Introduction
Radioactive iodine (131I) has been used
to treat differentiated thyroid carcinoma (DTC) for decades
(1), and satisfactory results have
been achieved. The sodium iodide symporter (NIS) is expressed in
DTC cell membranes and can specifically transport 131I
into cells. Thus, 131I is an ideal targeted internal
radiotherapy that primarily produces beta rays to mimic
radiotherapy. Furthermore, 131I exerts little damage to
surrounding tissues and causes fewer side effects (2–6).
Breast cancer comprises 33% of all cancer cases
among women and is responsible for 19% of all cancer-related deaths
(7). Improving the survival rate
and quality of life for individuals suffering from breast cancer is
of particular interest but has proven to be difficult (8). When advanced breast cancer
metastasizes to the liver, cancer cells often exhibit
chemotherapeutic and endocrine drug resistance (9,10).
Surgery, chemotherapy, radiotherapy and endocrine therapy cannot
effectively prevent such advanced cancer, and treatments often
fail. Thus, we questioned whether 131I can be used to
treat breast cancer to improve patient outcomes.
The specific absorption and binding affinity of
131I are exclusive to the thyroid in vivo. Breast
cancer cells are unable to specifically absorb 131I.
Thus, fully replicating 131I treatment of DTC in breast
cancer is impractical. However, based on recent progress, the
feasibility of incorporating 131I into breast cancer
treatment was examined as follows.
Relationship among estrogen, estrogen receptors and
breast cancer. Most breast cancers are hormone-dependent, and
estrogen receptors (ERs) are widely expressed in cancer cells and
on cell membranes. Estrogen can specifically bind with ERs in
vivo and promote tumor cell growth via a post-receptor effect.
This ligand-receptor binding reaction has high specificity
(11,12), and endocrine therapies that target
ER-positive(ER+) breast cancers are considerably
effective (13). Two common breast
cancer-based endocrine therapies include blocking ER activity and
estrogen production via antagonism of ERs. Drugs with chemical
structures similar to ERs can be used for competitive binding,
although such interactions do not induce a post-receptor effect. In
addition, such binding inhibits signal transduction cascades, which
interferes with the metabolism and growth of cancer cells (14,15).
Commonly used drugs include tamoxifen, toremifene, and fulvestrant
(16). Fulvestrant was approved in
the United States in 2002 and is primarily administered to
postmenopausal women who suffer cancer progression after
anti-estrogen therapy. Fulvestrant has become the first-choice
endocrine therapy for breast cancer in patients who develop drug
resistance to tamoxifen. Furthermore, fulvestrant exerts more
powerful endocrine and anticancer effects than tamoxifen and has a
300-fold stronger affinity for ERs than tamoxifen.
Progress in chemical modification of drugs. Chemical
modification refers to altering functional groups and maintaining
not only the basic structures of a drug but also its
physicochemical properties and biological effects. The most common
chemical modification methods are the Iodogen method, which is the
process of introducing iodine atoms into a compound, and the
chloramine T method. These approaches can be used to label
fulvestrant with 131I.
Once 131I-fulvestrant was successfully
synthesized, we sought to evaluate whether the compound binds to
ERs in hormone-dependent breast cancer cells. Thus, the aim of this
study was to assess both the radiotherapeutic and endocrine
therapeutic effects of 131I-fulvestrant on breast cancer
cells.
Materials and methods
Experimental materials
Main reagents and their
preparation
Fulvestrant (Rongda Pharm & Chem Co., Ltd.,
Hangzhou, China) was prepared as previously described (17–20): 2
mg fulvestrant was dissolved in 1 ml ethanol at a final
concentration of 2 g/l. Chloramine T and sodium metabisulfite were
obtained from the Chengdu Kelong Chemical Reagent Factory (Chengdu,
China). Chloramine T (2 mg) was dissolved in 1 ml of a 1:1 ethanol
and PBS solution (0.05 mmol/l, pH 7.5), and 2 mg sodium
metabisulfite was dissolved in 1 ml of a 1:1 ethanol and PBS
solution (0.05 mmol/l, pH 7.5) at a final concentration of 2 g/l. A
Na131I solution [Chengdu Gaotong Isotope Co., Ltd.
(CNNC), Chengdu, China], FN3 medium-speed chromatography paper
(Beijing Worthful Technology Co. Ltd., Beijing, China), ER ELISA
kit (Shanghai Yansheng Industrial Co., Ltd., Shanghai, China),
propidium lodide (Shang Hai Haoran Biological Technology Co., Ltd.,
Shanghai, China), Annexin V-FITC apoptosis detection kit
(Becton-Dickinson, Franklin Lakes, NJ, USA), SephadexG15 (Pharmacia
Company), RMPI-1640 and serum-free medium (HyClone) were used in
the experiments. MTT, DMSO, 100% ethanol, and PBS were all obtained
in China.
Main instruments and equipment. The following
equipment was used for the experiments: a microbench (SW-CJ-1F;
Suzhou Jiangdong Precision Istrument Co., Ltd., Suzhou, China), a
desk centrifuge (KA-1000/TGL-16G; Shanghai Precision Istrument Co.,
Ltd., Shangai, China), a constant temperature oven (MIR160; Sanyo,
Osaka, Japan), an electronic balance (Librorael-200; Shimadzu
Corporation, Kyoto, Japan), a vacuum desiccator (Christ/Alpha1-2;
Marin Christ, Osterode, Germany), a carbon dioxide incubator
(Thermo Scientific Forma CO2; Thermo Scientific Fisher,
Waltham, MA, USA), a liquid mass spectrometer (Agilent Technologies
6410, Triple Quad LC/MS; Agilent Technologies, Santa Clara, CA,
USA), a gamma counter (SN-684; Shanghai Hesuo Rihuan Photoelectric
Istrument Co., Ltd., Shangai, China), a radioactivity detector
(RM905a; Furui Hengchuang Technology Co., Ltd., Beijing, China), a
microvortex mixer (VXH-3; Shanghai Huxi Analysis Istrument Factory
Co., Ltd., Shangai, China), and an ultraviolet spectrophotometer
(UV-265; Shimadzu Corporation).
Cell lines and animals. MCF-7 and MDA-MB-231 cells
were obtained from the Typical Culture Preservation Committee Cell
Bank of the Chinese Academy of Sciences. Female Balb/c nude mice
(21 days old) were acquired from the Laboratory Animal Center at
Chongqing Medical University.
Experimental procedures
Fulvestrant radio-iodination using an
improved chloramine T (Ch-T) method
Step one: In a common EP tube, 50 µl fulvestrant was
mixed with 100 µl chloramine T. Then, 1 mCi Na131I was
added to the tube, which was mixed via rapid oscillation and
incubated at room temperature for 5 min. Step two: Sodium
metabisulfite (200 µl) was added to the tube to terminate the
iodination reaction. Referring to the methods described by Wang
et al (21), the reaction
was incubated at room temperature at pH 7.5 for 5 min and repeated
5 times.
Labeling rate detection by paper
chromatography
Chromatography paper was cut into a 1×20-cm section
and longitudinally folded. After centrifugation, the supernatant
and Na131I solution were suctioned using a capillary
tube until 1 cm of liquid remained. After drying, the bottom of the
paper was placed in ethanol approximately 0.5 cm deep and covered
with a 2000-ml beaker. The chromatography paper was removed when
the front edge of the spreading agent reached 10 cm from the sample
point. After drying the chromatography paper at 37°C in an oven,
the paper was cut into 0.5-cm strips beginning at 1 cm from the
bottom. The radioactivity (cpm) of the paper strips was detected by
γ counter, and the labeling rate was determined from the following
equation:
Labeling rate=Radioactivity of the
marker's paperRadioactivity of the all paper strips×100%
Separation and purification using
molecular sieve chromatography
Sephadex G15 dextran gel was used as the solid
phase, and 100% ethanol was the eluent. The outflow rate was 0.5
ml/min, and 10 drops of effluent were collected per test tube. The
radioactivity (cpm) of the effluent was immediately detected, and
the effluent with the highest radioactivity was defined as tube
A.
Detection of
131I-fulvestrant using mass spectrography
Fulvestrant powder (1 mg) was dissolved in 500 µl
methanol solution, and 20 µl of the solution was removed to detect
unlabeled fulvestrant. Another 50 µl of the fulvestrant solution
was removed after the iodination reaction, supplemented with pure
water, and centrifuged at 15000 rpm. The supernatant was discarded,
and 200 µl methanol was added to dissolve the precipitate. Then, 20
µl of this solution was used for mass spectrography.
131I-fulvestrant stability
detection by paper chromatography
Samples of tube A were collected at 24, 48, 72, 96,
and 120 h after incubation at room temperature and were assessed
using paper chromatography. The paper was cut into 1-cm strips, and
the radioactivity (cpm) of these fragments was detected. The
Na131I and original reactive solution groups were
established as control groups, and the radiochemical purity of all
the samples was calculated concurrently using the following
equation:
Radiochemical purity=Radioactivity of the
labeled compoundTotal radioactivity×100%
Detection of
131I-fulvestrant binding to ER using the cell binding
assay
ER+ MCF-7 cells were routinely cultured
on a 24-well plate, and the culture medium was removed when growth
reached approximately 50% confluency. Afterward, 1 ml serum-free
medium was added to each well with 6 different concentrations (0.5,
1, 2, 4, 8, and 16 µCi) of 131I-fulvestrant, and each
concentration was tested in triplicate. After the cells were
cultured for 24 h, the culture medium and cells were collected in
order based on the concentration added, and radioactivity was
detected.
Growth inhibition of
131I-fulvestrant-treated MCF-7 and MDA-MB-231 cells as
assessed via MTT assays
Group A: MCF-7 cells (1000 per well) were seeded
into four 96-well plates. Four different concentrations of
131I-fulvestrant (10, 20, 40, and 80 µCi) were added to
each well in triplicate. A blank control and negative control
treatment were also included on each plate, and one 96-well plate
was removed every 24 h to measure cell proliferation. Group B:
MDA-MB-231 cells underwent the same treatments as described for
group A. Group C: MCF-7 cells (5×105 cells in 4 ml) were
evenly aliquoted into 4 sterile centrifuge tubes. First, 0, 1, 3,
or 7 ml of serum-free medium was added to the tubes followed by the
addition of 0, 20, 40, or 80 µCi of 131I-fulvestrant.
After 1 h, the cells were centrifuged at 1000 rpm, and the
supernatant was removed. The cells were then washed with PBS,
resuspended in 2.4 ml ordinary culture medium and measured as
described for group A. Group D: MDA-MB-231 cells underwent the same
treatments as described for group C.
Coloration: One 96-well plate was removed every 24
h, and 20 µl MTT solution (5 mg/ml) was added to each well. After a
4 h of incubation, the culture medium was gently removed, and 150
µl DMSO was added to each well. The plate was then placed on a
shaker for 10 min. The absorbance (A) at 490 nm was measured using
an enzyme-linked immunometric meter. Blank wells were set as zero,
and the negative control group was established to calculate the
inhibition rate as follows:
Inhibitionrate(%)=(1-A490of experimental
groupA490of blank group)×100%
Growth inhibition of
131I-fulvestrant-treated MCF-7 cells as assessed via
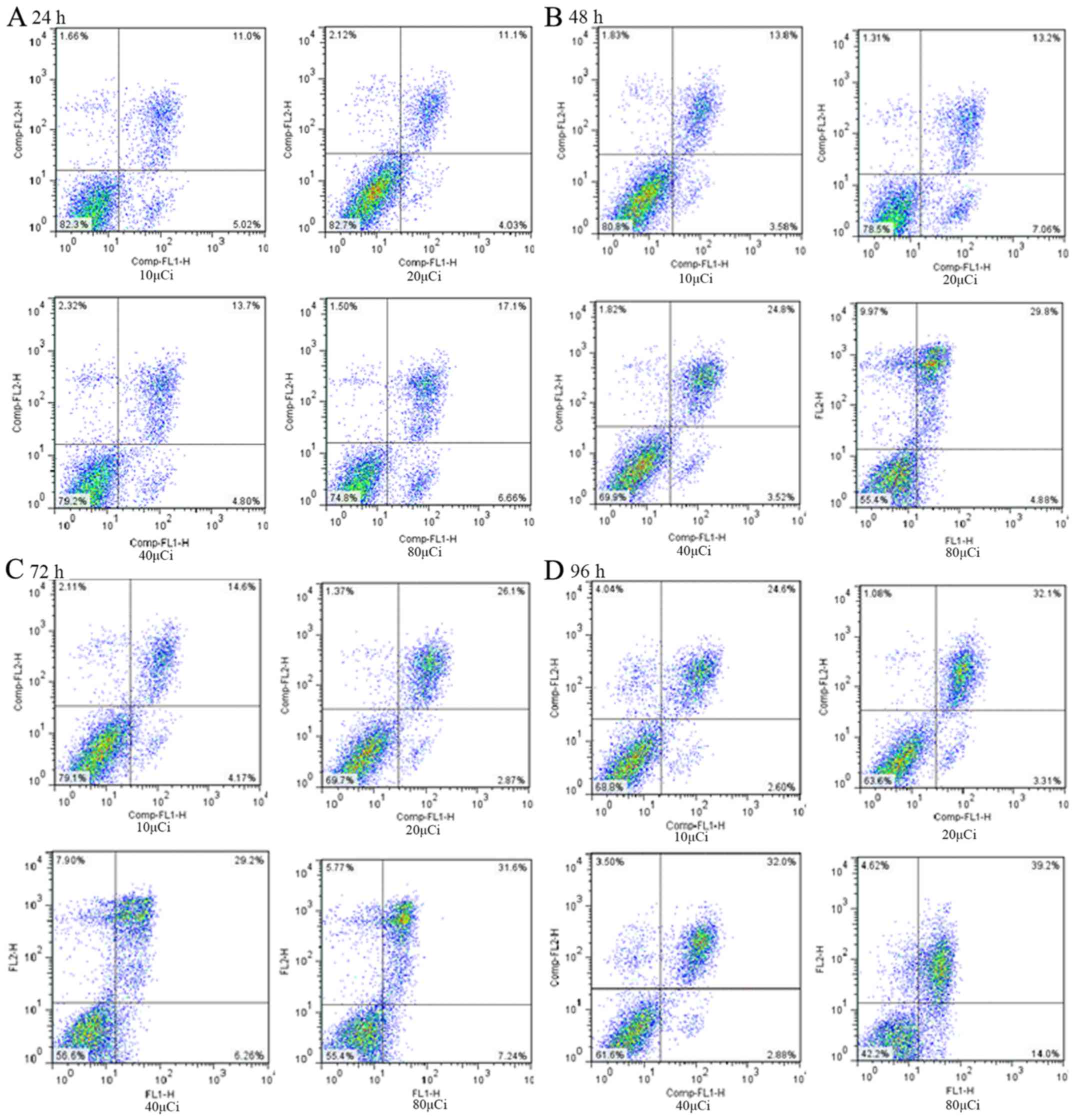
flow cytometry
MCF-7 cells were treated with
131I-fulvestrant at different concentrations (10, 20,
40, 80 µCi) and tested at 24, 48, 72 and 96 h. The negative control
group and the blank control group were analyzed after dyeing with
Annexin V-FITC and propidium iodide.
Establishment of MCF-7 cell xenografts
(22,23)
A single-cell suspension of MCF-7 cells (0.5 ml,
2×106/ml) was subcutaneously injected into the right
shoulders of 21-day-old female Balb/c nude mice. These nude mice
were maintained in an independent ventilation cage (IVC) system
within a clean level barrier animal facility.
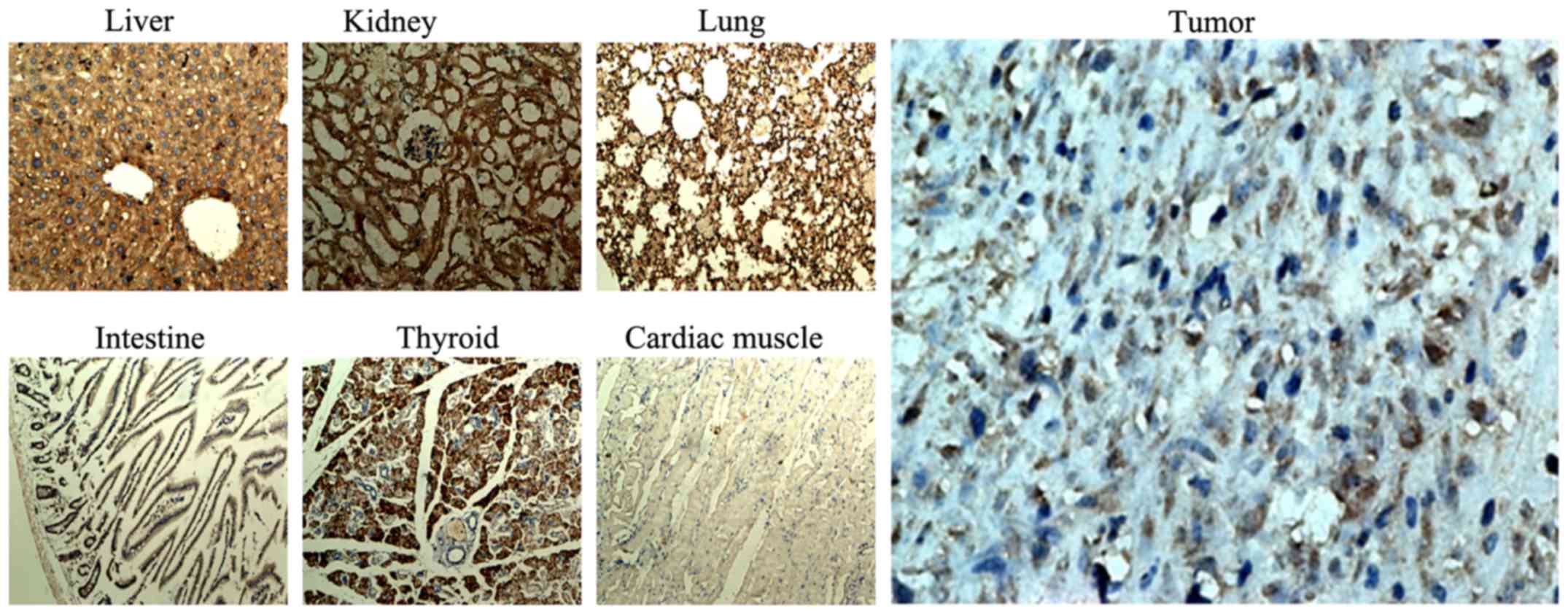
Distribution of ER in critical organs
and xenografts of nude mice detected by immunohistochemistry
Three female xenograft nude mice without any other
intervention were euthanized after inoculation with MCF-7 cells for
5 weeks, by which time xenografts had formed. Critical organs and
xenografts were immediately removed and fixed in 10% formaldehyde.
Referring to the methods described by Nishihara et al
(24), immunohistochemistry was
used to detect the ER distribution in these tissues.
Distribution of radionuclides in vivo and
the effects of intravenous administration of
131I-fulvestrant on xenograft tumors and organs
Whole-body ECT imaging of xenografts
and radioactivity in organs
One hundred microliters of 200 µCi
131I-fulvestrant was intravenously injected into mice.
After 1 h, the mice were administered general anesthesia followed
by ECT scanning with a detection time set at 300 sec. Two hours
later, 1 ml blood was collected retro-orbitally. The nude mice were
then euthanized, and their critical organs were immediately removed
and weighed. Radioactivity of the blood and organs was
detected.
Changes in xenograft tumors and organs
after intravenous injection of 131I-fulvestrant
Nine nude mice with xenografts were randomly divided
into 3 groups, groups E-G, and the volume (length × width × height)
of the xenografts was measured through the surface of the body. The
mice received intravenous injections of 200 µCi
131I-fulvestrant dissolved in 67% ethanol (group E,
approximately 50 µl), 400 µCi 131I-fulvestrant dissolved
in 67% ethanol (group F, approximately 100 µl) or 100 µl 67%
ethanol (group G). Tumor volumes were measured weekly, and the
general conditions of the mice were observed daily. After 4 weeks,
the mice were euthanized, and critical organs underwent H&E
staining and were observed under a light microscope.
Changes in xenograft tumors after
131I-fulvestrant injection into tumors
131I-fulvestrant (200 µCi) was injected
into the core and basal regions of tumors in 3 nude mice. After 24
h, the mice were placed under general anesthesia and subjected to
ECT scanning. After 1 week, changes in tumor size were observed,
and 1 ml blood was collected retro-orbitally. The nude mice were
then euthanized, and critical organs immediately were removed and
weighed. Radioactivity of the blood and organs was detected.
Statistical analysis
In this study, the measured data are expressed as
the mean ± standard deviation (SD), and numerical data are
expressed as rates. SPSS13.0 statistical software was used for
statistical tests. The measured data were statistically analyzed
using either the t-tests or analysis of variance, and significance
level was set at α=0.05.
Results
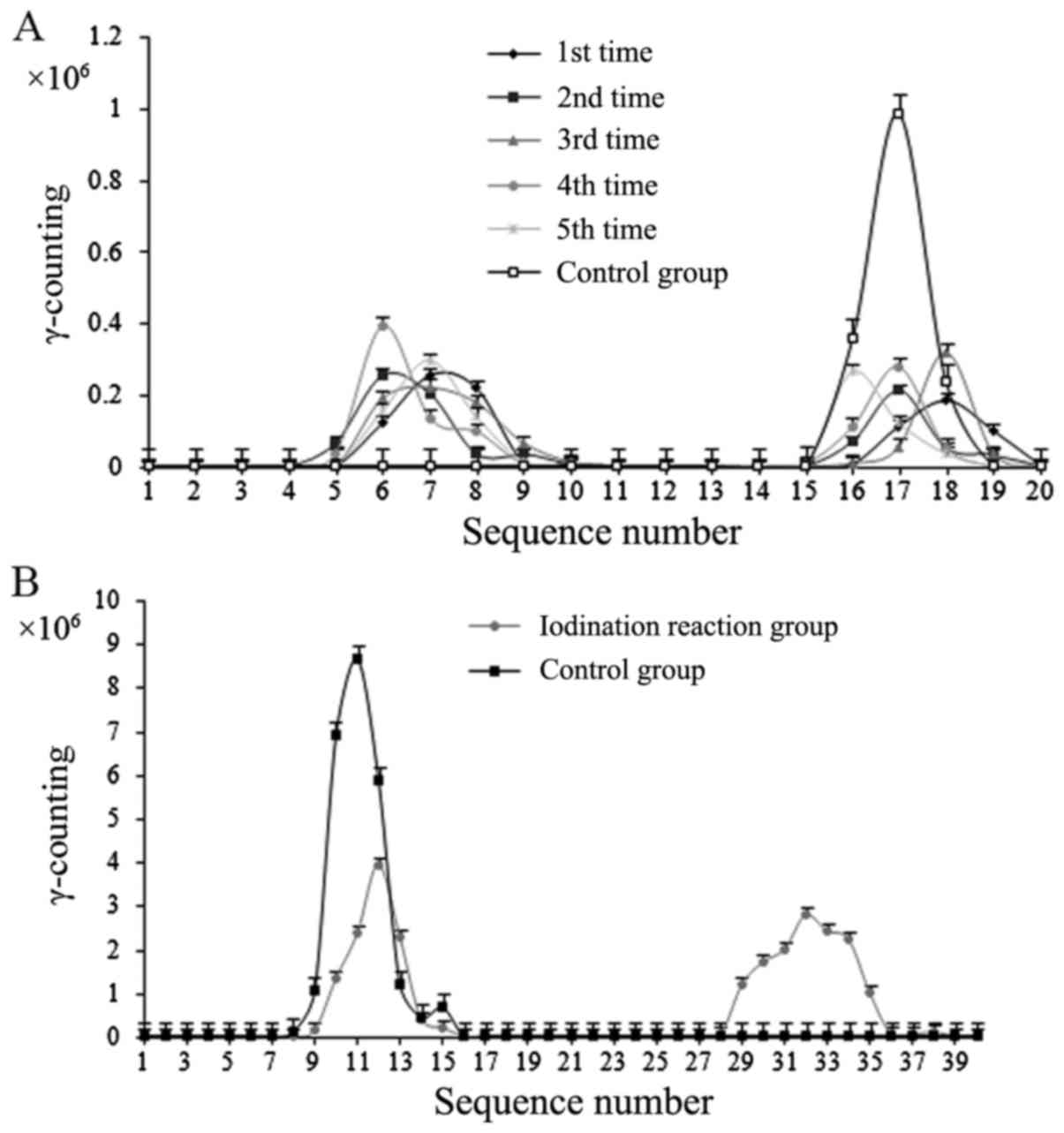
Radioactivity of the 131I
labeled compound and Na131I control groups
The iodination reaction generated a compound labeled
with 131I, and the labeling rate was 60.56±1.2%
(Fig. 1A).
Separation and purification
results
Gamma counting of the iodination reaction using the
eluate from molecular sieve chromatography revealed a double peak
(Fig. 1B). The first peak was
consistent with the position of the iodine peak of the control
group and was confirmed as iodine. The labeling rate was
62.34±1.8%, which was similar to the results of the paper
chromatography assessment. Thus, purified iodide was collected.
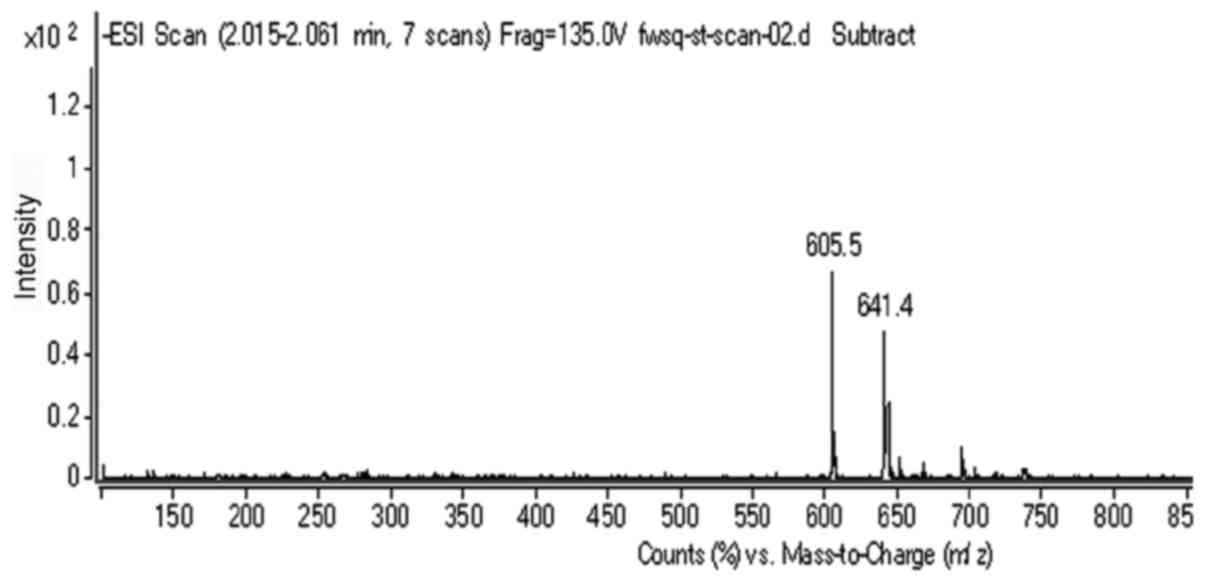
Mass spectrometry analysis using a
liquid mass spectrometer
The molecular weight of 131I is 131
(Fig. 2), and 131I
showed a peak at 130.9 (which was knocked down from
131I-fulvestrant). The molecular weight of fulvestrant
is 606.77, and fulvestrant showed a peak at 605.5. Additionally,
131I-fulvestrant presented a peak at 737.7. Because of
the negative ion detection, one hydrogen atom should be added to
the molecular weight, thus yielding 737.4 - 606.77 + 1 = 131.93. It
can be inferred that an iodide atom was successfully attached to
fulvestrant after the iodination reaction.
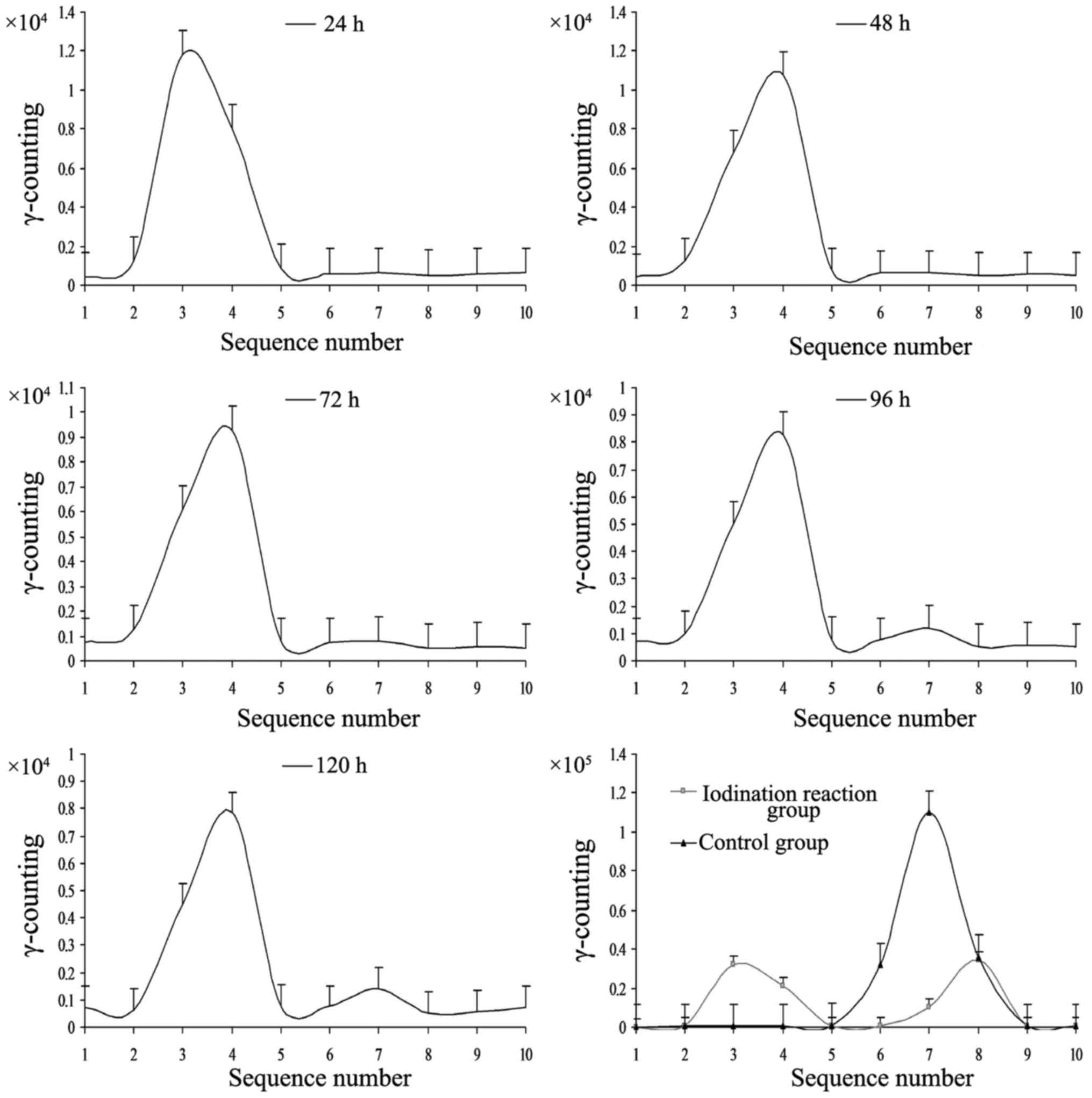
Detection of
131I-fulvestrant stability
The iodination reaction products at 24, 48, and 72 h
(Fig. 3) showed a single peak.
There was a weak second peak at 96 h, which was increased at 120 h.
The first peak in the control groups (Na131I group and
the original reactive solution group) corresponded to stable
131I-fulvestrant, and the second peak corresponded to
iodine (131I). The second peak appeared in the range of
iodine in the chromatographic band, similar to the band observed in
the control group (Na131I). These data indicate that
131I-fulvestrant was stable for 72 h and began to decay
at 96 h, which suggests that 131I-fulvestrant maintains
a stable chemical structure for 120 h after generation.
Radiochemical purity greater than 95% within 96 h after the
iodination reaction revealed that 131I-fulvestrant was
stable (Table I).
 | Table I.Radiochemical purity testing results
(n=3). |
Table I.
Radiochemical purity testing results
(n=3).
|
| 24 h | 48 h | 72 h | 96 h | 120 h |
|---|
| Radiochemical
purity (%) | 98.65±3.43 | 97.24±2.98 | 96.68±3.12 | 95.52±3.36 | 93.47±4.32 |
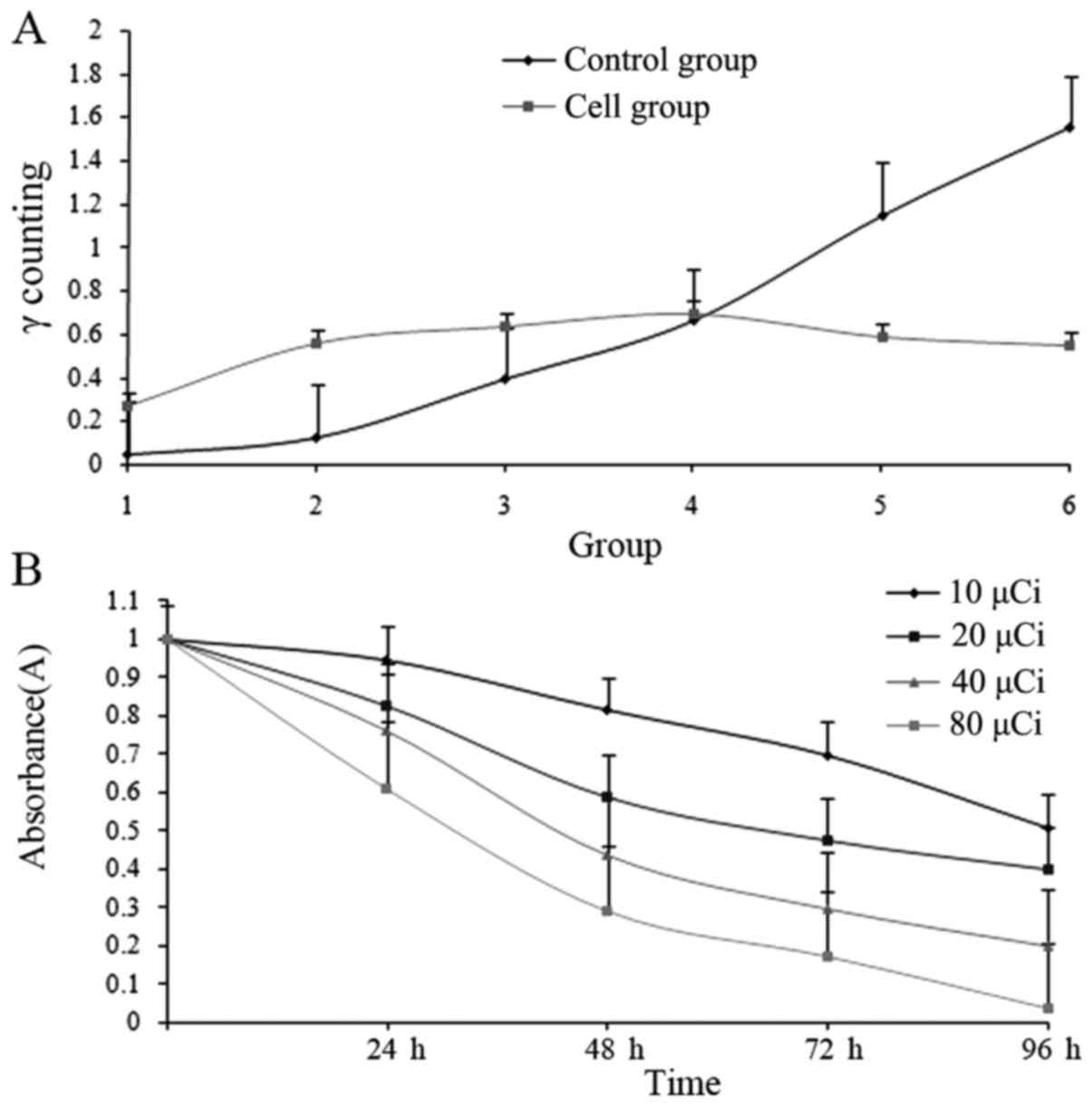
Binding affinity of
131I-fulvestrant in ER+ MCF-7 cells
131I-fulvestrant can bind to
ER+ MCF-7 cells, but the binding affinity did not
increase with increasing concentrations of
131I-fulvestrant (Fig.
4A); rather, the binding peaked and saturated at a dose of 4
µCi. In addition, the amount of bound ligand slightly decreased
with increasing doses. There was no significant difference between
these two groups (F=9.03, P=0.12).
Effect of 131I-fulvestrant
on growth inhibition of ER+ MCF-7 cells
The survival of ER+ MCF-7 cells was
assessed at various time points after treatment with different
doses of 131I-fulvestrant (Fig. 4B). There were significant
differences among all the groups (F=14.02, P=0.00), which indicated
that with increasing radiation doses and incubation times, the
number of surviving cells decreased, thus reflecting a marked
inhibitory effect. In addition, none of the groups reached a
measurable half maximal inhibitory concentration (IC50)
at 24 h, whereas at 48, 72, and 96 h, the IC50 values
were 35, 18, and 13 µCi, respectively.
Inhibitory effects of different
concentrations of 131I-fulvestrant on the growth of
human breast cancer cells
In Table II, there
was a significant difference between each concentration (P<0.05)
within groups A and B, reflecting marked inhibition, but there was
no significant difference between groups A and B (P>0.05). Group
C showed weaker inhibition with persistent
131I-fulvestrant treatment than groups A and B. After 1
h, 131I-fulvestrant exposure to MDA-MB-231 cells was
halted in group D, the radiation damage was terminated, and growth
inhibition at each concentration was diminished and did not reach
the IC50 values. However, there were significant
differences in group D compared with groups A-C.
 | Table II.Inhibition of
131I-fulvestrant on the growth of MCF-7 and MDA-MB-231
cells (mean ± SD, n=3). |
Table II.
Inhibition of
131I-fulvestrant on the growth of MCF-7 and MDA-MB-231
cells (mean ± SD, n=3).
| Group | 10 µCi
(absorbance) | 20 µCi
(absorbance) | 40 µCi
(absorbance) | 80 µCi
(absorbance) |
|---|
| Group A |
| 24
h |
0.945±0.062 |
0.813±0.045 |
0.696±0.056 |
0.509±0.046 |
| 48
h |
0.826±0.048 |
0.586±0.037 |
0.473±0.034 |
0.396±0.019 |
| 72
h |
0.763±0.033 |
0.437±0.026 |
0.297±0.042 |
0.197±0.025 |
| 96
h |
0.612±0.042 |
0.288±0.017 |
0.169±0.009 |
0.033±0.002 |
| Group B |
| 24
h |
0.965±0.012 |
0.828±0.017 |
0.736±0.062 |
0.582±0.035 |
| 48
h |
0.876±0.023 |
0.643±0.025 |
0.547±0.057 |
0.369±0.028 |
| 72
h |
0.812±0.048 |
0.512±0.067 |
0.435±0.074 |
0.235±0.032 |
| 96
h |
0.824±0.032 |
0.303±0.054 |
0.281±0.046 |
0.068±0.017 |
| Group C |
| 24
h |
0.954±0.021 |
0.908±0.025 |
0.832±0.031 |
0.795±0.025a |
| 48
h |
0.882±0.032 |
0.833±0.019 |
0.667±0.014 |
0.626±0.017a |
| 72
h |
0.816±0.043 |
0.676±0.017a |
0.553±0.018a |
0.525±0.024a |
| 96
h |
0.723±0.018 |
0.512±0.026a |
0.418±0.022a |
0.391±0.019a |
| Group D |
| 24
h |
0.972±0.022 |
0.965±0.015 |
0.943±0.011 |
0.835±0.023 |
| 48
h |
0.956±0.019 |
0.934±0.017 |
0.876±0.024 |
0.768±0.022 |
| 72
h |
0.923±0.056 |
0.907±0.025 |
0.828±0.016 |
0.742±0.016 |
| 96
h |
0.908±0.025 |
0.862±0.029 |
0.811±0.025 |
0.715±0.031 |
Results of growth inhibition of
131I-fulvestrant-treated MCF-7 cells as assessed via
flow cytometry
After treating with different concentrations of
131I-fulvestrant (10, 20, 40, and 80 µCi), the apoptosis
rates of MCF-7 cells were detected at 24, 48, 72 and 96 h. The
results are shown in Fig. 5. Flow
cytometry revealed that the apoptosis rate and the cell necrosis
rate increased with increasing doses and durations of
131I-fulvestrant treatment. A significant dose-time
dependence was found, which was consistent with the MTT assay. The
mechanism included both induction of apoptosis and cell necrosis
caused by cytotoxicity.
Animal model
Of the 15 nude mice inoculated, 14 successfully grew
xenografts after 4–6 weeks, which corresponds to a one-time success
rate of 93.33%. The lone nude mouse that did not grow a xenograft
was inoculated with 2×106/ml MCF-7 cells a second time,
and xenografts were successfully detected 4 weeks later. Xenografts
were used for experiments when their diameter reached approximately
1 cm.
Results of the distribution of ERs in
critical organs and xenografts of nude mice under light
microscopy
Immunohistochemical staining of critical organs and
xenografts was carried out (Fig.
6). As shown in the Fig. 6, the
positive rate and the strong positive rate of ER-α in the
xenografts, liver, lung, thyroid and kidney were high, while they
were low in cardiac muscle and small intestine. The positive rate
and the strong positive rate were analyzed by IPP 6.0 software, and
the results are shown in Table
III.
 | Table III.ER-α expression in xenografts in nude
mice and critical organs (mean ± SD, n=3). |
Table III.
ER-α expression in xenografts in nude
mice and critical organs (mean ± SD, n=3).
| Organs | Positive rate
(%) | Strong positive
rate (%) |
|---|
| Livera |
74.14±7.52 |
46.86±5.21 |
|
Xenograftsa |
68.33±6.45 |
44.57±5.37 |
|
Thyroida |
54.76±4.88 |
41.45±2.32 |
| Kidneya |
64.45±5.76 |
40.46±2.79 |
| Lunga |
68.23±3.79 |
43.65±2.18 |
| Small
intestineb |
27.24±2.12 |
5.24±0.46 |
| Heartb |
22.25±1.58 |
8.66±0.67 |
There was no significant difference in the positive
rate of ER-α between each organ within group E (P>0.05), and
there was a significant difference in the positive rate of ER-α
between groups E and F (P<0.05). The in vivo expression
of ER-α in nude mice with MCF-7 cell xenografts was not
significantly different than the ER expression of MCF-7 cells in
vitro. Thus, ER-α was expressed in all of the detected tissues;
the highest expression was observed in liver and the lowest in
small intestine.
Radionuclide distribution in vitro
after 131I-fulvestrant intravenous injection
At 2 h after intravenous injection of
131I-fulvestrant, radioactivity in the blood peaked and
corresponded to 20.76±2.54%, whereas the percentage of
radioactivity in the remaining organs was low (Table IV).
 | Table IV.Radioactivity of important organs
in vitro (% ID/g, mean ± SD, n=3). |
Table IV.
Radioactivity of important organs
in vitro (% ID/g, mean ± SD, n=3).
| Organs | Organ weight
(g) | Radioactivity
concentration (µCi/g) | Total radioactivity
(µCi) | Total radioactivity
rate (%) |
|---|
| Tumor |
1.15±0.16 |
5.79±0.31 |
6.67±0.42 |
4.33±0.28 |
| Liver |
2.8±0.22 |
4.74±0.52 |
13.28±1.15 |
8.62±0.47 |
| Blood |
1.2±0.04 |
26.64±2.88 |
31.97±3.11 |
20.76±2.54 |
| Kidney |
0.61±0.04 |
3.48±0.22 |
2.13±0.16 |
1.38±0.12 |
| Heart |
0.42±0.03 |
4.54±0.43 |
1.91±0.25 |
1.64±0.16 |
| Lungs |
1.26±0.02 |
3.08±0.27 |
3.88±0.28 |
2.52±0.21 |
| Small
intestine |
1.68±0.12 |
5.16±0.52 |
8.67±0.65 |
3.63±0.43 |
| Thyroid |
0.18±0.01 |
4.02±0.33 |
0.72±0.08 |
1.47±0.16 |
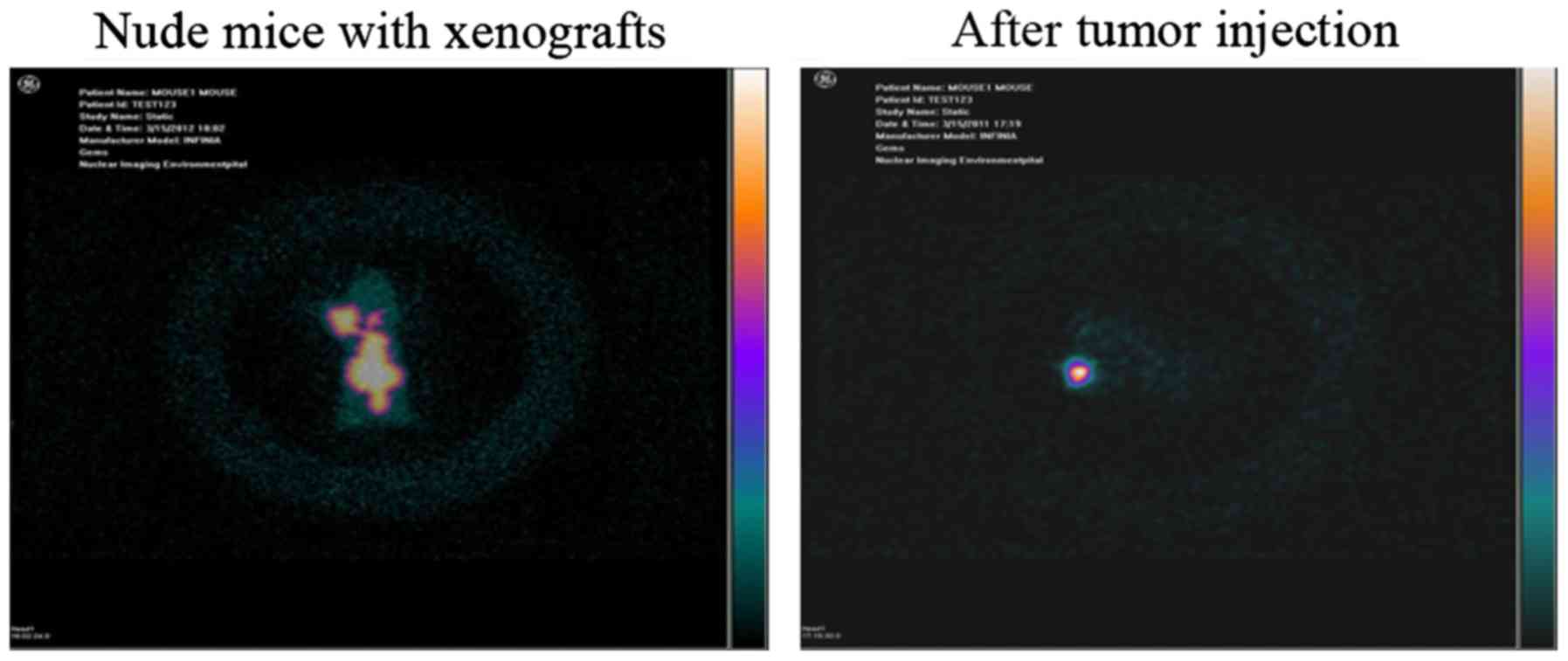
ECT whole-body imaging of nude mice
with xenografts
Radionuclide imaging was observed in tumors
(Fig. 7, left), and radioactivity
in the chest and abdominal organs was also found. However, no
apparent activity in the thyroid was observed.
Changes in xenografts and organs after intravenous
injection of 131I-fulvestrant. In Table V, the tumor volume in group E
gradually reduced within 2 weeks after 131I-fulvestrant
injection, but the volume recovered during the 3rd week. The tumor
volume in group F was gradually reduced within 3 weeks after
injection but began to increase during the 4th week. However, group
G maintained continuously increasing tumor growth.
 | Table V.Changes in tumor volume after
injection (mean ± SD, n=3). |
Table V.
Changes in tumor volume after
injection (mean ± SD, n=3).
| Group | 0
(cm3) | 1 week
(cm3) | 2 weeks
(cm3) | 3 weeks
(cm3) | 4 weeks
(cm3) |
|---|
| Group E |
1.24±0.21a |
1.04±0.15b |
0.85±0.13b |
0.92±0.14b |
1.06±0.12b |
| Group F |
1.35±0.25a |
0.67±0.18b |
0.52±0.09b |
0.48±0.06b |
0.69±0.08b |
| Group G |
1.22±0.19a |
1.34±0.26b |
1.49±0.31b |
1.62±0.35b |
1.85±0.38b |
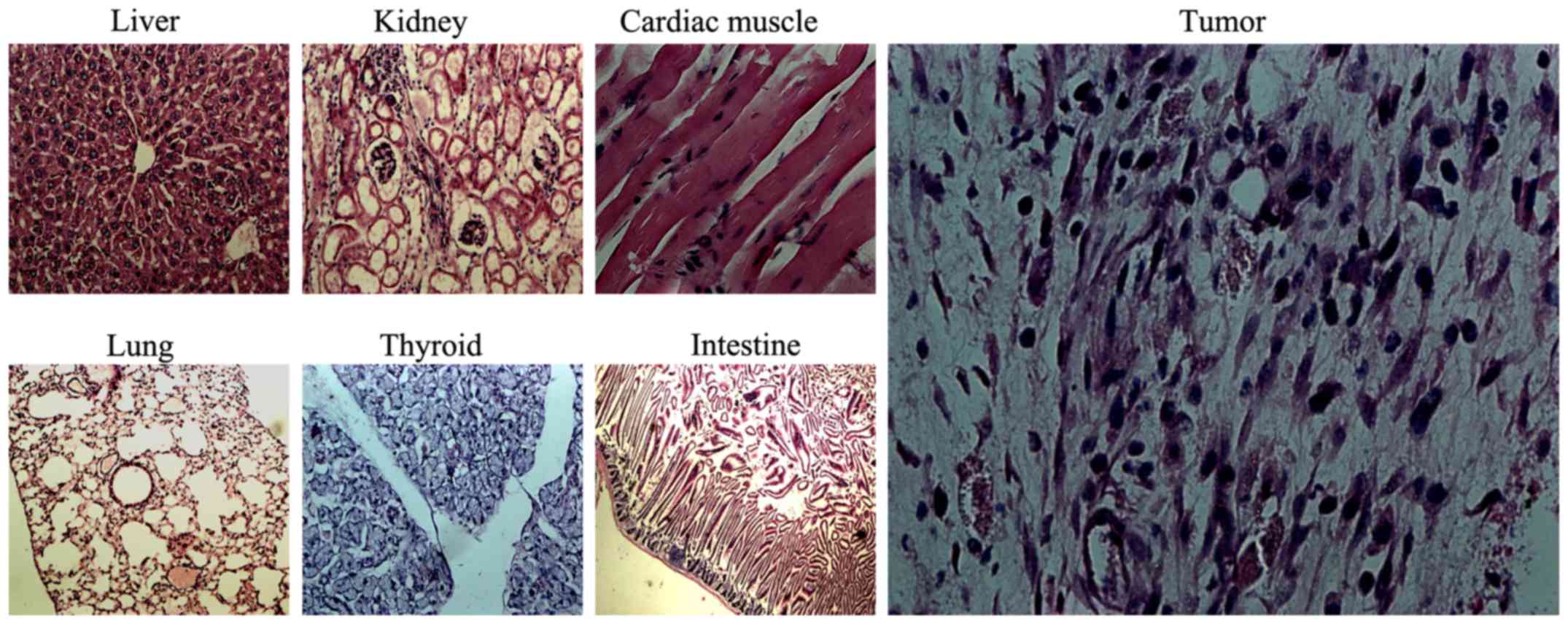
General conditions and morphological
changes in critical organs under light microscopy
Nude mice in all the groups tolerated the
intravenous injection and survived. Mice in groups E and F
exhibited anorexia and reduced activity the day after injection but
returned the normal after approximately 3 days. No abnormal
behaviors were observed in the mice in group G. After 4 weeks,
H&E staining of the xenografts and organs was performed. The
liver, lung, kidney, thyroid, heart, small intestine and other
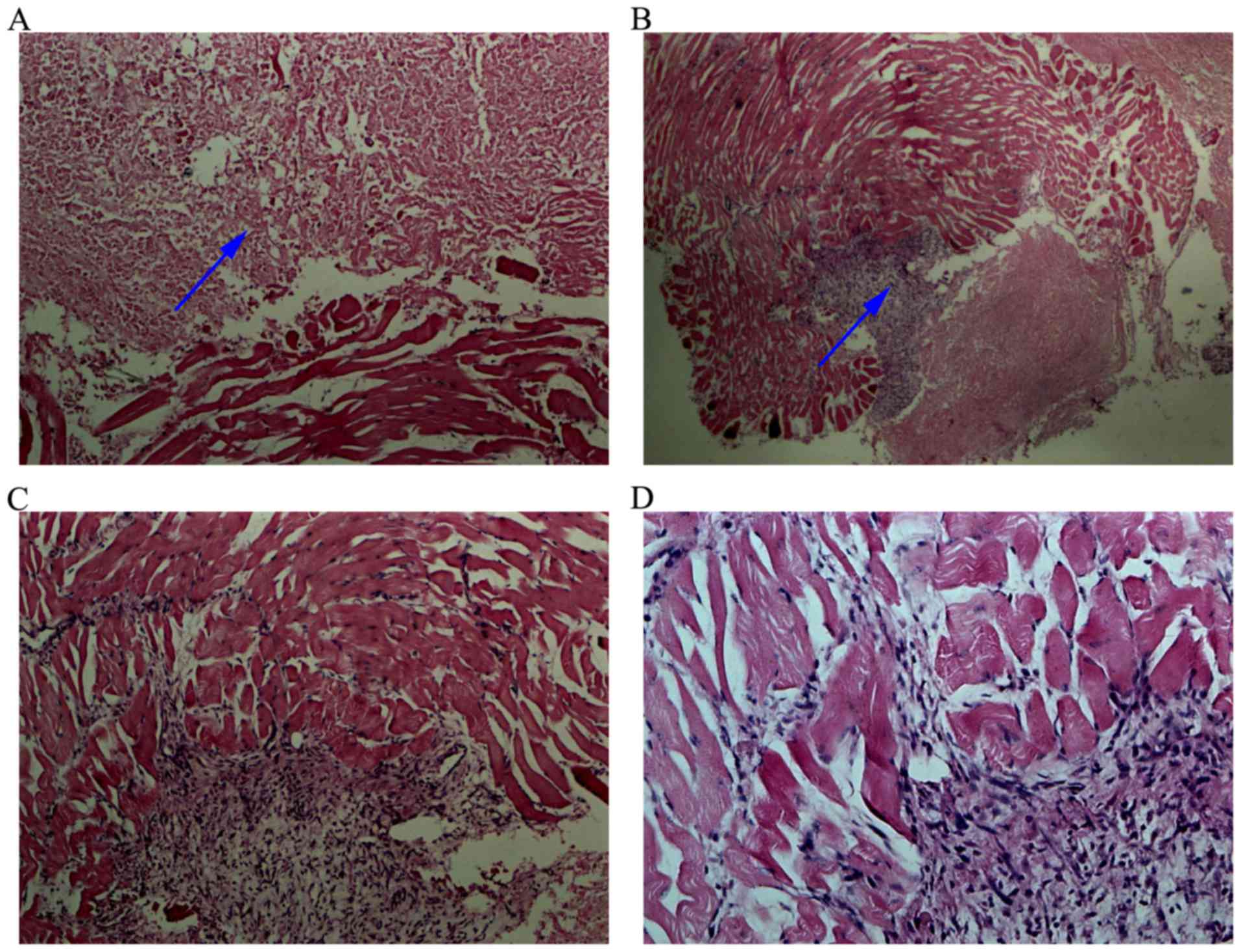
tissues did not exhibit obvious damage (Fig. 8), but the tumor tissue presented
necrosis.
Xenograft changes and ECT imaging results
after 131I-fulvestrant injection into tumors
Xenograft changes
After 131I-fulvestrant injection into the
tumor site, 3 nude mice showed good tolerance with no significant
changes in their general condition. Massive necrosis was observed
in the MCF-7 cell xenografts, which indicated the effect of
131I-fulvestrant against the tumors.
At 72 h after injection, the nude mice were
sacrificed, and the xenografts were observed via H&E staining.
The large-area tumor nuclei had disappeared (Fig. 9), cell morphology was altered, and
the xenografts appeared as amorphous homogeneous red tissues.
However, a few surviving MCF-7 cells were found in normal adjacent
tissue.
ECT whole-body imaging of nude mice with xenografts
after tumor injection. At 24 h after 131I-fulvestrant
injection at the tumor site, ECT scans showed that the radionuclide
was primarily confined to the tumor site (Fig. 7, right) and did not spread to other
parts of the body.
Discussion
To achieve targeted radiotherapy of malignant
tumors, many new radiotherapies have been explored, including
conformal radiotherapy, administration of radioactive particles,
radioactive iodine injection and monoclonal antibody technology.
Among these, the most encouraging approach has been 131I
radiation therapy for differentiated thyroid carcinoma. In this
approach, tumor cells actively absorb radioactive iodine, thus
achieving targeted radiotherapy. In addition, 131I
primarily relies on beta rays to induce radiotherapy with a range
of millimeters. Therefore, 131I radiation therapy
induces little damage to surrounding tissues while simultaneously
overcoming the ‘cross fire’ radiotherapy of tumor cells, which is
derived from heterogeneity in tumors that do not express ERs
(2–5).
This study aimed to incorporate 131I into
breast cancer treatments. Under normal conditions in vivo,
the specific absorption and binding affinity of 131I
have been exclusively studied in the thyroid. Therefore,
131I must be bound to an appropriate carrier. An
improved chloramine T method was used in this study based on
chemical synthesis, which is a widely accepted approach for
radioiodine labeling of proteins, polypeptides and other
macromolecules. This study aimed to attach 131I to
fulvestrant, a small organic molecule with a weight of 606.77 and
poor water solubility but high ethanol solubility. However,
chloramine T and sodium metabisulfite have high water solubility
but poor ethanol solubility. Thus, radio-iodination cannot be
implemented using the traditional chloramine T method because all
the substances are aqueous, and thus, a modified chloramine T
method was implemented. Fulvestrant was dissolved in ethanol, and
chloramine T and sodium metabisulfite were dissolved in 50%
ethanol. Therefore, the final ethanol concentration in the
iodination reaction system was 70%. This not only ensures the
dissolution of all the substances but also allows chloramine T to
release perchlorate radicals into the water to oxidize iodide to
atomic iodine.
This study showed that stable labeling of
fulvestrant with 131I could be implemented using an
improved chloramine T method. The radiochemical yield of
radioiodine labeling to fulvestrant was 62.34±1.8%, which was
detected using paper chromatography and molecular sieve
chromatography. 131I-fulvestrant had a stable chemical
structure as reflected by the results that the iodine moiety did
not dissociate from fulvestrant 72 h after labeling and was
subsequently released slowly. The results of the cell binding assay
demonstrated that 131I-fulvestrant could also interact
with estrogen-dependent breast cancer cells and exhibited
saturation, consistent with traditional ligand-receptor binding
theory. Increasing doses slightly decreased the amount of bound
ligand, which we interpreted as increased radiation damage to
breast cancer cells with higher radiation doses. It should further
be elucidated whether radiation damage in breast cancer cells is
related to decreased expression of estrogen at the molecular
level.
In breast cancer, ER expression is an important
marker of prognosis and primarily serves as a predictor of patient
responses to endocrine therapy, individuals who have ER+
tumors are more likely to exhibit a better response and prognosis
than those with ER− tumors (25). Human breast cancer MCF-7 cells
(ER+, estrogen receptor-positive) and MDA-MB-231 cells
(ER−, estrogen receptor-negative) were selected for this
investigation, because these two cell lines represent
ER+ and ER− breast cancer cells. Thus, we
selected these two cell lines for this experiment. Several studies
have shown that MCF-7 cells either overexpress or can induce
overexpression of ER-α (26–29).
Breast cancer cells express the NIS (30), which uses the transmembrane sodium
ion concentration gradient as the primary driving force to
transport iodine into cells (31–33).
Therefore, this protein provides a new target for improving
radioiodine treatment of breast cancer.
The results of the MTT assay showed that the growth
of these two types of breast cancer cells was inhibited by
131I-fulvestrant. Transient contact experiments showed
that the growth inhibition of breast cancer cells was decreased,
and this effect was significantly more pronounced in MCF-7 cells
than in MDA-MB-231 cells. Provided that a specific dose of
radiation is present in the culture medium, we suggest that MCF-7
and MDA-MB-231 cells will be subjected to radiation damage and
growth inhibition regardless of ER-α expression status. When the
radioactive substance was removed, MDA-MB-231 cells were completely
unaffected by the radioactive environment due to their inability to
absorb 131I-fulvestrant. However,
131I-fulvestrant remained bound to ERs in MCF-7 cells
and, thus, continued to cause radiation damage. This effect by
which 131I-fulvestrant induces tumor cell death
represents a combination of radiotherapy and endocrine therapy.
MCF-7 cells either overexpress or can induce the
overexpression of ER-α (26–29);
concurrent with this, ER-α has a characteristically wide
distribution throughout the body (34–36).
Fulvestrant has fewer side effects and is regarded as a pure
endocrine therapy-based antagonist of breast cancer. Studies have
shown that fulvestrant can even downregulate ER expression;
however, the mechanism of this activity is unclear (15,18,20).
After intravenous injection of
131I-fulvestrant, the growth of the xenografts in nude
mice was first reduced but later restored, which suggests that
growth inhibition by 131I-fulvestrant is due to a
combination of radiotherapy and endocrine therapy. Growth recovery
was subsequently shown to be related to the decrease in drug
concentration in vivo, including the decay of radioiodine
and the biological metabolism of fulvestrant. Nude mice with
xenografts initially presented anorexia and reduced activity
following 131I fulvestrant injection. Tumor exhibited
growth inhibition and tissue necrosis, but no significant organ
damage was found upon morphological examination. This dichotomy is
due to the fact that tumor cells and normal cells have different
sensitivities to radiation, as tumor cells are more susceptible to
radiation damage and have an inferior damage response compared to
normal tissue cells.
Massive necrosis occurred in the tumors after
131I-fulvestrant injection to the tumor site, and only a
few residual tumor cells were found in normal adjacent tissues. ECT
scanning showed that the radionuclide was primarily localized to
the tumor site and did not spread to other parts of the body. It is
understood that 131I-fulvestrant maintains the poor
aqueous solubility of fulvestrant. Thus, after local injection,
131I-fulvestrant locally precipitated into crystals,
which resulted in a high dose of localized radiation that could
maximize its ability to kill tumor cells. In addition, although the
crystalline solid cannot enter blood circulation, it is still
possible to change its physical location in the tissue space.
Therefore, 131I-fulvestrant can be used to fight tumor
cells over a larger local range. However, ECT scanning cannot be
set to a different time point, and the purpose of this experiment
was to verify the effects of 131I-fulvestrant. Once
injected, 131I-fulvestrant is slowly metabolized, and
nude mice cannot tolerate a repeated injection. We plan to continue
to investigate the in vivo pharmacokinetics of
131I-fulvestrant on larger experimental animals
(rabbits) in future studies.
Our goal was to prove that fulvestrant is stable
and is able to kill tumor cells. Adding fulvestrant to the cell
culture medium will produce a precipitate. Fulvestrant inhibited
the growth of tumor cells, in contrast to the active killing effect
of 131I fulvestrant on tumors. Thus, we did not set
fulvestrant as a control group. The lack of the fulvestrant control
group was a limitation of our investigation. We plan to continue to
compare fulvestrant with 131I-fulvestrant in future
investigation.
In conclusion, fulvestrant can be successfully
labeled with radioiodine using an improved chloramine T method. The
obtained product 131I-fulvestrant is chemically stable
and retains its binding affinity to estrogen-dependent breast
cancer cells. 131I-fulvestrant can precisely inhibit the
growth of estrogen-dependent breast cancer cells via the following
mechanisms: i) 131I radiation damage to MCF-7 cells via
delivery of 131I-fulvestrant; ii) binding to ER, which
blocks the tumorigenic effect of estrogen in MCF-7 cells; and iii)
downregulation of ER expression. Therefore, our investigation has
made a significative exploration concerning the use of
131I in the treatment of breast cancer, and lays the
foundation to support patients who undergo 131I
treatment.
Acknowledgements
This research was supported by the Natural Science
Foundation of Chongqing (grant no. cstc2012jjA10042) and the
Chongqing Municipal Public Health Bureau (grant no.
2011-2-175).
References
|
1
|
Pitoia F and Miyauchi A: 2015 American
thyroid association guidelines for thyroid nodules and
differentiated thyroid cancer and their implementation in various
care settings. Thyroid. 26:319–321. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Higashi T, Kudo T and Kinuya S:
Radioactive iodine (131I) therapy for differentiated
thyroid cancer in Japan: Current issues with historical review and
future perspective. Ann Nucl Med. 26:99–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sawka AM, Straus S, Gafni A, Meiyappan S,
David D, Rodin G, Brierley JD, Tsang RW, Thabane L, Rotstein L, et
al: Thyroid cancer patients' involvement in adjuvant radioactive
iodine treatment decision-making and decision regret: An
exploratory study. Support Care Cancer. 20:641–645. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haymart MR, Banerjee M, Stewart AK, Koenig
RJ, Birkmeyer JD and Griggs JJ: Use of radioactive iodine for
thyroid cancer. JAMA. 306:721–728. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tuttle RM, Rondeau G and Lee NY: A
risk-adapted approach to the use of radioactive iodine and external
beam radiation in the treatment of well-differentiated thyroid
cancer. Cancer Contr. 18:89–95. 2011. View Article : Google Scholar
|
|
6
|
Sacks W, Fung CH, Chang JT, Waxman A and
Braunstein GD: The effectiveness of radioactive iodine for
treatment of low-risk thyroid cancer: A systematic analysis of the
peer-reviewed literature from 1966 to April 2008. Thyroid.
20:1235–1245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hosseinzadeh M, Eivazi Ziaei J, Mahdavi N,
Aghajari P, Vahidi M, Fateh A and Asghari E: Risk factors for
breast cancer in Iranian women: A hospital-based case-control study
in tabriz, iran. J Breast Cancer. 17:236–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Howard-Anderson J, Ganz PA, Bower JE and
Stanton AL: Quality of life, fertility concerns, and behavioral
health outcomes in younger breast cancer survivors: A systematic
review. J Natl Cancer Inst. 104:386–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gradilone A, Raimondi C, Naso G, Silvestri
I, Repetto L, Palazzo A, Gianni W, Frati L, Cortesi E and Gazzaniga
P: How circulating tumor cells escape from multidrug resistance:
Translating molecular mechanisms in metastatic breast cancer
treatment. Am J Clin Oncol. 34:625–627. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen WJ, Wang H, Tang Y, Liu CL, Li HL and
Li WT: Multidrug resistance in breast cancer cells during
epithelial-mesenchymal transition is modulated by breast cancer
resistant protein. Chin J Cancer. 29:151–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dall G, Vieusseux J, Unsworth A, Anderson
R and Britt K: Low dose, low cost estradiol pellets can support
MCF-7 tumour growth in nude mice without bladder symptoms. J
Cancer. 6:1331–1336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang ZY, Wang MW, Zhang YP, Xu JY, Yuan HY
and Zhang YJ: The biodistribution and imaging of 16α-(18F)
fluroroestradiol (18F-FES) in rats and breast tumor-bearing nude
mice. Shanghai Medical Imaging. 20:234–238. 2011.
|
|
13
|
Turner NC, Neven P, Loibl S and Andre F:
Advances in the treatment of advanced oestrogen-receptor-positive
breast cancer. Lancet. 389:2403–2414. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dalmau E, Armengol-Alonso A, Muñoz M and
Seguí-Palmer MÁ: Current status of hormone therapy in patients with
hormone receptor positive (HR+) advanced breast cancer.
Breast. 23:710–720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
James R, Thriveni K, Krishnamoorthy L,
Deshmane V, Bapsy PP and Ramaswamy G: Clinical outcome of adjuvant
endocrine treatment according to Her-2/neu status in breast cancer.
Indian J Med Res. 133:70–75. 2011.PubMed/NCBI
|
|
16
|
Al-Mubarak M, Sacher AG, Ocana A,
Vera-Badillo F, Seruga B and Amir E: Fulvestrant for advanced
breast cancer: A meta-analysis. Cancer Treat Rev. 39:753–758. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wardell SE, Nelson ER, Chao CA and
McDonnell DP: Bazedoxifene exhibits antiestrogenic activity in
animal models of tamoxifen-resistant breast cancer: Implications
for treatment of advanced disease. Clin Cancer Res. 19:2420–2431.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mishra AK, Abrahamsson A and Dabrosin C:
Fulvestrant inhibits growth of triple negative breast cancer and
synergizes with tamoxifen in ERα positive breast cancer by
up-regulation of ERβ. Oncotarget. 7:56876–56888. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He S, Wang M, Yang Z, Zhang J and Zhang Y,
Luo J and Zhang Y: Comparison of 18F-FES, 18F-FDG, and 18F-FMISO
PET imaging probes for early prediction and monitoring of response
to endocrine therapy in a mouse xenograft model of ER-positive
breast cancer. PLoS One. 11:e01599162016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fernandes SA, Gomes GR, Siu ER,
Damas-Souza DM, Bruni-Cardoso A, Augusto TM, Lazari MF, Carvalho HF
and Porto CS: The anti-oestrogen fulvestrant (ICI 182,780) reduces
the androgen receptor expression, ERK1/2 phosphorylation and cell
proliferation in the rat ventral prostate. Int J Androl.
34:486–500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang MH, Xu YJ, Wang ZZ, Liu M, Li Z, Weng
W and Fan W: Radiolabeling of paclitaxel with 125I. J
Isotopes. 21:82–87. 2008.
|
|
22
|
Wang L, Mi C and Wang W: Establishment of
lymph node metastasis of MDA-MB-231 breast cancer model in nude
mice. Zhonghua Yi Xue Za Zhi. 95:1862–1865. 2015.(In Chinese).
PubMed/NCBI
|
|
23
|
Nofiele JT and Cheng HL: Establishment of
a lung metastatic breast tumor xenograft model in nude rats. PLoS
One. 9:e97950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishihara E, Nagayama Y, Inoue S, Hiroi H,
Muramatsu M, Yamashita S and Koji T: Ontogenetic changes in the
expression of estrogen receptor α and β in rat pituitary gland
detected by immunohistochemistry. Endocrinology. 141:615–620. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Giacinti L, Giacinti C, Gabellini C,
Rizzuto E, Lopez M and Giordano A: Scriptaid effects on breast
cancer cell lines. J Cell Physiol. 227:3426–3433. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu L, Ma H, Tang Y, Chen W, Lu Y, Guo J
and Duan JA: Discovery of estrogen receptor α modulators from
natural compounds in Si-Wu-Tang series decoctions using
estrogen-responsive MCF-7 breast cancer cells. Bioorg Med Chem
Lett. 22:154–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ko YM, Wu TY, Wu YC, Chang FR, Guh JY and
Chuang LY: Annonacin induces cell cycle-dependent growth arrest and
apoptosis in estrogen receptor-α-related pathways in MCF-7 cells. J
Ethnopharmacol. 137:1283–1290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mendoza RA, Enriquez MI, Mejia SM, Moody
EE and Thordarson G: Interactions between IGF-I, estrogen
receptor-α (ERα), and ERβ in regulating growth/apoptosis of MCF-7
human breast cancer cells. J Endocrinol. 208:1–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hong W, Chen L, Li J and Yao Z: Inhibition
of MAP kinase promotes the recruitment of corepressor SMRT by
tamoxifen-bound estrogen receptor alpha and potentiates tamoxifen
action in MCF-7 cells. Biochem Biophys Res Commun. 396:299–303.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kelkar MG, Senthilkumar K, Jadhav S, Gupta
S, Ahn BC and De A: Enhancement of human sodium iodide symporter
gene therapy for breast cancer by HDAC inhibitor mediated
transcriptional modulation. Sci Rep. 6:193412016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Renier C, Do J, Reyna-Neyra A, Foster D,
De A, Vogel H, Jeffrey SS, Tse V, Carrasco N and Wapnir I:
Regression of experimental NIS-expressing breast cancer brain
metastases in response to radioiodide/gemcitabine dual therapy.
Oncotarget. 7:54811–54824. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chatterjee S, Thaker N and De A: Combined
2-deoxy glucose and metformin improves therapeutic efficacy of
sodium-iodide symporter-mediated targeted radioiodine therapy in
breast cancer cells. Breast Cancer (Dove Med Press). 7:251–265.
2015.PubMed/NCBI
|
|
33
|
Poole VL and McCabe CJ: Iodide transport
and breast cancer. J Endocrinol. 227:R1–R12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jan KC, Ku KL, Chu YH, Hwang LS and Ho CT:
Tissue distribution and elimination of estrogenic and
anti-inflammatory catechol metabolites from sesaminol triglucoside
in rats. J Agric Food Chem. 58:7693–7700. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Younes M and Honma N: Estrogen receptor β.
Arch Pathol Lab Med. 135:63–66. 2011.PubMed/NCBI
|
|
36
|
Ur Rahman MS and Cao J: Estrogen receptors
in gastric cancer: Advances and perspectives. World J
Gastroenterol. 22:2475–2482. 2016. View Article : Google Scholar : PubMed/NCBI
|