Introduction
Gallbladder cancer (GBC) is a prevalent biliary
tract malignancy (1,2), and the seventh most common
gastrointestinal cancer worldwide (3,4). The
prognosis for GBC is extremely poor (5,6), and
GBC is usually diagnosed at a late stage due to the lack of
specific symptoms (7,8). The only curative therapy for GBC is
surgical resection (9). The
American College of Surgeons reported in 2010 that the 5-year
survival rate for stage IV GBC is ~4% (10), resulting from early infiltration by
lymphatic, perineural and hematogenous routes, as well as direct
invasion into the liver (11).
Thus, the identification of novel and promising agents for the
treatment of GBC is urgently needed.
Shikonin (Fig. 1) is
a compound isolated from the roots of Lithospermum
erythrorhizon, a traditional medicinal plant (12). Numerous studies have demonstrated
that shikonin exhibits antitumor effects such as inhibition of
cancer cell proliferation (13),
induction of apoptosis (14),
attenuation of invasion and migration (15), and inhibition of proteasome activity
(16), angiogenesis (17) and cancer cell glycolysis (18). However, no studies have been
conducted concerning the effects of shikonin on GBC cells and the
potential mechanisms involved. Therefore, the present study was
designed to investigate the effect of shikonin on GBC cells in
vitro and xenograft tumors in vivo, and explore the
underlying molecular mechanisms underlying the effects. The present
study may offer a promising agent for the treatment of GBC.
Materials and methods
Chemicals and reagents
Shikonin was obtained from the Shanghai Jinsui
Biotechnology Co., Ltd. (Shanghai, China). After being dissolved in
dimethyl sulfoxide (DMSO) at a stock solution (0.05 mol/l),
shikonin was stored at −20°C. Subsequent dilutions were conducted
with culture medium. An equal proportion of vehicle was added to
the control cells.
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT)
and Hoechst 33342 were obtained from Sigma Chemical Company (St.
Louis, MO, USA). An Annexin V/propidium iodide (PI) apoptosis kit
was obtained from Invitrogen (Carlsbad, CA, USA). Primary
antibodies against Bcl-2, Bax, cleaved caspase-9, cleaved
caspase-3, cleaved PARP, cyclin D1, CDK4, JNK, phosphorylated JNK
(p-JNK), GAPDH and secondary antibodies (goat anti-rabbit) were
obtained from Cell Signaling Technology (Danvers, MA, USA).
Cell lines and culture
NOZ and EHGB-1 (human GBC) cell lines were obtained
from the Shanghai Institute of Cell Biology, Chinese Academy of
Sciences and grown in high-glucose Dulbecco's modified Eagle's
medium (Gibco, Grand Island, NY, USA). The media contained 100 U/ml
penicillin (HyClone, Logan, UT, USA), and 10% fetal bovine serum
(FBS; Gibco) and 100 µg/ml streptomycin. The cells were grown at
37°C in an atmosphere with 5% CO2.
Cell viability assay
Cell viability was detected by the MTT assay. NOZ
and EHGB-1 cells (2×103/well) were seeded into 96-well
plates and incubated overnight. Then, the cells were treated with
shikonin at various concentrations of 0, 0.5, 1, 2, 3 and 4 µmol/l
for 24, 48 and 72 h. After treatment, 10 µl of MTT solution (5
mg/ml) was added to each well and incubation was carried out at
37°C for 4 h. The culture medium was then replaced with 100 µl of
DMSO. Absorbance of the solution at 490 nm was measured with a
microplate reader (BioTek, Winooski, VT, USA). The results
represent the average of three parallel samples.
Colony formation assay
NOZ and EHGB-1 cells were liquated as single cell
suspensions and 600 cells were seeded into each well of 6-well
plates. Cells were treated with shikonin (0, 0.1, 0.2 and 0.3
µmol/l for NOZ and EHGB-1), and cultured for ~14 days. The cells
were then fixed with 4% paraformaldehyde for 15 min, and stained
with 0.1% crystal violet (Sigma-Aldrich, St. Louis, MO, USA) for 30
min. The total number of colonies (>50 cells/colony) was
manually counted.
Cell apoptosis assay
Cells were seeded in 6-well plates and treated with
shikonin (0, 1, 2 and 3 µmol/l) for 48 h. After adherence, cells
were harvested and washed twice with cold phosphate-buffered saline
(PBS), then resuspended at a density of 1×106 cells/ml.
Next, 300 µl of binding buffer containing 5 µl of Annexin V-FITC
and 5 µl of PI (100 µg/ml) was added to the cells, followed by
incubation in the dark for 30 min. The samples were determined
using flow cytometry (BD Biosciences, San Diego, CA, USA).
Cell cycle analysis
NOZ and EHGB-1 cells were treated with shikonin in
different concentrations (0, 1, 2 and 3 µmol/l) for 48 h. Cells
were harvested, washed with cold PBS, and fixed in 70% ethanol
overnight. Then, the cells were washed, added with RNase and PI
(Sigma-Aldrich), and incubated in the dark for 30 min. The cells
were detected by flow cytometry (BD Biosciences). The percentages
of cells in each phase of the cell cycle were analyzed by CellQuest
acquisition software (BD Biosciences).
Hoechst 33342 staining
After treatment with shikonin (0, 1, 2 and 3 µmol/l)
for 48 h, the NOZ and EHGB-1 cells were washed in cold PBS and
added with methanol:acetic acid (3:1) for 10 min. The cells were
stained with Hoechst 33342 5 µg/ml for 10 min at 37°C and
subsequently observed with a fluorescence microscope (Leica
Biosystems, Wetzlar, Germany).
Western blot analysis
Cells were treated with shikonin (0, 1, 2 and 3
µmol/l) for 48 h, and then harvested, washed and lysed in RIPA
buffer (Beyotime Institute of Biotechnology, Beijing, China) and
protease inhibitor (Roche Applied Science, Indianapolis, IN, USA)
at 4°C for 5 min. The lysates were centrifugated at 14,000 × g for
5 min, the supernatant was collected, and the protein concentration
was detected by a bicinchoninic acid assay kit (Beyotime Institute
of Biotechnology). The proteins were loaded on a 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
nitrocellulose membranes (Millipore, Bedford, MA, USA). The
membranes were blocked with 5% skim milk, and incubated with
primary antibodies against Bcl-2, Bax, cleaved caspase-3, cleaved
caspase-9, cleaved PARP, cyclin D1, CDK4 and GAPDH overnight. The
membrane was washed with Tris-buffered saline with Tween-20 (TBST),
and then incubated with HRP-conjugated goat anti-rabbit secondary
antibodies (Abcam, Cambridge, UK) for 1.5 h. The bands were
detected with Gel Doc 2000 (Bio-Rad, Hercules, CA, USA).
In vivo tumor xenograft study
Four-week-old male athymic nude mice were purchased
from the Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai,
China). All procedures were approved by the Institutional Animal
Care and Use Committee of Shanghai Jiao Tong University. NOZ cells
were resuspended in PBS (1×106 cells in 0.2 ml), and
injected into the right flank of each mouse. Twenty-four hours
after inoculation, the mice were randomly divided into three groups
(5 mice/group). The control group was injected with vehicle (10%
DMSO and 90% PBS) and the others were injected with shikonin (1 or
3 mg/kg) every 2 days for up to 14 days. The body weight was
measured every 2 days. On day 15, the tumor tissues were removed
and weighed after sacrifice of the mice injected with lethal dose
of pentobarbital. The tumor volume (V) = 1/2 × length ×
width2.
Statistical analysis
All assays were performed three dependent times, and
data are expressed as means ± SD. The Student's t-test in GraphPad
Prism was applied to assess the difference between two groups
(GraphPad Software, San Diego, CA, USA). P<0.05 (*P<0.05,
**P<0.01, ***P<0.001) was considered to indicate a
statistically significant result.
Results
Shikonin suppresses proliferation and
colony forming of GBC cells
The cell proliferation was assessed by MTT assay. We
discovered an obvious reduction in the viability of
shikonin-treated cells (Fig. 2A).
The inhibitory concentration (IC)50 for NOZ and EHGB-1 cells was ~2
µmol/l at 48 h treat-ment. The ability of NOZ and EHGB-1 cells to
form colonies when treated with shikonin was demonstrated by the
colony formation assay (Fig. 2B).
The result suggested that shikonin exerted a negative influence on
colony formation ability. Furthermore, we found less amount of
clone formations in shikonin-treated groups than those in the
control groups (Fig. 2C). These
results indicated that shikonin obviously suppressed the
proliferation and colony forming of NOZ and EHGB-1 cells.
Shikonin induces
mitochondrial-dependent apoptosis via the JNK signaling pathway in
GBC cells
The impacts of shikonin on apoptosis in the NOZ and
EHGB-1 cells were detected using Annexin V/PI staining and flow
cytometry. As shown in Fig. 3A, the
percentage of surviving cells was reduced, whereas the percentage
of apoptotic cells was obviously increased (Fig. 3B).
To further confirm the cell apoptosis, we applied
Hoechst 33342 staining to examine the change in nuclear morphology.
The untreated cells were round with uniformity in chromatin
distribution, while vivid chromatin condensation and lobulated
nuclear fragmentation were revealed in cells treated with shikonin
(Fig. 3C). Furthermore, the
proportion of apoptotic nuclei that was obviously increased agreed
with the gradually increased shikonin concentration.
Proteins of the Bcl-2 and caspase family play major
roles in initiation and maintenance of mitochondrial apoptosis
(19). To further explore the
molecular mechanism underlying shikonin-mediated apoptosis, we
evaluated the quantitative levels of apoptosis-related proteins via
western blot analysis, including Bax, bcl-2, cleaved caspase-9,
cleaved caspase-3 and cleaved PARP. As shown in Fig. 4A, we observed increased expression
of Bax, cleaved caspase-9, cleaved caspase-3 and cleaved PARP and
downregulation of Bcl-2. Moreover, the Bcl-2/Bax ratio represents
apoptotic activity (20), as cell
apoptosis is promoted when the ratio decreases. In the present
study, we found a significant decrease in the ratio of Bcl-2/Bax in
the shikonin-treated cells (Fig.
4B). We also observed upregulation of p-JNK while no
significant change in the expression of JNK was noted.
Together, these results revealed that shikonin may
initiate mitochondrial-dependent apoptosis via the JNK signaling
pathway in GBC cells.
Shikonin triggers G0/G1 phase arrest
by regulating cell cycle-related proteins in GBC cells
The percentage of cells in each phase of the cell
cycle was determined via flow cytometry. We found an obvious
increase in the proportion of G0/G1 cells (Fig. 5B), indicating that shikonin
suppressed cell cycle progression in the NOZ and EHGB-1 cells
(Fig. 5A). We detected the
expression of cell cycle-related proteins cyclin D1 and CDK4, and
observed an obvious decrease in the NOZ and EHGB-1 cells (Fig. 5C). Therefore, shikonin may inhibit
GBC cell proliferation by triggering G0/G1 phase arrest.
Shikonin exhibits anticancer effects
in vivo
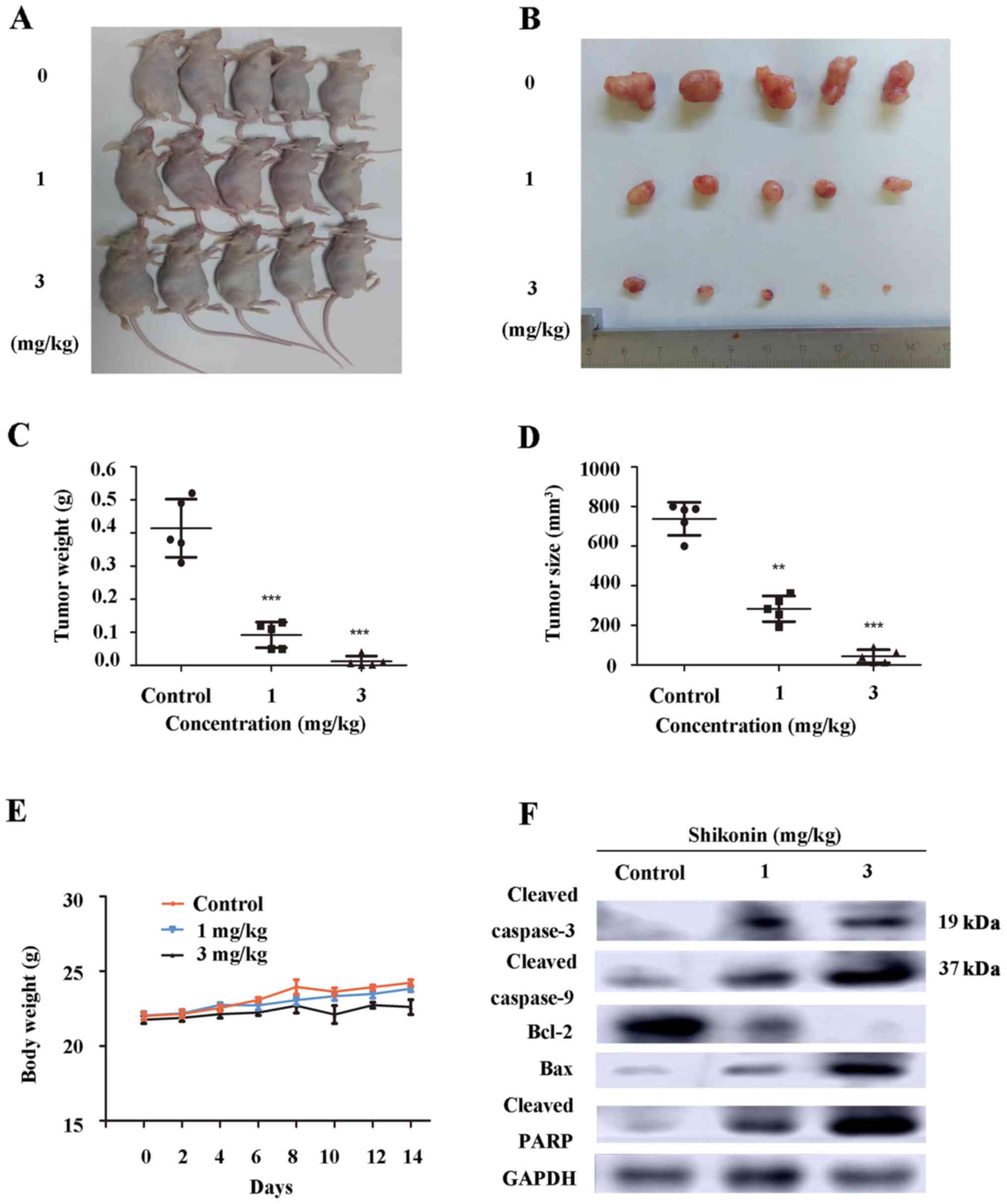
To further detect whether shikonin suppresses tumor
growth in vivo, nude mice with palpable tumor xenografts
were injected with vehicle (10% DMSO and 90% PBS) or shikonin (1
and 3 mg/kg) every other day. This dose was determined to be
effective (21,22) and a non-toxic level; LD50 of
shikonin in the mouse is 20 mg/kg when injected in an
intraperitoneal manner (23). After
injection, the bile and liver were previously found to contain the
highest levels of shikonin, and most of the excreted metabolite was
transformed (24). As shown in
Fig. 6A-D, the shikonin group
produced the smaller tumors when compared with the tumor volume of
the vehicle group. The results demonstrated that tumor growth was
obviously suppressed in the shikonin-treated mice in a
dose-dependent manner. Moreover, we found no significant difference
in regards to body weight between the vehicle and shikonin-treated
groups (Fig. 6E), suggesting that
shikonin had no side-effects in nude mice. As shown in Fig. 6F, we detected the upregulation of
Bax, cleaved caspase-9, cleaved caspase-3 and cleaved PARP and
downregulation of Bcl-2 in the tumor tissues by western blot
assay.
Discussion
Recently, various studies have shown that shikonin
acts as an anticancer agent. However, the effects of shikonin on
gallbladder cancer (GBC) cells required elucidation. In the present
study, we demonstrated that shikonin induced apoptosis and G0/G1
phase arrest in human GBC cells.
The cytotoxic effects of shikonin were assessed by
MTT and colony formation assays. We found that shikonin
significantly inhibited cell growth in a time- and dose-dependent
manner. To better understand the apoptotic effect of shikonin in
GBC cells, we performed flow cytometric analysis and Hoechst 33342
staining. The results showed that shikonin induced apoptosis in GBC
cells. To date, two pathways play crucial roles in cell apoptosis,
including the mitochondrial intrinsic pathway and the death
receptor-induced extrinsic pathway (25). In the present study, we demonstrated
the importance of the intrinsic pathway in shikonin-induced
apoptosis. Proteins in the Bcl-2 family are crucial regulators of
the mitochondrial-dependent apoptotic pathway (26). Increased expression levels of Bax
and reduced expression of Bcl-2, the dominant inhibitor of Bax,
were observed in the present study. Moreover, the ratio of
Bcl-2/Bax is a critical factor which determines the apoptosis
threshold (27). In the present
study, we detected that the Bcl-2/Bax ratio was significantly
decreased following treatment with shikonin. These results suggest
that shikonin induced apoptosis in the GBC cells.
We also detected the expression level of caspase and
cleaved PARP. Caspase-9 is activated in a mitochondrial-dependent
intrinsic pathway, which then mediates the activation of caspase-3
(28). Caspase-3 is an important
molecule to trigger the cleavage of downstream proteins, such as
PARP, in the caspase-dependent apoptosis pathway, eventually
leading to apoptosis (29). PARP
can be triggered in cells undergoing stress stimulus, resulting in
chromatin lysis and finally triggering apoptosis (30). In the present study, we observed an
obvious upregulation of cleaved caspase-3, and −9, and PARP.
Shikonin has been shown to induce cell apoptosis and
inhibit proliferation in dozens of cancers through various
signaling pathways, such as Erk (31), PI3K/AKT (32) and NF-κB signaling pathways (33). In the present study, we detected the
expression levels of JNK and p-JNK. JNK plays an essential role in
the intrinsic apoptotic pathway (34). After initially activated by
extracellular stimuli, JNK activates caspase-9 and inhibits the
anti-apoptotic protein Bcl-2 (35).
The data revealed upregulation of p-JNK and no obvious change in
JNK, suggesting that JNK was activated in the shikonin-treated GBC
cells. All in all, shikonin may trigger the apoptosis of GBC cells
via the JNK signaling pathway, subsequently enhancing cell
death.
Blockage of cell cycle progression has been
considered as an effective strategy for the treatment of human
malignancies (33). In the present
study, cell cycle analysis showed that the G0/G1 phase arrest of
GBC cells was induced by shikonin. The G0/G1 phase of the cell
cycle is regulated by cell cycle checkpoint proteins, such as
cycling D1 and CDK4 (36), which
was verified by the downregulation of CDK4 and cyclin D1 by western
blot analysis.
To further confirm the apoptotic effects of
shikonin, we established xenograft tumors in nude mice, and then
treated them with shikonin. According to the difference in tumor
volume, weight and apoptosis-related molecules in the tumors
removed from the sacrificed mice, the antitumor effect of shikonin
in vivo was verified. The insignificant change in body
weight among the three groups indicated the safety of shikonin in
the treatment of GBC.
In conclusion, the results demonstrated that
shikonin exhibited marked anticancer effects by inducing apoptosis
via the JNK signaling pathway and G0/G1 phase arrest. Moreover,
tumor growth was also inhibited in vivo with no
side-effects. Therefore, shikonin may be a novel and safe
chemotherapeutic agent for the treatment of GBC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 81372642).
References
|
1
|
Li Z, Yu X, Shen J, Law PT, Chan MT and Wu
WK: MicroRNA expression and its implications for diagnosis and
therapy of gallbladder cancer. Oncotarget. 6:13914–13921.
2015.PubMed/NCBI
|
|
2
|
Subbannayya T, Leal-Rojas P, Barbhuiya MA,
Raja R, Renuse S, Sathe G, Pinto SM, Syed N, Nanjappa V, Patil AH,
et al: Macrophage migration inhibitory factor - a therapeutic
target in gallbladder cancer. BMC Cancer. 15:8432015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shu YJ, Bao RF, Jiang L, Wang Z, Wang XA,
Zhang F, Liang HB, Li HF, Ye YY, Xiang SS, et al: MicroRNA-29c-5p
suppresses gallbladder carcinoma progression by directly targeting
CPEB4 and inhibiting the MAPK pathway. Cell Death Differ.
24:445–457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shu YJ, Weng H, Ye YY, Hu YP, Bao RF, Cao
Y, Wang XA, Zhang F, Xiang SS, Li HF, et al: SPOCK1 as a potential
cancer prognostic marker promotes the proliferation and metastasis
of gallbladder cancer cells by activating the PI3K/AKT pathway. Mol
Cancer. 14:122015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagaraja V and Eslick GD: Systematic
review with meta-analysis: The relationship between chronic
Salmonella typhi carrier status and gall-bladder cancer.
Aliment Pharmacol Ther. 39:745–750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carriaga MT and Henson DE: Liver,
gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 75
Suppl 1:S171–S190. 1995. View Article : Google Scholar
|
|
7
|
Jung W, Jang JY, Kang MJ, Chang YR, Shin
YC, Chang J and Kim SW: Effects of surgical methods and tumor
location on survival and recurrence patterns after curative
resection in patients with T2 gallbladder cancer. Gut Liver.
10:140–146. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li M, Zhang Z, Li X, Ye J, Wu X, Tan Z,
Liu C, Shen B, Wang XA, Wu W, et al: Whole-exome and targeted gene
sequencing of gallbladder carcinoma identifies recurrent mutations
in the ErbB pathway. Nat Genet. 46:872–876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao S, Cao Y, Liu SB, Wang XA, Bao RF,
Shu YJ, Hu YP, Zhang YJ, Jiang L, Zhang F, et al: The E545K
mutation of PIK3CA promotes gallbladder carcinoma progression
through enhanced binding to EGFR. JJ Exp Clin Cancer Res.
35:972016. View Article : Google Scholar
|
|
10
|
Marcano-Bonilla L, Mohamed EA, Mounajjed T
and Roberts LR: Biliary tract cancers: Epidemiology, molecular
pathogenesis and genetic risk associations. Linchuang Zhongliuxue
Zazhi. 5:612016.
|
|
11
|
Bao RF, Shu YJ, Hu YP, Wang XA, Zhang F,
Liang HB, Ye YY, Li HF, Xiang SS, Weng H, et al: miR-101 targeting
ZFX suppresses tumor proliferation and metastasis by regulating the
MAPK/Erk and Smad pathways in gallbladder carcinoma. Oncotarget.
7:22339–22354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang F, Chen Y, Duan W, Zhang C, Zhu H and
Ding J: SH-7, a new synthesized shikonin derivative, exerting its
potent antitumor activities as a topoisomerase inhibitor. Int J
Cancer. 119:1184–1193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh F, Gao D, Lebwohl MG and Wei H:
Shikonin modulates cell proliferation by inhibiting epidermal
growth factor receptor signaling in human epidermoid carcinoma
cells. Cancer Lett. 200:115–121. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu D, Qian J, Li W, Feng Q, Pan S and
Zhang S: β-hydroxyisovaleryl-shikonin induces human cervical cancer
cell apoptosis via PI3K/AKT/mTOR signaling. Oncol Lett.
10:3434–3442. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu JP, Liu D, Gu JF, Zhu MM and Cui L:
Shikonin inhibits the cell viability, adhesion, invasion and
migration of the human gastric cancer cell line MGC-803 via the
Toll-like receptor 2/nuclear factor-kappa B pathway. J Pharm
Pharmacol. 67:1143–1155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang H, Zhou P, Huang H, Chen D, Ma N, Cui
QC, Shen S, Dong W, Zhang X, Lian W, et al: Shikonin exerts
antitumor activity via proteasome inhibition and cell death
induction in vitro and in vivo. Int J Cancer. 124:2450–2459. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Komi Y, Suzuki Y, Shimamura M, Kajimoto S,
Nakajo S, Masuda M, Shibuya M, Itabe H, Shimokado K, Oettgen P, et
al: Mechanism of inhibition of tumor angiogenesis by
beta-hydroxyisovalerylshikonin. Cancer Sci. 100:269–277. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen J, Xie J, Jiang Z, Wang B, Wang Y and
Hu X: Shikonin and its analogs inhibit cancer cell glycolysis by
targeting tumor pyruvate kinase-M2. Oncogene. 30:4297–4306. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He G, He G, Zhou R, Pi Z, Zhu T, Jiang L
and Xie Y: Enhancement of cisplatin-induced colon cancer cells
apoptosis by shikonin, a natural inducer of ROS in vitro and in
vivo. Biochem Biophys Res Commun. 469:1075–1082. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu T, Li R, Zhao H, Deng J, Long Y, Shuai
MT, Li Q, Gu H, Chen YQ and Leng AM: eIF4E promotes tumorigenesis
and modulates chemosensitivity to cisplatin in esophageal squamous
cell carcinoma. Oncotarget. 7:66851–66864. 2016.PubMed/NCBI
|
|
21
|
Jeung YJ, Kim HG, Ahn J, Lee HJ, Lee SB,
Won M, Jung CR, Im JY, Kim BK, Park SK, et al: Shikonin induces
apoptosis of lung cancer cells via activation of FOXO3a/EGR1/SIRT1
signaling antagonized by p300. Biochim Biophys Acta.
1863:2584–2593. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Zhou Y, Jia G, Han B, Liu J, Teng
Y, Lv J, Song Z, Li Y, Ji L, et al: Shikonin suppresses tumor
growth and synergizes with gemcitabine in a pancreatic cancer
xenograft model: Involvement of NF-κB signaling pathway. Biochem
Pharmacol. 88:322–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hayashi M: Pharmacological studies of
Shikon and Tooki. (2) Pharmacological effects of the pigment
components, Shikonin and acetylshikonin. Nihon Yakurigaku Zasshi.
73:193–203. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen X, Yang L, Oppenheim JJ and Howard
MZ: Cellular pharmacology studies of shikonin derivatives.
Phytother Res. 16:199–209. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Call JA, Eckhardt SG and Camidge DR:
Targeted manipulation of apoptosis in cancer treatment. Lancet
Oncol. 9:1002–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Min R, Tong J, Wenjun Y, Wenhu D, Xiaojian
Z, Jiacai H, Jian Z, Wantao C and Chenping Z: Growth inhibition and
induction of apoptosis in human oral squamous cell carcinoma
Tca-8113 cell lines by Shikonin was partly through the inactivation
of NF-kappaB pathway. Phytother Res. 22:407–415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang W, Guo Q, You Q, Zhang K, Yang Y, Yu
J, Liu W, Zhao L, Gu H, Hu Y, et al: Involvement of bax/bcl-2 in
wogonin-induced apoptosis of human hepatoma cell line SMMC-7721.
Anticancer Drugs. 17:797–805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hill MM, Adrain C and Martin SJ: Portrait
of a killer: The mitochondrial apoptosome emerges from the shadows.
Mol Interv. 3:19–26. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nuñez G, Benedict MA, Hu Y and Inohara N:
Caspases: The proteases of the apoptotic pathway. Oncogene.
17:3237–3245. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, An R, Umanah GK, Park H, Nambiar
K, Eacker SM, Kim B, Bao L, Harraz MM, Chang C, et al: A nuclease
that mediates cell death induced by DNA damage and poly(ADP-ribose)
polymerase-1. Science. 354:3542016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jing H, Sun W, Fan J, Zhang Y, Yang J, Jia
J, Li J, Guo J, Luo S and Zheng Y: Shikonin induces apoptosis of
HaCaT cells via the mitochondrial, Erk and Akt pathways. Mol Med
Rep. 13:3009–3016. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang FY, Hu Y, Que ZY, Wang P, Liu YH,
Wang ZH and Xue YX: Shikonin inhibits the migration and invasion of
human glioblastoma cells by targeting phosphorylated β-catenin and
phosphorylated PI3K/Akt: A potential mechanism for the anti-glioma
efficacy of a Traditional Chinese Herbal Medicine. Int J Mol Sci.
16:23823–23848. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tian R, Li Y and Gao M: Shikonin causes
cell-cycle arrest and induces apoptosis by regulating the
EGFR-NF-κB signalling pathway in human epidermoid carcinoma A431
cells. Biosci Rep. 35:352015. View Article : Google Scholar
|
|
34
|
Schroeter H, Boyd CS, Ahmed R, Spencer JP,
Duncan RF, Rice-Evans C and Cadenas E: c-Jun N-terminal kinase
(JNK)-mediated modulation of brain mitochondria function: New
target proteins for JNK signalling in mitochondrion-dependent
apoptosis. Biochem J. 372:359–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakamura Y: Chemoprevention by
isothiocyanates: Molecular basis of apoptosis induction. Forum
Nutr. 61:170–181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakanishi M, Shimada M and Niida H:
Genetic instability in cancer cells by impaired cell cycle
checkpoints. Cancer Sci. 97:984–989. 2006. View Article : Google Scholar : PubMed/NCBI
|