Introduction
Trastuzumab is a humanized anti-HER2 monoclonal
antibody, which was approved for the treatment of HER2-positive
cancers. Although trastuzumab is effective in various clinical
cases, it eventually fails as primary and/or acquired resistance
occurs in up to 62% of patients during the first year of treatment
(1–3). For instance, many
HER2/neu-positive cancers do not respond to trastuzumab
treatment (de novo resistance), while many
trastuzumab-responsive patients develop resistance after continuous
trastuzumab infusion within 1 year (acquired resistance) (4–7).
Vascular endothelial growth factor (VEGF) is a valid
proangiogenic factor that stimulates endothelial cell
proliferation/growth, migration and increases vascular permeability
(8). Its significance has been
implicated in promoting solid tumor growth and metastasis via
stimulating tumor-associated angiogenesis. Thus, blocking the
activity of VEGF results in the starvation of tumors. Actually the
function of VEGF in cancer is not limited to angiogenesis or
vascular permeability as VEGF-mediated signaling also contributes
to tumorigenesis, including the function of cancer stem cells and
tumor initiation.
In our previous study, we induced an acquired
trastuzumab resistance cell model SKOV3-T by long-term trastuzumab
treatment of ovarian cancer cell line SKOV3 (9). In the present study, we found that the
proliferation of SKOV3-T cells was much more rapid than that noted
in SKOV3 both in vitro and in vivo. The microvessel
counts were significantly higher in the SKOV3-T tumor tissues. The
expression of VEGF was significantly upregulated in SKOV3-T. SP1,
known as a VEGF-upregulating molecule, was also found to be
upregulated in SKOV3-T cells. The results suggest a possible
SP1-VEGF axis which may be beneficial for angiogenesis and cell
growth/migration. Then, we explored the function of SP1 as well as
downstream VEGF in resistant SKOV3-T cells using both in
vitro assays. The results revealed that SP1 promoted tumor
angiogenesis and invasion by activating VEGF expression in the
acquired trastuzumab-resistant ovarian cancer model.
Materials and methods
Reagents
Trastuzumab (Herceptin®) was obtained
from F. Hoffmann-La Roche Ltd. (Shanghai, China). Antibodies of
HIF-α, STAT3, p-STAT3, P65, p-P65, SP1, histone H3, GAPDH and
corresponding secondary antibodies were purchased from Cell
Signaling Technology (Boston, MA, USA). Electrophoresis reagents
and hybridization nitrocellulose filter membranes were obtained
from Bio-Rad (Hercules, CA, USA). PE, DAPI, FITC and human VEGF-A
Platinum ELISA kit were obtained from eBioscience (San Diego, CA,
USA). Goat anti-human CD31 antibody was obtained from Abcam
Biotechnology (Cambridge, MA, USA). BCA protein assay kit and
enhanced chemiluminescent (ECL) reagents were purchased from Pierce
(Rockford, IL, USA). Cell culture medium Dulbeccos modified Eagles
medium (DMEM) and fetal bovine serum (FBS) were purchased from
HyClone (Logan, UT, USA). SP1 interference plasmids, SP1 shRNAs
(1–4), were purchased from GeneChem (Shanghai,
China). Female 6-week-old BALB/c nude mice were purchased from the
Vital River Laboratory (Beijing, China). TransiT-2020 transfection
reagent was purchased from Mirus Bio LLC (Madison, WI, USA).
Transwell chamber was obtained from Merck Millipore (Darmstadt,
Germany). All other chemicals were obtained from commercial sources
of analytical grade.
Cell culture
Human ovarian cancer cell line SKOV3 was obtained
from the American Type Culture Collection (ATCC; no. HTB-77)
(Manassas, VA, USA). Acquired trastuzumab-resistant ovarian cancer
cell line SKOV3-T was developed by continuously culturing SKOV3
cells in the presence of 20 µg/ml trastuzumab as previously
described (9). SKOV3-T cells were
maintained in the presence of 10 µg/ml trastuzumab (9). SKOV3 and SKOV3-T cells were cultured
in DMEM supplemented with 10% heat-inactivated FBS and 100 U/ml
penicillin and streptomycin. Cells were cultured at 37°C in 5%
CO2. Human umbilical vein endothelial cells (HUVECs)
were obtained from human umbilical veins as previously described
(10).
HUVEC proliferation assay
HUVECs were suspended at a density of
1×105/ml and were seeded in a 96-well plate (100
µl/well). After serum-free starvation overnight, the cells were
treated with 4 or 8 times diluted cell culture supernatant of SKOV3
or SKOV3-T cells. After cultivation for 10 h at 37°C, 10 µl/well of
Cell Counting Kit-8 (CCK8; Dojindo Laboratories, Kumamoto, Japan)
was added, and the plate was incubated for another 4 h. The
absorbance was measured using a spectrophotometer at 450 nm to
determine the cell viability.
Immunohistochemistry (IHC)
A week after the last observation, mice were
sacrificed, and the tumors were separated and fixed with 10%
formaldehyde. Paraffin-embedded tissue sections were processed,
deparaffinized, rehydrated and quenched for endogenous peroxidase
activity. Sections were stained with anti-CD31 antibody (dilution
1:100), and then incubated with horseradish peroxidase-conjugated
secondary antibody. Finally, the sections were developed with
diaminobenzidine and counterstained with hematoxylin. Images were
captured using an Olympus BX5 microscope with an UPlanFL N digital
camera (10×0.13 numeric aperture objective). Any single
brown-stained cell or cluster of endothelial cells that was clearly
separated from adjacent microvessels, tumor cells and other
connective tissue elements was considered a vessel. The number of
CD31-positive capillaries was counted from 5 randomly chosen
fields.
Transwell assay
The migration and invasion capacity of the SKOV3-T
and SKOV3 cells was quantified by Transwell assays using a
permeable membrane system plate with 8-µm pore size (Corning
Costar; Corning, Inc., Corning, NY, USA). SKOV3-T and SKOV3 cells
were starved in serum-free DMEM overnight and resuspended in DMEM
at a density of 4×105/ml. Then, 250 µl cells were seeded
in the top chambers; meanwhile, 750 µl FBS was added to the bottom
chamber. The cells were induced to migrate towards medium
containing 0, 10 or 100 µg/ml Avastin. After 6, 12 or 24 h of
incubation at 37°C, non-migrated cells on top of the upper membrane
were removed thoroughly with cotton swabs. The migrated cells below
the membrane were fixed with 4% paraformaldehyde, stained with
Giemsa solution, and then analyzed by a bright-field microscopy.
Cell images were captured using an Olympus BX5 microscope with an
UPlanFL N digital camera (10×0.13 numeric aperture objective). The
number of migrated cells was counted from 5 randomly chosen
fields.
SP1 silencing in SKOV3-T cells
SP1-silencing shRNA plasmids (1–4) were
used to knock down SP1 expression in the SKOV3-T cells. SKOV3-T
cells (2.5×105 cells/well) were seeded into a 6-well
plate and cultured overnight. SP1-silencing shRNA plasmid (2.5 µg)
and 7.5 µl TransIT-2020 transfection reagent were added into 250 µl
Opti-MEM I reduced-serum medium, mixed well and the mixture was
placed at room temperature (RT) for 15–30 min. Then, the
TransIT-2020 reagent-DNA complexes were added to the cell
supernatant for 72 h of incubation. The transfectants were treated
using 12 µg/ml puromycin (Sigma-Aldrich, St. Louis, MO, USA) for 7
days to obtain SP1 silenced clones.
Western blotting
SKOV3 cells were seeded into 6-well plates at a
density of 5×105 cells/ml. Meanwhile, SKOV3-T cells were
seeded into 6-well plates at a density of 3×105
cells/ml. After being serum-starved overnight, cells were collected
and the supernatant of the cell lysate was subjected to SDS-PAGE
analysis using 10% gel. Samples were transferred from the gel onto
a nitrocellulose membrane. The membrane was blocked with non-fat 5%
milk for 1 h at RT, and then incubated with anti-HIF-α, STAT3,
p-STAT3, P65, p-P65, SP1, GAPDH and histone H3 antibodies (1:1,000
dilution) overnight at 4°C, respectively. After washes with
Tris-buffered saline with Tween-20 (TBST) 3 times, the membranes
were incubated with the appropriate secondary antibodies conjugated
with HRP (dilution 1:2,500) for 1 h at RT. Signals on the membrane
were detected and visualized by enhanced chemilunimescence (ECL)
detection system and autoradiography. Relative protein expression
in whole cells or the cytoplasm was normalized to GAPDH intensity
and SP1 expression in the nucleus was normalized to H3
intensity.
RT-PCR
Total RNA was isolated from the cells using a total
RNA isolation kit (RNAgents; Promega, Madison, WI, USA). The total
RNA (1.0 µg) was reverse transcribed using an oligo(dT) 18-mer and
reverse transcription system (Promega). PCR was carried out using
selective primers for human VEGF (sense primer,
5′-GCTACTGCCATCCAATCGAG-3′ and antisense primer,
5′-TGCATTCACATTTGTTGTGC-3′); and GAPDH (sense primer,
5′-TCCAAGGGTCCGCTGCAGTC-3′ and antisense primer,
5′-CGTTCACCTTGATGAGCCCACGTTCACCTTGATGAGCCCATT-3′). PCR was carried
out for 35 cycles under the following conditions: denaturation at
95°C for 30 sec, annealing at 58°C for 30 sec, and elongation at
72°C for 30 sec.
Xenograft mouse model
Six-week-old female BALB/c nude mice were purchased
from Vital River Laboratory, housed in a barrier environment with
12:12 h light-dark cycle, and fed normal mouse laboratory diet.
Thirty-two mice were divided randomly into 4 groups (n=8). Each
mouse was subcutaneously inoculated on the back with
2×106/0.1 ml SKOV3-T, SKOV3, 1F10 or 3D7 cells on day 0.
Mice were observed twice a week and the perpendicular tumor
dimensions were measured using a Vernier scale caliper. The tumor
volumes were calculated according to the following equation: Tumor
volume (mm3) = 1/2 × (length) × (width)2. A
week after the last observation, the mice were sacrificed and the
tumors were separated for IHC analysis. All procedures were
administered in strict agreement with international guidelines for
the care and use of laboratory animals and approved by the Animal
Ethics Committee of the Institute of Basic Medical Sciences,
Academy of Military Medical Sciences, Beijing.
Enzyme-linked immunosorbent assay
(ELISA)
First, we prepared all reagents and standard samples
following the instructions of the human VEGF ELISA kit. ELISA
plates were blocked, diluted cultured media were added and antibody
was detected following 2 h of incubation at 37°C. After washing,
horseradish peroxidase-conjugated goat anti-human IgG was added for
45 min of incubation at RT. The peroxidase reaction was developed
with substrate solution provided in the ELISA kit and the light
absorbance was measured with an ELISA reader at 450 nm.
Confocal microscopy
Cell monolayers were washed with phosphate-buffered
saline (PBS) before being fixed with 4% paraformaldehyde for 10
min. After washing with PBS, the samples were permeabilized with
0.25% Triton-PBS for 8 min at RT. The culture dishes were blocked
with 10% FBS for 1 h at RT, and then incubated with various
antibodies (dilution 1:100) overnight at 4°C. Then, the samples
were incubated with secondary antibody with fluorescence (PE, DAPI)
for 30 min at RT. Finally, samples were washed and visualized by
laser scanning confocal microscope (LSCM).
Statistical analysis
Data are expressed as the mean ± SD. Pairwise
differences between several groups were compared. Significant
differences were analyzed by the one-way analysis of variance
(ANOVA), and P-values of <0.05 were taken as statistically
significant.
Results
Herceptin-resistant SKOV3-T cells
possess enhanced tumorigenesis and angiogenesis
HER2/neu-positive SKOV3 cells were
continuously cultured in the presence of 20 µg/ml trastuzumab for 8
months, resulting in trastuzumab-resistant SKOV3-T cells. The
xenograft model results revealed that the in vivo tumor
growth rate of SKOV3-T was significantly more rapid than that of
the SKOV3 cells. Meanwhile, the shape of SKOV3-T xenografts seemed
more irregular than SKOV3. The results suggested the possibility of
enhanced migration of SKOV3-T cells (Fig. 1A; P<0.001). Tumor sections were
then stained with anti-CD31 (a vascular endothelial marker)
antibody for IHC assay. As shown in Fig. 1B, a significant increase in
microvessel density was observed in the xenograft tumors of SKOV3-T
cells, which may facilitate the faster growth of SKOV3-T
xenografts. Therefore, we assumed that trastuzumab treatment may
induce the upregulation of angiogenesis factors in SKOV3-T cells in
order to accelerate vessel formation and tumor growth in
vivo.
Overexpression of VEGF in SKOV3-T
cells accelerates the migration of SKOV3-T cells
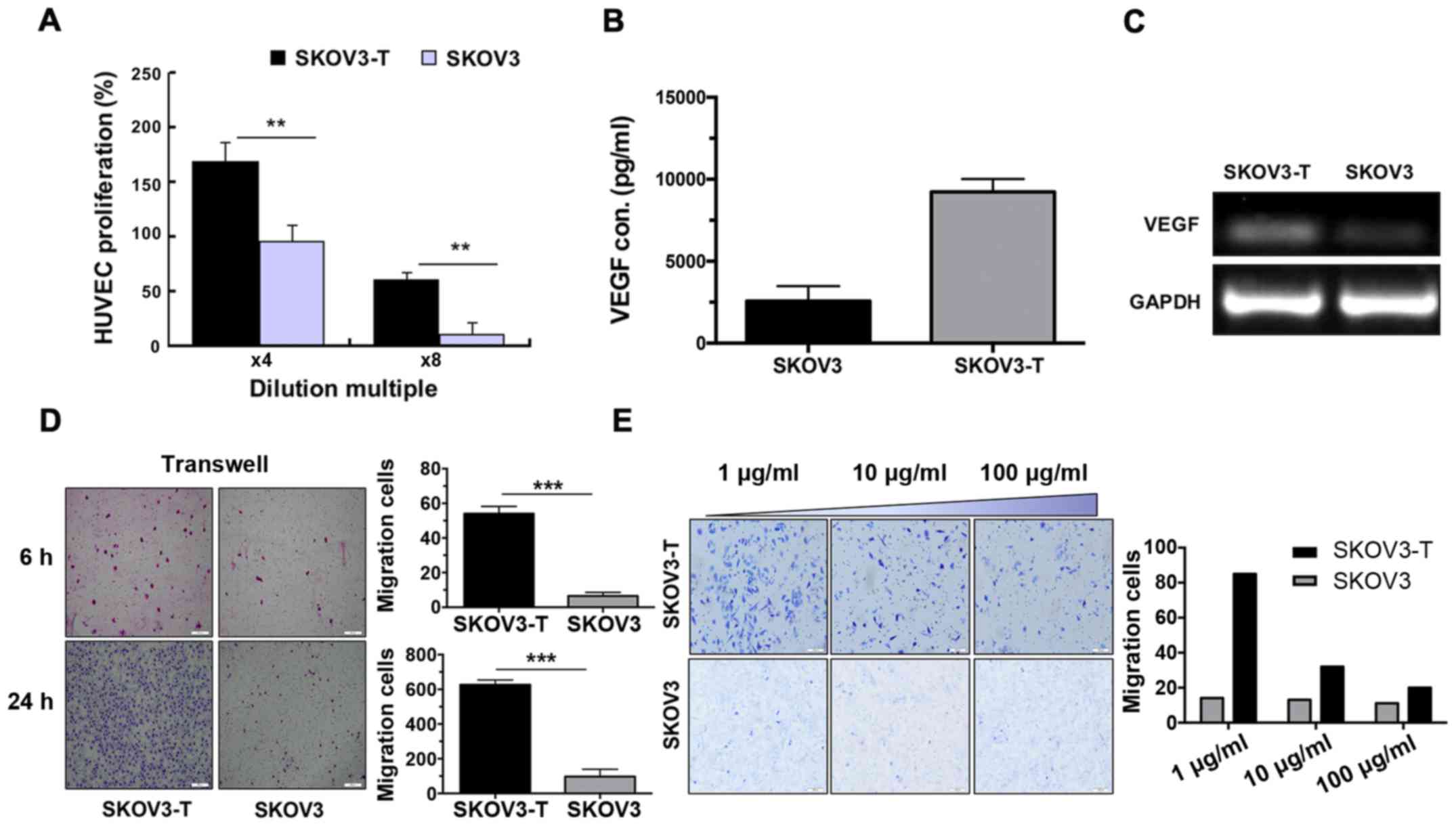
VEGF is the most important proangiogenic factor,
which enhances the proliferation of endothelial cells. As shown in
Fig. 2A, the proliferation of
HUVECs cultured in the SKOV3-T supernatant was markedly increased,
suggesting higher VEGF secretion in the supernatant of SKOV3-T
cells (P<0.01). ELISA assay results confirmed the VEGF
overexpression in the SKOV3-T cells, which was nearly 3 times
higher than that in the SKOV3 cells (Fig. 2B). RT-PCR analysis results (Fig. 2C) were consistent with the protein
detection results; VEGF was overexpressed in the SKOV3-T when
compared to the expression noted in the SKOV3 cells.
Endothelial cell migration is the
essential/functional process in angiogenesis
Thus, a Transwell assay was employed to evaluate the
difference in cell migration between the two cell lines. As shown
in Fig. 2D, SKOV3-T cells exhibited
a markedly stronger migration ability (P<0.001). The number of
migratory SKOV3-T cells was >6 times that of the SKOV3 cells
after 6 or 24 h. Meanwhile, along with the increased concentration
of Avastin, the migration capacity of the SKOV3-T cells was
inhibited in a dose-dependent manner (Fig. 2E). These results indicate that the
enhanced migration activity of the SKOV3-T cells was induced by the
overexpression of VEGF, which also explained why this cell line
proliferated much faster than SKOV3 as well as had enhanced
angiogenesis.
SP1 promotes VEGF overexpression in
SKOV3-T cells
The 5′-flanking region of the VEGF gene contains the
binding sites of several transcription factors, which play an
important role in regulating the transcription of the VEGF gene.
Western blot results revealed that SP1 expression was upregulated,
while the levels of other transcription factors were identical to
those in the SKOV3 cells (Fig. 3A).
SP1 seemed to respond to VEGF overexpression in the
trastuzumab-resistant ovarian cancer SKOV3-T cells. Furthermore,
the SP1 level in the cytoplasm appeared to be identical between the
SKOV3-T and SKOV3 cells (Fig. 3B,
left panel; P<0.01), and the level of SP1 localized in the
nucleus of SKOV3-T cells was much higher, suggesting its possible
function to enhance the transcription level of VEGF (Fig. 3B, right panel; P<0.01). Confocal
microscopy observation also showed the similar consequence of SP1
overexpression in SKOV3-T cells, and we observed more SP1 gathered
in the nucleus in contrast to that observed in the SKOV3 cells
(Fig. 3C).
SP1 knockdown inhibits VEGF-mediated
migration in SKOV3-T cells
In order to evaluate and confirm whether SP1
influences VEGF expression as well as VEGF-induced migration in
SKOV3-T cells, we synthesized SP1 shRNAs (1–4) and
transfected the SKOV3-T cells, respectively, to knock down SP1
expression. Then, the cells were collected for western blot
analysis to confirm the markedly reduced SP1 (Fig. 4A). Along with SP1 knockdown, the
VEGF expression level in the supernatant was lower in the SKOV3-T
cells, indicating the importance of SP1 to upregulate VEGF
(Fig. 4B; P<0.01); Furthermore,
SP1 knockdown by SP1 shRNA inhibited the migration capacity of the
SKOV3-T cells, which confirmed the presence of an ‘SP1-VEGF axis’
to induce enhanced migration following long-time trastuzumab
treatment of SKOV3 cells (Fig. 4C;
P<0.01).
After subcloning, we screened two SP1-knockdown
SKOV3-T cell clones, named 3D7 and 1F10, for next-step in
vivo assays. As shown in Fig.
4D, the SP1 expression in the 3D7 and 1F10 cells was low. The
clone 1F10 had a lower expression of SP1 compared with 3D7, thus
3D7 was set as a ‘partially knocked down’ clone with medium SP1
expression level between SKOV3-T and SKOV3 cells (Fig. 4D). The difference between the two
clones in regards to VEGF secretion was detected by ELISA. The
results revealed that the concentration of VEGF was higher in 1F10
than that in the 3D7 cell line (Fig.
4E; P<0.01, P<0.001).
SP1-knockdown inhibits the tumor
growth and angiogenesis of SKOV3-T cells in vivo
Six-weeks old female nude mice were randomly divided
into 4 groups with 8 mice/group (n=8), and were subcutaneously
inoculated with SKOV3-T, SKOV3, 1F10 or 3D7 cells
(2×106/mice) and the xenografts were allowed to grow for
28 days. The tumor size was measured twice a week. As shown in
Fig. 5A-C, the mean tumor
size/weight of the 1F10- and 3D7-derived tumors were significantly
smaller than the tumors derived from the SKOV3-T cells, indicating
that SP1-knockdown reversed trastuzumab-resistance to inhibit the
carcinogenicity of the SKOV3-T cells. However, the ‘partial
knockdown’ clone 3D7 xenografts were slightly larger than 1F10,
while 1F10 tumors were similar to those derived from the SKOV3
cells, which was consistent with the in vitro assay trend.
Furthermore, the microvessel density assay showed that SP1
knockdown inhibited the angiogenesis of tumors, confirming the
importance of the SP1-VEGF axis in trastuzumab-resistant SKOV3-T
cells (Fig. 5D).
Discussion
Ovarian cancer is the fourth common cancer in terms
of mortality among women, and is the leading cause of death from
gynecologic malignancies worldwide (11,12).
HER2 is overexpressed in ~25–30% of ovarian cancer patients, yet
anti-HER2 therapies display limited clinical response in the
treatment of HER2-positive ovarian cancer (13). It has been reported that many
HER2/neu-positive cancer patients indeed do not respond to
trastuzumab treatment (de novo resistance), or develop
resistance after continuous trastuzumab infusion within 1 year
(acquired resistance) (4–7), which may be the reason why trastuzumab
therapy for ovarian cancer patients usually fails in clinical
trials.
Previously, we established in vitro an
acquired trastuzumab-resistant ovarian cancer cell model, SKOV3-T,
as previously described (9).
SKOV3-T cells possessed enhanced proliferation and carcinogenesis
characteristic in vivo (Fig.
1A). We found overexpression of IGF-1R and HER3 in the
resistant cells (9). However,
IGF-1R and HER3 were not the key overexpressed biomarkers of the
resistant cell model. Anti-IGF-1R treatment only partially
inhibited the cell growth, and the treated cells maintained rapid
growth either in vitro or in vivo; therefore it
failed to reverse the trastuzumab resistance thoroughly (14).
Recently, we noted the enhanced angiogenesis and
migration characteristics in the in vivo xenograft assays,
by which we inferred the overexpression of VEGF in SKOV3-T cells.
It was reported that ovarian tumors usually contain a rich vascular
network and are highly dependent on VEGF-mediated angiogenesis
(15). Angiogenesis is the
progression of new blood vessel formation, which plays a key role
in physiological and pathological processes (16,17).
VEGF plays a pivotal role in human tumorigenesis and angiogenesis
of cancer. In the present study, as shown in Fig. 1B, the SKOV3-T xenograft tumors
contained more microvessels, as the density was higher than SKOV3.
The HUVEC-based cell proliferation assay indicated the presence of
higher VEGF in the SKOV3-T cell culture (Fig. 2A). ELISA and RT-PCR also provided
identical data (Fig. 2B and C). At
the same time, we also noted that the edges of the SKOV3-T tumors
were markedly more irregular, suggesting possible enhanced
migration activity of the SKOV3-T cells probably due to the
overexpression of VEGF. A Transwell assay was used to confirm the
assumption concerning cell migration (Fig. 2D), and anti-VEGF antibody, Avastin,
showed specific inhibitory function against migration in a
dose-dependent manner (Fig. 2E),
suggesting the importance of higher VEGF to induce stronger
migration activity in the SKOV3-T cells.
Furthermore, we analyzed the possible translational
factors of VEGF, e.g. HIF-1α, STAT3, P65 and SP1. According to
western blotting of whole cells (Fig.
3A), SP1 was upregulated, particularly in the nuclei of SKOV3-T
cells (Fig. 3B and C). Sp1 is
well-known as a transcription factor which binds to the promoters
of many target genes and is implicated in the regulation of
multiple essential biological processes, including cell apoptosis
and growth, cell cycle progression, angiogenesis and metastasis
(18–20). Sp1 not only regulates the expression
of multiple genes, but also the Sp1 gene itself. Sp1 levels often
correlate with tumor stage and poor prognosis (21). Constitutive activation of SP1 plays
a critical role in VEGF expression (22,23).
SP1 was found to enhance cell invasion by upregulating VEGF in lung
cancer and LKB1, an inhibitor of SP1, suppressed the invasion
(22). In colon cancers, SP1 was
confirmed as a predominant factor required for AKT-mediated
induction of VEGF (24). The
self-renewal ability, drug resistance, and metastasis potential of
colon CSCs may be partially due to preferentially high expression
of SP1 (24).
In the present study, we used shRNAs to knock down
SP1 (Fig. 4A) to identify the
function of the SP1-VEGF axis in cell migration and angiogenesis of
SKOV3-T cells, which was found to downregulate the corresponding
VEGF expression in SKOV3-T cells (Fig.
4B) and inhibit VEGF-induced migration in vitro
(Fig. 4C). Then, we screened two
SP1-knockdown clones, 3D7 and 1F10, in which 3D7 was set as a
‘partial knockdown’ clone (Fig.
4D). Similar to the trend in SP1 expression, the VEGF secretion
in the two clones was decreased; more importantly, VEGF in the 1F10
supernatant was lower than that in 3D7 (Fig. 4D), indicating that SP1 was pivotal
in the regulation of migration and VEGF, and influenced malignancy
and enhanced the angiogenesis of tumors.
In vivo experiments also confirmed SP1 as the
core factor to control VEGF-related tumor growth and microvessel
formation. Notably, the average tumor size of the 1F10 cell-derived
xenografts was almost as small as those derived from the SKOV3
cells, indicating that SP1 knockdown successfully inhibited the
growth of trastuzumab-resistant SKOV3-T xenografts (Fig. 5B). Meanwhile, IHC analysis showed
reduced microvessel density in the 1F10 tumors which showed that
SP1 knockdown inhibited VEGF-dependent angiogenesis, and therefore,
reversed the trastuzumab resistance in the ovarian cancer cells
(Fig. 5D). Although further
investigation is warranted to understand the related mechanism, the
in vitro and in vivo data provide evidence of the
SP1-VEGF axis that induced malignancy, angiogenesis and migration
in the acquired trastuzumab-resistant ovarian cancer cell
model.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 81272528).
References
|
1
|
Esteva FJ, Yu D, Hung MC and Hortobagyi
GN: Molecular predictors of response to trastuzumab and lapatinib
in breast cancer. Nat Rev Clin Oncol. 7:98–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Madrid-Paredes A, Cañadas-Garre M,
Sánchez-Pozo A and Calleja-Hernández MA: Non-HER2 signaling
pathways activated in resistance to anti-HER2 therapy in breast
cancer. Breast Cancer Res Treat. 153:493–505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Madrid-Paredes A, Cañadas-Garre M,
Sánchez-Pozo A and Calleja-Hernández MA: De novo resistance
biomarkers to anti-HER2 therapies in HER2-positive breast cancer.
Pharmacogenomics. 16:1411–1426. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kruser TJ and Wheeler DL: Mechanisms of
resistance to HER family targeting antibodies. Exp Cell Res.
316:1083–1100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fiszman GL and Jasnis MA: Molecular
mechanisms of trastuzumab resistance in HER2 overexpressing breast
cancer. Int J Breast Cancer. 2011:3521822011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Köninki K, Barok M, Tanner M, Staff S,
Pitkänen J, Hemmilä P, Ilvesaro J and Isola J: Multiple molecular
mechanisms underlying trastuzumab and lapatinib resistance in
JIMT-1 breast cancer cells. Cancer Lett. 294:211–219. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nahta R and Esteva FJ: HER2 therapy:
Molecular mechanisms of trastuzumab resistance. Breast Cancer Res.
8:2152006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia Y, Zhang Y, Qiao C, Liu G, Zhao Q,
Zhou T, Chen G, Li Y, Feng J, Li Y, et al: IGF-1R and ErbB3/HER3
contribute to enhanced proliferation and carcinogenesis in
trastuzumab-resistant ovarian cancer model. Biochem Biophys Res
Commun. 436:740–745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang J, Wang Q, Qiao C, Lin Z, Li X, Huang
Y, Zhou T, Li Y, Shen B, Lv M, et al: Potent anti-angiogenesis and
anti-tumor activity of a novel human anti-VEGF antibody, MIL60.
Cell Mol Immunol. 11:285–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nusrat O, Belotte J, Fletcher NM, Memaj I,
Saed MG, Diamond MP and Saed GM: The role of angiogenesis in the
persistence of chemoresistance in epithelial ovarian cancer. Reprod
Sci. 23:1484–1492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thibault B, Castells M, Delord JP and
Couderc B: Ovarian cancer microenvironment: Implications for cancer
dissemination and chemoresistance acquisition. Cancer Metastasis
Rev. 33:17–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bookman MA, Darcy KM, Clarke-Pearson D,
Boothby RA and Horowitz IR: Evaluation of monoclonal humanized
anti-HER2 antibody, trastuzumab, in patients with recurrent or
refractory ovarian or primary peritoneal carcinoma with
overexpression of HER2: A phase II trial of the Gynecologic
Oncology Group. J Clin Oncol. 21:283–290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang W, Zhang Y, Lv M, Feng J, Peng H,
Geng J, Lin Z, Zhou T, Li X, Shen B, et al: Anti-IGF-1R monoclonal
antibody inhibits the carcinogenicity activity of acquired
trastuzumab-resistant SKOV3. J Ovarian Res. 7:1032014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramakrishnan S, Subramanian IV, Yokoyama Y
and Geller M: Angiogenesis in normal and neoplastic ovaries.
Angiogenesis. 8:169–182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Huang Y, Huang Y, Xia X, Zhang J,
Zhou Y, Tan Y, He S, Qiang F, Li A, et al: JWA suppresses tumor
angiogenesis via Sp1-activated matrix metalloproteinase-2 and its
prognostic significance in human gastric cancer. Carcinogenesis.
35:442–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsu TI, Wang MC, Chen SY, Yeh YM, Su WC,
Chang WC and Hung JJ: Sp1 expression regulates lung tumor
progression. Oncogene. 31:3973–3988. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jungert K, Buck A, von Wichert G, Adler G,
König A, Buchholz M, Gress TM and Ellenrieder V: Sp1 is required
for transforming growth factor-beta-induced mesenchymal transition
and migration in pancreatic cancer cells. Cancer Res. 67:1563–1570.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vizcaíno C, Mansilla S and Portugal J: Sp1
transcription factor: A long-standing target in cancer
chemotherapy. Pharmacol Ther. 152:111–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang X, Li ZL, Jiang LL, Guo QQ, Liu MJ
and Nan KJ: Suppression of lung cancer cell invasion by LKB1 is due
to the downregulation of tissue factor and vascular endothelial
growth factor, partly dependent on SP1. Int J Oncol. 44:1989–1997.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hirose T and Horvitz HR: An Sp1
transcription factor coordinates caspase-dependent and -independent
apoptotic pathways. Nature. 500:354–358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Y, Zhang W, Guo Z, Ma F, Wu Y, Bai Y,
Gong W, Chen Y, Cheng T, Zhi F, et al: Inhibition of the
transcription factor Sp1 suppresses colon cancer stem cell growth
and induces apoptosis in vitro and in nude mouse xenografts. Oncol
Rep. 30:1782–1792. 2013. View Article : Google Scholar : PubMed/NCBI
|