Introduction
Breast cancer is the leading cause of death in women
in both developed and developing countries, with an estimated 1.7
million cases and 521,900 deaths in 2012 (1). Although advanced treatments such as
surgery, radiotherapy and chemotherapy have been developed, breast
cancer incidence and mortality rates continue to increase due to
the low efficacy, severe side effects, and lack of access to
treatment (2,3). Alternative strategies with fewer
adverse effects are therefore necessary to increase current
therapies for breast cancer treatment.
For several centuries, natural products have been
used to treat diseases in humans. Bacteria, plants and fungi are
the major sources of natural products being investigated for their
potential as anticancer drugs. An example of a natural product with
noteworthy biological activities is the fungal metabolite, terrein,
isolated from Aspergillus terreus Thom. Terrein was first
described in 1935 and was subsequently isolated from other strains
of Aspergillus as well as Emericella and
Penicilium (4–6). Terrein exhibits several biological
activities including anti-inflammation, anti-melanogenesis, and
anticancer properties. Treatment of keratinocytes, hepatoma and
lung cell lines with terrein does not stimulate apoptosis (7,8),
instead it appears to limit proliferation by promoting cell cycle
arrest. Similarly, terrein also inhibited growth and induced cell
cycle arrest in a human ovarian cancer cell line (9) and hepatoma Bel-7402 cells (8). However, the activity of terrein is
cell type-dependent and this compound not only inhibits cell
proliferation but also induces apoptosis. Activation of the
caspase-7 pathway and suppression of Akt signaling was observed
following exposure of ABCG-2-expressing MCF-7 breast cancer cells
to terrein (10). HeLa cervical
cancer cells were also sensitive to the anti-proliferation activity
of terrein which was mediated through induction of apoptosis
(11). In addition, terrein
attenuated angiogenin production in head and neck cancer cells
(12), which may be another
underlying mechanism in its anticancer effects. Although terrein
has been known for more than 80 years, its biological activities
are still poorly understood. Further investigation into its
anticancer mechanism, especially on cell migration and invasion
will be beneficial for the development of alternative therapeutic
measures for breast cancer treatment.
Cell migration and invasion are typically initiated
by changes of the extracellular matrix leading to alterations in
cell adhesion, the cytoskeleton as well as stimulation of
intracellular signaling pathways (13). Upon stimulation by a change in a
chemokine gradient, cell migration begins by altering the dynamics
of actin polymerization which is highly coordinated with cell
movement through the extracellular matrix. The matrix
metalloproteinases (MMPs) are crucial for degradaing the
extracellular matrix facilitating migration. In addition, the Rho
family of small GTPases play key roles in regulating the cell
migration process (14). Among the
22 Rho family proteins so far identified, RhoA/B/C, Rac1/2/3 and
Cdc42 are the best characterized and all have been implicated in
the regulation of cell migration (15–17).
In untransformed cells, Rho GTPases are involved in the control of
cell morphology and motility, while Cdc42 regulates the extension
of filopodia and lamellipodia formation at the
leading edge of the cell (18).
RhoA is involved in the generation of the contractile force as well
as moving the body and tail of the cell (18). RhoB appears to regulate membrane
trafficking, cell proliferation, DNA-repair, and apoptosis
(19). In addition, RhoC plays a
critical role in the dynamic of actin polymerization at the leading
edge of the cell, promoting cell motility (20). Rac1 which regulates the assembly of
actin filament networks has also been demonstrated to enhance cell
invasiveness upon activation (21).
In the present study, to better understand the
anticancer properties of terrein the contribution of Rho GTPases
and MMPs on cell migration and invasion in breast cancer cells were
examined.
Material and methods
Materials and chemicals
DMEM culture medium was purchased from Gibco/Thermo
Fisher Scientific, Inc. (Rockville, MD, USA). Fetal bovine serum
(FBS), penicillin-streptomycin cocktail, 0.25% (w/v) trypsin/1 mM
EDTA and SuperScript® III RNase H-RT kit were purchased
from Invitrogen/Thermo Fisher Scientific, Inc. (Carlsbad, CA, USA).
Fibronectin from human plasma, type IV collagen from human
placenta, 3-(4,5-dimethyl-2-thaizol)-2,5-diphenyltetrazolium
bromide (MTT) were supplied by Sigma-Aldrich (St. Louis, MO, USA).
Matrigel was obtained from BD Biosciences (Bedford, MA, USA). The
Annexin V/7AAD apoptosis detection kit was supplied by BioLegend,
Inc. (San Diego, CA, USA).
Cell culture
Human breast cancer cell lines, MCF-7 (cat no.
HTB-22) and MDA-MB-231 (cat. no. HT-B26; both from ATCC, Manassas,
VA, USA) were grown in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml
penicillin and 100 µg/ml streptomycin (complete DMEM) in a
humidified atmosphere containing 5% CO2 at 37°C. Cells
were sub-cultured two or three times a week to maintain their
viability.
Preparation of terrein
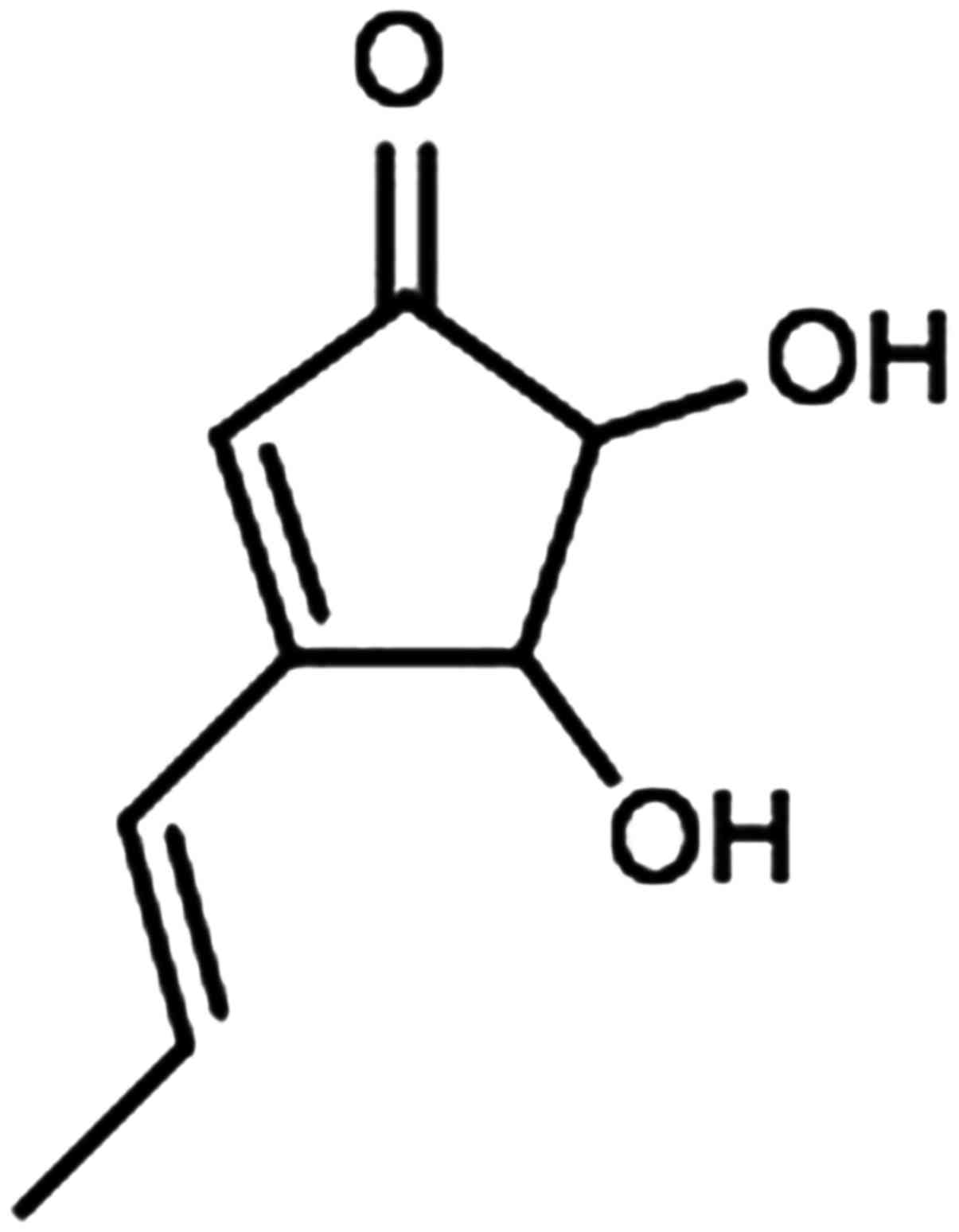
Isolation of terrein (Fig. 1) was performed as previously
described (11). Briefly, an ethyl
acetate (EtOAc) extract from the culture broth of Aspergillus
terreus CRI301 was concentrated in vacuo, fractioned and
purified by Sephadex™ LH-20 using MeOH as an eluent. The compound
was then characterized by spectroscopic analysis. The purity of
terrein was ~80% as determined by LCMS.
Cell viability assay
The effect of terrein on breast cancer cell
viability was determined using the MTT assay. MCF-7 and MDA-MB-231
cells were seeded at the density of 1×105 cells/well on
96-well plates. The following day, the cells were treated with
various concentrations of terrein and cultured for an additional 24
or 48 h. After the incubation period, DMEM was removed and MTT
solution (0.5 mg/ml) was added to each well and incubation followed
for 4 h at 37°C. The formazan product was solubilized by the
addition of 100 µl of DMSO and the absorbance (OD) at 540 nm was
assessed using a microplate reader. The ratio of the OD of treated
cells relative to those of the control cells was calculated and
expressed as the percentage of cell viability.
Cell adhesion assay
The 96-well plates were coated with either 10 µg/ml
of fibronectin or type IV collagen overnight at 4°C. Nonspecific
binding was blocked with 1% bovine serum albumin (BSA) for 1 h at
37°C, followed by washing three times with phosphate-buffered
saline (PBS). MCF-7 and MDA-MB-231 cells at 80% confluence were
treated with vehicle or terrein (25 and 75 µM) for 24 h. The cells
were then trypsinized, resuspended in medium without serum and
plated at the density of 1×104 cells into each well. The
cells were allowed to adhere at 37°C, in a 5% CO2
atmosphere for 30 and 60 min. Non-adherent cells were removed by
washing three times with 100 µl PBS. Adherent cells were fixed with
4% paraformaldehyde and stained with 2.5% crystal violet in
methanol. Cells were dissolved with 25% acetic acid and the
absorbance was assessed at 540 nm using VersaMax ELISA Microplate
Reader (Molecular Devices, Sunnyvale, CA, USA).
Wound healing assay
MCF-7 and MDA-MB-231 cells were seeded onto
60-mm2 dishes at a density of 5×105
cells/dish in DMEM containing 10% FBS. Cells at near confluence
were then starved with serum-free DMEM for 24 h. Wounding was
simulated by drawing a scratch on the cell monolayer with a 20 µl
pipette tip, and debris was removed by washing with serum-free
DMEM. Cells were then incubated with or without terrein (25 and 75
µM) for 24 h in complete DMEM. Images of cells migrating into the
wound area were captured from different fields at 0 and 24 h with a
light microscope at a magnification of ×400. The open wound areas
were assessed using TScratch software (22) and the results are presented as the
percentage of closed wound areas.
Cell migration assay
In vitro migration assays were performed
using a 24-well Transwell chamber with a polycarbonate membrane
containing 8.0-µm pores (Corning Costar, Cambridge, MA, USA). MCF-7
and MDA-MB-231 cells were grown to 80% confluence and serum-starved
overnight prior to migration experiments. Cells (2×105)
in DMEM serum-free media were plated into the upper chamber with or
without terrein (25 and 75 µM). The lower chamber contained DMEM
with 5% FBS which acted as a chemoattractant. After 24 h at 37°C in
5% CO2, the experiment was stopped by wiping the
non-migrated cells from the top of the membrane with a cotton swab.
Migrated cells on the bottom of the membrane were fixed with 4%
paraformaldehyde for 5 min, stained with 2.5% crystal violet in
methanol for 10 min and washed with water to remove the excess dye.
The migrated cells on the membrane were dissolved with 25% acetic
acid and quantitated by assessing the absorbance at 540 nm using
VersaMax ELISA Microplate Reader (Molecular Devices).
Cell invasion assay
In vitro invasion assay was also performed
using a 24-well Transwell chamber with a polycarbonate membrane
containing 8.0-µm pores. The method was identical to the migration
assay previously described with the exception that the inserts were
coated with 100 µl Matrigel® (BD Biosciences) diluted to
1 mg/ml.
Reverse transcription (RT) and
real-time PCR
Cells were lysed and total RNA was extracted with
the Illustra™ RNAspin mini kit (GE Healthcare, Little Chalfont, UK)
according to the manufacturer's instructions. Isolated RNA was used
as a template for cDNA synthesis employing the
SuperScript® III RNase H-RT kit (Invitrogen/Thermo
Fisher Scientific, Inc.). Real-time PCR was performed with a
Stratagene Mx3000P qPCR system (Agilent Technologies, Santa Clara,
CA, USA) utilizing RBC ThermOne® Real-Time Premix
(SYBR-Green) (RBC Bioscience Corp., New Taipei City, Taiwan). All
reactions were carried out using the following conditions: 10 min
at 95°C; 30 sec at 95°C, 30 sec at 60°C and 30 sec of 72°C (40
cycles). Determination of the expression of hypoxanthine-guanine
phosphoribosyltransferase (HPRT), a housekeeping gene, was included
in each experiment to correct for variations in template
concentrations. The amounts of PCR amplicons were analyzed using
Gel Doc 2000 and Quantity One software package (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Primers used for the
amplification of MMP-2, MMP-9 and the internal control HPRT have
been previously described (23,24)
with the following sense and antisense primer sets: MMP-2 forward,
5′-GTTCATTTGGCGGACTGTGACG-3′ and reverse,
5′-ATTCATTCCCTGCAAAGAACACAGC-3′; MMP-9 forward,
5′-CACGACGTCTTCCAGTACCGAGA-3′ and reverse,
5′-CATAGGTCACGTAGCCCACTTGGT-3′; and HPRT forward,
5′-TGTGATGAAGGAGATGGGAGG-3′ and reverse,
5′-AAGCTTGCGACCTTGACCATCT-3′. The method was used for determination
of relative fold of gene expression (25).
Apoptosis assay using flow
cytometer
MCF-7 and MDA-MB-231 cells (5×105) cells
were seeded onto 60-mm2 plates in DMEM containing 10%
FBS for 24 h prior to the apoptosis assay. Cells were then cultured
in the presence of DMSO or increasing concentrations of terrein for
24 h. For each condition, adherent cells were detached using
Trypsin-EDTA and pooled with the cells collected in the
corresponding culture supernatant. Cells were washed twice with
ice-cold PBS and subsequently stained with Annexin V-7AAD
(PerCP/Cy5.5) (BioLegend) in Annexin V binding buffer for 15 min.
Samples were analyzed using a Beckman-Coulter CytoFLEX
(Beckman-Coulter, Brea, CA, USA). Analysis was performed using
CytExpert software (Beckman-Coulter).
Western blot analysis
Small GTPases were analyzed by western blotting
using Rho-GTPase Antibody Sampler kit (cat no. 9968; Cell Signaling
Technology, Inc., Danvers, MA, USA). Equal amounts of total protein
were separated by SDS-PAGE and then transferred onto a PDVF
membrane. The membrane was incubated with the following primary
antibodies: Cdc42 (1:1,000; rabbit mAb; cat. no. 11A11),
phospho-Rac1/cdc42 (Ser71) (1:1,000; rabbit pAb), RhoA (1:1,000;
rabbit mAb; cat. no. 67B9), RhoB (1:1,000; rabbit pAb), RhoC
(1:1,000; rabbit mAb; cat. no. D40E4), or Rac1/2/3 (1:1,000; rabbit
pAb) for 1 h at room temperature, and the secondary antibody
(1:1,000; anti-rabbit IgG, HRP-linked) for 1 h at room temperature,
respectively. All lanes presented in one western blot strip were
derived and adjusted equally from the same corresponding blot using
β-actin (1:1,000; rabbit mAb; cat no. 4970; Cell Signaling
Technology, Inc.) as a normalizing control.
Statistical analysis
All assays were performed in triplicate. Statistical
analyses were performed using GraphPad Prism 5 software (GraphPad
Software, Inc., La Jolla, CA, USA). All data are presented as the
mean ± standard deviation (SD). Statistical differences were
determined by unpaired Student's t-test with Welch's correction.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of terrein on the viability of
breast cancer cells
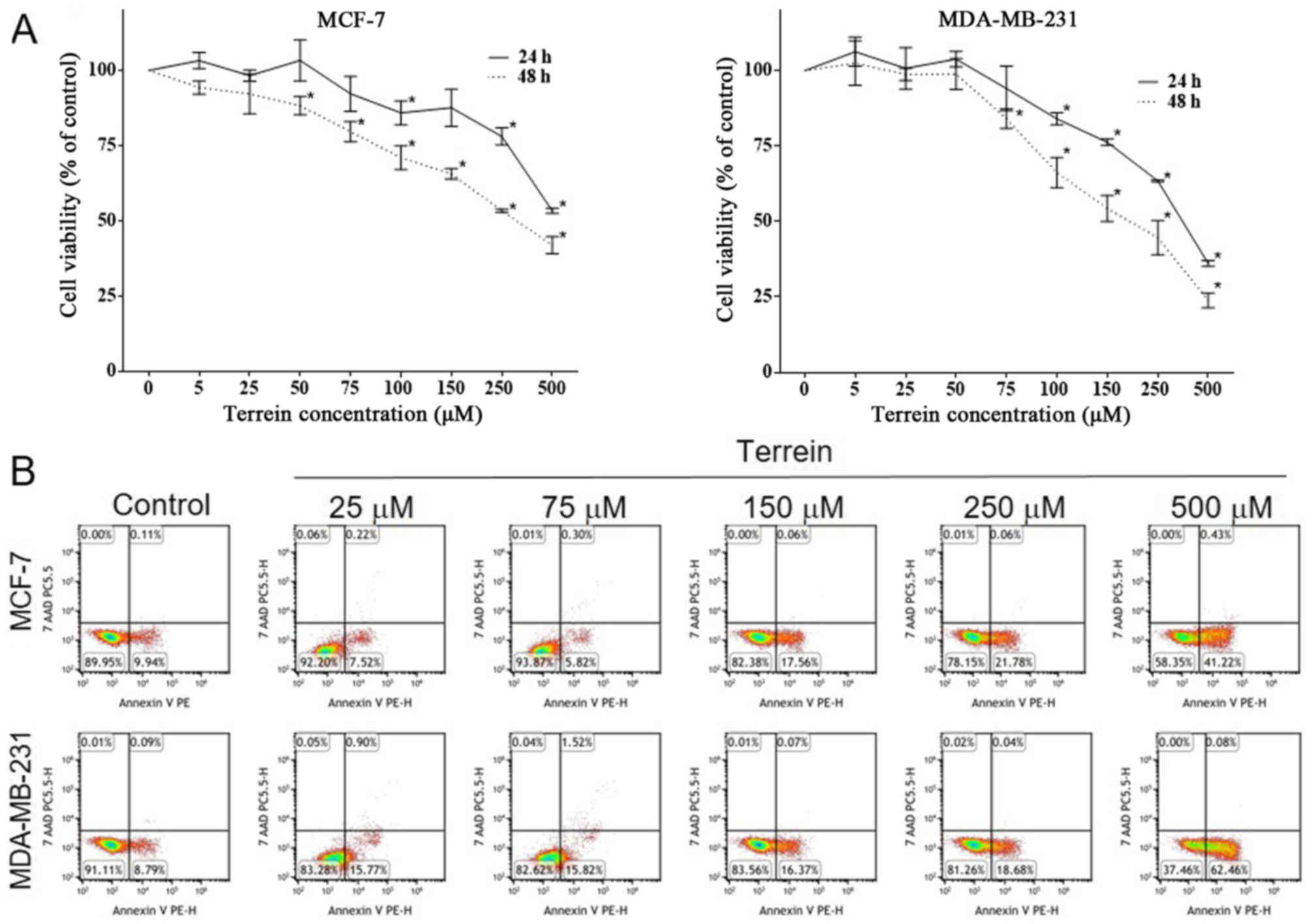
MCF-7 and MDA-MB-231 cells were treated with
increasing concentrations of terrein from 0 to 500 µM for 24 and 48
h. The cytotoxicity of terrein was evaluated using MTT assays. The
results demonstrated that terrein reduced the viability of these
cells in concentration- and time-dependent manners (Fig. 2A and Table I). Terrein exhibited similar effects
on both cell lines revealing a significant reduction in cell
viability following exposure to 100–500 and 50–500 µM at 24 and 48
h, respectively (Table I). The
IC50 values of terrein for MCF-7 and MDA-MB-231 cells
after 24 h of incubation were 2.34 mM and 700 µM, respectively
(data not shown). After 48 h of incubation, the IC50
value of terrein was 244.3 µM for MCF-7 and 244.5 µM for MDA-MB-231
cells (data not shown). Moreover, the effect of terrein on the cell
viability of Vero cells, (an African green monkey kidney cell
line), which were used as a normal control, was determined. At both
time-points, a significant decrease in cell viability of Vero cells
was observed with an estimated IC50 of 646 and 690.8 µM
at 24 and 48 h, respectively (data not shown).
 | Table I.Effects of terrein on viability of
breast cancer cells. |
Table I.
Effects of terrein on viability of
breast cancer cells.
|
| Cell viability (%
of control) |
|---|
|
|
|
|---|
|
| MCF-7 | MDA-MB-231 |
|---|
|
|
|
|
|---|
| Terrein (µM) | 24 h | 48 h | 24 h | 48 h |
|---|
|
5 |
103.274±2.621 |
94.342±2.244 |
106.246±4.820 |
102.495±7.324 |
| 25 |
98.305±1.848 |
92.211±6.667 |
100.780±6.892 |
98.737±2.025 |
| 50 |
103.342±6.775 |
88.261±2.985a |
103.812±2.611 |
98.810±5.138 |
| 75 |
92.263±5.822 |
79.650±3.324b |
94.050±7.422 |
84.064±3.242a |
| 100 |
85.894±3.954a |
70.998±3.972b |
84.042±2.005b |
66.231±5.003b |
| 150 |
87.593±6.205 |
65.649±1.752c |
76.294±1.027c |
54.368±4.322b |
| 250 |
78.099±2.840b |
53.384±0.568d |
63.480±0.328d |
44.623±5.688b |
| 500 |
53.398±0.856c |
41.970±2.830c |
36.144±0.951d |
23.863±2.463c |
To determine whether the reduction of cell viability
detected using MTT assays was due to cell death or the inhibition
of cell cycle progression, we performed flow cytometry and Annexin
V-7AAD staining. As shown in Fig.
2B, the percentage of cells undergoing apoptosis (Annexin
V-positive) increased in a concentration-dependent fashion for both
MCF-7 and MDA-MB-231 cells. Our results revealed that a high
concentration of terrein induced programmed cell death in both
invasive and non-invasive breast cancer cell lines. Thus, the
optimal non-toxic concentrations of terrein (25 and 75 µM) were
used for subsequent experiments.
Effect of terrein on the adhesion of
breast cancer cells
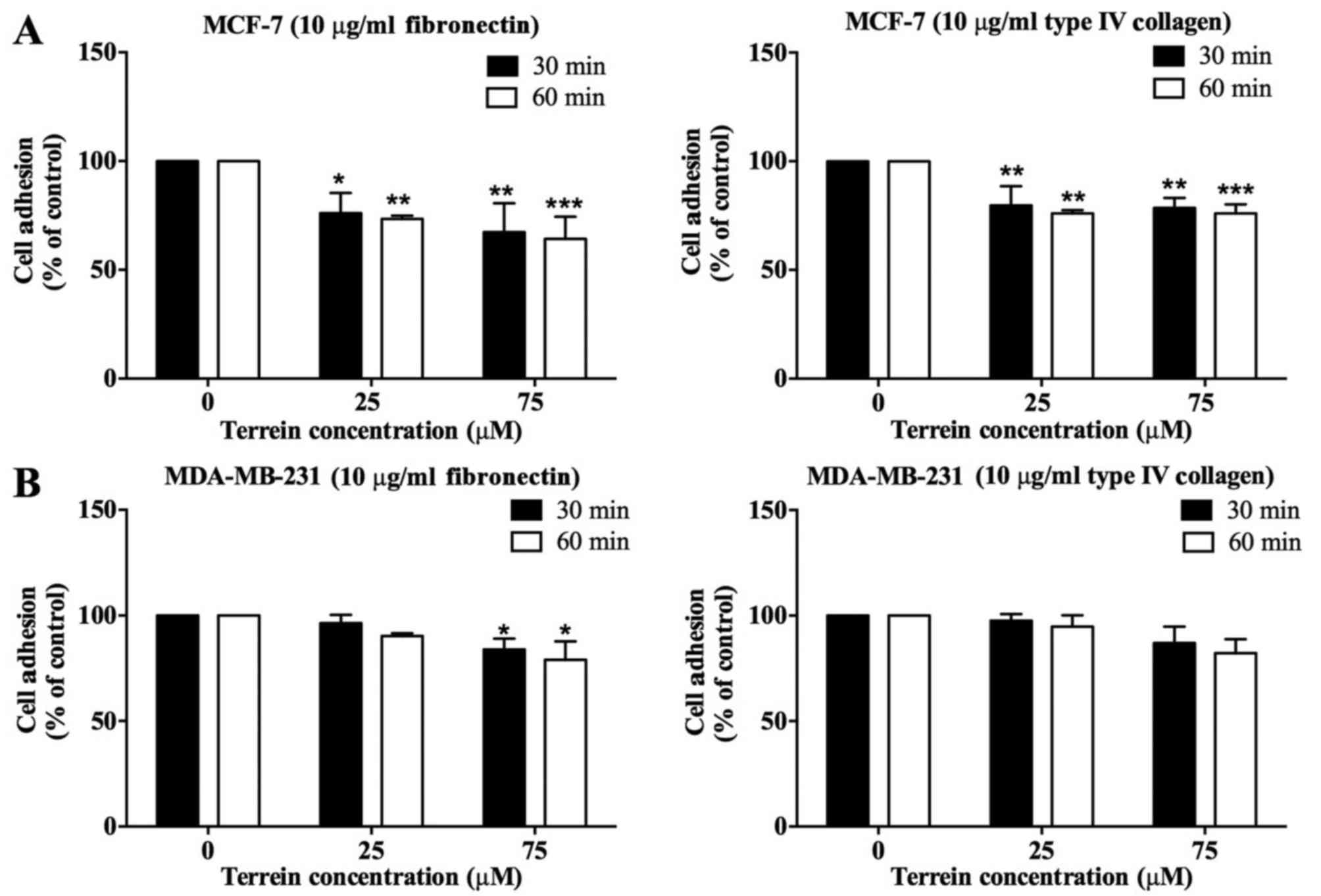
Adhesion is one of the essential steps in cancer
cell metastasis. We performed cell adhesion assays in order to
determine the effect of terrein on breast cancer cell adhesion.
MCF-7 and MDA-MB-231 cells were allowed to adhere on either a
fibronectin- or type IV collagen-coated surface for 30 and 60 min.
As shown in Fig. 3A, terrein
significantly decreased MCF-7 cell adhesion on fibronectin and type
IV collagen revealing ~30% inhibition. Although adhesion of
terrein-treated MDA-MB-231 cells on type IV collagen was not
impaired, cell adhesion on fibronectin was reduced by 20% for
MDA-MB-231 cells exposed to 75 µM of terrein (Fig. 3B). These results demonstrated the
selective inhibitory effect of terrein on the adhesion of breast
cancer cells.
Effect of terrein on the migration of
breast cancer cells
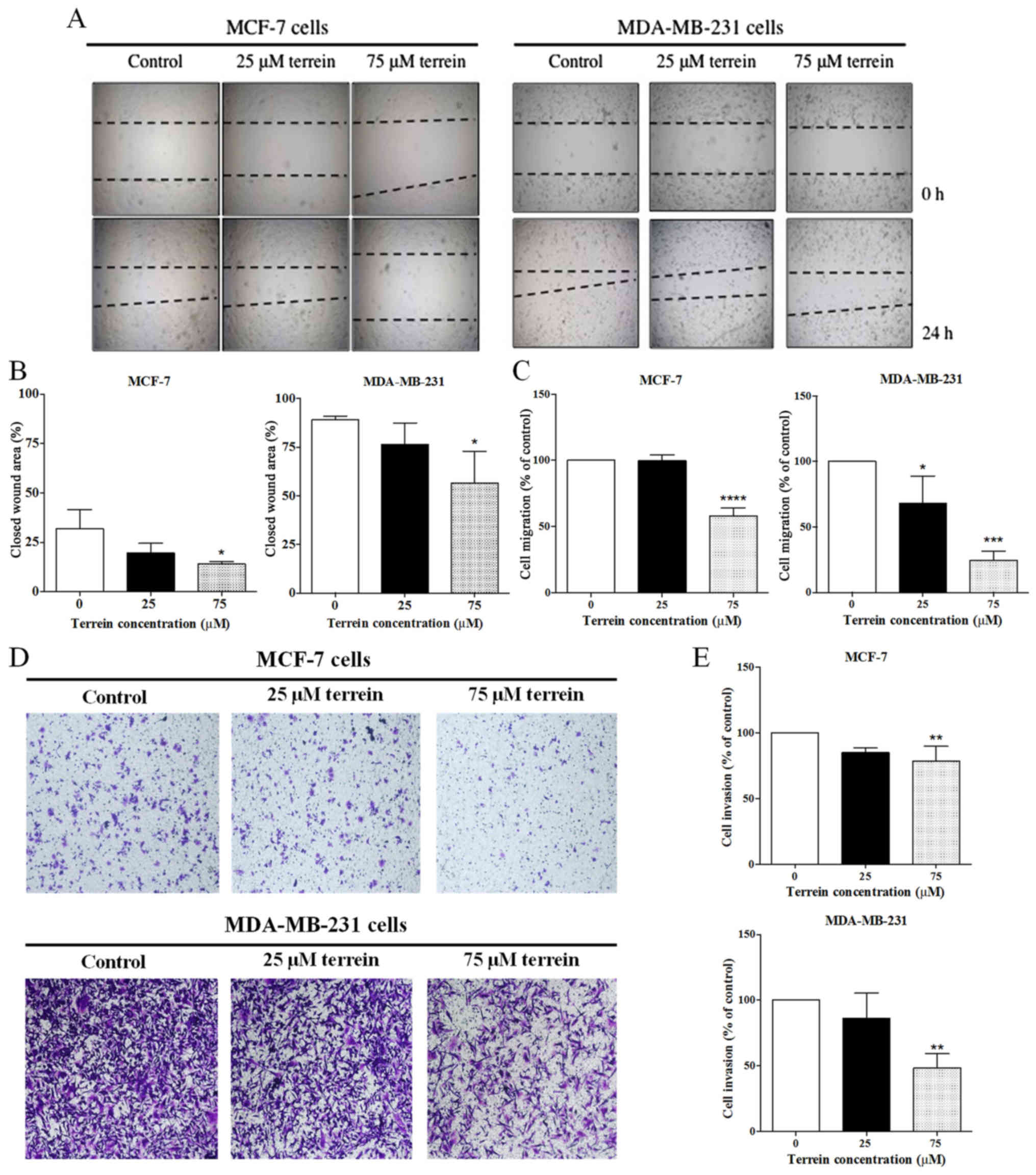
To evaluate the effect of terrein on breast cancer
cell migration, a wound-healing assay was performed. Scrape wounds
were created on slides containing confluent breast cancer cell
lines. After treatment with terrein, cancer cells were allowed to
migrate to the wound area for 24 h. As shown in Fig. 4A, untreated MDA-MB-231 cells almost
closed the wound area after 24 h of incubation (~85%), whereas
MCF-7 cells closed only ~25% of the wound area. As indicated by the
densitometric analyses (Fig. 4B),
terrein at 75 µM significantly decreased MCF-7 and MDA-MB-231 cell
migration after incubation for 24 h by ~10 and 40%,
respectively.
The ability of terrein to impair breast cancer cell
migration was further confirmed using Transwell assays with 5% FBS
as a chemoattractant. Migration of MCF-7 cells treated with 75 µM
terrein was significantly reduced after 24 h of incubation
(Fig. 4C). Notably, terrein
exhibited even stronger inhibitory effects on MDA-MB-231 cells,
reducing cell migration by 30 and 70% at 25 and 75 µM terrein,
respectively. These results indicated that terrein inhibited the
migration of breast cancer cells.
Effect of terrein on breast cancer
cell invasion
We further examined the effect of terrein on breast
cancer cell invasion using the Transwell assay containing a
Matrigel coating. MCF-7 and MDA-MB-231 cells were allowed to invade
through Matrigel in the presence of terrein for 24 h. The invasive
ability of MCF-7 cells exposed to terrein was significantly reduced
by ~20% (Fig. 4D and E). Although
at a low concentration (25 µM) terrein only slightly impaired
MDA-MB-231 cell invasion, a marked reduction of 50% was observed in
these cells treated with 75 µM of terrein, a non-toxic dose
(Fig. 4D and F). Therefore, these
results confirmed the inhibitory effects of terrein on breast
cancer cell migration and invasion.
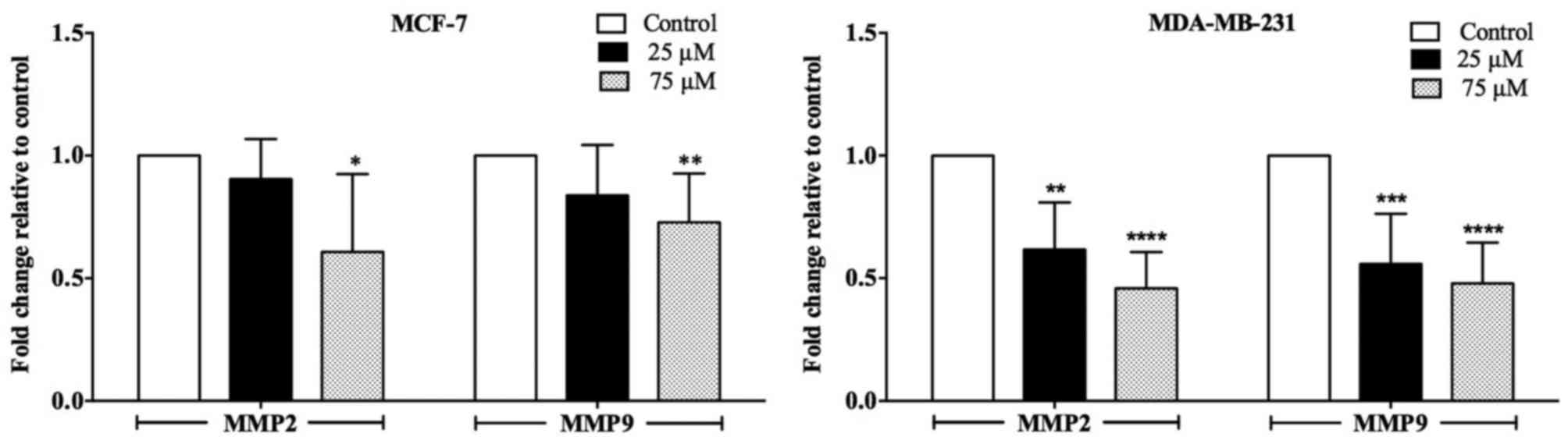
Effect of terrein on the expression of
MMP-2 and MMP-9
MMPs play an important role in cancer cell invasion
and metastasis, and MMP-2 and MMP-9 are known to be essential for
breast cancer progression (26).
Real-time PCR was used to examine whether the anti-invasive
property of terrein was correlated with altered expression levels
of MMP-2 and MMP-9. As revealed in Fig.
5, terrein decreased the mRNA levels of both MMP-2 and MMP-9 in
a concentration-dependent manner. Significant decreases were
observed in MDA-MB-231 cells treated with 25 and 75 µM of terrein,
and in the 75-µM terrein-treated MCF-7 cells.
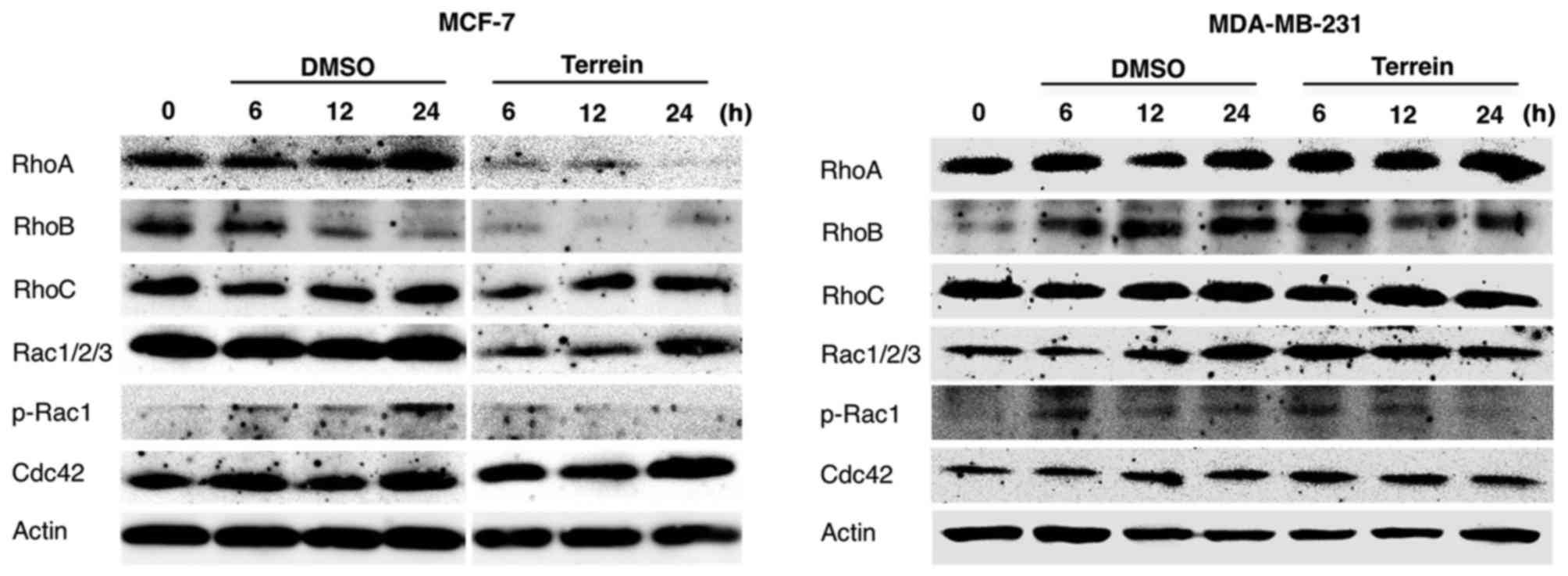
Effect of terrein on the expression of
RhoA, RhoB, RhoC, p-Rac1 and Cdc42
We next investigated the molecular mechanism through
which terrein inhibited breast cancer cell migration and invasion
at non-toxic concentrations. Rho GTPases regulate signaling
pathways that link cell surface receptors to a wide range of
cellular responses, including actin cytoskeleton dynamics (27). Rho regulates the assembly of
contractile, actin-myosin filaments. In contrast, Rac and Cdc42
regulate Arp2/3 complex-mediated actin polymerization controlling
the formation of cell protrusions (lamellipodia and
filopodia) which are involved in cell migration and invasion
(27). As shown in Fig. 6, RhoA and RhoB protein levels were
significantly decreased after 6 h of 75 µM terrein treatment in
MCF-7 cells. Phosphorylated (p)-Rac1 was also slightly decreased at
12 h. However, RhoC and Cdc42 levels remained unchanged. RhoB
protein levels and Rac1 phosphorylation were also decreased in
MDA-MB-231 cells in the presence of terrein, at 12 and 24 h,
respectively. Therefore, inhibition of RhoB and Rac1 by terrein may
disrupt actin cytoskeletal formation and arrangement leading to a
decrease in cell migration. This hypothesis was supported by our
observation that terrein treatment induced a scattered pattern of
migration for both MCF-7 and MDA-MB-231 cells (Fig. 4A). Thus, terrein-induced suppression
of RhoB and Rac1 expression appeared to disturb the overall
migration processes.
Discussion
Breast cancer cells often acquire more aggressive
phenotypes at later stages, with increased cell growth, migration,
and invasion capabilities. Suppression or prevention of these
properties could significantly inhibit cancer progression and
improve disease outcome. Terrein, a compound from Aspergillus
terreus, is a notable candidate for breast cancer treatment
with several noteworthy anticancer properties, and also exhibits
inhibitory activity on breast cancer resistance protein (28). However, its effects on cell
migration and invasion ability have not been examined.
In the present study, biological activities and the
molecular effects of terrein on adhesion, migration and invasion of
MCF-7 and MDA-MB-231 breast cancer cells were investigated in
vitro. Our results revealed that the anticancer activities of
terrein were mediated through several processes. Terrein exhibited
cytotoxic effects by inhibiting proliferation of both MCF-7 and
MDA-MB-231 breast cancer cells at a high concentration (150 µM).
However, using Annexin V staining and flow cytometry, we observed
that MCF-7 and MDA-MB-231 cells underwent apoptosis upon treatment
at a low concentration of terrein (75 µM). Cytotoxicity of this
metabolite has been previously reported in different cell lines.
Terrein induced apoptosis in HeLa cells due to the activation of
p53 and the intrinsic apoptosis pathway (11). Apoptosis observed in
ABCG2-expressing MCF7 cells was a consequence of the activation of
caspase-7 and the inhibition of Akt signaling (10). Terrein was also demonstrated to
induce cell-cycle arrest in ovarian cancer cells (9). Demasi et al revealed that
pro-apoptotic activity of terrein on the human tumoral cell line
NCI-H292 was due to the inhibition of the proteasome (29). Proteasome inhibitiors such as
botezomib, induce apoptosis and cell-cycle arrest, and have been
approved for the treatment of cancers (30). Notably, the inhibition of ERK or AKT
signaling is often observed in terrein-treated cells even in the
absence of apoptosis (7,31,32)
and the inhibition of ERK and Akt phosphorylation has been reported
following proteasome inhibition (33). One hypothesis is that the
differential outcome of terrein treatment on various cell lines may
be due to their susceptibility to inhibition of the proteasome.
At a non-toxic dose (75 µM), terrein could clearly
inhibit wound healing, cell migration and cell invasion of breast
cancer cells, and this was more clearly observed in aggressive
MDA-MB-231 cells. Cell adhesion assays revealed that terrein
significantly reduced adhesion mediated by both type IV collagen
and fibronectin, with a greater effect on MCF-7 cells. These
results indicated that terrein could suppress cancer progression at
several steps.
Rho GTPases have been reported to be essential for
both cancer initiation and progression during tumorigenesis
(34), implicating their potential
for use as a molecular target for cancer treatment. Although
mutations of RHO genes are rare in tumors, dysregulation of their
expression and activity is frequently reported. Overexpression of
RhoA was detected in colon, lung as well as breast cancers
(35). Moreover, depletion of Rac1
was reported to inhibit migration and invasion of carcinoma and
melanoma cells (36,37). The inhibition of Cdc42 abolished a
mesenchymal-amoeboid transition, subsequently impairing melanoma
cell invasion (37). Further
investigation into the RHO family demonstrated that terrein
exhibited a consistent inhibitory effect on the levels of RhoB and
Rac in both breast cancer cell lines. The ability of terrein to
inhibit migration and invasion of MCF-7 and MDA-MB-231 cells
appeared to be related to the reduction of protein levels for the
Rho GTPases. The effects of impaired Rho GTPases on cancer survival
and migration have previously been reported. Rac-specific inhibitor
targeting Rac-GEF suppressed prostate cancer cell proliferation and
invasion (38,39). Similarly, a Cdc42/Rac1 GTPase
inhibitor was revealed to significantly decrease the growth of
prostate cancer and improve survival in vivo (40). The increased scattered pattern of
migration of MCF-7 and MDA-MB-231 cells in the presence of terrein
suggested the possible disruption of cytoskeleton reorganization
and focal adhesions, which are mainly regulated by Rho GTPases.
Despite promising evidence, Rho GTPase inhibitors have not yet been
successfully adopted for clinical use. The effects of terrein
appear to be partly attributable to a decrease in the activity of
Rho and Rac GTPases in these breast cancer cells. This indicates
the potential of terrein as a therapeutic intervention as part of
breast cancer therapy.
In addition to Rho GTPases, matrix
metalloproteinases (MMPs) are also involved in migration, invasion,
proliferation, and apoptosis. Six subclasses of MMPs including
gelatinases, collagenases, stromelysins, matrilysins, membrane-type
MMPs and others are present in mammalian cells (41). Among these two gelatinases, MMP2 and
MMP9, are often upregulated and strongly associated with breast
cancer (42). Reduction of MMP2 and
MMP9 levels was revealed to suppress growth and invasion of MCF-7
breast cancer cells (43–46). We determined that at a non-toxic
dose (75 µM), terrein significantly reduced the number of invading
cells in both cell lines, but to a lesser degree in MCF-7 cells.
Downregulation of MMP-2 and MMP-9 transcription, as determined by
real-time PCR, in both MDA-MB-231 and MCF-7 cells support our
findings that terrein exerted an inhibitory effect on breast cancer
cell invasion. Whether terrein directly contributes to
transcriptional control of these genes is not clear and the precise
mechanism will require further investigation. In vivo
experiments on the activities of terrein would be more informative
in terms of the effects on tumor growth and metastasis under
physiological conditions and, therefore, need to be explored
further. In addition to cell migration and invasion, animal
experiments would also elucidate the effects of terrein on other
important processes, including tumor growth, angiogenesis, and
circulatory cancer cell survival.
Several links have been demonstrated between cell
migration and invasion processes. Matsumoto et al in 2001
used Rho modulators to demonstrate that the Rho pathway was
involved in MMP-2 activation. In addition, the regulation of Rho
levels could modulate both cell motility and matrix degradation in
osteosarcomas (47). Rac1 was also
shown to mediate collagen-induced MMP-2 activation in fibrosarcoma
cells (21). Consistent with these
studies, SDF-1α (or CXCL12), a chemokine known to stimulate cell
adhesion and migration after binding to its G-protein-coupled
receptor CXCR4, plays a key role in melanoma cell invasion
(48,49). The processes of migration and
invasion have been demonstrated to involve stimulation of both Rho
GTPases and MT1-MMP emphasizing their roles in cancer cells.
Therefore, blocking the activity chain of these enzymes has the
potential to inhibit cell invasiveness. The precise target of
terrein was not identified in this study; however, one possibility
is that terrein disrupts migration and invasion by interfering with
chemokine receptor interaction.
In conclusion, this study is the first to
demonstrate that terrein exhibits more than pro-apoptotic
properties against cancer. The present study revealed that terrein
inhibited the adhesion, migration, and invasion properties of
breast cancer cells at non-toxic concentrations. The potential use
of terrein in combination with other chemotherapeutic drugs to
suppress both cancer growth and metastasis appears promising
however it requires further investigation.
Acknowledgements
The present study was financially supported by the
MED-RES-200 (Basic/Applied Research) fund from the Faculty of
Medicine, Srinakharinwirot University (A.K.) and Mahidol University
(M.P. and T.K.). F.L. was supported by a New Research Grant
(A31/2556), Mahidol University, Thailand. We thank Rattanavinan
Hanchaina and Kritsada Jaisamak for excellent technical assistance
and Dr Sittirak Roitrakul for help in chemical analysis. We
sincerely thank Dr Laran Jensen for critical reading of the
manuscript.
References
|
1
|
Torre LA, Islami F, Siegel RL, Ward EM and
Jemal A: Global Cancer in Women: Burden and Trends. Cancer
Epidemiol Biomarkers Prev. 26:444–457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee S, Kim WG, Kim E, Ryoo IJ, Lee HK, Kim
JN, Jung SH and Yoo ID: Synthesis and melanin biosynthesis
inhibitory activity of (+/-)-terrein produced by Penicillium sp.
20135. Bioorg Med Chem Lett. 15:471–473. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malmstrøm J, Christophersen C, Barrero AF,
Oltra JE, Justicia J and Rosales A: Bioactive metabolites from a
marine-derived strain of the fungus Emericella variecolor. J Nat
Prod. 65:364–367. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Phattanawasin P, Pojchanakom K, Sotanaphun
U, Piyapolrungroj N and Zungsontiporn S: Weed growth inhibitors
from Aspergillus fischeri TISTR 3272. Nat Prod Res. 21:1286–1291.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim D-S, Lee H-K, Park S-H, Lee S, Ryoo
IJ, Kim WG, Yoo ID, Na JI, Kwon SB and Park KC: Terrein inhibits
keratinocyte proliferation via ERK inactivation and G2/M cell cycle
arrest. Exp Dermatol. 17:312–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang F, Mijiti M, Ding W, Song J, Yin Y,
Sun W and Li Z: (+)-Terrein inhibits human hepatoma Bel 7402
proliferation through cell cycle arrest. Oncol Rep. 33:1191–1200.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YF, Wang SY, Shen H, Yao XF, Zhang FL
and Lai D: The marine-derived fungal metabolite, terrein, inhibits
cell proliferation and induces cell cycle arrest in human ovarian
cancer cells. Int J Mol Med. 34:1591–1598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liao WY, Shen CN, Lin LH, Yang YL, Han HY,
Chen JW, Kuo SC, Wu SH and Liaw CC: Asperjinone, a nor-neolignan,
and terrein, a suppressor of ABCG2-expressing breast cancer cells,
from thermophilic Aspergillus terreus. J Nat Prod. 75:630–635.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Porameesanaporn Y,
Uthaisang-Tanechpongtamb W, Jarintanan F, Jongrungruangchok S and
Thanomsub Wongsatayanon B: Terrein induces apoptosis in HeLa human
cervical carcinoma cells through p53 and ERK regulation. Oncol Rep.
29:1600–1608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shibata A, Ibaragi S, Mandai H, Tsumura T,
Kishimoto K, Okui T, Hassan NM, Shimo T, Omori K, Hu GF, et al:
Synthetic terrein inhibits progression of head and neck cancer by
suppressing angiogenin production. Anticancer Res. 36:2161–2168.
2016.PubMed/NCBI
|
|
13
|
Cairns RA, Khokha R and Hill RP: Molecular
mechanisms of tumor invasion and metastasis: An integrated view.
Curr Mol Med. 3:659–671. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liotta LA and Stetler-Stevenson WG: Tumor
invasion and metastasis: An imbalance of positive and negative
regulation. Cancer Res. 51 Suppl 18:5054s–5059s. 1991.PubMed/NCBI
|
|
15
|
Sahai E and Marshall CJ: RHO-GTPases and
cancer. Nat Rev Cancer. 2:133–142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ridley AJ: Rho GTPases and actin dynamics
in membrane protrusions and vesicle trafficking. Trends Cell Biol.
16:522–529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ridley AJ: Rho GTPase signalling in cell
migration. Curr Opin Cell Biol. 36:103–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ridley AJ: Rho family proteins:
Coordinating cell responses. Trends Cell Biol. 11:471–477. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vega FM and Ridley AJ: The RhoB small
GTPase in physiology and disease. Small GTPases. Nov 22–2016.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bravo-Cordero JJ, Moshfegh Y, Condeelis J
and Hodgson L: Live cell imaging of RhoGTPase biosensors in tumor
cells. Methods Mol Biol. 1046:359–370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhuge Y and Xu J: Rac1 mediates type I
collagen-dependent MMP-2 activation. Role in cell invasion across
collagen barrier. J Biol Chem. 276:16248–16256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gebäck T, Schulz MM, Koumoutsakos P and
Detmar M: TScratch: A novel and simple software tool for automated
analysis of monolayer wound healing assays. Biotechniques.
46:265–274. 2009.PubMed/NCBI
|
|
23
|
Asano T, Tada M, Cheng S, Takemoto N,
Kuramae T, Abe M, Takahashi O, Miyamoto M, Hamada J, Moriuchi T, et
al: Prognostic values of matrix metalloproteinase family expression
in human colorectal carcinoma. J Surg Res. 146:32–42. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wanichwatanadecha P, Sirisrimangkorn S,
Kaewprag J and Ponglikitmongkol M: Transactivation activity of
human papillomavirus type 16 E6*I on aldo-keto reductase genes
enhances chemoresistance in cervical cancer cells. J Gen Virol.
93:1081–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koujan SE, Gargarib BP, Pirouzpanah S and
Khalili M: Matrix metalloproteinases and breast cancer. Thrita.
4:e219592015.
|
|
27
|
Prudnikova TY, Rawat SJ and Chernoff J:
Molecular pathways: Targeting the kinase effectors of RHO-family
GTPases. Clin Cancer Res. 21:24–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cherigo L, Lopez D and Martinez-Luis S:
Marine natural products as breast cancer resistance protein
inhibitors. Mar Drugs. 13:2010–2029. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Demasi M, Felicio A, Pacheco A, Leite H,
Limaa C and Andrade L: Studies on terrein as a new class of
proteasome inhibitors. J Braz Chem Soc. 21:299–305. 2010.
View Article : Google Scholar
|
|
30
|
Adams J and Kauffman M: Development of the
proteasome inhibitor Velcade (Bortezomib). Cancer Invest.
22:304–311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mandai H, Omori K, Yamamoto D, Tsumura T,
Murota K, Yamamoto S, Mitsudo K, Ibaragi S, Sasaki A, Maeda H, et
al: Synthetic (+)-terrein suppresses interleukin-6/soluble
interleukin-6 receptor induced-secretion of vascular endothelial
growth factor in human gingival fibroblasts. Bioorg Med Chem.
22:5338–5344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee YH, Lee SJ, Jung JE, Kim JS, Lee NH
and Yi HK: Terrein reduces age-related inflammation induced by
oxidative stress through Nrf2/ERK1/2/HO-1 signalling in aged HDF
cells. Cell Biochem Funct. 33:479–486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cirit M, Grant KG and Haugh JM: Systemic
perturbation of the ERK signaling pathway by the proteasome
inhibitor, MG132. PLoS One. 7:e509752012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vega FM and Ridley AJ: Rho GTPases in
cancer cell biology. FEBS Lett. 582:2093–2101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fritz G, Just I and Kaina B: Rho GTPases
are over-expressed in human tumors. Int J Cancer. 81:682–687. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chan AY, Coniglio SJ, Chuang YY,
Michaelson D, Knaus UG, Philips MR and Symons M: Roles of the Rac1
and Rac3 GTPases in human tumor cell invasion. Oncogene.
24:7821–7829. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gadea G, Sanz-Moreno V, Self A, Godi A and
Marshall CJ: DOCK10-mediated Cdc42 activation is necessary for
amoeboid invasion of melanoma cells. Curr Biol. 18:1456–1465. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nassar N, Cancelas J, Zheng J, Williams DA
and Zheng Y: Structure-function based design of small molecule
inhibitors targeting Rho family GTPases. Curr Top Med Chem.
6:1109–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Akbar H, Cancelas J, Williams DA, Zheng J
and Zheng Y: Rational design and applications of a Rac
GTPase-specific small molecule inhibitor. Methods Enzymol.
406:554–565. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Montalvo-Ortiz BL, Castillo-Pichardo L,
Hernández E, Humphries-Bickley T, De la Mota-Peynado A, Cubano LA,
Vlaar CP and Dharmawardhane S: Characterization of EHop-016, novel
small molecule inhibitor of Rac GTPase. J Biol Chem.
287:13228–13238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nagase H, Visse R and Murphy G: Structure
and function of matrix metalloproteinases and TIMPs. Cardiovasc
Res. 69:562–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brown GT and Murray GI: Current
mechanistic insights into the roles of matrix metalloproteinases in
tumour invasion and metastasis. J Pathol. 237:273–281. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mitropoulou TN, Tzanakakis GN, Kletsas D,
Kalofonos HP and Karamanos NK: Letrozole as a potent inhibitor of
cell proliferation and expression of metalloproteinases (MMP-2 and
MMP-9) by human epithelial breast cancer cells. Int J Cancer.
104:155–160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Farabegoli F, Govoni M, Spisni E and Papi
A: EGFR inhibition by (−)-epigallocatechin-3-gallate and IIF
treatments reduces breast cancer cell invasion. Biosci Rep.
37:BSR201701682017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lou C, Zhu Z, Zhao Y, Zhu R and Zhao H:
Arctigenin, a lignan from Arctium lappa L., inhibits metastasis of
human breast cancer cells through the downregulation of MMP-2/-9
and heparanase in MDA-MB-231 cells. Oncol Rep. 37:179–184. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bakar F, Kilic-Kurt Z, Caglayan MG and
Olgen S: The effects of 1,3,5-trisubstituted indole derivatives on
cell growth, apoptosis and MMP-2/9 mRNA expression of MCF-7 human
breast cancer cells. Anticancer Agents Med Chem. 17:762–767. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Matsumoto Y, Tanaka K, Harimaya K,
Nakatani F, Matsuda S and Iwamoto Y: Small GTP-binding protein,
Rho, both increased and decreased cellular motility, activation of
matrix metalloproteinase 2 and invasion of human osteosarcoma
cells. Jpn J Cancer Res. 92:429–438. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Aiuti A, Webb IJ, Bleul C, Springer T and
Gutierrez-Ramos JC: The chemokine SDF-1 is a chemoattractant for
human CD34+ hematopoietic progenitor cells and provides
a new mechanism to explain the mobilization of CD34+
progenitors to peripheral blood. J Exp Med. 185:111–120. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bartolomé RA, Molina-Ortiz I, Samaniego R,
Sánchez-Mateos P, Bustelo XR and Teixidó J: Activation of Vav/Rho
GTPase signaling by CXCL12 controls membrane-type matrix
metalloproteinase-dependent melanoma cell invasion. Cancer Res.
66:248–258. 2006. View Article : Google Scholar : PubMed/NCBI
|