Introduction
Breast cancer is the most frequently diagnosed
cancer in women. Globally, ~1.3 million new cases are diagnosed
annually (1). China accounted for
12.2% of all newly diagnosed global cases and 9.6% of all breast
cancer-related deaths worlwide in 2008 (2), and since 1990s the incidence in China
has increased more than twice as fast as that of global rates,
particularly in urban areas (3).
Although treatments of breast cancer have significantly been
improved in surgery, radiotherapy and molecular targeted therapy,
chemotherapy is still the major method. Chemotherapy reduced the
risk of disease recurrence and metastasis (4). However, the chemotherapy resistance is
still a major limitation in clinical treatment. Hence, new drugs or
new therapeutic combinations are required to enhance
chemosensitization and therapeutic efficacy (5).
Paclitaxel, one of the most important anticancer
drugs, has been widely used for chemotherapy in ovarian, non-small
cell lung cancer, and head and neck cancer, for almost 40 years,
and it is also the first-line chemotherapy drug used in breast
cancer (6,7). Paclitaxel prompts tubulin
polymerization by binding β-tubulin. By stabilizing the microtubule
polymer and preventing microtubules from disassembly, paclitaxel
arrests the cell cycle in the G2/M phases and induces cell death
(8–10). However, chemotherapy resistance
limits the effect of paclitaxel and influences the prognosis of
patients. Thus, it is particularly important to enhance the
sensitivity of cancer cells to paclitaxel.
Trametes robiniophila Murr. (Huaier), a type
of traditional Chinese medicine, has been widely used in China for
~1,600 years (11). Huaier extract
is the fungus extracted twice with hot water and the effective
ingredient is proteoglycan, containing 41.53% polysaccharides,
12.93% amino acids and 8.72% water (12–14).
However, the anticancer effect of proteoglycan is less effective
than that of the Huaier extract (15). It has been demonstrated that the
main anticancer mechanisms of Huaier are achieved by inhibiting the
growth and proliferation of cancer cells, inducing apoptosis,
decreasing drug resistance in cancer cells, and improving immunity
as well (16,17). In vitro experiments revealed
that Huaier extract inhibited the growth of hepatocarcinoma and
human lung adenocarcinoma cells (18,19).
Huaier extract could inhibit the growth of ERα-positive breast
cancer cells through the ERα/NF-κB pathway (20). A recent study reported that Huaier
extract synergized with tamoxifen to increase autophagy and cell
apoptosis in MCF-7 breast cancer cells, which was more effective
than monotherapy (21).
In 1986, NF-κB was discovered as a nuclear factor
that binds to the enhancer element of the immunoglobulin κ
light-chain of activated B cells (22). Aberrant activation of NF-κB is
frequently found in various solid tumors and hematological
malignancies. NF-κB family members and their regulated genes are
associated with tumor development, tumor cell proliferation,
survival, angiogenesis, invasion, metastasis and drug resistance
(23). IκB is the specific
intracellular inhibitor of NF-κB. In most cell types, NF-κB is
present in an inactive form, where it is complexed with IκB in the
cytoplasm (24). A previous study
observed persistent activation of NF-κB in hepatocellular carcinoma
cell lines, and suggested that inhibition of NF-κB signaling
markedly sensitized HCC cells to doxorubicin-induced apoptosis
(25). In the chemotherapy, drug
resistance is an important problem, as well as the expression
levels of multidrug resistant (MDR) proteins, apoptosis and
anti-apoptosis genes are the main factors of tumor chemosensitivity
(26). Moreover, NF-κB is closely
associated with apoptosis and mediates cell proliferation and
survival. NF-κB can activate a variety of anti-apoptosis genes,
such as Bcl-xL, Bfl/A1 and Bcl-2. Furthermore, the downregulation
of NF-κB and different Bcl-2 family members leads to enhanced
chemosensitization or radio-sensitization of multiple human cancers
(27).
In the present study, we demonstrated for the first
time that Huaier extract combined with paclitaxel induced cell
apoptosis and enhanced paclitaxel drug sensitivity in breast cancer
cells via the NF-κB pathway.
Materials and methods
Cell lines and reagents
ER-positive breast cancer cell line MCF-7 and
triple-negative breast cancer cell line MDA-MB-231 were obtained
from The Cell Bank of the Chinese Academy Sciences (Shanghai,
China). Huaier extract was kindly provided by Gaitianli Medicine
Co. Ltd. (Jiangsu, Chain). One gram Huaier extract was dissolved in
10 ml of complete RPMI-1640 medium and sterilized with a 0.22-µm
filter to obtain 100 mg/ml stock solution at 4°C. Paclitaxel was
purchased from Yangzijiang Medicine Co. Ltd. (Jiangsu, China). p65,
IκBα and c-Met monoclonal antibodies were purchased from Abcam
(Cambridge, UK). Rabbit anti-human polyclonal β-actin antibody was
purchased from Bioworld Technology Inc. (St. Louis, MO, USA). The
secondary anti-rabbit antibody was purchased from Qiaoyi
Biotechnics Co. Lit (Shanghai, China).
Cell culture
Both MCF-7 and MDA-MB-231 cells were cultured in
RPMI-1640 medium (Gibco-BRL, Thermo Fisher Scientific, Waltham, MA,
USA) with 10% fetal bovine serum (FBS; Biological Industries Israel
Beit-Haemek Ltd., Kibbutz Beit-Haemek, Israel), 100 U/ml penicillin
and 100 µg/ml streptomycin. MCF-7 and MDA-MB-231 cells were
routinely cultured at 37°C with 5% CO2 in a humidified
incubator.
Cell viability assay (CCK-8
assay)
Cell viability was determined by Cell Counting Kit-8
(CCK-8) assay. Both MCF-7 and MDA-MB-231 cells were seeded into
96-well plates at a density of 5×104 cells/well in 100
µl RPMI-1640 medium. After being cultured in 5% CO2, in
a 37°C incubator overnight, the RPMI-1640 medium in each well was
treated with different concentrations of solutions (0, 2, 4, 8, 10
and 16 mg/ml of Huaier extract; 0, 0.001, 0.01, 0.1, 1 and 10 µg/ml
of paclitaxel, or combined therapies) and incubated for 0, 24 or 48
h. Afterwards, 10 µl of CCK-8 solution was added to each well for
another 2 h at 37°C. The absorbance at 450 nm was read using a
microplate reader (BioTek, Winooski, VT, USA). The inhibitory rate
of cell growth was calculated based on the following equation: Cell
growth inhibition rate = (1 - experimental OD450/control OD450) ×
100%. Based on the statistical analysis of results, 4 and 8 mg/ml
of Huaier extract and 0.01 and 0.1 µg/ml of paclitaxel were used to
explore cell functional studies and mechanisms. For combined
therapies in vitro, the cells were treated with 4 mg/ml
Huaier extract + 0.01 µg/ml paclitaxel (H4 + P0.01) and 8 mg/ml
Huaier extract + 0.1 µg/ml paclitaxel (H8 + P0.1) for 48 h.
Experiments were repeated three times, independently.
Flow cytometric analysis of the cell
cycle and apoptosis
Cell apoptosis was performed using an FITC Annexin V
apoptosis detection kit (BD Biosciences, San Jose, CA, USA). All
groups of cells were strictly treated under manual processing and
analyzed using a Beckman Coulter Cytomics FC500 flow cytometer
(Beckman Coulter, Inc., Brea, CA, USA). The data were analyzed by
EXPO32 ADC analysis software.
Cell cycle analysis was performed using the standard
method with some modifications. Cells were fixed overnight with 75%
ethanol at 4°C. The following day, the fixed cells were washed with
phosphate-buffered saline (PBS). Then, the cells were suspended
with 200 µl RNase A at 37°C for 10 min, followed by the addition of
250 µl propidium iodide (PI) (100 µg/ml) to stain the DNA of cells
in the dark for 15 min. Finally, the cell cycle was analyzed with a
Beckman Coulter Cytomics FC 500 flow cytometer, and the data was
analyzed using MultiCycle AV for Windows (version 295)
software.
Real-time PCR analysis
Total RNA from treated cells was extracted using
TRIzol and was used to synthesize cDNA using PrimeScript RT reagent
kit (both from Takara, Dalian, China) according to the
manufacturer's instructions. Then, real-time PCR was carried out
using PowerUp SYBR-Green Master Mix (Life Technologies, Thermo
Fisher Scientific). The reaction was conducted using the following
parameters: 95°C for 30 sec, 40 cycles at 95°C for 5 sec and 60°C
for 30 sec. Internal control and primers for real-time PCR were
obtained from the reference. Real-time PCR primers were synthesized
by SBS Genentech Co. Ltd. (Shanghai, China). The specific primers
for P65, IκB, c-Met and the reference gene (β-actin) are listed as
follows: p65 forward, 5′-ACAACCCCTTCCAAGTTCCT-3′ and reverse,
5′-TGGTCCCGTGAAATACACCT-3′; IκB forward, 5′-TGAGGACCAGCAGTGTCTTG-3′
and reverse, 5′-CATCGTTGATCACAAGTCGG-3′; c-Met forward,
5′-CATCTCAGAACGGTTCATGCC-3′ and reverse,
5′-TGCACAATCAGGCTACTGGG-3′; β-actin forward,
5′-GCTACAGCTTCACCACCACAG-3′ and reverse,
5′-GGTCTTTACGGATGTCAACGTC-3′. The experiments were repeated at
least three times and the data were analyzed using
2−ΔΔCt.
Western blot analysis
MCF-7 and MDA-MB-231 cells were treated with
different concentrations of Huaier extract (4 and 8 mg/ml) and/or
paclitaxel (0.01 and 0.1 µg/ml) for 48 h. Τhe cells were lysed and
total protein was separated using 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred (300 mA, 2 h) onto polyvinylidene fluoride (PVDF)
membranes. After blotting with 5% non-fat milk, the membranes were
incubated with primary antibodies (anti p65 1:5,000, anti p-p65
1:1,000, anti IκBα 1:1,000, anti c-Met 1:1,000, β-actin 1:5,000) at
4°C overnight. Then, the membranes were washed using Tris-buffered
saline with Tween-20 (TBS-T) buffer and incubated with a secondary
horseradish peroxidase (HRP)-labeled anti-rabbit antibody at room
temperature for 1 h. Subsequently, the membranes were washed again
with TBS-T buffer three times (10 min each time). The target
proteins were visualized with a chemiluminescence system (Gene
Company Ltd., Shanghai, China) and normalized to β-actin from the
same membrane.
Xenograft tumorigenicity assay
MCF-7 and MDA-MB-231 cells (5×106 in 0.1
ml PBS) were subcutaneously injected into four week old female nude
mice. Treatments were started at the second week after injection.
The mice were randomly assigned to control (double-distilled
water), Huaier extract, paclitaxel or combined treatment groups.
The dose of Huaier extract was 250 mg/ml and paclitaxel was 10
mg/kg. The Huaier group was administered 50 mg of Huaier extract by
gavage once every two days, while paclitaxel was administered by
intraperitoneal injection (IP) once a week or both were
administered in the combined treatment group. Tumor growth was
assessed every week and the tumor volume was calculated using the
formula: 0.5 × (length × width2). After six weeks, the
mice were sacrificed and the xenografted tumors were removed for
flow cytometric and western blot analyses.
Statistical analysis
Statistical analyses were performed with SPSS for
Windows 21.0 (SPSS, Inc., Chicago, IL, USA). Student's t-tests and
one-way ANOVA were performed to determine statistical significance.
All data are presented as mean ± standard error of the mean (SEM)
of three experiments, and P<0.05 was considered statistically
significant.
Results
Combination of Huaier extract and
paclitaxel exhibits a more effective inhibitory effect on cell
proliferation compared with monotherapies
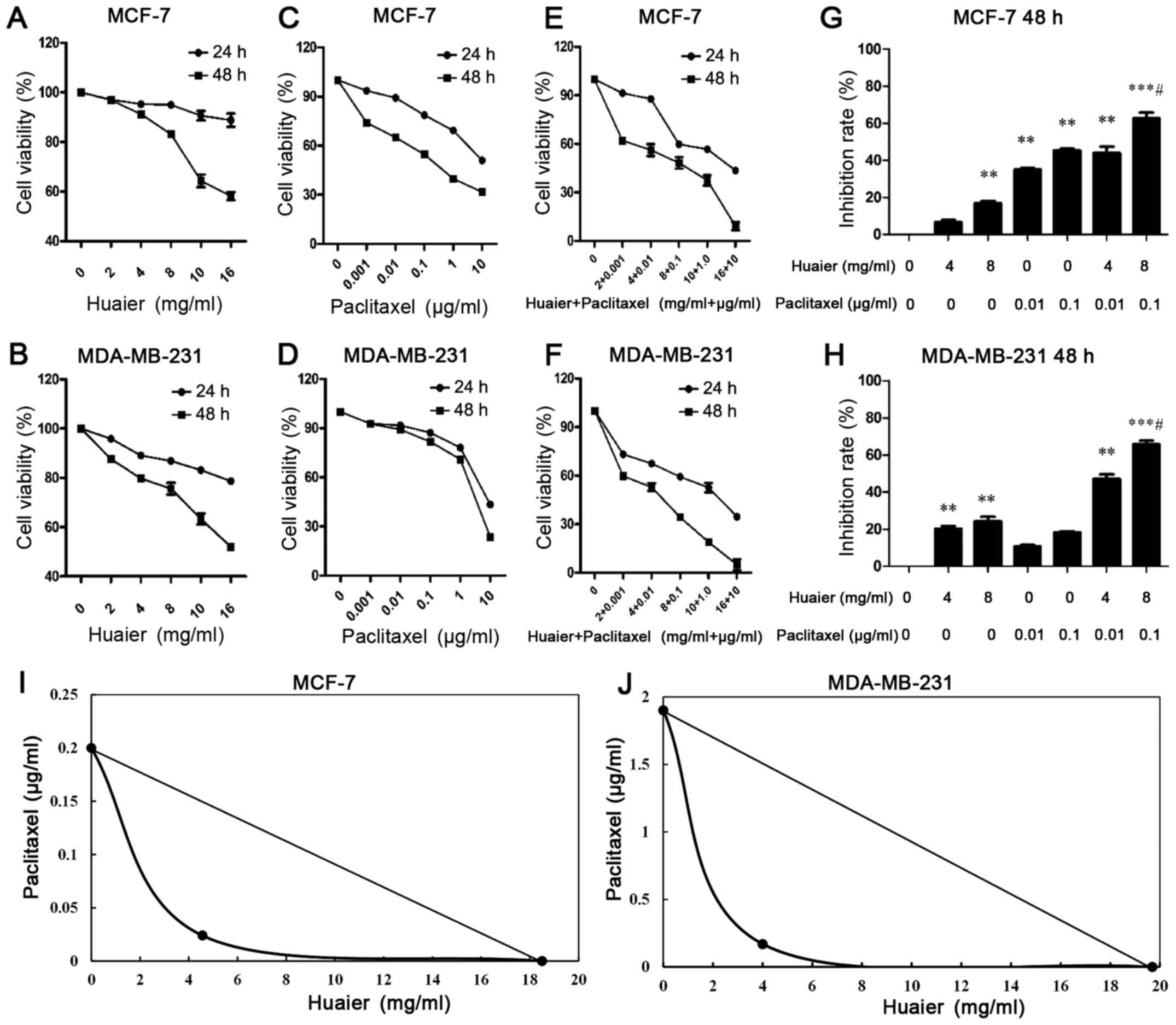
As shown in Fig. 1A and
B, Huaier extract reduced the viability of MCF-7 and MDA-MB-231
cells in a time- and concentration-dependent manner. Compared with
the control group, 10 and 16 mg/ml of Huaier extract significantly
decreased cell viability in both cell lines at 24 h (P<0.01). At
48 h, the dosing >4 mg/ml of Huaier extract significantly
reduced the cell viability in both cell lines (P<0.01). We
observed that the proliferation of MCF-7 and MDA-MB-231 cells was
significantly inhibited by paclitaxel treatment in a time- and
dose-dependent manner (Fig. 1C and
D). At 48 h, cell viability was significantly decreased by
paclitaxel treatment as low as 0.01 µg/ml (P<0.05). The combined
therapies sharply decreased the viability of both MCF-7 and
MDA-MB-231 cells, and the extent of reduced cell viability by
combined therapies was significantly greater than the monotherapies
of Huaier extract or paclitaxel treatment (Fig. 1E and F). Compared with the 0.1 µg/ml
of paclitaxel treatment alone, the inhibition rate of cell
viability in the combined treatments (H8P0.1) was 1.382±0.161 times
in the MCF-7 cells, and 2.83±0.751 times in the MDA-MB-231 cells
(Fig. 1G and H). In order to
establish a synergistic mode of action between Huaier and
paclitaxel, we performed an isobolometric analysis. The results
revealed that the point was on the left. This suggested that the
combined action was supra-additive in both cell lines (Fig. 1I and J).
Huaier extract combined with
paclitaxel to induce apoptosis in MCF-7 and MDA-MB-231 cells
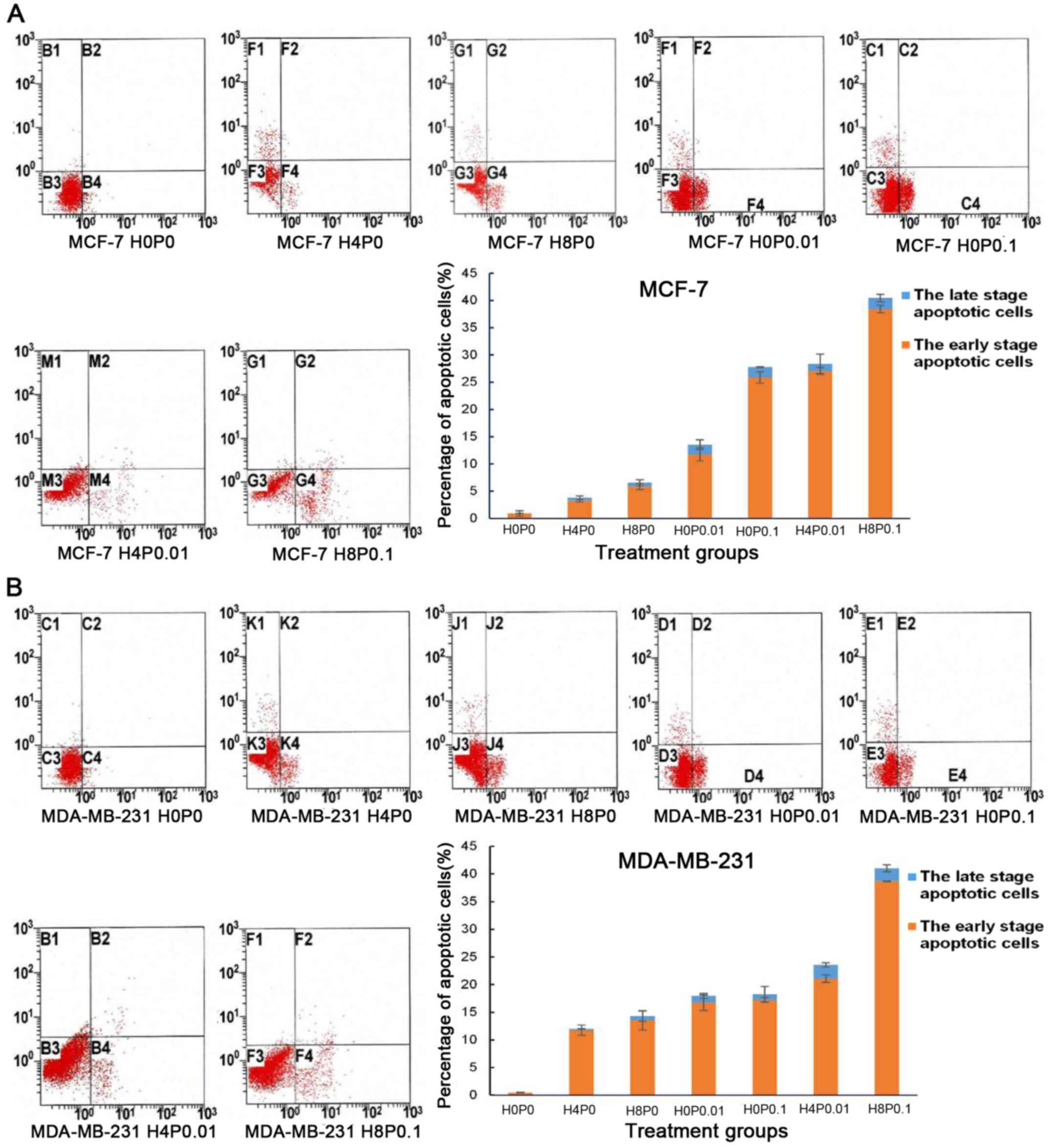
The four quadrants of the apoptosis results: upper
left represents cell debris, upper right represents late-stage
apoptotic, lower left represents living and lower right represents
early-stage apoptotic cells. Compared with the control group,
Huaier extract (H4P0 and H8P0) significantly increased cell
apoptosis in MCF-7 and MDA-MB-231 cells, and 8 mg/ml of Huaier
extract revealed a more significant effect on cell apoptosis than 4
mg/ml Huaier extract (Fig. 2A and
B; P<0.001). Similar to Huaier extract treatment, paclitaxel
treatment (H0P0.01 and H0P0.1) significantly induced cell apoptosis
in MCF-7 and MDA-MB-231 cells, and 0.1 µg/ml of paclitaxel
exhibited a more significant effect, particularly in the increase
of early-stage apoptosis (Fig. 2A and
B; P<0.001). The combined treatments of Huaier extract and
paclitaxel revealed a more significant effect on cell apoptosis
than the monotherapies (Fig. 2A and
B). Compared with paclitaxel treatment (H0P0.01 and H0P0.1)
alone, the apoptosis rate in the combined therapy group (H4P0.01
and H8P0.1) increased by 2.139 and 1.431 times, respectively, in
MCF-7 cells (P<0.001). Similarly, in MDA-MB-231 cells, the
apoptosis rate of the combined therapy group (H4P0.01 and H8P0.1)
increased by 1.131 (P<0.05) and 2.245 times (P<0.001),
respectively, compared with paclitaxel treatment alone (H0P0.01 and
H0P0.1).
Huaier extract synergizes with
paclitaxel to induce cell cycle arrest in MCF-7 and MDA-MB-231
cells
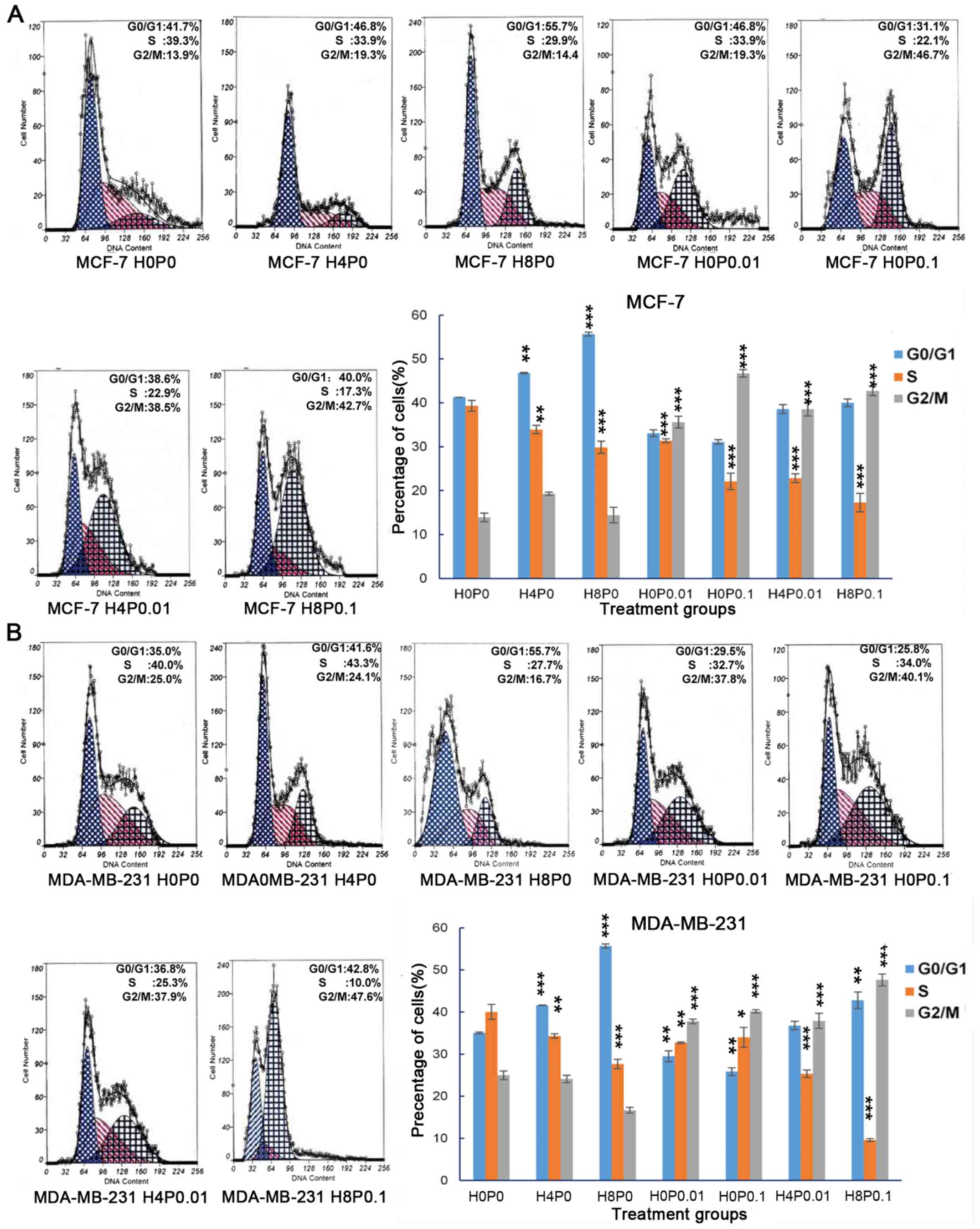
After being exposed to Huaier extract for 48 h, the
percentage of cells in the G0/G1 phase significantly increased in
both MCF-7 cells (from 41.268±0.11 to 55.704±0.45%; P<0.001) and
MDA-MB-231 cells (from 35.026±0.23 to 55.659±1.54%; P=0.001), while
the percentage of cells in the S phase was decreased (Fig. 3A and B). Moreover, a higher
concentration of Huaier extract (H8P0) exhibited a stronger effect
on cell cycle arrest in the G0/G1 phase in both cell lines compared
with the lower dose (H4P0). Notably, paclitaxel treatment (H0P0.01
and H0P0.1) also significantly induced cell cycle arrest in both
cell lines, but cell cycle arrest was observed in the G2/M phase
(Fig. 3A and B; P<0.001).
Similar to Huaier extract treatment, paclitaxel treatment reduced
the percentage of MCF-7 and MDA-MB-231 cells in the S phase
(P<0.001). Furthermore, we analyzed the cell cycle in MCF-7 and
MDA-MB-231 cells after combined therapies and found significant
arrest in both G0/G1 and G2/M phases while the proportion of S
phase was sharply decreased (P<0.001).
Huaier extract and paclitaxel target
p65, IκBα and c-Met in MCF-7 and MDA-MB-231 cells
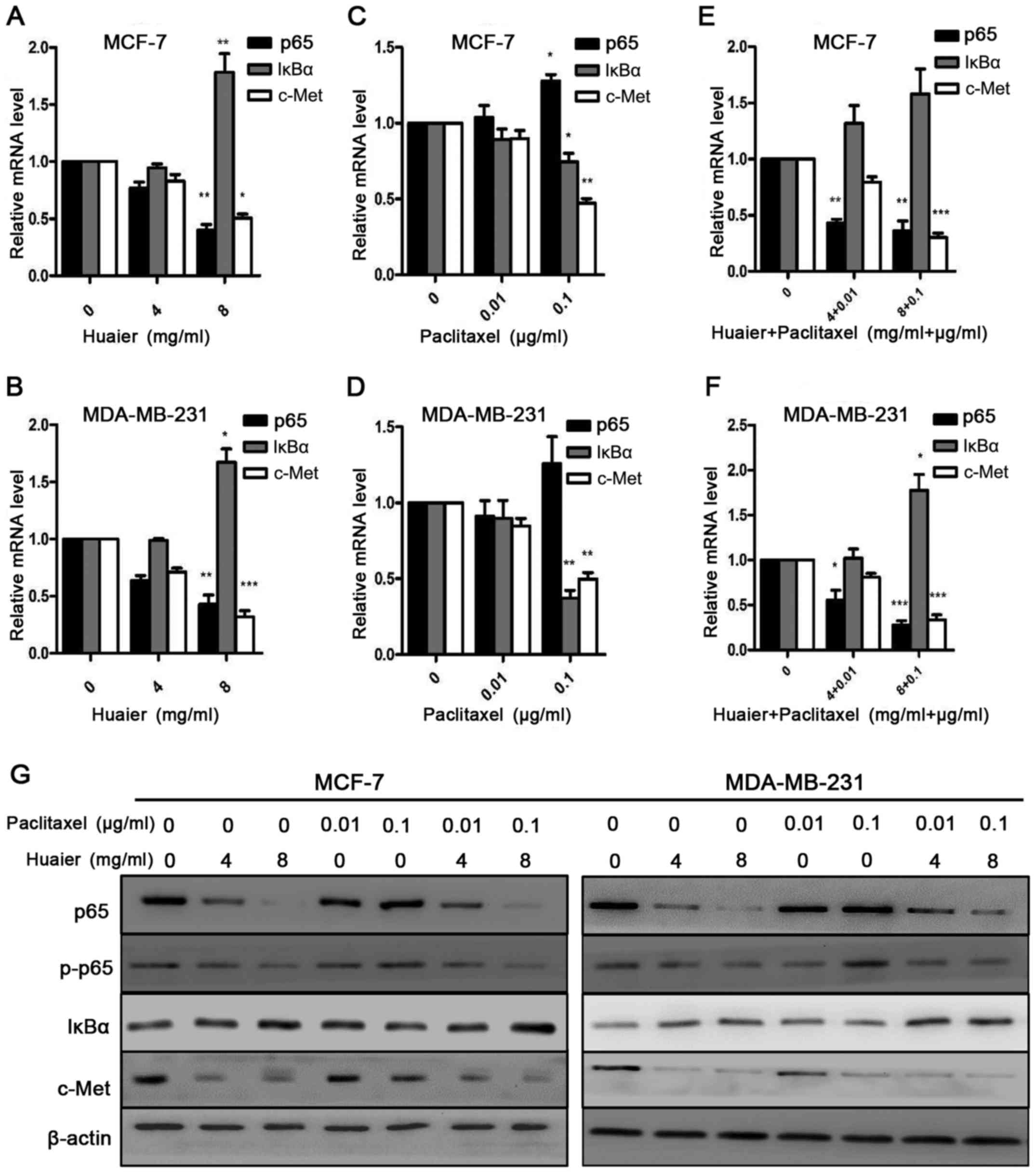
c-Met plays an important role in cell proliferation,
invasion and chemoresistance. The phosphorylation of c-Met
activates multiple downstream signaling pathways, including
Ras/MAPK, PI3K/Akt and NF-κB. Thus, we wanted to detect whether
Huaier and combined treatment mediated c-Met expression. As shown
in Fig. 4A and B and G, after being
treated with Huaier extract (H4P0 and H8P0) for 48 h, the
expression of p65 and c-Met at the mRNA and protein levels was
significantly decreased, while the expression of IκBα was
significantly increased after treatment with H8P0 for 48 h
(P<0.01). Therefore, Huaier extract suppressed the NF-κB/IκBα
pathway. In contrast to the Huaier extract treatment, paclitaxel
treatment (H0P0.01 and H0P0.1) for 48 h significantly increased the
expression of p65 at the mRNA and protein levels in MCF-7 and
MDA-MB-231 cells (P<0.05), while the expression of IκBα and
c-Met at the mRNA and protein levels was significantly reduced
(Fig. 4C, D and G). These results
implied that paclitaxel activates the NF-κB pathway. We also
examined the expression of p65, IκBα and c-Met in MCF-7 and
MDA-MB-231 cells after combined treatments with Huaier extract and
paclitaxel (Fig. 4E, F and G). The
combined treatments (H4 + P0.01 and H8 + P0.1) significantly
decreased the expression of p65 and c-Met at the mRNA and protein
levels, while the expression of IκBα mRNA and protein was
significantly increased in both cell lines after combined
treatments (P<0.05). These results were similar but more
significant than those of the Huaier extract treatment alone.
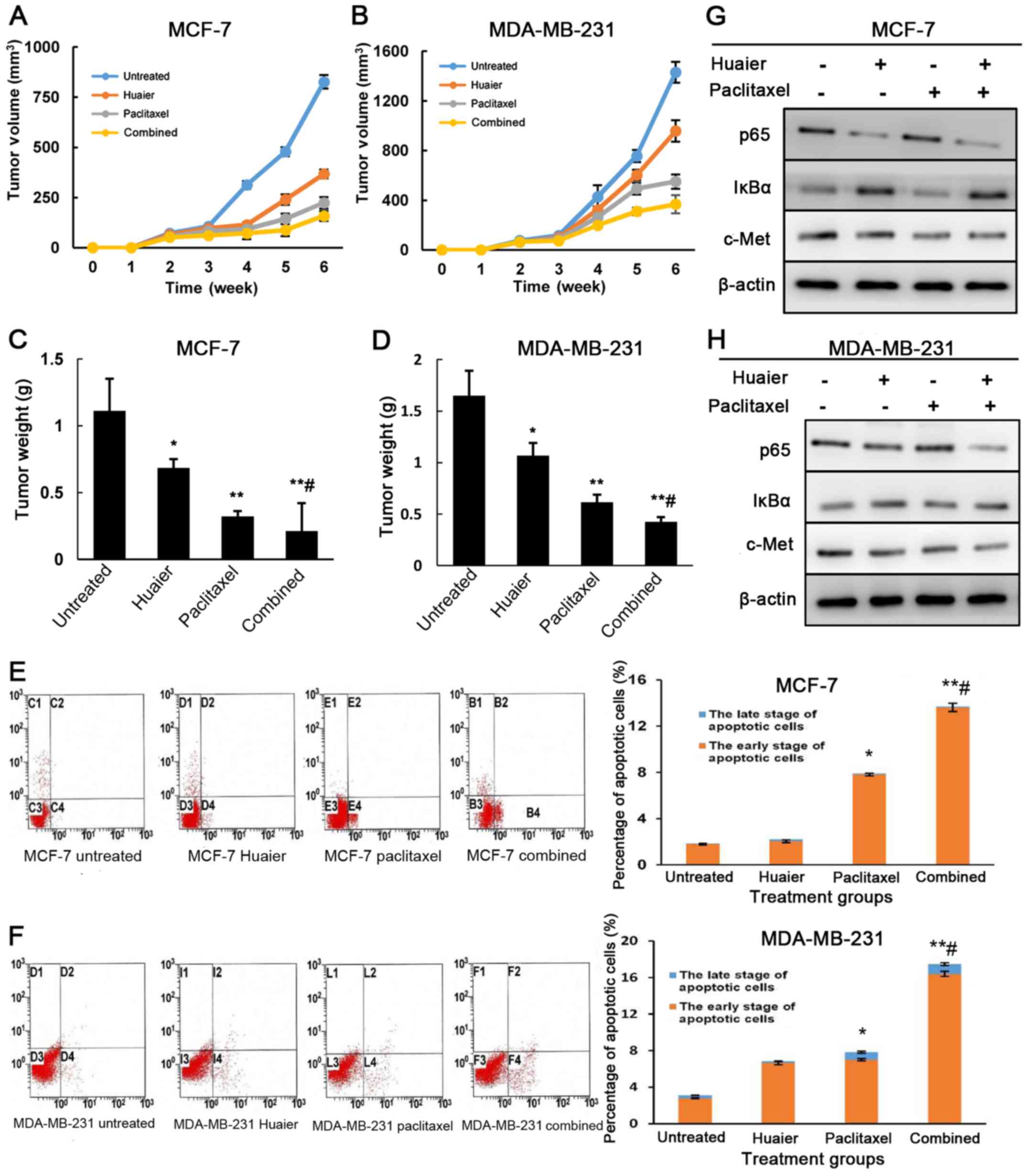
Combined Huaier extract and paclitaxel
treatments inhibit tumor growth in a xenograft model
To ascertain the inhibitory effect of Huaier extract
and paclitaxel on breast cancer cells, we examined the antitumor
effect of Huaier extract, paclitaxel and combined treatment in nude
mice. Compared with the control group (825.8±33.7 mm3),
the tumor volume in the Huaier (367.3±22.1 mm3;
P<0.05), paclitaxel (223.9±28.2 mm3; P<0.01) and
combined treatment group (158.1±25.2 mm3; P<0.01) was
significantly smaller in MCF-7 xenografted tumors (Fig. 5A). However, there was no significant
difference in the volume between the combined group and paclitaxel
group (P=0.144). Similar results were observed in the MDA-MB-231
xenografted tumors (Fig. 5B), but
the tumor volume in the combined group was smaller than that in the
paclitaxel group (P<0.05). After the mice were sacrificed, we
assessed the tumor weight (Fig. 5C and
D). The tumor weight in each group was consistent with that of
tumor volume in both cell lines (P<0.05). Moreover, compared
with the paclitaxel group, the tumor weight in the combined group
was lighter in both xenografted tumors (P<0.05). To examine the
antitumor mechanisms of the Huaier extract, paclitaxel and the
combined treatment in vivo, we analyzed the apoptosis and
the expression of p65 and IκBα in xenografted tumors. As shown in
Fig. 5E and F, the combined
treatments caused the most significant apoptosis compared to the
control and monotherapy groups. Furthermore, combined treatment
significantly decreased the expression of p65 and c-Met, but
increased IκBα expression in xenografted tumors compared with those
from monotherapies (Fig. 5G and
H).
Discussion
Breast cancer, as a highly heterogeneous tumor, is
significantly different in pathological type, molecular
classification and prognosis. Estrogen receptor-positive breast
cancer accounts for ~70% of breast cancer and triple-negative
breast cancer (TNBC) accounts for ~10–20% (28). Although ER-positive breast cancer
patients can benefit from endocrine therapy, chemotherapy is still
an important systemic therapy after surgery for preventing
recurrent and metastasis. Chemotherapy is the only treatment for
TNBC presently, since TNBC is invasive, recurs and metastasizes
easily and thus TNBC cannot benefit from endocrine and target
therapy.
Recently, traditional Chinese medicines (TCMs) have
been considered as anticancer drugs for their ability to kill
cancer cells, and reduce the side-effects of chemotherapy. Huaier
extract has been found to not only have an antitumor effect, but
also enhance the sensitivity of cancer cells to chemotherapy drugs
and improve immunity and long-term prognosis in cancer patients
(29,30). Bao et al (18) found that the Huaier extract
inhibited the proliferation of human hepatocellular carcinoma
Hep-G2 cells and induced cell apoptosis in a
concentration-dependent manner. In the present study, after
treating MCF-7 and MDA-MB-231 cells with Huaier extract for 24 and
48 h, we found that Huaier extract inhibited cell proliferation,
reduced cell viability and promoted cell apoptosis in a time- and
dose-dependent manner, results which were consistent with a study
from Zhang et al (14). We
also found that Huaier extract could induce cell cycle arrest in
the G0/G1 phase, which was in line with a study by Qi et al
(21). A previous study
demonstrated that paclitaxel treatment caused cell cycle arrest in
the G2/M phase, inhibited mitotic progression and induced apoptotic
cell death (31). In the present
study, we found that combined therapies of Huaier extract and
paclitaxel treatments in breast cancer cells induced cell cycle
arrest in the G0/G1 and G2/M phases, but reduced the percentage of
cells in the S phase. These results revealed that both Huaier
extract and paclitaxel treatments inhibited cell proliferation
activity and induced cell apoptosis in breast cancer cells, and the
combined therapies achieved the most effective efficacy.
Our research also studied the relationship between
the anticancer effect of paclitaxel and the NF-κB pathway. Current
studies have suggested that paclitaxel functions by binding to
tubulin, promoting the formation of stable microtubules and
inhibiting microtubule depolymerization. Thus, paclitaxel
interferes with the normal function of mitotic spindle formation,
arrests the cell cycle in the G2/M phase and induces cell death in
cancer (32). Our results revealed
that paclitaxel reduced IκBα expression, but increased p65
expression. As the inhibitory protein of NF-κB, the reduction of
IκBα expression was associated with NF-κB activation. Similar
results were also observed in a study in which paclitaxel induced
the degradation of the IκBα protein, which in turn activated NF-κB
and induced cell apoptosis (33).
Bava et al (34) reported
that paclitaxel treatment after 30 min may lead to the
translocation of NF-κB into the nucleus of HeLa cells. NF-κB
activation plays an important role in the resistance to cytotoxic
agents (such as doxorubicin), microtubule disrupting agents (such
as paclitaxel) and 5-fluorouracil (27,31).
Our data indicated that paclitaxel treatment for 48 h may induce
drug resistance via NF-κB activation.
In a previous study, the combination of Huaier
extract with chemotherapy drugs promoted tumor cell apoptosis and
improved sensitivity of tumor cells to chemotherapy drugs (15), but the mechanism is still not clear.
Zhang et al (35) reported
that Huaier increased the sensitivity of MCF-7/A breast cancer
cells to adriamycin (ADM) which was related to the decrease in the
expression of drug resistant gene MDR-1. Another study revealed
that Huaier extract synergized with tamoxifen (TAM) to increase
apoptosis and G0/G1 arrest in ER-positive breast cancer cells
(21). However, in the actively
proliferative cancer cells, the proportion of G0/G1 and S phase
cells was much higher than the proportion of G2/M phase cells.
Paclitaxel mainly induced cell cycle arrest in the G2/M phase,
while Huaier extract caused cell cycle arrest in the G0/G1 phase.
Therefore, paclitaxel and Huaier complemented each other and the
anticancer effect produced from their combination treatment was
significantly improved. Moreover, the inhibition of NF-κB (p65) was
more effective after combined therapies. A previous study suggested
that the inhibition of NF-κB can improve tumor cell sensitivity to
chemotherapy drugs (36). Our
results revealed that compared with monotherapies, the combined
therapies increased cell cycle arrest in the G0/G1 and G2/M phases,
more significantly promoted cell apoptosis, inhibited NF-κB and
enhanced antitumor efficacy.
The encoding product of proto-oncogene c-met
is the hepatocyte growth factor receptor (also known as c-Met),
which is a transmembrane tyrosine kinase. c-Met plays an important
role in cell proliferation, invasion and angiogenesis (37). In breast cancer tissue particularly
TNBC, c-Met was abnormally expressed (38). The phosphorylation of c-Met
activated multiple downstream signaling pathways, including
Ras/MAPK, PI3K/Akt and NF-κB (39).
Activation of the PI3K/Akt pathway increased cell survival and
inhibited apoptosis. Akt can regulate the NF-κB pathway. In a
future study we would like to further investigate the relationship
between drug resistance reversion by Huaier and c-Met/Akt/NF-κB. In
the present study, both the Huaier extract and paclitaxel inhibited
the expression of c-Met, and their combination in a high-dose group
revealed the most inhibitory effect on c-Met expression. These
results revealed that combined treatments may enhance the antitumor
effect of paclitaxel therapy in breast cancer cells.
Our results demonstrated that Huaier-induced
inhibition of the NF-κB pathway resulted in the suppression of cell
proliferation and the promotion of apoptosis. Paclitaxel-induced
NF-κB activation was associated with chemoresistance and a
decreased antitumor effect. Huaier reversed the effect of
paclitaxel on the activation of NF-κB, and thus, suppressed cell
proliferation and increased the antitumor effect. The NF-κB pathway
may play an important role in the combined treatment. In future,
further research into the molecular mechanisms and the proteins
involved in the cell cycle and proliferation as well as
resistance-associated genes regulated by NF-κB is warranted in
order to obtain more valuable insights.
In summary, Huaier extract synergized with
paclitaxel to suppress proliferation and increase apoptosis in
ER-positive breast cancer cells and TNBC. The mechanisms were
involved in inducing cell cycle arrest and cell apoptosis and
inhibition of the NF-κB pathway. Moreover, the combined treatments
of Hauier extract and paclitaxel were more effective on antitumor
activity than the monotherapies, which may increase the anticancer
effect of breast cancer cells to paclitaxel therapy.
Acknowledgements
The present study was supported by the Hebei
Province Natural Science Foundation (H2012206169).
References
|
1
|
Panieri E: Breast cancer screening in
developing countries. Best Pract Res Clin Obstet Gynaecol.
26:283–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan L, Zheng Y, Yu KD, Liu GY, Wu J, Lu
JS, Shen KW, Shen ZZ and Shao ZM: Breast cancer in a transitional
society over 18 years: Trends and present status in Shanghai,
China. Breast Cancer Res Treat. 117:409–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim YM, Tsoyi K, Jang HJ, Park EJ, Park
SW, Kim HJ, Hwa JS and Chang KC: CKD712, a synthetic isoquinoline
alkaloid, enhances the anti-cancer effects of paclitaxel in
MDA-MB-231 cells through regulation of PTEN. Life Sci. 112:49–58.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sonnenblick A, Eleyan F, Peretz T, Ospovat
I, Merimsky O, Sella T, Peylan-Ramu N and Katz D: Gemcitabine in
combination with paclitaxel for advanced soft-tissue sarcomas. Mol
Clin Oncol. 3:829–832. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wall ME, Wani MC and Taylor H: Plant
antitumor agents, 27. Isolation, structure, and structure activity
relationships of alkaloids from Fagara macrophylla. J Nat Prod.
50:1095–1099. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quispe-Soto ET and Calaf GM: Effect of
curcumin and paclitaxel on breast carcinogenesis. Int J Oncol.
49:2569–2577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wen G, Qu XX, Wang D, Chen XX, Tian XC,
Gao F and Zhou XL: Recent advances in design, synthesis and
bioactivity of paclitaxel-mimics. Fitoterapia. 110:26–37. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bharadwaj R and Yu H: The spindle
checkpoint, aneuploidy, and cancer. Oncogene. 23:2016–2027. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brito DA, Yang Z and Rieder CL:
Microtubules do not promote mitotic slippage when the spindle
assembly checkpoint cannot be satisfied. J Cell Biol. 182:623–629.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li LX, Ye SL, Wang YH and Tang ZZ:
Progress on experimental research and clinical application of
Trametes robiniophila. Bull Chin Cancer. 16:110–113.
2007.
|
|
12
|
Guo YW, Cheng PW, Chen YJ, et al: Studies
on the constituents of polysaccharide from the hyphae of
Trametes robiniophila (II) - identification of
polysaccharide from the hyphae of Trametes robiniophila and
determination of its molar ratio. J Chin Pharm U. 23:155–157.
1992.
|
|
13
|
Guo Y, Cheng P and Chen Y: Isolation and
analysis of the polysaccharide of Huaier mycelium. Chin J Biochem
Pharm. 63:56–59. 1993.
|
|
14
|
Zhang N, Kong X, Yan S, Yuan C and Yang Q:
Huaier aqueous extract inhibits proliferation of breast cancer
cells by inducing apoptosis. Cancer Sci. 101:2375–2383. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huo Q and Yang QF: Role of Huaier extract
as promising anticancer drug. Adaptive Med. 4:2076–944X. 2012.
|
|
16
|
Zhang T, Wang K, Zhang J, Wang X, Chen Z,
Ni C, Qiu F and Huang J: Huaier aqueous extract inhibits colorectal
cancer stem cell growth partially via downregulation of the
Wnt/β-catenin pathway. Oncol Lett. 5:1171–1176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng J, Li C, Wu X, Liu M, Sun X, Yang Y,
Hao M, Sheng S, Sun Y, Zhang H, et al: Astrocyte elevated gene-1
(AEG-1) shRNA sensitizes Huaier polysaccharide (HP)-induced
anti-metastatic potency via inactivating downstream P13K/Akt
pathway as well as augmenting cell-mediated immune response. Tumour
Biol. 35:4219–4224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bao H, Liu P, Jiang K, Zhang X, Xie L,
Wang Z and Gong P: Huaier polysaccharide induces apoptosis in
hepatocellular carcinoma cells through p38 MAPK. Oncol Lett.
12:1058–1066. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie HX, Xu ZY, Tang JN, Du YA, Huang L, Yu
PF and Cheng XD: Effect of Huaier on the proliferation and
apoptosis of human gastric cancer cells through modulation of the
PI3K/AKT signaling pathway. Exp Ther Med. 10:1212–1218. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Zhang N, Huo Q, Sun M, Lv S and
Yang Q: Huaier aqueous extract suppresses human breast cancer cell
proliferation through inhibition of estrogen receptor α signaling.
Int J Oncol. 43:321–328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qi W, Sun M, Kong X, Li Y, Wang X, Lv S,
Ding X, Gao S, Cun J, Cai C, et al: Huaier extract synergizes with
tamoxifen to induce autophagy and apoptosis in ER-positive breast
cancer cells. Oncotarget. 7:26003–26015. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sen R and Baltimore D: Multiple nuclear
factors interact with the immunoglobulin enhancer sequences. Cell.
46:705–716. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li F, Zhang J, Arfuso F, Chinnathambi A,
Zayed ME, Alharbi SA, Kumar AP, Ahn KS and Sethi G: NF-κB in cancer
therapy. Arch Toxicol. 89:711–731. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang W, Nag SA and Zhang R: Targeting the
NFκB signaling pathways for breast cancer prevention and therapy.
Curr Med Chem. 22:264–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao N, Wang R, Zhou L, Zhu Y, Gong J and
Zhuang SM: MicroRNA-26b suppresses the NF-κB signaling and enhances
the chemosensitivity of hepatocellular carcinoma cells by targeting
TAK1 and TAB3. Mol Cancer. 13:352014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pommier Y, Sordet O, Antony S, Hayward RL
and Kohn KW: Apoptosis defects and chemotherapy resistance:
Molecular interaction maps and networks. Oncogene. 23:2934–2949.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li F and Sethi G: Targeting transcription
factor NF-kappaB to overcome chemoresistance and radioresistance in
cancer therapy. Biochim Biophys Acta. 1805:167–180. 2010.PubMed/NCBI
|
|
28
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji D and Mai D: Effect of Huaier Granule
on immunity and quality of life in patient with gastric cancer
undergoing postoperative concurrent radiochemotherapy. China
Cancer. 19:73–76. 2010.
|
|
30
|
Song X, Li Y, Zhang H and Yang Q: The
anticancer effect of Huaier (Review). Oncol Rep. 34:12–21. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeong YJ, Kang JS, Lee SI, So DM, Yun J,
Baek JY, Kim SK, Lee K and Park SK: Breast cancer cells evade
paclitaxel-induced cell death by developing resistance to
dasatinib. Oncol Lett. 12:2153–2158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McGrogan BT, Gilmartin B, Carney DN and
McCann A: Taxanes, microtubules and chemoresistant breast cancer.
Biochim Biophys Acta. 1785:96–132. 2008.PubMed/NCBI
|
|
33
|
Huang Y, Johnson K, Norris J and Fan W:
NF-kB/IkB signaling pathways may contribute to the mediation of
paclitaxel-induced apoptosis in solid tumor cells. Cancer Res.
60:4426–4432. 2000.PubMed/NCBI
|
|
34
|
Bava SV, Sreekanth CN, Thulasidasan AK,
Anto NP, Cheriyan VT, Puliyappadamba VT, Menon SG, Ravichandran SD
and Anto RJ: Akt is upstream and MAPKs are downstream of NF-κB in
paclitaxel-induced survival signaling events, which are
down-regulated by curcumin contributing to their synergism. Int J
Biochem Cell Biol. 43:331–341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang YB, Zhang GQ, Wang JS and Zhang QF:
Function and clinical application of Huaier plaster in
comprehensive therapy of breast cancer. Chin J Clin Oncol Rehabil.
11:62004.
|
|
36
|
Uetsuka H, Haisa M, Kimura M, Gunduz M,
Kaneda Y, Ohkawa T, Takaoka M, Murata T, Nobuhisa T, Yamatsuji T,
et al: Inhibition of inducible NF-kappaB activity reduces
chemoresistance to 5-fluorouracil in human stomach cancer cell
line. Exp Cell Res. 289:27–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maroun CR and Rowlands T: The Met receptor
tyrosine kinase: A key player in oncogenesis and drug resistance.
Pharmacol Ther. 142:316–338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Raghav KP, Wang W, Liu S, Chavez-MacGregor
M, Meng X, Hortobagyi GN, Mills GB, Meric-Bernstam F, Blumenschein
GR Jr and Gonzalez-Angulo AM: cMET and phospho-cMET protein levels
in breast cancers and survival outcomes. Clin Cancer Res.
18:2269–2277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gherardi E, Birchmeier W, Birchmeier C and
Vande Woude G: Targeting MET in cancer: Rationale and progress. Nat
Rev Cancer. 12:89–103. 2012. View
Article : Google Scholar : PubMed/NCBI
|