Introduction
Leukemia is one of the most common hematological
malignancies in humans (1,2). Chemotherapy, target therapy,
radiotherapy and hematopoietic stem cell (HSC) transplantation are
common therapies for leukemia (3,4).
However, chemoresistance and severe side-effects caused by
high-dose chemotherapy drugs are current hurdles to effective
treatment (5,6). Therefore, the screening of
target-specific small-molecule drugs for leukemia treatment is
crucial.
2-Phenyl-4-quinolone derivatives have various
pharmacological effects including anti-inflammatory and anticancer
activities (7–10). We have designed and synthesized a
new series of 2-phenyl-4-quinolone derivatives as new anticancer
candidates (9). These derivatives
can act as anti-mitotic agents by inhibiting tubulin polymerization
and disrupting microtubule organization (9,11).
Previously, we demonstrated that CHM-1
(20-fluoro-6,7-methylenedioxy-2-phenyl-4-quinolone) induced DNA
damage and inhibited the expression of DNA repair genes in human
osteosarcoma U-2 OS cells (12).
CHM-1 induced cell cycle arrest at the G2/M phase and
mitochondrial-dependent apoptotic cell death in CT-26 murine
colorectal adenocarcinoma cells (13). Smh-3
(2-[3-(methylamino)-phenyl]-6-(pyrrolidin-1-yl) quinolin-4-one),
another 2-phenyl-4-quinolone derivative, was found to induce
G2/M cell cycle arrest and mitochondria-dependent
apoptotic cell death through inhibition of AKT activity in HL-60
human leukemia cells (14). In
human hepatocellular carcinoma Hep3B cells, Smh-3 also induced cell
cycle arrest in the G2/M phase and apoptotic cell death
through endoplasmic reticulum stress and mitochondria-dependent
signaling (15). Herein, we
investigated the anti-proliferative effects of 2-phenyl-4-quinolone
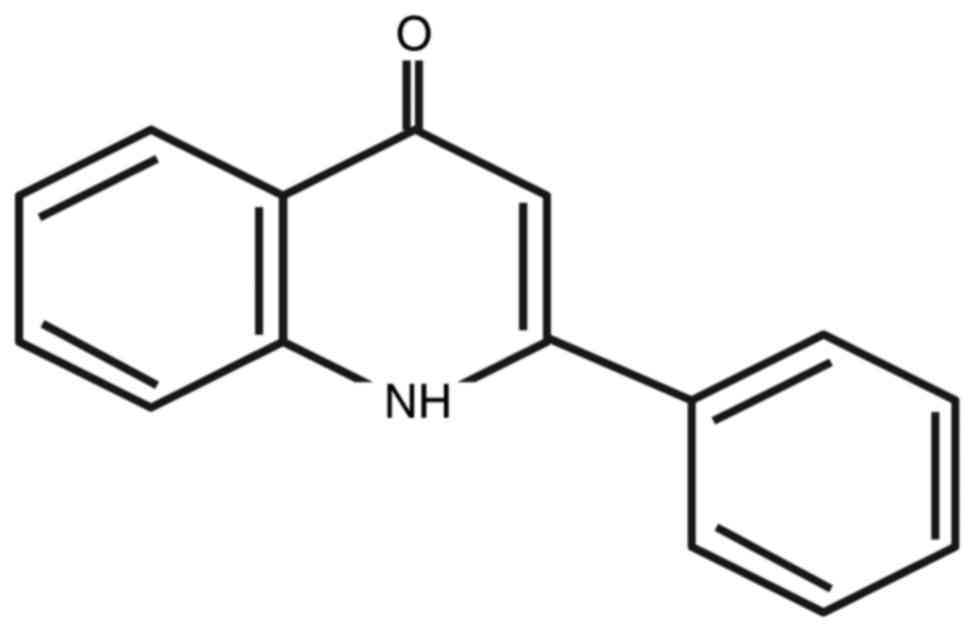
(YT-1) (Fig. 1) on leukemia or
normal cells and its underlying molecular mechanisms.
Materials and methods
Reagents and chemicals
YT-1 was provided by Professor Sheng-Chu Kuo and
synthesized as previously described (10). RPMI-1640 medium and
penicillin/streptomycin were obtained from Thermo Fisher Scientific
(Waltham, MA, USA). All antibodies used in this study and
anti-mouse (cat. no. sc-2005) and anti-rabbit (cat. no. sc-2004)
IgG peroxidase-conjugated secondary antibodies were obtained from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). All chemicals and
reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA)
unless otherwise stated.
Cell culture
Human leukemia cell lines U937 (lymphoma), HL-60
(promyelocytic leukemia), K562 (chronic myeloid leukemia) and WS-1
(normal skin fetal fibroblasts) were obtained from the Bioresource
Collection and Research Center (BCRC) (Hsinchu, Taiwan). All tested
cells were maintained in RPMI-1640 medium supplemented with 10%
fetal calf serum (HyClone Laboratories, Logan, UT, USA), 2 mM
L-glutamine and antibiotics (100 U/ml penicillin and 100 µg/ml
streptomycin) at 37°C in a humidified atmosphere of 5%
CO2.
Cell viability by flow cytometry and
microscopy observation
Cells were plated in a 24-well plate at a density of
2.5×105 cells/ml and cultivated for 24 h in the presence
of DMSO vehicle or 0.05, 0.1, 0.5, 1, 5 and 10 µM of YT-1. At each
time point, cell viability was determined by propidium iodide (PI)
exclusion method followed by flow cytometry (FACSCalibur flow
cytometer; Becton-Dickinson, Franklin Lakes, NJ, USA) as previously
described (16,17). In addition, following YT-1 treatment
for 24 h, the cells were also observed and photographed by a
phase-contrast microscope to monitor apoptotic characteristics
before being subjected to flow cytometry.
Analysis of DNA content by flow
cytometry
U937 cells (2×105 cells/well) in 12-well
plates were incubated with 1 µM YT-1 for 0, 2, 4, 6, 8, 10, 12, 14,
16, 18 and 20 h. Cells were then harvested and washed twice with
pre-chilled PBS. Cells were fixed in cold 70% ethanol overnight and
then re-suspended in 500 µl propidium iodide (PI) staining buffer
(0.1% Triton X-100, 100 µg/ml RNase A and 50 µg/ml PI in PBS) for
30 min as previously described (18,19).
Analyses of cell cycle profiling and apoptotic cells were performed
by flow cytometry (Becton Dickinson FACSCalibur flow cytometer) and
BD CellQuest Pro software (BD Biosciences, Franklin Lakes, NJ, USA)
as previously described (20–22).
Nuclear DAPI staining
After treatment with or without 1 µM YT-1 for 24 h,
U937 cells (1×105 cells/well) were sequentially washed
with PBS, fixed in 4% formaldehyde (Sigma-Aldrich) for 15 min and
permeabilized in 0.1% Triton X-100 for 15 min. Each sample was
stained with 200 µl DAPI solution (1 µg/ml) for 30 min at 37°C in
the dark. The integrity of nuclei and cells was visualized under a
fluorescence microscope (Nikon, Inc., Tokyo, Japan).
DNA fragmentation assay
After treatment with 1 µM YT-1 for 24 h, U937 cells
were harvested and lysed in 500 µl lysis buffer [20 mM Tris (pH
8.0), 10 mM EDTA and 0.2% Triton X-100] at 4°C for 30 min. Cell
lysates were then digested overnight with 100 µg/ml proteinase K at
50°C followed by 1 h of 50 µg/ml RNase A incubation at 37°C. Total
DNA was extracted with phenol/chloroform/isopropanol (24:25:1) and
then precipitated in 50% isopropanol. These samples were
electrophoresed on 1% agarose gel in 0.5X TBE buffer. DNA was
stained with 1 µg/ml ethidium bromide (EtBr) before the gel was
photographed under UV light.
Annexin V/PI double staining
U937 cells (2×105 cells/well) were
challenged with or without 1 µM YT-1 for 0, 3, 6, 9 and 12 h and
then incubated with Annexin V and PI solution (Annexin V-FITC
Apoptosis Detection kit, BD Biosciences Pharmingen, San Diego, CA,
USA). According to the manufacturer's protocol, the apoptotic cells
were detected by a Becton Dickinson FACSCalibur flow cytometer and
quantified by BD CellQuest Pro software.
Determination of mitochondrial
membrane potential (ΔΨm) by flow cytometry
U937 cells (2×105 cell/ml) in 24-well
plates were incubated with 1 µM YT-1 for 1, 2, 6, 12, 18 and 24 h.
Cells were harvested, washed twice with PBS and re-suspended in 500
µl of 50 nM 3,3′-dihexyloxacarbocyanine [DiOC6(3)]
solution (Thermo Fisher Scientific) at 37°C for 30 min. The level
of ΔΨm was determined and quantified by flow cytometry (Becton
Dickinson FACSCalibur flow cytometer).
Western blot analysis
U937 cells (1×107 cells) were plated in
75-T flasks in the presence of 1 µM YT-1 for 2, 4, 6, 8, 10, 12 and
18 h. Cell pellets were re-suspended in ice-cold lysis buffer (150
mM sodium chloride, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1%
sodium dodecyl sulfate, and 50 mM Tris, pH 8.0). Protein
concentration of each cell lysate was measured by Bradford assay
(Bio-Rad Laboratories, Hercules, CA, USA). Equal amount of protein
from each sample (40 µg protein/lane) was resolved by 12% SDS-PAGE.
The proteins were then electro-transferred onto a polyvinylidene
difluoride (PVDF) membrane. The membranes were immersed in PBS/0.1%
Tween-20 (PBST) containing 5% skim milk for 2 h at room temperature
as previously described (20,23,24).
Each blot was thereafter incubated overnight at 4°C with the
desired primary antibodies (1:1,000), including Bcl-2 (cat. no.
sc-509/mouse), Bax (cat. no. sc-70405/mouse), Bak (cat. no.
sc-517390/mouse), Bid (cat. no. sc-373939/mouse), cytochrome
c (cat. no. sc-13560/mouse), caspase-3 (cat. no.
sc-7272/mouse), caspase-9 (cat. no. sc-56076/mouse), PARP (cat. no.
sc-7150/rabbit), cyclin A (cat. no. sc-751/rabbit), cyclin B (cat.
no. sc-166210/mouse), and p21CIP1/WAF1 (cat. no.
sc-756/rabbit) and α-tubulin (cat. no. sc-5286/mouse) (all from
Santa Cruz Biotechnology). Protein bands were visualized using the
enhanced chemiluminescence (ECL) detection kit (Immobilon Western
HRP Substrate, Merck Millipore, Billerica, MA, USA) and Amersham
Hyperfilm ECL (GE Healthcare, Piscataway, NJ, USA). The blots were
stripped and reprobed with α-tubulin as the internal loading
controls.
Statistical analysis
All data are shown as the mean ± SD (n=3).
Statistical analysis of the results was carried out using the
Student's t-test. P<0.05 and P<0.01 were considered
statistically significant.
Results
YT-1 is cytotoxic to human leukemia
cell lines but not to normal cells
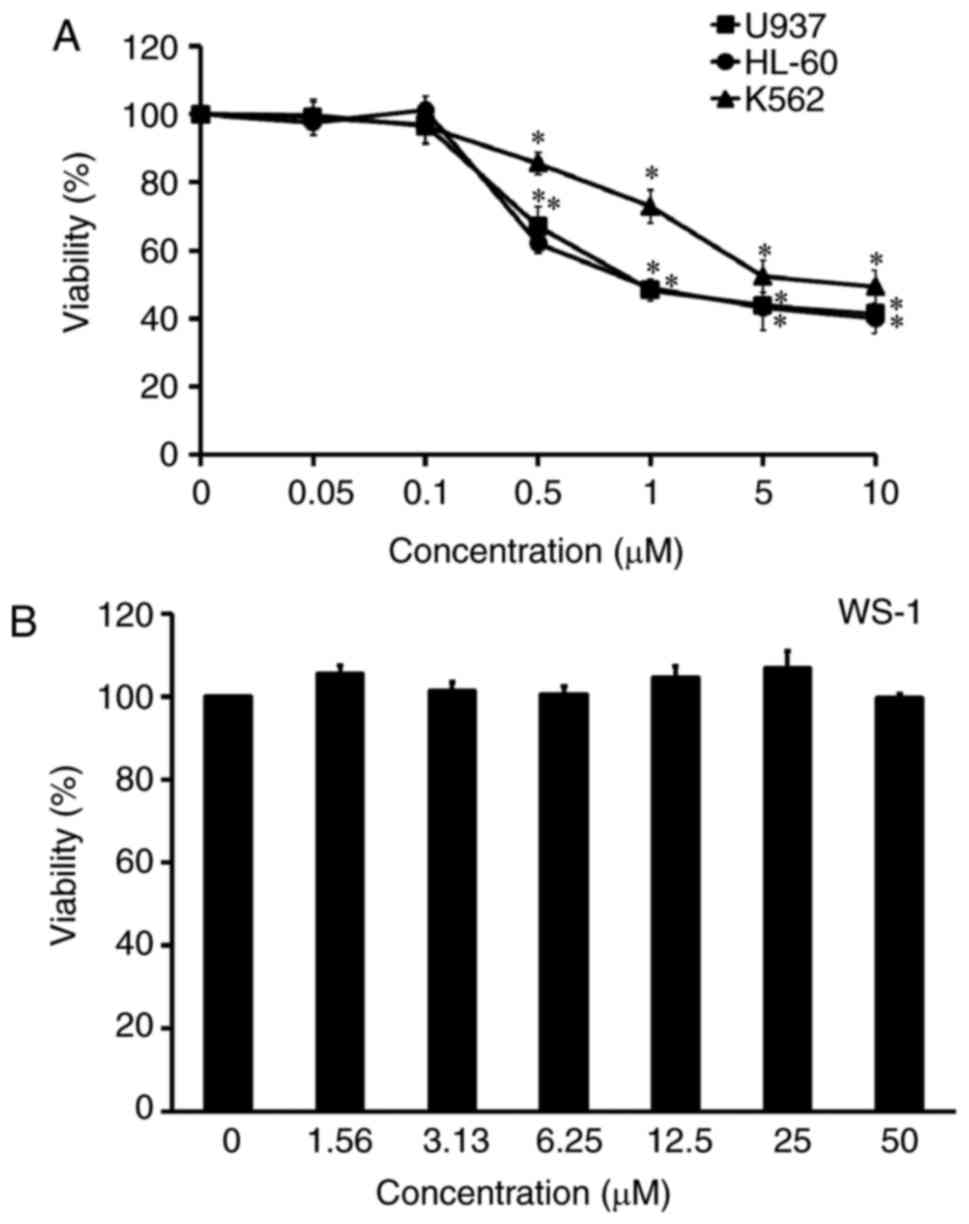
The cytotoxic effect of YT-1 was examined on various
human leukemia cells, including U937, HL-60 and K562 cells. After
treatment with 0.05–10 µM YT-1 for 24 h, leukemia cells showed
concentration-dependent sensitivity to YT-1. The IC50
values (the 50% inhibitory concentration of cell viability) were ~1
µM YT-1 (Fig. 2A). U937 was the
most sensitive cell line to YT-1. It is worth noting that no
significant cytotoxicity appeared in human fetal skin fibroblast
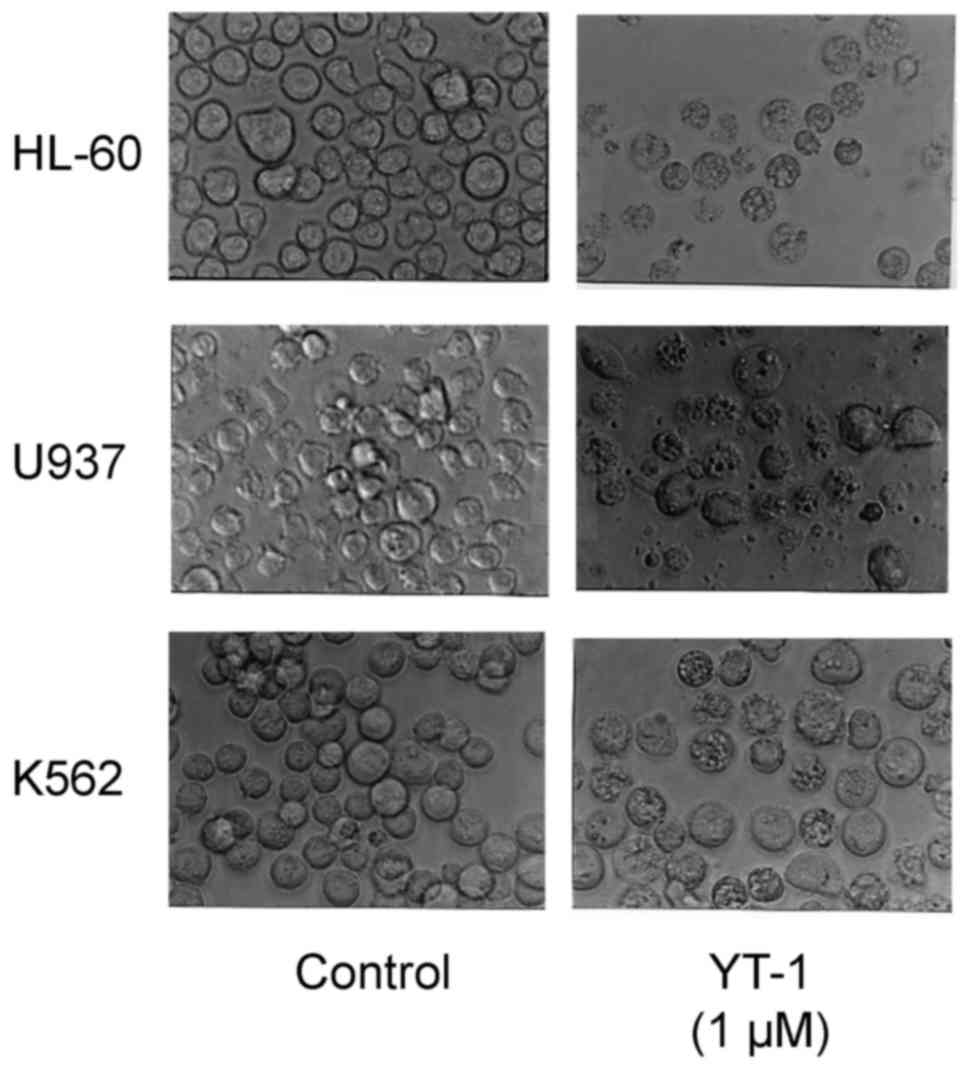
WS-1 cells after 1.56–50 µM YT-1 challenge (Fig. 2B). Furthermore, HL-60, U937 and K562
cells following 1 µM YT-1 treatment showed morphological changes
with apoptotic characteristics (cell shrinkage, rounding and
membrane blebbing) (Fig. 3). We
selected YT-1 at 1 µM to further investigate its effects on U937
cells.
YT-1 induces G2/M phase
arrest and apoptosis in U937 cells
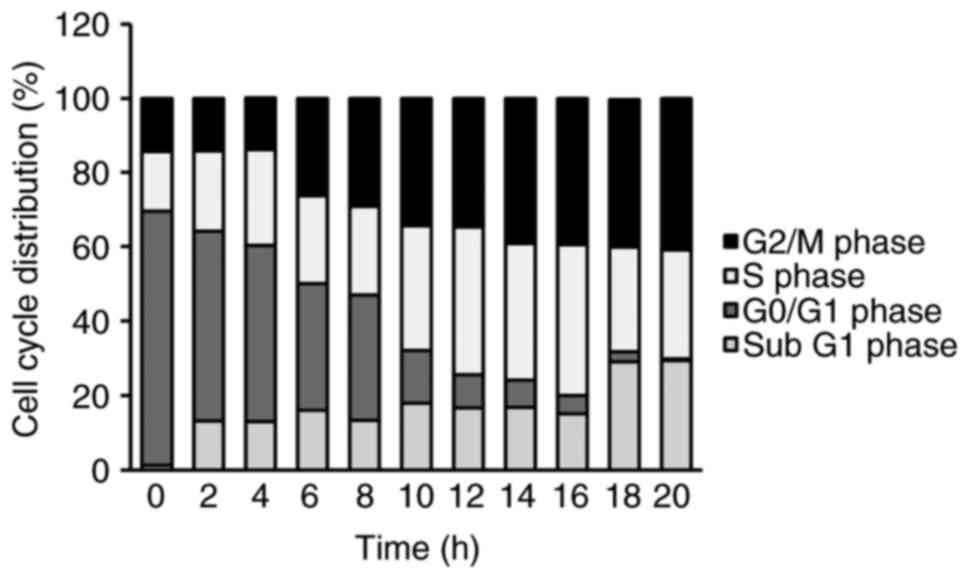
To investigate whether the cytotoxic effect of YT-1
was mediated by apoptotic machinery or/and interference of cell
cycle distribution, the cells were exposed to 1 µM YT-1 for 2, 4,
6, 8, 10, 12, 14, 16, 18 and 20 h. We monitored the percentage of
the sub-G1 population (apoptotic cells) and the cell cycle profile
via PI staining followed by flow cytometry. Following 6 to
20 h of treatment, YT-1 promoted G2/M phase arrest. It
also increased the sub-G1 population (apoptotic cells) after a 2-h
exposure (Fig. 4). In addition,
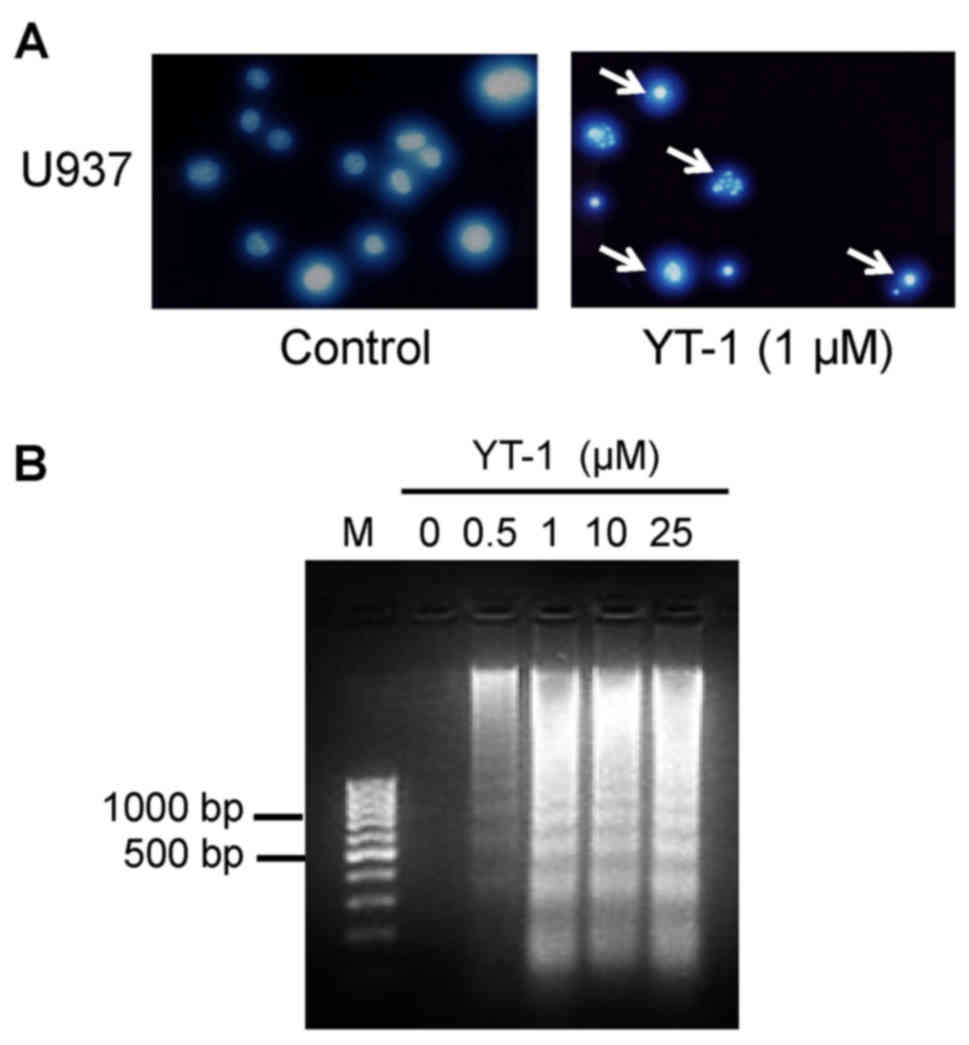
results from DAPI staining showed that YT-1 caused an increase in
the fluorescence intensity in nuclei and apoptotic bodies (Fig. 5A). These results indicated that YT-1
caused chromatin condensation and cleavage of nuclei in the U937
cells. In addition, YT-1 treatment at 1, 10 and 25 µM
concentrations provoked inter-nucleosomal DNA fragmentation in the
U937 cells (Fig. 5B). At indicated
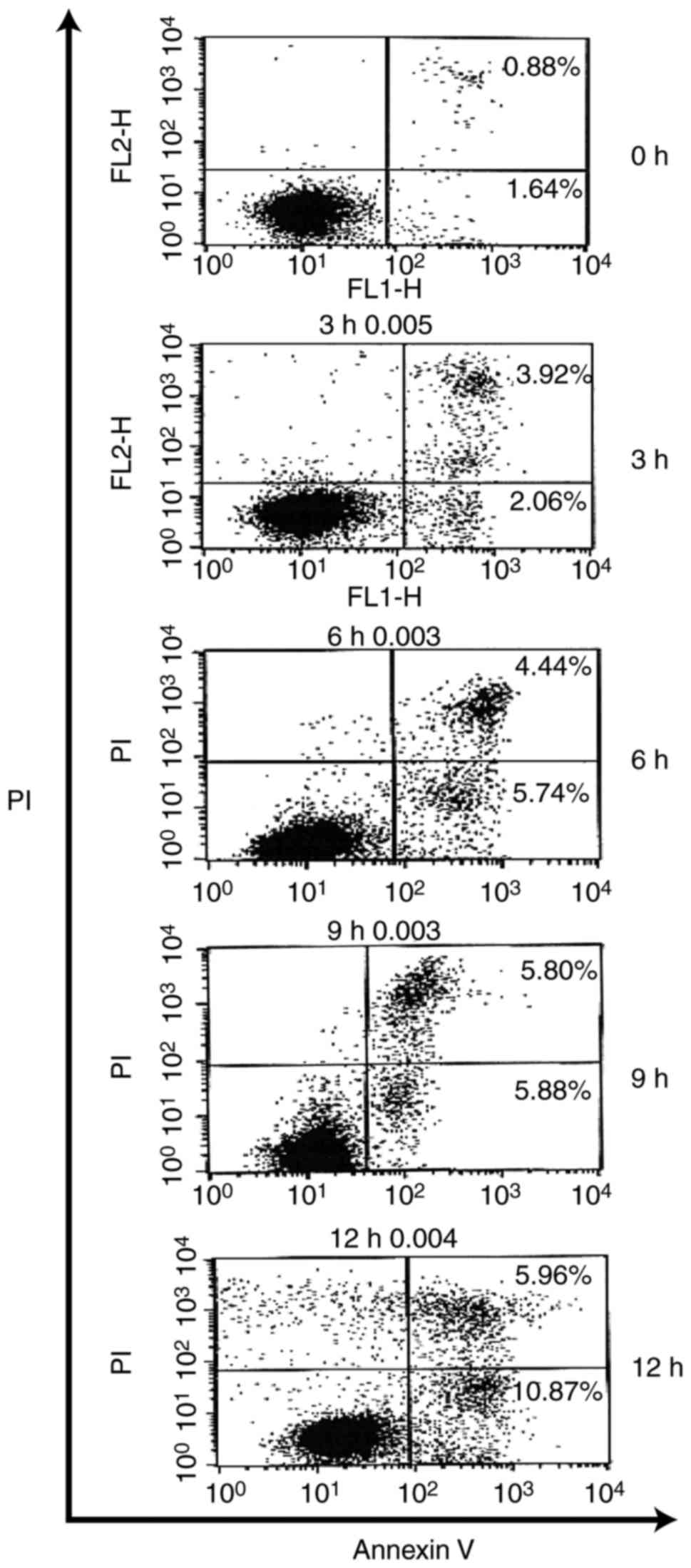
intervals of time, YT-1 triggered a concentration-dependent
increase in Annexin-positive/PI-negative (Annexin
V+/PI−) cell population, which is the cell
population with early signs of apoptosis. Approximately 11% of the
U937 cells were Annexin V+/PI− visualized at
a 12-h exposure (Fig. 6). Based on
these findings, the cytotoxic impact of YT-1 might result from
G2/M phase arrest and apoptotic death in U937 cells.
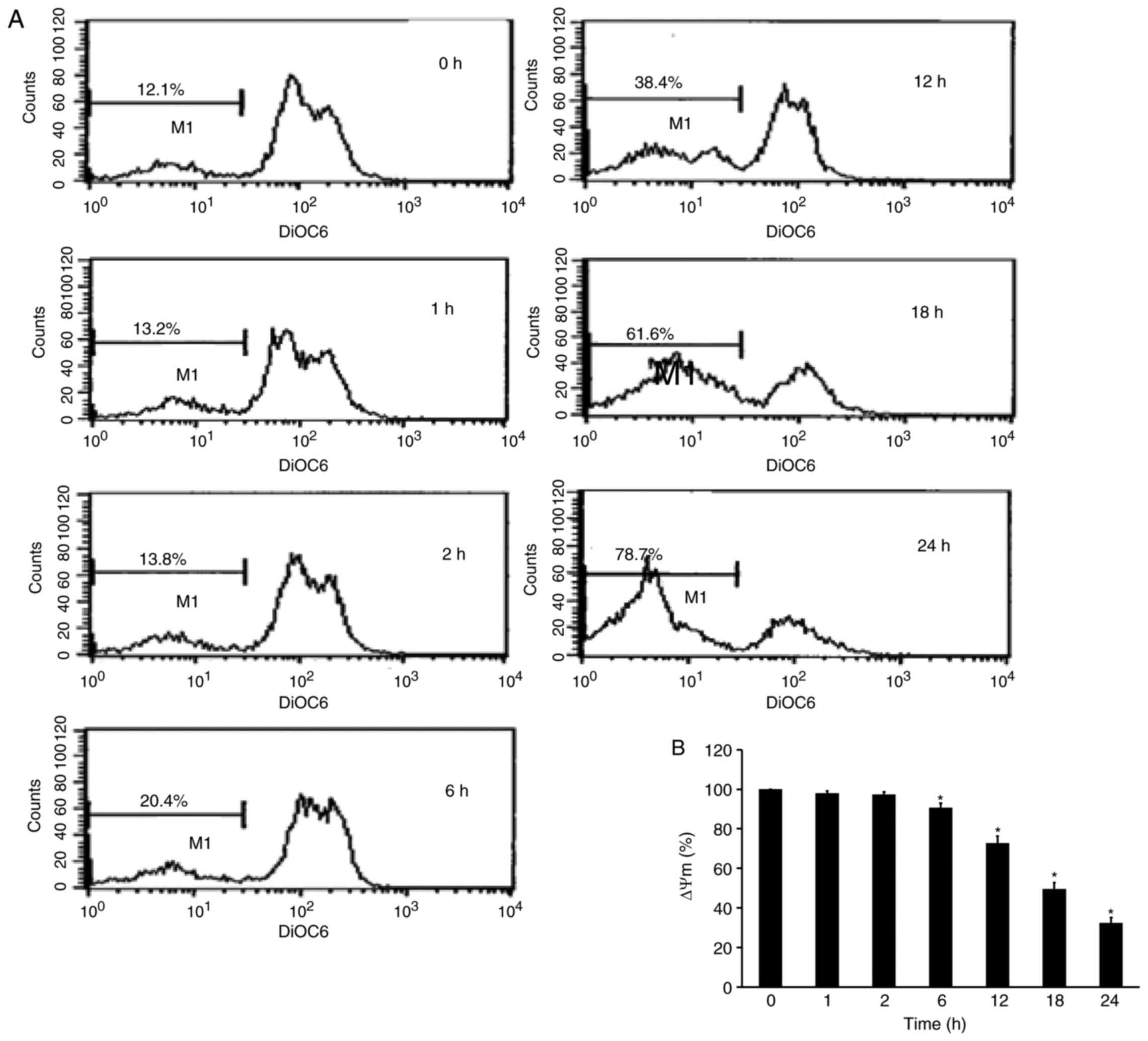
YT-1 disrupts the mitochondrial
membrane potential (ΔΨm) in U937 cells
To explore whether the apoptosis induced by YT-1 was
mediated by the mitochondrial pathway, we detected alterations in
ΔΨm. YT-1-treated U937 cells were analyzed by DiOC6(3)
incorporation. The resulting flow cytometric profile exhibited a
left-shifted fluorescent peak. Following 24 h of 1 µM YT-1
treatment, the loss of ΔΨm increased from 12.1 to 78.7%. The
time-dependent loss of ΔΨm indicated that YT-1-induced apoptosis
was mediated through mitochondrial regulation in U937 cells.
YT-1 triggers mitochondria-mediated
apoptosis in U937 cells
We next determined the effects of YT-1 on Bcl-2
family-regulated molecules and intrinsic caspase activation in U937
cells by western blotting. The results demonstrated that the
protein levels of Bax and Bak were increased, while those of Bcl-2
and Bid were decreased in a time-dependent manner (Fig. 8). Moreover, YT-1 increased the
expression of cytochrome c and proteolytically activated
caspase-3 and caspase-9 after exposure to 1 µM YT-1 (Fig. 8). These findings revealed that
mitochondria-dependent intrinsic apoptosis signaling was involved
in the YT-1-triggered U937 cell death.
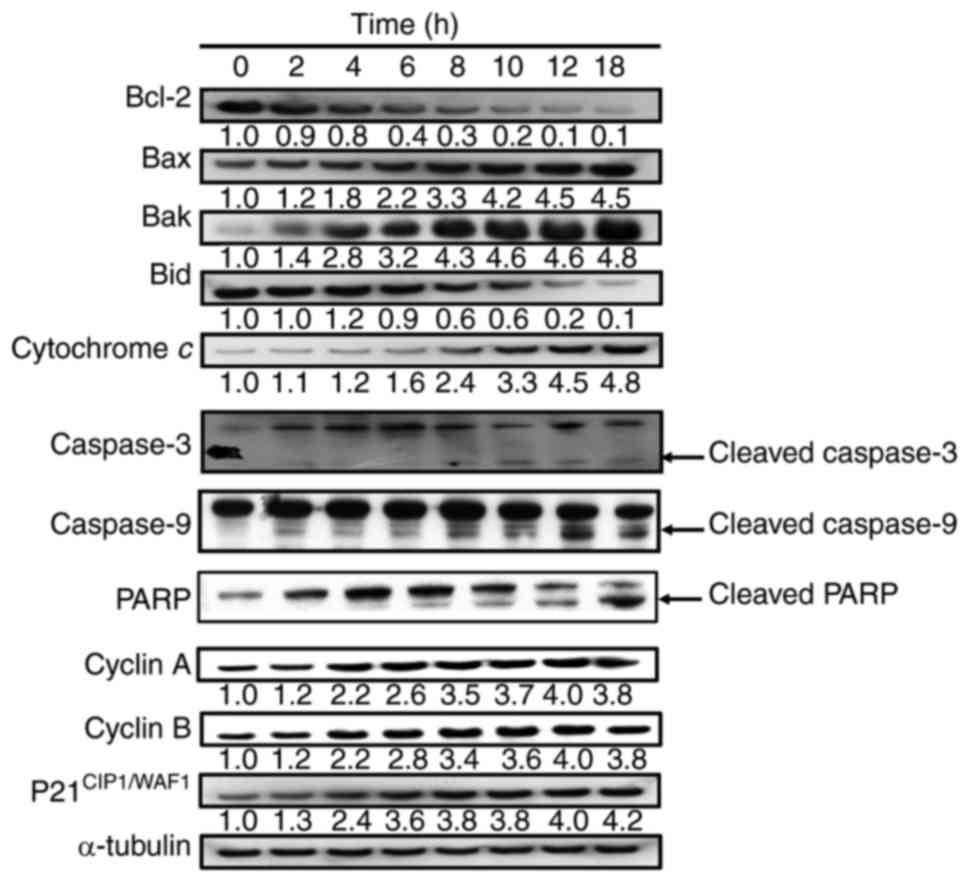 | Figure 8.YT-1 alters expression of the Bcl-2
family and mitochondria-dependent proteins in U937 cells. Cells
were incubated with 1 µM YT-1 for the indicated lengths of time.
Cell lysates were collected and blotted with antibodies for
mitochondria-regulated signals (Bcl-2, Bax, Bak, Bid, cytochrome c,
caspase-3, caspase-9, PARP, cyclin A, cyclin B, and
p21CIP1/WAF1). α-tubulin was used as the internal
control. |
Discussion
Previously we designed and synthesized
2-phenyl-4-quinolone derivatives to screen potential
anti-inflammatory and anticancer compounds (9,11–15).
In the present study, we investigated 2-phenyl-4-quinolone (YT-1)
for its effects on anti-leukemia activity and signaling
transduction associated with G2/M arrest and apoptosis
in leukemia cell lines. YT-1 significantly inhibited the cell
proliferation of U937, HL-60 and K562 cells (Fig. 2A). Importantly, YT-1 had only a weak
cytotoxic effect on normal human skin fibroblast WS-1 cells when
compared with its effect on U937, HL-60 and K562 cells (Fig. 2B).
Wang et al (25) showed that YT-1 inhibits neutrophil
O2˙− generation in vitro. YT-1
increased cellular cyclic AMP levels by inhibiting
phosphodiesterase (PDE) activity in
formylmethionyl-leucyl-phenylalanine (fMLP)-induced respiratory
burst in rat neutrophils, with an IC50 value of 60.7±8.2
µM. Kuo et al (10)
demonstrated that 2-phenyl-4-quinolone derivatives have significant
cytotoxic effects against human lung carcinoma (A-549), ileocecal
carcinoma (HCT-8), melanoma (RPMI-7951), epidermoid carcinoma of
the nasopharynx (KB) and two murine leukemia cell lines (P-388 and
L1210). We selected a dosage of 1 µM of lead compound YT-1 to
investigate its molecular mechanisms underlying its anti-leukemia
activity in vitro. The results are summarized as follows. i)
In HL-60, U937 and K562 cells, 6–20 h of YT-1 treatment caused
growth inhibition (Fig. 2A) and
cell cycle arrest at the G2/M phase (Fig. 4). ii) YT-1 increased protein levels
of cyclin A, cyclin B and CDK1 in U937 cells (Fig. 8). iii) YT-1 induced apoptotic body
formation (Fig. 3), chromatin
condensation (Fig. 5A), and DNA
fragmentation (Fig. 5B) in U937
cells (Fig. 8). iv) YT-1 induced
early apoptosis as demonstrated by increased Annexin V-positive
U937 cells (Fig. 6). v) YT-1
decreased the mitochondrial membrane potential in U937 cells
(Fig. 7). vi) YT-1 caused a
decrease in the protein level of Bcl-2 and Bid, but increased the
protein levels of Bax and Bak in U937 cells (Fig. 8). vii) YT-1 increased protein levels
of cleaved-activated caspase-3 and caspase-9 and the cleaved form
of PARP in U937 cells. Based on these results, we suggest that YT-1
induced apoptotic cell death through the mitochondria-dependent
pathway in U937 cells.
2-Phenyl-4-quinolone derivatives are potent
inhibitors of tubulin polymerization (9,11).
These activities are nearly comparable to those of the anti-mitotic
natural products colchicine, podophyllotoxin, and combretastatin
A-4 (26,27). Previous studies indicate that
microtubule-targeting agents (MTAs) promote CDK1 activity (28–30).
CDK1 plays a pro-apoptotic role in microtubule-targeting agents
(27). An increase in CDK1 activity
and induction of apoptosis have been demonstrated by paclitaxel
(Taxol) and vinca alkaloids (vinblastine, vincristine) in leukemia
cells (27,31). CDK1 can trigger mitochondrial
membrane permeabilization by targeting anti-apoptotic Bcl-2 protein
(27). Bcl-2 protein was
phosphorylated by CDK1 on Ser70 and was found to suppress its
anti-apoptotic activity, therefore leading to apoptosis (12). As to the G2/M arrest
induction by YT-1 (Fig. 4), our
results further demonstrated that YT-1 increased protein levels of
p21CIP1/WAF1, cyclin A, cyclin B and CDK1 (Fig. 8) in U937 cells. We suggest that YT-1
causes cell cycle arrest at the G2/M phase and apoptosis
through CDK1-mediated Bcl-2 phosphorylation.
Once anticancer drugs trigger mitochondria-dependent
apoptotic pathways, the mitochondrial outer membrane becomes
permeable, and then cytochrome c, Apaf-1, pro-caspase-9, AIF
and Endo G are released into the cytosol to sequentially activate
caspase-9 and caspase-3, eventually leading to apoptotic cell death
(16,17,32).
YT-1 decreased the mitochondrial membrane potential (Fig. 7), and reduced Bcl-2 and Bid protein
levels. In contrast, YT-1 upregulated Bax and Bak, activated
caspase-3 and caspase-9, and increased the proteolytic cleavage of
PARP in U937 cells (Fig. 8). The
results revealed that YT-1 triggers an mitochondria-dependent
apoptotic mechanism (an intrinsic pathway).
In conclusion, YT-1 showed significant cytotoxicity
against HL-60, U937 and K562 leukemia cells. However, YT-1 was less
toxic to normal human skin fibroblast WS-1 cells. The mechanisms
underlying the inhibitory effects of YT-1 in U937 human leukemia
cells included the promotion of G2/M phase arrest and
induction of the mitochondria-dependent apoptotic pathway.
Acknowledgements
This research was supported by a grant (CTCN-104-08)
from the Cardinal Tien Junior College of Healthcare and Management
(New Taipei, Taiwan).
References
|
1
|
Kohnken R, Porcu P and Mishra A: Overview
of the use of murine models in leukemia and lymphoma research.
Front Oncol. 7:222017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Allart-Vorelli P, Porro B, Baguet F,
Michel A and Cousson-Gélie F: Haematological cancer and quality of
life: A systematic literature review. Blood Cancer J. 5:e3052015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Medinger M, Zeiter D, Heim D, Halter J,
Gerull S, Tichelli A, Passweg J and Nigro N: Hypothyroidism
following allogeneic hematopoietic stem cell transplantation for
acute myeloid leukemia. Leuk Res. 58:43–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murakami K, Kohashi S, Sakurai M, Kato J,
Toyama T, Koda Y, Yamane Y, Hashida R, Abe R, Yamazaki R, et al:
Hyponatremia associated with human herpesvirus-6 (HHV-6)
encephalitis after allogeneic hematopoietic stem cell
transplantation: A presentation different from HHV-6 myelitis. Int
J Hematol. May 13–2017.(Epub ahead of print). View Article : Google Scholar
|
|
5
|
Tanoue S, Konuma T, Takahashi S, Watanabe
E, Sato N, Watanabe N, Isobe M, Kato S, Ooi J and Tojo A: Long-term
persistent donor-recipient mixed chimerism without disease
recurrence after myeloablative single-unit cord blood
transplantation in adult acute myeloid leukemia following
myelodysplastic syndrome. Leuk Lymphoma. 54:2973–2975. 2017.
View Article : Google Scholar
|
|
6
|
Busca A, Lessi F, Verga L, Candoni A,
Cattaneo C, Cesaro S, Dragonetti G, Delia M, De Luca A, Guglielmi
G, et al: SEIFEM 2010-E: Economic evaluation of posaconazole for
antifungal prophylaxis in patients with acute myeloid leukemia
receiving induction chemotherapy. Leuk Lymphoma. 58:2859–2864.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hour MJ, Huang LJ, Kuo SC, Xia Y, Bastow
K, Nakanishi Y, Hamel E and Lee KH: 6-Alkylamino- and
2,3-dihydro-3′-methoxy-2-phenyl-4-quinazolinones and related
compounds: Their synthesis, cytotoxicity, and inhibition of tubulin
polymerization. J Med Chem. 43:4479–4487. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xia Y, Yang ZY, Xia P, Bastow KF,
Tachibana Y, Kuo SC, Hamel E, Hackl T and Lee KH: Antitumor agents.
181. Synthesis and biological evaluation of
6,7,2′,3′,4′-substituted-1,2,3,4-tetrahydro-2-phenyl-4-quinolones
as a new class of antimitotic antitumor agents. J Med Chem.
41:1155–1162. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Wang HK, Kuo SC, Wu TS, Mauger A,
Lin CM, Hamel E and Lee KH: Antitumor agents. 155. Synthesis and
biological evaluation of 3′,6,7-substituted 2-phenyl-4-quinolones
as antimicrotubule agents. J Med Chem. 37:3400–3407. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuo SC, Lee HZ, Juang JP, Lin YT, Wu TS,
Chang JJ, Lednicer D, Paull KD, Lin CM, Hamel E, et al: Synthesis
and cytotoxicity of 1,6,7,8-substituted 2-(4′-substituted
phenyl)-4-quinolones and related compounds: Identification as
antimitotic agents interacting with tubulin. J Med Chem.
36:1146–1156. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu SC, Yang JS, Kuo CL, Lo C, Lin JP,
Hsia TC, Lin JJ, Lai KC, Kuo HM, Huang LJ, et al: Novel quinolone
CHM-1 induces apoptosis and inhibits metastasis in a human
osterogenic sarcoma cell line. J Orthop Res. 27:1637–1644. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen HY, Lu HF, Yang JS, Kuo SC, Lo C,
Yang MD, Chiu TH, Chueh FS, Ho HC, Ko YC and Chung JG: The novel
quinolone CHM-1 induces DNA damage and inhibits DNA repair gene
expressions in a human osterogenic sarcoma cell line. Anticancer
Res. 30:4187–4192. 2010.PubMed/NCBI
|
|
13
|
Chou LC, Yang JS, Huang LJ, Wu HC, Lu CC,
Chiang JH, Chen KT, Kuo SC and Chung JG: The synthesized
2-(2-fluorophenyl)-6,7-methylenedioxyquinolin-4-one (CHM-1)
promoted G2/M arrest through inhibition of CDK1 and induced
apoptosis through the mitochondrial-dependent pathway in CT-26
murine colorectal adenocarcinoma cells. J Gastroenterol.
44:1055–1063. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang SM, Yang JS, Tsai SC, Chen MH, Hsu
MH, Lin HY, Chou LC, Chinag JH, Lee KH, Huang LJ and Kuo SC: The
novel synthesized
2-(3-(methylamino)phenyl)-6-(pyrrolidin-1-yl)quinolin-4-one (Smh-3)
compound induces G2/M phase arrest and mitochondrial-dependent
apoptotic cell death through inhibition of CDK1 and AKT activity in
HL-60 human leukemia cells. Int J Oncol. 38:1357–1364. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu CY, Yang JS, Huang SM, Chiang JH, Chen
MH, Huang LJ, Ha HY, Fushiya S and Kuo SC: Smh-3 induces G(2)/M
arrest and apoptosis through calciummediated endoplasmic reticulum
stress and mitochondrial signaling in human hepatocellular
carcinoma Hep3B cells. Oncol Rep. 29:751–762. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu CC, Yang JS, Huang AC, Hsia TC, Chou
ST, Kuo CL, Lu HF, Lee TH, Wood WG and Chung JG: Chrysophanol
induces necrosis through the production of ROS and alteration of
ATP levels in J5 human liver cancer cells. Mol Nutr Food Res.
54:967–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chiang JH, Yang JS, Ma CY, Yang MD, Huang
HY, Hsia TC, Kuo HM, Wu PP, Lee TH and Chung JG: Danthron, an
anthraquinone derivative, induces DNA damage and caspase
cascades-mediated apoptosis in SNU-1 human gastric cancer cells
through mitochondrial permeability transition pores and
Bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiu YJ, Hour MJ, Lu CC, Chung JG, Kuo SC,
Huang WW, Chen HJ, Jin YA and Yang JS: Novel quinazoline HMJ-30
induces U-2 OS human osteogenic sarcoma cell apoptosis through
induction of oxidative stress and up-regulation of ATM/p53
signaling pathway. J Orthop Res. 29:1448–1456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang JS, Hour MJ, Kuo SC, Huang LJ and Lee
MR: Selective induction of G2/M arrest and apoptosis in HL-60 by a
potent anticancer agent, HMJ-38. Anticancer Res. 24:1769–1778.
2004.PubMed/NCBI
|
|
20
|
Lin CF, Yang JS, Lin C, Tsai FJ, Lu CC and
Lee MR: CCY-1a-E2 induces G2/M phase arrest and apoptotic cell
death in HL-60 leukemia cells through cyclin-dependent kinase 1
signaling and the mitochondria-dependent caspase pathway. Oncol
Rep. 36:1633–1639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen YF, Yang JS, Chang WS, Tsai SC, Peng
SF and Zhou YR: Houttuynia cordata Thunb extract modulates G0/G1
arrest and Fas/CD95-mediated death receptor apoptotic cell death in
human lung cancer A549 cells. J Biomed Sci. 20:182013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu SH, Hang LW, Yang JS, Chen HY, Lin HY,
Chiang JH, Lu CC, Yang JL, Lai TY, Ko YC and Chung JG: Curcumin
induces apoptosis in human non-small cell lung cancer NCI-H460
cells through ER stress and caspase cascade- and
mitochondria-dependent pathways. Anticancer Res. 30:2125–2133.
2010.PubMed/NCBI
|
|
23
|
Lai KC, Lu CC, Tang YJ, Chiang JH, Kuo DH,
Chen FA, Chen IL and Yang JS: Allyl isothiocyanate inhibits cell
metastasis through suppression of the MAPK pathways in epidermal
growth factorstimulated HT29 human colorectal adenocarcinoma cells.
Oncol Rep. 31:189–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma YS, Weng SW, Lin MW, Lu CC, Chiang JH,
Yang JS, Lai KC, Lin JP, Tang NY, Lin JG and Chung JG: Antitumor
effects of emodin on LS1034 human colon cancer cells in vitro and
in vivo: Roles of apoptotic cell death and LS1034 tumor xenografts
model. Food Chem Toxicol. 50:1271–1278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang JP, Raung SL, Huang LJ and Kuo SC:
Involvement of cyclic AMP generation in the inhibition of
respiratory burst by 2-phenyl-4-quinolone (YT-1) in rat
neutrophils. Biochem Pharmacol. 56:1505–1514. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lin JJ, Huang WW, Tsuzuki M, Lee TH and Chung JG: Novel
quinazolinone MJ-29 triggers endoplasmic reticulum stress and
intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits
leukemic mice. PLoS One. 7:e368312012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC
and Chung JG: MJ-29 inhibits tubulin polymerization, induces
mitotic arrest, and triggers apoptosis via cyclin-dependent kinase
1-mediated Bcl-2 phosphorylation in human leukemia U937 cells. J
Pharmacol Exp Ther. 334:477–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chan KS, Koh CG and Li HY:
Mitosis-targeted anti-cancer therapies: Where they stand. Cell
Death Dis. 3:e4112012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lee TH and Chung JG: Cell death caused by quinazolinone HMJ-38
challenge in oral carcinoma CAL 27 cells: Dissections of
endoplasmic reticulum stress, mitochondrial dysfunction and tumor
xenografts. Biochim Biophys Acta. 1840:2310–2320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chiang JH, Yang JS, Lu CC, Hour MJ, Chang
SJ, Lee TH and Chung JG: Newly synthesized quinazolinone HMJ-38
suppresses angiogenetic responses and triggers human umbilical vein
endothelial cell apoptosis through p53-modulated Fas/death receptor
signaling. Toxicol Appl Pharmacol. 269:150–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bates D, Feris EJ, Danilov AV and Eastman
A: Rapid induction of apoptosis in chronic lymphocytic leukemia
cells by the microtubule disrupting agent BNC105. Cancer Biol Ther.
17:291–299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu CC, Huang BR, Liao PJ and Yen GC:
Ursolic acid triggers nonprogrammed death (necrosis) in human
glioblastoma multiforme DBTRG-05MG cells through MPT pore opening
and ATP decline. Mol Nutr Food Res. 58:2146–2156. 2014. View Article : Google Scholar : PubMed/NCBI
|