Introduction
Lung cancer is ranked the first in terms of
morbidity among all human tumors, which also has short survival
(1). Although the treatment is
continually updated, the 5-year survival rate has not improved
significantly over the past 25 years, which is ~15%. Lung cancer
cells are resistant to chemotherapy, which is one of the major
causes short survival for patients (2). Certain factors are known to be
associated with tumor tolerance to chemotherapeutic agents. The
regulation of chemotherapeutic uptake and elimination by lung
carcinoma cells, which is mediated by membrane translocation
mechanism-related proteins (such as the P-glycoprotein and
multidrug resistance-associated proteins). In addition, other
important physiological activities of cells, such as apoptosis,
proliferation, intracellular environment abnormalities, are also
important ways to induce the occurrence of drug resistance
(2,3). The former is referred to as the
classical resistance pathway while the latter is as the atypical
pathway of drug resistance (4).
Cisplatin (CDDP) is a non-cell cycle-specific
cytotoxic drug. Its main mechanism is to form hydrates in the body
after crosslinking with DNA and replication and transcription
inhibition, thereby promoting tumor cell apoptosis to achieve the
purpose of killing tumor cells (5).
It is the first-line drug for clinical application. At present,
treatment for lung cancer is dominated by platinum-based
chemotherapy (such as cisplatin and carboplatin), which is
supplemented by other chemotherapeutic drugs (6). That lung cancer cells are resistant to
cisplatin is an important cause of chemotherapy failure, the
mechanism of which has not been fully elucidated yet.
Biological information data showed that miRNA
molecules control more than one third of human genes, which are
considered to be the most predominant factors of gene expression in
the eukaryotic genome (7). Abundant
evidence has shown that gene expression profile in different
tissues and at various differentiation stages is closely related to
tumor occurrence and development (8). It has been predicted that using miRNA
as target molecule for tumor biotherapy may be more effective than
coding gene as a target molecule (8). Therefore, treating cancer by
regulating miRNA expression may open up new roads for the targeted
therapy of lung cancer (7).
Abnormal miRNA expression may lead to the loss or
enhancement of miRNA function, thus affecting the expression levels
of the target protein (9).
Receptors affecting gene expression on drug uptake, metabolism and
distribution pathways, as well as targeting clinical function may
significantly affect the therapeutic effects of antitumor drugs
(10). It is of great significance
to elucidate the mechanism of action of miRNA in the metabolism of
antitumor drugs, so as to improve the efficacy and safety, to
reverse the drug resistance of the tumor and to guide the
personalized medication (11).
Studies have shown that the signal channels of Janus
kinase signal transducers 2 and activator of transcription 3
(JAK2/STAT3) can regulate the expression of pro-angiogenesis
factors, such as vascular endothelial growth factor (VEGF). In this
way, it can promote the formation of capillaries in lung cancer.
VEGF expression is closely related to the hypoxia inducible
factor-1α (HIF-1α) (12).
The PI3K/AKT pathway is a widely existing pathway
for signal transduction. It plays an important role in the cell
proliferation, cell apoptosis, as well as cell metabolism. In
recent years, it has been found that the PI3K/AKT pathway plays an
important role in the development and progression of tumors
(13). It mainly affects the energy
metabolism, growth as well as the proliferation of tumor cells,
inhibits apoptosis and affects invasion ability of tumor cells
(14). A variety of antitumor drugs
have been developed in clinical application with the key members
such as PI3K, AKT, mTOR, p70s6k as drug targets (13). The PI3K/AKT pathway also plays an
important role in the resistance of tumor cells to cisplatin. For
instance, the overexpression of AKT1 leads the resistance of lung
cancer cells to cisplatin. The inhibition of the AKT1 expression
can reverse the resistance of lung adenocarcinoma MDR cells
A549/CDDP to cisplatin (15).
Further studies have shown that AKT1-induced resistance of lung
cancer to cisplatin is mediated by the signal pathway mTOR-P70S6K1
(15). The present study
demonstrated anticancer activity and its mechanism of microRNA-133b
on cell proliferation of cisplatin-induced non-small cell lung
cancer cells.
Patients and methods
Patients
In the present study, NSCLC and para-carcinoma
tissues (>5 cm from cancer tissue) from patients (n=24) were
collected at operation. At every three months, we re-evaluated each
patient. All experimental procedures were performed in accordance
with the guidelines of Fourth Hospital of Hebei Medical University
(Hebei, China).
Cell culture and miRNA
transfection
A549/cisplatin cells were obtained from the Cell
Biology Tesearch Laboratory and Modern Analysis Testing Center of
Central South University (Changsha, China) and cultured in
RPMI-1640 medium (Gibco, Life Technologies, Grand Island, NY, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco, Grand Island,
NY, USA) with 2 mg/l cisplatin (Sigma, St. Louis, MO, USA) at 37°C
in a humidified atmosphere of 5% CO2. MicroRNA-133b and
negative control mimics were obtained synthesized by GenePharma
(Shanghai, China). A549/cisplatin was transfected using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA).
Cell viability assay
Cell proliferation was documented every 24 h after
transfection, and MTT solution was added into each well and
incubated at 37°C for 4 h. The purple colored precipitates of
formazan were dissolved in 200 µl dimethyl sulfoxide (DMSO) at 37°C
for 15 min. The absorbance was measured using an automatic
multi-well spectrophotometer (Bio-Rad, Richmond, CA, USA) at 490
nm.
Apoptosis assay
Cell proliferation was documented every 48 h after
transfection, and washed with phosphate-buffered saline (PBS). Cell
was stained with 10 µl Annexin V-FITC and 5 µl propidium iodide
(PI) apoptosis detection kit (BD Biosciences, San Jose, CA, USA)
for 15 min in the dark at 4°C. Analysis rate was carried out on
FACSCanto II (BD Biosciences).
Western blot analysis
The cells were harvested with ice-cold PBS. The
total protein was prepared using radioimmunoprecipitation assay
lysis buffer and determined using the Bradford protein assay kit
(Beyotime Institute of Biotechnology, Shanghai, China). Equal
quantities of protein (50 µg) were loaded onto an 8–10% SDS-PAGE
and transferred onto a polyvinylidene fluoride (PVDF) membrane
(Millipore, Billerica, MA, USA). The membranes were blocked in 5%
shimmed milk diluted with Tris-buffered saline with Tween-20 (TBST)
at 37°C for 1 h, and incubated overnight at 4°C with anti-EGFR,
anti-PI3K, anti-p-Akt, anti-p-JAK2, anti-p-STAT3, anti-cyclin D1,
anti-Bax and anti-GAPDH (all from Cell Signaling Technology, Inc.,
Danvers, MA, USA). The membranes were then incubated with a goat
anti-rabbit IgG-conjugated to horseradish peroxidase secondary
antibody (1:5,000; Cell Signaling Technology, Inc.) for 1 h at 37°C
and visualized using ECLplus reagents (Amersham Biosciences Corp.,
USA).
Statistical analysis
Data from each group are expressed as mean ±
standard error of the mean (SEM). Two-way ANOVA with Bonferroni
multiple comparison adjustments were used to assess differences
across the investigational groups. p<0.05 was considered to
indicate a statistically significant difference.
Results
Cisplatin-induced NSCLC microRNA-133b
expression
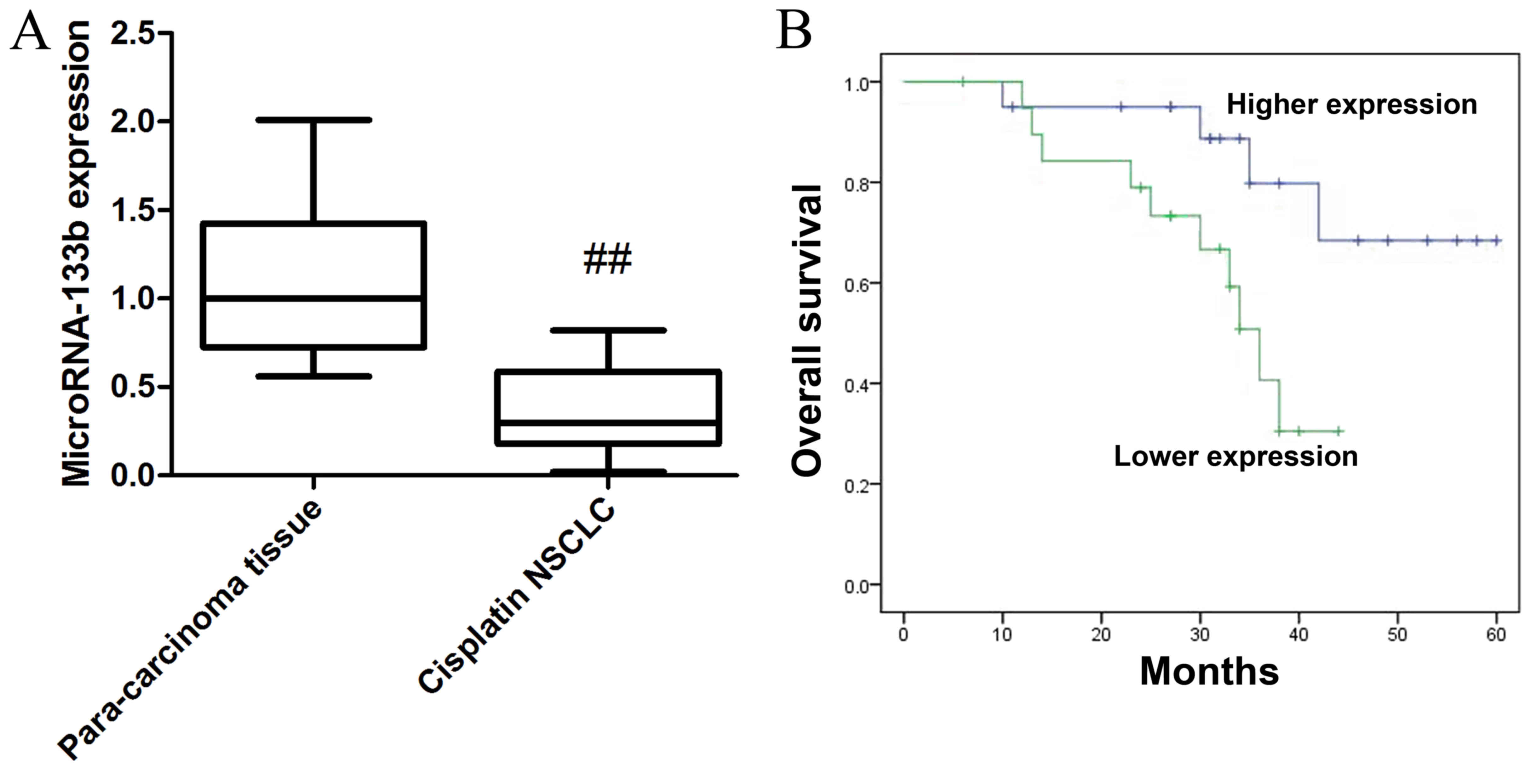
First the endogenous levels of microRNA-133b
expression in cisplatin-induced NSCLC were examined. Fig. 1 shows that the expression of
microRNA-133b cisplatin-induced non-small cell lung cancer (NSCLC)
tissue was lower than that of para-carcinoma tissue in patients.
Moreover, overall survival of higher expression in
cisplatin-induced NSCLC patients was higher than that of lower
expression in cisplatin-induced NSCLC patients.
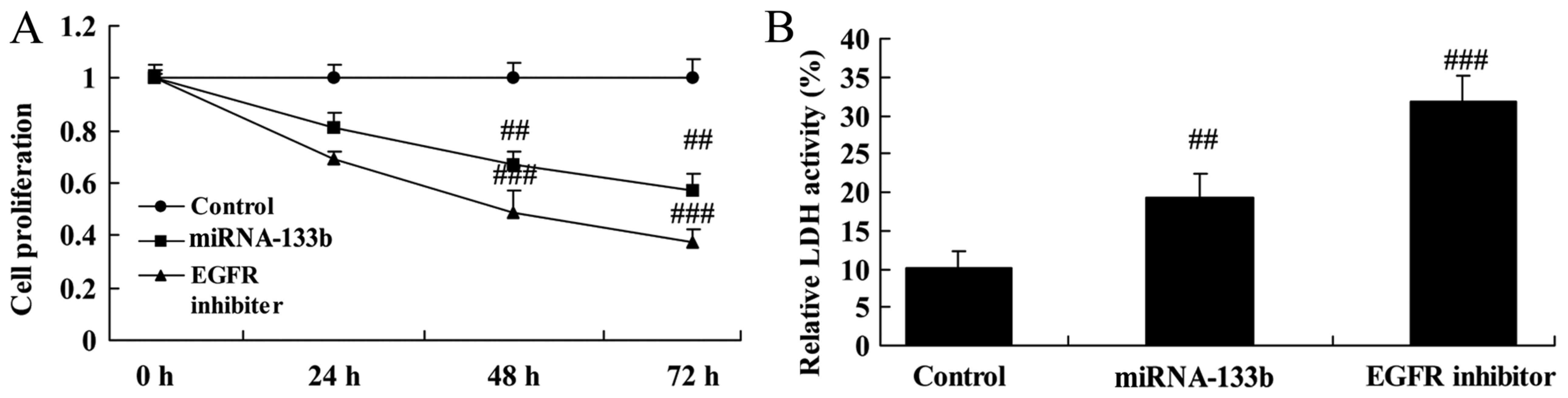
Over-regulation of microRNA-133b
inhibits cell proliferation and LDH activity in cisplatin-induced
NSCLC
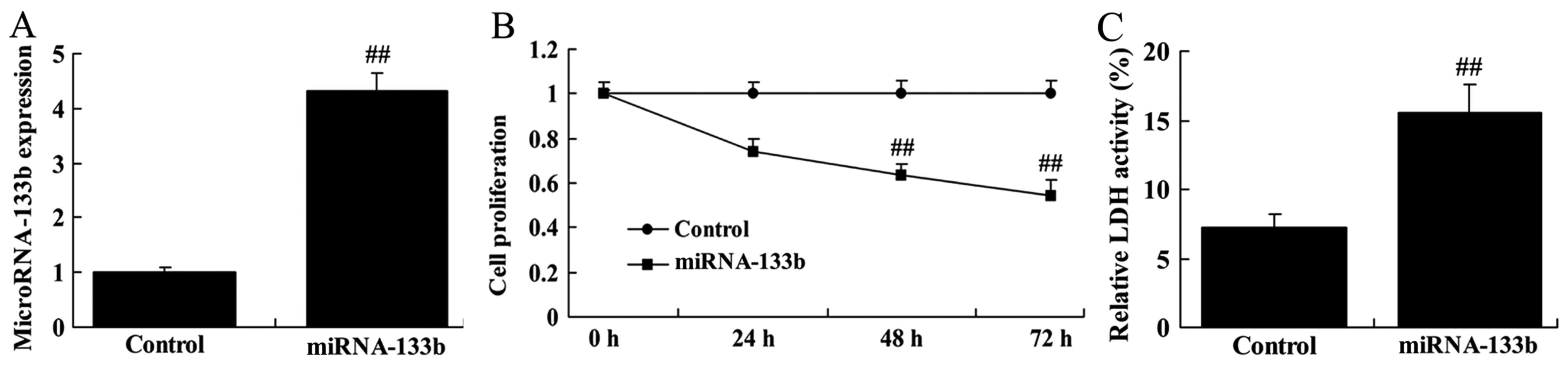
To investigate the roles of miRNA-133b in
cisplatin-induced NSCLC apoptosis, A549/cisplatin cells were
transfected with microRNA-133b mimics. As shown in Fig. 2, over-regulation of microRNA-133b
inhibited cell proliferation and increased LDH activity in
cisplatin-induced NSCLC.
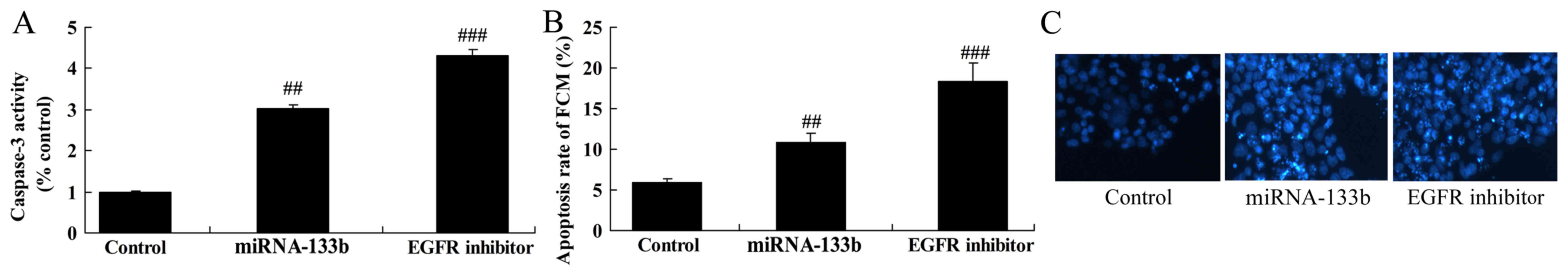
Over-regulation of microRNA-133b
induces apoptosis and caspase-3 activity in cisplatin-induced
NSCLC
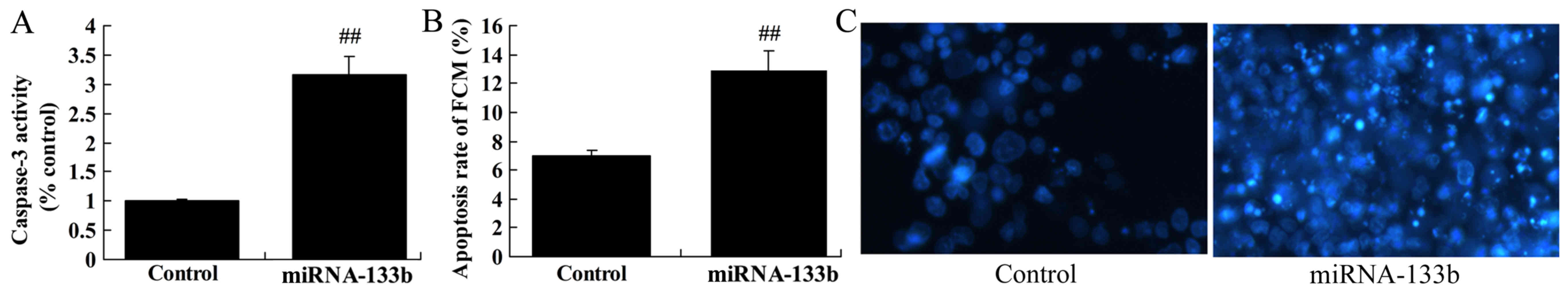
We measured apoptosis and caspase-3 activity in
cisplatin-induced NSCLC by microRNA-133b. Notably, the results from
the present study demonstrated over-regulation of
microRNA-133b-induced apoptosis and caspase-3 activity in
cisplatin-induced NSCLC (Fig.
3).
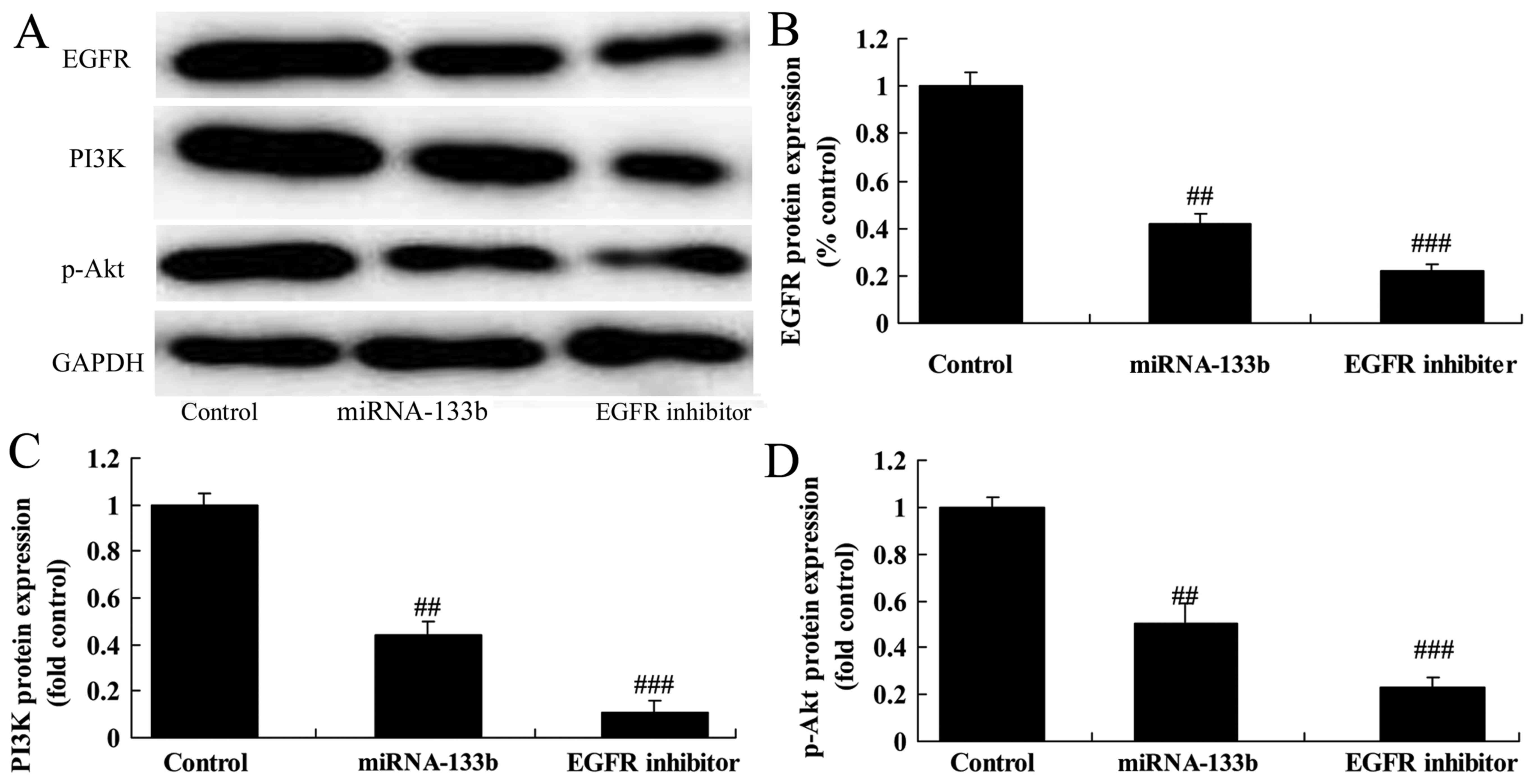
Over-regulation of microRNA-133b
suppresses EGFR, PI3K and p-Akt protein expression in
cisplatin-induced NSCLC
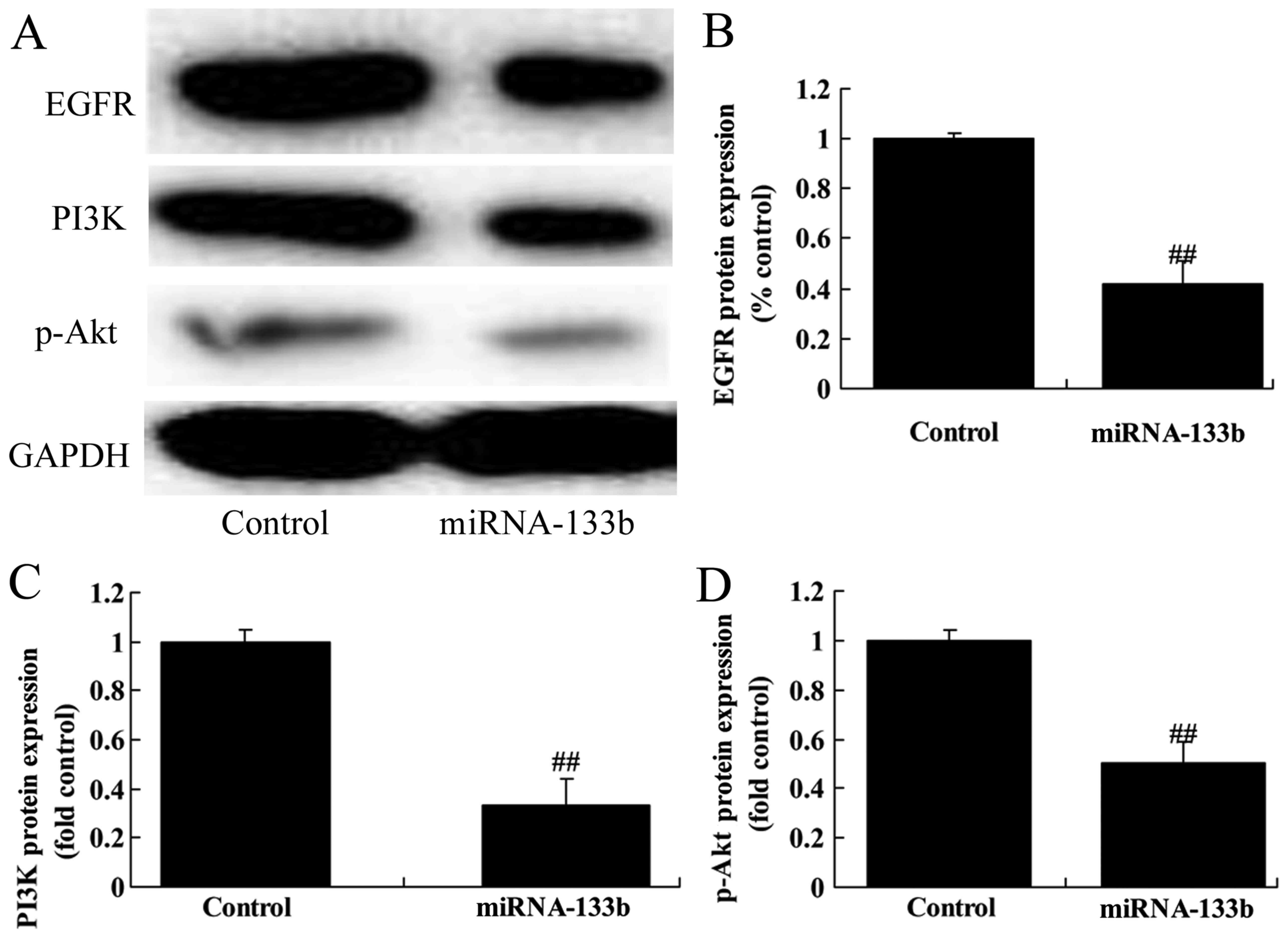
To investigate the mechanism of microRNA-133b on
cisplatin-induced NSCLC apoptosis, EGFR, PI3K and p-Akt protein
expression was measured using western blot analysis. The results of
western blot analysis showed that over-regulation of microRNA-133b
suppressed EGFR, PI3K and p-Akt protein expression in
cisplatin-induced NSCLC (Fig.
4).
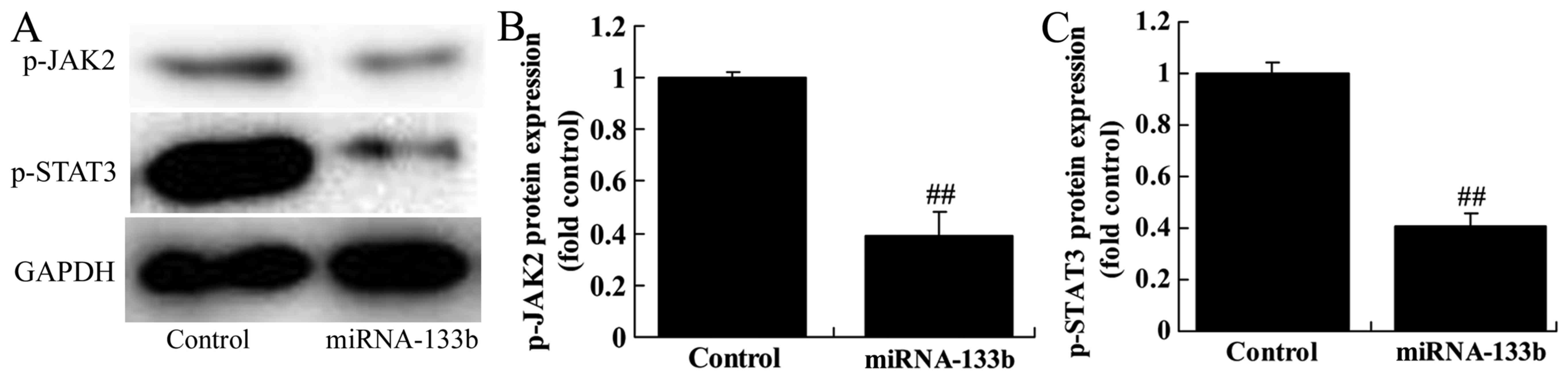
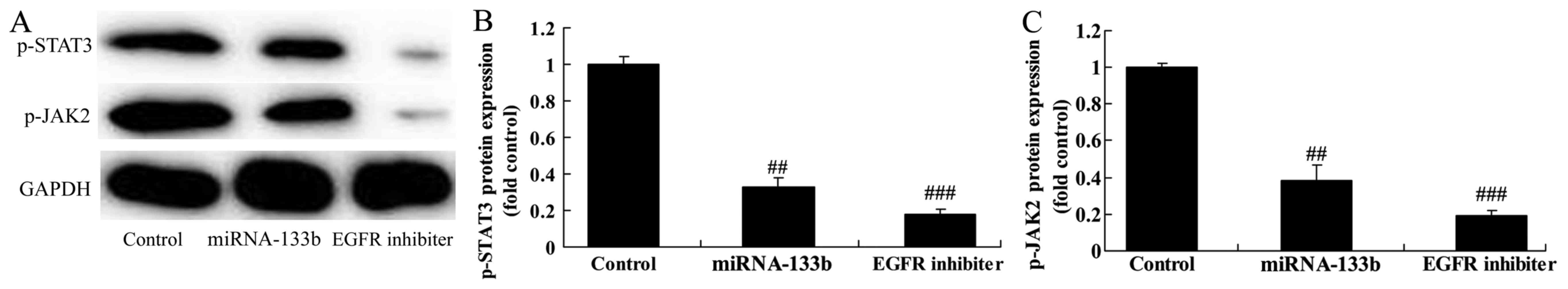
Over-regulation of microRNA-133b
suppresses p-JAK2 and p-STAT3 protein expression in
cisplatin-induced NSCLC
To verify the mechanism of microRNA-133b on
cisplatin-induced NSCLC apoptosis, JAK2/STAT3 signaling pathway was
selected and analyzed. As expected, p-JAK2 and p-STAT3 protein
expression in cisplatin induced NSCLC by microRNA-133b
over-regulation (Fig. 5).
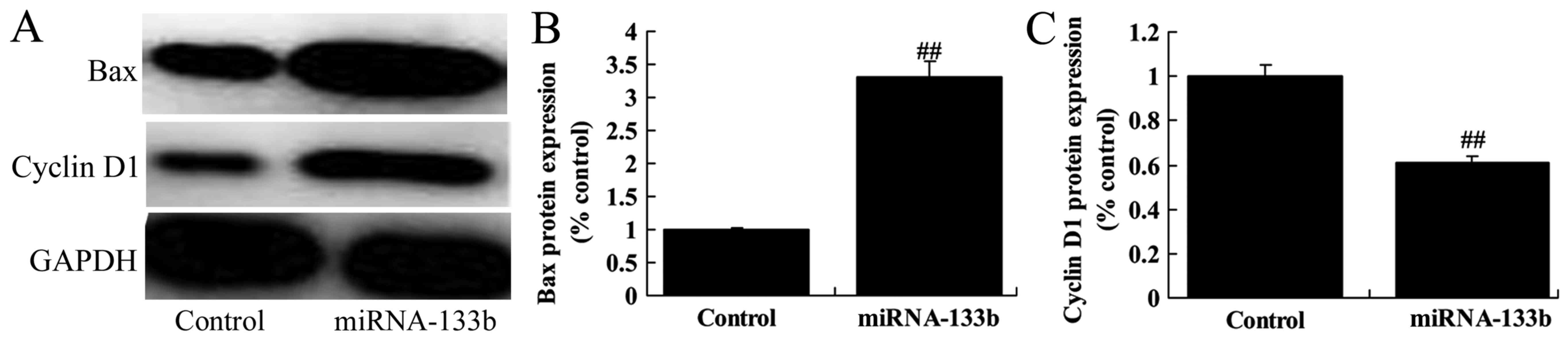
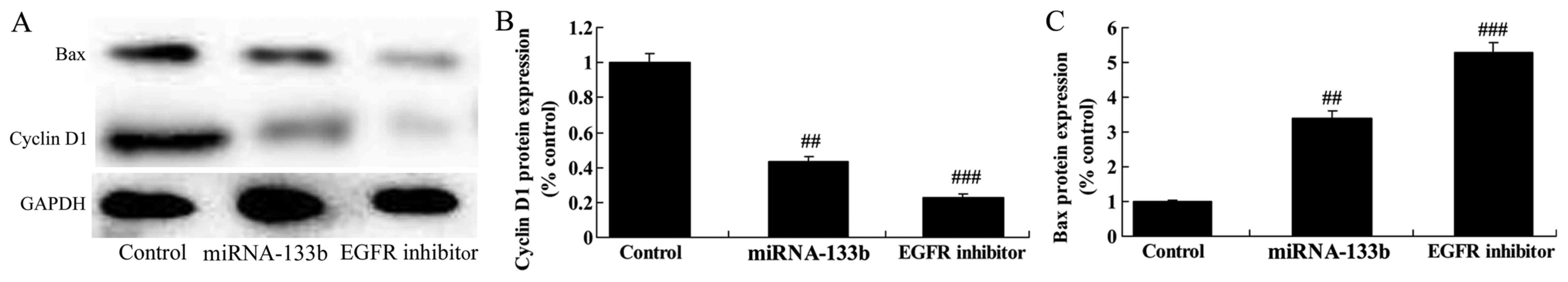
Over-regulation of microRNA-133b
suppressed cyclin D1 protein expression and induced Bax protein
expression in cisplatin-induced NSCLC
Next, we measured the function of microRNA-133b on
cisplatin-induced NSCLC apoptosis and cell cycle. As shown in
Fig. 6, over-regulation of
microRNA-133b suppressed cyclin D1 protein expression and induced
Bax protein expression in cisplatin-induced NSCLC, suggesting that
the upregulation of microRNA-133b on apoptosis and cell cycle of
cisplatin-induced NSCLC is subsequent to PI3K/Akt and JAK2/STAT3
signaling pathway by targeting EGFR.
The suppression of EGFR on cell
proliferation and LDH activity in cisplatin-induced NSCLC following
microRNA-133b
We investigated the role of EGFR in the function of
microRNA-133b on cell growth of cisplatin-induced NSCLC. As shown
in Fig. 7, the suppression of EGFR
(lapatinib, 5 nM) significantly inhibited cell proliferation and
increased LDH activity in cisplatin-induced NSCLC following
microRNA-133b, compared to that of microRNA-133b group.
The suppression of EGFR on apoptosis
and caspase-3 activity in cisplatin-induced NSCLC following
microRNA-133b
Then, the suppression of EGFR significantly also
induced apoptosis rate and caspase-3 activity in cisplatin-induced
NSCLC following microRNA-133b, compared to that of microRNA-133b
group (Fig. 8).
The suppression of EGFR on EGFR, PI3K
and p-Akt protein expression in cisplatin-induced NSCLC following
microRNA-133b
However, we measured EGFR, PI3K and p-Akt protein
expression using western blot analysis. As shown in Fig. 9, the suppression of EGFR
significantly also suppressed EGFR, PI3K and p-Akt protein
expression in cisplatin-induced NSCLC following microRNA-133b,
compared to that of microRNA-133b group (Fig. 9).
The suppression of EGFR on p-JAK2 and
p-STAT3 protein expression in cisplatin-induced NSCLC following
microRNA-133b
Our findings suggested that p-JAK2 and p-STAT3
protein expression in cisplatin-induced NSCLC following
microRNA-133b by EGFR suppression, compared to that of
microRNA-133b group (Fig. 10).
The suppression of EGFR on cyclin D1
and Bax protein expression in cisplatin-induced NSCLC following
microRNA-133b
Lastly, cyclin D1 protein expression was
significantly suppressed, and Bax protein expression significantly
induced in cisplatin-induced NSCLC following microRNA-133b by EGFR
suppression, compared to that of microRNA-133b group (Fig. 11).
Discussion
The resistance of lung cancer cells to chemotherapy
is one of the main reasons for the treatment failure in lung cancer
patients (3). Among the current
therapeutic schemes of lung cancer, cisplatin is the first-line
chemotherapeutic commonly used. Its main mechanism is to form
adducts with DNA, to inhibit cell transcription and translation,
and to promote apoptosis of tumor cells. In recent years, studies
have shown that lung cancer is susceptible to resistance to
cisplatin, leading to treatment failure (16). This is one of the hot spots of the
research on lung cancer, the main mechanism of which has not been
fully elucidated, yet (17). In the
present study, we explored the expression of microRNA-133b
cisplatin-induced non-small cell lung cancer (NSCLC) tissue and
para-carcinoma tissue in patients. Notably, overall survival of
higher expression in cisplatin-induced NSCLC patients was higher
than that of lower expression in cisplatin-induced NSCLC
patients.
In 1993, the first miRNA (miRNA-line4) was found in
the Caenorhabditis elegans, which was confirmed to play a
role in regulating the timing of cell development (18). Later, the researchers continued to
find the corresponding miRNA from fruit flies, human beings, plants
and other eukaryotes (9). Growing
evidence has suggested that they are mediators of the genetic
expression, modification, transcription as well as translation
(9). It was found that the miRNA
polymorphism, abnormal miRNA expression, as well as receptors
affecting gene expression on drug uptake, metabolism and
distribution pathways, as well as targeting clinical function may
significantly affect the therapeutic effects of antitumor drugs.
This may lead to the sustaining drug resistance of the tumor
(9). Our results confirmed that
overregulation of microRNA-133b inhibited cell proliferation and
LDH activity, induced apoptosis and caspase-3 activity, decreased
cyclin D1 and increased Bax protein expression in cisplatin-induced
A549 cells. Our results suggested that microRNA-133b played
critical roles in regulation of proliferation of NSCLC and may be
potential diagnostic and predictive biomarkers. Chen et al
showed that microRNA-133b may be used as valuable prognostic
biomarker for colorectal cancer (19). In the present study, we only used
cisplatin-induced A549 cells, which is a limitation in the present
study. We may use more cisplatin-induced NSCLC cell lines in
further study.
STAT3 is involved in the proliferation,
angiogenesis, migration, invasion and transformation of tumor cells
(12). STAT3 is an effective target
site to inhibit VEGF expression and tumor angiogenesis (20). It was found that there is a site for
binding to STAT3 protein on the VEGF promoter (21). The activation of STAT3 protein
allows VEGF to promote migration and angiogenesis of vascular
endothelial cells. It shows that the STAT3 protein is at the core
position in tumor angiogenesis (22). In the present study, we found that
over-regulation of microRNA-133b suppressed EGFR, p-JAK2 and
p-STAT3 protein expression in cisplatin-induced A549 cells. Zhou
et al demonstrated that microRNA-133b induces apoptosis of
human renal carcinoma cells via the JAK2/STAT3 signaling pathway
(23). Our findings revealed that
microRNA-133b may be associated with EGFR/JAK2/STAT3 signaling
pathway downstream in cisplatin-induced A549 cells.
The main members of the PI3K/AKT pathway are PI3K,
AKT, mTOR, p70S6K1 and PTEN. PI3K is activated by a variety of
factors, including the insulin-like growth factor (IGF-I). Later,
PIP2 is transformed into PIP3, which induces the translocation of
AKT into the cytoplasm. P-AKT is an important phosphorylase in
vivo that is known as phosphokinase B (PKB) (24). There are many downstream active
substrates (24). Phosphorylation
can directly or indirectly affect (activate or inhibit) downstream
mTOR as well as Bcl-2, thus playing extensive and complex
physiological effects. It is believed that the PI3K/AKT/mTOR
pathway is highly expressed in many tumor tissues (25). Moreover, many gene mutations can be
seen, which leads to the enhancement or abnormality. Thus, this may
affect the regulation of the proliferation, apoptosis as well as
invasion of tumor cells and other important physiological
activities (26). To be specific,
the upregulation of PI3K/AKT/mTOR pathway can promote the
proliferation of tumor cells, induce the invasiveness and inhibit
apoptosis of tumor cells, and promote tumor growth (27). Therefore, a variety of PI3K/AKT/mTOR
pathway inhibitors have been developed. Some of them affects
inhibition of tumor growth improving the 5-year survival rate in
animal and clinical experiments. This belongs to the new field of
tumor treatment (28). Our results
indicated that over-regulation of microRNA-133b suppressed PI3K and
p-AKT protein expression in cisplatin-induced A549 cells, which may
also clarify the molecular mechanisms of microRNA-133b on
suppression of NSCLC proliferation. Liu et al showed that
microRNA-133b inhibits proliferation of ovarian cancer cells via
Akt by EGFR (21). However, the
limitation of the study is not to sufficiently demonstrate that
microRNA-133b regulates EGFR, and we may use luciferase assay to
analyze the binding site of miR-133b in EGFR-3′UTR in further
study.
In conclusion, to the best of our knowledge, this is
the first study to demonstrate that expression of microRNA-133b
cisplatin-induced NSCLC tissue was lower than that of
para-carcinoma tissue in patients, and made a positive impact on
overall survival of cisplatin-induced NSCLC patients. Consequently,
over-regulation of microRNA-133b inhibits cell proliferation of
cisplatin-induced NSCLC by PI3K/Akt and JAK2/STAT3 signaling
pathway by targeting EGFR. These findings showed that microRNA-133b
may be exploited further for treatment of cisplatin-induced
NSCLC.
References
|
1
|
Levallet G, Bergot E, Antoine M, Creveuil
C, Santos AO, Beau-Faller M, de Fraipont F, Brambilla E, Levallet
J, Morin F, et al Intergroupe Francophone de Cancérologie
Thoracique (IFCT), : High TUBB3 expression, an independent
prognostic marker in patients with early non-small cell lung cancer
treated by preoperative chemotherapy, is regulated by K-Ras
signaling pathway. Mol Cancer Ther. 11:1203–1213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shao M, Jin B, Niu Y, Ye J, Lu D and Han
B: Association of POLK polymorphisms with platinum-based
chemotherapy response and severe toxicity in non-small cell lung
cancer patients. Cell Biochem Biophys. 70:1227–1237. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirsch FR, Herbst RS, Olsen C, Chansky K,
Crowley J, Kelly K, Franklin WA, Bunn PA Jr, Varella-Garcia M and
Gandara DR: Increased EGFR gene copy number detected by fluorescent
in situ hybridization predicts outcome in non-small-cell lung
cancer patients treated with cetuximab and chemotherapy. J Clin
Oncol. 26:3351–3357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Z, Xiao Y, Zhao J, Chen M, Xu Y,
Zhong W, Xing J and Wang M: Relationship between circulating tumour
cell count and prognosis following chemotherapy in patients with
advanced non-small-cell lung cancer. Respirology. 21:519–525. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsumoto M, Nakajima W, Seike M, Gemma A
and Tanaka N: Cisplatin-induced apoptosis in non-small-cell lung
cancer cells is dependent on Bax- and Bak-induction pathway and
synergistically activated by BH3-mimetic ABT-263 in p53 wild-type
and mutant cells. Biochem Biophys Res Commun. 473:490–496. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong Z, Zhong Z, Yang L, Wang S and Gong
Z: MicroRNA-31 inhibits cisplatin-induced apoptosis in non-small
cell lung cancer cells by regulating the drug transporter ABCB9.
Cancer Lett. 343:249–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishijima N, Seike M, Soeno C, Chiba M,
Miyanaga A, Noro R, Sugano T, Matsumoto M, Kubota K and Gemma A:
miR-200/ZEB axis regulates sensitivity to nintedanib in non-small
cell lung cancer cells. Int J Oncol. 48:937–944. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cui J, Mo J, Luo M, Yu Q, Zhou S, Li T,
Zhang Y and Luo W: c-Myc-activated long non-coding RNA H19
downregulates miR-107 and promotes cell cycle progression of
non-small cell lung cancer. Int J Clin Exp Pathol. 8:12400–12409.
2015.PubMed/NCBI
|
|
9
|
Yi Y, Lu X, Chen J, Jiao C, Zhong J, Song
Z, Yu X and Lin B: Downregulated miR-486-5p acts as a tumor
suppressor in esophageal squamous cell carcinoma. Exp Ther Med.
12:3411–3416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo J, Li H and Zhang C: MicroRNA-7
inhibits the malignant phenotypes of non-small cell lung cancer in
vitro by targeting Pax6. Mol Med Rep. 12:5443–5448. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Qian J, Qiang Y, Huang H, Wang C,
Li D and Xu B: Down-regulation of miR-4500 promoted non-small cell
lung cancer growth. Cell Physiol Biochem. 34:1166–1174. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao M, Gao FH, Wang JY, Liu F, Yuan HH,
Zhang WY and Jiang B: JAK2/STAT3 signaling pathway activation
mediates tumor angiogenesis by upregulation of VEGF and bFGF in
non-small-cell lung cancer. Lung Cancer. 73:366–374. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guerriero I, D'Angelo D, Pallante P,
Santos M, Scrima M, Malanga D, De Marco C, Ravo M, Weisz A,
Laudanna C, et al: Analysis of miRNA profiles identified miR-196a
as a crucial mediator of aberrant PI3K/AKT signaling in lung cancer
cells. Oncotarget. 8:19172–19191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang J, Feng X, Zhou W, Wu Y and Yang Y:
MiR-128 reverses the gefitinib resistance of the lung cancer stem
cells by inhibiting the c-met/PI3K/AKT pathway. Oncotarget.
7:73188–73199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang Y, Zhang C, Wu T, Wang Q, Liu J and
Dai P: Transcriptome sequencing reveals key pathways and genes
associated with cisplatin resistance in lung adenocarcinoma A549
Cells. PLoS One. 12:e01706092017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Checinska A, Hoogeland BS, Rodriguez JA,
Giaccone G and Kruyt FA: Role of XIAP in inhibiting
cisplatin-induced caspase activation in non-small cell lung cancer
cells: A small molecule Smac mimic sensitizes for
chemotherapy-induced apoptosis by enhancing caspase-3 activation.
Exp Cell Res. 313:1215–1224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ueda K, Kawashima H, Ohtani S, Deng WG,
Ravoori M, Bankson J, Gao B, Girard L, Minna JD, Roth JA, et al:
The 3p21.3 tumor suppressor NPRL2 plays an important role in
cisplatin-induced resistance in human non-small-cell lung cancer
cells. Cancer Res. 66:9682–9690. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang C, Chen YX, Wu NY, Yin JY, Li XP,
Huang HS, Zhang W, Zhou HH and Liu ZQ: MiR-488 inhibits
proliferation and cisplatin sensibility in non-small-cell lung
cancer (NSCLC) cells by activating the eIF3a-mediated NER signaling
pathway. Sci Rep. 7:403842017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Zhang Y, He J, Fu Y, Lin C and Li
X: MicroRNA-133b is regulated by TAp63 while no gene mutation is
present in colorectal cancer. Oncol Rep. 37:1646–1652. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Lan T, Zhang W, Dong L, Kang N,
Zhang S, Fu M, Liu B, Liu K and Zhan Q: Feed-forward reciprocal
activation of PAFR and STAT3 regulates epithelial-mesenchymal
transition in non-small cell lung cancer. Cancer Res. 75:4198–4210.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao D, Pan C, Sun J, Gilbert C,
Drews-Elger K, Azzam DJ, Picon-Ruiz M, Kim M, Ullmer W, El-Ashry D,
et al: VEGF drives cancer-initiating stem cells through
VEGFR-2/Stat3 signaling to upregulate Myc and Sox2. Oncogene.
34:3107–3119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Y, Hong Y, Xu Y, Liu P, Guo DH and Chen
Y: Inhibition of the JAK/STAT pathway with ruxolitinib overcomes
cisplatin resistance in non-small-cell lung cancer NSCLC.
Apoptosis. 19:1627–1636. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou W, Bi X, Gao G and Sun L: miRNA-133b
and miRNA-135a induce apoptosis via the JAK2/STAT3 signaling
pathway in human renal carcinoma cells. Biomed Pharmacother.
84:722–729. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Q, Yue J, Zhang C, Gu X, Chen H and
Xu L: Inactivation of M2 AChR/NF-κB signaling axis reverses
epithelial-mesenchymal transition (EMT) and suppresses migration
and invasion in non-small cell lung cancer (NSCLC). Oncotarget.
6:29335–29346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han J, Zhao F, Zhang J, Zhu H, Ma H, Li X,
Peng L, Sun J and Chen Z: miR-223 reverses the resistance of
EGFR-TKIs through IGF1R/PI3K/Akt signaling pathway. Int J Oncol.
48:1855–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu Z, Liu D, Fan C, Luan L, Zhang X and
Wang E: DIXDC1 increases the invasion and migration ability of
non-small-cell lung cancer cells via the PI3K-AKT/AP-1 pathway. Mol
Carcinog. 53:917–925. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sin TK, Wang F, Meng F, Wong SC, Cho WC,
Siu PM, Chan LW and Yung BY: Implications of microRNAs in the
treatment of gefitinib-resistant non-small cell lung cancer. Int J
Mol Sci. 17:2372016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu Q, Liang X, Dai J and Guan X:
Prostaglandin transporter, SLCO2A1, mediates the invasion and
apoptosis of lung cancer cells via PI3K/AKT/mTOR pathway. Int J
Clin Exp Pathol. 8:9175–9181. 2015.PubMed/NCBI
|