Introduction
Neuroblastoma, which is the most common extracranial
solid childhood tumor, accounts for 10% of pediatric cancers and
15% of childhood cancer-related deaths (1). In most children diagnosed with
neuroblastoma, the tumors are aggressive, resistant to chemotherapy
and metastasize to the bone. Improvements in clinical treatment in
recent decades have increased the survival rate of neuroblastoma
patients, but the long-term survival in children with high-risk
neuroblastoma is still only 30% (2). Therefore, there is a need for novel
therapeutic strategies against neuroblastoma to improve the outcome
in such patients.
P21-activated kinase 4 (PAK4), which belongs to
group II of the PAK family of proteins, was initially identified as
a regulator of cell polarization via mediation of filopodium
formation (3–6). PAK4 has been found to be involved in a
wide range of biological activities. Pak4 knockout results
in embryonic lethality in mice (7).
Thus, PAK4 may play a vital role in embryonic development. Indeed,
PAK4 has been found to be important for neuronal development
(7) and extra-embryonic tissue
development (8). Moreover, PAK4 has
been reported to promote premature senescence of cells via the ERK
signaling pathway (9).
Recent studies have shown that PAK4 also has
multiple roles in oncogenic processes. PAK4 is highly expressed in
most human cancers, including breast (10,11)
and gastric cancer (12,13), hepatocellular carcinoma (14), cervical (15) and pancreatic cancer (16), but it is expressed at low levels in
most normal tissues (17).
Moreover, PAK4 is thought to be involved in tumorigenesis via
regulation of cell polarization, adhesion (18,19),
proliferation and invasion (20,21)
and cell cycle control (17). In
addition, in vitro overexpression of PAK4 in mouse mammary
epithelial cells produced the tumor phenotype in these cells. Thus,
PAK4 may have the ability to induce oncogenic transformation in
normal cells (22). PAK4 may also
contribute to the progression and recurrence of cervical cancers by
conferring chemoresistance to cancer cells (15). A recent study showed that activated
PAK4 was implicated as a mediator dowmstream αvβ3 to suppress
p21-dependent senescence in glioblastoma cells (23). All these findings seem to indicate
that PAK4 is an oncogenetic protein that could be a potential
therapeutic target. However, the role of PAK4 in neuroblastomas
remains poorly understood.
PF-3758309 is a novel small-molecule inhibitor of
PAK4. It is defined as a potent, ATP-competitive pyrrolopyrazole
inhibitor of PAK4. PF-3758309 has been shown to inhibit
anchorage-independent proliferation in several tumor cell lines
in vitro and to block the growth of multiple tumor xenograft
models in vivo (24). In
addition, PF-3758309 exhibits an anti-migration effect via
downregulation of MMP-2/MMP-9 in human lung cancer cells (25).
In the present study, using high-throughput
small-molecule inhibitor screening, we attempted to evaluate the
antitumor effect and molecular mechanism of PF-3758309 in human
neuroblastoma. Our findings indicate that PAK4 could be a
therapeutic target in the treatment of neuroblastoma, and that
blocking PAK4 with PF-3758309 may be a potential therapeutic
strategy for neuroblastoma treatment.
Materials and methods
Cell lines and reagents
The human neuroblastoma cell lines were purchased
from JENNIO Biological Technology (Guangzhou, China) within 5
years. All cells were maintained as monolayer cultures in
RPMI-1640, Dulbecco's modified Eagles medium (DMEM) or DMEM/F12
medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA, USA),
penicillin (100 U/ml) and streptomycin (100 µg/ml) (Sigma, St.
Louis, MO, USA) in a humidified atmosphere of 5% CO2 at
37°C. All cells were tested routinely for Mycoplasma. The
small-molecular inhibitor PF-3758309 was purchased from Selleck
Chemicals (Houston, TX, USA).
High-throughput small-molecule
inhibitor screening
Eight NB cell lines were subjected to
high-throughput small-molecule inhibitor screening with a panel of
33 small-molecule inhibitors as previously described (26). Briefly, cells were seeded in
384-well plates with a seeding density of 500 cells/well and
exposed to graded concentrations of each compound. After 24 h
treatment, relative viability of cells was quantified by Cell
Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies,
Tokyo, Japan). All absorbance values from the CCK-8 assay were
normalized to the control group [absrobance measured in control
wells, containing dimethyl sulphoxide (DMSO)]. IC50
values were calculated by GraphPad Prism software (GraphPad Prism
Software Inc., San Diego, CA, USA) to assess the relative
sensitivity of each cell line to each drug.
Tumor samples and immunochemistry
Neuroblastoma specimens were obtained from 50 NB
patients at the time of diagnosis, who presented at the Children's
Hospital of Soochow University between 2000 and 2013. Ethical
approval was provided by the Children's Hospital of Soochow
University Ethics Committee (nos. SUEC2000-021 and SUEC2011-037).
All specimens were fixed in 10% neutral formalin, embedded in
paraffin and sections were cut at 4 µm. These sections were stained
by hematoxylin and eosin (H&E) to confirm their histological
diagnosis and other microscopic characteristics. The
tumor-node-metastasis (TNM) staging for each neuroblastoma was
evaluated according to Union Internationale Contre le Cancer
system. The immunochemical procedures were performed as described
previously (27). The primary
antibody anti-PAK4 was purchased from Abcam Trading Co. Ltd.
(Shanghai, China) (cat. ab62509; 1:200). One hundred cells were
randomly selected and counted from 5 representative fields of each
section blindly by two independent observers (Yunyun Xu and Yi Wu).
The expression of PAK4 was graded and counted as follows: 0,
negative; 1, 1–50%; 2, >50–74%; 3, ≥75%. The staining intensity
score was graded as follows: 1, weak; 2, intermediate; and 3,
strong.
Cell proliferation and viability
assay
Neuroblastoma cells (2×104/100 µl) were
seeded into 96-well plates overnight and incubated with DMSO, or
increasing concentrations of PF-3758309 (0.05–20 µM) for 24 h. The
same volume of DMSO was added to the wells as a control group. Each
drug concentration was replicated 3 times. Then, 10 µl CCK-8
solution was added to each well, incubated at 37°C for 2–4 h and
the optical density (OD) values were measured at 450 nm using a
scanning multi-well spectrophotometer (Bio-Rad Model 550; Bio-Rad,
Hercules, CA, USA). Relative survival rate was calculated from the
absorbance values compared with the control group. The
proliferation of cells was calculated as a percentage of the
DMSO-treated control wells with 50% inhibitory concentration
(IC50) values derived after plotting proliferation
values on a logarithmic curve. The IC50 value of
PF-3758309 was calculated by GraphPad Prism software.
Clone formation assay
SH-SY5Y or IMR-32 cells were seeded in 6-well plates
at a density of 200 cells/well and cultured at 37°C for 2 weeks.
Culture medium was changed every 3 days. After an incubation period
of 2 weeks, the cells were fixed with 100% methanol and the
colonies were visualized by staining with 0.04% crystal violet.
Cell cycle analysis
Cells (1×106 cells/well) were
synchronized by culturing them in serum-free media for 24 h.
Subsequently, cells were grown in regular medium for 24 h, washed,
trypsinized and fixed with 70% ethanol overnight at 4°C. After
fixation, cells were transparented with 0.5% Triton X-100 for 10
min. After that, cells were washed, stained with staining solution
containing 1.5 µmol/l propidium iodide (PI) (P4170; Sigma-Aldrich,
St. Louis, MO, USA) and 25 µg/ml RNase A. Then, samples were
analyzed by flow cytometry on a Beckman Gallios™ Flow Cytometer
(Beckman, Krefeld, Germany). The percentage of cell population in
various phases of the cell cycle was calculated using MultiCycle AV
DNA analysis software (Verity Software House, Topsham, ME,
USA).
Apoptosis assay
Apoptosis assay was performed using FITC-Annexin V
apoptosis detection kit (cat. 556420; BD Biosciences, Franklin
Lakes, NJ, USA). Briefly, cells (1×105 cells/well) were
seeded in 6-well plate and allowed to grow for 72 h. Thereafter,
the harvested cells were washed, resuspended in 1× binding buffer
at a concentration of ~1×106 cells/ml, incubated with
FITC-Annexin V and PI in the dark for 30 min at room temperature
and analyzed by flow cytometry within 1 h.
Western blot analysis
Lysates were extracted in 40 mM Tris-HCl (pH 7.4)
containing 150 mM NaCl and 1% (v/v) Triton X-100, supplemented with
protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA) for
western blot analysis. Protein samples were quantified using the
Pierce BCA kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA),
25–50 µg denatured protein was loaded onto a denaturing
SDS-polyacrylamide gel, and then transferred to a polyvinylidene
fluoride (PVDF) membrane (Millipore, Bedford, MA, USA). Blots were
blocked in 5% skim milk in Tris-buffered saline with Tween-20
(TBST) and probed with primary antibodies against caspase-3 (cat.
9661S; 1:1,000), PARP (cat. 9542S; 1:1,000), (both from Cell
Signaling Technology Inc., Danvers, MA, USA) PAK4 (cat. ab62509;
1:1,000; Abcam Trading Co. Ltd.), ERK (cat. 4695S; 1:1,000), p-ERK
(cat. 4370S; 1:1,000), Akt (cat. 4691S; 1:1,000), p-Akt (cat.
4060S; 1:1,000), P38 (cat. 9212S; 1:1,000), p-P38 (cat. 4551S;
1:1,000) (all from Cell Signaling Technology Inc.), AATF (cat.
ab39631; 1:1,000), BCL-2 (cat. ab7973; 1:1,000), Bax (cat. 32503;
1:1,000), BAD (cat. ab32445; 1:1,000), BAK1 (cat. ab69404; 1:1,000)
(all from Abcam Trading Co. Ltd.), CDKN1A (cat. 2947S; 1:1,000;
Cell Signaling Technology, Inc.), actin (cat. A5441; 1:5,000;
Sigma), GAPDH (cat. AP0063; 1:5,000; Bioworld Technology, Inc.).
After washing 3 times, the blots were incubated with horseradish
peroxidase-conjugated secondary antibodies for 1 h. Finally, the
protein bands were visualized with LAS 4010 (GE Healthcare Life
Sciences, Little Chalfont, UK) by an enhanced chemiluminescence kit
(Pierce, Rockford, IL, USA).
Real-time PCR array analysis
Samples from each group were submerged in 1 ml
TRIzol reagent (Invitrogen) for RNA extraction, and stored at −80°C
until further processing. cDNA synthesis was performed on 4 µg of
RNA in a 10 µl sample volume using SuperScript II reverse
transcriptase (Invitrogen) as recommended by the manufacturer.
Real-time PCR array (SABioscience Human Apoptosis PCR Array
PAHS-3012; SABiosciences, Frederick, MD, USA) analysis was
performed in a total volume of 20 µl including 2 µl of cDNA,
primers (0.2 mM each) and 10 µl of SYBR-Green Mix (Roche). For gene
expression quantification, a comparative Ct method was used. Gene
expression levels for each sample were normalized to the expression
level of the housekeeping gene encoding glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) within a given sample (−ΔCt); the relative
expression of each gene was calculated with 106 × log2
(−ΔCt). Statistical significance of gene expression was calculated
with the t-test using SPSS 11.5 software (SPSS, Inc., Chicago, IL,
USA).
Statistical analysis
Each experimental condition was performed 3 times,
and these replicates are presented in the results. All values are
presented as means ± SEM. Student's paired t-test was applied to
reveal statistical significant results. P-values <0.05 were
considered significant. Statistical analyses were performed using
SPSS software for Windows.
Results
High-throughput small-molecule
inhibitor screening of neuroblastoma cells
We first compiled a library of 33 small-molecule
inhibitors with a broad class of kinome targets, including
phosphoinositide-3 kinase (PI3K)/AKT, CDK4/6, PLK1, P65, MEK, JNK,
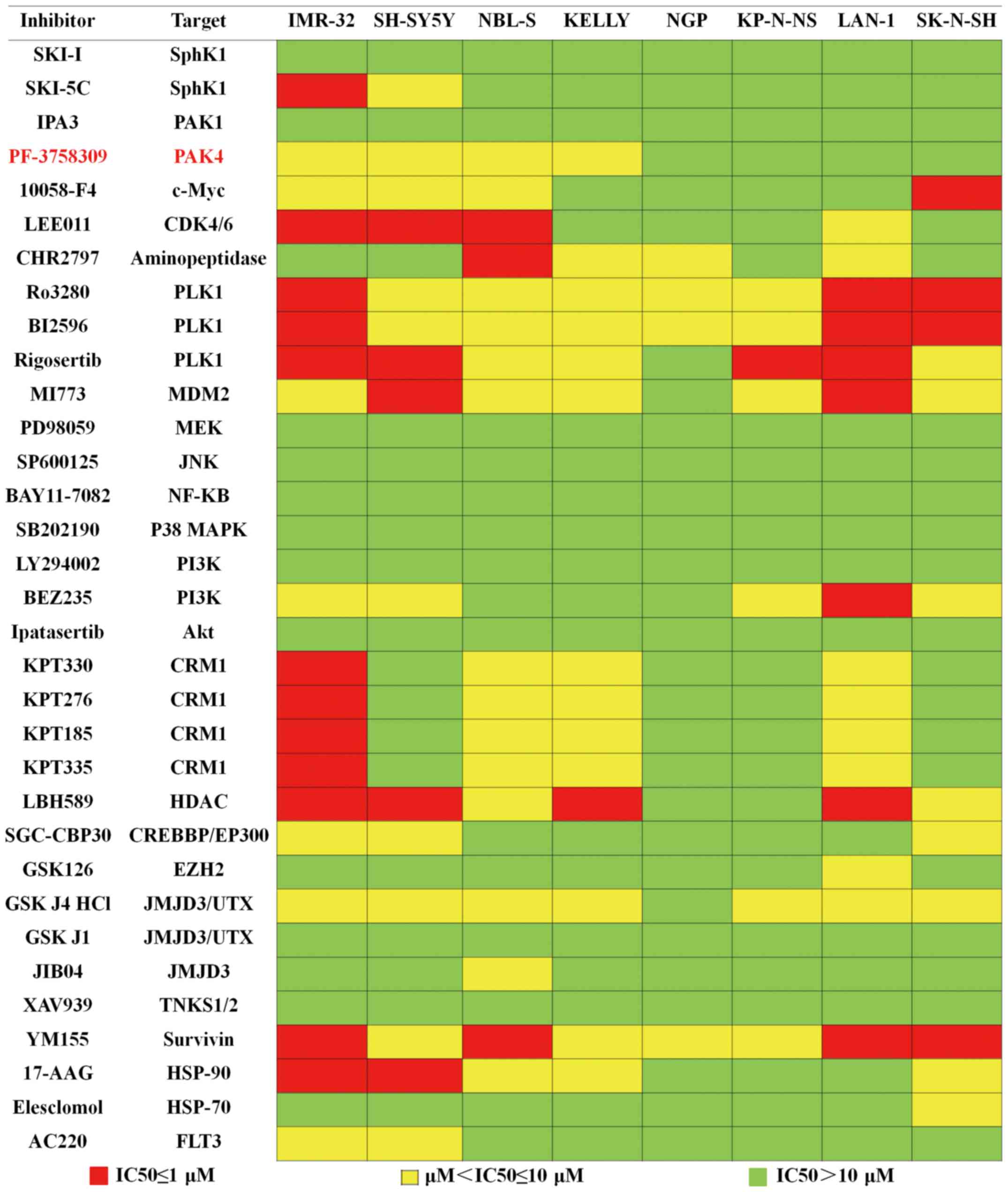
p38, the HSP family, HDAC and EZH2 (Fig. 1). Each inhibitor was plated at 4
graded concentrations, which were 0.5, 1, 5 and 10 µM, and 8
neuroblastoma cell lines were exposed to these inhibitors. The
results of the assay are summarized in Fig. 1. The colors of the data boxes
indicate the IC50 ranges of the inhibitor. Our results
showed that 21 inhibitors exhibited an antiproliferation effect in
at least 2 cell lines. Of these 21 inhibitors, we focused on
PF-3758309, a novel inhibitor of PAK4, since 4 of the neuroblastoma
cell lines were highly sensitive to this agent. Since PAK4 is known
to participate in many important cell processes, but is not as well
studied in neuroblastomas, we attempted to assess the antitumor
effect of PF-3758309 in neuroblastomas.
PAK4 overexpression in
neuroblastomas
First, we compared PAK4 expression in tumor tissues
from 50 neuroblastoma patients and 16 normal peripheral nerve
tissues by immunohistochemistry. The results showed high expression
of PAK4 in neuroblastoma tissues. By comparison, PAK4 was barely
detectable in the normal peripheral nerve tissues (Fig. 2A). Real-time PCR was also performed
to examine the mRNA transcript levels of PAK4 in 30 neuroblastoma
samples and 15 normal peripheral nerve tissues. The mRNA level of
PAK4 significantly elevated in the neuroblastoma tissues in
comparison with that noted in the normal peripheral nerve tissues
(Fig. 2B).
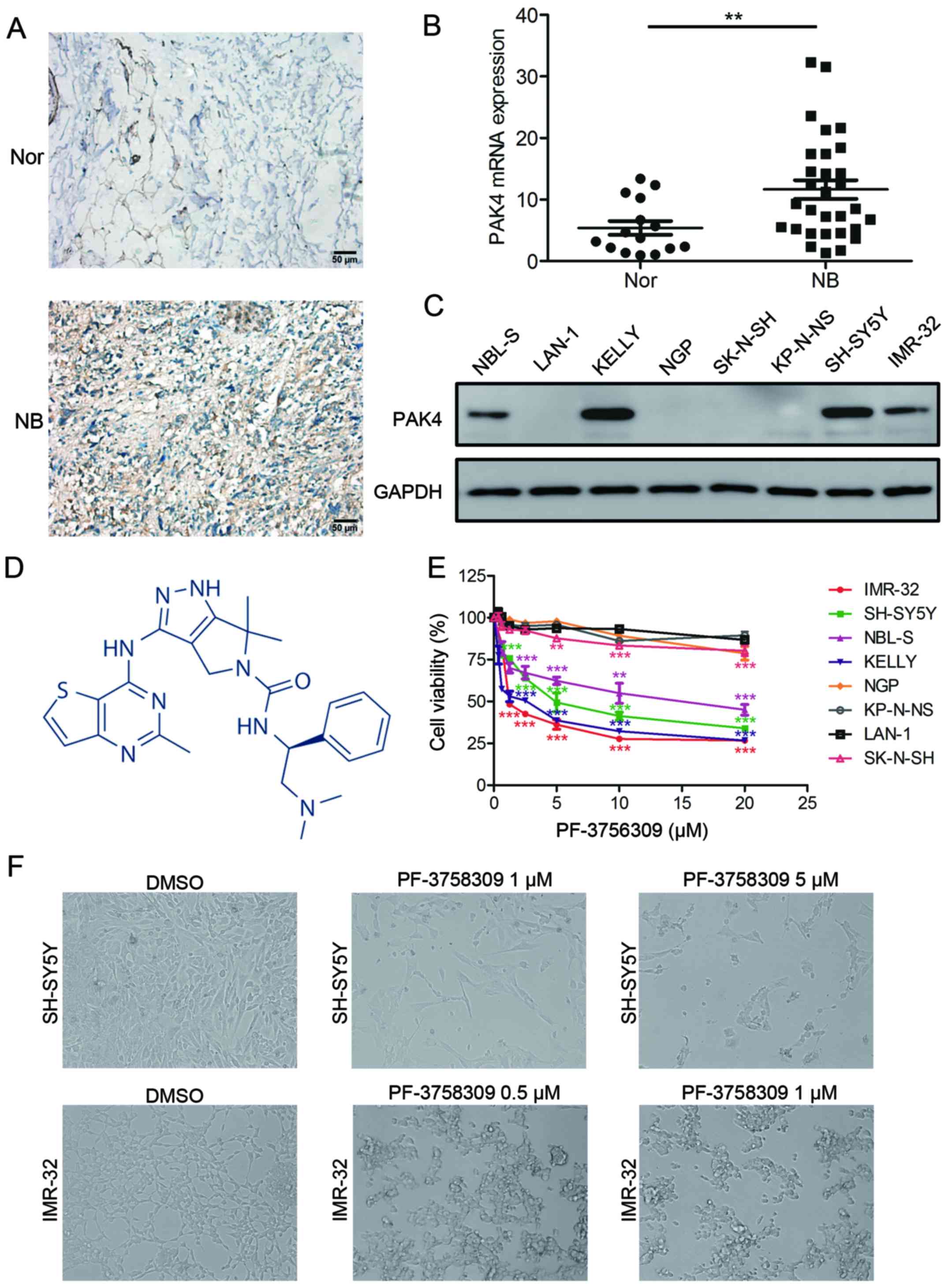 | Figure 2.PF-3758309 inhibits cell viability in
neuroblastoma cells. (A) Immunohistochemical staining of PAK4
expression in neuroblastoma (NB) and normal nerve (Nor) tissues.
Original magnifications, ×400; scale bar, 50 µm. (B) Quantification
of PAK4 mRNA expression in neuroblastoma and normal nerve tissues.
(C) Western blot analysis of PAK4 expression in neuroblastoma cell
lines. (D) Molecular structure of PF-3758309. (E) Proliferation and
IC50 analysis of PF-3758309 in 8 neuroblastoma cell
lines. Neuroblastoma cells (2×104) were seeded in
96-well plates overnight and incubated with DMSO or increasing
concentrations of PF-3758309 (0.625, 1.25, 2.5, 5, 10 or 20 µM) for
24 h. Cell proliferation was calculated as a percentage of the
DMSO-treated control wells. The IC50 values were derived
after plotting proliferation values on a logarithmic curve.
IC50 values: SH-SY5Y, 5.641; IMR-32, 2.214; NBL-S, 14.02
and KELLY, 1.846 µM. (F) Images of SH-SY5Y and IMR-32 cells
incubated with DMSO or PF-3758309 for 24 h; **P<0.01 and
***P<0.001. P-values were determined by two-tailed t-tests. All
data are representative of 3 independent experiments with
n=3–6/group and are means ± SEM. |
Next, we investigated the relationship between PAK4
expression and the clinical data of 50 neuroblastoma patients. PAK4
expression was closely associated with the grade of diagnosis and
TNM stage (including the degree of invasion of the primary tumor,
the number of regional lymph nodes with metastasis, and the extent
of distant metastasis) of the neuroblastoma samples. A high level
of PAK4 expression was strongly associated with an unfavorable
phonotype. This finding demonstrates that PAK4 is a biomarker of
poor prognosis (Table I).
 | Table I.Association of PAK4 expression with
clinical characteristics in 50 neuroblastoma samples. |
Table I.
Association of PAK4 expression with
clinical characteristics in 50 neuroblastoma samples.
|
|
| PAK4
expression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Cases | Low | High | P-value |
|---|
| Age (years) | 50 |
|
| 0.274 |
|
<30 | 44 | 8 | 36 |
|
|
≥30 | 6 | 4 | 2 |
|
| Sex |
|
|
| 0.877 |
|
Male | 24 | 6 | 18 |
|
|
Female | 26 | 6 | 20 |
|
| Stage |
|
|
| 0.013 |
| I | 6 | 3 | 3 |
|
|
II–III | 44 | 9 | 35 |
|
| Tumor invasion |
|
|
| 0.046 |
| T1 | 10 | 4 | 6 |
|
| T2 | 16 | 2 | 14 |
|
| T3 | 20 | 6 | 14 |
|
| T4 | 4 | 0 | 4 |
|
| LN metastasis |
|
|
| 0.027 |
|
Positive | 8 | 4 | 4 |
|
|
Negative | 42 | 8 | 36 |
|
To determine the expression of PAK4 in neuroblastoma
cell lines, we performed western blot analysis on a panel of
neuroblastoma cell lines. Aberrant expression of PAK4 was observed
in KELLY, NBL-S, SH-SY5Y and IMR-32 cells, but no expression was
detected in the other neuroblastoma cells (Fig. 2C). These results indicate that PAK4
is highly expressed in most neuroblastoma tissues and cells.
Inhibitory effect of PF-3758309 on the
growth of neuroblastoma cells
To evaluate the inhibitory effect of PF-3758309
(Fig. 2D) on neuroblastoma cells,
we treated 8 neuroblastoma cell lines with the PAK4 inhibitor
PF-3758309 (Fig. 2E).
Pharmacological inhibition of PAK4 by PF-3758309 treatment resulted
in significant inhibition of proliferation in neuroblastoma cells
with high expression of PAK4, that is, KELLY, NBL-S, SH-SY5Y and
IMR-32 cells. In contrast, cells with low levels of PAK4 expression
were less sensitive to PF-3758309 exposure. PF-3758309 treatment
was found to have a dose-dependent inhibitory effect on the growth
of neuroblastoma cells (Fig. 2E).
The IC50 value of PF-3758309 was determined in 4
neuroblastoma cell lines: SH-SY5Y, 5.461 µM; IMR-32, 2.214 µM;
NBL-S, 14.02 µM; KELLY 1.846 µM. The cells that were affected by
PF-3758309 presented with abnormal morphological features; most of
the cells had shrunk and lost their ability to adhere, and they
were observed to be floating (Fig.
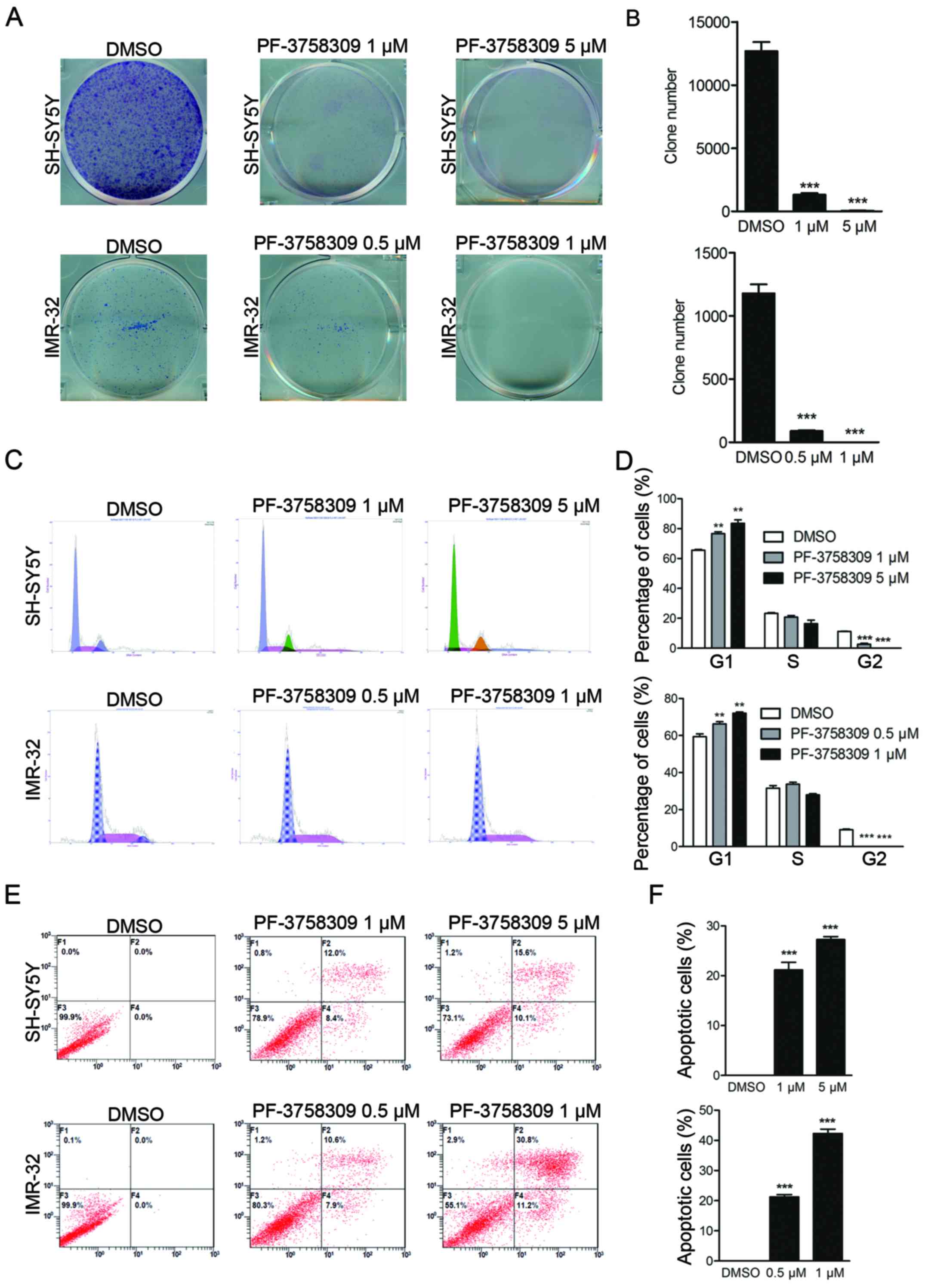
2F). In addition, clone formation assay showed that PF-3758309
caused a reduction in the number of both SH-SY5Y and IMR-32 cell
clones (Fig. 3A and B). These
results demonstrate that PF-3758309 effectively impairs the growth
potential of neuroblastoma cells.
Induction of cell cycle arrest in
neuroblastoma cells by PF-3758309
PI staining was performed to study the effect of
PF-3758309 on the cell cycle in SH-SY5Y and IMR-32 cells.
PF-3758309 significantly induced cell cycle arrest in both cell
lines in a dose-dependent manner. An increase in the population of
sub-G1 cells from 65.6 to 77.3% was observed in the SH-SY5Y cells
in response to treatment with 1 µM PF-3758309, and a decrease in
the G2 cell population from 10.8 to 1.5% was observed.
Furthermore, a higher dose of PF-3758309 (5 µM) induced a
significant increase in the sub-G1 population (Fig. 3C and D). PF-3758309 exhibited a
similar effect on IMR-32 cells (Fig. 3C
and D). These results indicate that PF-3758309 effectively
inhibits cell cycle progression in neuroblastoma cells by arresting
cells in the sub-G1 phase.
Induction of apoptosis in
neuroblastoma cells by PF-3758309
We further investigated whether PF-3758309 can
trigger cell apoptosis in neuroblastoma cell lines. Cell apoptosis
was evaluated by Annexin V/PI staining followed by flow cytometric
analysis. As shown in Fig. 3E,
after 24 h of treatment with PF-3758309, induction of early and
late apoptosis was observed in IMR-32 and SH-SY5Y cells. The
apoptotic cell population comprised 21.2 and 26.9% of the total
SH-SY5Y cell population following treatment with 1 and 5 µM
PF-3758309, respectively (Fig. 3E and
F). The apoptosis rate in the IMR-32 cells was 19.7 and 44.9%
in response to treatment with 0.5 and 1 µM PF-3758309, respectively
(Fig. 3E and F). We further
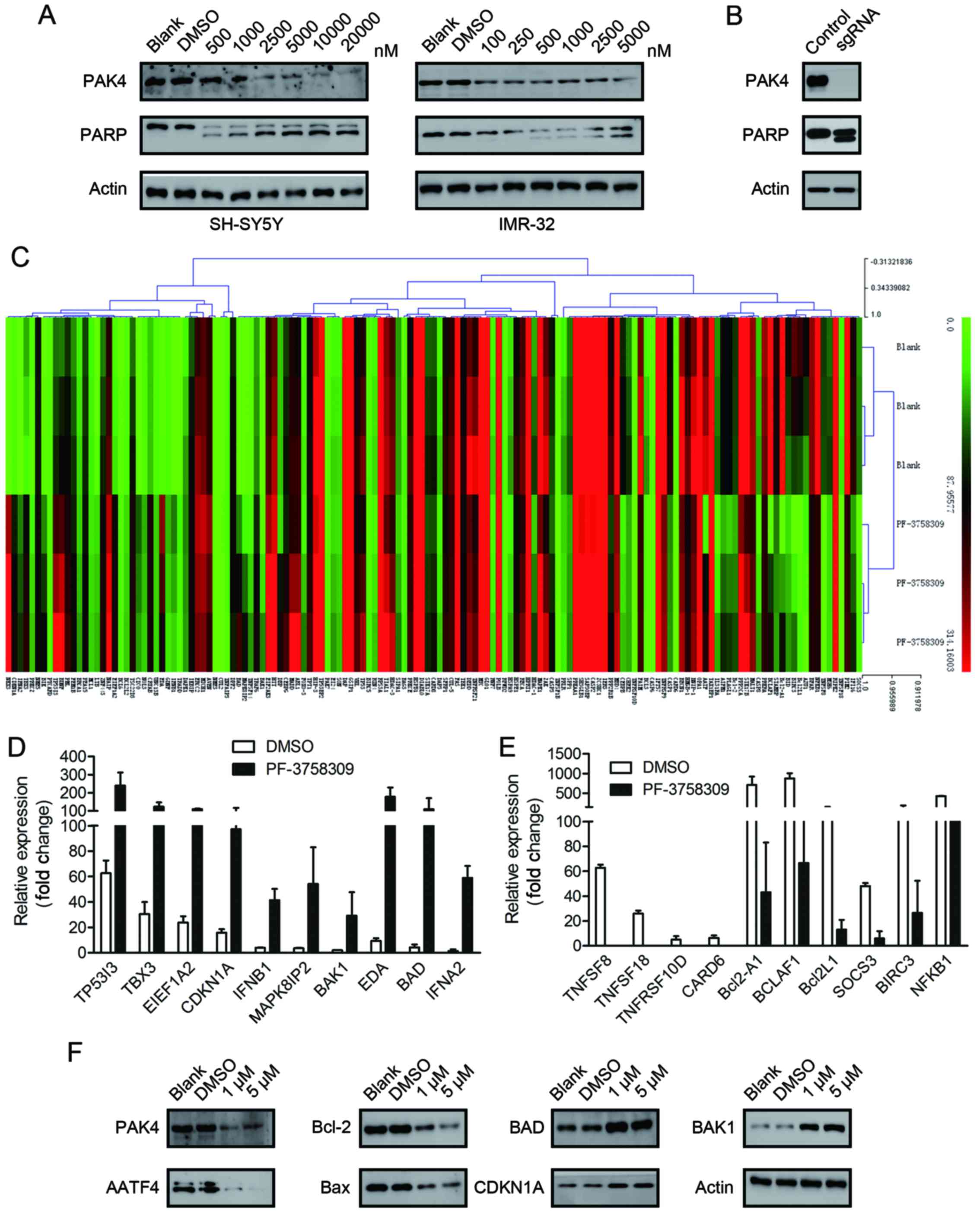
assessed the cell lysates for cleaved PARP, which is a classical
marker of cell apoptosis. A dose-dependent increase in the amount
of cleaved PARP was observed in the SH-SY5Y and IMR-32 cells
following treatment with a series of concentrations of PF-3758309
(Fig. 4A). However, we were unable
to clearly detect the cleaved bands of caspase-9, which is another
indicator of apoptosis activation (data not shown).
To confirm that the apoptotic effect of PF-3758309
was caused by PAK4 inhibition, we applied the CRISPR/Cas9 technique
to generate PAK4-knockout SH-SY5Y cells. The PAK4-knockout cells
exhibited apoptotic morphology. Furthermore, cleavage of PARP was
observed (Fig. 4B). These findings
are similar to those in the PF-3758309-treated cells. This
indicates that the apoptotic effect of PF-3758309 was brought about
via PAK4 inhibition.
Real-time PCR array analysis of the
target genes of PF-3758309
To investigate the molecular mechanism underlying
PF-3758309-induced cell cycle arrest and apoptosis, we analyzed the
target genes of PF-3758309 by SABioscience Human Apoptosis PCR
Array PAHS-3012Z. The expression of 370 key genes involved in
apoptosis was analyzed. The genes were grouped according to their
functional contribution to apoptosis: anti-apoptotic regulation or
the induction of apoptosis by death domain receptors, DNA damage,
oxidative stress or extracellular and intracellular signals. We
clustered 370 genes according to their expression in the DMSO- or
PF-3758309-treated groups (Fig.
4C). The PCR array data revealed that the expression of 23
genes was significantly upregulated (>2-fold) and the expression
of 20 genes was significantly downregulated (>2-fold) after 24 h
of PF-3758309 treatment in SH-SY5Y cells (Tables II and III). Expression of the following genes
was upregulated: TP53I3, TBX3, EEF1A2, CDKN1A, IFNB1, MAPK8IP2,
BAK1, EDA, BAD and IFNA2 (Fig. 4D).
Expression of the following genes was downregulated: TNFSF8,
TNFSF18, TNFRSF10D, CARD6, Bcl2-A1, BCLAF1, Bcl2L1, SOCS3, BIRC3
and NFKB1 (Fig. 4E). Furthermore,
the expression of AATF, Bcl-2, BAX, BAD, BAK1 and CDKN1A was
verified at the protein level by western blot analysis (Fig. 4F).
 | Table II.Genes upregulated in SH-SY5Y cells
treated with PF-3758309 compared with the DMSO control group. |
Table II.
Genes upregulated in SH-SY5Y cells
treated with PF-3758309 compared with the DMSO control group.
| Gene | Description | +DMSO | +PF-3758309 | Fold change | P-value |
|---|
| NKX3 | Homeobox protein
Nkx-3.1 | 0.001803 | 0.325538 | 180.5711 | 0.001869 |
| IFNA2 | Interferon α-2 | 1.410604 | 58.84984 | 41.71959 | 0.000504 |
| CD70 | CD70 antigen | 0.001643 | 0.050203 | 30.54704 | 0.000289 |
| BAD | Bcl2-associated
agonist of cell death | 0.00431 | 0.10939 | 25.38147 | 0.040529 |
| EDA |
Ectodysplasin-A | 0.931618 | 17.75593 | 19.05924 | 0.005116 |
| BIK | Bcl-2-interacting
killer | 4.232982 | 67.26735 | 15.89124 | 0.005184 |
| BAK1 | Bcl-2 homologous
antagonist/killer | 1.966254 | 29.21924 | 14.86036 | 0.063145 |
| MAPK8IP2 | JNK-interacting
protein 2 | 3.721264 | 54.2914 | 14.5895 | 0.038288 |
| IFNB1 | Interferon β | 4.028926 | 41.34035 | 10.26089 | 0.001974 |
| CDKN1A | Cyclin-dependent
kinase inhibitor 1 | 1573.162 | 9728.808 | 6.184238 | 0.002498 |
| LTB | Leukotriene B | 0.61312 | 3.435288 | 5.602959 | 0.002222 |
| TRADD | Tumor necrosis
factor receptor type 1-associated DEATH domain protein | 3.880523 | 20.69663 | 5.333463 | 0.002113 |
| EEF1A2 | Elongation factor
1-α 2 | 2374.489 | 11000.2 | 4.63266 | 1.53E-05 |
| BCL6 | B-cell lymphoma 6
protein | 3.452591 | 15.40138 | 4.460819 | 0.000277 |
| TBX3 | T-box transcription
factor TBX3 | 3.061456 | 12.38112 | 4.044192 | 0.002894 |
| TP53I3 | Tumor protein
p53-inducible protein 3 | 62.6146 | 240.1363 | 3.835148 | 0.013412 |
| TNFSF14 | Tumor necrosis
factor ligand superfamily member 14 | 8.970259 | 32.94411 | 3.672593 | 0.029409 |
| BDNF | Brain-derived
neurotrophic factor | 74.15095 | 271.9117 | 3.667002 | 0.019061 |
| CRYAB | α-crystallin B
chain | 35.13305 | 122.0248 | 3.473218 | 9.65E-06 |
| PRKCZ | Protein kinase C
zeta type | 10.70091 | 30.36617 | 2.83772 | 0.004804 |
| NOL3 | Nucleolar protein
3 | 1.524283 | 3.654028 | 2.397211 | 0.000416521 |
| TRAF6 | TNF
receptor-associated factor 6 | 45.48575 | 97.24143 | 2.137844 | 0.026841067 |
| BCL2L2 | Bcl-2-like protein
2 | 16.04355 | 32.47326 | 2.024069 | 0.000458933 |
 | Table III.Genes downregulated in SH-SY5Y cells
treated with PF-3758309 compared with the DMSO control group. |
Table III.
Genes downregulated in SH-SY5Y cells
treated with PF-3758309 compared with the DMSO control group.
| Gene | Description | +DMSO | +PF-3758309 | Fold change | P-value |
|---|
| TNFSF8 | Tumor necrosis
factor ligand superfamily member 8 | 6.279951 | 0.000828 | 0.000132 | 1.57936E-06 |
| TNFSF18 | Tumor necrosis
factor ligand superfamily member 18 | 2.608747 | 0.003663 | 0.001404 | 3.6821E-05 |
| TNFRSF10D | Tumor necrosis
factor receptor superfamily member 10D | 0.049302 | 0.000298 | 0.006039 | 0.042432232 |
| CARD6 | Caspase recruitment
domain-containing protein 6 | 6.316158 | 0.096765 | 0.01532 | 0.006827195 |
| Bcl2-A1 | Bcl-2-related
protein A1 | 709.7745 | 43.10898 | 0.060736 | 0.005756717 |
| BCLAF1 | Bcl-2-associated
transcription factor 1 | 876.6456 | 66.62478 | 0.076 | 0.000645046 |
| Bcl2L1 | Bcl-2-like protein
1 | 110.9282 | 13.04641 | 0.117611 | 0.0297663 |
| SOCS3 | Suppressor of
cytokine signaling 3 | 0.480132 | 0.060371 | 0.125739 | 0.000312779 |
| BIRC3 | Baculoviral IAP
repeat-containing protein 3 | 1.289468 | 0.265287 | 0.205733 | 0.064433748 |
| AZU1 | Azurocidin | 0.067689 | 0.015775 | 0.233046 | 0.03000673 |
| CASP6 | Caspase-6 | 9.207628 | 2.682053 | 0.291286 | 0.026410912 |
| BCL3 | B-cell lymphoma 3
protein | 32.88351 | 10.42351 | 0.316983 | 0.02903268 |
| NFKB1 | Nuclear factor
NF-κ-B p105 subunit | 428.9497 | 141.1446 | 0.329047 | 1.91951E-07 |
| CEBPB |
CCAAT/enhancer-binding protein β | 8624.573 | 2954.241 | 0.342538 | 0.003298518 |
| AATF |
Apoptosis-antagonizing transcription
factor | 243.7826 | 85.06575 | 0.348941 | 0.022581279 |
| STAMBP | STAM-binding
protein | 12.88956 | 4.622598 | 0.358631 | 0.000569316 |
| PIM2 |
Serine/threonine-protein kinase pim-2 | 405.8789 | 153.5832 | 0.378397 | 0.000267339 |
| RIPK2 |
Receptor-interacting
serine/threonine-protein kinase 2 | 1157.068 | 444.2396 | 0.383936 | 5.15526E-05 |
| CASP1 | Caspase-1 | 117.0643 | 45.73255 | 0.390662 | 0.013481275 |
| IFI16 |
γ-interferon-inducible protein 16 | 1394.752 | 569.8934 | 0.408598 | 0.001865793 |
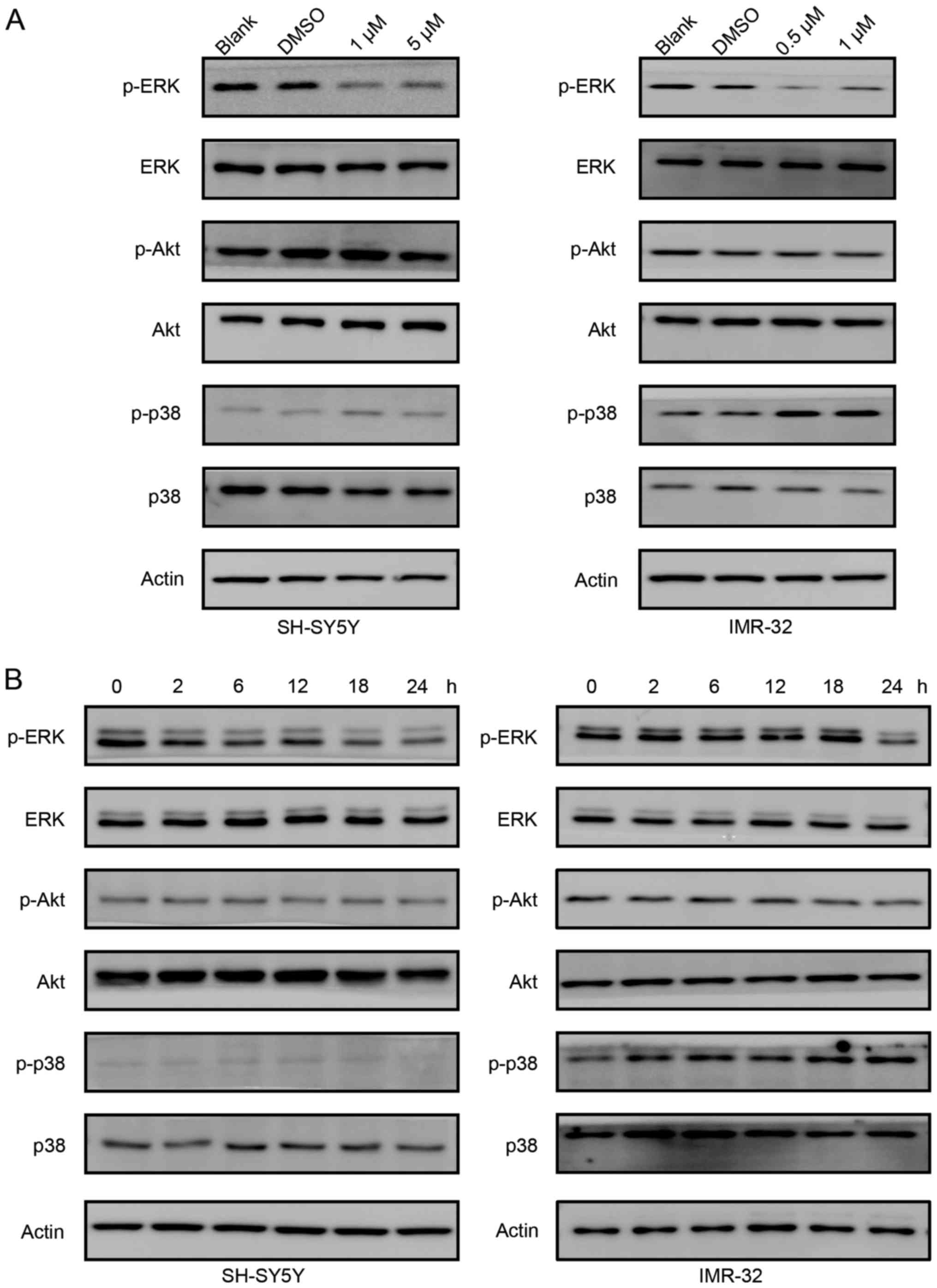
Role of the ERK signaling pathway in
the antiproliferation effects of PF-3758309
We studied the signaling pathways involved in
PF-3758309 treatment. As shown in Fig.
5A, the phosphorylation of ERK gradually decreased as the
concentration of PF-3758309 increased in both SH-SY5Y and IMR-32
cells. However, phosphorylation of Akt was not affected by
PF-3758309 treatment. However, the status of phosphorylation of p38
differs in SH-SY5Y and IMR-32 cells. In SH-SY5Y cells, PF-3758309
did not affect phosphorylation of p38. In contrast, phosphorylation
of p38 was induced in IMR-32 cells upon PF-3758309 treatment. Then,
we treated SH-SY5Y and IMR-32 cells with 1 µM PF-3758309 at
different time points. In both cell lines, PF-3758309 downregulated
the phosphorylation of ERK, but it did not have an effect on the
phosphorylation of Akt (Fig. 5B).
Similarly, PF-3758309 induced phosphorylation of p38 in IMR-32
cells, but not in SH-SY5Y cells. We suppose the different effect of
PF-3758309 on the phosphorylation of p38 is probably cell specific.
In all, these results indicate that the ERK signaling pathway
participates in PF-3758309-induced cell cycle control and
apoptosis.
Discussion
In the present study, we found that PAK4 expression
was elevated in neuroblastoma cell lines and tumor tissues, and
that PAK4 expression was closely associated with prognosis in
neuroblastoma patients. An increase in PAK4 expression was strongly
associated with an increased risk of lymph node invasion and
metastasis in neuroblastoma patients. This indicates that PAK4
expression may promote the malignant transformation of
neuroblastomas. Studies have shown that PAK4 expression is also
amplified in colon (28), prostate
(18), pancreatic (29), glioma (30) and breast cancer (11). Furthermore, PAK4 was found to have
prognostic and therapeutic significance in ovarian cancer (31). Additionally, a recent study showed a
close association between activation of PAK4 expression and
advanced tumor stage in gastric cancer patients (13). However, none of these previous
studies focused on neuroblastoma. The present study demonstrated,
for the first time, the expression and function of PAK4 in
neuroblastomas and provides evidence for PAK4 as a novel oncogene
target of neuroblastomas.
The prognostic value of PAK4 makes it an attractive
therapeutic target for the treatment of cancer. Recently, experts
have paid more attention to the development of compounds that
target PAK family kinases. Due to the low level of PAK4 in normal
tissue, compounds that target PAK4 can effectively distinguish
malignant tumor cells from normal cells and exert their effect with
few side-effects. To date, several PAK family inhibitors have been
investigated. For example, the group I PAK inhibitor FRAX1036 when
used in combination with taxane was observed to induce apoptosis in
breast cancer cells (32).
Furthermore, GNE-2861, a small molecule that selectively inhibits
group II PAKs, restores tamoxifen sensitivity via inhibition of
PAK4 in breast cancer cells (33).
In the present study, we used a novel PAK4 inhibitor, PF-3758309,
generated by Pfizer Oncology, which is currently being clinically
tested for its effectiveness against various solid tumors.
PF-3758309 has been profiled for its growth inhibitory activity in
a panel of 92 tumor cell lines, 46% of which exhibit
IC50 values <10 nM (24). In the present study, we demonstrated
that PF-3758309 effectively decreased PAK4 expression, inhibited
cell proliferation, and induced apoptosis in neuroblastoma cell
lines, SH-SY5Y and IMR-32. This indicates that PAK4 is a potential
therapeutic target for neuroblastomas and that PF-3758309 may be a
candidate for neuroblastoma treatment.
PF-3758309 exhibits inhibitory activity against both
group I and II PAKs (34). However,
in the present study, we specifically investigated the effect of
this inhibitor on cellular PAK4 expression by western blot analysis
and found that it significantly inhibited the cellular expression
of this group II PAK. In order to understand the mechanism
underlying PF-3758309-induced cell death, we established
PAK4-knockout cells with the CRISPR/Cas9 technique. The rate of
apoptosis in PAK4-knockout cells was similar to that in
PF-3758309-treated cells. This finding confirms that
PF-3758309-induced cell death is caused via PAK4 inhibition.
Accumulating data suggest that PAK4 plays a role in
cell proliferation and survival in tumors (35–37).
The present study found that pharmaceutical inhibition of PAK4 by
PF-3758309 drastically reduced neuroblastoma cell viability and
inhibited cell proliferation. Furthermore, PF-3758309 induced cell
cycle arrest at the G1 phase and cell apoptosis in neuroblastoma
cells. Similarly, another study reported that overexpression of
PAK4 is involved in the pathogenesis of gestational trophoblastic
disease via promotion of proliferation and cell migration and
invasion in choriocarcinoma cells (20). In pancreatic cancer cells, PAK4
expression was associated with an increase in the growth of
pancreatic cancer cells that resulted from promotion of cell cycle
progression and resistance to apoptosis (38). The role of PAK4 in cell cycle
control has been also demonstrated by Nekrasova and Minden. They
reported that PAK4 levels increased markedly in the early part of
the G1 phase and that the absence of PAK4 led to a reduction in the
amount of cells in the G1 phase and an increase in the amount of
cells in the G2/M phase (17). The
present study revealed for the first time the function of PAK4 in
neuroblastomas and provided evidence for the oncogenic role of PAK4
that is brought about via promotion of cell cycle progression and
apoptosis resistance in neuroblastomas. However, considering that
the expression of PAK4 was relative low in a small proportion of
neuroblastoma samples, and 3 NB cell lines with low level of PAK4
expression were less sensitive to PF-3758309 exposure, we
hypothesize that PAK4 inhibition can be combined with other
existing therapies to achieve an inhibitory effect on this type of
neuroblastoma. This is a topic for future studies.
Similar to other cellular events, cell cycle and
apoptosis are tightly regulated by specific proteins, such as
cyclins and their inhibitors, as well as anti/pro-apoptotic
proteins. To explore the molecular mechanism underlying PF-3758309
treatment, we used real-time PCR array and found that PAK4
inhibition led to altered expression of a considerable number of
genes associated with the cell cycle (CDKN1A) and apoptosis (Bax,
Bcl2 and BIRC3). This finding confirms those of previous studies
that increased PAK4 expression in the early G1 phase reduces p21
levels, and thus abrogates CDK4/CDK6 activity (17). PAK4 has been reported to protect
cells against apoptosis by increasing phosphorylation of the
pro-apoptotic protein Bad and inhibiting caspase activation
(39). The present study also
identified certain novel genes regulated by PAK4 inhibition, such
as AATF, TBX3 and CARD6. Furthermore, our research indicates that
in addition to the classical genes associated with apoptosis, PAK4
may also regulate AATF, TBX3 and CARD6 expression. Although the
regulatory mechanism of PAK4 is unclear, the present study provides
new insight into the molecular mechanism of PF-3758309-regulated
cell cycle arrest and apoptosis in neuroblastomas.
The researchers who firstly generated PF-3758309
demonstrated that it could significantly reduce p53 levels, but
that the p53 degradation inhibitor Nutlin-3 had no effect on PAK4
expression; this indicates that PAK4 is present upstream of p53
(24). However, in our experiment,
PF-3758309 did not cause a significant difference in p53 expression
at both the mRNA and protein level (data not shown). This is not
associated with the p53 status in the neuroblastoma cell lines we
used, since the original inventors had excluded the effect of p53
status on sensitivity to PF-3758309. Therefore, based on our
current findings, we presume that p53 may be present upstream of
PAK4, as this explains why PAK4 inhibition by PF-3758309 did not
influence p53 expression in the neuroblastoma cells. However, since
our results are contradictory to the previously reported one, it
may be useful to further study the effect of this inhibitor on p53
expression.
We found that PF-3758309 treatment inhibited
p-ERK/MEK expression in the neuroblastoma cells, which shows that
PAK4 regulates cell proliferation and apoptosis via the MEK/ERK
signaling pathway. In agreement with our findings, PAK4 has been
reported to promote ovarian cancer cell migration and invasion
through activation of c-Src and MEK-1 (31). Moreover, Tyagi et al
(38) reported that PAK4-induced
proliferation and survival of pancreatic cancer cells were mediated
through the action of ERK and Akt kinases. Furthermore, another
study showed that PAK4 conferred cisplatin resistance in gastric
cancer cells through activation of the PI3K/Akt and MEK/ERK
pathways (40).
This is the first study to report the overexpression
of PAK4 in neuroblastoma cells. Furthermore, PF-3758309, a potent
PAK4 inhibitor, was found to inhibit cell proliferation and
survival in neuroblastoma cells via inhibition of the MEK/ERK
pathway. The present study provides evidence that PAK4 is a
potential target in neuroblastoma treatment, and could be
considered in an alternative or complementary treatment
strategy.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation (nos. 81570125, 81370627,
81502500, 81501840, 81502157, 31500822, 81471488, 31600695 and
81602181), the Natural Science Foundation of Jiangsu Province
(BK20151207, BK20150293 and H201420), the 333 High-Level Personnel
Training Project of Jiangsu Province (BRA2016530, Jiangsu
Provincial Medical Talent (Professor Jian Pan), the ‘Six Talent
Peak’ High-Level Talent Project (2016-WSN-129, 2014-WSN-027), the
Universities Natural Science Foundation of Jiangsu Province (no.
16KJB310014), the Jiangsu Provincial Medical Youth Talent (nos.
QNRC2016762 and QNRC2016756), the Department of Pediatrics Clinical
Center of Suzhou (Szzx201504); the Talent's Subsidy Project in
Science and Education of Department of Public Health of Suzhou City
(no. kjxw2014016), and the Applied Foundational Research of Medical
and Health Care of Suzhou City (SYS201646 and SYS201642).
References
|
1
|
Brodeur GM: Neuroblastoma: Biological
insights into a clinical enigma. Nat Rev Cancer. 3:203–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bokoch GM: Biology of the p21-activated
kinases. Annu Rev Biochem. 72:743–781. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Radu M, Semenova G, Kosoff R and Chernoff
J: PAK signalling during the development and progression of cancer.
Nat Rev Cancer. 14:13–25. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abo A, Qu J, Cammarano MS, Dan C, Fritsch
A, Baud V, Belisle B and Minden A: PAK4, a novel effector for
Cdc42Hs, is implicated in the reorganization of the actin
cytoskeleton and in the formation of filopodia. EMBO J.
17:6527–6540. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dan C, Kelly A, Bernard O and Minden A:
Cytoskeletal changes regulated by the PAK4 serine/threonine kinase
are mediated by LIM kinase 1 and cofilin. J Biol Chem.
276:32115–32121. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qu J, Li X, Novitch BG, Zheng Y, Kohn M,
Xie JM, Kozinn S, Bronson R, Beg AA and Minden A: PAK4 kinase is
essential for embryonic viability and for proper neuronal
development. Mol Cell Biol. 23:7122–7133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tian Y, Lei L, Cammarano M, Nekrasova T
and Minden A: Essential role for the Pak4 protein kinase in
extraembryonic tissue development and vessel formation. Mech Dev.
126:710–720. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cammarano MS, Nekrasova T, Noel B and
Minden A: Pak4 induces premature senescence via a pathway requiring
p16INK4/p19ARF and mitogen-activated protein
kinase signaling. Mol Cell Biol. 25:9532–9542. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Minden A: The pak4 protein kinase in
breast cancer. ISRN Oncol. 2012:6942012012.PubMed/NCBI
|
|
11
|
Yang JX, Han YJ, Zheng H and Luo RC:
Expression of PAK4 in breast cancer and benign breast pathological
changes. Nan Fang Yi Ke Da Xue Xue Bao. 30:981–983. 2010.(In
Chinese). PubMed/NCBI
|
|
12
|
Wang C, Li Y, Zhang H, Liu F, Cheng Z,
Wang D, Wang G, Xu H, Zhao Y, Cao L, et al: Oncogenic PAK4
regulates Smad2/3 axis involving gastric tumorigenesis. Oncogene.
33:3473–3484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li D, Zhang Y, Li Z, Wang X, Qu X and Liu
Y: Activated Pak4 expression correlates with poor prognosis in
human gastric cancer patients. Tumour Biol. 36:9431–9436. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xue J, Chen LZ, Li ZZ, Hu YY, Yan SP and
Liu LY: MicroRNA-433 inhibits cell proliferation in hepatocellular
carcinoma by targeting p21 activated kinase (PAK4). Mol Cell
Biochem. 399:77–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shu XR, Wu J, Sun H, Chi LQ and Wang JH:
PAK4 confers the malignance of cervical cancers and contributes to
the cisplatin-resistance in cervical cancer cells via PI3K/AKT
pathway. Diagn Pathol. 10:1772015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tyagi N, Marimuthu S, Bhardwaj A, Deshmukh
SK, Srivastava SK, Singh AP, McClellan S, Carter JE and Singh S:
p-21 activated kinase 4 (PAK4) maintains stem cell-like phenotypes
in pancreatic cancer cells through activation of STAT3 signaling.
Cancer Lett. 370:260–267. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nekrasova T and Minden A: PAK4 is required
for regulation of the cell-cycle regulatory protein p21, and for
control of cell-cycle progression. J Cell Biochem. 112:1795–1806.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wells CM, Whale AD, Parsons M, Masters JR
and Jones GE: PAK4: A pluripotent kinase that regulates prostate
cancer cell adhesion. J Cell Sci. 123:1663–1673. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z, Lock JG, Olofsson H, Kowalewski JM,
Teller S, Liu Y, Zhang H and Strömblad S: Integrin-mediated cell
attachment induces a PAK4-dependent feedback loop regulating cell
adhesion through modified integrin alpha v beta 5 clustering and
turnover. Mol Biol Cell. 21:3317–3329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang HJ, Siu MK, Yeung MC, Jiang LL, Mak
VC, Ngan HY, Wong OG, Zhang HQ and Cheung AN: Overexpressed PAK4
promotes proliferation, migration and invasion of choriocarcinoma.
Carcinogenesis. 32:765–771. 2011.PubMed/NCBI
|
|
21
|
Paliouras GN, Naujokas MA and Park M:
Pak4, a novel Gab1 binding partner, modulates cell migration and
invasion by the Met receptor. Mol Cell Biol. 29:3018–3032. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Chen N, Cui X, Zheng X, Deng L,
Price S, Karantza V and Minden A: The protein kinase Pak4 disrupts
mammary acinar architecture and promotes mammary tumorigenesis.
Oncogene. 29:5883–5894. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Franovic A, Elliott KC, Seguin L, Camargo
MF, Weis SM and and Cheresh DA: Glioblastomas require integrin
αvβ3/PAK4 signaling to escape senescence. Cancer Res. 75:4466–4473.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murray BW, Guo C, Piraino J, Westwick JK,
Zhang C, Lamerdin J, Dagostino E, Knighton D, Loi CM, Zager M, et
al: Small-molecule p21-activated kinase inhibitor PF-3758309 is a
potent inhibitor of oncogenic signaling and tumor growth. Proc Natl
Acad Sci USA. 107:pp. 9446–9451. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ryu BJ, Lee H, Kim SH, Heo JN, Choi SW,
Yeon JT, Lee J, Lee J, Cho JY, Kim SH, et al: PF-3758309,
p21-activated kinase 4 inhibitor, suppresses migration and invasion
of A549 human lung cancer cells via regulation of CREB, NF-κB, and
β-catenin signalings. Mol Cell Biochem. 389:69–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tyner JW, Yang WF, Bankhead A III, Fan G,
Fletcher LB, Bryant J, Glover JM, Chang BH, Spurgeon SE, Fleming
WH, et al: Kinase pathway dependence in primary human leukemias
determined by rapid inhibitor screening. Cancer Res. 73:285–296.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu Y, Gu TT and Zheng PS: CIP2A cooperates
with H-Ras to promote epithelial-mesenchymal transition in
cervical-cancer progression. Cancer Lett. 356:646–655. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
John-Baptiste A, Huang W, Kindt E, Wu A,
Vitsky A, Scott W, Gross C, Yang AH, Schaiff WT and Ramaiah SK:
Evaluation of potential gastrointestinal biomarkers in a PAK4
inhibitor-treated preclinical toxicity model to address
unmonitorable gastrointestinal toxicity. Toxicol Pathol.
40:482–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen S, Auletta T, Dovirak O, Hutter C,
Kuntz K, El-ftesi S, Kendall J, Han H, Von Hoff DD, Ashfaq R, et
al: Copy number alterations in pancreatic cancer identify recurrent
PAK4 amplification. Cancer Biol Ther. 7:1793–1802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kesanakurti D, Chetty C, Maddirela D
Rajasekhar, Gujrati M and Rao JS: Functional cooperativity by
direct interaction between PAK4 and MMP-2 in the regulation of
anoikis resistance, migration and invasion in glioma. Cell Death
Dis. 3:e4452012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Siu MK, Chan HY, Kong DS, Wong ES, Wong
OG, Ngan HY, Tam KF, Zhang H, Li Z, Chan QK, et al: p21-activated
kinase 4 regulates ovarian cancer cell proliferation, migration,
and invasion and contributes to poor prognosis in patients. Proc
Natl Acad Sci USA. 107:pp. 18622–18627. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ong CC, Gierke S, Pitt C, Sagolla M, Cheng
CK, Zhou W, Jubb AM, Strickland L, Schmidt M, Duron SG, et al:
Small molecule inhibition of group I p21-activated kinases in
breast cancer induces apoptosis and potentiates the activity of
microtubule stabilizing agents. Breast Cancer Res. 17:592015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhuang T, Zhu J, Li Z, Lorent J, Zhao C,
Dahlman-Wright K and Strömblad S: p21-activated kinase group II
small compound inhibitor GNE-2861 perturbs estrogen receptor alpha
signaling and restores tamoxifen-sensitivity in breast cancer
cells. Oncotarget. 6:43853–43868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao ZS and Manser E: Do PAKs make good
drug targets? F1000 Biol Rep. 2:702010.PubMed/NCBI
|
|
35
|
Callow MG, Clairvoyant F, Zhu S, Schryver
B, Whyte DB, Bischoff JR, Jallal B and Smeal T: Requirement for
PAK4 in the anchorage-independent growth of human cancer cell
lines. J Biol Chem. 277:550–558. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li X and Minden A: PAK4 functions in tumor
necrosis factor (TNF) alpha-induced survival pathways by
facilitating TRADD binding to the TNF receptor. J Biol Chem.
280:41192–41200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Y, Xiao H, Tian Y, Nekrasova T, Hao X,
Lee HJ, Suh N, Yang CS and Minden A: The pak4 protein kinase plays
a key role in cell survival and tumorigenesis in athymic mice. Mol
Cancer Res. 6:1215–1224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tyagi N, Bhardwaj A, Singh AP, McClellan
S, Carter JE and Singh S: p-21 activated kinase 4 promotes
proliferation and survival of pancreatic cancer cells through AKT-
and ERK-dependent activation of NF-kappaB pathway. Oncotarget.
5:8778–8789. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gnesutta N, Qu J and Minden A: The
serine/threonine kinase PAK4 prevents caspase activation and
protects cells from apoptosis. J Biol Chem. 276:14414–14419. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fu X, Feng J, Zeng D, Ding Y, Yu C and
Yang B: PAK4 confers cisplatin resistance in gastric cancer cells
via PI3K/Akt- and MEK/ERK-dependent pathways. Biosci Rep. 34:59–67.
2014. View Article : Google Scholar
|