Introduction
Thyroid cancer is the most prevalent endocrine
malignancy in humans. The incidence of thyroid cancer has increased
in recent years. Papillary thyroid cancer (PTC) is a major type
(80–85%) of thyroid cancer (1).
Along with the improvement of diagnostic approaches for thyroid
cancer, more and more PTC cases have been diagnosed (2). Although the prognosis of most PTC
patients is optimistic, the recurrence rate was found to be
relatively high after a 15-year follow-up (3–5). A
small group of PTC patients appear to have a higher risk of
recurrence and metastasis (6). To
distinguish the population of patients with higher risk is an
important task in the clinic. Appropriate biomarkers could be used
to help evaluate the recurrence and metastasis risk of PTC.
However, there are no effective biomarkers currently used in the
clinic. New molecular biomarkers are urgently needed for the
identification of the PTC patients with higher recurrence and
metastasis risk.
Genetic research, which has been used as a molecular
method for genetic discovery, has played a vital role in
understanding the process of tumorigenesis.
BRAFV600E mutation, by far, is the most common
genetic event found in PTC, occurring in 20–50% of cases (7–11).
Yet, PTC tumorigenesis may be regulated by epigenetic events as
well. DNA methylation is the most common epigenetic regulatory
mechanism in tumorigenesis. Evaluating the methylation status of
DNA could be useful for the diagnosis, prognostic evaluation and
predicting the risk for recurrence and metastasis of PTC (12–15).
In previous studies, we performed methylated DNA
immunoprecipitation sequencing (MethylCap-seq) assay and
established a database of the genome-wide DNA methylation profile
of PTC. HOXD10 was one of the candidate genes that were
aberrantly hypermethylated in PTC (Fig.
1). In the present study, we aimed to analyze the function and
methylation status of the HOXD10 gene in PTC and to elucidate the
relationship between HOXD10 methylation, HOXD10
expression, BRAF mutation and clinicopathological
characteristics.
Materials and methods
Clinical samples
Human primary PTCs and adjacent non-tumor tissues (2
cm away from the tumor edge) were collected from patients who were
initially surgically treated at the Department of Head and Neck
Surgery, Fudan University Shanghai Cancer Center (Shanghai, China).
All the patients had received lobectomy and isthmectomy plus
ipsilateral central lymph node dissection. Additional modified
lateral lymph node dissection was performed in patients with
clinically suspicious lateral lymph node metastasis. All of the
samples were pathologically confirmed. Totally 152 PTC patients
were enrolled from April 2014 to December 2014. All the samples
were stored at −80°C. Informed consent for the use of the tissues
for clinical research was obtained before surgery, and the study
protocol and consent form were approved by the Ethics Committee of
Fudan University Shanghai Cancer Center. The tumor-node-metastasis
(TNM) stages were determined according to the American Joint Cancer
Committee (AJCC) TNM grading system, 7th edition. The
clinicopathological data of the patients enrolled are summarized in
Table I.
 | Table I.Clinical characteristics of the PTC
patients. |
Table I.
Clinical characteristics of the PTC
patients.
| Characteristic | Data |
|---|
| Patients, n | 152 |
| Age (years) |
|
|
Mean | 42.72±13.309 |
|
>45 | 69 (45.4) |
|
≤45 | 83 (54.6) |
| Sex, n (%) |
|
|
Male | 33 (21.7) |
|
Female | 119 (78.3) |
| Invasion, n
(%) |
|
|
Yes | 29 (19.1) |
| No | 123 (80.9) |
| Size (cm) |
|
|
Mean | 1.419±0.849 |
|
>1 | 94 (61.8) |
| ≤1 | 56 (36.8) |
| Multifocality, n
(%) |
|
|
Yes | 50 (32.9) |
| No | 102 (67.1) |
| Bilaterality, n
(%) |
|
|
Yes | 30 (19.7) |
| No | 121 (79.6) |
| Hashimoto's
thyroiditis, n (%) |
|
|
Yes | 29 (19.1) |
| No | 123 (80.9) |
| Surgery, n (%) |
|
|
Lobectomy + isthmectomy +
CLND | 111 (73.0) |
|
Lobectomy + isthmectomy + CLND
+ LLND | 41 (27.0) |
| Central lymph node
metastasis, n (%) |
|
|
Yes | 79 (52.0) |
| No | 73 (48.0) |
| Lateral lymph node
metastasis, n (%) |
|
|
Yes | 41 (27.0) |
| No | 111 (73.0) |
Cell culture and 5-Aza-2-deoxycytidine
treatment
Human PTC cell lines TPC-1, BCPAP, K1, W3 were used
for the present study (16). The
cell lines TPC-1 and BCPAP were routinely cultured at 37°C in
RPMI-1640 medium with 10% fetal bovine serum (FBS). K1 and W3 cells
were cultured in DMEM/Hams F-12 medium (Invitrogen Life
Technologies, Inc., Carlsbad, CA, USA). All the media were
supplemented with penicillin/streptomycin. In some experiments,
tumor cells were treated with 5 µm/ml 5-Aza-2′-deoxycytidine
(5-Aza) for 72 h as a demethylation treatment. Media and 5-Aza were
replenished every 24 h.
Plasmid construction and cell
transfection
The HOXD10-overexpressing plasmid was
constructed by cloning of the full-length HOXD10 open
reading frame into the mammalian expression vector pcDNA3.1 with
BamHI and XhoI restriction enzyme sites. The
sequences were confirmed by DNA sequencing. PTC TPC-1 cells were
cultured into a 6-well plate for 24 h and transfected with
pcDNA3.1-HOXD10 or empty vector pcDNA3.1 using Lipofectamine
2000 (Invitrogen, Carlsbad, CA, USA). After confirming the
transfection efficiency by RT-PCR and western blotting in the
surviving colonies, cells were transferred into a 6-well plate, and
cultivated for further use.
Cell migration assay
PTC cells transfected with pcDNA3.1 vector or
pcDNA3.1-HOXD10 were used for cell migration assays. Cell
migration was assessed by modified Boyden Transwell chambers assay
(Corning, Corning, NY, USA). Briefly, 2×104 cells/well
were plated into 100 µl of no FBS medium in the upper chamber, and
500 µl of medium containing 10% FBS was added to the lower chamber.
The cells were incubated for 12 h. The nonmigratory cells in the
upper chamber were removed with a cotton swab. The cells on the
bottom of the membrane were fixed and stained with polyfluoroalkoxy
(PFA) and crystal violet stain solution (0.5%). The number of
visible cells was counted by fluorescence microscope in 5 random
high power fields. All the experiments were repeated 3 times.
Cell apoptosis and cell cycle
Analysis of cell apoptosis was performed using the
PE Annexin V apoptosis detection kit (BD Biosciences, Franklin
Lakes, NJ, USA) by flow cytometric analysis (FCA). Briefly, stably
transfected PTC cell line TPC-1 was suspended in annexin binding
buffer, Alexa Fluor 488 Annexin V and propidium iodide (PI) working
solution were added in sequence. The stained cells were finally
analyzed by FACScan flow cytometry (BD Biosciences). Cell cycle
distribution was detected by the Cycletest™ Plus DNA Reagent kit
(BD Biosciences). Briefly, transfected cells were harvested and
washed in PBS. Cellular DNA was stained with 125 µg/ml PI for 20
min at 4°C in dark. The cells were then sorted by FACSCalibur, and
cell cycle distribution was determined using the ModFit LT software
(Verity Software House, Topsham, ME, USA).
Western blot analysis
Total proteins were extracted from the stably
transfected cells using RIPA lysis buffer. Lysates were resolved on
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) gel and transferred to polyvinylidene difluoride (PVDF)
membranes (Millipore, Billerica, MA, USA). Primary antibodies were
used as follows: HOXD10 (1:1,000; Abcam, Cambridge, MA, USA)
and Tubulin (1:1,000; Proteintech Group, Chicago, IL, USA).
The blots were developed using chemiluminescence with Las 4000
imaging system (Fujifilm, Tokyo, Japan).
DNA extraction and bisulphite
conversion
Fresh-frozen tissue specimens and PTC cell lines
were homogenized using a bead homogenizer and genomic DNA was
extracted using the Genomic DNA Extraction kit (Tiangen, Beijing,
China) according to the manufacturer. The DNA concentration was
determined by NanoDrop 1000. The bisulfite conversion is described
by Yu et al (17). The DNA
sample was then stored at-20°C until further use.
Quantitative methylation-specific PCR
(Q-MSP)
Q-MSP assay was performed to analyze the methylation
level of the HOXD10 gene in PTC. We used a plasmid vector to
construct a standard curve for absolute quantification PCR. The
repetitive DNA element ALU was used as an internal
reference. Briefly, quantitative PCR was carried out in a final
reaction mixture of 20 µl containing 4 µl bisulfite-treated DNA,
500 nM of each primer, 250 nM TaqMan probe, 1.875 mM
MgCl2, 200 µM deoxyguanosine triphosphate and 0.5 U
platinum Taq polymerase in the King Hot Start Taq polymerase
reaction system (Ruian Biotech, China). The reaction involved an
initial pre-denaturation for 3 min at 94°C, followed by 40 cycles
with denaturation for 15 sec at 94°C, annealing and extension for
60 sec at 60°C in an ABI 7500 Fast Real-Time instrument. The final
results are presented as methylated gene copies
(HOXD10/ALU*100).
The specific primers and TaqMan probes for the
target gene HOXD10 and the internal reference gene
ALU are presented in Table
II. The HOXD10 gene and the promoter CpG island were
searched for using the UCSC Human Genome Browser and PubMed
(Fig. 1). The primers and TaqMan
probes of HOXD10 for Q-MSP assay were designed by JIELI
Biotechnology (Shanghai, China). The specific primers and TaqMan
probes for the ALU gene were previously described by
Weisenberger et al (18).
 | Table II.Primer sequences for RT-PCR, Q-MSP
and BRAF sequencing. |
Table II.
Primer sequences for RT-PCR, Q-MSP
and BRAF sequencing.
| Reaction | Primer sequences
(5′-3′) |
|---|
|
HOXD10-RT-F |
CTGAGGTCTCCGTGTCCAGT |
|
HOXD10-RT-R |
CTGAGGTCTCCGTGTCCAGT |
| GAPDH-RT-F |
GGCCTCCAAGGAGTAAGACC |
| GAPDH-RT-R |
CAAGGGGTCTACATGGCAAC |
|
HOXD10-Q-MSP-F |
TGGAGAGGCGGACAGGAG |
|
HOXD10-Q-MSP-R |
GGGTAAGCACGGACAACAGAGC |
|
HOXD10-Q-MSP-probe |
6FAM-CCAGCGCGCACTATCGCGG-TAMRA |
|
ALU-Q-MSP-F |
GGTTAGGTATAGTGGTTTATATTTGTAATTTTAGTA |
|
ALU-Q-MSP-R |
ATTAACTAAACTAATCTTAAACTCCTAACCTCA |
|
ALU-Q-MSP-probe |
6FAM-CCTACCTTAACCTCCC-MGB |
|
BRAFV600E-F |
CATAATGCTTGCTCTGATAGGAAAATG |
|
BRAFV600E-R |
CTGATGGGACCCACTCCAT |
BRAF mutational screening
BRAF gene mutational status was analyzed in
both clinical samples and PTC cell lines. BRAF mutational
screening was analyzed by PCR followed by DNA sequencing at Boshang
Biotechnology (Shanghai, China). The specific PCR primers for the
BRAFV600E mutation region are presented in
Table II.
RNA extraction and quantitative
real-time PCR
Fresh-frozen tissue specimens were homogenized using
a bead homogenizer. Genomic RNA from cell lines and tissues was
extracted with TRIzol reagent (Invitrogen) according to the
manufacturer's protocol.
The expression of the HOXD10 gene was
analyzed in both clinical samples and PTC cell lines by RT-PCR.
Reverse transcription reaction was performed using 1 µg of total
RNA with PrimeScript RT reagent kit with gDNA Eraser (Perfect
Real-Time; RR047A; Takara, Dalian, Japan). The expression level of
HOXD10 was determined by RT-PCR using SYBR Premix Ex Taq
(Tli RNaseH Plus; RR420A; Takara). The PCR reaction was performed
in a 20-µl volume containing up to 100 ng of template cDNA in a
Cyclelight 480 PCR system. The reaction involved an initial
denaturation for 3 min at 94°C, followed by 40 cycles with
denaturation for 5 sec at 94°C, annealing for 60 sec at 60°C.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as
an internal control. The expression level of HOXD10 was
calculated using the 2−ΔCt method. The primers for
RT-PCR were designed using the website http://www.embnet.sk/cgi-bin/primer3_www.cgi. The
RT-PCR primer sequences are shown in Table II.
Statistical analysis
Statistical analyses were performed by Student's and
paired t-tests, and Chi-square test. The odds ratios (ORs) for
relationships between each variable and HOXD10 methylation
were calculated by univariate logistic regression analysis.
Multivariate logistic regression analysis was used to analyze the
relationship between invasion and other clinicopathological
characteristics including the methylation status of HOXD10.
All confidence intervals (CIs) were stated at the 95% confidence
level. A value of P<0.05 was considered to be statistically
significant. SPSS 19.0 was used for data analysis (SPSS, Inc.,
Chicago, IL, USA). Figures were constructed using GraphPad Prism 5,
Adobe Illustrator CS4 and Stata/SE 12.0.
Results
HOXD10 promoter is hypermethylated in
PTC tissues
Q-MSP was designed to detect the methylation level
of HOXD10 in PTC tissues. PTC and adjacent normal thyroid
tissues (152 pairs) were tested by Q-MSP assay. The results of
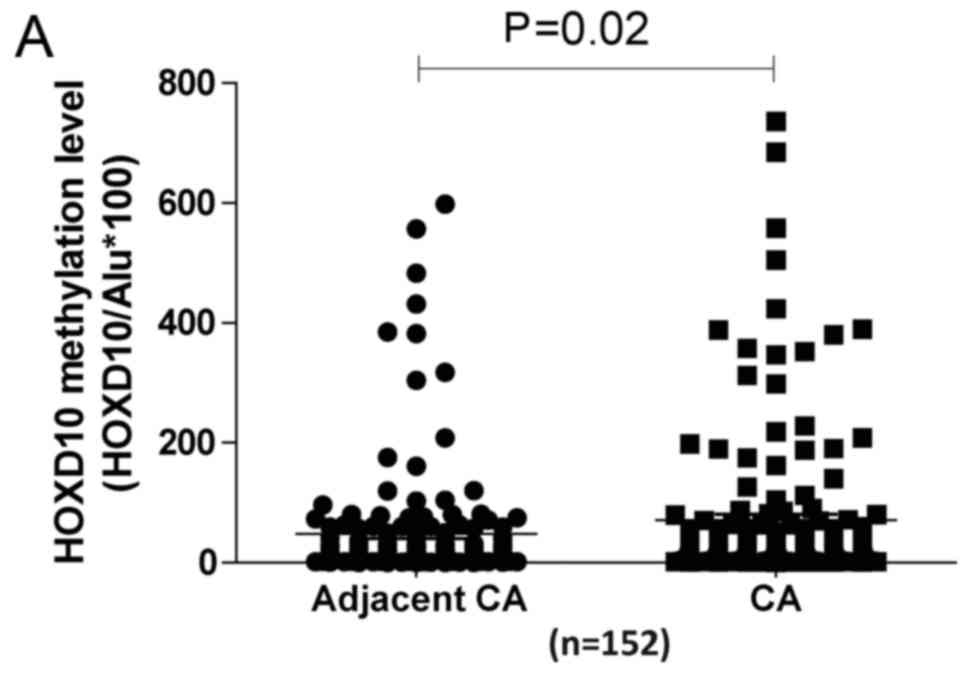
Q-MSP are shown in Fig. 2A. The
overall methylation levels of the HOXD10 promoter were
significantly higher in PTC tissues than levels in the adjacent
normal thyroid tissues (P=0.02). Our findings showed that the
promoter region of the HOXD10 gene was hypermethylated in
17.76% (27/152) of PTC tissues and in 10.53% (16/152) of adjacent
normal thyroid tissues, using a cut-off value of 88
(HOXD10/ALU*100) (Fig.
2B).
HOXD10 mRNA expression is decreased in
PTC tissues
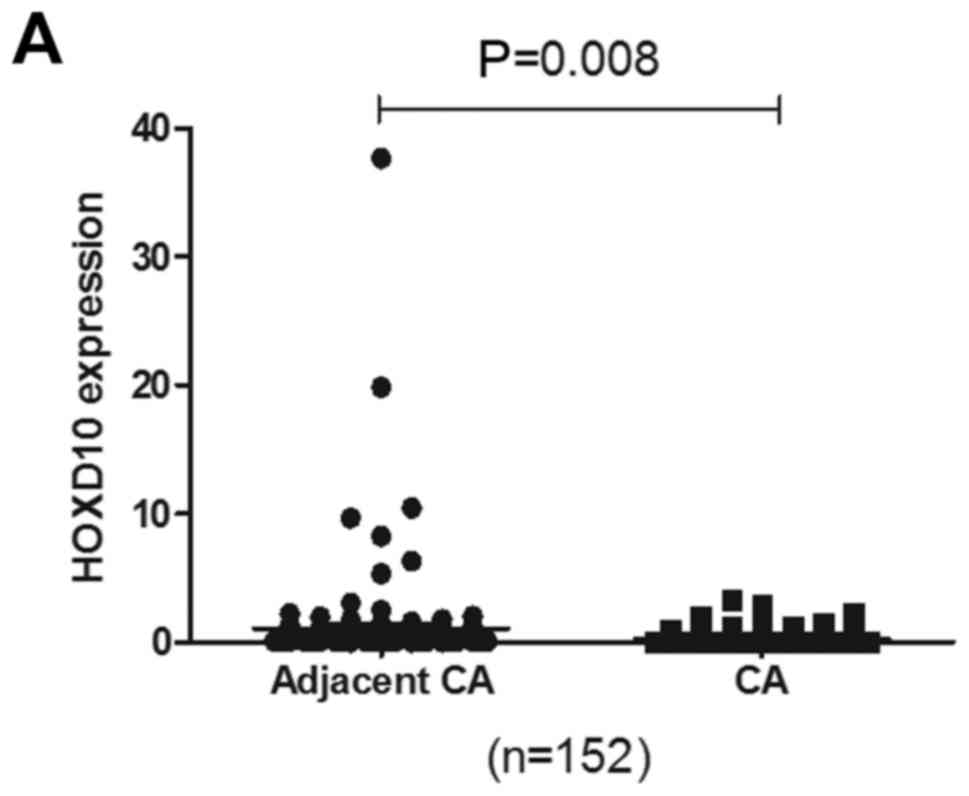
To determine the relationship between the
methylation status of the HOXD10 gene and its expression
level, we evaluated the HOXD10 mRNA levels in 152 pairs of
PTC tissue samples by RT-PCR. The result showed that the expression
level of the HOXD10 gene was significantly decreased in the
PTC tissues when compared with the level in the adjacent normal
thyroid tissues (P=0.008) (Fig.
3A). Low expression of HOXD10 was found in 46.7%
(71/152) of the PTC tissues and in only 13.8% (21/152) of the
adjacent normal thyroid tissues (Table
V), using a cut-off value of 0.06 (relative value).
 | Table V.Association between
clinicopathological factors of the PTC cases and HOXD10
expression. |
Table V.
Association between
clinicopathological factors of the PTC cases and HOXD10
expression.
|
Characteristics | High expression n
(%) | Low expression n
(%) | P-value |
|---|
| Patients | 81 (53.3) | 71 (46.7) |
|
| Age (years) |
|
|
0.022a |
|
>45 | 37 (44.6) | 46 (55.4) |
|
|
≤45 | 44 (63.8) | 25 (36.2) |
|
| Sex |
|
| 0.333 |
|
Male | 15 (45.5) | 18 (54.5) |
|
|
Female | 66 (55.5) | 53 (44.5) |
|
| Invasion |
|
| 0.839 |
|
Yes | 16 (55.2) | 13 (44.8) |
|
| No | 65 (52.8) | 58 (47.2) |
|
| Size (cm) |
|
| 0.237 |
|
>1 | 47 (50.0) | 47 (50.0) |
|
| ≤1 | 34 (60.7) | 22 (39.3) |
|
| Multifocality |
|
| 0.490 |
|
Yes | 29 (58.0) | 21 (42.0) |
|
| No | 52 (51.0) | 50 (49.0) |
|
| Bilaterality |
|
| 0.839 |
|
Yes | 15 (50.0) | 15 (50.0) |
|
| No | 65 (53.7) | 56 (46.3) |
|
| Hashimoto's
thyroiditis |
|
| 0.829 |
|
Yes | 14 (56.0) | 11 (44.0) |
|
| No | 67 (52.8) | 60 (47.2) |
|
| Lymph nodes
metastasis |
|
| 0.254 |
|
Yes | 41 (48.8) | 43 (51.2) |
|
| No | 40 (58.8) | 28 (41.2) |
|
| Central lymph node
metastasis |
|
| 0.197 |
|
Yes | 38 (38.1) | 41 (51.9) |
|
| No | 43 (58.9) | 30 (41.1) |
|
| Lateral lymph node
metastasis |
|
| 0.857 |
|
Yes | 23 (54.8) | 19 (45.2) |
|
| No | 58 (52.7) | 52 (47.3) |
|
| BRAF mutation |
|
|
0.022a |
|
Yes | 27 (42.2) | 23 (57.8) |
|
| No | 54 (61.4) | 34 (38.6) |
|
Hypermethylation and low expresssion
of HOXD10 is associated with BRAFV600E mutation in PTC
tissues
To analyze the relationship between DNA methylation
and BRAF mutation, we tested the BRAFV600E
mutation in 4 PTC cell lines (TPC-1, BCPAP, K1 and W3) and 152 PTC
clinical samples. Our results showed that
BRAFV600E mutation occurred in 64 (42.1%) PTC
patients and in 2 PTC cell lines (BCPAP and K1). In accordance with
previous studies, no BRAF mutation was found in TPC-1 and W3
cell lines. Moreover, we observed that the hypermethylation and
low-expresssion of HOXD10 was related to
BRAFV600E mutation in PTC tissues. The expression
of HOXD10 was significantly lower in PTC tissues with
BRAFV600E mutation than in those without the
mutation (P=0.022) (Table V).
However, the methylation status of HOXD10 did not show
statistical difference between PTC tissues with and without
BRAFV600E mutation (P=0.669) (Table III). However, further analysis in
PTC tissues with BRAFV600E mutation showed that
the methylation levels of HOXD10 were significantly higher
in tumor tissues than levels in the adjacent normal thyroid tissues
(P=0.01). While in PTC tissues without BRAFV600E
mutation such a significant difference was not found (P=0.50)
(Fig. 2C).
 | Table III.Association between
clinicopathological factors and HOXD10 methylation. |
Table III.
Association between
clinicopathological factors and HOXD10 methylation.
|
Characteristics | Hyper-methylated n
(%) | Hypo-methylated n
(%) | P-value |
|---|
| Patients | 27 (17.8) | 125 (82.2) |
|
| Age (years) |
|
|
0.003a |
|
>45 | 20 (27.4) | 53 (72.6) |
|
|
≤45 | 7 (8.9) | 72 (91.1) |
|
| Sex |
|
| 1.000 |
|
Male | 6 (18.2) | 27 (81.8) |
|
|
Female | 21 (17.6) | 98 (82.4) |
|
| Invasion |
|
|
0.027a |
|
Yes | 9 (33.3) | 18 (66.7) |
|
| No | 18 (14.4) | 107 (85.6) |
|
| Size (cm) |
|
| 0.170 |
|
>1 | 16 (15.0) | 91 (85.0) |
|
| ≤1 | 11 (24.4) | 34 (75.6) |
|
| Multifocality |
|
| 1.000 |
|
Yes | 8 (17.0) | 39 (83.0) |
|
| No | 19 (18.1) | 86 (81.9) |
|
| Bilaterality |
|
| 0.306 |
|
Yes | 5 (16.7) | 25 (83.3) |
|
| No | 22 (18.0) | 100 (82.0) |
|
| Hashimoto's
thyroiditis |
|
| 0.776 |
|
Yes | 5 (20.0) | 20 (80.0) |
|
| No | 22 (17.3) | 105 (82.7) |
|
| Lymph node
metastasis |
|
| 0.523 |
|
Yes | 13 (15.5) | 71 (84.5) |
|
| No | 14 (20.6) | 54 (79.4) |
|
| Central lymph node
metastasis |
|
| 0.405 |
|
Yes | 12 (17.8) | 67 (84.8) |
|
| No | 15 (20.5) | 58 (79.5) |
|
| Lateral lymph node
metastasis |
|
| 0.636 |
|
Yes | 6 (14.3) | 36 (85.7) |
|
| No | 21 (19.1) | 89 (80.9) |
|
| BRAF mutation |
|
| 0.669 |
|
Yes | 10 (15.6) | 54 (84.4) |
|
| No | 17 (19.3) | 71 (80.7) |
|
5-Aza-2-deoxycytidine treatment
reverts the expression of the HOXD10 gene in PTC cell lines
To further verify the relationship between the
methylation status of the HOXD10 gene and its expression
level, we detected the expression level of HOXD10 and
performed 5-Aza treatment in 4 PTC cell lines: TPC-1, W3
(BRAF wild-type) and BCPAP, K1 (BRAFV600E
mutation) to ascertain whether the changes in the methylation level
influence the expression of HOXD10. The results are shown in
Fig. 3B. HOXD10 mRNA was
found to be weakly expressed in the TPC-1 and W3 cell lines, while
the expression of HOXD10 in the BCPAP and K1 cell lines
showed no significant decrease compared with the normal thyroid
tissues. After a 72 h treatment of 5-Aza, the expression of
HOXD10 in the TPC-1 and W3 cell lines was significantly
increased (57 and 396 times, respectively), while there were no
significant changes in the expression of HOXD10 in the BCPAP
and K1 cell lines.
HOXD10 suppresses the migration and
induces the apoptosis of PTC cells
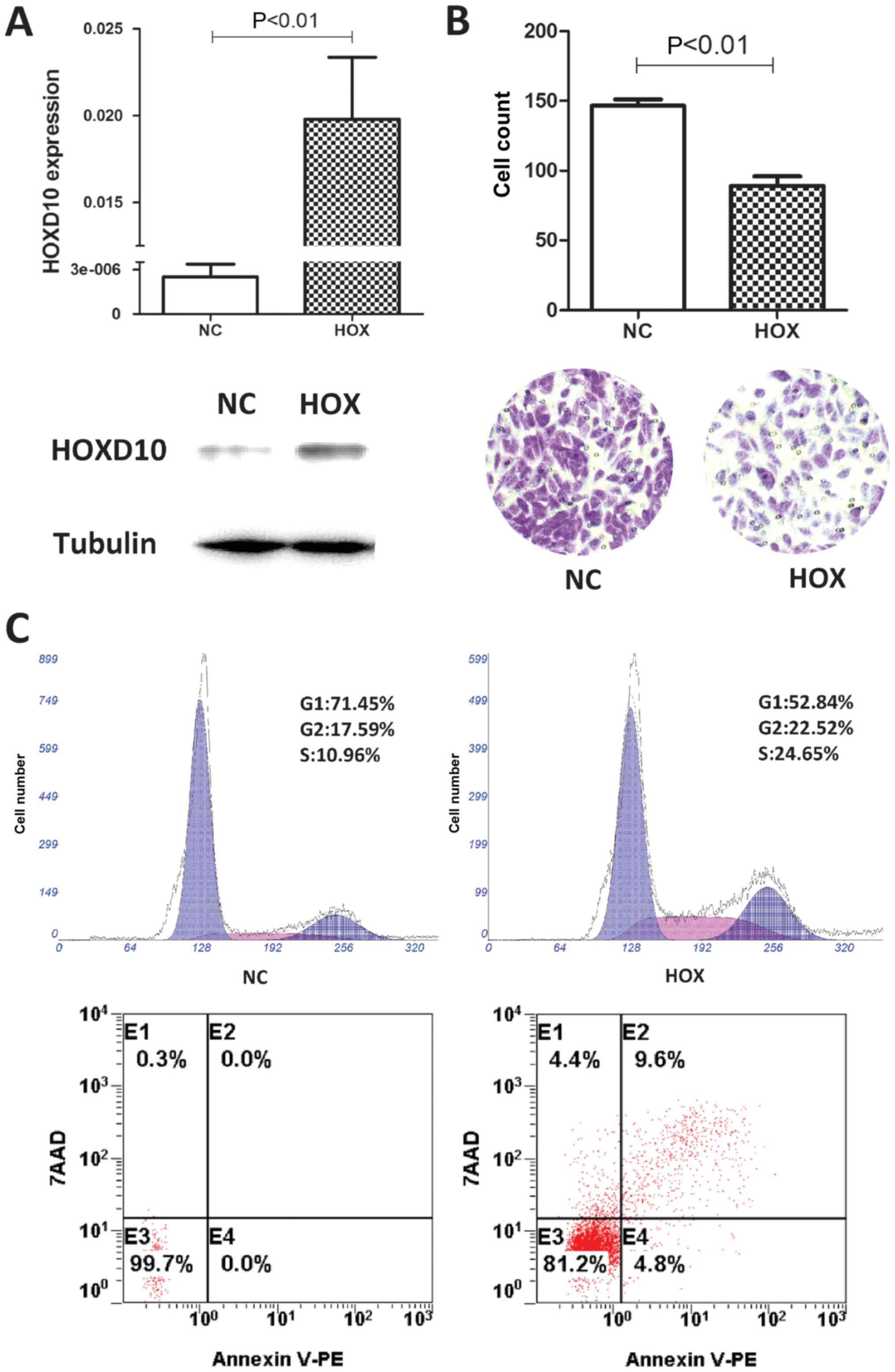
To understand the potential functions of
HOXD10 in PTC, we overexpressed HOXD10 in the TPC-1
cell line. TPC-1 cells were transfected with pcDNA3.1-HOXD10
or pcDNA3.1 vector. The transfection efficiency was confirmed by
RT-PCR and western blotting (Fig.
4A). We observed that the overexpression of HOXD10
suppressed TPC-1 cell migration significantly compared to the
control vector transfectants through a Transwell assay (P<0.01;
Fig. 4B). To explore the mechanisms
underlying the inhibition of cell proliferation by the
overexpression of HOXD10, we assessed cell apoptosis and
cell cycle by flow cytometry. The overexpression of HOXD10
induced the apoptosis of TPC-1 cells when compared to the empty
vector-transfected cells. Additionally, we observed that the
HOXD10-overexpressing TPC-1 cells showed higher S and G2
phase populations in comparison to the empty vector transfectants
(Fig. 4C). In summary, the present
study showed that the overexpression of HOXD10 inhibited the
migration of TPC-1 cells and also induced the apoptosis in
vitro. The results implied that HOXD10 may act as a
tumor-suppressor in PTC.
The aberrant hypermethylation of the
HOXD10 gene is associated with clinicopathological
characteristics
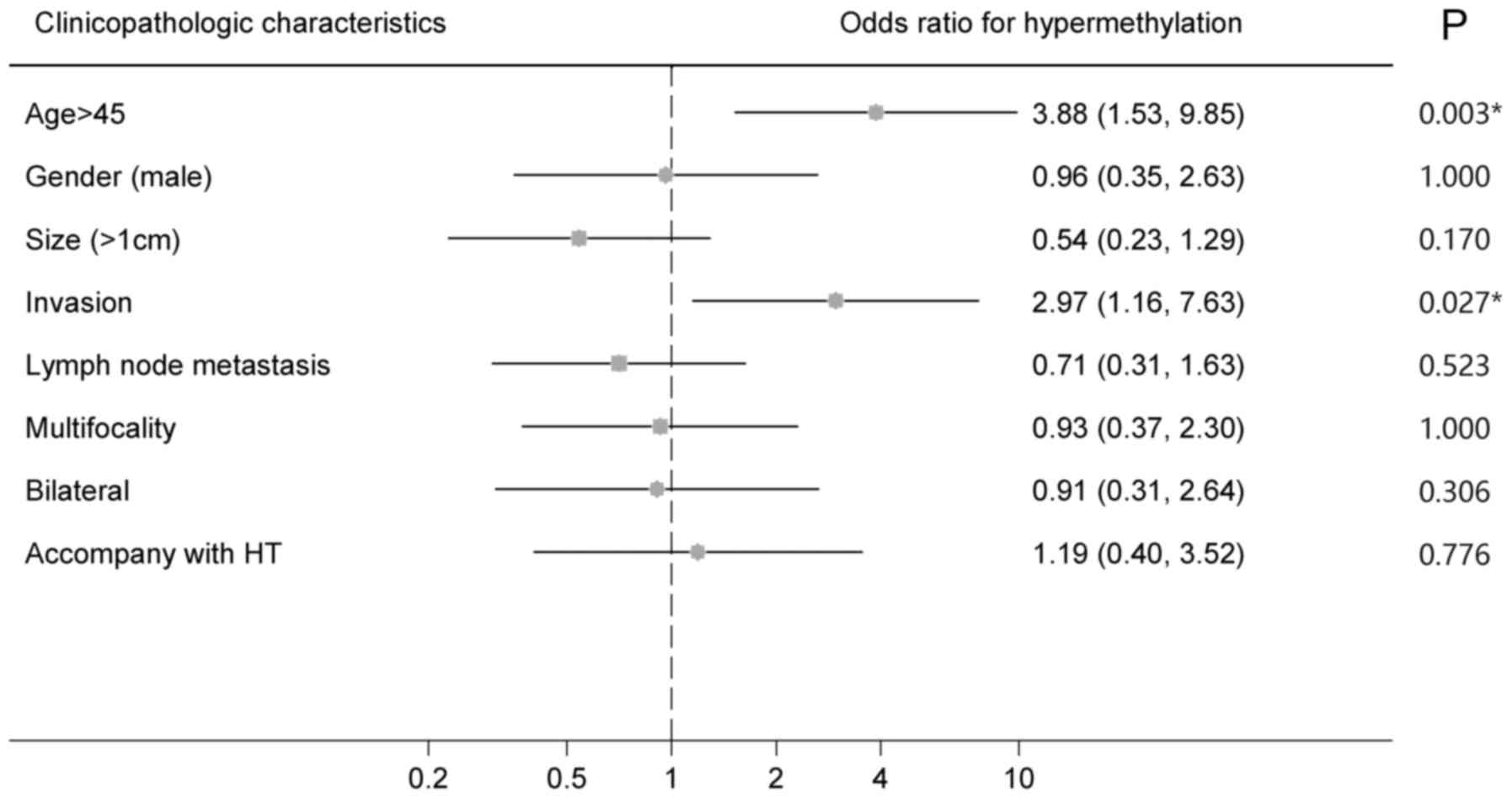
The relationship between the HOXD10
methylation status and clinicopathological characteristics was
analyzed to evaluate the prognostic value of the HOXD10 gene
as a biomarker of PTC. Chi-square analysis and univariate logistic
regression analysis revealed that age >45 (OR 3.881, 95% CI
1.930–9.847; P=0.003) and invasion (OR 2.972, 95% CI 1.157–7.633;
P=0.027) were associated with the hypermethylation status of
HOXD10 (Table III and
Fig. 5), while, no relationship was
found between HOXD10 hypermethylation status and sex, tumor
size, multifocality, bilaterality, lymph node metastasis or
Hashimoto's thyroiditis. The multivariate regression analysis was
also performed to find the correlationship between invasion and
other clinicopathological characteristics including methylation
status of HOXD10. The result indicated that the
hypermethylation of HOXD10 (OR 3.779, 95% CI 1.283–11.128;
P=0.016) as well as tumor size >1 cm (OR 7.456, 95% CI
1.484–37.459; P=0.015) were independent risk factors of invasion in
PTC (Table IV). In a word, our
results showed the potential clinical value of HOXD10
methylation in PTC as a biomarker.
 | Table IV.Multivariate regression analysis of
invasion in PTC. |
Table IV.
Multivariate regression analysis of
invasion in PTC.
|
Characteristics | OR (95% CI) | P-value |
|---|
| Sex (male) | 1.035
(0.341–3.141) | 0.951 |
| Age (years) | 2.044
(0.773–5.408) | 0.150 |
| Size >1 cm | 7.456
(1.484–37.459) |
0.015a |
| Lymph node
metastasis | 1.579
(0.556–4.483) | 0.391 |
| Multivariate | 0.262
(0.065–1.054) | 0.059 |
| Bilateral | 2.432
(0.565–10.474) | 0.233 |
| Hashimotos
thyroiditis | 0.593
(0.119–2.968) | 0.525 |
| HOXD10
hypermethylation | 3.779
(1.283–11.128) |
0.016a |
The relationship between HOXD10 expression
and other clinicopathological characteristics including
BRAFV600E mutation was also analyzed. Age >45
(P=0.022) and BRAFV600E mutation (P=0.022) were
found to be associated with low expression of HOXD10
(Table V). However, no relationship
was found between HOXD10 expression status and sex, tumor
size, invasion, multifocality, bilaterality, lymph node metastasis
or Hashimoto's thyroiditis. In addition, no significant correlation
was found between BRAFV600E mutation and other
clinicopathological characteristics.
Discussion
DNA methylation is one of the most common molecular
events in cancers, along with genetic alterations, leading to
carcinogenesis. Evaluating the status of DNA methylation could be
useful for the diagnosis and prognostic evaluation of cancers and
may be helpful in clarifying the process of tumorigenesis (12,19–22).
We have paid close attention to DNA methylation in thyroid cancer
in recent years. Our previous studies established a genome-wide DNA
methylome database of PTC by MethylCap-seq, demonstrating that the
HOXD10 gene was aberrantly hypermethylated in PTC.
Previous studies recognized HOXD10 as a
sequence-specific transcription factor, mainly involved in cell
differentiation and limb development (23,24).
In recent years, its function in tumorigenesis has been gradually
recognized (25,26). The homebox superfamily plays an
important role in cell differentiation and morphogenesis. The
dysregulation of the HOX gene may affect various pathways
and play roles in tumorigenesis and metastasis (25,27).
Several HOX genes (such as HOXB13, HOXA5 and
HOXC6) have been found aberrantly expressed through promoter
methylation in malignancies including lung, breast and
gastrointestinal cancer (25,26,28–33).
Emerging studies have found that the expression of HOXD10 is
decreased in various tumors (such as breast and gastric cancer),
and have considered HOXD10 as a candidate tumor-suppressor
gene (34–39). However, the methylation and
expression status of the HOXD10 gene, and its biological
significance in PTC have not been identified.
In the present study, 152 pairs of PTC samples were
collected for relative research, including Q-MSP, RT-PCR and
BRAF mutation sequencing. Cytology experiments with 4 PTC
cell lines were carried out to explore the relationship between the
methylation and expression status of HOXD10. Overexpression
transfection of HOXD10 in TPC-1 cells was designed to
research the function of HOXD10 in PTC. The results showed
that the methylation level of the HOXD10 gene was
significantly higher in PTC tissues, compared with that noted in
the adjacent normal thyroid tissues (P=0.02). RT-PCR assay showed
that the expression of HOXD10 was significantly decreased in
PTC cell lines and tumor tissues than that observed in the adjacent
normal thyroid tissues (P=0.008), which was in accordance with the
Q-MSP results. 5-Aza treatment reverted the expression of the
HOXD10 gene in PTC cell lines, which demonstrated that the
decreased expression of HOXD10 was caused by aberrant
promoter hypermethylation. Moreover, the overexpression of
HOXD10 suppressed the migration of TPC-1 cells, and promoted
the cell apoptosis, implying that HOXD10 may act as a tumor
suppressor in PTC. In addition, statistical analysis showed the
potential clinical value of HOXD10 methylation as a
biomarker. Besides, further stratified analysis showed that low
expression of HOXD10 was related to
BRAFV600E mutation (P=0.022). Also, the
HOXD10 methylation level of PTC was significantly higher
than that of adjacent normal thyroid tissues in patients with
BRAFV600E mutation (P=0.01). In conclusion, we found
that the HOXD10 gene was downregulated through promoter
hypermethylation in PTC and HOXD10 may act as a tumor
suppressor. Moreover, the aberrant hypermethylation and low
expession of HOXD10 were associated with
BRAFV600E mutation in PTC.
It is widely accepted that PTC with
BRAFV600E mutation is a flag of high-risk.
Detection of BRAFV600E mutation is an important
clinical application for the diagnosis and prognostic prediction of
PTC. In addition, the present study suggests that HOXD10 may
be a candidate tumor suppressor and it also has interactions with
the BRAF gene. The results showed that the low expression of
HOXD10 was associated with BRAFV600E
mutation (P=0.022). In addition, in PTCs with
BRAFV600E mutation, the methylation levels of
HOXD10 were significantly higher in PTC tissues than in
adjacent normal thyroid tissues. While no significant difference
was observed in PTCs without BRAFV600E mutation.
In addition, the PTC cell lines with BRAFV600E
mutation (K1, BCPAP) showed relatively high expression of
HOXD10 and low sensitivity to 5-Aza treatment. On the
contrary, the PTC cell lines without BRAFV600E
mutation (TPC-1, W3) showed relatively low expression of
HOXD10 and high sensitivity to 5-Aza treatment. The results
indicated the possible interaction between HOXD10 gene and
BRAFV600E mutation. One possible hypothesis to
explain the results was that the hypermethylation of HOXD10
may be an accompanied event in BRAFV600E-mutated
PTC, where BRAFV600E mutation plays the major
role in tumorigenesis. While in wild-type PTC, HOXD10
hypermethylation may play an important role in tumorigenesis.
Combining the detections of HOXD10 methylation and
BRAF mutation may be a good choice to improve clinical
diagnostic and prognostic accuracy.
Our results showed that the HOXD10 gene may
be involved in PTC tumorigenesis and it may act with
BRAFV600E mutation. However, the underlying
mechanism of HOXD10 is still not clear. Wang et al
(39) reported that HOXD10
regulates multiple downstream genes including IGFBP3 in
gastric cancer. Reintroduction of HOXD10 upregulated
IGFBP3, activated caspase-3 and caspase-8, and subsequently
induced cell apoptosis. Yang et al (40) claimed that HOXD10 acted as a
tumor suppressor via the inhibition of RHOC/AKT/MAPK pathway in
cholangiocellular carcinoma. The upregulation of HOXD10 led
to the dephosphorylation of AKT and ERK, implying that the PI3K/AKT
and MAPK pathways were significantly inactivated. According to the
above studies, MAPK pathways may be a key point for the interaction
between BRAF and HOXD10, since BRAF is one of the
most important regulatory gene in the MAPK pathways. However,
further research is needed to clarify the mechanisms of the
interaction between BRAF and the HOXD10 gene.
Combined with clinical data, the hypermethylation
status of the HOXD10 promoter was significantly correlated
with age >45 (OR 3.881, 95% CI 1.930–9.847; P=0.003) and
invasion (OR 2.972, 95% CI 1.157–7.633; P=0.027). These 2 clinical
characteristics usually predict a worse prognosis of PTC. Patients
with an age >45 years and patients with primary tumor invasion
may have a higher chance of recurrence and metastasis, leading to
worse survive (41–43). Our analysis indicated that the
hypermethylation of the HOXD10 gene was an independent risk
factor for invasion in PTC. This indicates that the HOXD10
gene may play a role in PTC tumorigenesis and its methylation
status could be used to predict the prognosis of PTC as a
biomarker.
Recently, the incidence of thyroid cancer
particularly PTC has significantly increased (2,44). It
is well known that patients with PTC usually have good prognosis,
but a small population of patients have a relatively higher
recurrence risk (5,42). New biomarkers are urgently needed
for the diagnosis and prognostic prediction of PTC. Currently,
several biomarkers have been used to improve the diagnostic
accuracy in PTC, such as the detection of
BRAFV600E mutation (45–49).
DNA methylation of tumor suppressors (such as Rassf1A and
RARβ2) and thyroid-specific genes (such as TSHR) have
been determined to be associated with BRAFV600E
mutation, which may also play a role in PTC tumorigenesis (50–52).
However, apart from genetic biomarkers, research must provided new
ideas to search for viable epigenetic biomarkers to improve the
diagnostic and prognostic accuracy in PTC. According to the present
study, the hypermethylation of HOXD10 may be a promising
biomarker for the diagnosis and prognostic prediction of PTC.
In summary, the present study firstly studied the
methylation profile of HOXD10 and explored its functions in
PTC. HOXD10 may act as a tumor suppressor in PTC. The
decreased expression of the HOXD10 gene caused by aberrant
hypermethylation was shown in PTCs particularly in those with
BRAFV600E mutation. The epigenetic suppression of
the HOXD10 gene may play a role in the tumorigenesis of PTC, and it
may be a prospective biomarker for the diagnosis and prognostic
prediction of PTC.
Acknowledgements
The present study was sponsored by the National
Natural Science Foundation of China (81372368) and the Natural
Science Foundation of Shanghai (12ZR1406800). The authors are
grateful to Q.-H.J. for kindly providing the PTC cell lines TPC-1,
K1, BCPAP and W3.
References
|
1
|
Kilfoy BA, Zheng T, Holford TR, Han X,
Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, et al:
International patterns and trends in thyroid cancer incidence,
1973–2002. Cancer Causes Control. 20:525–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiang J, Wu Y, Li DS, Shen Q, Wang ZY, Sun
TQ, An Y and Guan Q: New clinical features of thyroid cancer in
eastern China. J Visc Surg. 147:e53–e56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noguchi S, Noguchi A and Murakami N:
Papillary carcinoma of the thyroid. I. Developing pattern of
metastasis. Cancer. 26:1053–1060. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mazzaferri EL and Jhiang SM: Long-term
impact of initial surgical and medical therapy on papillary and
follicular thyroid cancer. Am J Med. 97:418–428. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hundahl SA, Cady B, Cunningham MP,
Mazzaferri E, McKee RF, Rosai J, Shah JP, Fremgen AM, Stewart AK
and Hölzer S: Initial results from a prospective cohort study of
5583 cases of thyroid carcinoma treated in the united states during
1996. U.S. and German Thyroid Cancer Study Group. An American
College of Surgeons Commission on Cancer Patient Care Evaluation
study. Cancer. 89:202–217. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patron V, Bedfert C, Le Clech G, Aubry K
and Jegoux F: Pattern of lateral neck metastases in N0 papillary
thyroid carcinoma. BMC Cancer. 11:82011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xing M: BRAF mutation in thyroid cancer.
Endocr Relat Cancer. 12:245–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
da Silva RC, de Paula HS, Leal CB, Cunha
BC, de Paula EC, Alencar RC, Meneghini AJ, Silva AM, Gontijo AP,
Wastowski IJ, et al: BRAF overexpression is associated with
BRAFV600E mutation in papillary thyroid carcinomas. Genet Mol Res.
14:5065–5075. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schulten HJ, Alotibi R, Al-Ahmadi A, Ata
M, Karim S, Huwait E, Gari M, Al-Ghamdi K, Al-Mashat F, Al-Hamour
O, et al: Effect of BRAF mutational status on expression profiles
in conventional papillary thyroid carcinomas. BMC Genomics. 16
Suppl 1:S62015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xing M, Alzahrani AS, Carson KA, Shong YK,
Kim TY, Viola D, Elisei R, Bendlová B, Yip L, Mian C, et al:
Association between BRAFV600E mutation and recurrence of papillary
thyroid cancer. J Clin Oncol. 33:42–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yarchoan M, LiVolsi VA and Brose MS: BRAF
mutation and thyroid cancer recurrence. J Clin Oncol. 33:7–8. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jones PA and Baylin SB: The epigenomics of
cancer. Cell. 128:683–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kondo Y and Issa JP: DNA methylation
profiling in cancer. Expert Rev Mol Med. 12:e232010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baylin SB and Ohm JE: Epigenetic gene
silencing in cancer - a mechanism for early oncogenic pathway
addiction? Nat Rev Cancer. 6:107–116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jaenisch R and Bird A: Epigenetic
regulation of gene expression: How the genome integrates intrinsic
and environmental signals. Nat Genet. 33 Suppl:S245–S254. 2003.
View Article : Google Scholar
|
|
16
|
Schweppe RE, Klopper JP, Korch C,
Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow LA, Copland
JA, Smallridge RC, et al: Deoxyribonucleic acid profiling analysis
of 40 human thyroid cancer cell lines reveals cross-contamination
resulting in cell line redundancy and misidentification. J Clin
Endocrinol Metab. 93:4331–4341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu J, Zhang HY, Ma ZZ, Lu W, Wang YF and
Zhu JD: Methylation profiling of twenty four genes and the
concordant methylation behaviours of nineteen genes that may
contribute to hepatocellular carcinogenesis. Cell Res. 13:319–333.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weisenberger DJ, Campan M, Long TI, Kim M,
Woods C, Fiala E, Ehrlich M and Laird PW: Analysis of repetitive
element DNA methylation by MethyLight. Nucleic Acids Res.
33:6823–6836. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xing M: Gene methylation in thyroid
tumorigenesis. Endocrinology. 148:948–953. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Samowitz WS, Albertsen H, Sweeney C,
Herrick J, Caan BJ, Anderson KE, Wolff RK and Slattery ML:
Association of smoking, CpG island methylator phenotype, and V600E
BRAF mutations in colon cancer. J Natl Cancer Inst. 98:1731–1738.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hou P, Ji M and Xing M: Association of
PTEN gene methylation with genetic alterations in the
phosphatidylinositol 3-kinase/AKT signaling pathway in thyroid
tumors. Cancer. 113:2440–2447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Porra V, Ferraro-Peyret C, Durand C,
Selmi-Ruby S, Giroud H, Berger-Dutrieux N, Decaussin M, Peix JL,
Bournaud C, Orgiazzi J, et al: Silencing of the tumor suppressor
gene SLC5A8 is associated with BRAF mutations in classical
papillary thyroid carcinomas. J Clin Endocrinol Metab.
90:3028–3035. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lance-Jones C, Omelchenko N, Bailis A,
Lynch S and Sharma K: Hoxd10 induction and regionalization in the
developing lumbosacral spinal cord. Development. 128:2255–2268.
2001.PubMed/NCBI
|
|
24
|
Gurnett CA, Keppel C, Bick J, Bowcock AM
and Dobbs MB: Absence of HOXD10 mutations in idiopathic clubfoot
and sporadic vertical talus. Clin Orthop Relat Res. 462:27–31.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Samuel S and Naora H: Homeobox gene
expression in cancer: Insights from developmental regulation and
deregulation. Eur J Cancer. 41:2428–2437. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shah N and Sukumar S: The Hox genes and
their roles in oncogenesis. Nat Rev Cancer. 10:361–371. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Botas J: Control of morphogenesis and
differentiation by HOM/Hox genes. Curr Opin Cell Biol. 5:1015–1022.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jung C, Kim RS, Zhang H, Lee SJ, Sheng H,
Loehrer PJ, Gardner TA, Jeng MH and Kao C: HOXB13 is downregulated
in colorectal cancer to confer TCF4-mediated transactivation. Br J
Cancer. 92:2233–2239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raman V, Martensen SA, Reisman D, Evron E,
Odenwald WF, Jaffee E, Marks J and Sukumar S: Compromised HOXA5
function can limit p53 expression in human breast tumours. Nature.
405:974–978. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Friedmann Y, Daniel CA, Strickland P and
Daniel CW: Hox genes in normal and neoplastic mouse mammary gland.
Cancer Res. 54:5981–5985. 1994.PubMed/NCBI
|
|
31
|
Rauch T, Wang Z, Zhang X, Zhong X, Wu X,
Lau SK, Kernstine KH, Riggs AD and Pfeifer GP: Homeobox gene
methylation in lung cancer studied by genome-wide analysis with a
microarray-based methylated CpG island recovery assay. Proc Natl
Acad Sci USA. 104:pp. 5527–5532. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shiraishi M, Sekiguchi A, Terry MJ, Oates
AJ, Miyamoto Y, Chuu YH, Munakata M and Sekiya T: A comprehensive
catalog of CpG islands methylated in human lung adenocarcinomas for
the identification of tumor suppressor genes. Oncogene.
21:3804–3813. 2002. View Article : Google Scholar : View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shiraishi M, Sekiguchi A, Oates AJ, Terry
MJ and Miyamoto Y: HOX gene clusters are hotspots of de novo
methylation in CpG islands of human lung adenocarcinomas. Oncogene.
21:3659–3662. 2002. View Article : Google Scholar : View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carrio M, Arderiu G, Myers C and Boudreau
NJ: Homeobox D10 induces phenotypic reversion of breast tumor cells
in a three-dimensional culture model. Cancer Res. 65:7177–7185.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vardhini NV, Rao PJ, Murthy PB and
Sudhakar G: HOXD10 expression in human breast cancer. Tumour Biol.
35:10855–10860. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sekar P, Bharti JN, Nigam JS, Sharma A and
Soni PB: Evaluation of p53, HoxD10, and E-Cadherin status in breast
cancer and correlation with histological grade and other prognostic
factors. J Oncol. 702527:20142014.
|
|
37
|
Hakami F, Darda L, Stafford P, Woll P,
Lambert DW and Hunter KD: The roles of HOXD10 in the development
and progression of head and neck squamous cell carcinoma (HNSCC).
Br J Cancer. 111:807–816. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Myers C, Charboneau A, Cheung I, Hanks D
and Boudreau N: Sustained expression of homeobox D10 inhibits
angiogenesis. Am J Pathol. 161:2099–2109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang L, Chen S, Xue M, Zhong J, Wang X,
Gan L, Lam EK, Liu X, Zhang J, Zhou T, et al: Homeobox D10 gene, a
candidate tumor suppressor, is downregulated through promoter
hypermethylation and associated with gastric carcinogenesis. Mol
Med. 18:389–400. 2012. View Article : Google Scholar : View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang H, Zhou J, Mi J, Ma K, Fan Y, Ning J,
Wang C, Wei X, Zhao H and Li E: HOXD10 acts as a tumor-suppressive
factor via inhibition of the RHOC/AKT/MAPK pathway in human
cholangiocellular carcinoma. Oncol Rep. 34:1681–1691. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Baek SK, Jung KY, Kang SM, Kwon SY, Woo
JS, Cho SH and Chung EJ: Clinical risk factors associated with
cervical lymph node recurrence in papillary thyroid carcinoma.
Thyroid. 20:147–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wada N, Masudo K, Nakayama H, Suganuma N,
Matsuzu K, Hirakawa S, Rino Y, Masuda M and Imada T: Clinical
outcomes in older or younger patients with papillary thyroid
carcinoma: Impact of lymphadenopathy and patient age. Eur J Surg
Oncol. 34:202–207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ito Y, Hirokawa M, Jikuzono T, Higashiyama
T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Kuma K and
Miyauchi A: Extranodal tumor extension to adjacent organs predicts
a worse cause-specific survival in patients with papillary thyroid
carcinoma. World J Surg. 31:1194–1201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Oh CM, Jung KW, Won YJ, Shin A, Kong HJ
and Lee JS: Age-period-cohort analysis of thyroid cancer incidence
in Korea. Cancer Res Treat. 47:362–369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park KS, Oh YL, Ki CS and Kim JW:
Evaluation of the Real-Q BRAFV600E detection assay in fine-needle
aspiration samples of thyroid nodules. J Mol Diagn. 17:431–437.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zou M, Baitei EY, Alzahrani AS, BinHumaid
FS, Alkhafaji D, Al-Rijjal RA, Meyer BF and Shi Y: Concomitant RAS
RET/PTC, or BRAF mutations in advanced stage of papillary thyroid
carcinoma. Thyroid. 24:1256–1266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hwang TS, Kim WY, Han HS, Lim SD, Kim WS,
Yoo YB, Park KS, Oh SY, Kim SK and Yang JH: Preoperative RAS
mutational analysis is of great value in predicting follicular
variant of papillary thyroid carcinoma. Biomed Res Int.
2015:6970682015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Armstrong MJ, Yang H, Yip L, Ohori NP,
McCoy KL, Stang MT, Hodak SP, Nikiforova MN, Carty SE and Nikiforov
YE: PAX8/PPARγ rearrangement in thyroid nodules predicts
follicular-pattern carcinomas, in particular the encapsulated
follicular variant of papillary carcinoma. Thyroid. 24:1369–1374.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gómez Sáez JM: Diagnostic and prognostic
markers in differentiated thyroid cancer. Curr Genomics.
12:597–608. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hoque MO, Rosenbaum E, Westra WH, Xing M,
Ladenson P, Zeiger MA, Sidransky D and Umbricht CB: Quantitative
assessment of promoter methylation profiles in thyroid neoplasms. J
Clin Endocrinol Metab. 90:4011–4018. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hu S, Ewertz M, Tufano RP, Brait M,
Carvalho AL, Liu D, Tufaro AP, Basaria S, Cooper DS, Sidransky D,
et al: Detection of serum deoxyribonucleic acid methylation
markers: A novel diagnostic tool for thyroid cancer. J Clin
Endocrinol Metab. 91:98–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Smith JA, Fan CY, Zou C, Bodenner D and
Kokoska MS: Methylation status of genes in papillary thyroid
carcinoma. Arch Otolaryngol Head Neck Surg. 133:1006–1011. 2007.
View Article : Google Scholar : PubMed/NCBI
|