Introduction
Lung cancer is the leading cause of cancer-related
death with an increasingly higher mortality and incidence among
younger individuals worldwide and it has become one of the most
serious threats to the quality of human life (1,2). In
recent decades, the treatment of lung cancer has made great
progress yet the prognosis remains poor with a 5-year survival rate
less than 15%, and a median survival time of 16 to 18 months in
China (3,4). Considering the status quo, it is
necessary to pursue research on non-small cell lung cancer (NSCLC),
its pathogenesis and progression, so that we can provide a more
effective and reliable approach for disease prevention, treatment
and improvement of patient prognosis.
Anticancer drugs play crucial roles in many
different processes, such as inducing tumor cell apoptosis,
cytotoxicity, proliferation, and inhibition of the invasion and
metastasis of tumor cells. Inhibition of tumor cells itself mainly
occurs through two mechanisms: promotion of apoptosis and cell
cycle arrest. We found that serum and glucocorticoid-regulated
kinase 1 (SGK1) after activation through substrate phosphorylation
not only regulates cell proliferation and differentiation, but also
promotes cell survival and inhibition of apoptosis. SGK1 is
upregulated in most types of tumor tissues but it has also been
observed to be downregulated in several other tumor tissues such
prostate cancer (5), ovarian cancer
(6), liver cancer (7), and adrenal tumors (8). It has been reported that in NSCLC
tissue, SGK1 mRNA levels were upregulated and related to various
clinical prognosis parameters (9,10),
although the SGK1 protein expression was not significantly
increased. In most tumor tissues, apart from paracancer tissues,
SGK1 protein levels show a trend of inconsistency, which still
remains ambiguous.
SGK1 is expressed in many different tissues of
mammals and it belongs to the AGC protein kinase family which
includes PKB/Akt and protein kinase C (PKC). Unlike constitutive
expression of PKB/AKT, expression of SGK1 is more likely to be
induced by external stimuli. SGK1 can be activated by serum and
glucocorticoid; it can be also attained by regulation of other
hormones or cytokines, such as mineralocorticoid hormones, insulin,
vasopressin and growth factors (11–14).
In the human body a vast number of signaling molecules are involved
in the regulation of the transcription level of SGK1, including
PKC, mitogen-activated protein kinase 1 (MAPK1) and
phosphatidylinositol 3-kinase (PI3K) (15,16).
Therefore, SGK1 is involved in various pathophysiological
processes, including regulation of ion channels, cell survival,
cell proliferation and migration, and is an important member in
various intracellular signalling pathways, including the PI3K,
p38MAPK (ie, MEKKs-MKK6, MKK3-p38MAPK) and JNK pathways. SGK1
participates in the regulation through downstream substrates of
multiple pathways and through pathways within them, and it is the
intersection of a variety of functions in vivo
phosphorylation cascades. Recently, multiple downstream substrates
of SGK1 such as GSK-3, FOXO3a, p21WAF-1, CREB and so on have been
reported, and most of these substrates are apoptosis regulatory
factors or nuclear transcription factors (17,18).
The signal transfer dynamics mode is another
mechanism of transmitting signals within the cells by key
signalling molecules. p53 gene is a tumor suppressor gene (TSG)
containing 393 amino acids, which is capable of binding to specific
DNA sequences and activating transcription. p53 gene includes two
types: the wild-type and the mutated type; the former can inhibit
tumor formation through the regulation of cell growth and by
inducing apoptosis in mutated or senescent cells, while the latter
losses the competency for inhibition of cell proliferation, DNA
repair and induction of apoptosis, so that excessive cell
proliferation, apoptosis reduction, abnormal transformation and
accumulation occurs.
Additionally, it has been reported that
double-stranded DNA damage occurs after cells are exposed to γ-ray
stimulation and then ‘tumor suppressor’ p53 protein levels present
a series of similar ringing digital pulse dynamic fluctuation,
while a comparable dynamical result in response to the UV
irradiation exposure leads to single-strand DNA damage, and the p53
expression presents a single pulse fluctuations. The dynamic
performance of p53 protein, such as fluctuation numbers and
amplitude, are the key influencing factors of cellular fate
decisions (cell cycle arrest, senescence and apoptosis), and they
are directly linked to DNA damage modes
(single-strand/double-strand breaks) and the degree of their damage
(19,20).
After cells are exposed to outside pressure, DNA
damage occurs and p53 is activated in vivo and
extracellular-regulated kinase (ERK) is phosphorylated. High
expression of SGK1 induced by ERK1/2 can facilitate their
transportation from the nucleus to the cytoplasm by promoting the
phosphorylation of FOXO3a and thereby blocking the transcription of
downstream target genes, further inducing cell cycle arrest and
apoptosis (21–23). p53 can activate transcription of
SGK1 while SGK1 enables the phosphorylation of MDM2, which can bind
p53 inhibitor, thereby inhibiting the degradation of p53 protein,
and finally activating the MDM2-dependent ubiquitination of p53
protein degradation. The p53 protein expression levels decreases
and then causes cell proliferation, survival and differentiation
process. Thus, there exists a negative feedback pathway between
SGK1 and p53 in vivo, and the SGK1 expression is closely
related to p53 protein levels. We examined the expression of
intracellular SGK1 protein exposed to the γ-ray stimulation to find
out whether similar dynamics exist with p53 fluctuation phenomenon
and to observe its relationship with apoptosis.
It was reported that a method for altering p53
dynamics exposed to γ-irradiation by switching p53 natural pulses
into a sustained p53 signal is held at the peak pulse amplitude.
Sustained p53 signaling appears to accelerate the expression of
senescence genes, while pulsed p53 delays gene expression and
thereby protects cells from prematurely committing to an
irreversible fate. It was also reported that even for similar
cumulative p53, sustained p53 signaling led to higher expression of
its target genes than pulsed p53, suggesting that it is the
dynamics of p53 rather than its accumulated levels that control
gene expression.
In a recent research study it was demonstrated that
mRNA expression of SGK1 is significantly high in squamous cell
carcinomas of the lungs and was found to be correlated with several
clinical parameters. SGK1 was elevated in high-grade tumors and in
tumors with large size and advanced stage, but the protein
expression was of no significance. Therefore in the present study
we conducted a different approach to explore the dynamic
fluctuation of SGK1 in NSCLC (10),
we carried out our study exclusively in Chinese patients.
In our previous work we examined the dynamic changes
of non-small cell lung cancer A549 cells under stimulation at
different time points, and found that SGK1 protein expression
presents a series of dynamic pulses of reverse fluctuations after
the double-strand DNA in cells was damaged following stimulation by
γ-rays, and explored the influence of Wogonin with SGK1 expression
in human NSCLC cells (24). We
chose to use GSK650394 as an SGK1 inhibitor (25) as it was reported in some studies to
stimulate the cells and successfully found that it is possible to
exert a dynamic fluctuation behavior of SGK1 protein expression
similar to that of γ-ray irradiation.
Since in vitro, the drug concentration
remains unchanged for a certain time period after achieving the
concentration platform, it can only alter the absorption rate of
cells but not the elimination rate and it is known that
experimentally it is not able to simulate the process of drugs
in vivo. Therefore administered medium was designed to be
removed at different time points during this experiment and bovine
serum albumin (BSA) was added to serum-free medium (40 g/l) to
simulate the serum albumin level in cancer patients, which made the
cell culture medium more close to the patient's metabolism
environment (26). Meanwhile, BSA
promotes drug efflux metabolism, resulting in the elimination
process of intracellular drugs.
We designed this experiment in such a way to explore
SGK1 dynamics in cells treated differentially and to explore
downstream apoptotic gene expression under the influence of SGK1
dynamics.
Materials and methods
Patients and tissue samples
Formalin-fixed, paraffin-embedded NSCLC samples
(n=224) and matched adjacent tumor specimens (n=103) from 224
patients who underwent surgery were collected from the Clinical
Biobank of The Affiliated Hospital of Nantong University, Jiangsu,
China from 2004 to 2009. At the time of surgery, patient age ranged
from 35 to 83 years, with a median of 62.9 years. No patient
received chemotherapy or radiotherapy before surgery.
Clinical data were obtained by reviewing medical
records in the archives room at the hospital. The data included
patient sex, age, smoking status, tumor size, tumor
differentiation, histological type, tumor status (T), lymph node
metastasis (N), distant metastasis (M), and TNM stage. Follow-up
data were obtained through telephone investigation. The last
follow-up was on May 30, 2013. Cancer stage was classified
according to the guidelines of the 7th edition of TNM staging in
lung cancer (27). Informed consent
was obtained from all patients before surgery, and the study
protocol was approved by the Research Ethics Committee of The
Affiliated Hospital of Nantong University.
Tissue microarray (TMA) construction
and immunohistochemistry
TMA construction and immunohistochemistry of the
NSCLC and matched adjacent tumor tissues were prepared and used for
TMAs. The TMAs were assembled using a tissue arraying instrument
(Quick-Ray, UT06; UNITMA, Seoul, Korea). Core tissue samples (2-mm
in diameter) were taken from individual paraffin-embedded sections
and deposited in recipient paraffin blocks. TMA specimens were cut
into 4-µm sections and were kept on super frost-charged glass
microscope slides before immunohistochemical processing.
Immunohistochemical analysis was performed as previously described.
The slides were incubated with the primary antibodies against SGK1
(ab32374, 1:200; Abcam, Cambridge, MA, USA) at 4°C overnight.
Horseradish-peroxidase-conjugated rabbit IgG (Abcam) was applied as
the secondary antibody for SGK1. The binding of the primary
antibody was detected using diaminobenzidine solution. Slides in
which the primary antibody was omitted were used as the negative
control group, while a breast cancer sample known to be
SGK1-positive was included as a positive control. For the
semi-quantification of positive strength, the intensity (0, 1, 2,
3) and proportion of positive cells (0–100%) were both recorded.
Thus, the score range was from 0 (no cell was stained) to 300 (all
were strongly stained). We then used X-tile software (Rimm
Laboratory at Yale University, New Haven, CT, USA; http://www.tissuearray.org/rimmlab) to determine
a reasonable cut-off point to separate SGK1 protein expression into
low and high expression.
Cell culture
Human lung carcinoma A549 cell line was obtained
from the American Type Culture Collection (ATCC, Rockville, MD,
USA). A549 were grown in RPMI-1640 (Gibco, Grand Island, NY, USA)
medium. All the cells were cultured in the respective medium
supplemented with 10% fetal bovine serum, 100 µg/ml penicillin, and
100 µg/ml streptomycin at 37°C in a humidified atmosphere with 5%
CO2.
Western blot analysis
The cell samples used for LC-MS/MS assays were also
prepared in parallel for western blot analysis. Equal amount of
cell cytosolic protein (50 µg) was loaded and separated by
SDS-PAGE. After electrophoresis, proteins were transferred to a
PVDF membrane (Pall Corp., Port Washington, NY, USA). Blots were
blocked with 5% skim milk in TBST buffer and incubated overnight at
4°C with specific primary antibody. After washing with TBST, the
membrane was incubated with HRP-conjugated secondary antibody
(KeyGen, Nanjing, China) for 1 h. The signal was visualized by an
enhanced chemiluminescence (ECL) system (Millipore, Billerica, MA,
USA). The protein expression levels were normalized with GAPDH to
correct any experimental handing errors.
Gene expression analysis
RNA was isolated from human cells using RNeasy Mini
kit (74106, Qiagen, Valencia, CA, USA). Human blood was collected
in PaxGene Tubes (762165, Qiagen) and RNA was isolated using the
PAXGene Blood RNA kit (Qiagen, 762174). RNA was reverse-transcribed
with SuperScript III (18080044, Invitrogen, Carlsbad, CA, USA)
before quantitative real-time PCR (qRT-PCR). Microarray was
performed using Human GenomeU219 Array on the Affymetrix GeneAtlas
platform. Partek software (Ariadne) was used for quality control
and to identify signaling pathways.
ELISA analysis
We used ELISA analysis to detect the expression of
IFN-γ, IL-7 and IL-9 in NSCLC A549 cells. The cell suspension was
diluted with PBS (pH 7.2) and the cell concentration reached
~106/ml. The tissue protein extraction reagent was added
to disrupt the cells and centrifuged at 3000 rpm for 20 min. The
concentrations of the cytokines from the supernatant were measured
using ELISA kits following the manufacturer's instructions.
Statistical analysis
The survival curves were calculated using the
Kaplan-Meier method and the log-rank test was used for survival
analysis. Factors shown related to prognosis with the univariate
Cox regression model were evaluated with the multivariate Cox
regression model. Differences were regarded as statistically
significant at P<0.05. All statistical analyses were performed
using SPSS version 20.0 statistical software (SPSS Inc., Chicago,
IL, USA).
Results
Relative SGK1 mRNA expression in NSCLC
tissues
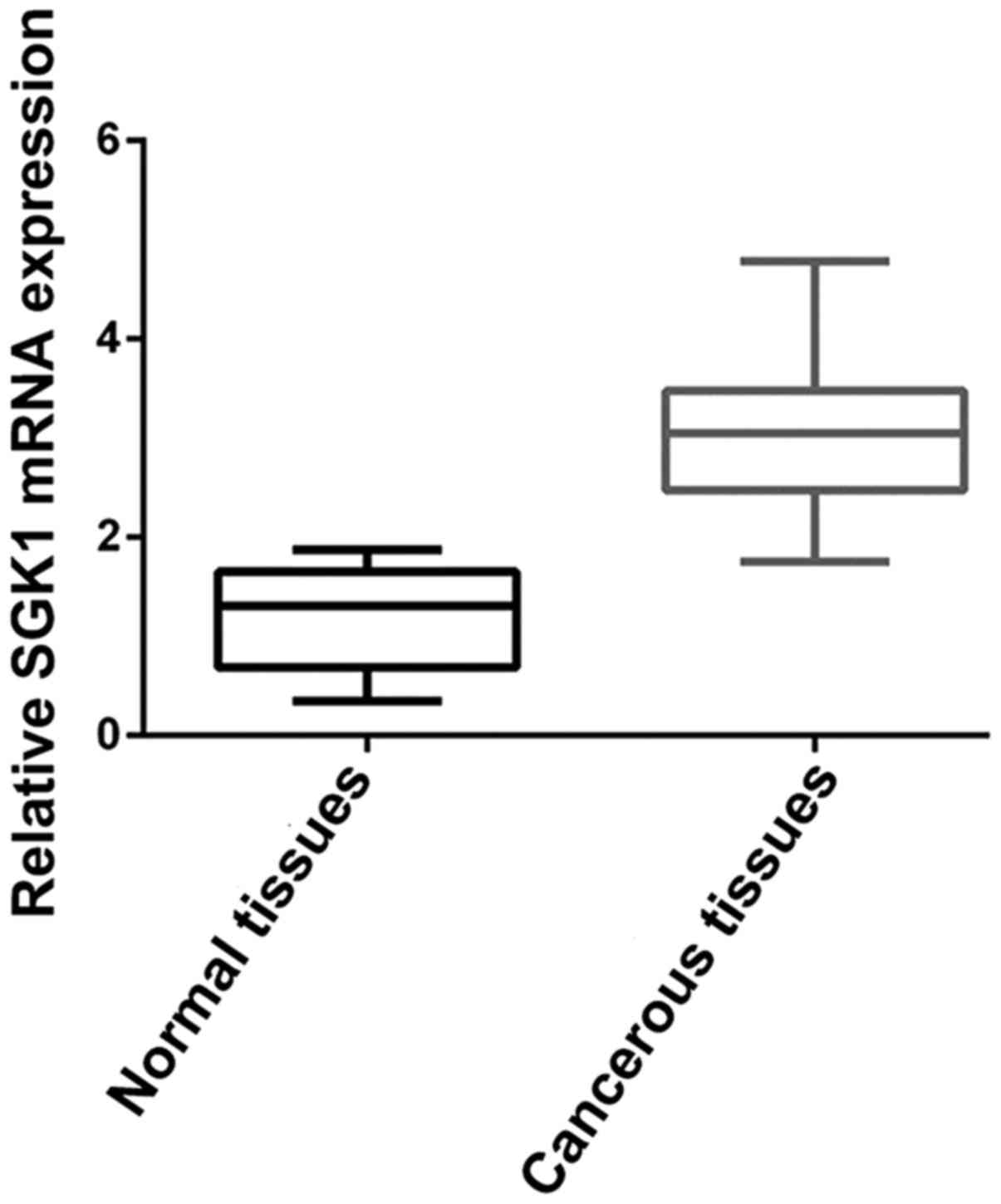
We determined SGK1 mRNA expression level in 30 pairs
of fresh frozen NSCLC tumorous and adjacent non-tumorous tissues.
Relative SGK1 mRNA expression level was normalized to the
expression of housekeeping gene GAPDH. We were able to observe
relatively high expression of SGK1 mRNA in cancerous tissues
compared to that noted in the adjacent non-cancerous tissues
(P<0.001) (Fig. 1), which
suggests higher SGK1 expression is related to the cancerous change
of NSCLC.
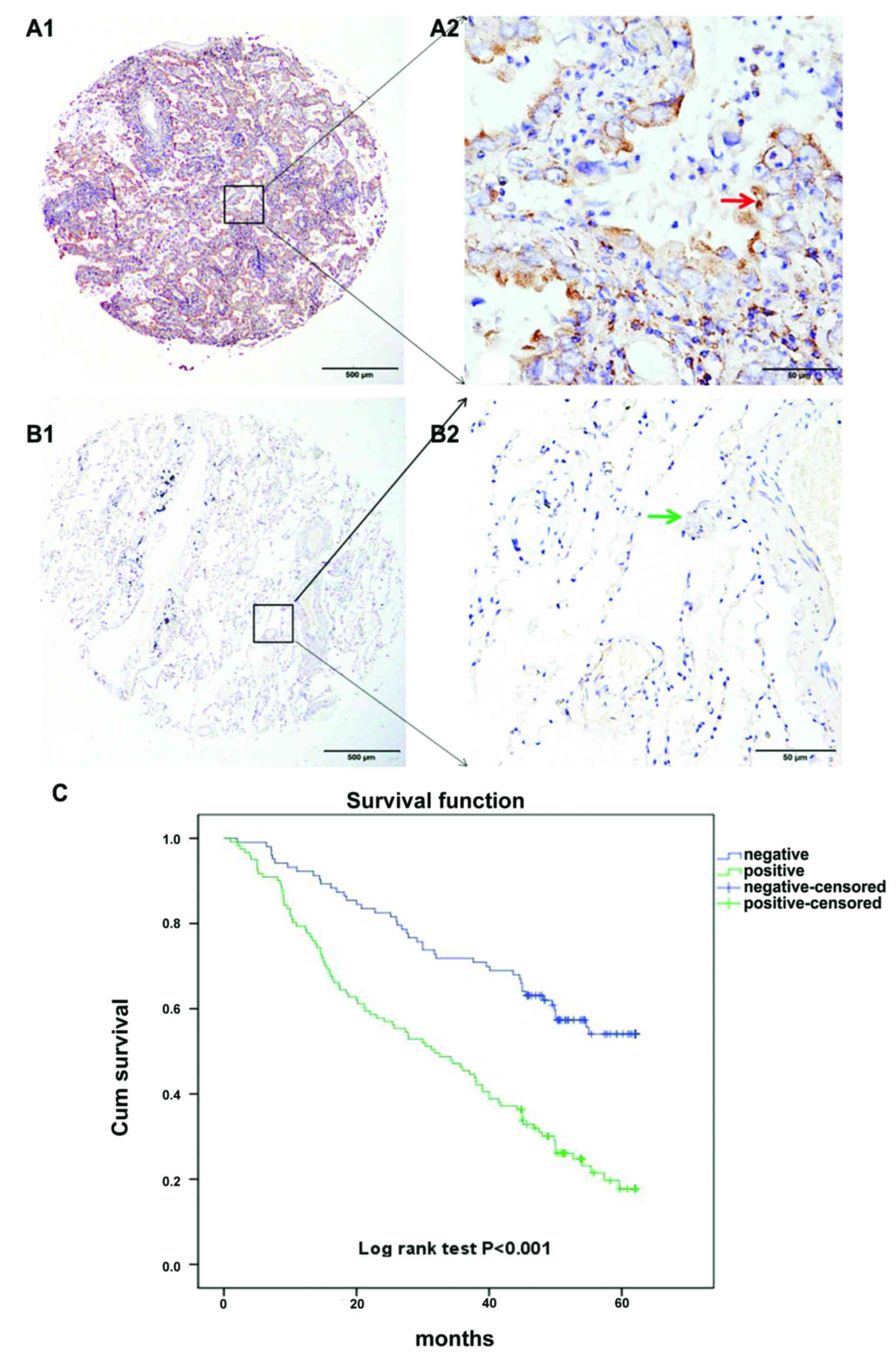
Analysis of SGK1 expression in NSCLC
patients by TMA-IHC
Two hundred and twenty-four cases of NSCLC were
collected and prepared, in which 103 cases of non-cancerous
adjacent tumor tissue samples were obtained. All samples belonged
to patients with a well-documented clinical history. Finally, we
analyzed prognostic factors in NSCLC patients using both univariate
and multivariate analyses.
Our results suggest that high SGK1 expression in
NSCLC patients predicts a reduced survival time. Likewise analyzing
the survival curve it was revealed that the percentage of surviving
NSCLC patients with positive SGK1 expression, after surgical
intervention was significantly lower than that of NSCLC patients
with negative SGK1 expression, which suggests the key role of SGK1
in predicting poor prognosis. The log-rank test revealed positive
SGK1 expression is associated with poor prognosis in NSCLC patients
(Fig. 2).
Correlation between SGK1 expression
and the clinicopathological characteristics of the NSCLC
patients
Next, we correlated SGK1 protein expression with the
following clinical characteristics of the NSCLC patients, which
included sex, age at diagnosis, tumor size, histopathological
grade, lymph node metastasis, smoking history and TNM stage. High
SGK1 protein expression was significantly associated with
differentiation (Pearson's χ2=5.279, P=0.022) and
histological type (Pearson's χ2=4.127, P=0.042)
(Table I). The statistical analysis
showed that high expression of SGK1 was not correlated with lung
cancer lymph node metastasis and advanced TNM staging.
 | Table I.Correlation of SGK1 expression in
tumor tissues with clinicopathological characteristics of the NSCLC
patients. |
Table I.
Correlation of SGK1 expression in
tumor tissues with clinicopathological characteristics of the NSCLC
patients.
|
|
| SGK1
expression |
|---|
|
|
|
|
|---|
| Groups | N | High (%) | Pearson's
χ2 | P-value |
|---|
| Total | 224 | 121 (54.02) |
|
|
| Age (years) |
|
| 0.201 | 0.654 |
|
<60 | 73 | 41
(56.16) |
|
|
|
≥60 | 151 | 80
(52.98) |
|
|
| Sex |
|
| 0.559 | 0.455 |
|
Male | 171 | 90
(52.63) |
|
|
|
Female | 53 | 31
(58.49) |
|
|
| Smoking
history |
|
| 0.058 | 0.81 |
|
Yes | 47 | 24
(51.06) |
|
|
| No | 92 | 45
(48.91) |
|
|
|
Unknown | 85 | 52
(61.18) |
|
|
|
Differentiation |
|
| 5.279 | 0.022a |
| Low
grade | 60 | 40
(66.67) |
|
|
| Middle
and high grade | 164 | 81
(49.39) |
|
|
| Histological
type |
|
| 4.127 | 0.042a |
|
Squamous cell carcinoma | 66 | 41
(62.12) |
|
|
|
Adenocarcinoma | 104 | 48
(46.15) |
|
|
|
Others | 54 | 32 |
|
|
| T |
|
| 0.594 | 0.743 |
|
Tis+T1 | 74 | 39
(52.70) |
|
|
| T2 | 126 | 72
(57.14) |
|
|
|
T3+T4 | 20 | 10
(50.00) |
|
|
|
Unknown |
4 | 0 |
|
|
| N |
|
| 2.982 | 0.225 |
| N0 | 122 | 60
(49.18) |
|
|
| N1 | 58 | 33
(56.90) |
|
|
| N2 | 44 | 28
(63.64) |
|
|
| TNM stage |
|
| 1.800 | 0.406 |
|
0–I | 90 | 45
(50.00) |
|
|
| II | 76 | 43
(56.58) |
|
|
|
III–IV | 54 | 32
(59.26) |
|
|
|
Unknown |
4 | 0 |
|
|
Findings from the univariate and multivariate
analyses of prognostic variables for the 5-year survival rate of
NSCLC patients are shown in Table
II. Univariate Cox regression analyses for all variables
suggested that the high expression of SGK1 [hazard ratio (HR)
1.926; 95% confidence interval (CI) 1.452–2.903; P<0.001] was a
significant negative prognostic factor for 5-year survival in
patients with NSCLC. Kaplan-Meier survival curves further confirmed
that SGK1 expression was significantly associated with the survival
of NSCLC patients, which was gradually reduced with increasing SGK1
expression (log-rank test, P<0.001, Fig. 2C). The survival curve corresponding
to SGK1 expression showed a good discrimination without much
overlap, indicating the success of the score standard for assessing
SGK1 staining, and the reliability of SGK1 as a prognostic
factor.
 | Table II.Univariate and multivariate analyses
of the prognostic variables for the 5-year survival rate of NSCLC
patients. |
Table II.
Univariate and multivariate analyses
of the prognostic variables for the 5-year survival rate of NSCLC
patients.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | P-value | 95% CI | HR | P-value | 95% CI |
|---|
| SGK1
expression | 1.926 |
<0.001a | 1.452–2.903 | 1.726 |
<0.001a | 1.396–2.865 |
| High
vs. low |
|
|
|
|
|
|
| Smoke | 0.846 | 0.821 | 0.576–1.463 |
|
|
|
| Yes vs.
no |
|
|
|
|
|
|
| Age (years) | 0.847 | 0.796 | 0.501–1.243 |
|
|
|
| <60
vs. ≥60 |
|
|
|
|
|
|
| Sex | 0.814 | 0.766 | 0.532–1.315 |
|
|
|
| Male
vs. female |
|
|
|
|
|
|
|
Differentiation | 1.117 | 0.215 | 0.729–1.587 |
|
|
|
| Low
grade vs. middle and high grade |
|
|
|
|
|
|
| Histological
type | 0.821 | 0.411 | 0.693–1.016 |
|
|
|
| Sq vs.
Ad vs. others |
|
|
|
|
|
|
| T | 1.103 | 0.137 | 0.845–1.507 |
|
|
|
| Tis+T1
vs. T2 vs. T3+T4 |
|
|
|
|
|
|
| N | 1.270 |
<0.001a | 1.053–1.559 |
|
|
|
| N0 vs.
N1 vs. N2 |
|
|
|
|
|
|
| TNM stage | 1.369 |
<0.001a | 0.957–1.655 | 1.281 |
<0.001a | 1.075–1.742 |
| 0–I vs.
II vs. III–IV |
|
|
|
|
|
|
Finally, multivariate Cox regression analysis of the
same set of NSCLC patients further demonstrated that high
expression of SGK1 (HR, 1.726; 95% CI 1.396–2.865; P<0.001) is
an independent prognostic factor for the 5-year survival of
patients with NSCLC (Table
II).
SGK1 dynamics following γ-ray
irradiation and treatment of GSK650394 at each time point
We aimed to observe the regulation of SGK1 produced
in tumor cells. For this we adopted the simulation of radiation
treatment with γ-radiation (10 Gy), and examined the protein
expression changes after irradiation in A549 cells. At the same
time we adopted a specific inhibitor of SGK1 (GSK650394) to
stimulate the cells and collected the cell samples hourly. Finally
we obtained the fluctuation expression curve of SGK1 protein with
western blot method.
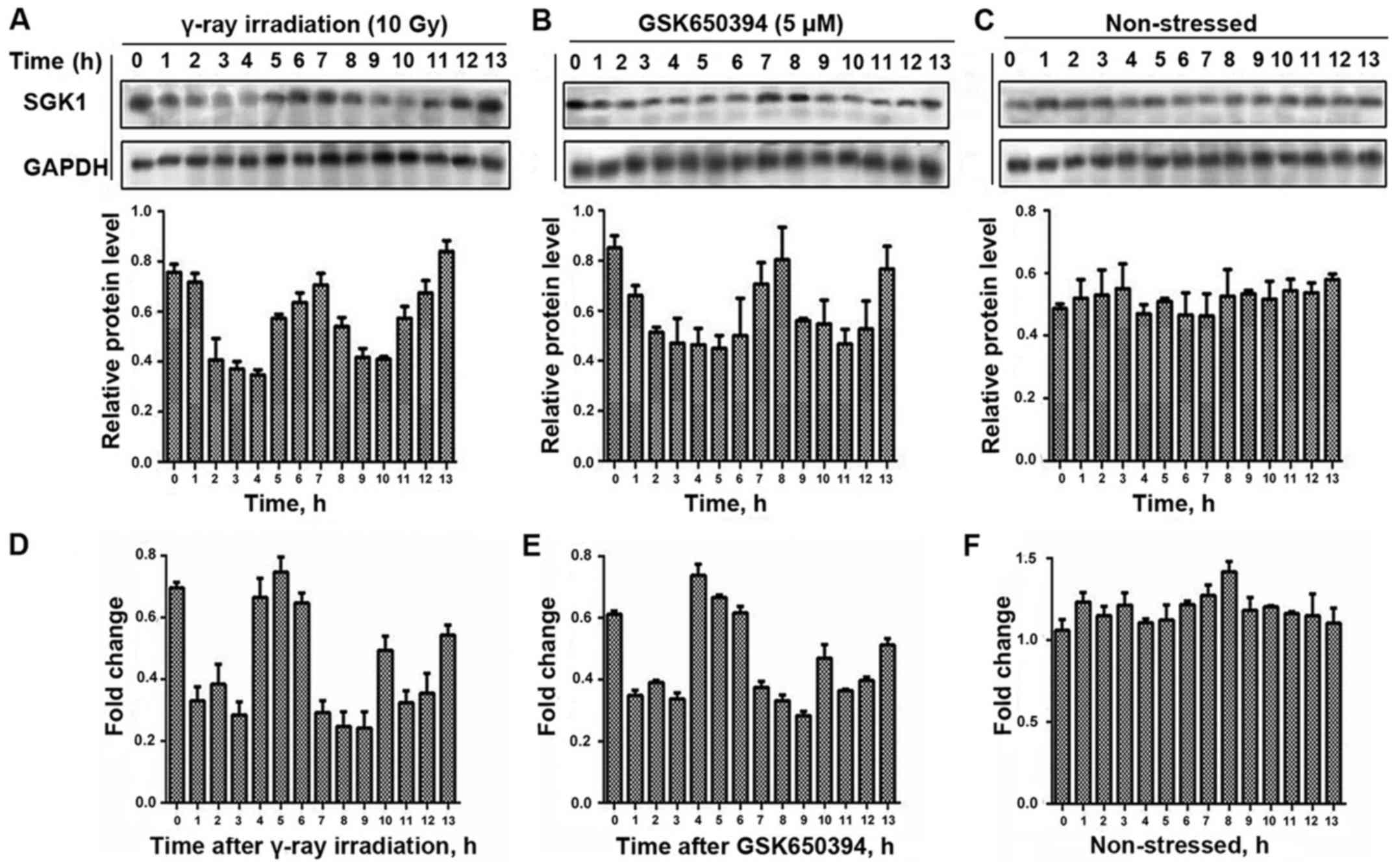
Following the exposure of A549 cells to γ-ray
irradiation and treatment with GSK650394, we measured their protein
level hourly and we did the same to non-stressed cells (Fig. 3). We were able to detect the highest
amplitude of fluctuation for the cells exposed to γ-radiation at 13
h and the highest amplitude of fluctuation for the cells after
treatment with GSK650394 was at 8 h while no such noticeable
fluctuations occurred in the non-stressed cells.
We were also able to detect mRNA dynamic fluctuation
for A549 cells exposed to γ-ray irradiation as well as for cells
after treatment with GSK650394, and did the same with the
non-stressed cells. Moreover, we detected the highest amplitude of
mRNA expression for the cells exposed to γ-radiation at 5 h and the
highest amplitude of fluctuation for the cells after treatment with
GSK650394 at 4 h and no such noticeable fluctuations occurred in
the non-stressed cells (Fig. 3).
Thus, we were able to prove the possibilities of dynamical
fluctuations in the cells through applying stress in two different
aspects.
Apoptosis-related protein expression
dynamics following stimulating of SGK1 inhibitor and γ-ray
irradiation
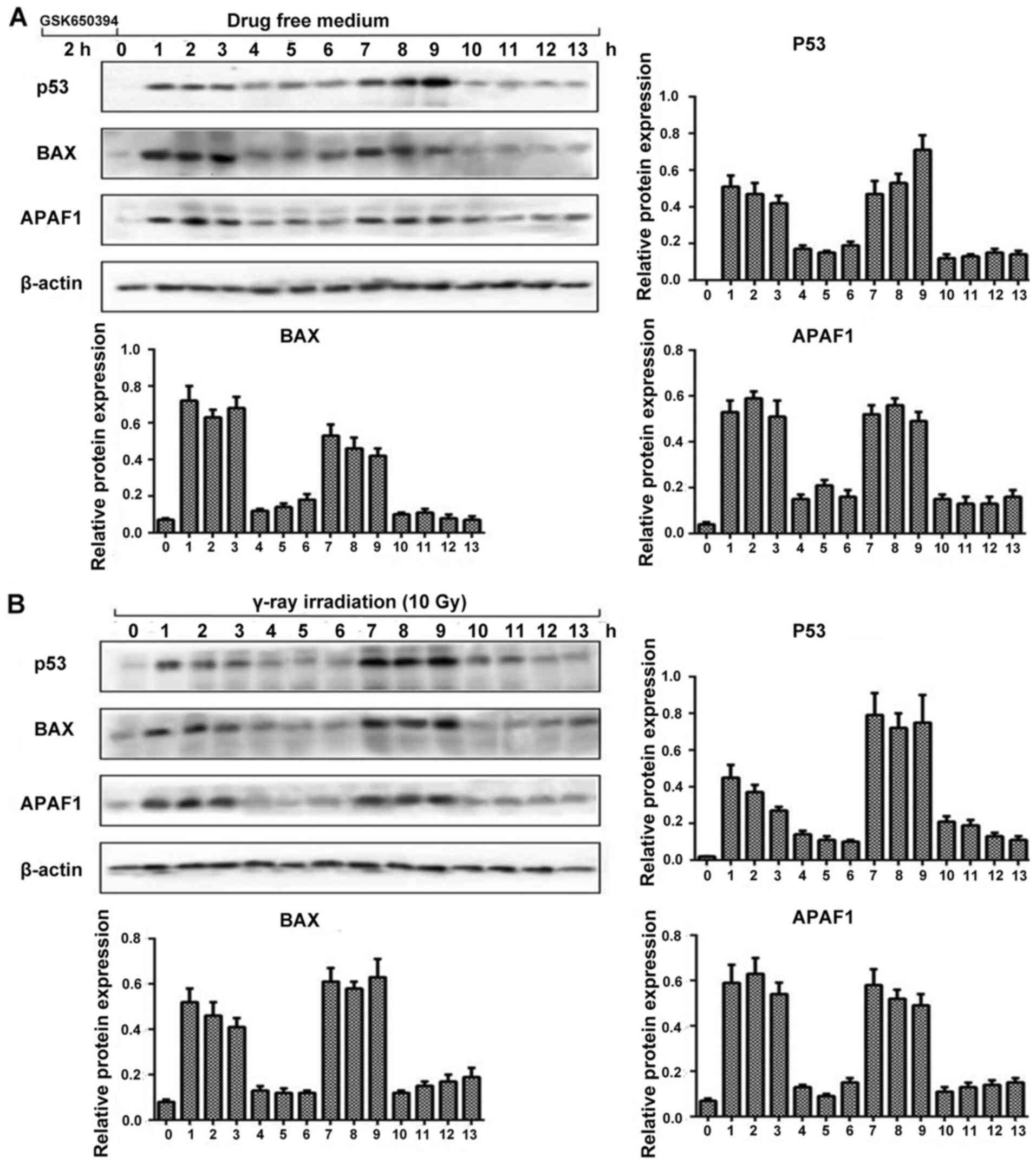
Next we performed western blot analysis to observe
the dynamic fluctuation of SGK1/p53 protein level with its
downstream apoptotic proteins following stimulation with SGK1
inhibitor GSK650394 and γ-ray irradiation. We chose p53, BAX, APAF1
along with β-actin. As shown in Fig.
4, by inducing stress, we observed the relative protein
expression levels every hour. The expression of p53, BAX and APAF1
showed almost a similar trend of fluctuation with their protein
expression levels. The fluctuation trend was in such a way that at
0 h there was minimum amplitude followed by elevated amplitude at
(1, 2 and 3 h) flowed by depressed amplitude at 4, 5 and 6 h again
followed by elevated amplitude at 7, 8 and 9 h which continued with
a depressed amplitude until 13 h, which was the pre-determined time
limit for our experiment. It also revealed that the expression
level of the p53 pathway including its downstream apoptotic
proteins following stimulation with SGK1 inhibitor GSK650394 and
γ-ray irradiation could also present a dynamic process of
fluctuation. This confirms a new method to control cell fate
decisions.
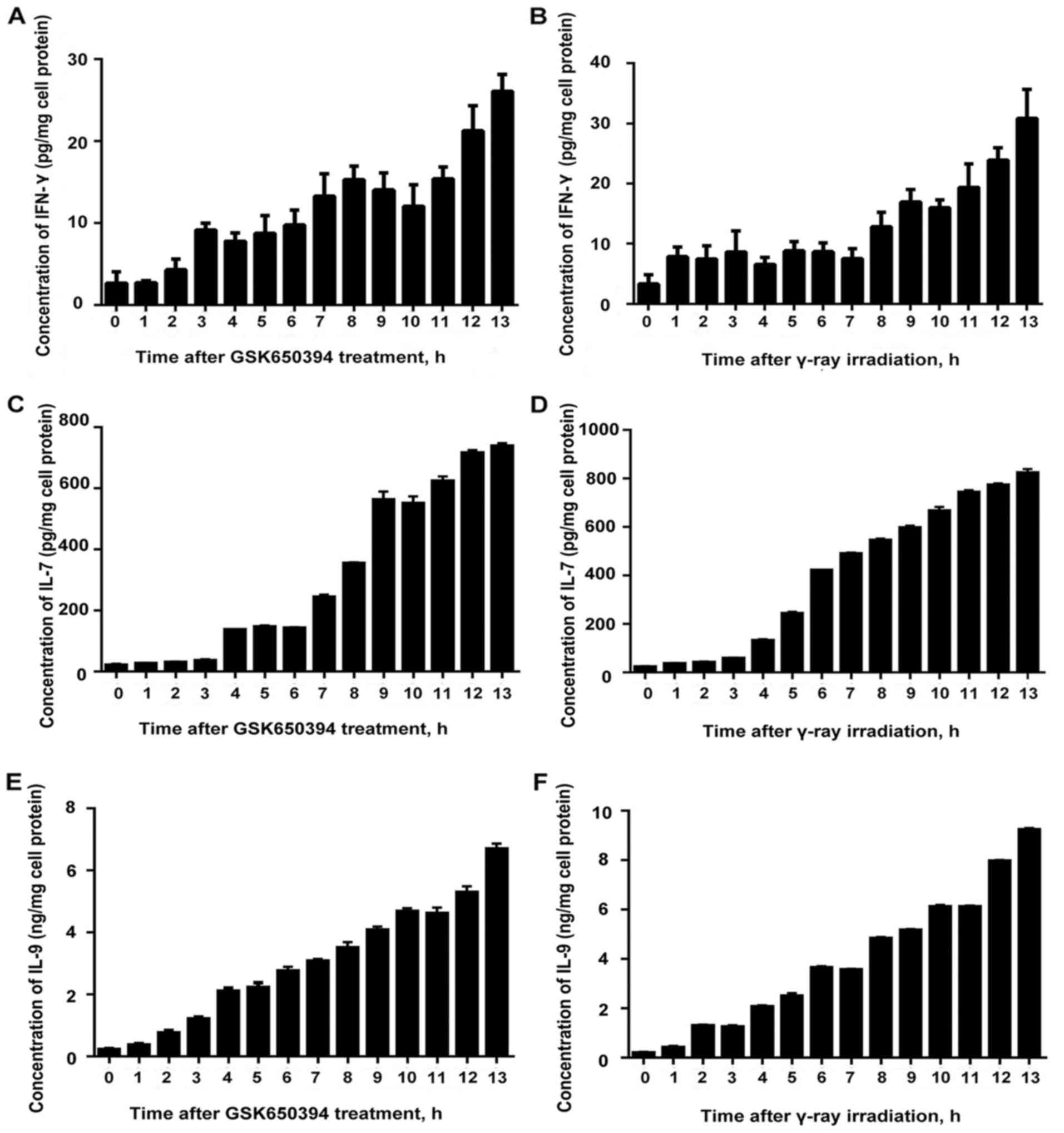
Accumulation of IFN-γ, IL-7 and IL-9
following stimulation with an SGK1 inhibitor and γ-ray
irradiation
We were able to detect an ascending trend of
fluctuation with least amplitude of fluctuation at 0 h and highest
amplitude at 13 h for both after treatment with SGK1 inhibitor
GSK650394 and after γ-ray irradiation. The results revealed that
SGK1 inhibitor inhibited the expression of SGK1 activity and was
capable of inducing the expression of IFN-γ and the concentration
accumulated, which could enhance the cellular immune response and
the capability to promote tumor cell apoptosis. Similar results
were obtained for IL-7 and IL-9 (Fig.
5).
Discussion
SGK1 was originally identified in a differential
screen for glucocorticoid-responsive genes in a mammary tumor cell
line. It is known to undergo both transcriptional and
posttranslational regulation dynamically in response to a variety
of stimulations such as glucocorticoids, hormones, cell volume and
growth factors (28). Under
non-pathological conditions, the expression of SGK1 was detected to
be low in several tissues and in fact not required for basic
functions. On the other hand, SGK1 is rapidly upregulated under
specific stress and pathological conditions (29). The expression of SGK1 is strongly
related to cellular stress. For this reason, the highest expression
was found in high-grade tumors, as these are usually characterized
by higher rates of energy metabolism, which expose them to relative
hypo-oxygenation and paradoxically to higher oxidative stress due
to the Warburg effect (30–32).
In response to DNA breakage caused by γ-irradiation,
the levels of p53 exhibit a series of pulses with fixed amplitude
and frequency (33). Mice were able
to produce p53 dynamics after their body was exposed wholly to
γ-ray irradiation (34). In this
case, the kinetics-exhibited phenotype of cells can be
distinguished, and such cells are likely able to become a target
for small-molecular drugs. SGK1 dynamics can reveal a potential
unrecognized regulation mode. Considering this, small-molecular
drugs can be used for the treatment together with intracellular
signaling dynamics, regulation of dynamic modes to change the fate
of cells, which can provide hope for future research. We concede
that our knowledge of the role of SGK1 with its specific
small-molecule inhibitors in tumors is at an early stage, and
further studies are urgently required to indicate the most
appropriate use for the prognostic or predictive evaluation of
NSCLC patients.
In the present study we explored the role of SGK1,
the most studied and represented member of the SGK family of
serine/threonine kinases. By analyzing various studies related to
SGK1 and through our own study we confirmed the role of SGK1 in
NSCLC. We observed that high SGK1 mRNA expression appeared to be a
worse prognostic indicator, nevertheless the SGK1 protein
expression was not significant, more specifically with the TNM
stage. Compared with protein expression, SGK1 mRNA expression was
more pronounced for the process of transcription to translation as
well as post-translational modification is very complex, and the
expression of protein at the translation level often lags the
expression of mRNA at the transcriptional level, so there is always
a difference between the two. Moreover, we were able to detect
remarkable dynamic fluctuation for SGK1 using γ-ray irradiation and
SGK1 inhibitor, which revealed great possibility in the treatment
of NSCLC. Hence this dynamic fluctuation can be used to regulate
the dose of anticancer drugs and to control the cell fate. With the
proper use of this dynamic fluctuation, there are great
possibilities to provide better treatment for patients in the
future. Therefore, there is urgent need of further studies to
explain more about how this fluctuation should be used to approach
lung cancer treatment.
The mechanism of action of kinetic signals can
provide a new way for the cells to adjust to the response of
external stimuli in a controlled manner, and this research could
bring new approaches to change cell fate and to achieve new drugs
for treatment. The advantage of this treatment in disturbing the
signal dynamics for therapeutic purposes is that, it is
non-invasive and can precisely control the instantaneous signal
transmission especially when it is necessary to distinguish
dynamics between normal cells and tumor cells (35). In a recent study, it was revealed
that SGK1 is essential for limiting and regulating cell survival,
proliferation and differentiation through phosphorylation of MDM2,
which controls p53 ubiquitylation and proteosomal degradation
(36). As mentioned above, several
studies have confirmed that there exists a negative feedback
pathway between SGK1 and p53, and SGK1 expression is closely
related to the p53 protein level (20–24).
Our study detected changes in apoptosis-related indices including
p53, and we believe that these cells underwent a different cell
fate which was associated with p53.
The detection of ideal predictive tumor biomarkers
is a complicated process, and currently the best choice for the
identification of such biomarkers appears to be a compromise
between the results obtained from high-throughput technologies and
hypothesis-driven analyses (37,38).
We conducted this explorative study to identify the role of SGK1
dynamics in NSCLC cells, and we successfully demonstrated a great
regulating possibility of the apoptosis of tumor cells.
Current research is focused on the immunotherapy of
cancer, and IFN-γ has become a focus of study in research of the
immune therapy of tumors (39).
IFN-γ functions via its potent antitumor activity through cell
growth inhibition, induction of cell apoptosis, killing of tumor
cells through activation of the immune system and enhancing
antitumor immunity of the microenvironment (40). Researchers at Johns Hopkins Kimmel
Cancer Center found that SGK1-knockout melanoma mice produced a
significant increase in interferon IFN-γ expression, than mice with
SGK1 normal expression, and at the same time the enhance in the
immune response resulted in half the chance of lung cancer than the
normal group (41). IL-7 treatment
exerted an anticancer function accompanying the increased level of
IFN-γ (42), and an intriguing
interaction between IFN-γ/IFN-γR and IL-7/IL-7R pathways
effectively reversed immune suppression and eliminated tumors
(43). Lu et al stated that
IL-9 promoted Tc9 cell migration into the tumor site to exert vital
function (44) and neutralization
of IL-9 in mice promoted tumor growth (45). Consequently, we realized that the
SGK1 enzyme is a key for the regulation of the immune response
in vivo and it is possible to apply SGK1 competitive
inhibitor GSK650394 in animal experiments, which may be able to
partially mimic the effect of the absence of the enzyme SGK1. This
may induce the increased expression of immune-related indicators to
enhance the cellular immune response and promote tumor cell
apoptosis.
Acknowledgements
We thank the staff of the Clinical Biobank of The
Affiliated Hospital of Nantong University. This work was supported
by grants from the National Natural Science Foundation of China
(81503143), Chinese Postdoctoral Science Foundation (no.
2015M581846); Six Talent Peaks Project in Jiangsu Province
(2015-WSN-062); Science and Technology Plan of Nantong
(MS22015113).
References
|
1
|
Hamra GB, Laden F, Cohen AJ,
Raaschou-Nielsen O, Brauer M and Loomis D: Lung cancer and exposure
to nitrogen dioxide and traffic: A systematic review and
meta-analysis. Environ Health Perspect. 123:1107–1112. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neal JW and Wakelee HA: Elusive target of
angiogenesis in small-cell lung cancer. J Clin Oncol. 35:1269–1271.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shu W, Yang H, Zhang L, Lu MM and Morrisey
EE: Characterization of a new subfamily of winged-helix/forkhead
(Fox) genes that are expressed in the lung and act as
transcriptional repressors. J Biol Chem. 276:27488–27497. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu H, Wang B, Borde M, Nardone J, Maika S,
Allred L, Tucker PW and Rao A: Foxp1 is an essential
transcriptional regulator of B cell development. Nat Immunol.
7:819–826. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rauhala HE, Porkka KP, Tolonen TT,
Martikainen PM, Tammela TL and Visakorpi T: Dual-specificity
phosphatase 1 and serum/glucocorticoid-regulated kinase are
downregulated in prostate cancer. Int J Cancer. 117:738–745. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chu S, Rushdi S, Zumpe ET, Mamers P, Healy
DL, Jobling T, Burger HG and Fuller PJ: FSH-regulated gene
expression profiles in ovarian tumours and normal ovaries. Mol Hum
Reprod. 8:426–433. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nasir O, Wang K, Föller M, Gu S, Bhandaru
M, Ackermann TF, Boini KM, Mack A, Klingel K, Amato R, et al:
Relative resistance of SGK1 knockout mice against chemical
carcinogenesis. IUBMB Life. 61:768–776. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ronchi CL, Sbiera S, Leich E, Tissier F,
Steinhauer S, Deutschbein T, Fassnacht M and Allolio B: Low SGK1
expression in human adrenocortical tumors is associated with
ACTH-independent glucocorticoid secretion and poor prognosis. J
Clin Endocrinol Metab. 97:E2251–E2260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiaobo Y, Qiang L, Xiong Q, Zheng R,
Jianhua Z, Zhifeng L, Yijiang S and Zheng J: Serum and
glucocorticoid kinase 1 promoted the growth and migration of
non-small cell lung cancer cells. Gene. 576:339–346. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abbruzzese C, Mattarocci S, Pizzuti L,
Mileo AM, Visca P, Antoniani B, Alessandrini G, Facciolo F, Amato
R, D'Antona L, et al: Determination of SGK1 mRNA in non-small cell
lung cancer samples underlines high expression in squamous cell
carcinomas. J Exp Clin Cancer Res. 31:42012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun JY, Li C, Shen ZX, Zhang WC, Ai TJ, Du
LJ, Zhang YY, Yao GF, Liu Y, Sun S, et al: Mineralocorticoid
receptor deficiency in macrophages inhibits neointimal hyperplasia
and suppresses macrophage inflammation through SGK1-AP1/NF-κB
pathways. Arterioscler Thromb Vasc Biol. 36:874–885. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mansley MK, Watt GB, Francis SL, Walker
DJ, Land SC, Bailey MA and Wilson SM: Dexamethasone and insulin
activate serum and glucocorticoid-inducible kinase 1 (SGK1) via
different molecular mechanisms in cortical collecting duct cells.
Physiol Rep. 4:42016. View Article : Google Scholar
|
|
13
|
Arteaga MF and Canessa CM: Functional
specificity of Sgk1 and Akt1 on ENaC activity. Am J Physiol Renal
Physiol. 289:F90–F96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu T, Zhang W and Wang DX: Insulin
up-regulates epithelial sodium channel in LPS-induced acute lung
injury model in rats by SGK1 activation. Injury. 43:1277–1283.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lang F, Böhmer C, Palmada M, Seebohm G,
Strutz-Seebohm N and Vallon V: (Patho)physiological significance of
the serum- and glucocorticoid-inducible kinase isoforms. Physiol
Rev. 86:1151–1178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lang F, Görlach A and Vallon V: Targeting
SGK1 in diabetes. Expert Opin Ther Targets. 13:1303–1311. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simon P, Schneck M, Hochstetter T,
Koutsouki E, Mittelbronn M, Merseburger A, Weigert C, Niess A and
Lang F: Differential regulation of serum- and
glucocorticoid-inducible kinase 1 (SGK1) splice variants based on
alternative initiation of transcription. Cell Physiol Biochem.
20:715–728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bai JA, Xu GF, Yan LJ, Zeng WW, Ji QQ, Wu
JD and Tang QY: SGK1 inhibits cellular apoptosis and promotes
proliferation via the MEK/ERK/p53 pathway in colitis. World J
Gastroenterol. 21:6180–6193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Purvis JE and Lahav G: Encoding and
decoding cellular information through signaling dynamics. Cell.
152:945–956. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Purvis JE, Karhohs KW, Mock C, Batchelor
E, Loewer A and Lahav G: p53 dynamics control cell fate. Science.
336:1440–1444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brunet A, Park J, Tran H, Hu LS, Hemmings
BA and Greenberg ME: Protein kinase SGK mediates survival signals
by phosphorylating the forkhead transcription factor FKHRL1
(FOXO3a). Mol Cell Biol. 21:952–965. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
You H, Jang Y, You-Ten AI, Okada H, Liepa
J, Wakeham A, Zaugg K and Mak TW: p53-dependent inhibition of
FKHRL1 in response to DNA damage through protein kinase SGK1. Proc
Natl Acad Sci USA. 101:pp. 14057–14062. 2004; View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng Z, Liu L, Zhang C, Zheng T, Wang J,
Lin M, Zhao Y, Wang X, Levine AJ and Hu W: Chronic restraint stress
attenuates p53 function and promotes tumorigenesis. Proc Natl Acad
Sci USA. 109:pp. 7013–7018. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi G, Wang Q, Zhou X, Li J, Liu H, Gu J,
Wang H, Wu Y, Ding L, Ni S, et al: Response of human non-small-cell
lung cancer cells to the influence of Wogonin with SGK1 dynamics.
Acta Biochim Biophys Sin (Shanghai). 49:302–310. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sherk AB, Frigo DE, Schnackenberg CG, Bray
JD, Laping NJ, Trizna W, Hammond M, Patterson JR, Thompson SK,
Kazmin D, et al: Development of a small-molecule serum- and
glucocorticoid-regulated kinase-1 antagonist and its evaluation as
a prostate cancer therapeutic. Cancer Res. 68:7475–7483. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sung J, Bochicchio GV, Joshi M, Bochicchio
K, Costas A, Tracy K and Scalea TM: Admission serum albumin is
predicitve of outcome in critically ill trauma patients. Am Surg.
70:1099–1102. 2004.PubMed/NCBI
|
|
27
|
Webster MK, Goya L, Ge Y, Maiyar AC and
Firestone GL: Characterization of sgk, a novel member of the
serine/threonine protein kinase gene family which is
transcriptionally induced by glucocorticoids and serum. Mol Cell
Biol. 13:2031–2040. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lang F and Görlach A: Heterocyclic
indazole derivatives as SGK1 inhibitors, WO2008138448. Expert Opin
Ther Pat. 20:129–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakashima RA, Paggi MG and Pedersen PL:
Contributions of glycolysis and oxidative phosphorylation to
adenosine 5′-triphosphate production in AS-30D hepatoma cells.
Cancer Res. 44:5702–5706. 1984.PubMed/NCBI
|
|
30
|
Wallace DC: Mitochondria and cancer:
Warburg addressed. Cold Spring Harb Symp Quant Biol. 70:363–374.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pedersen PL: Warburg, me and Hexokinase 2:
Multiple discoveries of key molecular events underlying one of
cancers' most common phenotypes, the Warburg Effect, i.e., elevated
glycolysis in the presence of oxygen. J Bioenerg Biomembr.
39:211–222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lahav G, Rosenfeld N, Sigal A,
Geva-Zatorsky N, Levine AJ, Elowitz MB and Alon U: Dynamics of the
p53-Mdm2 feedback loop in individual cells. Nat Genet. 36:147–150.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hamstra DA, Bhojani MS, Griffin LB, Laxman
B, Ross BD and Rehemtulla A: Real-time evaluation of p53
oscillatory behavior in vivo using bioluminescent imaging. Cancer
Res. 66:7482–7489. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Francisco DC, Peddi P, Hair JM, Flood BA,
Cecil AM, Kalogerinis PT, Sigounas G and Georgakilas AG: Induction
and processing of complex DNA damage in human breast cancer cells
MCF-7 and nonmalignant MCF-10A cells. Free Radic Biol Med.
44:558–569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Amato R, D'Antona L, Porciatti G, Agosti
V, Menniti M, Rinaldo C, Costa N, Bellacchio E, Mattarocci S,
Fuiano G, et al: Sgk1 activates MDM2-dependent p53 degradation and
affects cell proliferation, survival, and differentiation. J Mol
Med (Berl). 87:1221–1239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bianchi F, Nicassio F and Di Fiore PP:
Unbiased vs. biased approaches to the identification of cancer
signatures: The case of lung cancer. Cell Cycle. 7:729–734. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guan P, Huang D, He M and Zhou B: Lung
cancer gene expression database analysis incorporating prior
knowledge with support vector machine-based classification method.
J Exp Clin Cancer Res. 28:1032009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heikamp EB, Patel CH, Collins S, Waickman
A, Oh MH, Sun IH, Illei P, Sharma A, Naray-Fejes-Toth A, Fejes-Toth
G, et al: The AGC kinase SGK1 regulates TH1 and TH2 differentiation
downstream of the mTORC2 complex. Nat Immunol. 15:457–464. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rearden R, Sah A, Doff B, Kobayashi T,
McKee SJ, Leggatt GR and Mattarollo SR: Control of B-cell lymphoma
by therapeutic vaccination and acquisition of immune resistance is
independent of direct tumour IFN-gamma signalling. Immunol Cell
Biol. 94:554–562. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lugade AA, Sorensen EW, Gerber SA, Moran
JP, Frelinger JG and Lord EM: Radiation-induced IFN-gamma
production within the tumor microenvironment influences antitumor
immunity. J Immunol. 180:3132–3139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Goldstraw P: The 7th edition of TNM in
lung cancer: What now? J Thorac Oncol. 4:671–673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yuan CH, Yang XQ, Zhu CL, Liu SP, Wang BC
and Wang FB: Interleukin-7 enhances the in vivo anti-tumor activity
of tumor-reactive CD8+ T cells with induction of
IFN-gamma in a murine breast cancer model. Asian Pac J Cancer Prev.
15:265–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shi LZ, Fu T, Guan B, Chen J, Blando JM,
Allison JP, Xiong L, Subudhi SK, Gao J and Sharma P: Interdependent
IL-7 and IFN-γ signalling in T-cell controls tumour eradication by
combined α-CTLA-4+α-PD-1 therapy. Nat Commun. 7:123352016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lu Y, Hong B, Li H, Zheng Y, Zhang M, Wang
S, Qian J and Yi Q: Tumor-specific IL-9-producing CD8+
Tc9 cells are superior effector than type-I cytotoxic Tc1 cells for
adoptive immunotherapy of cancers. Proc Natl Acad Sci USA. 111:pp.
2265–2270. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lu Y, Hong S, Li H, Park J, Hong B, Wang
L, Zheng Y, Liu Z, Xu J, He J, et al: Th9 cells promote antitumor
immune responses in vivo. J Clin Invest. 122:4160–4171. 2012.
View Article : Google Scholar : PubMed/NCBI
|