Introduction
Ovarian cancer (OC) is the most fatal gynecological
malignant tumor, causing 151,900 deaths worldwide in 2012 (1). According to recent statistics, OC is
still the fifth leading cause of cancer-related female deaths in
the United States (2). Because
there are no effective methods to screen OC, 75% of cases are
diagnosed at an advanced stage, with tumor cells spreading widely
throughout the abdominal cavity (3). Although many therapeutic methods,
including surgery and cisplatin-based chemotherapy, have been used,
severe metastasis and chemoresistance lead to a 5-year overall
survival rate in no more than 25–35% of cases (3,4). EOC
accounts for 90% of OC (5);
therefore, it is important to investigate how this cancer
progresses.
Although cancer cells unlimitedly proliferate and
survive, a tumor microenvironment (TME) is required for the
formation and growth of clinically relevant tumors (6). The TME, comprising mesenchymal stem
cells, cancer-associated fibroblasts (CAFs), myeloid cells,
mesothelial cells and factors released by these cells, contributes
to tumor growth, immune escape, distant metastasis and
chemoresistance of cancer (6–8). For
example, CAF-derived exosomes (tiny vesicles formed during
endocytosis and 30–150 nm in size) can promote the survival and
proliferation of pancreatic cancer cells, thus affecting responses
to the standard chemotherapeutic agent gemcitabine (9). Mesothelial cells stimulated with TGF-β
can promote OC cell attachment and proliferation by activating the
promoters of matrix metalloprotein-2 and matrix metalloprotein-9
(10).
TAMs, the most common immune cells in the TME
(11), primarily refer to
macrophages infiltrating into tumor tissues (12). These cells are derived from
circulating monocytes and induced to differentiate through
alternative pathways through various factors in the TME, mainly due
to the activation of the Notch pathway (11,12).
In addition to the microenvironment within tumor tissues, TAMs are
also distributed in some special organs and lymph nodes, associated
with metastasis to these regions (13,14).
TAMs infiltrate into OC tissues in large numbers (15–17),
contributing to the progression of OC (18,19),
thus negatively affecting the progression-free survival rates and
overall survival rates of these patients (16). However, the precise mechanism of how
TAMs contribute to the progression of OC remains unclear.
The IGF1 pathway comprises three receptor tyrosine
kinases [IGF-1R, insulin-like growth factor-2 receptor (IGF-2R),
and insulin receptor (IR)], three ligands (insulin, IGF-1, and
IGF-2), and six serum insulin-like growth factor binding proteins
(IGFBPs), which are important regulators of this pathway (20). This pathway can enhance the
proliferation and development of cells by initiating the
anti-apoptotic PI3K/Akt/mTOR and the mitogenic Ras/Raf/Mek/Erk
pathways (21,22). The IGF1 pathway is associated with
the growth, metastasis and clinical outcome of various cancers,
including prostate cancer, gastric cancer, lung cancer and breast
cancer (23–27). For example, CAFs can increase the
invasion ability of pancreatic cancer cells via paracrine
IGF1/IGF1R signaling, particularly under hypoxia (27), and IGF1R is highly expressed in
chronic lymphocytic leukemia cells, whereas the inhibition of IGF1R
can enhance the death of CLL cells (28).
The IGF1 pathway has also been associated with the
progression of OC. Women carrying haplotype 2C of the IGF1 gene
have a decreased risk of OC, whereas those carrying haplotype 1D or
2D have an increased risk (29).
Serous ovarian carcinoma cells are strongly positive for IGF1, and
IGF1 could downregulate the expression of E-cadherin and upregulate
Snail and Slug expression, thereby promoting the epithelial to
mesenchymal transition of human OC cells (30,31).
Low IGFBP-3 expression is clinically correlated with high tumor
grade, advanced stage and poor survival of ovarian endometrioid
cancer patients (32). The
IGF1/PI3K/NFκB/Erk pathway was found to be upregulated in ovarian
specimens of patients demonstrating relative resistance compared
with those demonstrating sensitivity (33). Furthermore, high circulating IGF-1
levels have been correlated with decreased OC risk, and overall and
progression-free survival were significantly prolonged in patients
with higher serum IGF1 levels (34–36).
However, the underlying reason for the abnormality of IGF1 in OC
patients remains unknown.
In the present study, we showed that TAMs enhanced
the proliferation and migration of mouse OC ID8 cells by
upregulating IGF1, and inhibition of the IGF1 pathway using an IGF1
inhibitor effectively suppressed the proliferation and migration of
ID8 cells exposed to TAM-conditioned medium (CM). These results
indicate that targeting the IGF1 pathway is a promising EOC
therapy.
Materials and methods
TAM model establishment
A TAM model was established according to Lin et
al (37), and the protocols for
the treatment of animals were approved by the Ethics Committee of
Tongji University prior to the study. The C57 mice were euthanized,
and their hind legs were removed and placed in 75% alcohol for 5
min. Soft tissues were removed, a 26-G needle attached to a 1-cc
syringe was inserted into the bone marrow cavity to wash out cells
with RPMI-1640 (Gibco, Foster city, CA, USA) containing 10% fetal
bovine serum (FBS; Gibco) until the bone became white. The medium
with bone marrow cells was passed through a cell strainer with
70-µm pores (Merck Millipore, Billerica, MA, USA) into a 50-ml
centrifuge tube. The cells were centrifuged for 10 min at 1,350
rpm, and the supernatant was subsequently discarded. Next, the
cells were resuspended in 5 ml RPMI-1640 supplemented with 10% FBS
and centrifuged for 10 min at 1,350 rpm. The cells were resuspended
in RPMI-1640 containing 10% FBS and 1% penicillin-streptomycin
(Gibco) to a final concentration of 5×106 cells/ml.
Then, 1×107 cells/well were plated in 6-well plates.
M-CSF (10 ng/ml; R&D Systems, Minneapolis, MN, USA) was added
to the medium for M0 cell formation, or M-CSF, IL-4, IL-13, and
IL-10 (10 ng/ml; R&D Systems) were added to the medium for TAM
formation. The cells were incubated at 37°C in a humidified, 5%
CO2 incubator, and medium containing cytokines was
changed daily for 4 days.
Cell line
ID8 mouse EOC cells were purchased from Fuheng
Biotechnology Co., Ltd. (Shanghai, China) and maintained in DMEM
(Gibco) medium supplemented with 10% FBS and 1%
penicillin-streptomycin. The cells were cultured at 37°C in a
humidified, 5% CO2 incubator.
TAM CM preparation, transfer and
coculture
The TAM CM transfer was performed according to
Richards et al (9). TAM
medium was centrifuged at 2,500 rpm for 30 min to remove cell
debris and then mixed with complete DMEM at the ratio of 1:1.
Complete RPMI-1640, and DMEM was mixed at the ratio of 1:1 as
normal medium. TAM CM or normal medium was transferred to plates
with ID8 cells for coculture daily. IGF1 neutralizing antibody
(monoclonal, rabbit to mouse; Abcam, Cambridge, MA, USA) was
dissolved in PBS and linsitinib (Selleck, Houston, TX, USA) was
dissolved in DMSO before use.
RNA collection and quantitative
real-time PCR (qRT-PCR)
Total RNA was extracted from macrophages and ID8
cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's instructions. Reverse transcription
was performed using a Prime Script™ II 1st Strand cDNA Synthesis
kit (Takara Biotechnology Co., Ltd., Dalian, China) according to
the manufacturer's instructions. qRT-PCR was performed using Talent
qPCR PreMix (SYBR-Green) (Tiangen Biotech Co., Ltd., Beijing,
China) and the StepOnePlus™ Real-time PCR system (Invitrogen)
according to the manufacturer's instructions. The following primer
sequences were used: CD204 forward, 5′-TGGAGGAGAGAATCGAAAGCA-3′ and
reverse, 5′-CTGGACTGACGAAATCAAGGAA-3′; IGF1 forward,
5′-CACATCATGTCGTCTTCACACC-3′ and reverse,
5′-GGAAGCAACACTCATCCACAATG-3′; and GAPDH forward,
5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse, 5′-GGGTCGTTGATGGCAACA-3′.
The final concentration of all reagents was 2X Talent qPCR PreMix
(with SYBR-Green I), 1X; 50X ROX Reference Dye, 5X; and forward and
reverse primers: 0.3 µM. The PCR reactions cycling conditions
included an initial cycle at 95°C for 15 sec, followed by 40 cycles
of 95°C for 5 sec and 60°C for 15 sec. The data were calculated
using the 2−∆∆Cq method, with GAPDH as an internal
normalization control.
Western blot analyses
Total protein was collected from M0 cells, TAMs, ID8
cells treated with or without TAM CM or linsitinib using RIPA lysis
buffer (Beyotime, Shanghai, China). A BCA kit (Thermo Fisher,
Rockford, IL, USA) was used for protein quantification according to
the manufacturer's instructions. Protein was separated on 10%
SDS-PAGE gels (EpiZyme, Shanghai, China) at a consistent voltage of
80 V in the stacking gel and 120 V in the separating gel and
subsequently transferred onto polyvinylidene difluoride membranes
at consistent current of 300 mA for 80 min. The membranes were
blocked in TBST buffer with 5% bovine serum albumin (BSA) for 2 h
at room temperature. Then, the membranes were incubated with the
following primary antibodies at 4°C overnight: IGF1 (cat. no.
ab9572; 1:1,000, monoclonal, rabbit to mouse; Abcam), CD204 (cat.
no. ab15707; 1:1,000, polyclonal, rabbit to mouse; Abcam), IGF1R
(cat. no. 9750; 1:1,000, monoclonal, rabbit to mouse; Cell
Signaling Technology, Inc., Danvers, MA, USA), phospho-IGF1R (cat.
no. 3918; 1:1,000, monoclonal, rabbit to mouse; Cell Signaling
Technology, Inc.), Akt (cat. no. 4685; 1:1,000, monoclonal, rabbit
to mouse; Cell Signaling Technology, Inc.), phospho-Akt (cat. no.
ab81283; 1:1,000, monoclonal, rabbit to mouse; Abcam), Erk (cat.
no. 4695; 1:1,000, monoclonal, rabbit to mouse; Cell Signaling
Technology, Inc.), phospho-Erk (cat. no. 4370; 1:1,000, monoclonal,
rabbit to mouse; Cell Signaling Technology, Inc.), GAPDH (cat. no.
AB0036; 1:5,000; AB2000, monoclonal, rabbit to mouse; Abways,
Shanghai, China), and the HRP-conjugated rabbit secondary antibody
(cat. no. 16402-1-AP; 1:5,000; Proteintech, Wuhan, China) was used
to incubate the membranes at room temperature for 1 h. The protein
signals were detected using enhanced chemiluminescent HRP substrate
(Merck Millipore).
Immunohistochemistry (IHC)
Ovarian benign tumor specimens (patient ages ranged
from 21 to 70 years) and EOC specimens (patient ages ranged from 33
to 74 years) used for tissue microarrays were obtained from the
specimen repository of Shanghai First Maternity and Infant
Hospital, after obtaining consent from each patient and the Ethics
Committee of Tongji University. IHC was performed using an IGF1
primary antibody (1:125, monoclonal, rabbit to mouse; Abcam).
Specimens (4-µm thick) were subjected to heat-induced epitope
retrieval and then dewaxed in xylene and hydrated through a graded
series of alcohol. The specimens were incubated in 0.3%
H2O2 for 30 min to inactivate the endogenous
peroxidase. Next, the specimens were incubated with 1% goat serum
(Invitrogen) for 20 min at room temperature, followed by incubation
with IGF1 antibody overnight at 4°C and then rabbit secondary
antibody for 1 h at 37°C. A Vectastain ABC Elite kit (Vector
Laboratories, Burlingame, CA, USA) was used according to the
manufacturer's instructions for color development. The specimens
were counterstained with hematoxylin and then dehydrated through a
graded series of alcohol, cleared in xylene and mounted. Tumor
cells with immunohistochemical expression in the cytoplasm were
regarded as IGF1-positive, and the expression level was determined
using the IRS system by multiplication of the staining intensity
(0, no; 1, weak; 2, moderate; and 3, strong staining) and the
percentage of positively stained cells (0, no staining; 1, <10%
of cells; 2, 11–50% of cells; 3, 51–80% of cells; and 4, >81% of
cells stained), according to Remmele and Stegner (38). The total score per sample therefore
ranged from 0 to 12; a score <6 indicates low expression,
whereas a score of 6–12 indicates high expression. The slides were
examined in a blinded manner by two experienced investigators.
MTS proliferation assay
ID8 cells (1.5×103) were seeded onto
96-well plates and cultured with 100 µl of medium for 72 h. Then,
20 µl of MTS Solution Reagent (PR Omega Biosciences, USA) was added
to each well followed by incubation at 37°C in 5% CO2
for 2 h. The absorbance was measured at 490 nm using a 96-well
plate reader. Each group had six duplicates.
Cell migration assays
Transwell chambers (Corning, Glendale, AZ, USA)
containing 8-µm inserts were used to measure the migration of tumor
cells. ID8 cells (5×104 cells) in 200 µl DMEM containing
2% FBS was plated in the top chambers. The bottom of the wells was
filled with 800 µl complete DMEM containing 10% FBS. Calcein AM
(Invitrogen) was used to stain the cells in the bottom of the
filter membrane. The images of cells were captured using a
fluorescence microscope at ×100. ImageJ software was used to count
the cells that had migrated to the bottom of the filter membrane.
Each group had two duplicates, and five images of different fields
for each duplicate were captured.
Statistical analysis
All experiments were independently performed three
or more times. Statistical analysis was performed using SPSS 19.0
(SPSS, Inc., Chicago, IL, USA). The data are expressed as the means
± SEMs or means ± SDs and analyzed using Student's t-test or the
Mann-Whitney test. The correlation of IGF1 expression and clinical
and pathological factors was analyzed using the Chi-square test or
the Fisher's exact test. P-value <0.05 was considered
statistically significant.
Results
The TAM model is established using
mouse bone marrow monocytes
TAMs have been characterized as a popularized M2
macrophage phenotype different from the M1 type, which
differentiates through a classic pathway. In the present study, a
TAM model was established using isolated primary mouse bone marrow
cells. Monocytes were induced to become undifferentiated M0 cells
using MCSF. To induce M0 cells to differentiate into TAMs, IL-10,
IL-13, and IL-4 were continuously used.
To determine whether the TAM model was successful,
analyses of cell morphology and the expression level of the
TAM-specific marker CD204 was used. As shown, TAMs were larger and
more stacked in morphology (Fig.
1A). The expression of CD204 was higher in TAMs at both the
mRNA (Fig. 1B) and protein levels
(Fig. 1C).
TAM CM enhances the proliferation and
migration of ID8 cells
As TAMs are associated with the enhanced progression
of various cancers, including OC, and the number of TAMs in OC
specimens is correlated with poor prognosis, we assessed whether
TAMs similarly affect the physiological activity of mouse EOC
cells. We first studied the effect of TAMs on the proliferation of
ID8 cells, observing that TAM CM promoted the proliferation of ID8
cells (Fig. 2A). We next determined
whether TAMs could change the migration of ID8 cells, and the
results showed that the migration of ID8 cells treated with TAM CM
was significantly increased compared with the control group
(Fig. 2B). Taken together, these
data showed that TAMs could accelerate the proliferation and
migration of ID8 cells.
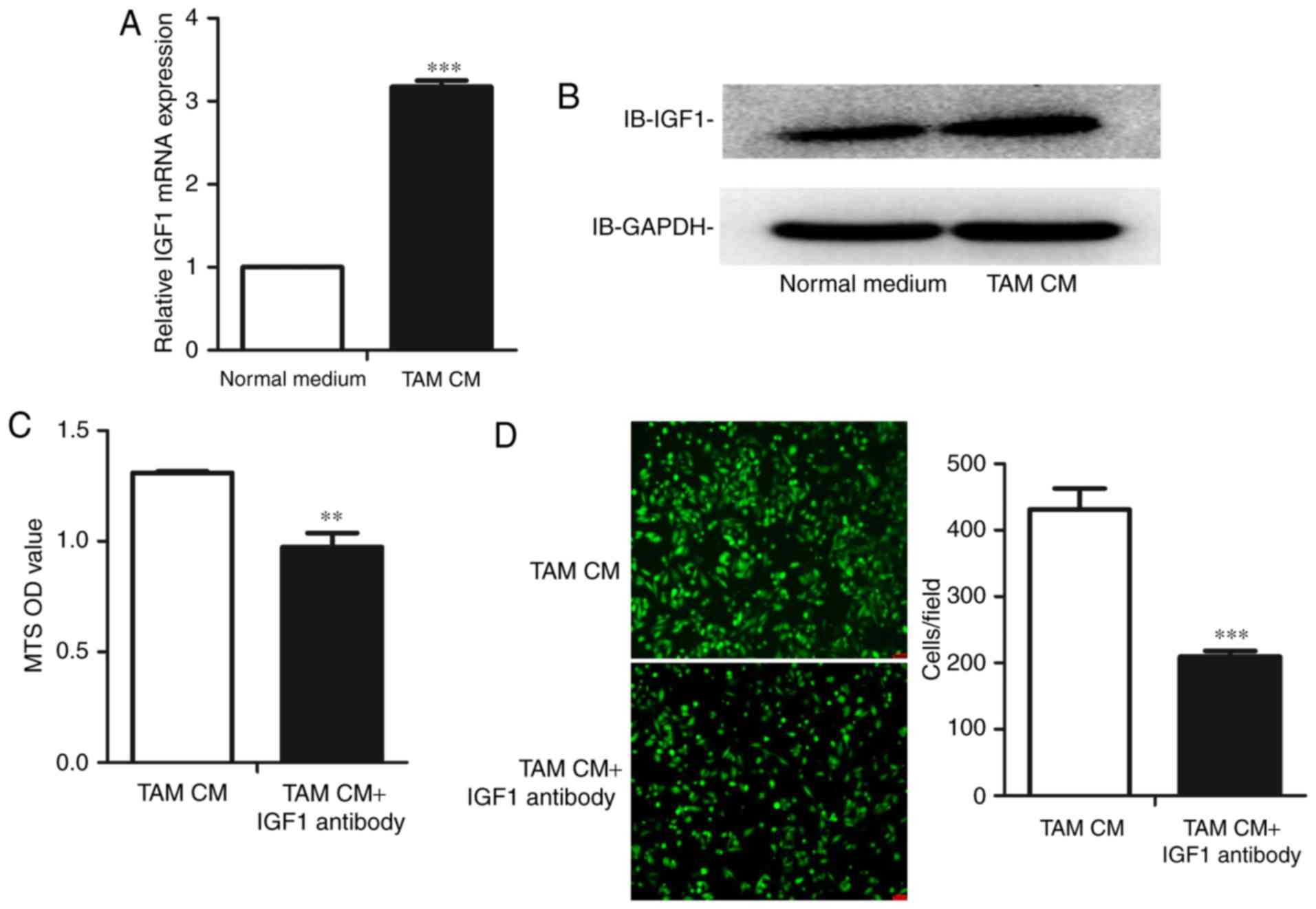
TAMs express higher levels of IGF1
than M0 cells
In a previous study, we performed a gene chip
analysis to compare the expression profile of human TAMs and M0
cells, and observed that TAMs expressed significantly higher levels
of IGF1 than M0 cells. We identified this result at both the mRNA
and protein levels in mouse TAMs. Consistent with the gene chip
analysis, the expression level of IGF1 was higher in mouse TAMs at
both the mRNA (Fig. 3A) and protein
levels (Fig. 3B).
Human EOC specimens express higher
levels of IGF1
The IGF1 level is higher in prostate cancer and in
gastric cancer compared with corresponding benign tumors (24,25);
thus, we examined whether EOC would have a similar phenomenon.
First, we used western blot analysis to assess the level of IGF1 in
fresh ovarian specimens, and observed that EOC specimens expressed
higher levels of IGF1 compared with levels in the benign ovarian
tumors (Fig. 4A).
Next, we used IHC staining to assess the expression
level of IGF1 in tissue microarrays of benign ovarian tumors and
EOC. The results showed that the mean IRS score of EOC specimens
was significantly higher than that of the benign ovarian tumors
(Fig. 4B). Then, we classified the
EOC specimens into high and low expression groups, and used the
Chi-square test or Fisher's exact test to identify the correlation
between clinical and pathological factors and IGF1 expression
levels. The median age of the patients was 56 years. Patients with
a high IGF1 expression were in a more advanced stage and tended to
have undergone liver metastasis more frequently (Table I). These results indicated that high
IGF1 expression may enhance the progression of EOC.
 | Table I.Correlation of IGF1 expression level
with clinical and pathological factors. |
Table I.
Correlation of IGF1 expression level
with clinical and pathological factors.
| Parameters | n | Low expression | High
expression | P-value |
|---|
| Age years) |
|
|
| 0.749 |
|
≥56 | 21 | 9 | 12 |
|
|
<56 | 20 | 9 | 11 |
|
| Tumor size |
|
|
| 0.169 |
| ≥7 | 26 | 9 | 17 |
|
|
<7 | 14 | 8 | 6 |
|
| Pathologic
grade |
|
|
| 0.848 |
|
I+II | 12 | 6 | 6 |
|
|
III | 13 | 6 | 7 |
|
| Clinical stage |
|
|
| 0.020 |
|
I+II | 14 | 10 | 4 |
|
|
III+IV | 27 | 9 | 18 |
|
| Ascites |
|
|
| 0.951 |
|
Yes | 23 | 10 | 13 |
|
| No | 18 | 8 | 10 |
|
| Liver
metastasis |
|
|
| 0.036 |
|
Yes | 11 | 2 | 9 |
|
| No | 29 | 16 | 13 |
|
TAM CM upregulates the expression of
IGF1 in ID8 cells
Based on the preceding results, we determined
whether TAMs could alter the proliferation and migration of mouse
OC cells by upregulating the tumor-promoting gene IGF1. Therefore,
we treated ID8 cells with TAM CM. As expected, the expression level
of IGF1 in ID8 cells increased following culture with TAM CM at
both the mRNA (Fig. 5A) and protein
levels (Fig. 5B).
Blockade of IGF1 reverses the
alteration of proliferation and migration of ID8 cells
After observing that TAMs accelerate the
proliferation and migration of ID8 cells and simultaneously
upregulate IGF1, we finally assessed whether the blockade of IGF1
in TAM CM would reverse the changes in proliferation and migration.
An IGF1 neutralizing antibody was added to TAM CM to block the IGF1
pathway. We observed that the blockade of IGF1 reduced the
proliferation of ID8 cells (Fig.
5C). We then determined whether the IGF1 neutralizing antibody
had the same effect on the migration of ID8 cells, observing that
blockade of IGF1 reversed the increase in ID8 cell migration
(Fig. 5D).
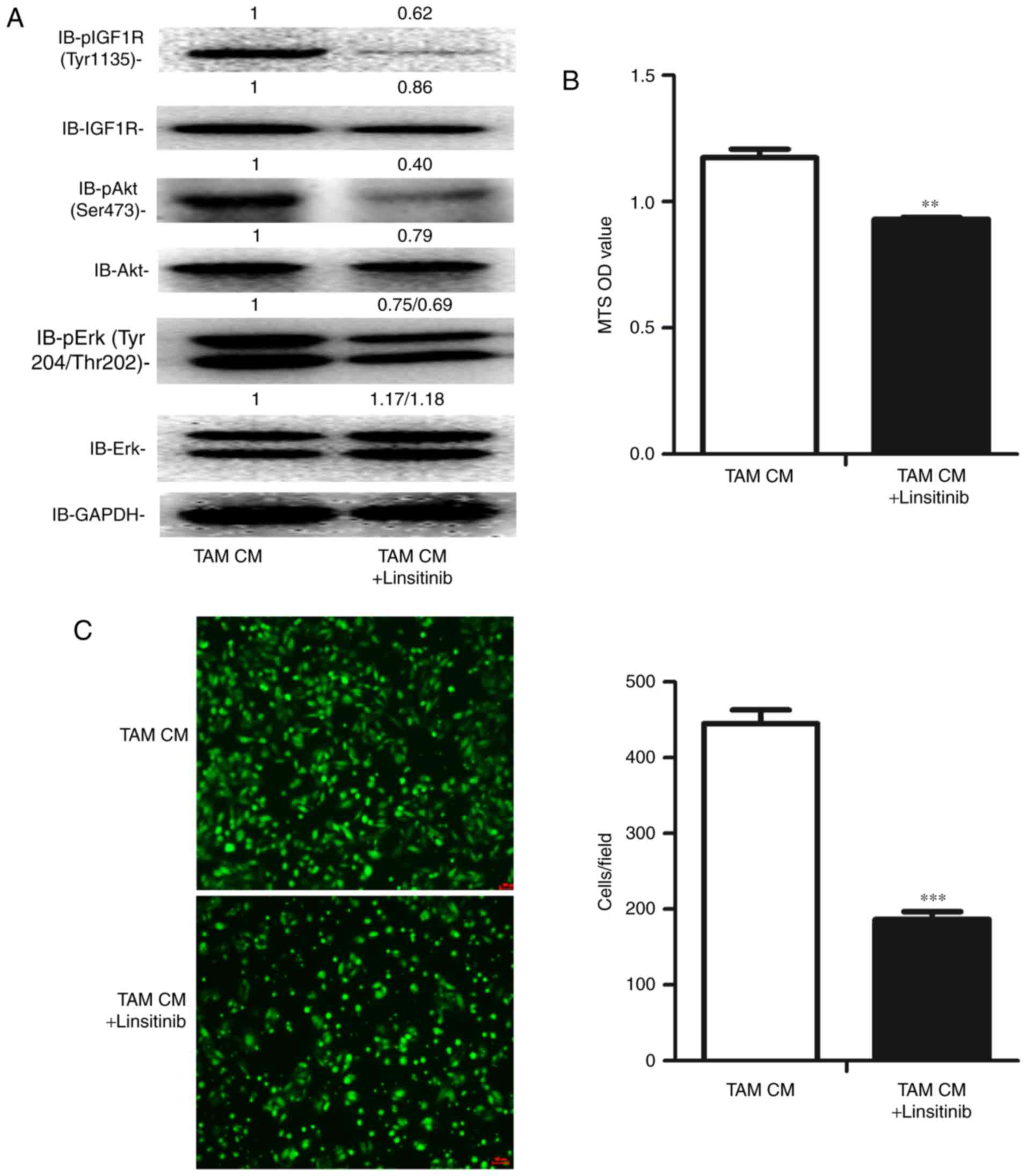
Inhibition of the activation of IGF1R
suppresses the proliferation and migration of ID8 cells exposed to
TAM CM
To validate the value of IGF1 pathway inhibition in
EOC therapy, we used the IGF1R inhibitor linsitinib to inhibit the
IGF1 pathway. As expected, the phosphorylation of IGF1R, Akt and
Erk was significantly inhibited (Fig.
6A). Additionally, the proliferation and migration of ID8 cells
were both significantly suppressed by linsitinib (Fig. 6B and C).
Taken together, these data suggest that IGF1 may be
a key regulator by which TAMs promote the proliferation and
migration of OC cells, indicating the potential of IGF1 inhibition
in EOC therapy.
Discussion
In the present study, we showed that IGF1 is
expressed at higher levels in mouse TAMs than in M0 cells, TAMs
were able to enhance the proliferation and migration of ID8 cells
by upregulating IGF1, and inhibition of the IGF1R pathway
effectively suppressed the proliferation and migration of ID8 cells
exposed to TAM CM.
We established a mouse TAM model and observed that
TAM CM accelerated the proliferation and migration of ID8 cells,
supporting the role of TAMs in the progression of OC (Figs. 1 and 2). The TME is a supportive and receptive
tissue microenvironment undergoing a series of molecular and
cellular changes to form metastatic-designated sites, or the
fertile soil in preparation for metastatic tumor cell seed
colonization, thus supporting tumor settlement in distant organs
and promoting tumor metastasis (6–8). As
the most common immune cells in the TME, TAMs infiltrate the TME in
OC in large numbers and play an important role in the formation of
the OC TME and education of OC cells to develop to become more
malignant by secreting various factors, such as IL-6 and IDO
(16,18,19,39,40).
Indeed, therapeutic methods targeting TAMs have been demonstrated
as effective for controlling OC. An inhibitor of IDO, which is
associated with the formation of TAMs, can control the growth of OC
in vivo (40). An MCSF
inhibitor was found to reduce the infiltration of TAMs, and promote
the survival of peritoneal blood vessels, thereby reducing the
production of ascites in mice transplanted with OC cells (41).
IGF1 is a cancer progression-related gene that is
associated with the growth, metastasis and chemoresistance of
various cancers (23–27). In the present study, we confirmed
that TAMs significantly expressed higher IGF1 levels than M0 cells
as demonstrated in a previous gene chip analysis (Fig. 3). More importantly, the level of
IGF1 was high in EOC specimens and was related to more advanced
clinical stage and liver metastasis (Fig. 4; Table
I). Thus, we demonstrated that TAMs promoted the progression of
EOC by upregulating IGF1. As expected, these results showed that
TAM CM upregulated IGF1, whereas an IGF1 neutralizing antibody
reversed the alteration of proliferation and migration (Fig. 5). Additionally, these results
provide evidence that IGF1 may be a key factor in the promotion of
EOC progression through TAMs, explaining its high expression in
EOC. Finally, we propose that the mechanism of the upregulation of
IGF1 in ID8 cells through TAMs may involve exosomes, which could
transfer microRNAs, mRNAs, DNA fragments and proteins, particularly
miRNA, from donor cells to recipient cells (42) or secrete cytokines, which also play
an important role in the communication between different cells
(43). However, these hypotheses
need further experimental validation.
Targeting the IGF1 pathway has been demonstrated as
effective in the therapy of various cancers (28,44). A
typical example is that IGF1R inhibitors decreased the viability of
chronic lymphocytic leukemia cells in a microenvironment context
(28). Theranostic nanoparticles
IGF1-IONP-Dox were found to significantly inhibit the growth of
pancreatic tumors (44). Indeed,
knockdown of IGF-1 using siRNA decreased the migration and invasion
of ES-2 cells (45). In the present
study, we used the IGF1R inhibitor linsitinib to block the
phosphorylation activation of the IGF1/IGF1R/Akt and IGF1/IGF1R/Erk
pathways. Remarkably, IGF1 pathway inhibition reduced the
proliferation and migration of ID8 cells, even those exposed to TAM
CM, suggesting that the IGF1 pathway is potentially involved in EOC
therapy, even in the TME (Fig. 6).
However, whether IGF1 is effective in vivo and whether it
will be more effective combined with other targets requires
additional studies.
In conclusion, these results showed that TAMs play
an active role in promoting the proliferation and migration of EOC,
and in upregulating the expression of IGF1, whereas the blockade of
IGF1 reversed the changes in proliferation and migration. These
findings may represent a new mechanism related to the growth and
metastasis of EOC, and currently available IGF1 inhibitors may be
potential tools for EOC intervention.
Acknowledgements
The authors thank Wang Kai from the Central Lab of
Shanghai First Maternity and Infant Hospital for excellent
technical assistance. This study was supported by grants from the
National Natural Science Foundation of China (81072136, 81372787),
the Shanghai Municipal Bureau of Health (20134033), and the
Shanghai Health system joint research project (2013ZYJB0201).
Glossary
Abbreviations
Abbreviations:
|
OC
|
ovarian cancer
|
|
EOC
|
epithelial ovarian cancer
|
|
IGF1
|
insulin-like growth factor-1
|
|
IGF1R
|
insulin-like growth factor-1
receptor
|
|
TAMs
|
tumor-associated macrophages
|
|
CM
|
conditioned medium
|
|
TME
|
tumor microenvironment
|
|
CAFs
|
cancer-associated fibroblasts
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colombo PE, Fabbro M, Theillet C, Bibeau
F, Rouanet P and Ray-Coquard I: Sensitivity and resistance to
treatment in the primary management of epithelial ovarian cancer.
Crit Rev Oncol Hematol. 89:207–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagaraj AB, Joseph P and DiFeo A: miRNAs
as prognostic and therapeutic tools in epithelial ovarian cancer.
Biomarkers Med. 9:241–257. 2015. View Article : Google Scholar
|
|
5
|
Naora H and Montell DJ: Ovarian cancer
metastasis: Integrating insights from disparate model organisms.
Nat Rev Cancer. 5:355–366. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Son B, Lee S, Youn HS, Kim EG, Kim W and
Youn BH: The role of tumor microenvironment in therapeutic
resistance. Oncotarget. 8:3933–3945. 2017.PubMed/NCBI
|
|
7
|
Liu Y and Cao X: Characteristics and
Significance of the Pre-metastatic Niche. Cancer Cell. 30:668–681.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu JS, Sheng SR, Liang XH and Tang YL: The
role of tumor microenvironment in collective tumor cell invasion.
Future Oncol. 13:991–1002. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Richards KE, Zeleniak AE, Fishel ML, Wu J,
Littlepage LE and Hill R: Cancer-associated fibroblast exosomes
regulate survival and proliferation of pancreatic cancer cells.
Oncogene. 36:1770–1778. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sugiyama K, Kajiyama H, Shibata K, Yuan H,
Kikkawa F and Senga T: Expression of the miR200 family of microRNAs
in mesothelial cells suppresses the dissemination of ovarian cancer
cells. Mol Cancer Ther. 13:2081–2091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim J and Bae JS: Tumor-associated
macrophages and neutrophils in tumor microenvironment. Mediators
Inflamm. 2016:60581472016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sica A, Larghi P, Mancino A, Rubino L,
Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P and Mantovani
A: Macrophage polarization in tumour progression. Semin Cancer
Biol. 18:349–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Zhang S, Yao J, Lowery FJ, Zhang
Q, Huang WC, Li P, Li M, Wang X, Zhang C, et al:
Microenvironment-induced PTEN loss by exosomal microRNA primes
brain metastasis outgrowth. Nature. 527:100–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kurahara H, Takao S, Maemura K, Mataki Y,
Kuwahata T, Maeda K, Sakoda M, Iino S, Ishigami S, Ueno S, et al:
M2-polarized tumor-associated macrophage infiltration of regional
lymph nodes is associated with nodal lymphangiogenesis and occult
nodal involvement in pN0 pancreatic cancer. Pancreas. 42:155–159.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reinartz S, Schumann T, Finkernagel F,
Wortmann A, Jansen JM, Meissner W, Krause M, Schwörer AM, Wagner U,
Müller-Brüsselbach S, et al: Mixed-polarization phenotype of
ascites-associated macrophages in human ovarian carcinoma:
Correlation of CD163 expression, cytokine levels and early relapse.
Int J Cancer. 134:32–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lan C, Huang X, Lin S, Huang H, Cai Q, Wan
T, Lu J and Liu J: Expression of M2-polarized macrophages is
associated with poor prognosis for advanced epithelial ovarian
cancer. Technol Cancer Res Treat. 12:259–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takaishi K, Komohara Y, Tashiro H, Ohtake
H, Nakagawa T, Katabuchi H and Takeya M: Involvement of
M2-polarized macrophages in the ascites from advanced epithelial
ovarian carcinoma in tumor progression via Stat3 activation. Cancer
Sci. 101:2128–2136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carroll MJ, Kapur A, Felder M, Patankar MS
and Kreeger PK: M2 macrophages induce ovarian cancer cell
proliferation via a heparin binding epidermal growth factor/matrix
metalloproteinase 9 intercellular feedback loop. Oncotarget.
7:86608–86620. 2016.PubMed/NCBI
|
|
20
|
Iams WT and Lovly CM: Molecular pathways:
Clinical applications and future direction of insulin-like growth
factor-1 receptor pathway blockade. Clin Cancer Res. 21:4270–4277.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dyer AH, Vahdatpour C, Sanfeliu A and
Tropea D: The role of insulin-like growth factor 1 (IGF-1) in brain
development, maturation and neuroplasticity. Neuroscience.
325:89–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baserga R: The contradictions of the
insulin-like growth factor 1 receptor. Oncogene. 19:5574–5581.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang M, Hu Z, Huang J, Shu Y, Dai J, Jin
G, Tang R, Dong J, Chen Y, Xu L, et al: A 3′-untranslated region
polymorphism in IGF1 predicts survival of non-small cell lung
cancer in a Chinese population. Clin Cancer Res. 16:1236–1244.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soulitzis N, Karyotis I, Delakas D and
Spandidos DA: Expression analysis of peptide growth factors VEGF
FGF2, TGFB1, EGF and IGF1 in prostate cancer and benign prostatic
hyperplasia. Int J Oncol. 29:305–314. 2006.PubMed/NCBI
|
|
25
|
Xu L, Zhou R, Yuan L, Wang S, Li X, Ma H,
Zhou M, Pan C, Zhang J, Huang N, et al: IGF1/IGF1R/STAT3
signaling-inducible IFITM2 promotes gastric cancer growth and
metastasis. Cancer Lett. 393:76–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pacher M, Seewald MJ, Mikula M, Oehler S,
Mogg M, Vinatzer U, Eger A, Schweifer N, Varecka R, Sommergruber W,
et al: Impact of constitutive IGF1/IGF2 stimulation on the
transcriptional program of human breast cancer cells.
Carcinogenesis. 28:49–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hirakawa T, Yashiro M, Doi Y, Kinoshita H,
Morisaki T, Fukuoka T, Hasegawa T, Kimura K, Amano R and Hirakawa
K: Pancreatic fibroblasts stimulate the motility of pancreatic
cancer cells through IGF1/IGF1R sgnaling under hypoxia. PLoS One.
11:e01599122016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yaktapour N, Übelhart R, Schüler J, Aumann
K, Dierks C, Burger M, Pfeifer D, Jumaa H, Veelken H, Brummer T, et
al: Insulin-like growth factor-1 receptor (IGF1R) as a novel target
in chronic lymphocytic leukemia. Blood. 122:1621–1633. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Terry KL, Tworoger SS, Gates MA, Cramer DW
and Hankinson SE: Common genetic variation in IGF1, IGFBP1 and
IGFBP3 and ovarian cancer risk. Carcinogenesis. 30:2042–2046. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lau MT and Leung PC: The PI3K/Akt/mTOR
signaling pathway mediates insulin-like growth factor 1-induced
E-cadherin down-regulation and cell proliferation in ovarian cancer
cells. Cancer Lett. 326:191–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Poljicanin A, Filipovic N, Vukusic Pusic
T, Soljic V, Caric A, Saraga-Babic M and Vukojevic K: Expression
pattern of RAGE and IGF-1 in the human fetal ovary and ovarian
serous carcinoma. Acta Histochem. 117:468–476. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Torng PL, Lee YC, Huang CY, Ye JH, Lin YS,
Chu YW, Huang SC, Cohen P, Wu CW and Lin CT: Insulin-like growth
factor binding protein-3 (IGFBP-3) acts as an invasion-metastasis
suppressor in ovarian endometrioid carcinoma. Oncogene.
27:2137–2147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koti M, Gooding RJ, Nuin P, Haslehurst A,
Crane C, Weberpals J, Childs T, Bryson P, Dharsee M, Evans K, et
al: Identification of the IGF1/PI3K/NF κB/ERK gene signalling
networks associated with chemotherapy resistance and treatment
response in high-grade serous epithelial ovarian cancer. BMC
Cancer. 13:5492013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang YF, Cheng WF, Wu YP, Cheng YM, Hsu
KF and Chou CY: Circulating IGF system and treatment outcome in
epithelial ovarian cancer. Endocr Relat Cancer. 21:217–229. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Li Y, Zhang J, Zheng C, Zhu H, Yu H
and Fan L: Circulating insulin-like growth factor-1 level and
ovarian cancer risk. Cell Physiol Biochem. 38:589–597. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gianuzzi X, Palma-Ardiles G,
Hernandez-Fernandez W, Pasupuleti V, Hernandez AV and Perez-Lopez
FR: Insulin growth factor (IGF) 1, IGF-binding proteins and ovarian
cancer risk: A systematic review and meta-analysis. Maturitas.
94:22–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin Y, Wei C, Liu Y, Qiu Y, Liu C and Guo
F: Selective ablation of tumor-associated macrophages suppresses
metastasis and angiogenesis. Cancer Sci. 104:1217–1225. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Remmele W and Stegner HE:
Immunhistochemischer Nachweis von Oestrogenrezeptoren (ER-ICA) in
Mammakarzinomen. Frauenarzt. 28:41–43. 1987.(In German).
|
|
39
|
Coward J, Kulbe H, Chakravarty P, Leader
D, Vassileva V, Leinster DA, Thompson R, Schioppa T, Nemeth J,
Vermeulen J, et al: Interleukin-6 as a therapeutic target in human
ovarian cancer. Clin Cancer Res. 17:6083–6096. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Koblish HK, Hansbury MJ, Bowman KJ, Yang
G, Neilan CL, Haley PJ, Burn TC, Waeltz P, Sparks RB, Yue EW, et
al: Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase
potently suppress systemic tryptophan catabolism and the growth of
IDO-expressing tumors. Mol Cancer Ther. 9:489–498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Moughon DL, He H, Schokrpur S, Jiang ZK,
Yaqoob M, David J, Lin C, Iruela-Arispe ML, Dorigo O and Wu L:
Macrophage blockade using CSF1R inhibitors reverses the vascular
leakage underlying malignant ascites in late-stage epithelial
ovarian cancer. Cancer Res. 75:4742–4752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Z, Chen JQ, Liu JL and Tian L:
Exosomes in tumor microenvironment: Novel transporters and
biomarkers. J Transl Med. 14:2972016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Goyne HE, Stone PJ, Burnett AF and Cannon
MJ: Ovarian tumor ascites CD14+ cells suppress dendritic
cell-activated CD4+ T-cell responses through IL-10 secretion and
indoleamine 2,3-dioxygenase. J Immunother. 37:163–169. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhou H, Qian W, Uckun FM, Wang L, Wang YA,
Chen H, Kooby D, Yu Q, Lipowska M, Staley CA, et al: IGF1 Receptor
targeted theranostic nanoparticles for targeted and image-guided
therapy of pancreatic cancer. ACS Nano. 9:7976–7991. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ukaji T, Lin Y, Banno K, Okada S and
Umezawa K: Inhibition of IGF-1-mediated cellular migration and
invasion by migracin A in ovarian clear cell carcinoma cells. PLoS
One. 10:e01376632015. View Article : Google Scholar : PubMed/NCBI
|