Introduction
Epidermal growth factor receptor (EGFR), a tyrosine
kinase receptor of ErbB family, overexpressing on the plasma
membrane of most human malignant tumor cells, including those of
the lung, head and neck, and pancreas, is regarded as a promising
and validated molecular target in cancer drug therapy (1,2). A
serial of anti-EGFR agents including small-molecule kinase
inhibitors and monoclonal antibodies have been applied for
preclinical or clinical therapeutics for carcinomas in the form of
drug combination (3,4). For example, 43.7% of patients with
non-small cell lung cancers treated with gefitinib as first-line
therapy and platinum-based chemotherapy as second-line therapy were
documented stable disease in NEJ002 (5). The combined application of erlotinib
and bevacizumab remarkably promoted the proportion of 16-week
progression-free survival (PFS16), the median PFS event and the
median overall survival in advanced hepatocellular carcinomas
(6). Afatinib-cetuximab
demonstrated excellent clinical efficacy and manageable safety
profile in heavily pretreated patients in advanced EGFR-mutant lung
cancer with acquired resistance to erlotinib/gefitinib (7).
CD13 (aminopeptidase N, APN) is a glycoprotein that
has been observed on the plasma membrane of vascular endothelial
cells and tumor cells (8–10). CD13 boosts invasion of tumor cells
through degradation of extracellular matrix and promotion of
angiogenesis by promoting vascular endothelial cell invasion and
adjusting related growth factors and cytokines (11,12).
Due to its special role in tumor progression, CD13 is an attractive
target for the modulation of tumor microenvironment. Therefore,
integration of EGFR and CD13 in cancer targeted therapy, the
EGFR/CD13 bi-targeting strategy, might be of interest, as it acts
on both tumor cells and tumor microenvironment. When EGFR and CD13
were considered together in the design of a recombinant fusion
protein, the bi-targeting strategy can be realized by exerting
potent antitumor efficacy through multiple biological modulations
in cancer cells and surrounding microenvironment simultaneously
with a single agent.
In our previous study, an EGFR/CD13 bi-targeted
fusion protein ER(Fv)-LDP-NGR composed of a single chain Fv (scFv)
targeting EGFR, a tri-cyclic NGR motif against CD13 and an
apoprotein LDP serving as ‘scaffold’ had been prepared by DNA
recombination. ER(Fv)-LDP-NGR at the dose of 10 mg/kg markedly
inhibited the growth of MCF-7 xenografts in athymic mice (13). In addition, the enediyne-energized
analogue of this fusion protein showed highly potent cytotoxicity
to cancer cells. However, in the previous study, the efficacy of
the non-enediyne-energized fusion protein ER(Fv)-LDP-NGR was not
evaluated. The present study was set to investigate the mechanism
of action of the EGFR/CD13 bi-targeted fusion protein
ER(Fv)-LDP-NGR, including its effect on signaling pathways,
induction of apoptosis and cell cycle arrest, inhibition of
migration and invasion, and suppression of tube formation.
Accordingly, the effects of ER(Fv)-LDP-NGR on tumor progression
were evaluated and discussed.
Materials and methods
Cell culture
Human breast cancer MCF-7 cells and lung carcinoma
A549 cells were cultured in RPMI-1640-Glutamax-I medium (Invitrogen
Life Technologies, Carlsbad, CA, USA), containing 10% fetal bovine
serum (Gibco/BRL, New York, NY, USA) and penicillin and
streptomycin at 100 U/ml and 100 µg/ml respectively. Human
microvascular endothelial cell HMEC-1 was grown in DMEM-Glutamax-I
medium (Invitrogen Life Technologies, Carlsbad, CA, USA), including
20% fetal bovine serum, 100 U/ml of penicillin and 100 µg/ml
streptomycin.
Protein extraction and western blot
analysis
The expression of target proteins and the influence
of ER(Fv)-LDP-NGR on cell signaling pathway including EGFR
signaling pathway, cell cycle pathway and apoptotic pathway were
analyzed by western blotting. Tumor cells were lysed in cell lysis
buffer (Beyotime Institute of Biotechnology, Haimen, China)
supplemented with 1 mM phenylmethylsulfonyl fluoride. Cellular
proteins was clarified by high-speed centrifugation at 4°C, and
quantified with BCA protein assay kit (Thermo Fisher Scientific,
Bremen, Germany). For each sample, 50 µg of protein were applied in
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and
electrophoretically transferred onto polyvinylidene difluoride
(PVDF) membranes (Merck Millipore, Belford, MA, USA). After being
blocked with TBST (5% skim milk, 20 mM Tris-HCl, 150 mM NaCl and
0.1% Tween-20), the membranes were incubated with the primary
antibodies (Cell Signaling Technology, Boston, MA, USA), and then
with the HRP-conjugated secondary antibodies (Zhongshan Golden
Bridge Biotechnology, Beijing, China). The specific bands were
visualized with Immobilon Western Chemiluminescent HRP Substrate
(Merck Millipore).
Cell proliferation assay
MTT assay was employed to examine proliferation
activity of tumor cells. MCF-7, A549 and HMEC-1 cells were plated
on 96-well plates at a density of 5,000 cells/well and placed at
37°C in 5% CO2 overnight for adhesion, then were
incubated with different concentrations of fusion protein
ER(Fv)-LDP-NGR for another 48 h. After incubation with 20 µl of MTT
(5 mg/ml) for 4 h, the culture supernatant was removed, and 150 µl
of DMSO was added into the wells. The cell plates were read at 570
nm with a microplate reader (Thermo Fisher Scientific), then the
survival rate and the 50% inhibitory concentration
(IC50) was calculated.
Determination of EGFR and CD13 mRNA
levels
Reverse transcription-polymerase chain reaction
(RT-PCR) was applied to assess mRNA levels of targeted protein in
tumor cells. MCF-7, A549 and HMEC-1 cells were incubated in
complete medium with 0.1 or 1 µM ER(Fv)-LDP-NGR for 48 h. Total RNA
of tumor cells was isolated with TRIzol reagent (Takara Bio Inc,
Dalian, China), and mRNA was purified with PolyATract mRNA
Isolation system (Promega Corp., Madison, WI, USA). cDNA was
synthesized by reverse transcription and EGFR, CD13 and
β-actin gene fragments were further amplified by polymerase
chain reaction using the primers EGFR (sense,
5′-TCCCCGTAATTATGTGGTGACAGATC-3′; antisense,
5′-ACCCCTAAATGCCACCGGC-3′), CD13 (sense, 5′-CCTTCAACCTGGCCAGTGC-3′;
antisense, 5′-CGTCTTCTCCAGGGCTTGCTCCAG-3′) and β-actin (sense,
5′-CTGGGACGACATGGAGAAAA-3′; antisense, 5′-AAGGAAGGCTGGAAGAGTGC-3′)
(Invitrogen Life Technologies, Shanghai, China), respectively. The
amplified DNA fragments were separated in 1% agarose gel with
GoodView nucleic acid stain (SBS Genetech Corp., Beijing, China)
and visualized under UV.
Cell cycle analysis
Cell cycle of MCF-7 and A549 cells treated with
ER(Fv)-LDP-NGR was detected by flow cytometry (Becton-Dickinson and
Co., Franklin Lakes, NJ, USA). The cells treated with 0.1 or 1 µM
ER(Fv)-LDP-NGR for 48 h were collected and fixed with 70% ethanol,
and then resuspended in dye containing 100 µg/ml of RNaseA and 25
µg/ml of propidium iodide (PI). Cell cycle analysis was performed
following a light proof dyeing at room temperature.
Apoptosis analysis
The effect of fusion protein on apoptosis of tumor
cells was examined by Hoechst 33385 staining and Annexin V-FITC/PI
analysis, respectively. MCF-7 and A549 cells seeded on coverslips
were incubated with 1 µM ER(Fv)-LDP-NGR for 48 h. Fixed with 4%
paraformaldehyde at room temperature, the cells were dyed with
Hoechst 33385 (Sigma-Aldrich, Santa Clara, CA, USA) for 10 min,
then were observed under a fluorescence microscope with ×200
magnification and recorded by a camera (Nikon Corp., Tokyo,
Japan).
For Annexin V-FITC/PI analysis, tumor cells with
ER(Fv)-LDP-NGR treatment were collected at 50,000 cells/tube
density. After washing three times with cold PBS, the cell pellets
were resuspended with binding buffer, followed by staining with
Annexin V-FITC/PI (Biosea Biotechnology, Beijing, China). The
fluorescence was determined by flow cytometry in an hour.
In vitro cell migration and cell
invasion assay
Transwell permeable supports (Corning Inc., Corning,
NY, USA) with polyester filter membrane were used to assess
migration and invasion capacity of tumor cells. The Transwell
compartments, 24-well format, with 8 µm pore size insert, were
incubated with RPMI-1640 medium for an initial equilibrium.
RPMI-1640 (0.1 ml) with 2% FBS containing 1×105 tumor
cells and 0.6 ml of RPMI-1640 with 20% FBS were added into the
wells and the Transwell inserts, respectively. After ER(Fv)-LDP-NGR
was added, the cells were incubated in the Transwell plates at
37°C, 5% CO2 for 24 h, which enabled the cells to
migrate toward the underside of the insert filters. The inserts
were carefully taken out and cells were fixed with methanol for 3
min and stained with 0.1% crystal violet for 30 min. Cells
remaining on the upper side of filter membrane were gently removed
with a cotton swab after washing the insert with PBS, then the
inserts were observed under a microscope and recorded by a camera.
The membrane was cut and immersed into 33% acetic acid to dissolve
crystal violet. The absorbance at 570 nm was read with a microplate
reader, and the relative migration of tumor cells was
calculated.
Transwell permeable supports were coated with
pre-cooled Matrigel (Becton-Dickinson and Co.) before cell invasion
assay. Matrigel melted at 4°C was added into the Transwell inserts
and incubated at 37°C for polymerization on filter membrane. The
subsequent experimental operation manipulation and calculation of
the relative invasion were carried out as cell migration assay.
Tube formation assay
Tube formation is a fast and easy assay to examine
the angiogenic/anti-angiogenic properties of drugs, which can be
conveniently performed in any cell culture lab. Pre-cooled Matrigel
was transferred to a 48-well plate and incubated at 37°C for
solidification. HMEC-1 cells (4×105) were counted and
suspended in 200 µl culture media with fusion protein, then were
transferred to the 48-well plate. After incubation at 37°C, 5%
CO2 for 48 h, the number of endothelial tubes and their
size were observed under a microscope and photographed. Tube
formation was quantified by measuring tubes per well using
Image-Pro Plus software.
Statistical analysis
The data are presented as the mean ± standard
deviation (SD). Statistical comparisons between groups were
analyzed by Student's t-test, and a significant difference was set
at P<0.05.
Results
Expression of EGFR and CD13 in tumor
cells and microvascular endothelial cells
The expression of the target proteins EGFR and CD13
in MCF-7, A549 and HMEC-1 cells was analyzed by western blotting.
Overexpression of EGFR and CD13 was observed in both tumor cells
and microvascular endothelial cells, while CD13 isoform in HMEC-1
cells replaced CD13 in MCF-7 and A549 cells (Fig. 1).
Effect of the bispecific fusion
protein on cell proliferation
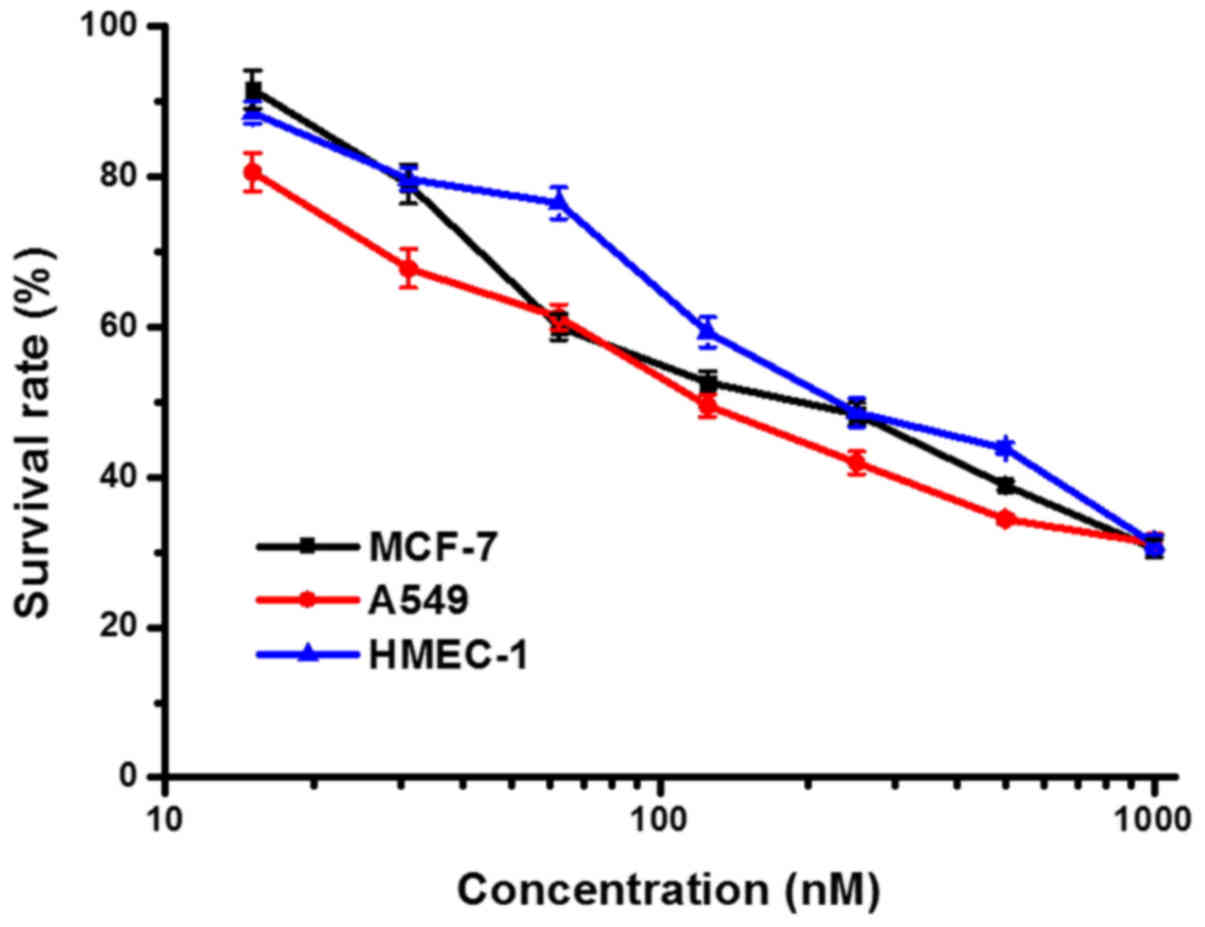
The anti-proliferative activity of ER(Fv)-LDP-NGR
was measured by MTT assay. As shown in Fig. 2, ER(Fv)-LDP-NGR at micromolar
concentration could inhibit the proliferation of tumor cells and
microvascular endothelial cells with IC50 values of
10−7-10−6 M.
Downregulation of the mRNA and protein
levels of EGFR and CD13
The regulation of EGFR and CD13 gene
transcription and expression was assessed by RT-PCR and western
blotting, respectively. As Fig. 3
shows, ER(Fv)-LDP-NGR caused a dose-dependent decrease in mRNA and
protein levels in both tumor cells and microvascular endothelial
cells, while no obvious change of β-actin was observed.
Regulation of signal transduction
pathways
The regulations of ER(Fv)-LDP-NGR on EGFR, cell
cycle and apoptosis signaling pathways in tumor cells were analyzed
by western blotting (Fig. 4). For
EGFR-positive MCF-7 cells, ER(Fv)-LDP-NGR dose-dependently
downregulated the expression level of EGFR and its phosphorylation.
In addition, the phosphorylation of corresponding protein kinases,
including Akt, Raf, ERK, MAPK, was accordingly reduced, except
SAPK/JNK, which could be induced by cellular stress response.
ER(Fv)-LDP-NGR upregulated the phosphorylation of
ATM/ATR kinase, which was followed by activation of checkpoint
kinases (Chk1 and Chk2) and p53, and then downregulation of
Cdc2/Cyclin B1 complexes. No obvious decrease was observed in
CDK2/Cyclin A, which are associated with S phase.
As shown in Fig. 4C,
a slight increased apoptosis induction of ER(Fv)-LDP-NGR resulted
from the reverse effects of intrinsic apoptotic pathway and
extrinsic apoptotic pathway. Although ER(Fv)-LDP-NGR inhibited the
extrinsic pathway that presented as decrease of caspase-10, it
activated the intrinsic pathway characterized by the
permeabilisation of the mitochondria and release of cytochrome
c into the cytoplasm. Cellular stress response and DNA
damage activated proapoptotic protein Bax, which subsequently
resulted in the activation of a series of cysteinyl aspartate
specific proteinases (the initiator caspase-9 and the executioner
protein caspase-3, and −7) and PARP cleavage. The hydrolysis of
protein finally led to DNA fragmentation, cell shrinkage, and
membrane bleb formation.
Effect of the bispecific fusion
protein on cell cycle progression
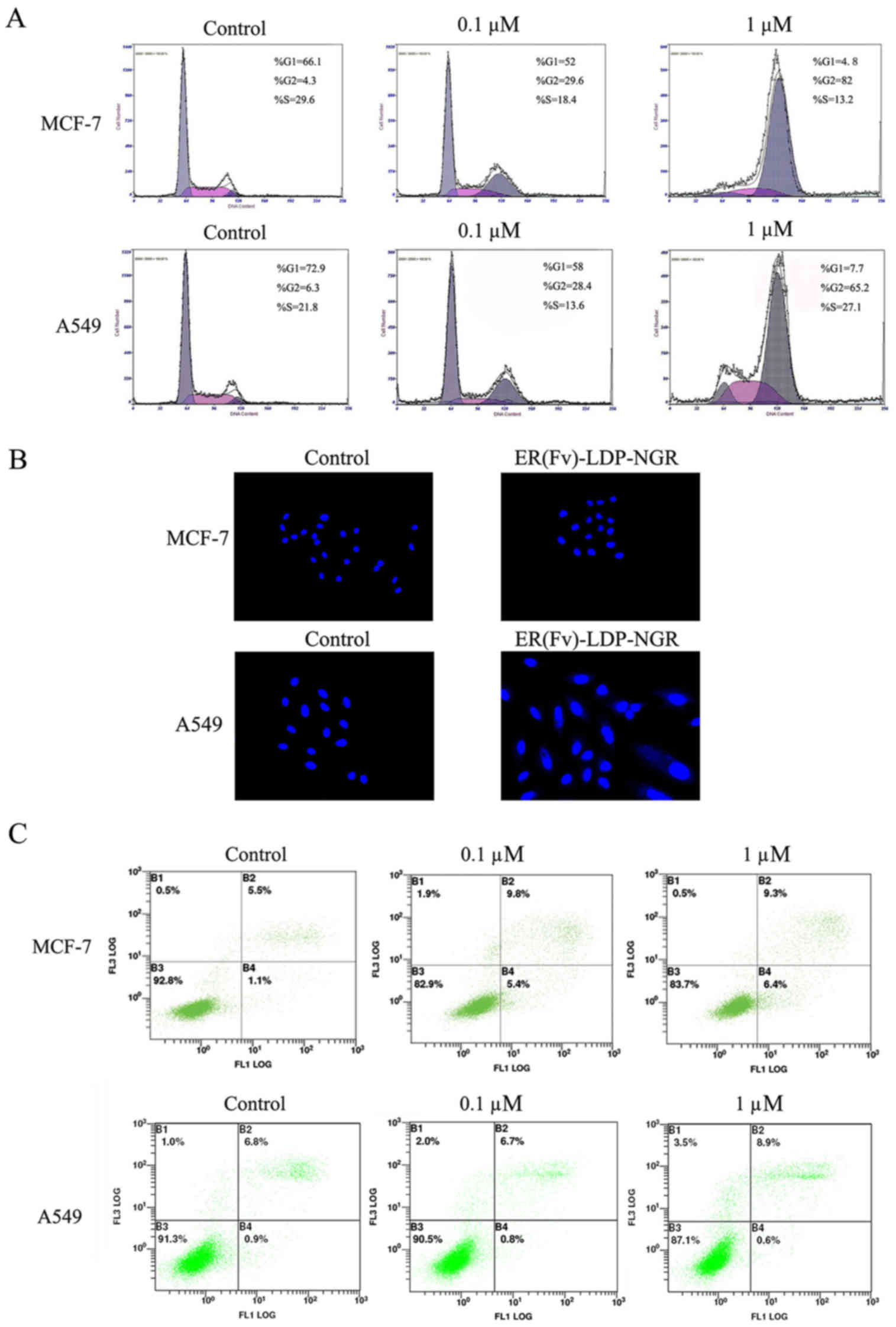
The cell cycle distribution of tumor cells treated
with ER(Fv)-LDP-NGR was detected by measuring PI fluorescence with
flow cytometry. ER(Fv)-LDP-NGR caused G2/M phase arrest of tumor
cells, and the extent of arrest was concentration-dependent.
Treated with 1 µM ER(Fv)-LDP-NGR, 82% of MCF-7 cells and 65.2% of
A549 cells were, respectively blocked at G2 phase, and a decrease
of proportion MCF-7 cells in S phase was observed (Fig. 5A).
Induction of apoptosis by the
bispecific fusion protein
As the result of Hoechst 33385 (Fig. 5B) and Annexin V-FITC/PI staining
(Fig. 5C), higher concentrations of
ER(Fv)-LDP-NGR induced early and late apoptosis in tumor cells.
Compared with the control, a part of MCF-7 and A549 cells treated
with 1 µM ER(Fv)-LDP-NGR showed enlarged cells with a single giant
nucleus and condensed nuclei.
Inhibition of migration and invasion
by the bispecific fusion protein
Transwell assay was applied to assess the migration
and invasion capacity of tumor cells. ER(Fv)-LDP-NGR at 1 µM
inhibited tumor cells migrating from the upper surface of polyester
filter membrane to the lower surface, with an inhibitory rate of
33.7% in MCF-7 cells and 32.8% in A549 cells, respectively
(Fig. 6A and B). ER(Fv)-LDP-NGR
also suppressed the invasion capacity of tumor cells, and reduced
the number of cells passing through Matrigel. Only 58.0% of MCF-7
cells and 56.3% of A549 cells incubated with ER(Fv)-LDP-NGR could
reach the lower surface of filter membrane (Fig. 6C and D).
Effects of the bispecific fusion
protein on microvascular endothelial cells
Tube formation assay, a classical angiogenesis assay
in vitro, was employed to detect the anti-angiogenesis
activity of the fusion protein ER(Fv)-LDP-NGR. As shown in Fig. 7, HMEC-1 cells normally aligned and
formed tube-like structures, while a decrease in the number of
tubes and branch points was observed following treatment with the
fusion protein, indicating that ER(Fv)-LDP-NGR could suppress the
angiogenic capacity of microvascular endothelial cells.
Discussion
The occurrence and development of tumors are the
result of imbalances in both tumor cells and surrounding
microenvironment, such as extracellular matrix, angiogenesis,
vascular permeability and immunocytes. The integrated treatment
targeting both cancer cells and tumor microenvironment has been
considered a promising therapeutic strategy. Accordingly,
bi-functional or multi-functional tyrosine kinase inhibitors,
bispecific monoclonal antibodies, antibody-based conjugates and
recombinant fusion proteins have been developed and reported as new
antitumor drugs. Among the potential drug targets, EGFR and CD13
have attracted much attention for the reason that they can be
combined with other targets to produce bi-targeted therapeutics. As
reported, the bispecific diphtheria toxin-based recombinant
cytotoxin (DTEGF13) targeting human EGFR and interleukin-13
selectively killed the human glioblastoma U87-luc cells and other
glioblastoma cells in vitro, and two injections of DTEGF13
at a dose of 0.5 mg/kg resulted in eradication of the aggressive
brain tumor in 50% of the rats that could survive with tumor-free
status for >110 days after tumor inoculation (14). Compared with unsubstituted zinc(II)
phthalocyanine, erlotinib-zinc(II) phthalocyanine conjugates could
effectively target EGFR-overexpressing tumor cells, and showed
significantly high photo-cytotoxicity toward HepG2 cells (15). A bispecific antibody (BsAb),
consisting of anti-CD3 Fab' and anti-CD13 Fab', was shown to react
with both CD3 T cells and CD13+ acute myeloid leukemia
(AML) cells. Cytokine-stimulated peripheral blood mononuclear cells
combining with BsAb exhibited enhanced cytotoxicity against
CD13+ AML cells, suggesting that BsAb might be effective
in the ex vivo purging of CD13+ AML cells in
autologous bone marrow transplantation (16). A large number of studies on
EGFR-based or CD13-based bi-targeted conjugates or fusion proteins
have been documented; however, few of them are related to EGFR/CD13
bi-targeting simultaneously in a single agent.
ER(Fv)-LDP-NGR is an EGFR/CD13 bi-targeting fusion
protein with multiple antitumor activities. EGFR is regarded as one
the most important targets on the plasma membrane of cancer cells
and has been validated as a drug target for cancer therapeutics.
CD13 expressed on the membrane of both tumor cells and vascular
endothelial cells is associated with the invasion of tumor cells
and tumor angiogenesis. This study showed that ER(Fv)-LDP-NGR
reduced the phosphorylation of EGFR by blocking the binding of the
receptor to its ligand and affected the hydrolysis capacity of
CD13. The fusion protein not only decreased the mRNA and protein
levels of EGFR and CD13 but also weakened their biological
activity, implying that ER(Fv)-LDP-NGR suppress the transcription
and translation of target genes. The multiple antitumor capability
of the fusion protein was embodied in anti-proliferation, invasion
blocking and tube formation inhibition. For cancer cells,
ER(Fv)-LDP-NGR could interfere with the intracellular EGFR
signaling pathway and related pathways by downregulating the
expression and phosphorylation of EGFR, resulting in proliferation
inhibition, cell cycle arrest, apoptosis induction, and blockade of
cell migration. Due to the decrease in CD13 expression, the
capacity of the cells degrade extracellular matrix was weakened;
consequently, cancer cell invasion was inhibited. In addition, the
downregulation of CD13 by ER(Fv)-LDP-NGR could interfere with the
formation of tube network structures of microvascular endothelial
cells, which would lead to the suppression of angiogenesis.
The fusion protein ER(Fv)-LDP-NGR containing an
anti-EGFR scFv and a tri-cyclic NGR peptide is endowed with the
capability to aim at two targets, the EGFR and CD13. In the present
study, the scFv against EGFR was obtained by multi-round screening
of the recombinant phage antibody library in our laboratory, and it
displays specific binding activity to purified EGFR protein and
EGFR on the membrane of carcinoma cells (17). The phage peptide library screening
product CNGRC shows a greater affinity for CD13 than GNGRG and
linear NGR with the same function (18), and improves therapeutic efficacy of
immunotherapeutic and antiangiogenic molecules (19,20).
The scFv and CNGRC portions in the fusion protein blocks EGFR and
CD13, respectively, and simultaneously exert antitumor efficacy by
growth inhibition and microenvironment modulation. Additionally,
the scFv and the NGR peptide constitute an efficient
EGFR/CD13-targeted delivery carrier, which can transport a variety
of potent ‘warhead’ drugs (such as lidamycin, maytansine, and
auristatin) into solid tumors to realize targeted therapy of cancer
(21–23).
In conclusion, the EGFR/CD13 bi-targeted fusion
protein ER(Fv)-LDP-NGR can simultaneously affect tumor cells and
tumor microenvironment through inhibition of cancer cell
proliferation, induction of apoptosis, blockade of invasion and
suppression of tube formation. In addition, ER(Fv)-LDP-NGR may be
used as an EGFR/CD13 bi-targeted carrier for various ‘warhead’
agents to construct antibody-based and ligand-based conjugates with
high therapeutic efficacy.
Acknowledgements
This study was supported by grants from ‘Significant
New Drug Development’ Major Science and Technology Projects of
China (nos. 2012ZX09301002-001-024-05 and 2013ZX09102064).
Glossary
Abbreviations
Abbreviations:
|
scFv
|
single-chain variable fragment
|
|
NGR
|
a tripeptide consisting of asparagine,
glycine and arginine
|
|
EGFR
|
epidermal growth factor receptor
|
|
CD13
|
aminopeptidase N
|
References
|
1
|
Levitzki A: EGF receptor as a therapeutic
target. Lung Cancer. 41:S9–S14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brand TM, Iida M, Li C and Wheeler DL: The
nuclear epidermal growth factor receptor signaling network and its
role in cancer. Discov Med. 12:419–432. 2011.PubMed/NCBI
|
|
3
|
Fury MG, Xiao H, Sherman EJ, Baxi S,
Smith-Marrone S, Schupak K, Gewanter R, Gelblum D, Haque S, Schoder
H, et al: Phase II trial of bevacizumab + cetuximab + cisplatin
with concurrent intensity-modulated radiation therapy for patients
with stage III/IVB head and neck squamous cell carcinoma. Head
Neck. 38:E566–E570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han JY, Lee SH, Lee GK, Yun T, Lee YJ,
Hwang KH, Kim JY and Kim HT: Phase I/II study of gefitinib
(Iressa(®)) and vorinostat (IVORI) in previously treated
patients with advanced non-small cell lung cancer. Cancer Chemother
Pharmacol. 75:475–483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyauchi E, Inoue A, Kobayashi K, Maemondo
M, Sugawara S, Oizumi S, Isobe H, Gemma A, Saijo Y, Yoshizawa H, et
al: North-East Japan Study Group: Efficacy of chemotherapy after
first-line gefitinib therapy in EGFR mutation-positive advanced
non-small cell lung cancer-data from a randomized Phase III study
comparing gefitinib with carboplatin plus paclitaxel (NEJ002). Jpn
J Clin Oncol. 45:670–676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thomas MB, Morris JS, Chadha R, Iwasaki M,
Kaur H, Lin E, Kaseb A, Glover K, Davila M and Abbruzzese J: Phase
II trial of the combination of bevacizumab and erlotinib in
patients who have advanced hepatocellular carcinoma. J Clin Oncol.
27:843–850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Janjigian YY, Smit EF, Groen HJ, Horn L,
Gettinger S, Camidge DR, Riely GJ, Wang B, Fu Y, Chand VK, et al:
Dual inhibition of EGFR with afatinib and cetuximab in kinase
inhibitor-resistant EGFR-mutant lung cancer with and without T790M
mutations. Cancer Discov. 4:1036–1045. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tokuhara T, Hattori N, Ishida H, Hirai T,
Higashiyama M, Kodama K and Miyake M: Clinical significance of
aminopeptidase N in non-small cell lung cancer. Clin Cancer Res.
12:3971–3978. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ranogajec I: Gelatinase (MMP-2, MMP-9) and
Aminopeptidase N/CD13 in Breast Carcinoma. LAP Lambert Academic
Publishing; 2014
|
|
10
|
Nohara S, Kato K, Fujiwara D, Sakuragi N,
Yanagihara K, Iwanuma Y and Kajiyama Y: Aminopeptidase N (APN/CD13)
as a target molecule for scirrhous gastric cancer. Clin Res Hepatol
Gastroenterol. 40:494–503. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saiki I, Fujii H, Yoneda J, Abe F,
Nakajima M, Tsuruo T and Azuma I: Role of aminopeptidase N (CD13)
in tumor-cell invasion and extracellular matrix degradation. Int J
Cancer. 54:137–143. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukasawa K, Fujii H, Saitoh Y, Koizumi K,
Aozuka Y, Sekine K, Yamada M, Saiki I, Nishikawa K, et al:
Aminopeptidase N (APN/CD13) is selectively expressed in vascular
endothelial cells and plays multiple roles in angiogenesis. Cancer
Lett. 243:135–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sheng W, Shang Y, Li L and Zhen Y: An
EGFR/CD13 bispecific fusion protein and its enediyne-energized
analog show potent antitumor activity. Anticancer Drugs. 25:82–91.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oh S, Ohlfest JR, Todhunter DA, Vallera
VD, Hall WA, Chen H and Vallera DA: Intracranial elimination of
human glioblastoma brain tumors in nude rats using the bispecific
ligand-directed toxin, DTEGF13 and convection enhanced delivery. J
Neurooncol. 95:331–342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang FL, Huang Q, Liu JY, Huang MD and
Xue JP: Molecular-target-based anticancer photosensitizer:
Synthesis and in vitro photodynamic activity of erlotinib-zinc(II)
phthalocyanine conjugates. ChemMedChem. 10:312–320. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaneko T, Fusauchi Y, Kakui Y, Masuda M,
Akahoshi M, Teramura M, Motoji T, Okumura K, Mizoguchi H and Oshimi
K: A bispecific antibody enhances cytokine-induced killer-mediated
cytolysis of autologous acute myeloid leukemia cells. Blood.
81:1333–1341. 1993.PubMed/NCBI
|
|
17
|
Sheng WJ, Shang BY, Miao QF and Zhen YS:
Construction of a single-chain Fv antibody against epidermal factor
receptor and its antitumor activity. Chung Kuo Yao Hsueh Tsa Chih.
46:1393–1398. 2011.(In Chinese).
|
|
18
|
Colombo G, Curnis F, De Mori GM, Gasparri
A, Longoni C, Sacchi A, Longhi R and Corti A: Structure-activity
relationships of linear and cyclic peptides containing the NGR
tumor-homing motif. J Biol Chem. 277:47891–47897. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Curnis F, Sacchi A, Borgna L, Magni F,
Gasparri A and Corti A: Enhancement of tumor necrosis factor alpha
antitumor immunotherapeutic properties by targeted delivery to
aminopeptidase N (CD13). Nat Biotechnol. 18:1185–1190. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang W, Jin G, Ma D, Wang F, Fu T, Chen
X, Chen X, Jia K, Marikar FM and Hua Z: Modification of cyclic NGR
tumor neovasculature-homing motif sequence to human plasminogen
kringle 5 improves inhibition of tumor growth. PLoS One.
7:e371322012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang JL, Qin Y, Li L, Cao CY, Wang Q, Li
Q, Lv YF and Wang Y: Apoptotic melanoma B16-F1 cells induced by
lidamycin could initiate the antitumor immune response in BABL/c
mice. Oncol Res. 23:79–86. 2016. View Article : Google Scholar
|
|
22
|
Tang X, Dai H, Zhu Y, Tian Y, Zhang R, Mei
R and Li D: Maytansine-loaded star-shaped folate-core PLA-TPGS
nanoparticles enhancing anticancer activity. Am J Transl Res.
6:528–537. 2014.PubMed/NCBI
|
|
23
|
Burns KE, Robinson MK and Thévenin D:
Inhibition of cancer cell proliferation and breast tumor targeting
of pHLIP-monomethyl auristatin E conjugates. Mol Pharm.
12:1250–1258. 2015. View Article : Google Scholar : PubMed/NCBI
|