Introduction
TTF1 (5,2′,4′-trihydroxy-6,7,5′-trimethoxyflavone)
is a flavonoid compound derived from Sorbaria sorbifolia,
which grows in the Changbai Mountains and was used as a medicinal
plant to treat swelling and inflammation in ancient China (1). TTF1 is the major bioactive anticancer
compound in S. sorbifolia: It has been shown to inhibit
angiogenesis induced by HepG2 cells in chick embryo chorioallantoic
membranes and to induce apoptosis through a mitochondrial pathway
in HepG2 cells (2,3). However, the use of TTF1 as an
anticancer drug has been limited by its high biodegradability. TTF1
nanoparticles (TTF1-NP) are prepared by an emulsion
evaporation-solidification method at a low temperature and are more
soluble and susceptible to absorption (1). We previously demonstrated that TTF1-NP
has an anti-hepatoma effect associated with induction of apoptosis
and inhibition of angiogenesis, migration and invasion in human
hepatoma cells and tissues (4,5).
Similar to apoptosis, autophagy plays an important
role in the regulation of cancer development and may be a target
for cancer therapies. Autophagy is the process by which eukaryotic
cells degrade and recycle misfolded proteins and impaired
organelles (6). It begins with a
semi-closed isolation membrane that subsequently develops into a
phagosome. In response to drugs or environmental stimuli, the
phagosome is converted into an autophagosome with a double-membrane
that contains broken organelles or cytosol. Autophagosomes then
fuse with lysosomes and their contents are degraded (7). In recent years, many efforts have been
made to identify autophagy-related genes with the aim of
elucidating the mechanism of autophagosome formation. Several
autophagy-related genes have been identified in yeast and orthologs
have been found in higher eukaryotes. However, the relationship
between apoptosis and autophagy in tumors is complex. Various
studies have implicated that autophagy is involved in the control
of both cell survival and death in different cell types or in
response to diverse drug treatments (8–10).
The apoptosis and autophagy responses to diverse
stressors share many pathways. For example, the protein kinase
B/mammalian target of rapamycin (Akt/mTOR) pathway and the
mitogen-activated protein kinase (MAPK) pathway that includes
extracellular signal-regulated kinase (ERK), p38 MAPK and
c-Jun-N-terminal kinase (JNK) play vital roles not only in
apoptosis, but also in autophagy in cancer cells (11,12).
Several studies have shown that the Akt/mTOR and MAPK pathways are
essential to regulation of autophagy in liver cancer cells
(13,14). However, studies of TTF1-NP-induced
autophagy and the interplay between autophagy and apoptosis in
human hepatoma are scarce. Thus, we studied the effect of TTF1-NP
on autophagy and the relationship between autophagy and apoptosis,
and examined potential mechanisms, focusing on the Akt/mTOR and
MAPK pathways in liver cancer cells.
Materials and methods
Preparation of TTF1-NP
TTF1-NP (Fig. 1A)
was prepared based on previous studies (1–3). TTF1
(380 mg) was obtained from 10 kg of Sorbaria sorbifolia
(collected from Yanji, China) by using water extraction and alcohol
precipitation (2). TTF1 was
encapsulated in stearic acid solid lipid nanostructured carrier
(average particle diameter, 195.2±35.2 nm; average entrapment rate,
64.57±8.21%) in an emulsion evaporation-solidification method at
low temperature (1). TTF1-NP with
stearic acid solid lipid nanostructure was more soluble than TTF1
(1,3).
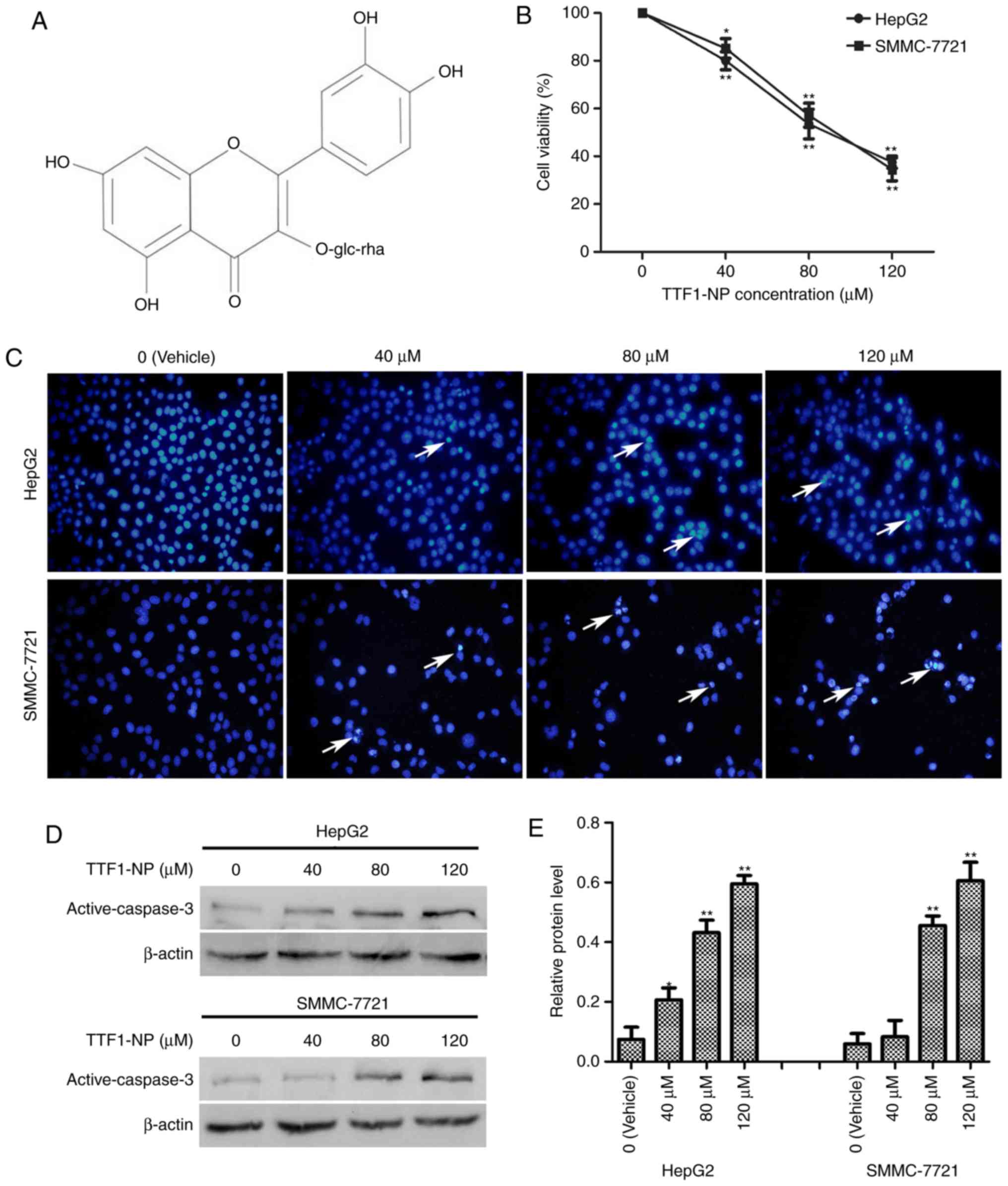 | Figure 1.TTF1-NP inhibits cell viability and
induces apoptosis in HepG2 and SMMC-7721 cells. (A) Chemical
structure of TTF1-NP. (B) HepG2, SMMC-7721 were treated with 0, 40,
80, and 120 µM TTF1-NP for 24 h, and cell viability was measured by
MTT assay. *P<0.05, **P<0.01 vs. Vehicle. (C) HepG2 and
SMMC-7721 cells were stained with Hoechst and observed by
fluorescence microscopy (magnification, ×400). Arrows indicate
apoptotic cells. (D) HepG2 and SMMC-7721 cells were treated with
TTF1-NP (0, 40, 80, 120 µΜ) for 24 h and the expression of
active-caspase-3 was detected by western blotting. (E) Densitometry
analysis of data in (D). Active-caspase-3 levels relative to
β-actin were determined. Data presented are means ± SD. *P<0.05,
**P<0.01 vs. Vehicle. |
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS) and penicillin-streptomycin were purchased from
Gibco-BRL (Grand Island, NY, USA). Rabbit-polyclonal antibodies of
LC3B І/II, Beclin-1, p-Akt (Ser473), Akt, p-mTOR (Ser2448), mTOR,
p-JNK (Thr183/Tyr185) were purchased from Abcam. Rabbit-polyclonal
antibodies of p-p38 MAPK (Thr180/Tyr182), p-ERK1/2 (Thr202/Tyr204),
active-caspase-3, p62 and β-actin were purchased from Cell
Signaling Technology (Danvers, MA, USA). The chemicals used were
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(Sigma, St. Louis, MO, USA), MDC (KeyGen Co. Ltd., Nanjing, China),
3-methyladenine (MedChemExpresss, Monmouth Junction, NJ, USA),
Hoechst 33258 (Beyotime, Jiangsu, China), insulin (Beyotime),
SP600125 (Beyotime), and U0126 (Beyotime).
Cell culture and viability assay
HepG2 and SMMC-7721 cells were purchased from KeyGen
Co. Ltd. and cultured in DMEM with 10% FBS, 100 U/ml
penicillin-streptomycin at 37°C in a humidified 5% CO2
incubator. Cells were plated in 96-well plates
(5×103/100 µl/well) and incubated with TTF1-NP (0, 40,
80, 120 µM). MTT (5.0 mg/ml, 20 µl) was added after the cells were
incubated for 24 h, then 150 µl DMSO was added into each well. The
optical density (OD) was measured at 490 nm, respectively. The cell
viability value was calculated using the following formula: OD
sample/OD blank ×100%.
Fluorescent staining
HepG2 and SMMC-7721 cells grown in 6-well plates
were treated with TTF1-NP for 24 h, incubated with 1 mM MDC for 30
min or Hoechst 33258 for 20 min and washed with phosphate-buffered
saline (PBS). Fluorescence images were captured by fluorescence
microscope (Olympus, Tokyo, Japan).
Transmission electron microscopy
(TEM)
Cells were treated with TTF1-NP (80 µM), trypsinized
and washed with PBS before fixed in fixative buffer. Subsequently,
cells were collected at 1,000 r/min for 10 min, and then incubated
in 3% glutaraldehyde. The next day, the samples were treated with
2% osmium tetroxide, followed by dehydrating through a graded
series of acetone, and embedded in resin. Finally, the samples were
sliced into 60-nm sections and prepared for TEM (Hitachi, Ltd.,
Tokyo, Japan).
Annexin V/PI double staining
HepG2 and SMMC-7721 cells were seeded into a 6-well
plate (3×105 cells/well), and treated with the indicated
amounts of TTF1-NP for 24 h. Cells were collected, washed in cold
PBS and resuspended in binding buffer at a concentration of
1×106 cells/ml. Then, 5 µl Annexin V-FITC and 10 µl PI
were added in 100 µl cell suspension and incubated for 15 min.
After added 400 µl PBS, the samples were detected by flow
cytometry. The results were analyzed with the BD FACSCalibur™
system.
Transfection of green fluorescent
protein-light chain 3 (GFP-LC3) plasmid
The GFP-LC3 plasmid was kindly provided by Dr Quan
Zhang (Yangzhou University, Yangzhou, China). According to
manufacturer's protocol of Lipofectamine 2000 (Invitrogen), HepG2
and SMMC-7721 cells were transfected with GFP-LC3 plasmid. After
indicated treatment of TTF1-NP, the cells with GFP-LC3 puncta were
detected under fluorescence microscope (Olympus, Tokyo, Japan).
Western blot analysis
After treatment HepG2 and SMMC-7721 cells with
various concentrations of TTF1-NP, the total protein was obtained
by RIPA lysis buffer (Solarbio, Beijing, China). The proteins were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) (100 V, 120 min) and transferred to a
polyvinylidene fluoride (PVDF) membrane (100 V, 30–90 min).
Subsequently, the membranes were blocked with skimmed milk (5%) and
then incubated overnight at 4°C with the following antibodies: Akt
(1:1,000), mTOR (1:1,500), p-Akt (1:5,000), p-mTOR (1:2,000), LC3B
І/II (1:500), Beclin-1 (1:1,000), p62 (1:5,000), p-JNK (1:1,000),
p-ERK1/2 (1:1,000), p-p38 MAPK (1:1,000), β-actin (1:1,000) and
active-caspase-3 (1:1,000). The next day, the membranes were
incubated with an anti-rabbit secondary antibody (1:1,500) for 2 h
at room temperature. After ECL incubation, the target proteins were
tested using Bio-Rad imaging system (Bio-Rad, Hercules, CA,
USA).
Statistical analysis
The experiments were repeated three times. The data
are expressed as the mean ± SD, and differences between groups were
analyzed by one-way analysis of variance (ANOVA) and Student's
t-test. The results were considered statistically significant when
the P-value was <0.05.
Results
TTF1-NP induces apoptosis in HepG2 and
SMMC-7721 cell lines
We previously reported TTF1-NP cytotoxicity in
various human liver cancer cell types (5). Herein, to examine the effect of
TTF1-NP on cell viability, HepG2 and SMMC-7721 were treated with
0–120 µM TTF1-NP for 24 h, and then MTT assays were performed.
TTF1-NP decreased HepG2 and SMMC-7721 cell viability in a
dose-dependent manner (Fig. 1B).
The IC50 values were 88.27 µM for HepG2 and 90.39 µM for
SMMC-7721 cells at 24 h. Hoechst staining and western blotting
showed that apoptosis-related bright blue fluorescence in the
nuclei of cells and levels of the apoptosis-related protein active
caspase-3, respectively, increased with increasing TTF1-NP
concentration in HepG2 and SMMC-7721 cells (Fig. 1C-E). These results indicate that
TTF1-NP induced apoptosis in a dose-dependent manner in HepG2 and
SMMC-7721 cells.
TTF1-NP induces autophagy in HepG2 and
SMMC-7721 cell lines
To determine whether TTF1-NP induces autophagy in
HepG2 and SMMC-7721 cells, monodansylcadaverine (MDC) staining was
used to detect preliminary autophagosomes. We observed increasing
levels of positive staining for autophagic puncta with increasing
TTF1-NP concentration in both cell lines (Fig. 2A). For further monitoring of
autophagosome formation, HepG2 and SMMC-7721 cells were examined by
transmission electron microscopy (TEM) or transfected with green
fluorescent protein (GFP)-light chain 3 (LC3) plasmid. The results
showed that TTF1-NP induced formation of autophagosomes with
double-layered membranes, and that the average number of GFP-LC3
puncta per cell was increased in both cell lines, compared with
vehicle-treated cells (Fig. 2B-D).
Furthermore, western blot analysis of the autophagy-related
proteins LC3B I/II, Beclin-1, and p62 showed that TTF1-NP increased
the protein levels of LC3B-II and Beclin-1, but decreased p62
levels (Fig. 2E and F).
Collectively, these results indicate that TTF1-NP induced autophagy
in HepG2 and SMMC-7721 cells.
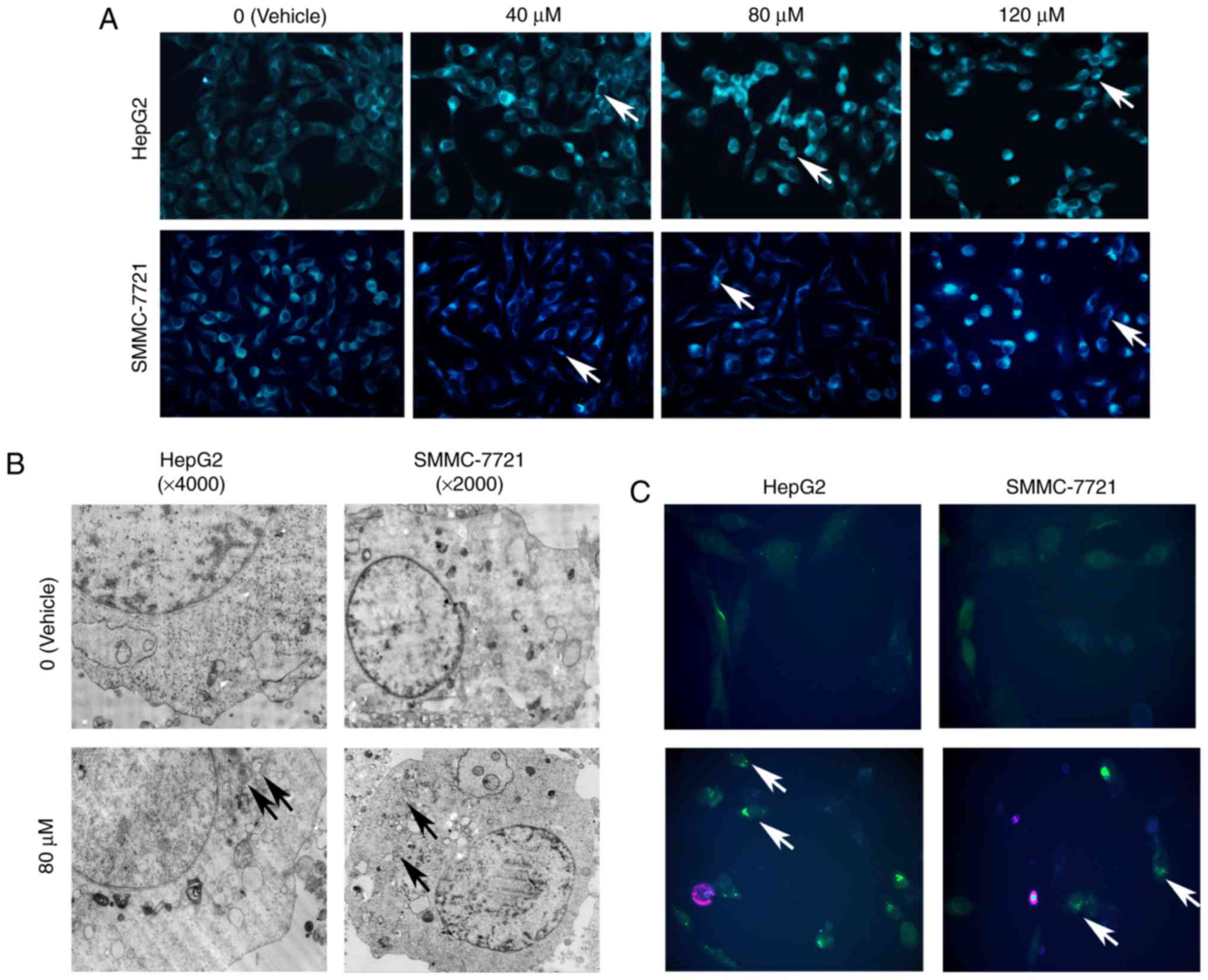 | Figure 2.TTF1-NP induces autophagy in HepG2 and
SMMC-7721 cells. (A) HepG2 and SMMC-7721 cells were treated with
TTF1-NP (0, 40, 80, 120 µΜ) for 24 h, stained with MDC and observed
by fluorescence microscopy (magnification, ×400). (B) The formation
of autophagosomes were observed in TTF1-NP-treated cells under
transmission electron microscopy (TEM). (C) Cells were transfected
with GFP-LC3 plasmid and treated with TTF1-NP for 24 h. GFP-LC3
puncta were observed by a fluorescence microscopy (magnification,
×400). (D) The number of GFP-LC3 puncta was counted. Data represent
the mean ± SD. **P<0.01 vs. Vehicle. (E) HepG2 and SMMC-7721
cells were treated with TTF1-NP (0, 40, 80, 120 µΜ) for 24 h and
the expression of LC3B I/II, Beclin-1, p62 was detected by western
blotting. (F) Densitometry analysis of data in (E). LC3B-II,
Beclin-1 and p62 levels relative to β-actin were determined. Data
presented are means ± SD. *P<0.05, **P<0.01 vs. Vehicle. |
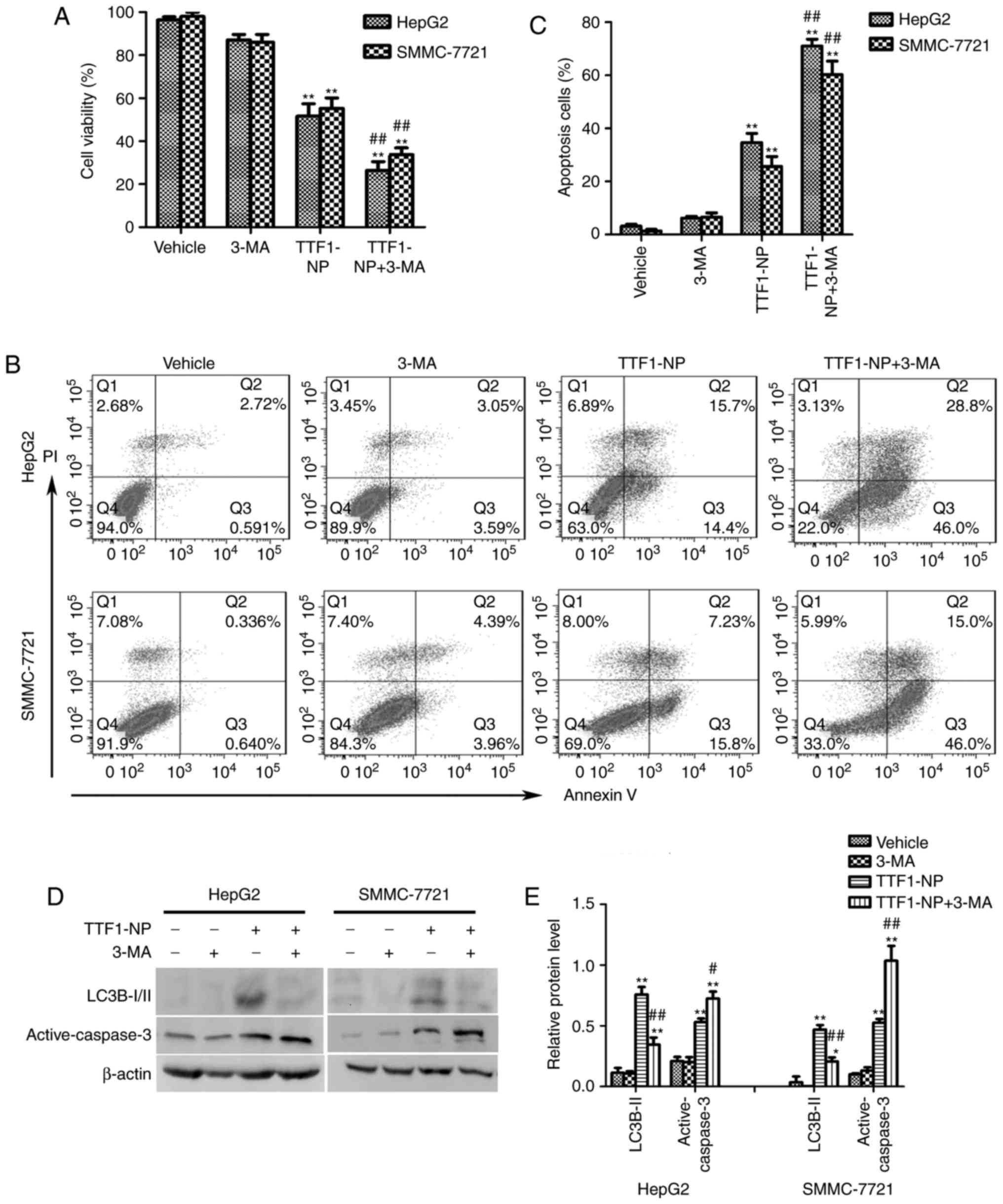
Autophagy inhibition enhanced
TTF1-NP-induced apoptosis in HepG2 and SMMC-7721 cells
It is reported that autophagy had a paradoxical
effect on death or survival in cancer cells (8–10). To
clarify the role of autophagy in TTF1-NP-induced apoptosis, we used
the autophagy inhibitor 3-MA to prevent autophagy and co-treated
with TTF1-NP for 24 h. Compared with TTF1-NP group, TTF1-NP
co-treatment with 3-MA decreased cell viability (Fig. 3A). Furthermore, the result of flow
cytometry assay showed that inhibition of autophagy significantly
increased apoptotic cells in TTF1-NP+3-MA group comparing with
TTF1-NP treatment group (Fig. 3B,
C). Additionally, TTF1-NP-induced caspase-3 activation was
enhanced by 3-MA (Fig. 3D and E).
Taken together, these results suggested that autophagy could have a
protective effect in TTF1-NP-induced apoptosis in HepG2 and
SMMC-7721 cells, prevention of autophagy could enhance the
anticancer effect of TTF1-NP in human liver cancer cells.
TTF1-NP-induced autophagy and
apoptosis are related to the Akt/mTOR pathway in HepG2 and
SMMC-7721 cell lines
The Akt/mTOR pathway plays an important role in cell
proliferation, apoptosis, and autophagy. To determine whether
TTF1-NP-induced apoptosis and autophagy were involved in this
signaling pathway, we examined the expression of Akt,
phosphorylated (p)-Akt (Ser473), mTOR and p-mTOR (Ser2448). The
results showed that the levels of the p-Akt (Ser473) and p-mTOR
(Ser2448) decreased in a dose-dependent manner in HepG2 and
SMMC-7721 cells (Fig. 4A-C).
Moreover, we used insulin to activate the Akt/mTOR pathway and
investigated the relationship between this pathway and autophagy or
apoptosis. As shown in Fig. 4D-F,
insulin significantly increased levels of p-Akt (Ser473) and p-mTOR
(Ser2448) in both cell lines (P<0.05). When cells were
pre-treated with TTF1-NP and then stimulated with insulin, the
phosphorylation of Akt and mTOR increased compared with cells
treated with TTF1-NP only, while levels of LC3B-II and active
caspase-3 decreased in response to insulin (Fig. 4C, D and F). Together, these results
showed that the TTF1-NP-induced autophagy and apoptosis in HepG2
and SMMC-7721 cells may be mediated by the Akt/mTOR pathway.
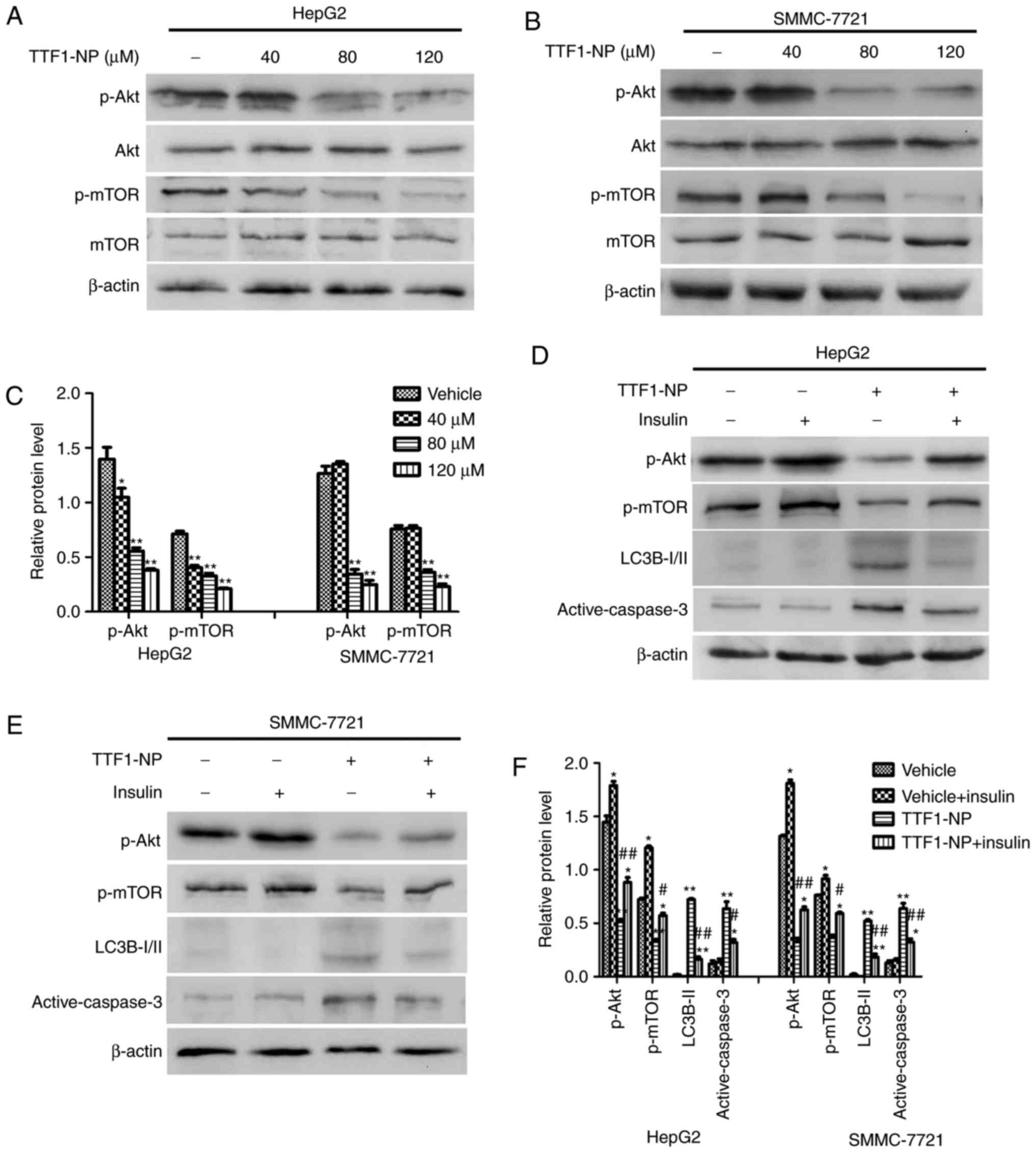 | Figure 4.AKT/mTOR pathway is involved in
TTF1-NP-induced autophagy and apoptosis in HepG2 and SMMC-7721
cells. (A and B) HepG2 and SMMC-7721 cells were treated with
TTF1-NP (0, 40, 80, 120 µΜ) for 24 h. Western blot analysis for the
expression of Akt, p-Akt, mTOR and p-mTOR was detected. (C)
Densitometry analysis of data in (A, B), p-Akt and p-mTOR levels
relative to β-actin was performed. *P<0.05, **P<0.01 vs.
Vehicle. (D and E) HepG2 and SMMC-7721 cells were treated with 80
µΜ of TTF1-NP or insulin, western blot analysis for the expression
of p-Akt, p-mTOR, LC3B І/II and active-caspase-3 were detected. (F)
Densitometry analysis of data in (D and E), p-Akt, p-mTOR, LC3B-II
and active-caspase-3 levels relative to β-actin was performed.
*P<0.05, **P<0.01 vs. Vehicle; #P<0.05,
##P<0.01 vs. TTF1-NP group. |
TTF1-NP induces autophagy and
apoptosis regulated by JNK and ERK1/2
Many studies have reported that the MAPK pathway
influences apoptosis and autophagy (15,16).
To examine the effect of TTF1-NP on the MAPK pathway, we evaluated
the phosphorylation of JNK, ERK1/2 and p38 MAPK by western
blotting. Levels of p-JNK (Thr183/Tyr185) increased and levels of
p-ERK1/2 (Thr202/Tyr204) decreased in a concentration-dependent
manner in response to TTF1-NP treatment (Fig. 5A and B). However, the level of p-p38
MAPK (Thr180/Tyr182) did not change (Fig. 5A and B). To further investigate
whether TTF1-NP-induced autophagy and apoptosis relied on JNK and
ERK1/2, we pretreated cells with SP600125 and U0126 (a JNK and an
ERK1/2 inhibitor, respectively) for 4 h followed by treatment with
TTF1-NP for 24 h. The results showed that levels of LC3B-II and
active caspase-3 decreased in the TTF1-NP- and SP600125-treated
cells, compared with cells treated with TTF1-NP only. However,
U0126 enhanced active caspase-3, but had no significant effect on
TTF1-NP-induced LC3B-II expression (Fig. 5C-F). Taken together, these results
suggest that JNK was associated with the effect of TTF1-NP on
autophagy and apoptosis, while ERK1/2 may be related to
TTF1-NP-induced apoptosis in HepG2 and SMMC-7721 cells.
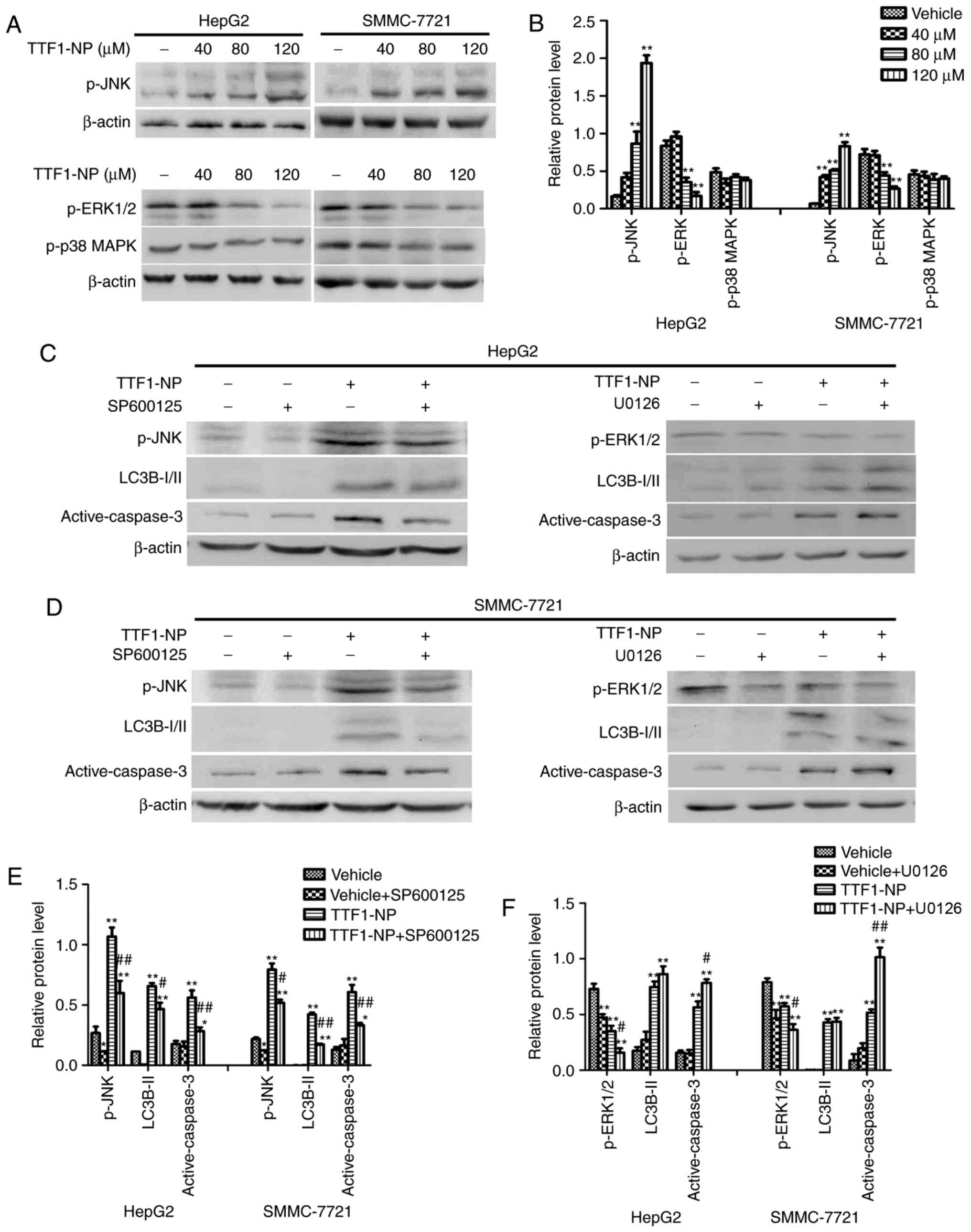 | Figure 5.The JNK and ERK1/2 are involved in
TTF1-NP-induced autophagy and apoptosis in HepG2 and SMMC-7721
cells. (A) HepG2 and SMMC-7721 cells were treated with TTF1-NP (0,
40, 80, 120 µΜ) for 24 h. Western blot analysis for the expression
of p-JNK, p-ERK and p-p38 MAPK were detected. (B) Densitometry
analysis of p-JNK, p-ERK and p-p38 MAPK were performed. *P<0.05,
**P<0.01 vs. Vehicle. (C) HepG2 were pre-treated with SP600125,
U0126 or treated with 80 µΜ of TTF1-NP, western blot analysis for
the expression of p-JNK, p-ERK, LC3B І/II and active-caspase-3 were
detected. (D) SMMC-7721 were pre-treated with SP600125, U0126 or
treated with 80 µΜ of TTF1-NP, western blot analysis for the
expression of p-JNK, p-ERK, LC3B І/II and active-caspase-3 were
detected. (E and F) Densitometry analysis of data in (C and D) were
performed in HepG2 and SMMC-7721 cells. *P<0.05, **P<0.01 vs.
Vehicle; #P<0.05, ##P<0.01 vs. TTF1-NP
group. |
Discussion
Hepatocellular carcinoma is the second leading cause
of cancer-related mortality worldwide (17). Traditional postoperative
chemotherapy is the current standard clinical treatment strategy.
However, many drugs show limited efficacy, so there is an urgent
need to develop more effective and lower-toxicity drugs or
treatment approaches. We previously reported that TTF1-NP inhibited
HepG2 cell proliferation by inducing apoptosis and inhibiting
angiogenesis, migration and invasion in vitro and in
vivo (4,5). However, whether TTF1-NP induces
autophagy in human hepatoma and the relationship between autophagy
and apoptosis remain unknown.
Autophagy and apoptosis are topics of considerable
interest in tumor research (18–20).
In our study, TTF1-NP induced apoptosis, including changes to cell
morphology and activation of the apoptosis-related protein
caspase-3. Moreover, TTF1-NP induced autophagy. The number of
puncta staining positive with MDC increased with increasing
concentrations of TTF1-NP. However, MDC is not a specific dye for
autophagosomes. TEM, the standard method for detecting autophagy
(21,22), is a more specific approach to
examining autophagy. Our TEM results clearly show that
autophagosomes with double-layered membranes appeared after TTF1-NP
treatment. Furthermore, our data revealed that TTF1-NP increased
the number of GFP-LC3B puncta in HepG2 and SMMC-7721 cells.
Autophagy-related proteins such as LC3, Beclin-1 and p62 are
important in the autophagy process. LC3 is homologous with Atg8 in
yeast and is conjugated to phosphatidyl ethanolamine to form
LC3-II, which is located in the autophagosome double membrane and
positively correlated with autophagosome formation (23–25).
Beclin-1 (Atg6) binds with class III PI3K as a complex to help form
autophagosome structures (26). As
another monitor of autophagic flux, p62 binds directly to LC3, and
they are degraded during autophagy (27). Our study showed that TTF1-NP
upregulated LC3B-II and Beclin-1 levels and decreased expression of
p62. Collectively, these results suggest that TTF1-NP induced
autophagy in both HepG2 and SMMC-7721 cells.
To further investigate the dual role of
TTF1-NP-induced autophagy, 3-MA (an autophagy inhibitor) was used
to prevent autophagy in both HepG2 and SMMC-7721 cells. Our results
show that inhibiting autophagy increased the proportion of
apoptotic cells and active caspase-3 expression, which suggests
that TTF1-NP-induced autophagy may play a protective role in
TTF1-NP-induced apoptosis. Therefore, inhibition of autophagy may
improve the efficacy of TTF1-NP treatment in human liver cancer
cells.
The Akt/mTOR pathway plays a critical role in
tumorigenesis. High expression of Akt frequently occurs in various
tumors including hepatoma (13).
mTOR is activated by Akt and negatively regulates autophagy
(28). Extensive research has
indicated that inhibiting the Akt/mTOR pathway contributes to
autophagy in cancer cells (29,30).
In our study, TTF1-NP inhibited the Akt/mTOR pathway and decreased
p-Akt (Ser473) and p-mTOR (Ser2448) levels in a dose-dependent
manner. Furthermore, we used insulin to verify the influence of
Akt/mTOR pathway on apoptosis and autophagy. As an activator,
insulin binds with the insulin receptor and promotes PI3K
conformational interconversion, which ultimately activates the
Akt/mTOR pathway (31). Insulin
significantly suppressed TTF1-NP-induced expression of LC3B-II and
active caspase-3, suggesting that the Akt/mTOR pathway may play an
important role in TTF1-NP-induced autophagy and apoptosis in HepG2
and SMMC-7721 cells.
Many studies have shown that the MAPK pathway is
involved in cell proliferation, apoptosis and autophagy (32,33).
Our experiments showed that TTF1-NP increased expression of p-JNK
(Thr183/Tyr185) and decreased p-ERK1/2 (Thr202/Tyr204) in a
concentration-dependent manner, but did not change p-p38 MAPK
(Thr180/Tyr182) levels. Furthermore, TTF1-NP co-treatment with the
JNK inhibitor SP600125 decreased caspase-3 activation, whereas
co-treatment with the ERK inhibitor U0126 increased caspase-3
activation. Moreover, JNK inhibition reduced LC3-II levels, whereas
U0126 did not affect TTF1-NP-induced LC3-II expression. The results
suggest that effects on JNK may account for TTF1-NP-induced
autophagy and apoptosis and ERK1/2 may account mainly for apoptosis
in HepG2 and SMMC-7721 cells.
In conclusion, our study showed for the first time
that TTF1-NP induced autophagy, and revealed the relationship
between autophagy and apoptosis. Our results show that TTF1-NP
induced a protective, apoptosis-related autophagy by regulating the
Akt/mTOR pathway and JNK in HepG2 and SMMC-7721 cells. Co-treatment
with an autophagy inhibitor reduced the effective treatment
concentration of TTF1-NP. Thus, autophagy may be a potential
therapeutic target of TTF1-NP in human hepatoma.
Acknowledgements
This study was supported by the NSFC (National
Natural Science Foundation of China (code: 81460617).
References
|
1
|
Li Z, Cui FD and Zhang XW: Preparation
technology of Sorbaria sorbifolia solid lipid nanoparticles.
Lishizhen Med Materia Med Res. 23:2549–2550. 2012.(In Chinese).
|
|
2
|
Liu C, Li XW, Cui LM, Li LC, Chen LY and
Zhang XW: Inhibition of tumor angiogenesis by TTF1 from extract of
herbal medicine. World J Gastroenterol. 17:4875–4882. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Bian L, Cui F, Li L and Zhang X:
TTF1-induced apoptosis of HepG-2 cells through a mitochondrial
pathway. Oncol Reps. 26:651–657. 2011.
|
|
4
|
Xiao B, Lin D and Zhang X, Zhang M and
Zhang X: TTF1, in the form of nanoparticles, inhibits angiogenesis,
cell migration and cell invasion in vitro and in vivo in human
hepatoma through STAT3 regulation. Molecules. 21(pii): E15072016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao B, Liu C, Liu BT, Zhang X, Liu RR and
Zhang XW: TTF1-NPs induce ERS-mediated apoptosis and inhibit human
hepatoma cell growth in vitro and in vivo. Oncol Res. 23:311–320.
2016. View Article : Google Scholar
|
|
6
|
Yue H, Li W, Liu P, Gao J, Miao J and Zhao
J: Inhibition of autophagy promoted sphingosylphosphorylcholine
induced cell death in non-small cell lung cancer cells. Biochem
Biophys Res Commun. 453:502–507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan HX, Russell RC and Guan KL:
Regulation of PIK3C3/VPS34 complexes by MTOR in nutrient
stress-induced autophagy. Autophagy. 9:1983–1995. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim KY, Park KI, Kim SH, Yu SN, Park SG,
Kim YW, Seo YK, Ma JY and Ahn SC: Inhibition of autophagy promotes
salinomycin-induced apoptosis via reactive oxygen species-mediated
PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human
prostate cancer cells. Int J Mol Sci. 18(pii): E10882017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Wang H, Zhu J, Xu J and Ding K:
Mollugin induces tumor cell apoptosis and autophagy via the
PI3K/AKT/mTOR/p70S6K and ERK signaling pathways. Biochem Biophys
Res Commun. 450:247–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ko A, Kanehisa A, Martins I, Senovilla L,
Chargari C, Dugue D, Mariño G, Kepp O, Michaud M, Perfettini JL, et
al: Autophagy inhibition radiosensitizes in vitro, yet reduces
radioresponses in vivo due to deficient immunogenic signaling. Cell
Death Differ. 21:92–99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao G, Gao B, Gao Y, Yang X, Cheng X and
Ou Y: Phycocyanin inhibits tumorigenic potential of pancreatic
cancer cells: Role of apoptosis and autophagy. Sci Rep.
6:345642016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Z, Wang F, Zhou ZW, Xia HC, Wang XY,
Yang YX, He ZX, Sun T and Zhou SF: Alisertib induces G2/M arrest,
apoptosis, and autophagy via PI3K/Akt/mTOR- and p38 MAPK-mediated
pathways in human glioblastoma cells. Am J Transl Res. 3:845–873.
2017.
|
|
13
|
Gong L, Di C, Xia X, Wang J, Chen G, Shi
J, Chen P, Xu H and Zhang W: AKT/mTOR signaling pathway is involved
in salvianolic acid B-induced autophagy and apoptosis in
hepatocellular carcinoma cells. Int J Oncol. 49:2538–2548. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He JD, Wang Z, Li SP, Xu YJ, Yu Y, Ding
YJ, Yu WL, Zhang RX, Zhang HM and Du HY: Vitexin suppresses
autophagy to induce apoptosis in hepatocellular carcinoma via
activation of the JNK signaling pathway. Oncotarget. 7:84520–84532.
2016.PubMed/NCBI
|
|
15
|
Hsieh MJ, Lin CW, Chen MK, Chien SY, Lo
YS, Chuang YC, Hsi YT, Lin CC, Chen JC and Yang SF: Inhibition of
cathepsin S confers sensitivity to methyl protodioscin in oral
cancer cells via activation of p38 MAPK/JNK signaling pathways. Sci
Rep. 7:450392017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsieh MJ, Chien SY, Lin JT, Yang SF and
Chen MK: Polyphyllin G induces apoptosis and autophagy cell death
in human oral cancer cells. Phytomedicine. 13:1545–1554. 2016.
View Article : Google Scholar
|
|
17
|
Shen L and Zhang G, Lou Z, Xu G and Zhang
G: Cryptotanshinone enhances the effect of Arsenic trioxide in
treating liver cancer cell by inducing apoptosis through
downregulating phosphorylated-STAT3 in vitro and in vivo. BMC
Complement Altern Med. 17:1062017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu S, Fei W, Shi Q, Li Q, Kuang Y, Wang
C, He C and Hu X: CHAC2, downregulated in gastric and colorectal
cancers, acted as a tumor suppressor inducing apoptosis and
autophagy through unfolded protein response. Cell Death Dis.
8:e30092017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan J, Jiang X, Yin G, He L, Liu J, Long
Z, Jiang Z and Yao K: Anacardic acid induces cell apoptosis of
prostatic cancer through autophagy by ER stress/DAPK3/Akt signaling
pathway. Oncol Rep. 38:1373–1382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Tan X, Yang Q, Zeng X, Zhou Y, Luo
W, Lin X, Song L, Cai J, Wang T and Wu X: Inhibition of autophagy
promotes apoptosis and enhances anticancer efficacy of adriamycin
via augmented ROS generation in prostate cancer cells. Int J
Biochem Cell Biol. 77:80–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang KF, Yang H, Jiang WQ, Li S and Cai
YC: Puquitinib mesylate (XC-302) induces autophagy via inhibiting
the PI3K/AKT/mTOR signaling pathway in nasopharyngeal cancer cells.
Int J Mol Med. 36:1556–1562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao R, Chen M, Jiang Z, Zhao F, Xi B,
Zhang X, Fu H and Zhou K: Platycodin-D induced autophagy in
non-small cell lung cancer cells via PI3K/Akt/mTOR and MAPK
signaling pathways. J Cancer. 6:623–631. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsieh MJ, Chen MK, Chen CJ, Hsieh MC, Lo
YS, Chuang YC, Chiou HL and Yang SF: Glabridin induces apoptosis
and autophagy through JNK1/2 pathway in human hepatoma cells.
Phytomedicine. 23:359–366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim YJ, Kang KS, Choi KC and Ko H:
Cardamonin induces autophagy and an antiproliferative effect
through JNK activation in human colorectal carcinoma HCT116 cells.
Bioorg Med Chem Lett. 25:2559–2564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mizushima N and Yoshimori T: How to
interpret LC3 immunoblotting. Autophagy. 3:542–545. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mastorci K, Montico B, Faè DA, Sigalotti
L, Ponzoni M, Inghirami G, Dolcetti R and Dal Co J: Phospholipid
scramblase 1 as a critical node at the crossroad between autophagy
and apoptosis in mantle cell lymphoma. Oncotarget. 7:41913–41928.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reis FS, Lima RT, Morales P, Ferreira IC
and Vasconcelos MH: Methanolic extract of ganoderma lucidum induces
autophagy of AGS Human gastric tumor cells. Molecules.
20:17872–17882. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ge D, Han L, Huang S, Peng N, Wang P,
Jiang Z, Zhao J, Su L, Zhang S, Zhang Y, et al: Identification of a
novel MTOR activator and discovery of a competing endogenous RNA
regulating autophagy in vascular endothelial cells. Autophagy.
10:957–971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao D, Wang W, Wang H, Peng H, Liu X, Guo
W, Su G and Zhao Z: PKD knockdown inhibits pressure
overload-induced cardiac hypertrophy by promoting autophagy via
AKT/mTOR pathway. Int J Biol Sci. 13:276–285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qin Y, Meng L, Fu Y, Quan Z, Ma M, Weng M,
Zhang Z, Gao C, Shi X and Han K: SNORA74B gene silencing inhibits
gallbladder cancer cells by inducing PHLPP and suppressing Akt/mTOR
signaling. Oncotarget. 8:19980–19996. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yao H and Han X and Han X: The
cardioprotection of the insulin-mediated PI3K/Akt/mTOR signaling
pathway. Am J Cardiovasc Drugs. 14:433–442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J, Chang F, Li F, Fu H, Wang J, Zhang
S, Zhao J and Yin D: Palmitate promotes autophagy and apoptosis
through ROS-dependent JNK and p38 MAPK. Biochem Biophys Res Commun.
463:262–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang C, Jia X, Wang K, Bao J, Li P, Chen
M, Wan JB, Su H, Mei Z and He C: Polyphyllin VII induces an
autophagic cell death by activation of the JNK pathway and
inhibition of PI3K/AKT/mTOR pathway in HepG2 cells. PLoS One.
11:e01474052016. View Article : Google Scholar : PubMed/NCBI
|