiple mechanisms. Cell Death Differ.
20:1698–1708. 2013. View Article : Google Scholar : PubMed/NCBI
|