Introduction
Infantile hemangioma (IH) affects approximately
4–10% of infants, and induces serious morbidity and mortality
(1,2). Although Hemangeol™ (propranolol
hydrochloride oral solution), the first US Food and Drug
Administration (FDA) approved drug for IH, is effective, its
adverse effects include aggravated respiratory tract infections
(3,4). Its twice daily oral administration
also reduces the compliance of patients. Thus, it is necessary to
develop novel alternative drugs to treat IH.
Urea, a normal body metabolite, is crucial for the
metabolism of nitrogenous compounds (5). Significantly, urea is highly soluble
in water, and practically non-toxic (LD50 is 15 g/kg for
rats). Urea-containing creams are widely used in various skin
diseases (6). Since 1970s, local
injection of urea has been used in the treatment of IH only in
China (7). In our hospital (Henan
Provincial People's Hospital, Zhengzhou, China), local injection of
urea has been used in the treatment of IH for 20 years (8–11). In
a clinical trial consisting of 869 cases of IH in our hospital
during 2006–2009, we demonstrated that 54.6% of the cases were
cured, and 31.4% were improved after urea injection, without
observing severe complications (8).
In a clinical trial consisting of 167 cases of IH in our hospital
during 2009–2011, we demonstrated that 87.4% of the cases were
cured, and 12.6% were improved after urea injection, without
observing severe complications (10). The mechanisms underlying the
therapeutic efficacy of urea against IH include direct cytotoxic
effects towards hemangioma cells and protein denaturation (7,11).
Since urea is injected topically, its side-effects are rare
(7–11). The primary side-effect of urea
injection for the treatment of IH is skin irritation, which could
disappear spontaneously in 3–5 days, and does not need special
treatments (7–11). Thus, urea is expected to be an
effective and well-tolerated treatment for IH. Although urea local
injection has achieved superior therapeutic effects in the
treatment of IH, daily injection significantly reduce the
compliance of patients (7–11). Thus, it is urgent to reduce the high
frequency of administration of urea in the treatment of IH.
To overcome the daily administration, a topical
controlled delivery modality could be used for the treatment of IH.
The topical administration could target diseases directly, and
minimize unpredictable absorption and side-effects (12,13).
Furthermore, topical delivery modalities could realize controlled
release of drugs, resulting in long-lasting potential therapeutic
efficacy, with reduced drug doses and administration frequency
(14). Thus, we hypothesized that a
topical controlled release systems could decrease the high
administration frequency of urea.
Liposomes, featured by their good biocompatibility
and long in vivo circulation, have been widely used as a
controlled release system (15–17).
Nevertheless, since liposomes are soft, liposomal sustained drug
release is not good (18,19). The biocompatible
poly(lactic-co-glycolic acid) (PLGA) microspheres, which are
more rigid, have been commonly used as a controlled delivery system
(20). Notably, some researchers
have realized that the combination of liposomes and PLGA
microspheres (liposomes-in-microspheres, LIM), could exert their
advantages and avert their disadvantages (21,22).
After being coated with PLGA, the stability and drug loading of
liposomes could be improved, since both the polymer and liposomal
bilayers could retard drug release. Furthermore, by the existence
of the lipids on the surface, the biocompatibility of PLGA
microspheres could be improved. It is conceivable that liposomes
could still act as drug sustained release reservoirs after the
liposomes are released from the microspheres. Several researchers
have developed LIM for successful sustained release of various
drugs suffered from quick drug release from liposomes (21,22).
To reduce the daily administration of urea, we
firstly utilized urea-loaded liposomes-in-microspheres (ULIM) as a
novel topical controlled release system to realize the sustained
release of urea. ULIM were developed from encapsulating urea-loaded
liposomes in microspheres made of poly(lactic-co-glycolic
acid)-b-poly(ethylene glycol)-b-poly(lactic-co-glycolic acid)
copolymers. The characteristics, activity and mechanism against IH
of ULIM were examined in vitro and in vivo.
Materials and methods
Reagents and cell culture
Poly(lactic-co-glycolic
acid)-b-Poly(ethylene
glycol)-b-Poly(lactic-co-glycolic acid) copolymers
(Mw 11,900, PLGA-PEG-PLGA, 1:1 LA:GA, 70 kDa-4.6 kDa-70 kDa) was
purchased from Akina, Inc. (West Lafayette, IN, USA). Hydro soy
phosphatidylcholine (HSPC) and cholesterol were provided by Avanti
Polar Lipids (Alabaster, AL, USA). Propranolol (hydrochloride
salt), urea, poly(vinyl alcohol) (PVA, Mw 30,000–70,000), and
chitosan (hydrochloride salt) were provided by Sigma-Aldrich (St.
Louis, MO, USA). Matrigel was provided by Becton-Dickinson
(Franklin Lakes, NJ, USA). All organic reagents were of analytical
grade and purchased from China Sinopharm International Co., Ltd.
(Shanghai, China).
The human hemangioma endothelial cells (HemECs)
derived from the IH of patients were isolated as previously
described (23,24). The present study was approved by the
Research Ethics Committee of Henan Provincial People's Hospital
(Zhengzhou, China). Written informed consent was obtained from all
the patients. All specimens were handled and made anonymous
according to the ethical and legal standards. First, IH specimens
were obtained in Henan Provincial People's Hospital, and the
clinical diagnosis was confirmed at the Department of Pathology in
Henan Provincial People's Hospital. The excised IH specimens were
stored in Endothelial Cell Growth Medium-2 (EGM-2; Lonza,
Walkersville, MD USA) at 4°C. Then the specimen was cut and
trypsinized by 0.25% trypsin at 37°C. Then EGM-2 with 10% fetal
bovine serum (FBS) was added to block the trypsinization. The
tissue blocks were inoculated in culture plates coated with
gelatin. After inoculation, EGM-2 with 10% FBS was added to the
tissue block, and the culture medium was replaced once every three
days. The cells were subcultured at a 1:3 ratio when the cells
reached confluence.
Preparation of urea-loaded
liposomes-in-microspheres
Urea-loaded liposomes were prepared by the reverse
evaporization method as previously described (25). Briefly, specific amounts of lipids
(20 µmol, HSPC:cholesterol = 55:45, molar ratios) were dissolved in
3 ml diethyl ether. One milliliter of urea solution (4 mg/ml) was
added to the above lipid solution. The mixture was sonicated for 10
min to form stable W/O (water in oil) emulsion. The diethyl ether
was then removed by reduction vaporization at 25°C. After then,
distilled water was added, and the resultant multilamellar
liposomes (MLL) were obtained. Unilamellar liposomes (ULL) were
obtained by the extrusion of the MLL using a LiposoFast™ extruder
(Avestin, Ottawa, ON, Canada) with 200-nm pore size membranes
(Whatman® Nuclepore™ membrane; Thermo Fisher Scientific)
with 10 cycles at 65°C. Sephadex G-50 Gel filtration separated the
ULL from the non-encapsulated urea. For the coating of liposomes
with chitosan, 1 ml liposome solution was mixed with 2 ml 1.5%
chitosan solution and incubated at 4°C overnight.
Subsequently, urea-loaded liposomes-in-microspheres
(ULIM) were prepared. After urea-loaded liposome solution (0.5 ml)
was dispersed into an organic phase (20 mg PLGA-PEG-PLGA dissolved
in 5 ml ethyl acetate) by vortex mixing, the W/O emulsion was
injected drop-by-drop into 50 ml 2% PVA solution and underwent
mechanical stirring (1,000 rpm for 5 min). The resultant W/O/W
emulsion was poured into 2% PVA aqueous solution (450 ml). After
then, the solution was stirred (500 rpm, 3 h) to evaporate the
organic solvent. The final microspheres were obtained after
filtration, washing and freeze-drying. Urea-loaded microspheres
(UM) were prepared in the same way as ULIM, except that urea-loaded
liposome solution was replaced with urea solution (0.5 ml, 4 mg/ml)
as the water phase.
The following abbreviations are used: urea loaded
liposomes (UL), urea-loaded microspheres (UM), and urea-loaded
liposomes-in-microspheres (ULIM). Drug-free liposomes or
microspheres are designated as blank liposomes or microspheres.
Size and zeta potential
The size and zeta potential were analyzed by a
Zetasizer Nano S (Malvern Instruments Ltd., Malvern, UK). The size
and distribution of microspheres were tested by a Malvern
Mastersizer 2000 particle size analyzer (Malvern Instruments).
Drug encapsulation efficiency and
loading of urea
The encapsulation efficacy and drug loading of urea
in the formulations were determined as described below. After 0.2
ml of the liposome solution was dissolved in methanol, the clear
solution was analyzed by high performance liquid chromatography
(HPLC L-2000; Hitachi). Alternatively, 5 mg of microspheres were
dissolved in 1 ml dichloromethane. After dichloromethane was
evaporated, 1 ml methanol was added for HPLC analysis. A Hypersil
NH2 column (250 × 4.6 mm, 5 µm) was equipped in the
HPLC, and the mobile phase was acetonitrile:water (95:5, v/v). The
flow rate was 1 ml/min. The detection wavelength was 190 nm. The
column temperature was 25°C. The encapsulation efficacy of urea =
the mass of encapsulated urea/the mass of total added urea × 100%.
The drug loading of urea = the mass of encapsulated urea/the mass
of liposomes or microspheres × 100%.
In vitro drug release
Two milliliters liposomes or 10 mg microspheres was
transferred to a Spectra/Por® dialysis membrane (MWCO
1000). The sealed tube was put into a vial with 200 ml
phosphate-buffered saline (PBS) (pH 7.4, with or without 10% FBS).
The vial was put in a water bath at 37°C with stirring (100 rpm). A
total of 2 ml of an aliquot of dialysate was taken out at different
time-points. The concentration of urea was determined as described
above.
CCK-8 assays
HemECs (10,000/well) were seeded at subconfluency on
a fibronectin-coated 96-well plate in EGM-2 with 10% FBS. Twelve
hours later, the media were removed and the cells were treated with
various concentrations of urea, liposomes or microspheres. After 72
or 120 h, the cell viability was determined using the CCK-8 kit
according to the manual.
Cell treatments and transfections
HemECs (200,000/well) were seeded on a
fibronectin-coated 6-well plate in EGM-2 with 10% FBS. Twelve hours
later, the medium was removed, and the cells were treated with urea
(0, 10, 20 and 50 µg/ml) and incubated for a period of time (48, 72
or 96 h). For gene knockdown or overexpression, specific siRNA
(Santa Cruz Biotechnology, Santa Cruz, CA, USA) or recombinant
lentiviral vectors (Shanghai GenePharma Co., Ltd., Shanghai, China)
were transfected to the cells and incubated for 48 h, before
collection for analysis.
Western blot analysis
The cellular proteins were extracted, and separated
by SDS-polyacrylamide gel electrophoresis. After the proteins were
transferred onto polyvinylidene fluoride membranes, the membrane
were incubated with the primary antibodies including anti-human
HIF-1α or VEGF and horseradish peroxidase conjugated to goat
anti-mouse IgG as the secondary antibody (Santa Cruz
Biotechnology). The GAPDH antibody (Santa Cruz Biotechnology) was
used as the internal control antibody. The bands were detected with
the Enhanced Chemiluminiscence kit (GE Healthcare) and visualized
with the ChemiDoc XRS system (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Analysis of vascular endothelial
growth factor (VEGF)-A
HemECs (200,000/well) were seeded on a
fibronectin-coated 6-well plate in EGM-2 with 10% FBS. Twelve hours
later, the medium was removed and the cells were treated with
various concentrations of the drugs. After a period of time, the
VEGF-A concentration in the cellular supernatant was measured by
VEGF-A ELISA kits (R&D Systems, Minneapolis, MN, USA) according
to the manufacturer's instructions. In brief, 200 µl of the sample
was added to the plate and incubated for 2 h. The sample was
aspirated and washed. Afterwards, 200 µl of conjugate was added and
incubated for 2 h. Finally, the substrate solution and stop
solution was added sequentially. The absorbance was measured at
450/540 nm using a BioTek ELx800 Universal microplate reader.
Animal studies
The mice were purchased from the Shanghai
Experimental Animal Center of the Chinese Academy of Sciences
(Shanghai, China). All procedures were approved by the Committee on
Animals of the Second Military Medical University (Shanghai, China)
and all procedures were performed in accordance with the guidelines
of the Committee on Animals of the Second Military Medical
University (Shanghai, China).
A xenograft mouse model of IH was used to study the
effects of urea on IH in vivo. HemECs (1×107)
suspended in Matrigel were implanted subcutaneously into the flanks
of female nude mice (6–8 weeks, ~20 g). After the hemangioma
reached ~25 mm3 on day 0, mice were treated with single
intratumoral (i.t.) injections of either formulation (UL, UM or
ULIM, 2 mg urea/kg), free urea (2 mg urea/kg) or blank LIM (80
mg/kg). The treatment was performed once every 5 days for 7 times
(days 0, 5, 10, 15, 20, 25 and 30). Propranolol was administered
orally (2 mg/kg, daily) for 30 days. Hemangioma was measured with a
caliper, and the hemangioma volume = (width2 ×
length)/2. The mice were euthanized on day 35. The hemangioma was
embedded in paraffin and stained with H&E. The analysis of
microvessel density (MVD) in the sections was performed as
previously described (26).
Statistical analysis
Data were analyzed with the software SPSS 13.0
(SPSS, Inc., Chicago, IL, USA). A direct comparison between two
groups was performed with the Students non-paired t-test. One-way
ANOVA with the Dunnetts or Newman Keuls post-test was used to
compare the means of three or more groups. A P<0.05 was
considered statistically significant: *P<0.05; **P<0.01;
***P<0.001; n.s. represents not significant (P>0.05).
Results
Preparation of urea-loaded
liposomes-in-microspheres (ULIM)
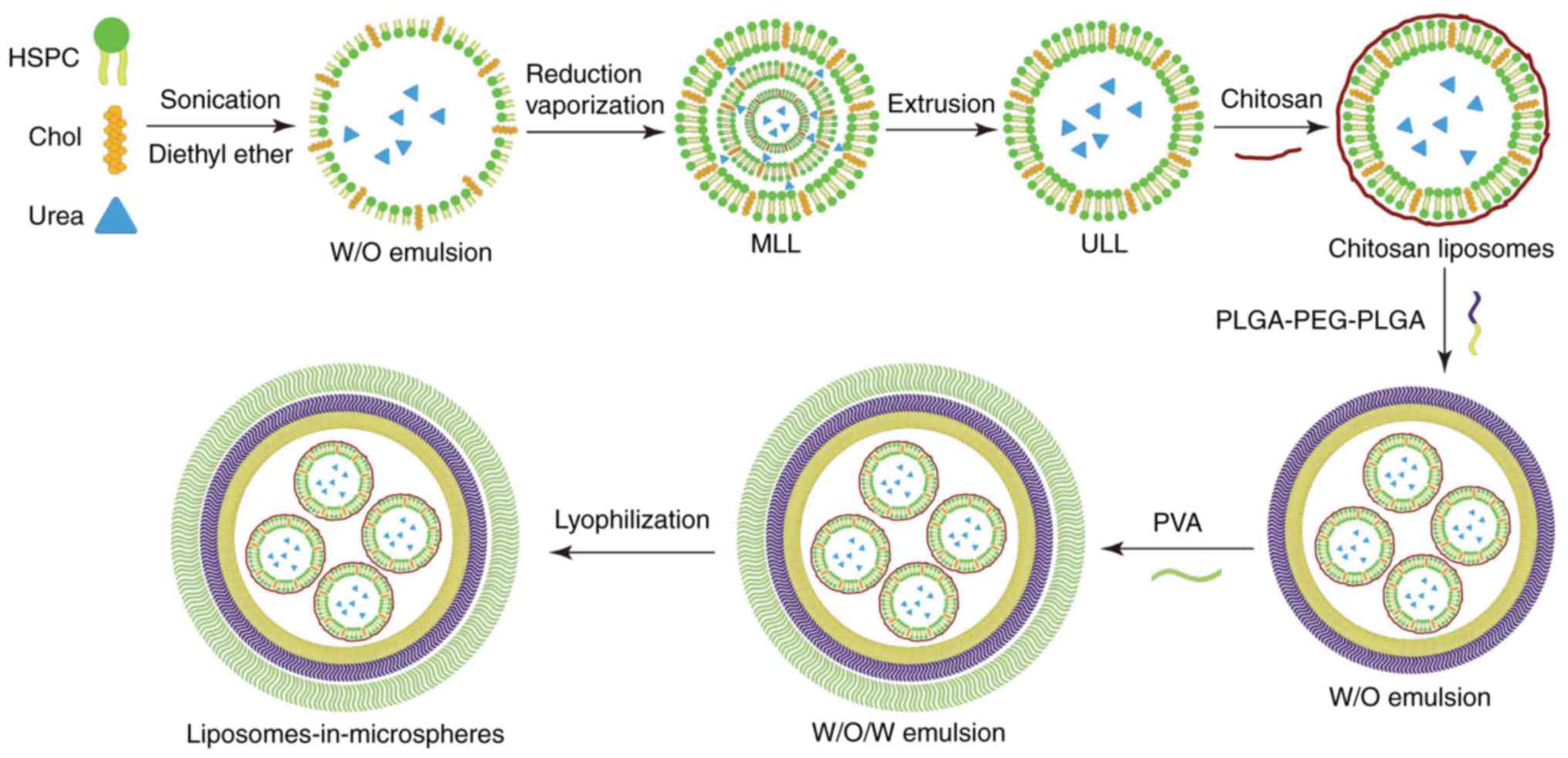
As described in Fig.
1, we firstly used the reverse evaporization method to develop
urea-loaded liposomes (UL), and then encapsulated UL into
microspheres to develop urea-loaded liposomes-in-microspheres
(ULIM). We chose HSPC as the phospholipids in liposome preparation
since it is saturated, neutral and has high phase transition
temperature. Reverse vaporization was chosen to load urea in
liposomes, since it is well regarded to encapsulate hydrophilic
drugs in liposomes with high encapsulation efficacy.
Although the hydrophilic head groups protect
liposomes, the liposomes could be readily damaged by the organic
solvent used for the preparation of ULIM. Thus, chitosan was used
to coat the liposomes to protect the
liposomes-in-microspheres. Double emulsions are commonly used for
encapsulating hydrophilic drugs suffering from low encapsulation
efficiency due to rapid drug partitioning into the external aqueous
phase when using single emulsions. Thus, the double emulsion method
was chosen to encapsulate hydrophilic liposomes in the
microspheres.
Characteristics of UL and ULIM
Table I summarizes
the characteristics of the liposomes and microspheres. The size of
UL was 186 nm, with a low PDI of 0.16 (suggesting a homogeneous
size distribution). UL showed a negative zeta potential of −6.8 mV.
The encapsulation efficacy (EE) of urea in UL is 31.7%, and the
drug loading of UL is 10.3%, suggesting that reverse vaporization
is a suitable approach to encapsulate urea in liposomes. ULIM have
a size of 62.4 µm, which is similar to that of UM (53.8 µm). The EE
of urea in ULIM is 51.5%, which is similar to that of UM
(55.2%).
 | Table I.Characterization of nanoparticles and
microspheres.a |
Table I.
Characterization of nanoparticles and
microspheres.a
|
| Size (nm/µm) | Zeta potential
(mV) | PDI | EE (%) | Drug loading
(%) |
|---|
| UL | 186.3±24.7 nm | −6.8±2.4 | 0.16±0.04 | 31.7±8.7 | 10.3±3.1 |
| ULIM | 62.4±21.3 µm | – | – | 51.5±8.6 | 2.5±0.5 |
| UM | 53.8±25.9 µm | – | – | 55.2±4.2 | 3.4±2.7 |
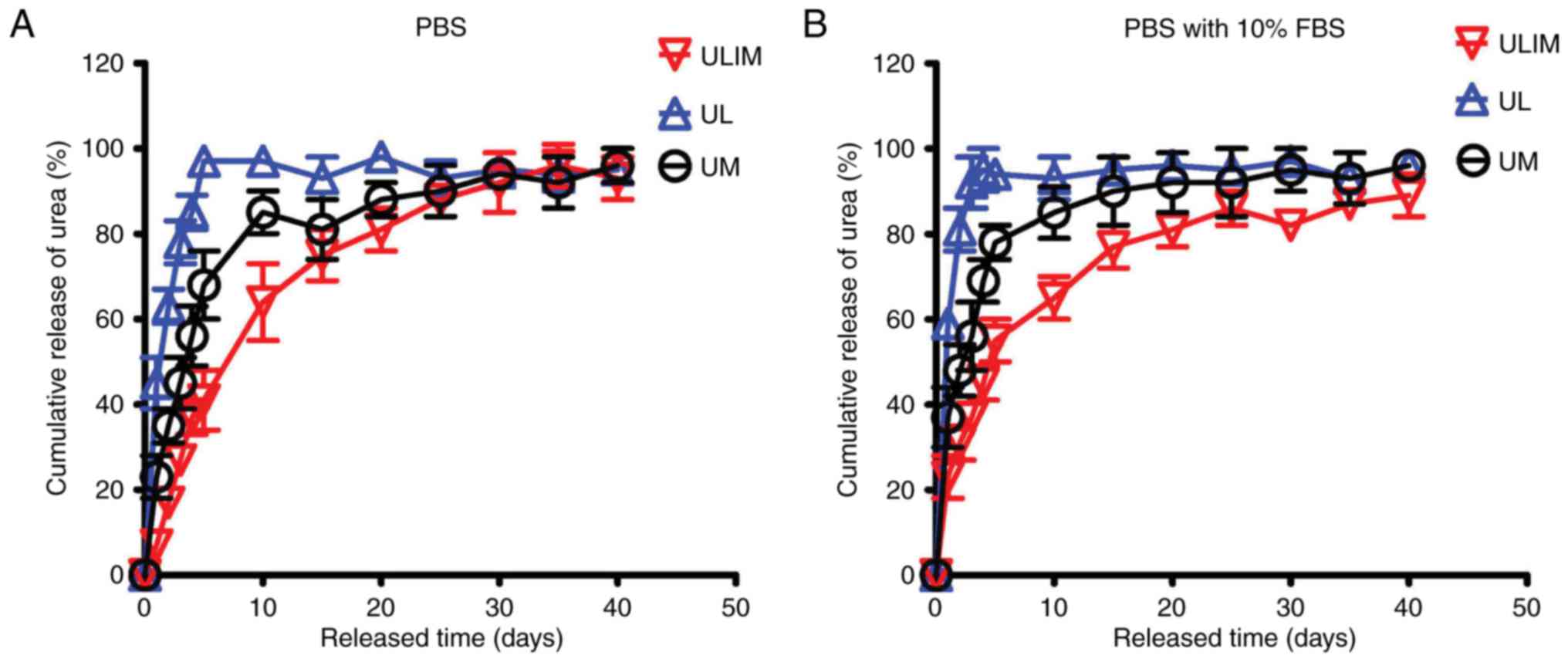
The drug release profiles of UL, UM and ULIM were
evaluated in PBS and PBS with 10% FBS. In PBS, UL showed a quick
urea release (45% of urea was released at day 1, and 85% at day 4)
(Fig. 2A). The reason why UL
quickly released urea is that hydrophilic drugs tend to permeate
across the liposomal membrane quickly (19). In contrast, UM showed a reduced
release (23% of urea was released at day 1 and 56% at day 4).
Significantly, ULIM showed the slowest release (only 7% of urea was
released at day 1 and 37% at day 4). It took 4, 10 and 20 days for
UL, UM and ULIM to release >80% of urea, respectively.
Similar results were obtained in the urea release in
PBS with 10% FBS. UL showed a quick urea release (59% of urea was
released at day 1 and 95% at day 4) (Fig. 2B). In contrast, UM showed a reduced
release (37% of urea was released at day 1 and 69% at day 4).
Significantly, ULIM showed the slowest release (only 23% of urea
was released at day 1 and 45% at day 4). It took 2, 10 and 20 days
for UL, UM and ULIM to release >80% of urea, respectively.
Thus, the urea release was significantly reduced in
ULIM compared with UL and UM, suggesting that encapsulation of
urea-loaded liposomes-in-microspheres significantly retard the
release of urea from liposomes or microspheres.
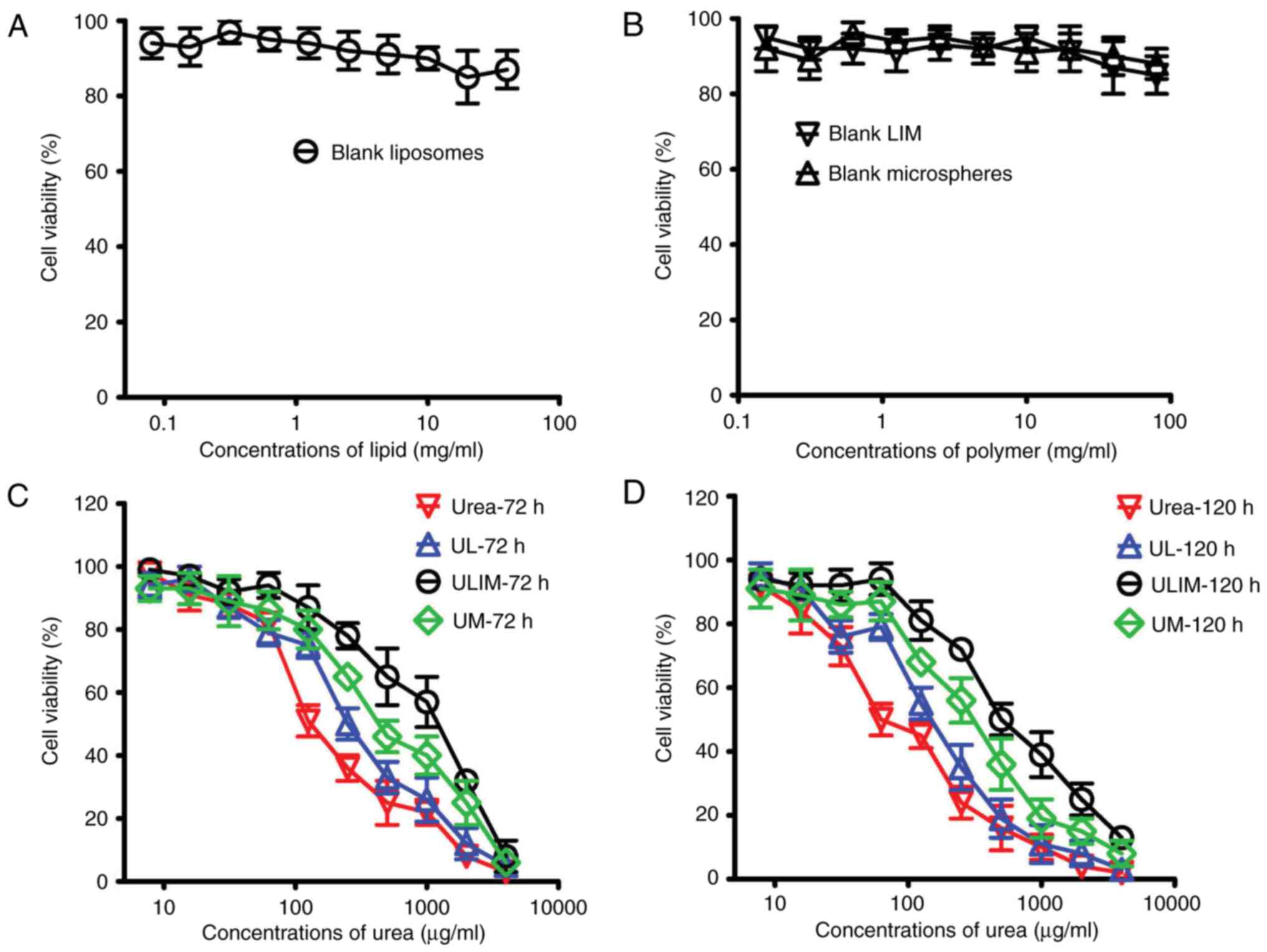
Cytotoxicity towards HemECs
To assess the biocompatibility of our prepared
formulations, we evaluated the cytotoxicity of the blank liposomes,
microspheres and LIM. As shown in Fig.
3A and B, all the blank formulations showed little toxicity to
HemECs (reflected by the cell viability which exceeded 85%).
On the contrary, urea, UL, UM and ULIM showed a
dose-dependent cytotoxicity towards HemECs (Fig. 3C and D). Their IC50
values are shown in Table II. The
72 h IC50 values of urea, UL, UM and ULIM were found to
be 168, 218, 502 and 1238 µg/ml, respectively, suggesting that the
cytotoxic effect of urea was significantly decreased when urea is
loaded in microspheres or liposomes-in-microspheres. Similar
results were obtained in the 120 h IC50 values. The 120
h IC50 values of urea, UL, UM and ULIM were found to be
98, 133, 389 and 631 µg/ml, respectively. We speculate that the
significantly reduced cytotoxic effects of UM and ULIM are
attributed to their slow release of urea.
 | Table II.IC50 values in HemECs
following 72 and 120 h of treatment.a |
Table II.
IC50 values in HemECs
following 72 and 120 h of treatment.a
| IC50,
µg/ml | 72 h | 120 h |
|---|
| Urea | 168±29 | 98±21 |
| UL | 218±36 | 133±32 |
| UM | 502±52 | 389±76 |
| ULIM | 1238±252 | 631±139 |
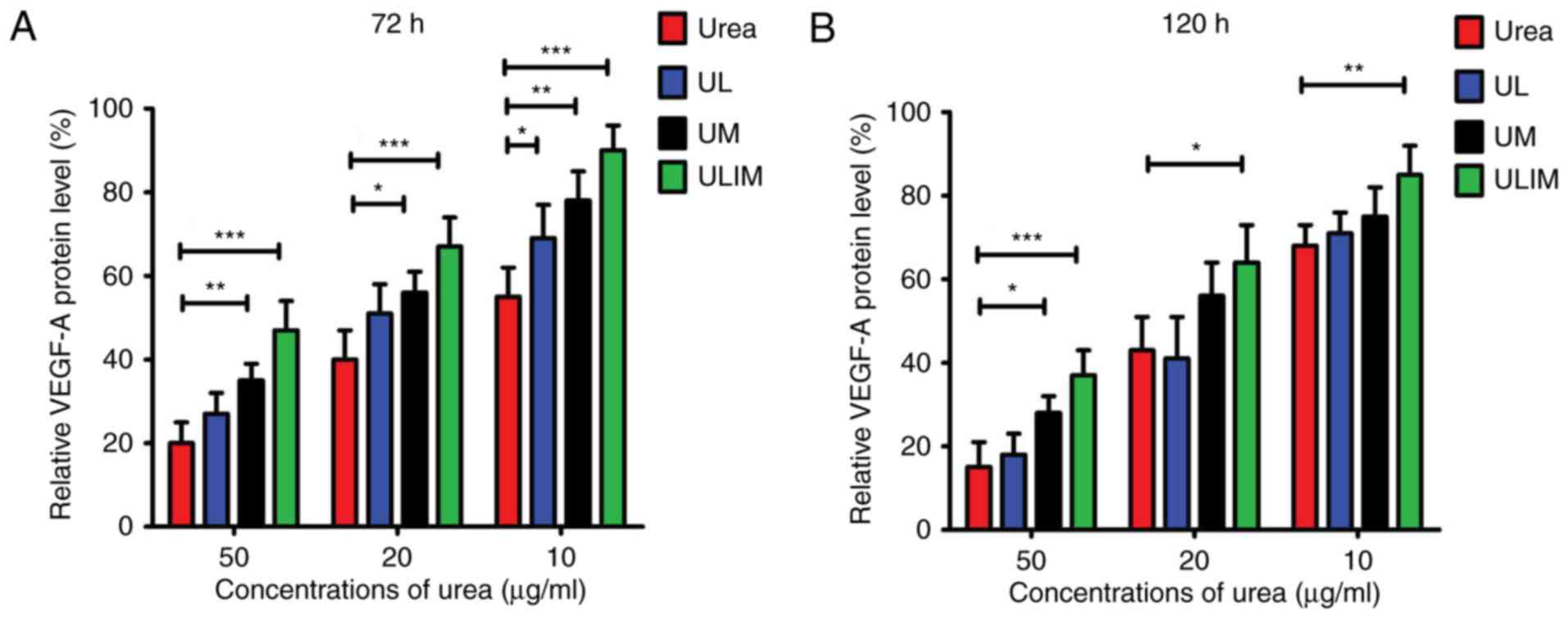
VEGF-A expression level in HemECs
after treatment
The heparin-binding growth factor VEGF-A is able to
induce angiogenesis (27). Fig. 4A shows that urea, UL, UM and ULIM
exerted dose-dependent inhibition of VEGF-A protein expression in
HemECs at 72 h. At the concentrations of urea ranging from 10 to 50
µg/ml, urea was more efficient than UM and ULIM in the inhibition
of VEGF-A expression (P<0.05 for UM, P<0.001 for ULIM). Urea
at 50 µg/ml inhibited VEGF-A expression by ~80%, whereas ULIM only
inhibited by ~60%. Consistently, urea was more effective at
inhibiting VEGF-A expression than ULIM at the concentrations of
urea ranging from 10 to 50 µg/ml at 120 h (P<0.05; Fig. 4B). At 50 µg/ml, urea inhibited
VEGF-A expression by >80%, whereas ULIM inhibited VEGF-A
expression by ~60%. Taken together, urea was more effective in
inhibiting both VEGF-A expression than ULIM. We speculate that the
significant reduced activity of ULIM against inhibition of VEGF-A
expression is attributed to its slow release of urea.
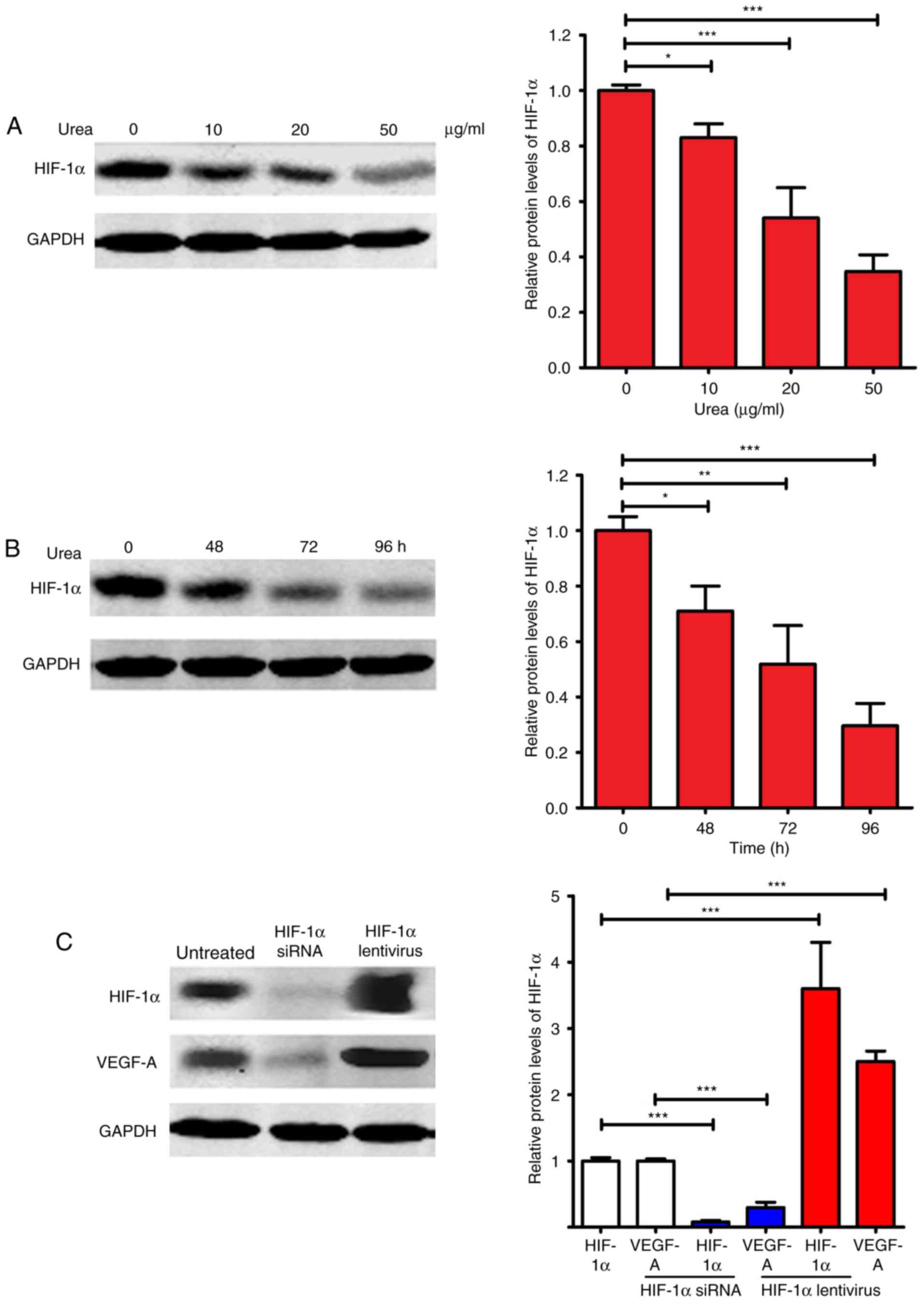
HIF-1α expression level in HemECs
after treatment
Hypoxia-inducible factor-1α (HIF-1α) and VEGF-A are
critical factors in promoting angiogenesis (28,29).
HIF-1α is a master regulator of VEGF-A, and could activate
transcription of the VEGF-A gene (29). We evaluated the expression of HIF-1α
after urea treatment. As shown in Fig.
5A and B, urea inhibited the expression of HIF-1α in a dose-
and time-dependent manner. At 50 µg/ml, urea inhibited HIF-1α
expression by ~70% relative to the untreated control (P<0.001).
At 96 h, urea at 20 µg/ml inhibited HIF-1α expression by ~75%
relative to the untreated control (P<0.001). Since the
expression of VEGF-A is directly regulated by HIF-1α, we
investigated whether regulated expression of HIF-1α affects VEGF-A
expression (Fig. 5C). After the
expression of HIF-1α was knocked down by HIF-1α siRNA, the
expression of VEGF-A was significantly repressed (P<0.001). In
contrast, the expression of VEGF-A was significantly upregulated
(P<0.001) after the overexpression of HIF-1α by transfection of
HIF-1α lentivirus. Taken together, these results suggested that the
downregulated VEGF-A expression after urea treatment was at least
partially caused by the urea-induced inhibition of HIF-1α
expression.
Inhibition of subcutaneous hemangioma
growth in vivo
The therapeutic effect of the various formulations
was examined in mice bearing subcutaneous hemangioma. As shown in
Fig. 6A, on day 35, blank
liposomes-in-microspheres (LIM) did not show significant antitumor
activity, and the blank LIM-treated hemangioma progressed rapidly.
As expected, oral administration of propranolol achieved superior
therapeutic efficacy, and resulted in a 78% decrease in hemangioma
volume. Notably, ULIM treatment resulted in a 95% decrease in
hemangioma volume, whereas UM, UL and urea treatment only resulted
in a 70, 60 and 35% decrease, respectively. At the end point,
compared with the initial volume (25 mm3), the
hemangioma volume in the propranolol-treated mice had increased by
2.4-fold, whereas the hemangioma volume in the ULIM-treated mice
did not increase but gradually decreased. The hemangioma volume of
the ULIM-treated group was significantly smaller than that of other
groups (saline, 278 mm3; blank LIM, 209 mm3;
urea, 180 mm3; UL, 111 mm3; UM, 84
mm3; ULIM, 13 mm3; ULIM vs. saline:
P<0.001; ULIM vs. urea, P<0.001; ULIM vs. UL, P<0.001;
ULIM vs. UM, P<0.001; ULIM vs. propranolol, P<0.01; ULIM vs.
blank LIM, P<0.001) (Fig.
6B).
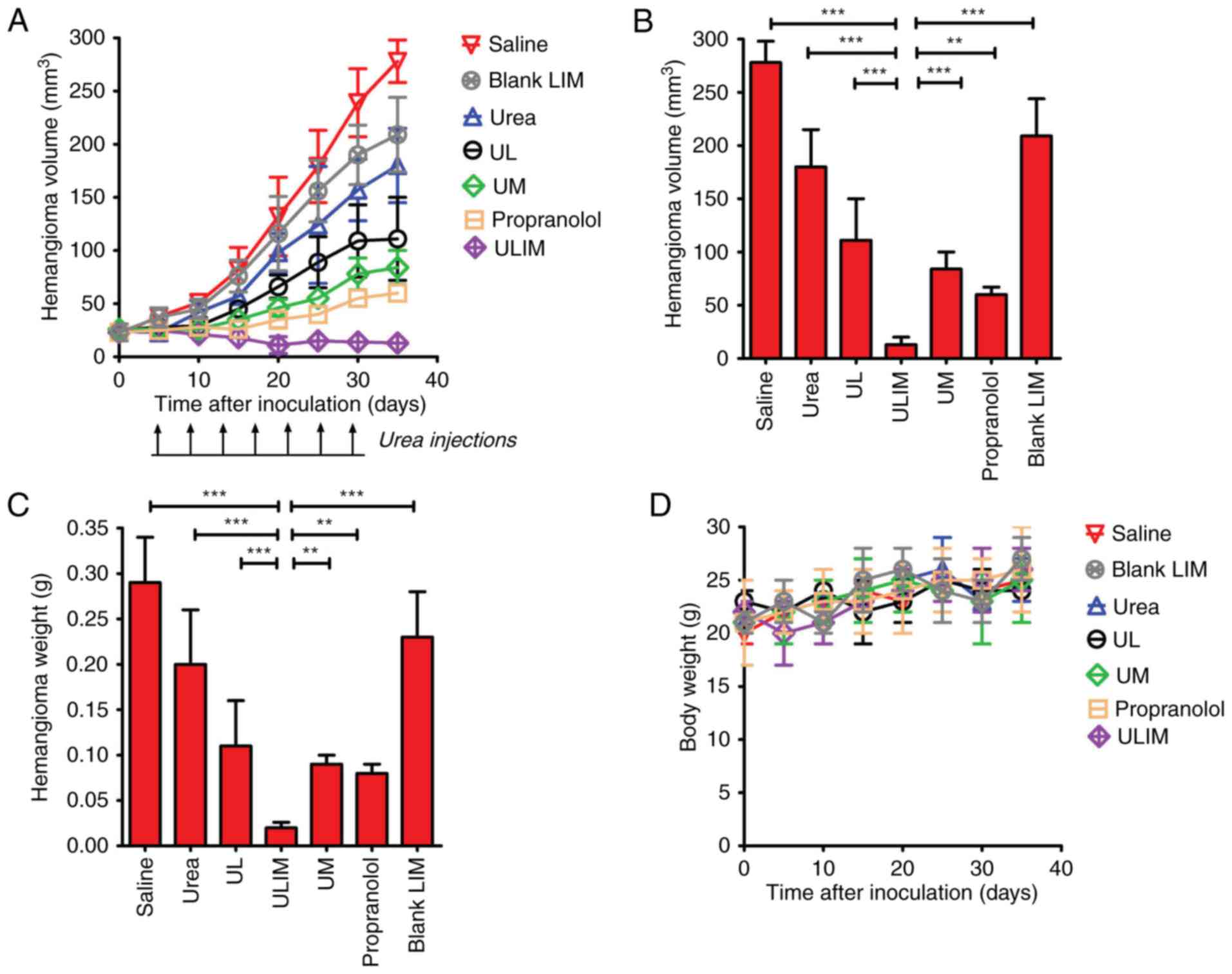 | Figure 6.Therapeutic effect of urea in mice
bearing subcutaneous IH xenografts. When the hemangiomas had
reached ~25 mm3 in size (day 0), mice were treated with
single intratumoral injections of either formulation (UL, UM and
ULIM, 2 mg urea/kg), free urea (2 mg urea/kg) or blank LIM (80
mg/kg). Propranolol was administrated orally (2 mg/kg, daily) for
30 days. Treatments were carried out on days 0, 5, 10, 15, 20, 25
and 30 (indicated by black arrows). (A) The emangioma growth curve.
(B) Hemangioma volume at the end point (day 35). (C) The excised
tumors were weighed at the end point. The tumor volume or weight of
the ULIM-treated group was compared with that of other groups by
one-way ANOVA with the Dunnett's post-test. *P<0.05;
**P<0.01; ***P<0.001. (D) The weight change of the mice
during the treatment. The body weight of the mice was monitored
once every five days. Data are expressed as mean ± SD (n=8). UL,
urea-loaded liposomes; UM, urea-loaded microspheres; ULIM,
urea-loaded liposomes-in-microspheres. |
The hemangioma was weighed at the endpoint (Fig. 6C). The mean hemangioma weight of the
ULIM-treated group was significantly lower than that of other
groups (saline, 0.28 g; urea, 0.20 g; UL, 0.11 g; ULIM, 0.02 g; UM,
0.09 g; propranolol, 0.08 g; blank LIM, 0.23 g; ULIM vs. saline,
P<0.001; ULIM vs. urea, P<0.001; ULIM vs. UL, P<0.001;
ULIM vs. UM, P<0.01; ULIM vs propranolol, P<0.05; ULIM vs.
blank LIM, P<0.001).
The toxicity of all treatments was measured by
observing any physical or behavioral changes post treatment and by
monitoring the weight of mice. In the urea-treated group, skin
irritation, as reflected by the red and swollen skin, was observed
in some mice, but all cases of skin irritation disappeared
spontaneously in 3 days and need no special treatments. None of the
treated mice showed any behavioral changes. None of the treated
mice showed significant change in weight compared to the saline
control (Fig. 6D). Taken together,
the results showed that all the treatments were well-tolerated by
mice bearing hemangioma.
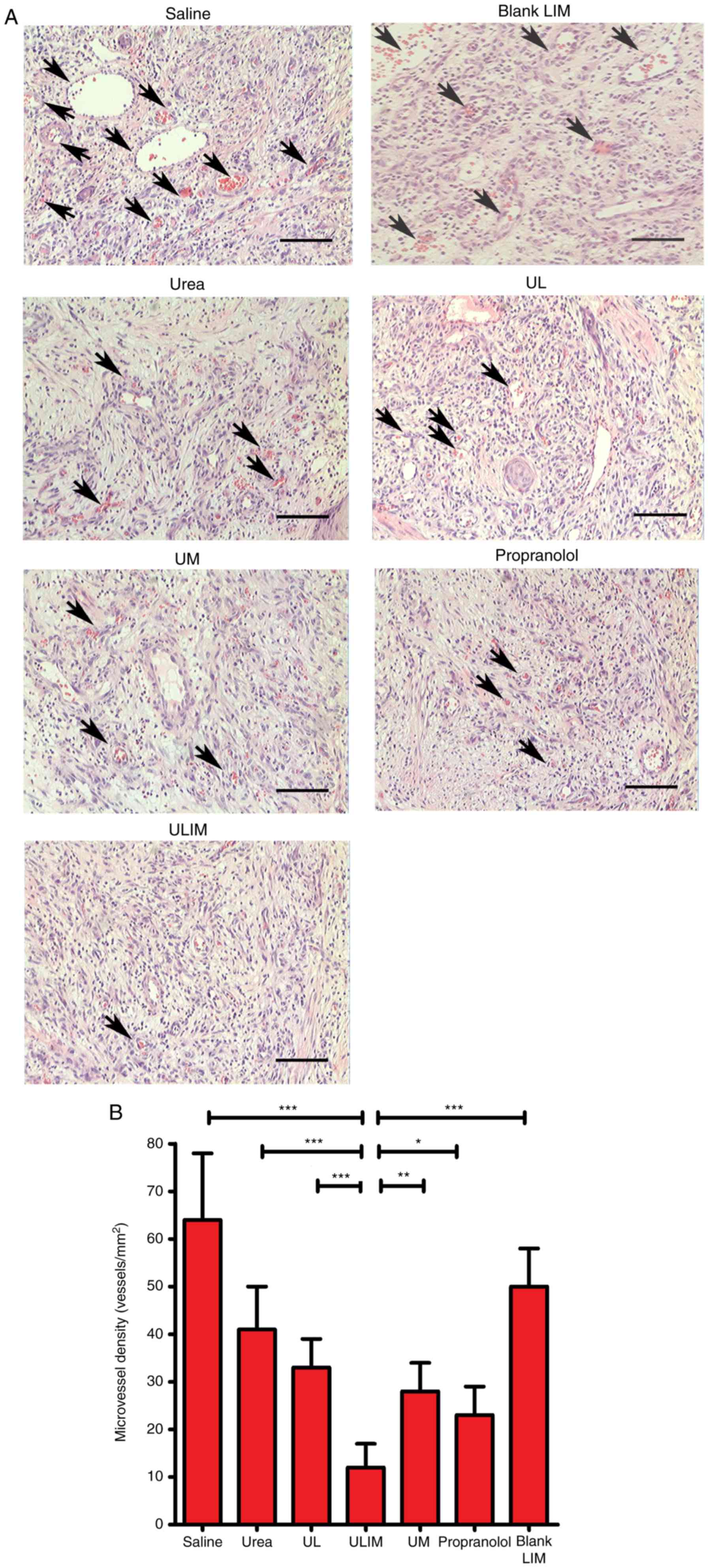
The hemangiomas excised from the mice were stained
with hematoxylin and eosin (H&E) (Fig. 7A), and microvessel density (MVD)
analysis of the histological sections was performed (Fig. 7B). The mean MVD of the ULIM-treated
group was significantly lower than that of other groups (saline, 64
vessels/mm2; urea, 41 vessels/mm2; UL, 33
vessels/mm2; ULIM, 12 vessels/mm2; UM, 28
vessels/mm2; propranolol, 23 vessels/mm2;
blank LIM, 50 vessels/mm2; ULIM vs. saline: P<0.001,
ULIM vs. urea: P<0.001; ULIM vs. UL: P<0.001; ULIM vs. UM:
P<0.01; ULIM vs. propranolol: P<0.05; ULIM vs. blank LIM:
P<0.001), suggesting that ULIM was the most effective at
inhibiting the vascularization of hemangioma among all the
groups.
Discussion
IH is a benign pediatric tumor, and rapid growth of
IH can result in serious morbidity and even mortality. We
demonstrated that urea is an effective and well-tolerated treatment
for IH (8–11). To reduce the daily administration of
urea in the treatment of IH, we firstly utilized ULIM as a topical
controlled release system to realize the sustained release of urea.
This study demonstrated that ULIM achieved superior therapeutic
efficacy compared with urea and propranolol, and reduced the daily
administration frequency of urea.
The selection of an anti-hemangioma is critically
important for the superior activity of our prepared ULIM. Urea is
an organic compound and a normal body metabolite, and is widely
used in various skin diseases (5,6).
Importantly, urea has been used in the treatment of IH in China
since 1970s (7). Our hospital,
Henan Provincial People's Hospital (Zhengzhou, China), has used
urea in the treatment of IH for 20 years, and we have demonstrated
that urea is an effective drug for the treatment of IH with few
side-effects (8–11). Thus, the superior effectiveness and
few side-effects of urea, coupled with the immediate availability
of the medication, led to a rapid and wide use of urea for IH. As
should be, the application of urea for IH will be more convenient
if its frequency of administration could be reduced.
Changing the route of administration could enhance
the therapeutic efficacy and reduce the side-effects of drugs. To
overcome the high frequency of urea administration, we developed a
practical sustained release system defined as ULIM to release urea.
The data presented here confirmed that ULIM showed a significantly
slower release of urea, compared with UL and UM, as reflected by
the fact that it took 4, 10 and 20 days for UL, UM and ULIM to
release >80% of urea, respectively. Feng et al (22) elucidated the mechanism underlying
the drug release from liposomes-in-microspheres. First, liposomes
must diffuse through tortuous water channels of microspheres. As
time passes, the degradation of the polymer matrix causes expansion
of the tortuous water channels, thereby leading to a sustained
release of liposomes. When the liposomes are released, they still
act as a sustained release reservoir of drugs. Thus, the mechanism
underlying the significantly slower release of urea by ULIM
compared with UL and UM could be clarified as follows. Since
liposomes are soft and easily ruptured, the release of urea from UL
is quick. PLGA microspheres which possess a more rigid structure
show relatively slower urea release after the gradual degradation
of the polymer matrix. As regards as ULIM, the release of urea
undergoes two stages. UL must slowly diffuse through tortuous water
channels of microspheres. After UL is released from ULIM, UL still
serves as a reservoir of urea and urea is gradually released from
UL. Thus, urea is released at a very slow rate from ULIM since it
needs to conquer two barriers consisting of the liposome membrane
and the PLGA polymer matrix.
Although ULIM were less efficient in the inhibition
of the proliferation of HemECs and VEGF expression than urea, UL
and UM in vitro, its therapeutic effect was the best in
vivo, as reflected by significantly reduced hemangioma volume,
weight and MVD. Notably, ULIM were superior to daily administration
of propranolol which is the only FDA approved drug for the
treatment of IH. We speculate that the discrepancy of ULIM could be
clarified as follows. When tested in vitro, all the drugs
are restricted to cell culture plates, and the activity of urea
depends on the concentration of urea released from the
formulations. The slow release of urea from ULIM results in a
reduced cytotoxic effect, compared with urea, UL and UM. The quick
release of urea from UL will surely induce superior cytotoxic
effects. However, after intratumor injection in vivo, urea
and UL undergo quick elimination of urea from hemangioma, resulting
in poor therapeutic efficacy for hemangioma, whereas the prolonged
and sustained release of urea from ULIM and UM significantly retard
the angiogenesis of hemangioma.
We investigated the effect of urea on the expression
of VEGF-A and HIF-1α which promote angiogenesis (28,29).
It is noteworthy that urea significantly repressed the expression
of VEGF-A and HIF-1α in a dose-dependent manner. Since HIF-1α is a
master regulator of VEGF-A, and activates transcription of the
VEGF-A gene (29), we investigated
whether regulated expression of HIF-1α affects VEGF-A expression.
VEGF-A expression was significantly repressed after HIF-1α was
knocked down, whereas VEGF-A expression was upregulated after
HIF-1α overexpression, suggesting that downregulated VEGF-A
expression after urea treatment was at least partially caused by
the urea-induced inhibition of HIF-1α expression. Propranolol has
been also reported to be able to inhibit IH by suppressing VEGF-A
in an HIF-1α-dependent manner (30).
The safety of a drug delivery systems is important
in the clinic (31). The components
of our prepared ULIM include liposomes, chitosan and PLGA, which
are rather biocompatible materials. Furthermore, our data revealed
that none of the treated mice showed behavioral changes, severe
side-effects and weight loss. Thus, the safety of ULIM should be
superior in clinical use. The detailed in vivo distribution
and further safety data of ULIM should be investigated in further
studies. Furthermore, the intratumor injection approach of our
microspheres is safe in the clinic. Based on our 10-year clinical
experience for IH, we can safely conclude that needle injection
into hemangioma does not induce bleeding even in large hemangiomas,
and the slight bleeding that occurs can be easily suppressed by
hand compression.
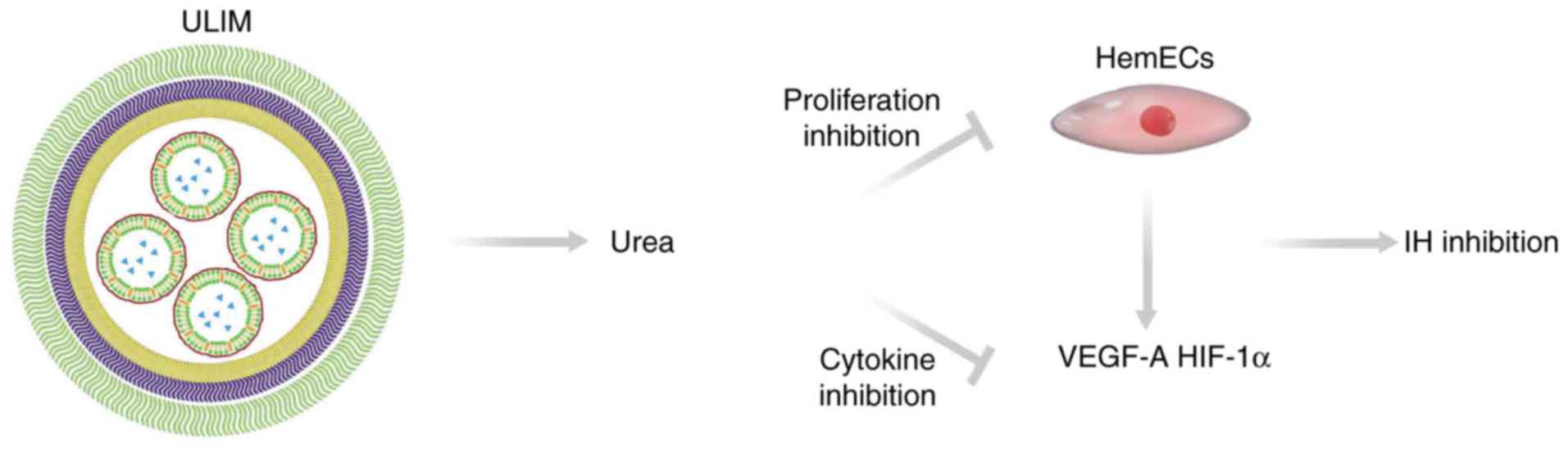
Our results elucidated the mechanism of the
anti-hemangioma activity of ULIM (Fig.
8). ULIM tends to accumulate in IH after intratumor injection.
After being released from ULIM urea inhibits the proliferation of
HemECs and reduces expression of angiogenesis factors including
VEGF-A and HIF-1α. The reduced expression of VEGF-A and HIF-1α
would also significantly retard the angiogenesis in IH. On the
contrary, when free urea is injected into IH, it is eliminated
quickly by the blood circulation, resulting in poor therapeutic
efficacy of IH.
In conclusion, we demonstrated that urea is an
effective and well-tolerated treatment of IH, whereas its frequent
administration reduces the compliance of patients. We hereby
firstly utilized ULIM as a topical controlled release system to
realize the sustained release of urea. ULIM have been demonstrated
to show sustained release of urea, achieving superior therapeutic
efficacy compared with urea and propranolol, and significantly
reducing the administration frequency of urea. Moreover, the safety
of ULIM is rather promising. Thus, our findings show that ULIM is a
promising treatment for IH.
Acknowledgements
The present study was supported by the Henan Medical
Provincial Science and Technology Plan Project (project number:
142102310080 and 201702202).
References
|
1
|
Kilcline C and Frieden IJ: Infantile
hemangiomas: how common are they? A systematic review of the
medical literature. Pediatr Dermatol. 25:168–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen TS, Eichenfield LF and Friedlander
SF: Infantile hemangiomas: an update on pathogenesis and therapy.
Pediatrics. 131:99–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Castaneda S, Melendez-Lopez S, Garcia E,
De la Cruz H and Sanchez-Palacio J: The role of the pharmacist in
the treatment of patients with infantile hemangiomas using
propranolol. Adv Ther. 33:1831–1839. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khorsand K, Backus S and Sidbury R: What's
new in pediatric dermatology. Current Dermatology Reports.
3:187–190. 2014. View Article : Google Scholar
|
|
5
|
Long CL, Jeevanandam M and Kinney JM:
Metabolism and recycling of urea in man. Am J Clin Nutr.
31:1367–1382. 1978.PubMed/NCBI
|
|
6
|
Fredriksson T and Gip L: Urea creams in
the treatment of dry skin and hand dermatitis. Int J Dermatol.
14:442–444. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li G and Gu J: Infantile Hemangioma. 1st.
Shaanxi Science and Technology Press; Xian: 1991, (In Chinese).
|
|
8
|
Liang X, Dong C, Ma Y, Lei H, Liu D, Qiao
J and Xiao L: The treatment of spongy infantile hemangioma on body
surface by local urea injection. Chin Hosp Pharm J. 31:1396–1397.
2011.(In Chinese).
|
|
9
|
Chang Y, Dong C and Zheng W: The clinical
observation of 80 cases of infantile hemangioma by urea local
injection. Clin Med. 19:52–53. 1999.(In Chinese).
|
|
10
|
Lei H, Huang J, Meng X, Zhang W, Dong C,
Sun B and Ma Y: Carbonyldiamide injection therapy for 167 cases of
infant hemangiomas at peculiar region. J Medical Forum. 35:3–5.
2014.(In Chinese).
|
|
11
|
Guo X, Zhu X, Dong C and Ma Y: Clinical
analysis of 32 cases of vulvar sponge hemangioma receiving
intratumoral urea injection combined with surgery. Chin J Obstet
Gynecol. 50:221–223. 2015.(In Chinese).
|
|
12
|
Müller-Goymann CC: Physicochemical
characterization of colloidal drug delivery systems such as reverse
micelles, vesicles, liquid crystals and nanoparticles for topical
administration. Eur J Pharm Biopharm. 58:343–356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Almeida H, Amaral MH, Lobao P, Frigerio C
and Lobo JM Sousa: Nanoparticles in ocular drug delivery systems
for topical administration: promises and challenges. Curr Pharm
Des. 21:5212–5224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Freiberg S and Zhu XX: Polymer
microspheres for controlled drug release. Int J Pharm. 282:1–18.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao J, Xia Y, Chen H, Yu Y, Song J, Li W,
Qian W, Wang H, Dai J and Guo Y: Polymer-lipid hybrid nanoparticles
conjugated anti-EGFR antibody for targeted drug delivery to
hepatocellular carcinoma. Nanomedicine (Lond). 9:279–293. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Van Slooten ML, Boerman O, Romøren K,
Kedar E, Crommelin DJ and Storm G: Liposomes as sustained release
system for human interferon-gamma: biopharmaceutical aspects.
Biochim Biophys Acta. 1530:134–145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao C, Qi X, Maitani Y and Nagai T:
Sustained release of cisplatin from multivesicular liposomes:
potentiation of antitumor efficacy against S180 murine carcinoma. J
Pharm Sci. 93:1718–1724. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bittman R and Clejan S: Kinetics of
cholesterol and phospholipid exchange between mycoplasma membranes
and lipid vesicles. Isr J Med Sci. 23:398–402. 1987.PubMed/NCBI
|
|
19
|
New RRC: Liposomes: A Practical Approach.
Publisher: Oxford University Press; Revised edition. 1990
|
|
20
|
Shive MS and Anderson JM: Biodegradation
and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv
Rev. 28:5–24. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dass CR, Walker TL, Kalle WH and Burton
MA: A microsphere-liposome (microplex) vector for targeted gene
therapy of cancer. II. In vivo biodistribution study in a solid
tumor model. Drug Deliv. 7:15–9. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng SS, Ruan G and Li QT: Fabrication and
characterizations of a novel drug delivery device
liposomes-in-microspheres (LIM). Biomaterials. 25:5181–5189. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Medici D and Olsen BR: Rapamycin inhibits
proliferation of hemangioma endothelial cells by reducing
HIF-1-dependent expression of VEGF. PLoS One. 7:e429132012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shuming C, Shaoquan C, Zaizhong Z,
Chengjin L, Yin X, Chen L, Qingjin H and Lie W: Effects of topical
rapamycin hydrochloride gel for treatment of infantile hemangiomas
in nude mice). Zhonghua Zheng Xing Wai Ke Za Zhi. 31:446–450.
2015.(In Chinese). PubMed/NCBI
|
|
25
|
Wang Z, Li J, Xu X, Duan X and Cao G: Urea
immunoliposome inhibits human vascular endothelial cell
proliferation for hemangioma treatment. World J Surg Oncol.
11:3002013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Greenberger S, Yuan S, Walsh LA, Boscolo
E, Kang KT, Matthews B, Mulliken JB and Bischoff J: Rapamycin
suppresses self-renewal and vasculogenic potential of stem cells
isolated from infantile hemangioma. J Invest Dermatol.
131:2467–2476. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mason JC, Lidington EA, Ahmad SR and
Haskard DO: bFGF and VEGF synergistically enhance endothelial
cytoprotection via decay-accelerating factor induction. Am J
Physiol Cell Physiol. 282:578–587. 2002. View Article : Google Scholar
|
|
28
|
Manalo DJ, Rowan A, Lavoie T, Natarajan L,
Kelly BD, Ye SQ, Garcia JG and Semenza GL: Transcriptional
regulation of vascular endothelial cell responses to hypoxia by
HIF-1. Blood. 105:659–669. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Déry MA, Michaud MD and Richard DE:
Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic
activators. Int J Biochem Cell Biol. 37:535–540. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li P, Guo Z, Gao Y and Pan W: Propranolol
represses infantile hemangioma cell growth through the
β2-adrenergic receptor in a HIF-1α-dependent manner. Oncol Rep.
33:3099–3107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nystrom AM and Fadeel B: Safety assessment
of nanomaterials: implications for nanomedicine. J Control Release.
161:403–408. 2012. View Article : Google Scholar : PubMed/NCBI
|